Abstract
Preventing the contamination of processed cells is required for achieving reproducible manufacturing. A droplet is one of the potential causes contamination in cell manufacturing. The present study elucidates the formation mechanism and characteristics of droplets based on the observation and detection of droplets on the base surface of the biological safety cabinet (BSC) where cell processing is conducted under unidirectional airflow. Pouring fluorescent solution into the vessel using a measuring pipette was conducted to visualize the formation of droplets by videos as well as visual detection by blacklight irradiation on the base surface of the BSC. The experiments revealed that airborne and non-airborne droplets emerged from bursting bubbles, which formed when the entire solution was pushed out of the measuring pipette. Therefore, the improving procedure of pouring technique when entire solution was not pushed out of the pipette realized no formation of the droplets due to the prevention of emergence of bubble. In addition, an alternative procedure in which the entire solution was poured into the deep point of the test tube prevented the flying of non-airborne droplets outside the tube, while airborne droplets that escaped the tube rode the airflow of BSC. These results suggested a method for the prevention of the droplet formation, as well as the deposit control of droplets onto the surface in BSC, leading to cleanup area in the BSC for changeover with environment continuity.
Keywords: Changeover, Airborne and non-airborne droplets, Pouring the solution, Bursting bubble
Abbreviations: BSC, biological safety cabinet; CPA, cell processing area
Highlights
-
•
The droplets were formed by bursting bubbles while pouring the solution.
-
•
Improved procedure for the prevention of emergence of droplets was proposed.
-
•
Classification of droplets proposes the procedure for cleanup the BSC.
-
•
Changeover was categorized based on the status for environment continuity.
1. Introduction
Cell processing is one of the most critical steps in manufacturing processed cells. The development of a stable manufacturing system is required in order to maintain the cleanliness in an aseptic environment during processing, resulting in the prevention of microbial and non-microbial contaminations to achieve reproducible manufacturing [1], [2], [3], [4], [5]. To prevent the extrinsic contamination from the outside of cell processing area, clean air is supplied through high efficiency particulate air (HEPA) filter and, physical and air barriers are constructed [6], [7]. In addition, management of the remaining contaminants such as liquid droplets and particle which formed inside of cell processing area is required for maintaining the cleanliness because most of the cell processing have the process which contains dealing with the solution such as culture media and buffers.
The contamination during cell processing using the biological safety cabinet (BSC) is categorized into four types: i) the use of nonsterile materials for starter cells in the cell processing area (CPA) in the BSC [6], [7]; ii) introduction into the CPA of vessels and hands that contaminants can be adhered outside the BSC [5]; iii) operations that cross the boundary between the inside and outside of the BSC [8]; and iv) setup of the CPA after operation [9], [10], [11]. Understanding the sources of pollution and their routes is an important activity [4], [12], and cell processing based on proper hygiene and manufacturing managements are required to prevent contamination.
In addition, the practical management of small-batch-size cell manufacturing is required [3], [13], which enables multipurpose manufacturing using a single BSC with frequent changeovers between cell manufacturing processes. This has raised concerns about cross-contamination due to insufficient cleanup after a given process. In order to start manufacturing processed cells, reprocessing, which was defined as the all steps that are necessary to make the equipment ready for its intended use in the field of medical device, was required for hygiene management [14]. During manufacturing of processed cells, maintaining the cleanliness of the CPA is essential to prevent contamination based on the manufacturing management. In particular, the liquid droplets formed during the process are one of the potential causes of contamination in later processes through the transferring of droplets by the stamping and release. Therefore, understanding the formation and behavior of droplets is necessary for developing a process to prevent contamination in cell manufacturing.
The present study examines the formation and behavior of droplets through observations and detection on the base surface of the BSC. Based on the categorization of changeover and understanding the process of formation and characteristics of the droplets, it was suggested the operation to maintain cleanliness in the CPA by pouring the solution and consideration of cleanup for changeover with environment continuity during cell manufacturing to reduce the contamination risks.
