Abstract
Suspension culture of three-dimensional (3D) spheroid of human induced pluripotent stem cells (hiPSCs) has been known as a potential method to enhance the scalability of hepatic differentiation of hiPSCs. However, the impact of size-related factor of initial formed spheroid were not largely considered. To address this problem, we evaluate the impact of different specific spheroid size of hiPSCs by forming the individual spheroid from different number of hiPSCs and differentiated into hiPSCs-derived hepatocytes (iHeps). The results showed that larger spheroid exhibit enhanced capability to differentiated into hepatic lineage by increasing the expression marker albumin, CYP3A4 and lower expression of fetal hepatic marker AFP. Several factor such as the tendency of cystic like structure forming, the necrotic area of the large dense spheroid, and interference of WNT/β-catenin signaling was significantly affecting the resulted iHeps. In this study, we suggest that the optimal spheroid size for hepatic differentiation can be attained from 500 to 600 μm diameter spheroid formed from 12,500–25,000 hiPSCs. This size can be potentially applied for various practical use of hepatic differentiation in scalable suspension culture.
Keywords: hiPSCs, iHeps, Spheroid, Size-dependent, Hepatic differentiation
1. Introduction
Liver is the vital organ which originally performs many important in vivo functions, such as metabolism, plasma protein synthesis, glycogen storage, bile secretion, and detoxification. The in vivo propagation of fresh hepatocyte isolated from adult liver was provided the opportunity for many applications [1]. However, to obtain the hepatocytes the invasive procedure is required and the limitation in the shortage of the donor organ still be a problem [2]. The human induced pluripotent stem cells (hiPSCs) derived-hepatocyte (iHeps) represent a potentially novel source for both clinical application, industrial utilization, and toxicological analysis. For these practical applications, it is necessary not only to generate the higher number of iHeps but also similar functional properties compared to in vivo primary hepatocyte as ideally original liver counterparts. For example, some application in tissue engineering such as bioartificial liver required at least around 1010 hepatocytes [3], [4]. To realize a large amount of the cell number, the surface area dependent-limitation of conventional two-dimensional (2D) monolayer culture need to be overcome.
One of the potential methods to increase the yield production of iHeps is by cultured and performed the differentiation in three-dimensional (3D) culture. Generating hepatocyte using 3D suspension culture of hiPSCs was known as the promising method to increase the functional ability and physiological performance of iHeps [5]. Despite the development of differentiation methods in 3D spheroid form, the production of functional iHeps from hiPSC cells still technically challenging. It remains difficult to obtain the similar level of physiological function as adult hepatocyte. This problem arose by insufficient maturation and the heterogeneous population of different lineage during hepatic differentiation [6], [7], [8]. Moreover, the emergence of the resulted 3D cyst-like structure population in hepatic differentiation among dense iHeps spheroid were often reported in 3D hepatic differentiation using spheroid form. This cystic morphology was reported to decreased the hepatic differentiation capability by shifting its characteristics into other lineage [9].
During 3D spheroid culture, size-related factor can be affecting both quality and quantity of the resulted cell yield. For example, the limitation of oxygen and nutrition transfer in larger spheroid can give rise into necrotic area at the center of spheroid. On the other side, initiate the differentiation from smaller spheroid could potentially decrease the amount of native cellular interaction which support the microenvironment inside the spheroid. This phenomenon were indicated the importance to controlling the spheroid size in various application such as hepatic differentiation, mainly when using larger scale culture system. Currently, there are several study which performing 3D hepatic differentiation with several types of bioreactor, such as stirred tank bioreactor [9], [10], perfusion culture [11], or rotary culture [12]. However, the optimization study of initial spheroid size for long term hepatic differentiation were still few.
