Abstract
Introduction
Routine C4d staining in renal transplantation has stimulated its use in kidney biopsies with glomerulonephritis (GN). Methodical description on staining patterns in the native kidney is not available.
Methods
We retrospectively evaluated C4d staining in formalin-fixed paraffin-embedded sections from 519 native kidney biopsies (bx) with and without glomerular disease.
Results
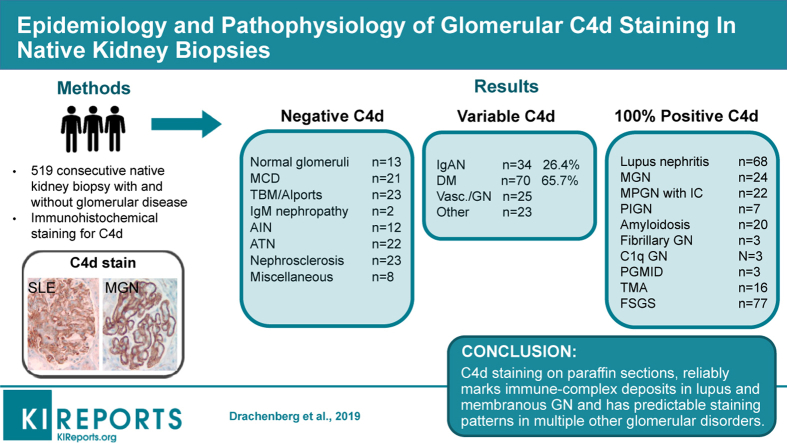
Strong C4d staining was consistently present in immune-complex GN, including lupus nephritis (LN) (n = 68), membranous GN (n = 24), membranoproliferative glomerulonephritis (MPGN) pattern (n = 22), fibrillary GN (n = 3), and proliferative GN with monoclonal IgG (n = 3). C4d stained all cases of postinfectious GN (n = 7) amyloidosis (n = 20) and C1q GN (n = 3). In contrast, IgA nephropathy (IgAN) (n = 34), was negative in 62% of bx, with the rest staining variably. The E1 Oxford classification score correlated with capillary wall C4d staining (P = 0.05). C4d marked the glomerular and arteriolar lesions in thrombotic microangiopathy (TMA; n = 16), the glomerular sclerotic segments in focal segmental glomerulosclerosis (FSGS; n = 77), and marked areas of necrosis in crescentic GN (n = 21). In diabetic glomerulopathy (n = 70), C4d marked advanced insudative lesions but was negative otherwise. C4d weakly stained the mesangium, or was negative in normal biopsies (n = 13), minimal change disease (MCD; n = 21), thin basement membrane disease (n = 20), Alport (n = 3), IgM nephropathy (n = 2), C3 glomerulopathy (n = 5), acute interstitial nephritis (n = 12), acute tubular necrosis (n = 22), ischemic glomerulopathy/nephrosclerosis (n = 23), and other miscellaneous processes (n = 14). Staining in tubular basement membranes and peritubular capillaries was most common in lupus.
Conclusion
Based on reliable staining in lupus and membranous GN, C4d staining is potentially useful as a screening and diagnostic tool, if only paraffin-embedded tissue is available. Knowledge of C4d staining patterns in normal and pathological tissues enhances its diagnostic value.
Keywords: complement deposition, diabetes mellitus, glomerulonephritis, IgA nephropathy, immune deposits, lupus nephritis, membranous glomerulopathy
Graphical abstract
Evaluation of complement components is essential in the clinical practice of nephrology and renal pathology.1 A complement-focused approach has influenced the pathological classification of glomerulopathies, and provides invaluable mechanistic insights on these disorders.1, 2
Histological assessment of the complement split product C4d has been extensively studied in renal allografts and is useful for the diagnosis of acute antibody-mediated rejection.3, 4, 5 C4d is generated by C4 activation in both, the classical (CP) and the Mannose-binding lectin (MBLP) complement pathways. C4d is inactive but lingers in the site of activation by binding covalently to tissue components.4 Assessment of C4d deposition in various solid organ transplants is done with immunofluorescence (IF) using fresh tissue, or with immunohistochemical (IHC) stains in formalin-fixed paraffin-embedded tissue. IF is more sensitive for the demonstration of capillary staining (i.e., peritubular capillaries),6 but a side-by-side comparison of the 2 methods showed no significant difference for glomerular staining.3, 7 Both methods have been successfully used in native kidney samples.8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19
C4d deposition in the native kidney has been recognized for several decades19 and is increasingly used to evaluate various GNs. C4d deposition can be useful as a marker of veiled or masked immune deposits14 and aids in the evaluation of proliferative GN. More specifically, in immune-complex–mediated GN, identification of both C4d and C1q on IF indicates activation of the CP, but presence of C4d without C1q is more consistent with MBLP activation. Correspondingly, in primary activation of the alternative pathway, C4d and C1q are both typically absent.15 A form of complement-mediated GN characterized by primary deposition of C4d but absence of significant C3, C1q, or immunoglobulin is also recognized.16, 20, 21 A review of C4d in native glomerular diseases has been published recently.22
As expected, various studies of membranous glomerulopathy and LN have shown, evidence of C4d deposition associated with immunoglobulin components.10, 23, 24, 25, 26, 27, 28 Glomerular capillary wall C4d staining also is observed in conditions characterized by glomerular basement membrane remodeling including donor-recipient size mismatch29 and TMA.30 In addition, C4d staining commonly marks arteriolar hyalinosis (AH)3, 31 and can outline tubular basement membranes in BK nephropathy.32
Whereas multiple studies of specific diseases exist, a methodical study of patterns of C4d immunostaining in native biopsies with and without glomerular disorders is not available. The current study is based on the evaluation of C4d staining in consecutive native kidney biopsies using the IHC technique. The main goal of the study was to (i) identify glomerular and extraglomerular C4d staining for correlation with the main pathology diagnosis, and (ii) compare these findings with previous studies, and assess the range of staining patterns in various diseases to set the basis for future potential application of the IHC C4d stain in cases lacking sufficient tissue for standard IF and/or electron microscopy (EM) studies.
Methods
Consecutive native kidney biopsies (bx) performed at the University of Maryland Medical Center between January 1, 2012, and December 31, 2014, were stained for C4d with the IHC technique (polyclonal rabbit anti-human C4d, 1:200 dilution, automated BenchMark, ultraview DAB; ARP, Belmont, MA). A total of 519 adequate bx with sufficient clinical information were included. Criteria for tissue adequacy included ≥8 glomeruli for light microscopy, ≥2 glomeruli for IF, and ≥1 glomerulus for EM. Clinical data included demographics, renal function and proteinuria, serologies for lupus, viral infections (hepatitis C and B, HIV) and protein electrophoresis for dysproteinemia in adults (>21 years old). Excluded were 33 bx for inadequate tissue sample, 25 for insufficient clinical information, and 3 for uninterpretable C4d stain (diffuse interstitial-vascular background staining).
Bx evaluation was based on current recommendations,33, 34 including hematoxylin-eosin, periodic acid–Schiff, Jones silver, Masson trichrome, and Congo red, in addition to C4d. IF stains included IgG, IgA, IgM, C3, C1q, kappa and lambda light chains, fibrinogen, and albumin. DNAJB9 stain confirmed fibrillary GN and IHC for amyloid A protein and mass spectrometry were used for characterization of amyloid (Mayo Clinic, reference laboratory).
Glomerular C4d was evaluated regarding location and pattern (global, segmental, mesangial, capillary walls, granular, linear, globular, and smudgy). Extraglomerular staining in tubular basement membranes (TBMs; ≥5% of bx surface), peritubular capillaries (PTCs; ≥5% of bx surface), casts, proximal tubular reabsorption droplets (RDs; ≥5% of bx surface), arteries, arterioles, and thrombi was also recorded. Positive staining was photographed for subsequent comparisons.
Glomerular processes were given priority (primary diagnosis) (Table 1). C4d was correlated with the morphological classifications for specific glomerulopathies.35, 36, 37, 38, 39, 40, 41 Uncommon entities were diagnosed according to published criteria.42, 43, 44, 45, 46, 47, 48, 49, 50, 51 Two-sided t test and χ2 test were used for statistical calculations. The study was approved by the institutional review board.
Table 1.