2. Materials and methods
2.1. Observation of droplet formation
Observation of droplets formed while pouring the solution was carried out by obtaining images with 29.97 frames per second using a video camera (Eye Scope; Shin Nippon Air Technologies, Tokyo, Japan) with the visualization of the particle by light emitting diode (Parallel Eye D, Shin Nippon Air Technologies). Videos were made from the obtained images using a software (Particle Eye, Shin Nippon Air Technologies).
2.2. Detection of droplets on base surface of the cell processing area
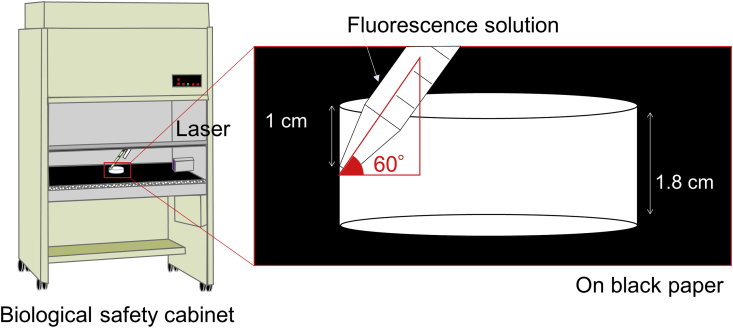
A culture dish (100 mm dish; Sumitomo Bakelite, Tokyo, Japan) or centrifuge tube (50 ml tube, Sumitomo Bakelite) was placed on the surface of the workbench in a BSC (MHE-131AJ; Panasonic Healthcare, Tokyo, Japan) covered with black paper. A fluorescent solution (Supergrow DF-300 LIQUID; Marktech, Tokyo, Japan) was poured into the culture dish using the measuring pipettes (10 ml disposable pipette; Sumitomo Bakelite), as shown in Fig. 1. The solution was poured at a height of 1.0 or 9.0 cm from the top of the dish or tube. The pouring rate and angle were 5.4 cm/s and 60°, respectively. With regard to the given quantity poured, either the entire solution or just a portion of the solution was poured. After the solution was poured 10 times, UV-light (365 nm UV-LED light, Tokyo, Japan) was used to observe the attached droplets.
Fig. 1.
Schematic illustration of experimental design.
Schematic illustration of the experimental design to confirm the attached droplets on the base surface of a biological safety cabinet.
3. Results
3.1. Characteristics of airborne and non-airborne droplets formed while pouring the solution