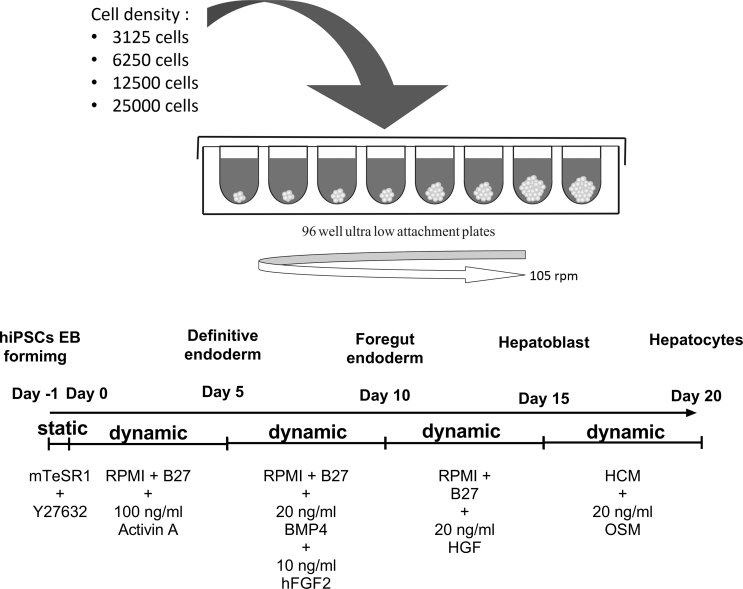
To overcome this problem, we perform the size-dependent hepatic differentiation of hiPSCs spheroid in order to identify the effect of initial spheroid size and obtain the optimal initial spheroid size specified for long term hepatic differentiation. In order to analysed the independent hiPSCs spheroid in size specific manner, we perform the single spheroid forming and hepatic differentiation in suspension culture using each well of 96 well U shape low attachment well plates.
2. Methods
2.1. hiPSCs maintenance on matrigel coated tissue culture dish
The TkDN4-M human hiPSCs cell line (provided by Stem Cell Bank, Center for Stem Cell Biology and Regenerative Medicine, The University of Tokyo) [13] were cultured using the mTeSR 1 medium (STEMCELL Technologies, Vancouver, Canada) in Matrigel (Corning, New York, USA) coated-6 well plates tissue culture dish (AGC Techno Glass, Shizuoka, Japan). The medium refreshment was carried out every 24 h.
2.2. Hepatic differentiation in 2D monolayer culture
105 hiPSCs were seeded in matrigel coated-6 well plates tissue culture dish (AGC Techno Glass) and maintain under regular monolayer hiPSCs culture conditions until it reaches around 80% confluency. Afterward, the culture medium was replaced by differentiation medium every 24 h following previous monolayer differentiation protocols by Si-Tayeb et al. [1] (Fig. 1).
Fig. 1.
The hepatic differentiation protocol for hiPSCs in single spheroid form using 96 well ultra low attachment plates. The stepwise hepatic differentiation of medium and growth factors was adapted from Si-Tayeb et al. [1].
2.3. Hepatic differentiation in suspension culture of hiPSCs in single 3D spheroid
The hiPSCs were harvested from monolayer culture and dissociated using Trypsin–EDTA solution (Life Technologies, California, US). The single cells obtained by passing the hiPSCs clumps through the cell strainer. To generate various size of spheroid, the dissociated hiPSCs was counted by eosinophil counter (SLGC, Saitama, Japan) and a different number of cells suspended in the 200 μl of mTeSR 1 medium solution was seeded into each well of ultra-low attachment round bottom-96 well plates (Corning) for 24 h at 37 °C incubator in static condition. Afterward, the hepatic differentiation was initiated by replacing the medium with 200 μl of differentiation medium with the same formulation as described in monolayer hepatic differentiation (Fig. 1). The medium replacement was carried out in every 24 h. In order to slightly mixing the medium, the plates were placed under 105 rpm rotary shaker during the spheroid forming and differentiation period.
2.4. Morphological analyses of hiPSCs spheroid during differentiation
The morphological analyses conducted by direct observation using the light microscope (Olympus, Tokyo, Japan). Afterward, the pictures were taken and the spheroid size was analyzed by using Fiji (NIH, Maryland, USA) image analysis.
2.5. Glucose measurement in culture medium
To analyze the glucose consumption during hepatic differentiation, 200 μl of the culture medium were isolated every day and measured using multipurpose analyzer (YSI, Ohio, USA).