Primary diagnosis represented in the 519 biopsies evaluated with C4d IHC staining
| Immune-complex GN (n = 158) | |
| Lupus nephritis | 68 |
| Lupus-like HIV nephropathy | 1 |
| IgA nephropathy | 34 |
| Membranous GN | 24 |
| MPGN Type 1 | 22 |
| Postinfectious GN | 7 |
| IgM nephropathy | 2 |
| Vasculitides/crescentic–necrotizing GN (n = 25) | |
| ANCA-associated microscopic polyangiitis | 17 |
| ANCA-associated small vessel vasculitis | 1 |
| Granulomatosis + microscopic polyangiitis | 2 |
| Eosinophilic granulomatosis with polyangiitis | 1 |
| Anti-GBM GN | 4 |
| Podocytopathies (n = 98) | |
| Minimal change disease | 21 |
| Focal segmental glomerulosclerosis | 77 |
| Diabetic nephropathy (n = 70) | |
| Idiopathic nodular GS | 1 |
| GBM abnormalities (n = 23) | |
| Thin basement membrane nephropathy | 20 |
| Alport syndrome | 3 |
| GN with dominant complement deposition (n = 9) | |
| C3 glomerulopathy | 3 |
| Postinfectious GN with C3 only | 3 |
| C1q nephropathy | 3 |
| Glomerulopathies with organized deposits (n = 27) | |
| AL amyloidosis | 10 |
| AA amyloidosis | 9 |
| ALECT-2 amyloidosis | 1 |
| Fibrillary GN | 3 |
| Immunotactoid GN | 4 |
| Plasma cell dyscrasias—monoclonal gammopathy (excluding amyloidosis) (n = 14) | |
| Cast nephropathy | 4 |
| Proximal light chain tubulopathy | 2 |
| Light chain deposition | 4 |
| Heavy and light chain deposition | 1 |
| Proliferative GN with monoclonal IgG deposits | 3 |
| TMA (n = 16) | |
| Atypical HUS | 9 |
| Hypertensive TMA | 3 |
| Other TMA | 4 |
| Tubulointerstitial processes (n = 34) | |
| Acute interstitial nephritis | 12 |
| Acute tubular injury | 22 |
| Hypertensive nephropathy (nephrosclerosis) (n = 23) | |
| Miscellaneous pathologies (n = 8) | |
| Urologic disease (n = 2), cardiorenal syndrome, calcineurin inhibitor toxicity, IgG4 disease, nephronophthisis, Warfarin nephropathy and familial hypocalciuric hypercalemia (1 each) | |
| Normal biopsy or insignificant histological changes (n = 13) | |
AA, serum amyloid A protein; AL, Ig light chain; ALECT-2, leukocyte chemotactic factor 2; ANCA, anti-neutrophil cytoplasmic antibodies; GBM, glomerular basement membrane; GN, glomerulonephritis; HUS, hemolytic uremic syndrome; MPGN, membranoproliferative glomerulonephritis; TMA, thrombotic microangiopathy.
Results
Of 519 bx studied, 450 (86.7%) were from adults with a mean age of 51.3 years (±15, range 22–87, 221 men). The remaining 69 bx (13.3%) were pediatric with a mean age of 13.7 (±4.6 years, range 2–21,42 boys). African American and white individuals were equally represented (46.6% and 48.5%, respectively), with a minority of Asian individuals (4.9%). Evaluation for living donation was the reason for bx in 22 adult bx (4.23%). Table 1 lists the diagnoses represented. Tables 2, 3, and 4 summarize the incidence of C4d positivity in various diseases.
Table 2.
Pathological processes with consistent glomerular C4d staining
| Diagnosis | Cases, n | Glom. C4d, %/strengtha | % Diffuse global | % Mesangial >2+ | % Segmental | % Extraglomerular | %b Neg/mesangial ≤2+ |
|---|---|---|---|---|---|---|---|
| Lupus nephritisc | 68d | 100/3–4+ | 88.3 | 11.7 | – | 47 | – |
| MGN | 24 | 100/3–4+ | 100 | – | – | 8.3 | – |
| MPGN with IC | 22 | 100/2–4+ | 72.7 | – | – | 9 | – |
| Acute postinfectious GN | 7 | 100/2–3+ | 14.2 | 85e | – | – | – |
| Amyloidosis | 20 | 100/2–3+ | 90 | – | 2 | 100 | – |
| Fibrillary GN | 3 | 100/3–4+ | 100 | – | – | – | – |
| C1q GN | 3 | 100/3+ | 33.3 | 66.6 | – | – | – |
| PGMID | 3 | 100/3–4+ | 66.6 | 33.3 | – | – | – |
| TMAf | 16 | 56.2/2–3+ | 31.2 | – | 50 | 31.2 | – |
| FSGSg | 77 | 97.4/2–3+ | 14.2 | – | 83.2 | 22 | 57.1/42.9 |
FSGS, focal segmental glomerulosclerosis; GN, glomerulonephritis; IC, immune complexes; ISN, International Society of Nephrology; MGN, membranous glomerulonephritis; MPGN, membranoproliferative glomerulonephritis; PGMID, proliferative glomerulonephritis with monoclonal IgG deposits; RPS, Renal Pathology Society; TMA, thrombotic microangiopathy.
Percentage of cases with glomerular staining/average strength of staining.
Includes staining in tubular basement membranes, peritubular capillaries, lymphoid aggregates, arteriolar hyalinosis, thrombotic lesions.
Refers to histologically normal glomeruli. Negative or minimal C4d is seen in ≈60% of glomeruli with no GN (see Table 3).
ISN/RPS class II (11.7%), class III (14.7%), class III + V (5.9%), class IV (30.9%), class IV + V (5.98%), class V (30.9%).
Mesangial and coarse granular positivity (equivalent to “humps”).
All TMA lesions (glomerular and extraglomerular) were marked by the C4d stain.
Diffuse staining was noted in the collapsing variant of FSGS. FSGS lesions were not present on the C4d stain in 2 cases.
Table 3.
Pathological processes with variable glomerular C4d staining
| Diagnosis | Cases, n | Glom. C4d, %/strengtha | % Diffuse-global | % Mesangial >2+ | % Segmental | % Extraglomerular | %b Neg/mesangial ≤2+ |
|---|---|---|---|---|---|---|---|
| IgAN | 34 | 26.4/2–3+ | 8.8 | 38.2 | 26.3 | 2.9 | 20.6/41.2 |
| DMc | 70 | 65.7/2–4+ | 24.2 | 20 | 21.6 | 40 | – |
| ANCA GN | 17 | 100/2+ | 5.8 | – | 100d | 5.8 | – |
ANCA, anti-neutrophil cytoplasmic antibodies; DM, diabetic nephropathy; GN, glomerulonephritis; IgAN, IgA nephropathy.
Percentage of cases with glomerular staining/average strength of staining.
Includes staining in tubular basement membranes, peritubular capillaries, lymphoid aggregates, arteriolar hyalinosis, thrombotic lesions.
Strong C4d staining is associated with exudative/insudative diabetic glomerular lesions.
Variable segmental staining in necrotizing and sclerosing lesions.
Table 4.
Pathological processes with insignificant glomerular C4d staining
| Diagnosis | Cases, n | Negative | Mesangial 1 to ≤2+a | Extraglomerularb |
|---|---|---|---|---|
| C3 glomerulopathy | 5c | 5/100% | Nonapplicable | None |
| Normal bx | 13 | 8/61.5% | 5/38.5% | None |
| MCD | 21 | 13/62% | 8/38% | 1/4.7% |
| TBM nephropathy | 20 | 11/55% | 9/45% | 4/20% |
| IgM nephropathy | 2 | – | 2/100% | None |
| AIN | 12 | 9/75% | 3/25% | 2/16.6% |
| ATN | 22 | 13/59% | 9/41% | 14/63.6% |
| Nephrosclerosis | 23 | 19/82.6% | 4/17.4% | 1/4.3% |
AIN, acute interstitial nephritis; ATN, acute tubular necrosis; MCD, minimal change disease; TBM, tubular basement membranes.
Minimal, variable C4d staining in mesangial areas is considered within the normal spectrum of positivity. See Tables 2 and 3.
Includes staining in TBM, peritubular capillaries, lymphoid aggregates, arteriolar hyalinosis, and necrotic casts.
Of the 6 cases of C3 dominant GN (Table 1), 1 with strong C4d staining was reclassified after pronase digestion unmasked significant IgG deposition.
Normal Renal Tissue
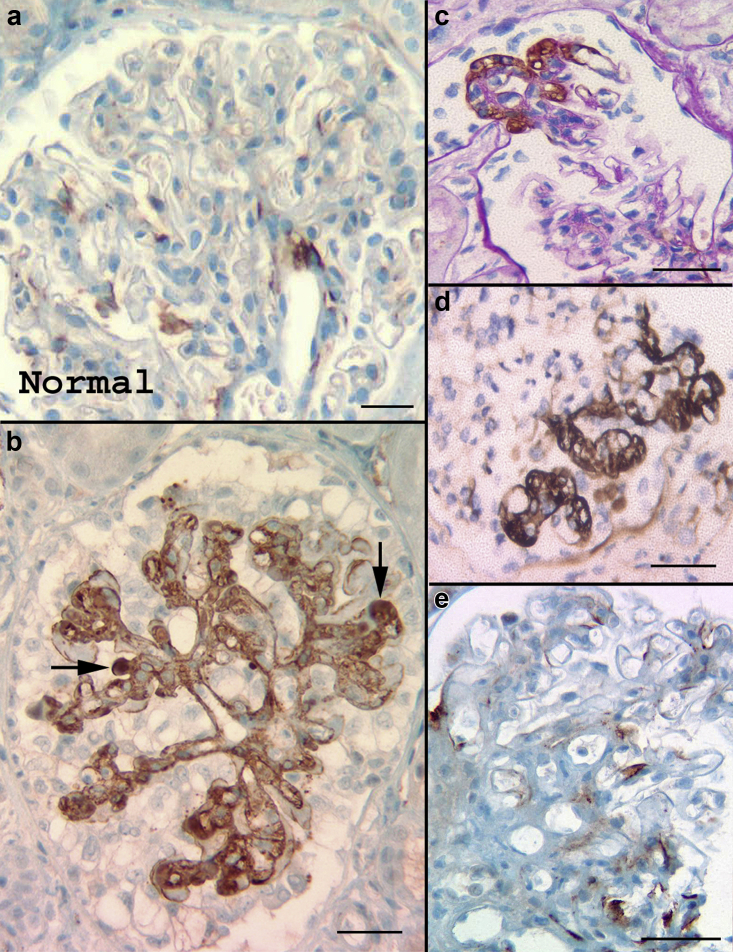
Of 13 cases with normal parenchyma (n = 10), or showing mild mesangioproliferative features but negative IF and EM (n = 3), the C4d was negative in 8 (n = 8/61.5%). The remaining 5 (38.5%) showed mesangial/hilar positivity (up to 1+) (Figure 1a) and no extraglomerular staining. Cause for bx was persistent mild proteinuria (500 mg: 1.5 g/24 hours); 6 patients were potential donors.