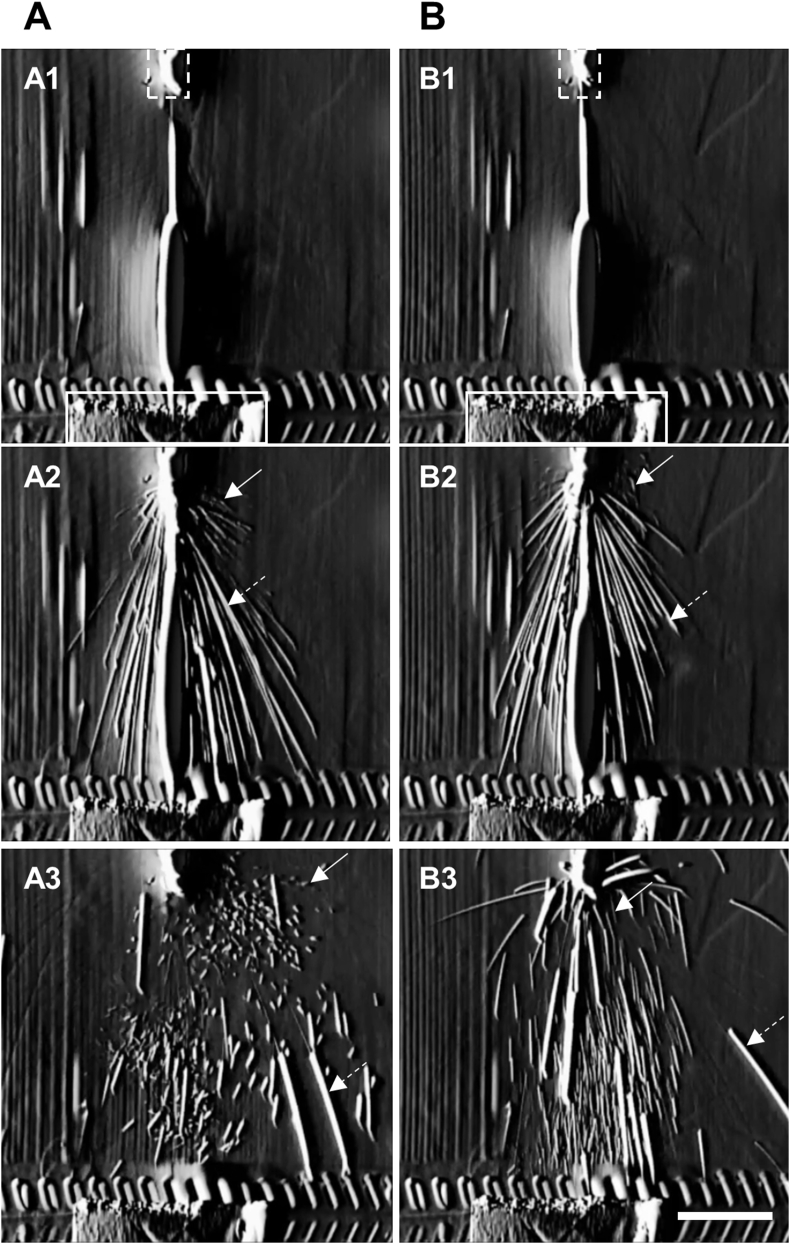
To understand the characteristics of the droplets that formed while pushing out the entire solution with turning off and on the airflow in BSC, we observed the process of pouring the solution by visualization of the particle. The observations revealed that the droplets did not form (Fig. 2A1 and B1, and Video S1). However, the droplets formed by bursting bubble that splashed in all directions when the airflow in the BSC was turned off and on (Fig. 2A2 and 2B2). When the airflow in the BSC was turned off, the droplets changed directions irregularly and others settled in a parabolic shape, indicating airborne and non-airborne droplets, respectively (Fig. 2A3, and Video S1). On the other hand, when the airflow in the BSC was turned on, airborne droplets were formed and rapidly changed direction by moving toward the base surface, while non-airborne droplets settled in a parabolic shape, similar to the case when airflow was turned off (Fig. 2B3).
Fig. 2.
Observations of droplet formation.
Still images of the observations of droplets during pouring of the solution with the airflow turned off (A) and airflow turned on (B) in the biological safety cabinet. Scale bars: 50 μm. Closed and dot squares indicated the tips of pipet and culture dish, respectively. Solid and dotted arrows indicated the typical airborne and non-airborne droplets, respectively.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.reth.2019.04.002.
The following is the supplementary data related to this article:
The observations of the droplets during pouring of the solution with the airflow turned off (A) and on (B) in the biological safety cabinet.