Figure 1.
C4d staining in normal glomeruli and focal segmental glomerulosclerosis (FSGS). (a) Minimal C4d mesangial staining (mesangial specks), sometimes with accentuation in the hilum, is found in 40% to 50% of histologically normal glomeruli. (b) C4d stain highlights the mesangium and abnormal tufts in the collapsing variant of FSGS. Stronger staining is seen in areas of hyalinosis (arrows). (c) The FSGS lesion (tip variant) is marked by the C4d stain (periodic acid–Schiff counterstain). (d) Several abnormal tufts are marked in this example of FSGS, not otherwise specified. (e) Mild C4d staining (specks, exudative lesions) is common in perihilar FSGS. Bar = 50 μm.
Minimal Change Disease
Of 21 bx with MCD, C4d was negative in 13 (62%). The remaining 8 (38%) showed multifocal mesangial positivity (1–2+). The pattern of C4d staining in individual glomeruli was not useful for distinguishing normal bx from cases of MCD. One case had PTC staining (4.7%).
Focal Segmental Glomerulosclerosis
There were 77 bx with FSGS, including 41 (53.2%) secondary/maladaptive, 24 (31.1%) consistent with primary FSGS, 10 (13%) associated with viral infections and 2 (2.6%) drug related.52, 53
All visualized sclerotic lesions were positive for C4d (100%), albeit in a heterogeneous manner ranging from specks of positivity to strong staining marking the sclerotic/abnormal segments and areas of hyalinosis (Figure 1b–e). (In 2 bx the segmental sclerotic lesions did not appear on the C4d stain.) Strongest C4d staining occurred in collapsing FSGS (Figure 1b), whereas weak, variable staining was noted in perihilar FSGS (Figure 1e). The affected segments in 2 cases of cellular variant stained weakly in contrast to those in the tip and not otherwise specified variants (Figure 1c and d). C4d in glomeruli not involved by FSGS, was negative in 44 bx (57.1%), whereas the remaining cases showed mesangial staining ranging from specks to 1 to 2+, similar to staining in normal glomeruli and MCD. In addition, C4d marked AH (n = 14, 18,1%), PTC (n = 3, 3.9%), and RD in podocytes (n = 8, 10.3%). PTC and TBM staining correlated with inflammatory classes (III and IV), P = 0.019.
Lupus Nephritis
Sixty-eight (n = 68) bx had LN, corresponding to International Society of Nephrology/Renal Pathology Society37 class II: 8 (11.74%), class III: 10 (7%, 2 inactive), class III+class V: 4 (5.88%), class IV: 21 (30.9%), class IV+V: 4 (5.88%), and class V: 21 (30.9%). Strong, granular glomerular C4d staining was noted in all cases. The staining pattern correlated with the class. Specifically, class II showed staining confined to the mesangium (Figure 2a), whereas classes III and IV showed a combination of granular mesangial staining with irregular, granular or confluent staining in peripheral capillary walls (Figure 2b–e). Staining was heterogeneous in segments with necrotizing lesions or marked hypercellularity. In class V with or without concurrent classes III or IV, C4d consistently highlighted granular membranous staining (Figure 2c and e). C4d closely correlated with the intensity of IF deposits for immunoglobulins, kappa and lambda light chains, and C1q.
Figure 2.
C4d staining in lupus nephritis (LN). Glomerular C4d staining characterizes all classes of LN. (a) Mesangial, class II, (b) class III, (c) class IV + V, (d) class IV, and (e) class V. (f) Extraglomerular staining is common in tubular basement membrane (arrowheads) and peritubular capillaries (arrow). Bar = 50 μm.
Extraglomerular positivity was common, consisting of granular staining in TBM (n = 16, 23.5%) and PTC (n = 6, 8.8%) (Figure 2f), as well as in tubular RD (n = 15, 22%). Also positive were segments of Bowman’s capsule (n = 3, 4.4%), the interstitium in lymphoid aggregates (n = 2, 2.9%), and lesions of TMA (n = 1, 1.4%). One case of lupus-like HIV nephropathy showed strong, diffuse, granular glomerular staining.
IgA Nephropathy
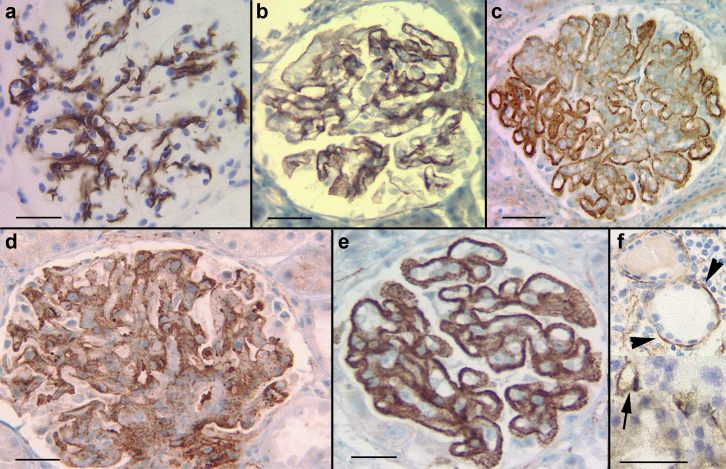
There were 34 bx with IgAN. In 21 cases (61.8%) C4d staining showed minimal mesangial positivity (specks to 1+), similar to normal bx. The remaining 13 (38.2%) showed moderate to marked mesangial positivity (Figure 3a) with additional positivity in capillary walls segmentally or diffusely in 9 (26.4%) (Figure 3b). Overall strength of C4d staining, and mesangial staining did not correlate with any of the Oxford scores: M (mesangial hypercellularity), E (endocapillary hypercellularity), S (segmental glomerulosclerosis), and T (tubular atrophy/interstitial fibrosis). However, capillary wall staining of any extent, correlated with the presence of endocapillary hypercellularity (E1, P = 0.05). C4d stained segmental sclerotic lesions in 5 cases (26.3%). Podocyte RD and AH were highlighted in 1 (2.9%) and 2 (5.8%) cases, respectively. IgM positivity ≥2+ on IF was identified in 12 cases (35.2%) and this did not correlate with the intensity or pattern of C4d. Extraglomerular staining was insignificant.
Figure 3.
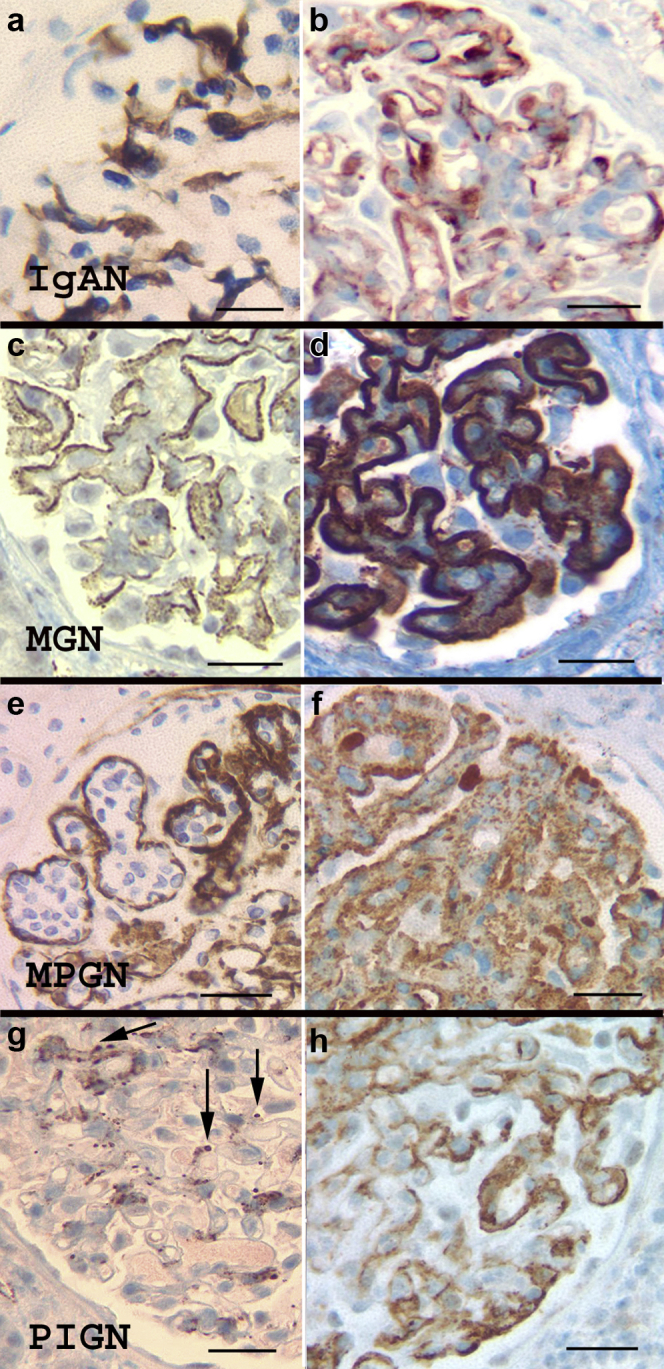
C4d staining in IgA nephropathy (IgAN), membranous glomerulonephritis (MGN), membranoproliferative glomerulonephritis (MPGN), and acute postinfectious glomerulonephritis (PIGN). (a,b) IgAN shows variable staining for C4d, ranging from negative, to mesangial (a) and mesangial with capillary wall staining (b). Capillary wall staining correlates with the E1 Oxford score. (c,d) MGN: C4d stain consistently marks the glomerular basement membranes in MGN. Early and advanced MGN are demonstrated in (c) and (d), respectively. (e,f) MPGN pattern of injury, with immune-complex deposits typically showing strong C4d staining, outlining the loops with endocapillary proliferation (e) or with a diffuse granular pattern (f). (g,h) Acute PIGN: The pattern of C4d staining varies in acute PIGN. There may be mesangial staining with prominent granules (corresponding to “humps,” arrows) (g), or, in cases with a “garland” immunofluorescence pattern, the C4d stain may also highlight capillary walls (h). Bar = 50 μm.
Membranous Glomerulonephritis
A total of 24 bx had membranous glomerulonephritis (MGN), of which 2 (8.3%) were associated with viral hepatitis and 1 (4.1%) with pancreatic cancer. The remaining cases were considered to be idiopathic/primary but anti-phospholipase-A2-receptor studies (PLA2R) were not consistently available. In all cases (100%), there was diffuse membranous, granular C4d staining. In most cases (n = 22, 91.6%), the intensity of the C4d correlated with the strength of IF staining for IgG, as well as with the Churg ultrastructural stages of MGN54 (Figure 3c and d). Regarding the 2 discrepant cases, one was very early MGN with strong C4d staining (3+) but weak finely granular IgG IF staining (1+) and small deposits on EM. The other case had weak (1+) granular membranous staining on C4d but very strong (4+) IgG IF staining with confluent epimembranous deposits on EM. Prominent podocyte staining in RD was noted in 1 (4.1%), proximal tubular RD in 6 (25%), and AH in 2 (8.3%).
MPGN With Immune Complexes
A total of 22 bx had an MPGN pattern of injury with immune deposition, the vast majority corresponding to the previously called type I (n = 20, 91%) and the remaining with features consistent with the former type III (n = 2, 9%).1 Fourteen cases (n = 14, 63.6%) were secondary to hepatitis C infection. All cases (n = 22, 100%) showed glomerular positivity in the mesangium and along the peripheral capillary walls, often highlighting the periphery of lobules expanded by the endocapillary hypercellularity (Figure 3e and f). The staining was strong, diffuse in 72.7% of cases (n = 16) and was variable in the rest. MPGN with abundant membranous deposits on EM showed coarse granular, membranous staining. Segments of Bowman’s capsule stained in 3 bx (13.6%) with TBM and staining in AH seen in 1 bx each (4.5%).
Acute Postinfectious GN
A total of 7 bx had clinical and histological features of acute postinfectious GN, all showing significant but uneven glomerular staining for C4d. In most cases (n = 6, 85.7%) there was variable mesangial positivity and irregularly distributed well-defined coarse granules located toward the urinary space (corresponding to humps seen on EM; Figure 3g). One case (n = 1, 14.3%) with a “garland pattern” on IF, showed diffuse equivalent staining on the C4d stain (Figure 3h). Extraglomerular staining was insignificant.
IgM Nephropathy
Two bx from pediatric patients and 1.0 to 1.5 g of proteinuria had IgM nephropathy.42 The C4d stains showed only mild mesangial positivity (1+) similar to normal and MCD bx.
Vasculitides/Crescentic–Necrotizing GN
A total of 25 bx had vasculitides (Table 1), as defined previously.55
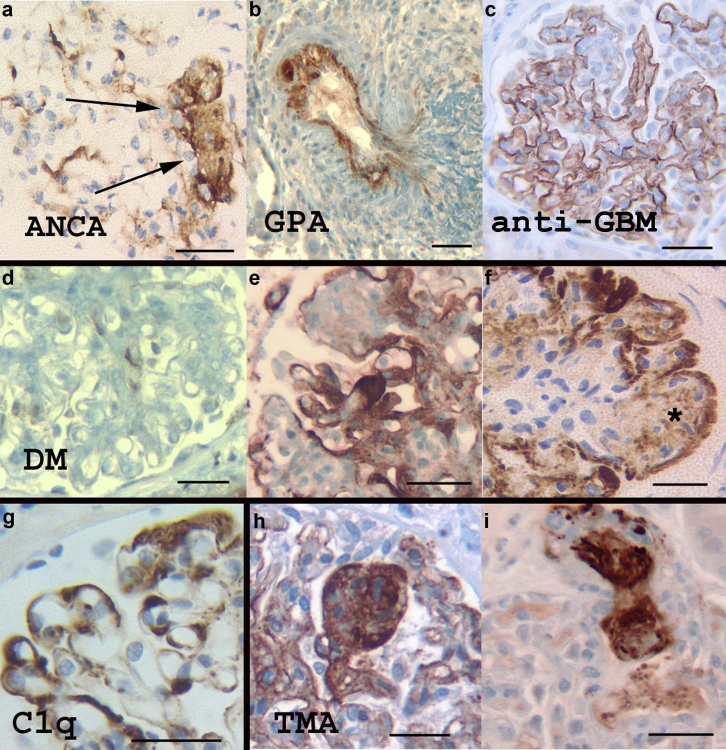
In all 17 (100%) cases of anti-neutrophil cytoplasmic antibodies (ANCA)–associated microscopic polyangiitis, the foci of glomerular tuft necrosis stained positive for C4d, albeit heterogeneously and with variable intensity (Figure 4a). Fibrous and fibroepithelial crescents were essentially negative (n = 7, 28%), whereas epithelial crescents showed irregular foci of positivity (n = 5, 20%). Glomerular tuft sclerosing/organizing lesions, as well as viable but abnormal tufts were irregularly positive for C4d (n = 4, 16%) resembling staining in FSGS lesions. Extraglomerular staining was noted in tubular RD and PTC in 2 bx (11.7% each).
Figure 4.
C4d staining in vasculitis, diabetic nephropathy (DM), C1q glomerulonephritis (GN), and thrombotic microangiopathy (TMA). (a) Anti-neutrophil cytoplasmic antibody (ANCA)–necrotizing GN. Necrotizing (arrows) and evolving sclerosing lesions in crescentic GN are often marked with the C4d stain. (b) C4d staining in an artery in granulomatosis with polyangiitis (GPA). (c) In this example of anti–glomerular basement membrane (GBM) disease all glomerular capillary walls were marked linearly with the C4d stain, but this finding is inconsistent from case to case. (d–f) Mesangial expansion in DM does not stain significantly with the C4d stain (d); however, exudative/insudative lesions in progressive DM are strongly stained (e,f). Simple mesangial nodules are not stained significantly with the C4d stain unless there is diabetic fibrillosis (f, asterisk). (g) Strong staining for C4d is typical in C1q nephropathy, following the distribution of C1q deposits on immunofluorescence and electron microscopy. (h,i) The C4d stain marks all lesions of thrombotic microangiopathy in glomerular tufts (h) and arterioles (i). The glomerular tufts overall show variable C4d staining depending on the severity of the endothelial injury and the extent of remodeling (chronic TMA). Bar = 50 μm.
Two cases of granulomatosis and microscopic polyangiitis showed identical staining in the glomerular lesions as ANCA-associated GN, but the C4d stain also highlighted fibrinoid necrosis in one artery with arteritis (Figure 4b). Strong arterial wall staining was present in a case of ANCA-associated small vessel vasculitis. In contrast, arteritic lesions and glomeruli were negative in eosinophilic granulomatosis with polyangiitis (Churg-Strauss vasculitis).
Four cases of anti–glomerular basement membrane (GBM) GN showed C4d staining in necrotizing lesions similar to ANCA-associated GN. In addition, smooth, linear C4d staining was noted along the GBMs in 2 cases, involving 100% and 30% of the loops in each case, respectively (Figure 4c). Complete absence of GBM C4d staining was noted in the 2 other anti-GBM cases. TBMs were not marked by the C4d stain.