3.2. Inhibition of droplet formation due to pouring
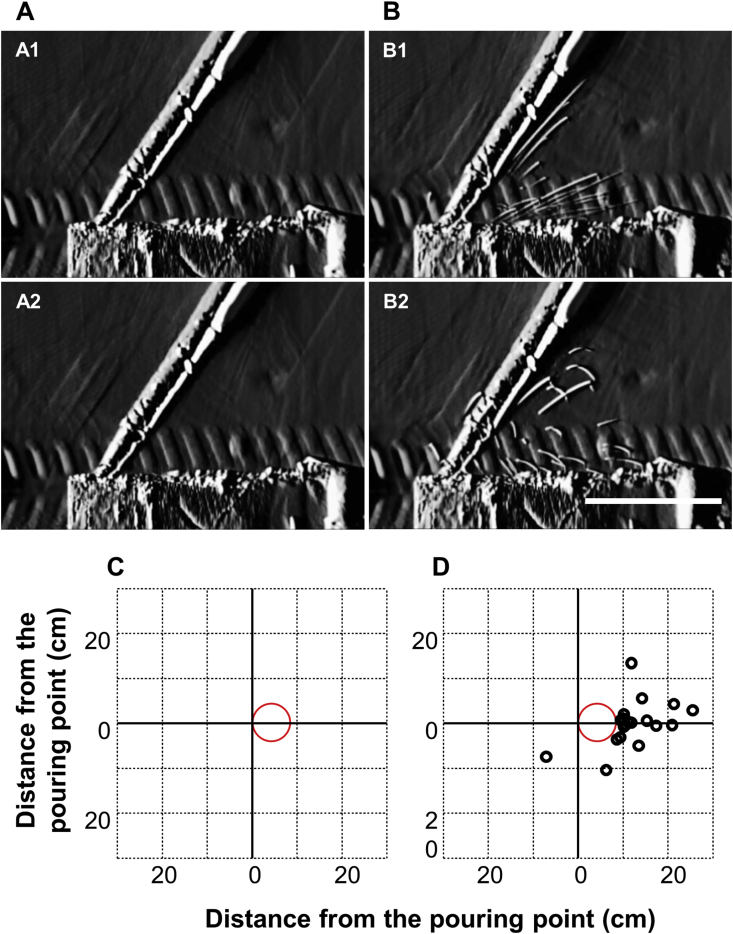
To confirm the effect of pouring on droplet formation, observations were made for the cases without or with pushing out the entire solution at the walls of dish with the visualization of the particle. The observations revealed that the droplets were not formed without pushing entire solution (Fig. 3A and Video S2). When droplets formed, these splashed in the direction opposite from the direction of pouring while pushing out the entire solution (Fig. 3B). The airborne droplets rapidly changed direction by moving downward, while the non-airborne droplets settled in a parabolic shape (Fig. 3B).
Fig. 3.
Effect of pushing out on droplet formation.
Still images of the observations of droplets without (A) and with (B) pushing out the entire solution. Scale bars: 50 μm. Detection of the droplets on the surface of the workbench in the biological safety cabinet without (C) and with (D) pushing out the entire solution. Red circles indicate the points where the solution was poured.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.reth.2019.04.002.
The following is the supplementary data related to this article:
The observations of the droplets while pouring a portion of the solution (A) and the entire solution (B)
To understand the effects of pouring on the attachment of droplets to the base surface of the BSC, the droplets that attached to the paper were observed with and without pushing out the entire solution. The droplets attached on the paper without pushing out were not detected (Fig. 3C), while those during pushing out the entire solution were distributed in the direction opposite the pouring direction (Fig. 3D).
3.3. Inhibition of attachment of non-airborne droplets onto base surface of CPA
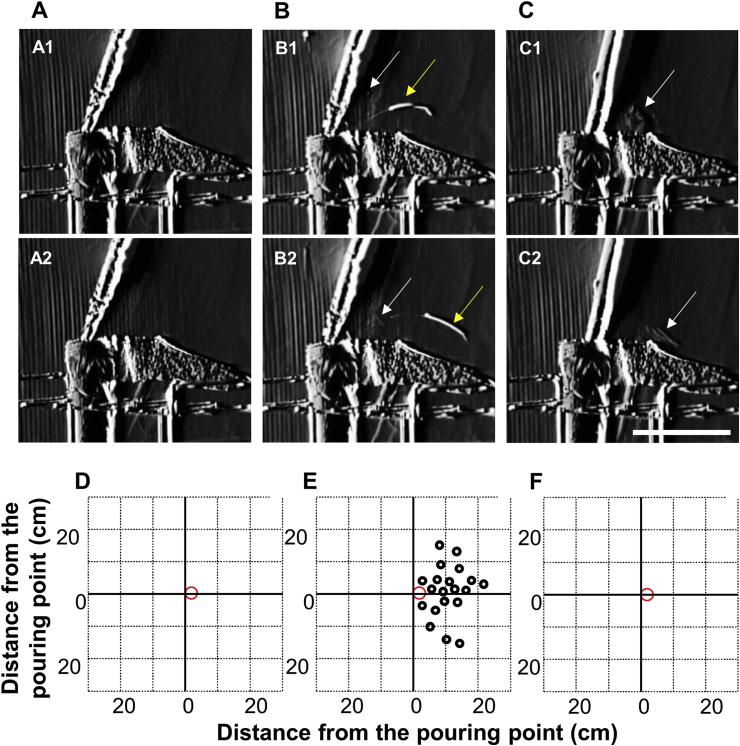
To understand the effect of the wall on attachment of non-airborne droplets onto the base surface of BSC, observations were carried out with and without completely pouring the entire solution at the shallow and deep points of the tube by visualization of the particle. When the solution was poured at the shallow point of the tube, the observations revealed that droplets did not form while pushing out the entire solution, similar to that observed with the dish (Fig. 4A and Video S3). On the other hand, droplets formed and splashed in the direction opposite the pouring direction when pushing out the entire solution (Fig. 4B). The airborne droplets splashed and changed direction by moving toward the base surface, while the non-airborne droplets settled in a parabolic shape. However, when pouring the solution at the deep point in the tube, the observations revealed that non-airborne droplets were not observed outside the tube, while the airborne droplets rose from the top of tube and changed direction by moving toward the bottom (Fig. 4C).
Fig. 4.
Effect of wall on droplet behaviors.