Diabetic Nephropathy
A total of 70 bx had diabetic nephropathy (DM), corresponding to DM class II: 11 (15.8%), class III: 55 (78.5%), and class IV: 4 (5.7%).38 C4d was negative in the diffuse, mesangial lesions characteristic of class IIa (n = 2) and class IIb (n = 9) (Figure 4d). Similar lack of staining was noted in simple nodular lesions. C4d stain, in contrast, strongly marked the exudative lesions, particularly hyalinotic exudative/insudative deposits including fibrin caps, capsular drops, and overall advanced degenerative, sclerosing glomerular changes. In advanced nodular changes, C4d showed coarse outlining of the periphery of most nodules (Figure 4e and f). Organizing microaneurysms stained heterogeneously with accentuation of the exudative lesions. The central areas of the nodules were negative with the exception of 5 cases (7.14%) that had diabetic fibrillosis on EM (Figure 4f). AH was marked in 16 cases (22.8%), TBM in 3 (4.28%), PTC in 2 (2.8%), and tubular RD in 2 (2.8%). One case of idiopathic nodular glomerulosclerosis was negative for C4d.
Thin Basement Membrane Nephropathy and Alport Syndrome
Twenty (n = 20) bx had thin basement membrane nephropathy, of which 11 (55%) were negative on the C4d stain. Specks of mesangial positivity (up to 1+) were noted in the remaining 9 bx (45%). AH stained in 4 bx (20%).
Of the 3 cases of Alport syndrome, weak mesangial C4d staining was found in 2 (66.6%), with the remaining case being negative.
GN With Dominant Complement Deposition
Six bx had GN with dominant C3 deposition as defined by IF and EM findings,40 3 of them with clinical and EM features characteristic of acute postinfectious GN. Five (n = 5, 83.3%) of the 6 cases were completely negative for the C4d stain. The remaining case was strongly positive along the peripheral capillary walls as seen in MPGN with immune complex (IC) deposition. Repeat IF studies after pronase digestion confirmed the presence of IgG (consistent with masked IC deposition) (Table 4).
Three cases of C1q nephropathy45 were strongly, diffusely positive in the mesangium with additional segmental staining in capillary walls in 2 cases (Figure 4g). C4d staining paralleled the C1q deposition on IF and EM.
Glomerulopathies With Organized Deposits
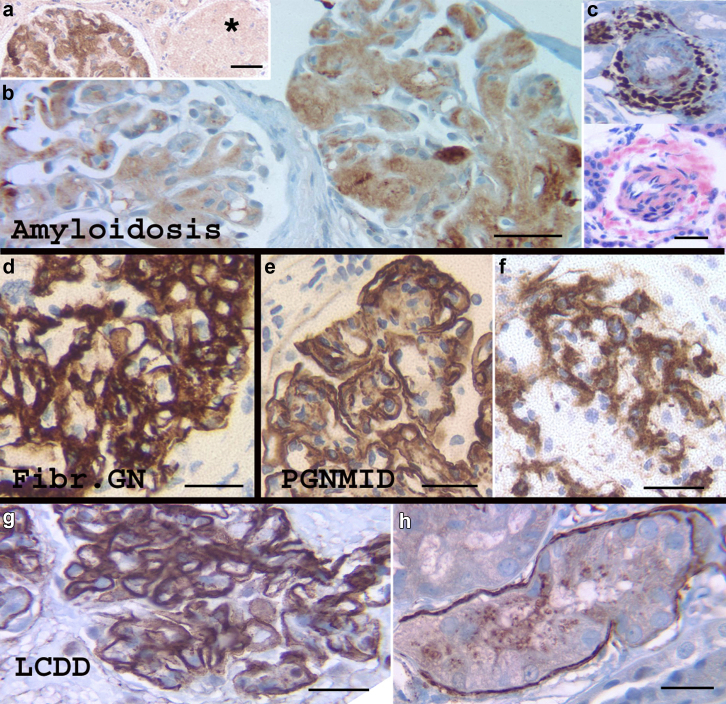
A total of 20 cases of renal amyloidosis were examined, 10 of Ig light chain (AL) type, 9 of serum amyloid A protein (AA) type, and 1 of leukocyte chemotactic factor 2 (ALECT-2) amyloidosis. C4d staining was seen in all cases. In comparison with the Congo red stain, C4d staining was heterogeneous but consistently marked glomerular (n = 20, 100%) and arteriolar amyloidosis (n = 14, 70%). C4d staining was weaker and inconsistent in interstitial/peritubular amyloidosis seen in 7 cases (35%) (Figure 5a–c).
Figure 5.
C4d staining in organized deposits and monoclonal gammopathies. (a) Leukocyte chemotactic factor 2 (ALECT-2) amyloidosis shows C4d positivity in the glomerulus with amyloidosis (right), but an obsolescent glomerulus (asterisk) that was also positive for Congo red stain is negative for C4d, demonstrating the heterogeneity of C4d positivity in amyloidosis. (b) Ig light chain (AL) amyloid with smudgy and granular staining of variable intensity in 2 glomeruli. (c) Small artery in serum amyloid A protein (AA) amyloidosis showing comparable staining of a C4d stain and Congo red stain. (d) All cases of fibrillary glomerulonephritis (Fibr. GN) stained strongly for C4d with a pattern corresponding to the fibrillary deposition. (e,f) Strong positivity for C4d was noted in cases of proliferative glomerulonephritis with monoclonal IgG deposits (PGNMID). Membranoproliferative glomerulonephritis (MPGN) and mesangial morphological patterns are highlighted in (e) and (f), respectively. (g,h) A C4d stain marked a case of light chain deposition disease (LCDD), both along the glomerular capillary walls (g) and occasional tubular basement membranes (h). Bar = 50 μm.
Three bx with fibrillary GN, showed strong positivity for C4d in the mesangium and capillary walls with a smudgy to coarse granular heterogeneous pattern corresponding to the IgG deposits on IF (Figure 5d).
Four bx with proliferative GN associated with cryoglobulinemia and immunotactoid deposits were evaluated, 2 related to hepatitis C infection and 2 to autoimmune disorders. C4d staining was strongly positive in the glomeruli in 2 bx, outlining the capillary walls with a pattern similar to staining in MPGN with IC and also marked PTC in both bx. C4d staining only highlighted scattered thrombi in glomerular capillaries in the 2 remaining cases.
Plasma Cell Dyscrasias and Monoclonal Gammopathies (Excluding Organized Deposits)
Four cases of light chain cast nephropathy were negative on the C4d stain or showed weak staining in occasional casts. Similarly, 2 cases of proximal light chain tubulopathy were negative, except for specks of mesangial positivity and weak staining in casts.
There were 3 cases of light chain deposition disease, one that appeared negative on the C4d stain. In contrast the 2 remaining cases showed strong C4d staining outlining the glomerular capillary walls (Figure 5g). Strong positivity in thickened TBMs was noted in 1 case (Figure 5h).
Three (n = 3) cases of heavy (IgG) and light chain deposition GN, all showed C4d staining in expanded mesangial areas, and irregular staining in glomerular capillary walls.
Three cases of proliferative GN with monoclonal IgG deposits showed strong glomerular staining with C4d according to the morphological pattern of GN, mesangial in 1 case and mesangioproliferative in the other 2 cases (Figure 5e and f). Extraglomerular staining was insignificant.
Thrombotic Microangiopathy
Sixteen cases of TMA were evaluated, 9 (56.2%) of atypical hemolytic uremic syndrome, 3 (18.75%) due to malignant hypertension, 2 (12.5%) associated with pregnancy, and 2 due to anti-vascular endothelial growth factor effect and radiation nephritis, respectively (6.25% each). The glomerular and/or arteriolar TMA lesions stained positive for C4d in all cases (100%) (Figure 4h and i). Independently of the obvious TMA lesions, the C4d stain marked segmentally or globally the glomerular capillary walls in 9 cases (n = 9, 56.25%).
Tubulointerstitial Diseases
C4d staining in 12 bx with acute interstitial nephritis was essentially negative. Mild mesangial glomerular positivity (specks) was noted in 3 cases (25%). In addition, 1 (8.3%) had prominent staining in PTC and the interstitium, whereas lymphoid aggregates were highlighted in another (n = 1, 8.3%).
C4d staining in 22 bx with acute tubular injury/acute tubular necrosis showed specks of mesangial positivity in 9 (41%). The C4d stain highlighted dense/necrotic tubular casts in 8 (36.4%). In addition, clusters of PTC and AH were highlighted in 3 bx (13.6%) each.
Chronic Hypertensive Nephropathy (Nephrosclerosis)
Twenty-three bx showed changes only secondary to chronic hypertension. C4d staining was either negative or showed mesangial specks in a minority of cases (n = 4, 17.4%).
AH and TBM were marked in 4 cases each (17.3%, each) and tubular RD and PTC were highlighted in 1 case each (4.34%, each).