Still images of the observations of the droplets during pouring of the solution without (A) and with (B) pushing out the entire solution at the shallow point, and completely pouring the solution at deep point in the tube (C). White and yellow arrows indicate the typical airborne and non-airborne droplets, respectively. Scale bars: 50 μm. Detection of the droplets on the base surface of the biological safety cabinet without (D) and with (E) pushing out the entire solution at the shallow point, and pushing out the entire solution at the deep point in the tube (F). Red circles indicate the points where the solution was poured.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.reth.2019.04.002.
The following is the supplementary data related to this article:
The observations of the droplets while pouring a portion of the solution (A) and entire solution (B) at shallow point, and pouring the entire solution at deep point of the tube (C)
The attachment of droplets to the paper was also determined in cases where the entire solution was and was not pushed out at the shallow and deep points of the tube. In the case of pouring the solution at the shallow point, the droplets attached to the paper without pushing out were not detected (Fig. 4D), while those in pushing out the entire solution were distributed in the direction opposite to the pouring (Fig. 4E). In the case of pouring the solution at the deep point, the droplets attached to the paper while pushing out the entire solution were not detected (Fig. 4F).
4. Discussion
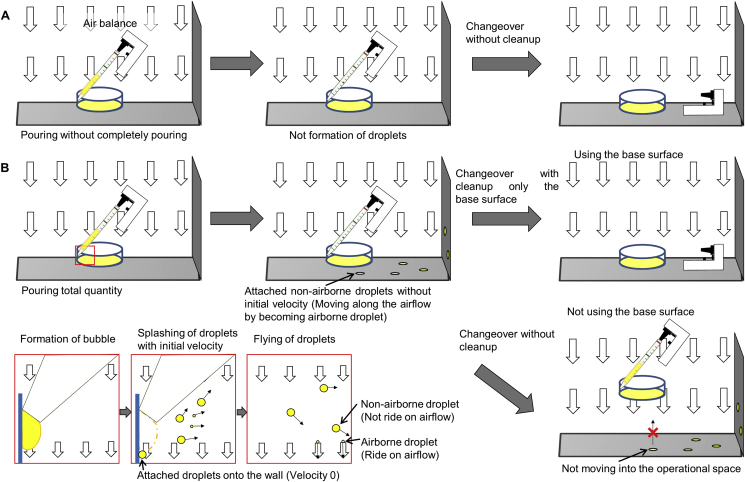
The prevention of droplet formation is a critical issue in developing manufacturing processes for aseptic environments to maintain the cleanliness of the CPA. It had been reported that droplets were formed by bursting bubbles caused by the thinning of the liquid film owing to air ejection [15], [16], [17], [18], [19]. In the present study, the droplet formation mechanism when measuring the volume of a solution using a pipette was clarified to prevent the formation as follows (Fig. 5): i) bubble formation - at the end of pouring the solution, the air in the pipette is blown out creating a single bubble at the tip of pipette; ii) droplet formation - overexpansion of the bubble causes it to burst and form droplets; iii) flying - the emerged droplets have the initial velocity, and are categorize into droplets which ride on the airflow or not, defining the airborne and non-airborne droplets, respectively. This mechanism leads to proposals of preventing random droplet dispersion onto the base surface of the BSC. The first step in preventing bubble formation is to cease pushing out the entire solution from the pipette (Fig. 2). The second step is to set the location for bubble blustering in the deeper part of vessel to prevent the dispersion of non-airborne droplets on the outside of the vessel (Fig. 3). The third step is to ensure airborne droplets that leak from the vessel go toward the leeward side of unidirectional airflow in BSC and land on a limited surface area or are blown out through the filter (Fig. 3).