Miscellaneous Pathologies
Various nonglomerular processes, including urologic disorders (n = 2), cardiorenal syndrome, calcineurin inhibitor toxicity, IgG4 nephropathy, nephronophthisis, warfarin-induced nephropathy, and familial hypomagnesemia with hypercalciuria and nephrocalcinosis (1 each), showed specks of mesangial positivity in only 4 cases (50%). In addition, C4d staining was noted in the interstitial space in lymphoid aggregates in IgG4 nephropathy.
Discussion
Widespread utilization of the C4d staining in allografts has generated interest in its potential application to native biopsies and glomerular diseases.4, 22 In this context, identification of C4d deposits produced after C4 cleavage in the CP and MBLP pathways but not in the alternative pathway, can be a useful diagnostic tool.1, 2, 14, 15
In the current study, we evaluated C4d staining in a series of consecutive native kidney biopsies, independently of the cause of biopsy or the presence of glomerular disease, with the purpose of characterizing potentially meaningful and reproducible staining in normal and diseased tissue compartments. Similar to a previous epidemiological study, ours contained a significant proportion of noninflammatory glomerular processes including FSGS and DM.56
C4d staining in histologically normal renal tissue and biopsies lacking glomerular disease is insignificant, with two-thirds of cases being negative, and the remaining showing only patchy mesangial positivity. Furthermore, a variety of glomerular processes, including MCD, thin basement membrane nephropathy, early DM, C3 glomerulopathy, and ischemic glomerular disease (nephrosclerosis) also show insignificant C4d staining.
In contrast, glomerular processes characterized by abundance of Ig deposits with CP complement activation, are strongly and consistently positive for C4d. These include LN, MGN, MPGN with IC, acute postinfectious GN, fibrillary GN, and proliferative glomerulonephritis with monoclonal IgG deposits (PGMID), for which evaluation of C4d staining could be potentially useful as an adjunct tool in special circumstances. More specifically, the reliability of the C4d stain to demonstrate immune deposits in LN and MGN makes it a useful screening tool when fresh tissue is not available.
Previous studies have addressed the value of C4d staining for LN and MGN.8, 23, 24, 25, 26, 27, 28, 31, 57, 58, 59, 60, 61 As anticipated, we also observed C4d staining in these 2 diseases with distribution mirroring the IC deposition on IF and EM. In addition, C4d highlighted extraglomerular staining in LN, in both TBM and PTC that correlated with the inflammatory classes (III, IV).58, 62 In LN, PTC C4d staining is typically more granular in comparison with the linear staining characteristic of antibody-mediated rejection in renal allografts.63 TBM staining that can occur in renal allografts, often with BK virus infection,32 also has been described in IgAN.64
In sharp contrast to other GNs with immune deposits, IgAN has variable C4d staining, ranging from absent (62%) to global (9%), probably corresponding to the clinical and histological heterogeneity of this disease. The significance of glomerular C4d staining in IgAN has generated substantial interest, as there is increasing recognition of the role of the complement system in this disease.11, 12, 13, 64, 65, 66, 67, 68, 69 C4d deposition in IgAN has been associated with aggressive histology and worse clinical course.11, 12, 67, 69 Similar to a previous study, we found that C4d deposition involving not only the mesangium but also the capillary walls, correlated with endocapillary proliferation (E1 lesion in the MEST-C score).70
Our study confirms reliable C4d staining in acute and chronic TMA lesions.8, 30, 31, 71 In a study including various types of TMA, C4d deposition and evidence of CP activation was identified in most cases supporting both a role for complement activation in TMA and the potential usefulness of the C4d stain to highlight the TMA process.22, 71 The fact that C4d consistently marks thrombotic and necrotizing vasculitic lesions is at least in part explained by the known interaction between the MBLP and fibrin/fibrinogen that are known to enhance the activity of this pathway.72
An interesting finding in our study is the identification of C4d in advanced, exudative lesions of DM. This is consistent with the increasingly recognized role for complement activation in this disease. Specifically, advanced glycation of proteins and formation of neo-epitopes results in heightened MBLP activation. Hyperglycemia is also associated with dysfunction or inactivation of several proteins that regulate complement functions.73 Both in experimental and clinical studies, increase in levels of Mannose-binding lectin, the protein that activates the MBLP, was associated with progression of DM nephropathy.73
We also identified C4d staining in the necrotizing lesions in all forms of crescentic GN, as well as in the abnormal and sclerotic segments in FSGS. Complement activation appears to play a role in pauci-immune crescentic GN74; however, it is unclear if C4d staining in the necrotizing lesions is pathogenetically meaningful or represents secondary complement deposition in areas of tissue damage.75 A similar question arises in FSGS, for which complement activation is not known to play a significant role. C4d marked all segmental sclerotic lesions in FSGS but was most pronounced in the collapsing variant, the most aggressive form of the disease. Although activation of the MBLP typically occurs due to contact with pathogens, the process also can be triggered by recognition of hidden self-antigens that are exposed by tissue injury or pathology.76
Staining for C4d in amyloid raises interesting questions about the pathogenetic significance of this association. Inflammatory reactions, as well as complement activation, are triggered by amyloid components in degenerative plaques in the brain,77, 78 but these phenomena have not been studied in the kidney. Due to its predictable deposition in amyloidosis, it was previously stated that C4d is as reliable as the Congo red stain79; however, this does not have a real practical value except for the recognition of this staining property of amyloid.
Of physiopathological interest, native biopsies lack significant C4d staining in the endothelium of larger arteries, a feature that is common (50%–65%) in renal transplants.3
This study has the limitations of a single-center study, including population and clinical practice biases, as well as lack of representation of some rare diseases. Furthermore, staining for IgG isotypes was not performed and new tools for the etiologic classification of membranous glomerulopathy were not available. This study also is limited by its morphological nature, with no available clinical correlations and follow-up.
In summary, the description of C4d staining patterns in this systematic study, as well as in other studies, supports the potential utility of the IHC C4d stain for evaluation of paraffin-embedded renal biopsies from patients with LN and membranous glomerulopathy, when material for IF and/or EM studies is lacking or insufficient. In addition, this study highlights C4d deposition in a variety of other processes, including DM, TMA, amyloidosis, and FSGS, possibly indicating activation of the MBLP. Elucidation of the pathogenetic role of C4d deposition in this broad range of processes will require further studies.
Elucidation of the precise diagnostic utility of the stain in native renal biopsies will require a study comparing diagnoses reached with and without C4d staining. This tool could be potentially useful when there is a need to evaluate the glomeruli in renal tissue that has only been preserved in paraffin, as may occur with autopsy samples and in nephrectomy specimens for renal tumors.80 Equally important is the potential use of IHC C4d staining in renal allograft biopsies for diagnosis of immune-complex GN, such as the early recognition of recurrent membranous GN.26
Disclosure
All the authors declared no competing interests.