Fig. 5.
Schematic illustration of changeover with environment continuity.
Schematic illustration of changeover based on the treatment of droplets with pouring method and classification. Changeover with environment continuity by inhibition of droplet formation (A). Changeover with environment continuity after treatment using the base surface according to the classification of the droplets (B).
Understanding the behavior of droplets formed during the operation is important for establishing cleanup methods. Airborne droplets created inside the CPA in the BSC rode the unidirectional airflow [8]. The droplets formed during the operation traveled according to the initial velocity, gravity, and airflow due to the size of droplets [15], [20], [21]. The non-airborne droplets settled on the surface inside the BSC regardless of airflow. In the field of infectious disease transmission, airborne, contact, and droplet routes are classified according to the characteristics of the droplets transmitted by airflow or direct contact [21]. Airborne and droplet transmissions result from the inhalation of droplet nuclei (≤5 μm in diameter) propelled at long distances, and droplets (>5 μm in diameter) propelled at short distances from an infectious source, respectively.
In general manufacturing, the changeover is referred to the operation required to switch from the process of a given product to another one, and consists of cleanup and setup operations [22], [23]. In the case of cell production, long-term cell manufacturing with variety of intermittent processing is required. To enhace the occupancy of the CPA, the parallel manufacturing of several processed cells in the same CPA can be performed with prompt changeover.
Changeover was categorized into four types according to the status of cell processing area and its surrounding environment as follows (Table 1): i) Changeover with environment continuity; ii) Changeover with environment continuity after treatment in cell processing area; iii) Changeover with environment continuity after treatment in both cell processing area and its surrounding environment; iv) Changeover after reprocessing. An adequate procedure in the CPA for the changeover with environment continuity after treatment is proposed as shown in Fig. 5, depending on the emergence extent of airborne or non-airborne droplet. The changeover with environment continuity can be performed in continuing status of the CPA and its surrounding environment (Fig. 5A). The changeover with environment continuity after treatment in CPA requires the operation of cleanup through the physical removal of contaminants to prevent cross-contamination of the next process, keeping the controlled status for environment continuity in the surrounding environment of the BSC (Fig. 5B). As the status of surrounding environment is deviated, changeover with environment continuity after treatment in both CPA and its surrounding environment requires the operation of cleanup and disinfection. On the other hand, uncontinuable status for environment continuity in CPA requires reprocessing including the cleanup, disinfection, and checkup, leading to the changeover after reprocessing. The practical action of wiping inside the BSC plays a fundamental role in the physical removal of droplets attached to the critical surface [24]. In addition, the base surface of the BSC is critical because of the risk of droplets transferring from the base surface to other vessels through droplet stamping and release (Fig. 5B). On the other hand, the release of airborne droplets that adhered to the wall during the operation could lead to riding of airborne droplets on the airflow in the BSC, which generates a parallel current to prevent the horizontal mixing of air [2], [8]. This current control prevents of the airborne droplets close to the wall from flowing into the center region for the aseptic operation. This reduces the risk of cross-contamination caused by the droplets adhered to the wall. These findings suggest that the base surface, rather than the wall surface inside the BSC is the critical surface that should be wiped clean before the changeover with environment continuity after treatment in cell processing area.
Table 1.
Category of changeover based on the status for environment continuity.
| Category | Status for environment continuity |
Requirement of operation |
|||
|---|---|---|---|---|---|
| Cell processing area | Surrounding environment | Cleanup | Disinfection | Checkup | |
| A: Changeover with environment continuity | Continuing | Controlled | |||
| B: Changeover with environment continuity after treatment in cell processing area | Continuable | Controlled | ○ | ||
| C: Changeover with environment continuity after treatment in both cell processing area and its surrounding environment | Continuable | Deviated | ○ | ○ | |
| D: Changeover after reprocessing | Uncontinuable | Deviated | ○ | ○ | ○ |
5. Conclusions
The present study proposed a treatment for contaminant droplets based on an understanding of the formation mechanism and behavior of these droplets. The droplets were formed by bursting bubbles, which suggests that bubble formation should be prevented by not pushing out the entire solution from the pipette. Based on the classification of the droplets into airborne and non-airborne droplets, it was suggested to a method for prevent the random droplet dispersion onto base surface in BSC. The dispersion of non-airborne droplets to the outside of vessels was prevented by performing the bubble bursting process in the deeper point of the vessels. The airborne droplets that leaked from the vessel went toward the leeward side of unidirectional airflow in the safety cabinet, and landed on a limited surface area or was blown out through the filter. From these results, understanding the formation and behaviors of droplets permit establishing the operation procedure for changeover with environment continuity after treatment in cell processing area.
Acknowledgments
This work was supported by the “Development of Cell Production and Processing Systems for Commercialization of Regenerative Medicine” and “Formulation of Regenerative Medicine National Consortium which renders Nationwide Assistance to Clinical Researches” projects of the Japan Agency for Medical Research and Development, AMED.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2019.04.002.
Declarations of interest
Nothing declared.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Houkin K., Shichinohe H., Abe K., Arato T., Dezawa M., Honmou O. Accelerating cell therapy for stroke in Japan: regulatory framework and guidelines on development of cell-based products. Stroke. 2018;49:e145–e152. doi: 10.1161/STROKEAHA.117.019216. [DOI] [PubMed] [Google Scholar]
- 2.Hu S.C., Shiue A., Tu J.Z., Lin H.Y., Chiu R.B. Validation of cross-contamination control in biological safety cabinet for biotech/pharmaceutical manufacturing process. Environ Sci Pollut Res. 2015;22:19264–19272. doi: 10.1007/s11356-015-5091-5. [DOI] [PubMed] [Google Scholar]
- 3.Kino-oka M., Taya M. Recent developments in processing systems for cell and tissue cultures toward therapeutic application. J Biosci Bioeng. 2009;108:267–276. doi: 10.1016/j.jbiosc.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Okada K., Koike K., Sawa Y. Consideration of and expectations for the pharmaceuticals, medical devices and other therapeutic products act in Japan. Regen Ther. 2015;1:80–83. doi: 10.1016/j.reth.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang W., Ignatius A.A., Thakkar S.V. Impact of residual impurities and contaminants on protein stability. J Pharm Sci. 2014;103:1315–1330. doi: 10.1002/jps.23931. [DOI] [PubMed] [Google Scholar]