References
- 1.Bomback A.S., Markowitz G.S., Appel G.B. Complement-mediated glomerular diseases: a tale of 3 pathways. Kidney Int Rep. 2016;1:148–155. doi: 10.1016/j.ekir.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berger S.P., Daha M.R. Complement in glomerular injury. Semin Immunopathol. 2007;29:375–384. doi: 10.1007/s00281-007-0090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batal I., Girnita A., Zeevi A. Clinical significance of the distribution of C4d deposits in different anatomic compartments of the allograft kidney. Mod Pathol. 2008;21:1490–1498. doi: 10.1038/modpathol.2008.152. [DOI] [PubMed] [Google Scholar]
- 4.Cohen D., Colvin R.B., Daha M.R. Pros and cons for C4d as a biomarker. Kidney Int. 2012;81:628–639. doi: 10.1038/ki.2011.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murata K., Baldwin W.M., 3rd Mechanisms of complement activation, C4d deposition, and their contribution to the pathogenesis of antibody-mediated rejection. Transplant Rev (Orlando) 2009;23:139–150. doi: 10.1016/j.trre.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solez K., Colvin R.B., Racusen L.C. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753–760. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki T., Horita S., Kadoya K. C4d Immunohistochemistry in glomerulonephritis with different antibodies. Clin Exp Nephrol. 2007;11:287–291. doi: 10.1007/s10157-007-0496-1. [DOI] [PubMed] [Google Scholar]
- 8.Cohen D., Koopmans M., Kremer Hovinga I.C. Potential for glomerular C4d as an indicator of thrombotic microangiopathy in lupus nephritis. Arthritis Rheum. 2008;58:2460–2469. doi: 10.1002/art.23662. [DOI] [PubMed] [Google Scholar]
- 9.Cook H.T. C4d staining in the diagnosis of C3 glomerulopathy. J Am Soc Nephrol. 2015;26:2609–2611. doi: 10.1681/ASN.2015040433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Espinosa-Hernandez M., Ortega-Salas R., Lopez-Andreu M. C4d as a diagnostic tool in membranous nephropathy. Nefrologia. 2012;32:295–299. doi: 10.3265/Nefrologia.pre2012.Feb.11224. [DOI] [PubMed] [Google Scholar]
- 11.Fabiano R.C.G., de Almeida Araujo S., Bambirra E.A. Mesangial C4d deposition may predict progression of kidney disease in pediatric patients with IgA nephropathy. Pediatr Nephrol. 2017;32:1211–1220. doi: 10.1007/s00467-017-3610-y. [DOI] [PubMed] [Google Scholar]
- 12.Sahin O.Z., Yavas H., Tasli F. Prognostic value of glomerular C4d staining in patients with IgA nephritis. Int J Clin Exp Pathol. 2014;7:3299–3304. [PMC free article] [PubMed] [Google Scholar]
- 13.Segarra A., Romero K., Agraz I. Mesangial C4d deposits in early IgA nephropathy. Clin J Am Soc Nephrol. 2018;13:258–264. doi: 10.2215/CJN.02530317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sethi S., Hernandez L.H., Alexander M.P., Fervenza F.C. C4d as a marker for masked immune deposits. Kidney Int. 2016;90:223–224. doi: 10.1016/j.kint.2016.02.041. [DOI] [PubMed] [Google Scholar]
- 15.Sethi S., Nasr S.H., De Vriese A.S., Fervenza F.C. C4d as a diagnostic tool in proliferative GN. J Am Soc Nephrol. 2015;26:2852–2859. doi: 10.1681/ASN.2014040406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sethi S., Quint P.S., O'Seaghdha C.M. C4 Glomerulopathy: a disease entity associated with C4d deposition. Am J Kidney Dis. 2016;67:949–953. doi: 10.1053/j.ajkd.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Wagrowska-Danilewicz M., Danilewicz M. The utility of glomerular C4d immunostaining in renal biopsies in patients with immunoglobulin A nephropathy. A clinicopathological study. Pol J Pathol. 2017;68:148–152. doi: 10.5114/pjp.2017.69691. [DOI] [PubMed] [Google Scholar]
- 18.Xing G.Q., Chen M., Liu G. Differential deposition of C4d and MBL in glomeruli of patients with ANCA-negative pauci-immune crescentic glomerulonephritis. J Clin Immunol. 2010;30:144–156. doi: 10.1007/s10875-009-9344-2. [DOI] [PubMed] [Google Scholar]
- 19.Zwirner J., Felber E., Herzog V. Classical pathway of complement activation in normal and diseased human glomeruli. Kidney Int. 1989;36:1069–1077. doi: 10.1038/ki.1989.302. [DOI] [PubMed] [Google Scholar]
- 20.Ali A., Schlanger L., Nasr S.H. Proliferative C4 dense deposit disease, acute thrombotic microangiopathy, a monoclonal gammopathy, and acute kidney failure. Am J Kidney Dis. 2016;67:479–482. doi: 10.1053/j.ajkd.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 21.Park J.M., Lee H., Song S. Primary glomerulonephritis with unique C4d deposition and concurrent non-infectious intermediate uveitis: a case report and literature review. J Korean Med Sci. 2018;33:e136. doi: 10.3346/jkms.2018.33.e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chandra P. C4d in native glomerular diseases. Am J Nephrol. 2019;49:81–92. doi: 10.1159/000496059. [DOI] [PubMed] [Google Scholar]
- 23.Kim M.K., Maeng Y.I., Lee S.J. Pathogenesis and significance of glomerular C4d deposition in lupus nephritis: activation of classical and lectin pathways. Int J Clin Exp Pathol. 2013;6:2157–2167. [PMC free article] [PubMed] [Google Scholar]
- 24.Kim S.H., Jeong H.J. Glomerular C4d deposition indicates in situ classic complement pathway activation, but is not a marker for lupus nephritis activity. Yonsei Med J. 2003;44:75–80. doi: 10.3349/ymj.2003.44.1.75. [DOI] [PubMed] [Google Scholar]
- 25.Kusunoki Y., Itami N., Tochimaru H. Glomerular deposition of C4 cleavage fragment (C4d) and C4-binding protein in idiopathic membranous glomerulonephritis. Nephron. 1989;51:17–19. doi: 10.1159/000185234. [DOI] [PubMed] [Google Scholar]
- 26.Rodriguez E.F., Cosio F.G., Nasr S.H. The pathology and clinical features of early recurrent membranous glomerulonephritis. Am J Transplant. 2012;12:1029–1038. doi: 10.1111/j.1600-6143.2011.03903.x. [DOI] [PubMed] [Google Scholar]
- 27.Shen Y., Chen X.W., Sun C.Y. Association between anti-beta2 glycoprotein I antibodies and renal glomerular C4d deposition in lupus nephritis patients with glomerular microthrombosis: a prospective study of 155 cases. Lupus. 2010;19:1195–1203. doi: 10.1177/0961203310368409. [DOI] [PubMed] [Google Scholar]
- 28.Val-Bernal J.F., Garijo M.F., Val D. C4d immunohistochemical staining is a sensitive method to confirm immunoreactant deposition in formalin-fixed paraffin-embedded tissue in membranous glomerulonephritis. Histol Histopathol. 2011;26:1391–1397. doi: 10.14670/HH-26.1391. [DOI] [PubMed] [Google Scholar]
- 29.Gougeon F., Mikhailov A.V., Gibson K. C4d-expressing glomerulopathy and proteinuria post transplantation of a too-big-for-size mismatched kidney allograft: an unusual case with good outcome. Clin Nephrol. 2017;88:364–370. doi: 10.5414/CN109296. [DOI] [PubMed] [Google Scholar]
- 30.Gasim A.H., Chua J.S., Wolterbeek R. Glomerular C4d deposits can mark structural capillary wall remodelling in thrombotic microangiopathy and transplant glomerulopathy: C4d beyond active antibody-mediated injury: a retrospective study. Transpl Int. 2017;30:519–532. doi: 10.1111/tri.12936. [DOI] [PubMed] [Google Scholar]
- 31.Brinkerhoff B.T., Houghton D.C., Troxell M.L. Renal pathology in hematopoietic cell transplant recipients: a contemporary biopsy, nephrectomy, and autopsy series. Mod Pathol. 2016;29:637–652. doi: 10.1038/modpathol.2016.61. [DOI] [PubMed] [Google Scholar]
- 32.Batal I., Zainah H., Stockhausen S. The significance of renal C4d staining in patients with BK viruria, viremia, and nephropathy. Mod Pathol. 2009;22:1468–1476. doi: 10.1038/modpathol.2009.118. [DOI] [PubMed] [Google Scholar]
- 33.Sethi S., D'Agati V.D., Nast C.C. A proposal for standardized grading of chronic changes in native kidney biopsy specimens. Kidney Int. 2017;91:787–789. doi: 10.1016/j.kint.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 34.Sethi S., Haas M., Markowitz G.S. Mayo Clinic/Renal Pathology Society consensus report on pathologic classification, diagnosis, and reporting of GN. J Am Soc Nephrol. 2016;27:1278–1287. doi: 10.1681/ASN.2015060612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D'Agati V.D., Fogo A.B., Bruijn J.A., Jennette J.C. Pathologic classification of focal segmental glomerulosclerosis: a working proposal. Am J Kidney Dis. 2004;43:368–382. doi: 10.1053/j.ajkd.2003.10.024. [DOI] [PubMed] [Google Scholar]
- 36.Trimarchi H., Barratt J., Cattran D.C. Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney Int. 2017;91:1014–1021. doi: 10.1016/j.kint.2017.02.003. [DOI] [PubMed] [Google Scholar]
- 37.Bajema I.M., Wilhelmus S., Alpers C.E. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018;93:789–796. doi: 10.1016/j.kint.2017.11.023. [DOI] [PubMed] [Google Scholar]
- 38.Tervaert T.W., Mooyaart A.L., Amann K. Pathologic classification of diabetic nephropathy. J Am Soc Nephrol. 2010;21:556–563. doi: 10.1681/ASN.2010010010. [DOI] [PubMed] [Google Scholar]
- 39.Berden A.E., Ferrario F., Hagen E.C. Histopathologic classification of ANCA-associated glomerulonephritis. J Am Soc Nephrol. 2010;21:1628–1636. doi: 10.1681/ASN.2010050477. [DOI] [PubMed] [Google Scholar]