- 6.Kozlowska-Skrzypczak M., Bembnista E., Kubiak A., Matsuzak P., Schneider A., Komarnicki M. Microbial contamination of peripheral blood and bone marrow hematopoietic cell products and environmental contamination in a stem cell bank: a single-center report. Transplant Proc. 2014;46:2873–2876. doi: 10.1016/j.transproceed.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Mizutani M., Samejima H., Terunuma H., Kino-oka M. Experience of contamination during autologous cell manufacturing in cell processing facility under the Japanese medical Practitioners Act and the Medical Care Act. Regen Ther. 2016;5:25–30. doi: 10.1016/j.reth.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kruse R.H., Puckett W.H., Richardson J.H. Biological safety cabinetry. Clin Microbiol Rev. 1991;4:207–241. doi: 10.1128/cmr.4.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falagas M.E., Thomaidis P.C., Kotsantis I.K., Sgouros K., Samonis G., Karageorgopoulos D.E. Airborne hydrogen peroxide for disinfection of the hospital environment and infection control: a systematic review. J Hop Infect. 2011;78:171–177. doi: 10.1016/j.jhin.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Imai K., Watanabe S., Ohima Y., Kokubo M., Akers J.E. A new approach to decontamination of isolators and clean rooms by vapor phase hydrogen peroxide. Pharm Tech Japan. 2006;22:967–972. [Google Scholar]
- 11.Grosh A., Dey S. Overview of cleaning validation in pharmaceutical industry. Int J Pharm Qual Assur. 2010;2:26–30. [Google Scholar]
- 12.Sandle T. Risk assessment for intervention scoring in relation to aseptic processing. J Validation Technol. 2016;22 [Google Scholar]
- 13.Kikuchi T., Kino-oka M., Wada M., Kobayashi T., Kato M., Takeda S. A novel, flexible and automated manufacturing facility for cell-based health care products: tissue Factory. Regen Ther. 2018;9:89–99. doi: 10.1016/j.reth.2018.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization and Pan American Health Organization . 2016. Decontamination and reprocessing of medical devices for health-care facilities. [Google Scholar]
- 15.Zhang J., Chen J.J.J., Zhou N. Characteristics of jet droplet produced by bubble bursting on the free liquid surface. Chem Eng Sci. 2012;68:151–156. [Google Scholar]
- 16.Walls P.L.L., Henaux L., Bird J.C. Jet drops from bursting bubbles: how gravity and viscosity couple to inhibit droplet production. Phys Rev E. 2015;92 doi: 10.1103/PhysRevE.92.021002. [DOI] [PubMed] [Google Scholar]
- 17.Ghabache E., Antkowiak A., Josserand C., Seon T. On the physics of fizziness: how bubble bursting controls droplets ejection. Phys Fluid. 2014;26:121701. [Google Scholar]
- 18.Liu J., Vu H., Yoon S.S., Jepsen R., Aguilar G. Splashing phenomena during liquid droplet impact. Atomization Sprays. 2010;2 0:297–310. [Google Scholar]
- 19.Bigg E.K., Leck C. The composition of fragments of bubbles bursting at the ocean surface. J Geophys Res. 2008;113:D11209. [Google Scholar]
- 20.Xie X., Li Y., Chwang A.T.Y., Ho P.L., Seto W.H. How far droplets can move in indoor environments – revisiting the wells evaporation-falling curve. Indoor Air. 2007;17:211–225. doi: 10.1111/j.1600-0668.2007.00469.x. [DOI] [PubMed] [Google Scholar]
- 21.Jones M.R., Brosseau M.L. Aerosol transmission of infectious disease. J Occup Environ Med. 2015;57:501–508. doi: 10.1097/JOM.0000000000000448. [DOI] [PubMed] [Google Scholar]
- 22.Gungor E.Z., Evans S. Understanding the hidden cost and identifying the root causes of changeover impacts. J Clean Prod. 2017;167:1138–1147. [Google Scholar]
- 23.Zhou F., Blocher D.J., Hu X., Heese S. Optimal single machine scheduling of products with components and changeover cost. Eur J Oper Res. 2014;233:75–83. [Google Scholar]
- 24.Boyce J.M. Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrob Resist Infect Contr. 2016;5:10. doi: 10.1186/s13756-016-0111-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The observations of the droplets during pouring of the solution with the airflow turned off (A) and on (B) in the biological safety cabinet.
The observations of the droplets while pouring a portion of the solution (A) and the entire solution (B)
The observations of the droplets while pouring a portion of the solution (A) and entire solution (B) at shallow point, and pouring the entire solution at deep point of the tube (C)