- 40.Pickering M.C., D'Agati V.D., Nester C.M. C3 glomerulopathy: consensus report. Kidney Int. 2013;84:1079–1089. doi: 10.1038/ki.2013.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ravindran A., Fervenza F.C., Smith R.J.H. C3 glomerulopathy: ten years' experience at Mayo Clinic. Mayo Clin Proc. 2018;93:991–1008. doi: 10.1016/j.mayocp.2018.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Connor T.M., Aiello V., Griffith M. The natural history of immunoglobulin M nephropathy in adults. Nephrol Dial Transplant. 2017;32:823–829. doi: 10.1093/ndt/gfw063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sethi S., Fervenza F.C., Zhang Y. Atypical postinfectious glomerulonephritis is associated with abnormalities in the alternative pathway of complement. Kidney Int. 2013;83:293–299. doi: 10.1038/ki.2012.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stillman I.E., Karumanchi S.A. The glomerular injury of preeclampsia. J Am Soc Nephrol. 2007;18:2281–2284. doi: 10.1681/ASN.2007020255. [DOI] [PubMed] [Google Scholar]
- 45.Vizjak A., Ferluga D., Rozic M. Pathology, clinical presentations, and outcomes of C1q nephropathy. J Am Soc Nephrol. 2008;19:2237–2244. doi: 10.1681/ASN.2007080929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Larsen C.P., Bell J.M., Harris A.A. The morphologic spectrum and clinical significance of light chain proximal tubulopathy with and without crystal formation. Mod Pathol. 2011;24:1462–1469. doi: 10.1038/modpathol.2011.104. [DOI] [PubMed] [Google Scholar]
- 47.Haas M. Alport syndrome and thin glomerular basement membrane nephropathy: a practical approach to diagnosis. Arch Pathol Lab Med. 2009;133:224–232. doi: 10.5858/133.2.224. [DOI] [PubMed] [Google Scholar]
- 48.Brodsky S.V., Satoskar A., Chen J. Acute kidney injury during warfarin therapy associated with obstructive tubular red blood cell casts: a report of 9 cases. Am J Kidney Dis. 2009;54:1121–1126. doi: 10.1053/j.ajkd.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 49.Nasr S.H., Dogan A., Larsen C.P. Leukocyte cell-derived chemotaxin 2-associated amyloidosis: a recently recognized disease with distinct clinicopathologic characteristics. Clin J Am Soc Nephrol. 2015;10:2084–2093. doi: 10.2215/CJN.12551214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cornell L.D. IgG4-related kidney disease. Semin Diagn Pathol. 2012;29:245–250. doi: 10.1053/j.semdp.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 51.Nasr S.H., D'Agati V.D. Nodular glomerulosclerosis in the nondiabetic smoker. J Am Soc Nephrol. 2007;18:2032–2036. doi: 10.1681/ASN.2006121328. [DOI] [PubMed] [Google Scholar]
- 52.De Vriese A.S., Sethi S., Nath K.A. Differentiating primary, genetic, and secondary FSGS in adults: a clinicopathologic approach. J Am Soc Nephrol. 2018;29:759–774. doi: 10.1681/ASN.2017090958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sethi S., Glassock R.J., Fervenza F.C. Focal segmental glomerulosclerosis: towards a better understanding for the practicing nephrologist. Nephrol Dial Transplant. 2015;30:375–384. doi: 10.1093/ndt/gfu035. [DOI] [PubMed] [Google Scholar]
- 54.Churg J., Sobin L.H. IGAKU-SHOIN Medical Publishers, Inc.; Tokyo: 1982. Renal Disease: Classification and Atlas of Glomerular Disease. [Google Scholar]
- 55.Jennette J.C. Overview of the 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Clin Exp Nephrol. 2013;17:603–606. doi: 10.1007/s10157-013-0869-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.O'Shaughnessy M.M., Hogan S.L., Poulton C.J. Temporal and demographic trends in glomerular disease epidemiology in the southeastern United States, 1986–2015. Clin J Am Soc Nephrol. 2017;12:614–623. doi: 10.2215/CJN.10871016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Batal I., Liang K., Bastacky S. Prospective assessment of C4d deposits on circulating cells and renal tissues in lupus nephritis: a pilot study. Lupus. 2012;21:13–26. doi: 10.1177/0961203311422093. [DOI] [PubMed] [Google Scholar]
- 58.Li S.J., Liu Z.H., Zen C.H. Peritubular capillary C4d deposition in lupus nephritis different from antibody-mediated renal rejection. Lupus. 2007;16:875–880. doi: 10.1177/0961203307083279. [DOI] [PubMed] [Google Scholar]
- 59.Meehan S.M., Chang A., Khurana A. Pauci-immune and immune glomerular lesions in kidney transplants for systemic lupus erythematosus. Clin J Am Soc Nephrol. 2008;3:1469–1478. doi: 10.2215/CJN.00790208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sahin O.Z., Gurses S., Tasli F. Glomerular c4d staining can be an indicator of disease activity in lupus nephritis. Ren Fail. 2013;35:222–225. doi: 10.3109/0886022X.2012.743916. [DOI] [PubMed] [Google Scholar]
- 61.Espinosa-Hernández M., Ortega-Salas R., López-Andreu M. C4d as a diagnostic tool in membranous nephropathy. Nefrologia. 2012;32:295–299. doi: 10.3265/Nefrologia.pre2012.Feb.11224. [DOI] [PubMed] [Google Scholar]
- 62.Lerut E., Kuypers D., Van Damme B. C4d deposition in the peritubular capillaries of native renal biopsies. Histopathology. 2005;47:430–432. doi: 10.1111/j.1365-2559.2005.02122.x. [DOI] [PubMed] [Google Scholar]
- 63.Kikic Z., Kozakowski N., Regele H. Clinicopathological relevance of granular C4d deposition in peritubular capillaries of kidney allografts. Transpl Int. 2014;27:312–321. doi: 10.1111/tri.12254. [DOI] [PubMed] [Google Scholar]
- 64.Maeng Y.I., Kim M.K., Park J.B. Glomerular and tubular C4d depositions in IgA nephropathy: relations with histopathology and with albuminuria. Int J Clin Exp Pathol. 2013;6:904–910. [PMC free article] [PubMed] [Google Scholar]
- 65.Coppo R. C4d deposits in IgA nephropathy: where does complement activation come from? Pediatr Nephrol. 2017;32:1097–1101. doi: 10.1007/s00467-016-3575-2. [DOI] [PubMed] [Google Scholar]
- 66.Espinosa M., Ortega R., Sanchez M. Association of C4d deposition with clinical outcomes in IgA nephropathy. Clin J Am Soc Nephrol. 2014;9:897–904. doi: 10.2215/CJN.09710913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Floege J., Daha M.R. IgA nephropathy: new insights into the role of complement. Kidney Int. 2018;94:16–18. doi: 10.1016/j.kint.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 68.Heybeli C., Unlu M., Yildiz S. IgA nephropathy: association of C4d with clinical and histopathological findings and possible role of IgM. Ren Fail. 2015;37:1464–1469. doi: 10.3109/0886022X.2015.1077319. [DOI] [PubMed] [Google Scholar]
- 69.Yoshimura M., Kida H., Abe T. Significance of IgA deposits on the glomerular capillary walls in IgA nephropathy. Am J Kidney Dis. 1987;9:404–409. doi: 10.1016/s0272-6386(87)80143-9. [DOI] [PubMed] [Google Scholar]
- 70.Rath A., Tewari R., Mendonca S. Oxford classification of IgA nephropathy and C4d deposition; correlation and its implication. J Nephropharmacol. 2016;5:75–79. [PMC free article] [PubMed] [Google Scholar]
- 71.Chua J.S., Baelde H.J., Zandbergen M. Complement factor C4d is a common denominator in thrombotic microangiopathy. J Am Soc Nephrol. 2015;26:2239–2247. doi: 10.1681/ASN.2014050429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Endo Y., Nakazawa N., Iwaki D. Interactions of ficolin and mannose-binding lectin with fibrinogen/fibrin augment the lectin complement pathway. J Innate Immun. 2010;2:33–42. doi: 10.1159/000227805. [DOI] [PubMed] [Google Scholar]
- 73.Flyvbjerg A. The role of the complement system in diabetic nephropathy. Nat Rev Nephrol. 2017;13:311–318. doi: 10.1038/nrneph.2017.31. [DOI] [PubMed] [Google Scholar]
- 74.Sethi S., Zand L., De Vriese A.S. Complement activation in pauci-immune necrotizing and crescentic glomerulonephritis: results of a proteomic analysis. Nephrol Dial Transplant. 2017;32(suppl 1):i139–i145. doi: 10.1093/ndt/gfw299. [DOI] [PubMed] [Google Scholar]
- 75.Markiewski M.M., Lambris J.D. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol. 2007;171:715–727. doi: 10.2353/ajpath.2007.070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takahashi K., Ip W.E., Michelow I.C., Ezekowitz R.A. The mannose-binding lectin: a prototypic pattern recognition molecule. Curr Opin Immunol. 2006;18:16–23. doi: 10.1016/j.coi.2005.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schwab C., Steele J.C., McGeer E.G., McGeer P.L. Amyloid P immunoreactivity precedes C4d deposition on extracellular neurofibrillary tangles. Acta Neuropathol. 1997;93:87–92. doi: 10.1007/s004010050586. [DOI] [PubMed] [Google Scholar]
- 78.Stoltzner S.E., Grenfell T.J., Mori C. Temporal accrual of complement proteins in amyloid plaques in Down's syndrome with Alzheimer's disease. Am J Pathol. 2000;156:489–499. doi: 10.1016/S0002-9440(10)64753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sung W.J., Maeng Y., Jo J. C4d deposits and a new diagnostic modality for amyloidosis. Int J Clin Exp Pathol. 2016;9:10593–10597. [Google Scholar]
- 80.Henriksen K.J., Meehan S.M., Chang A. Nonneoplastic kidney diseases in adult tumor nephrectomy and nephroureterectomy specimens: common, harmful, yet underappreciated. Arch Pathol Lab Med. 2009;133:1012–1025. doi: 10.5858/133.7.1012. [DOI] [PubMed] [Google Scholar]