2.6. Gene expression analyses by qRT PCR
The Spheroid was dissociated using Trypsin–EDTA solution (Life Technologies, California, US). The RNA isolation were performed using Trizol reagents (Life Technologies). The purified RNA solution was reverse transcribed by using ReverTra Ace qPCR RT Master Mix (Toyobo, Osaka, Japan) following qPCR analysis using Thunderbird SYBR qPCR Mix (Toyobo) with primer set as mentioned in Table 1. The gene expression analysis performed by using Pikoreal Real-Time PCR System (ThermoFisher Scientific). All of the procedure was performed by using the manufacturer protocols.
Table 1.
The list of primer sequences for gene expression analysis by qRT-PCR.
| Gene | Primer sequence |
|
|---|---|---|
| Forward | Reverse | |
| Human GATA4 | AGCACACTGCATCTCTCCTGTG | CTCCGCTTGTTCTCAGATCCTC |
| Human HNF4a | CGTCATCGTTGCCAACACAAT | GGGCCACTCACACATCTGTC |
| Human Axin 2 | GAGAGTGAGCGGCAGAGC | CGGCTGACTCGTTCTCCT |
| Human Albumin | CCTGCTGACTTGCCTTCATTAG | TGGCATAGCATTCATGAGGA |
| Human CYP3A4 | ACATAGCCCAGCAAAGAGCAAC | GTCTGGGATGAGAGCCATCACT |
| Human AFP | TGGGACCCGAACTTTCCA | GGCCACATCCAGGACTAGTTTC |
| Human CK19 | GCCACTACTACACGACCATCCA | AGAGCCTGTTCCGTCTCAAACT |
2.7. Albumin concentration measurements by ELISA
The 100 μl solution containing 1 g/L human albumin primary antibodies (Cat. No. A80-129A; Bethyl, US) were immobilized in each well of 96 well plates by incubation at 37 °C for 3 h. Afterward, the plates were washed with 200 μl/well PBS-Tween (FUJIFILM Wako Pure Chemical, Osaka, Japan), then the 100 μl/well of blocking buffer was added and incubated for 1 h at room temperature. The 100 μl of a serial dilution of the medium sample and standard solution were plated in each well and incubated 2 h at room temperature. The plates were washed with PBS Tween (FUJIFILM Wako Pure Chemical) and 100 μl/well of 1 g/L diluted HRP labeled-human albumin secondary antibodies (Cat. No. A80–129P; Bethyl, US) added and incubated for 2 h at 37OC. To obtain the color reaction, the plates washed by PBS tween and 100 μl of color solution consist of 2.5 mg 0-phenylene diammonium chloride (FUJIFILM Wako Pure Chemical) and 0.5 μl H2O2 (FUJIFILM Wako Pure Chemical) per ml citrate buffer (FUJIFILM Wako Pure Chemical) were added to each well following incubation for 30 min in room temperature. The color reaction was stopped afterward by adding 50 μl/well H2SO4 (4N) (FUJIFILM Wako Pure Chemical) without removing the previous solution. The fluorescence intensity was measured at 490–650 nm fluorescent wavelength by Wallac Arvo SX 1420 multilabel counter (PerkinElmer, Massachusetts, US).
2.8. Histological analyses of thin cross Sectioned-iHeps spheroid
The spheroid was collected and fixed by 4% paraformaldehyde solution (FUJIFILM Wako Pure Chemical) in PBS for 30 min at room temperature, washed in PBS and replaced with 30% PBS buffered sucrose solution at 4 °C overnight. The sucrose solution was discharged, and the Spheroid placed in the cryomold following embedding with Optimal Cutting Temperature (OCT) Compound (Sakura Finetek, Tokyo, Japan) at −20 °C until hardening. The 10 μm thin sections were taken by CM3050 cryostat (Leica, Wetzlar, Germany) and mounted onto glass slides for hematoxylin-eosin staining.
2.9. Statistical analyses
The statistical analysis was performed by using GraphPad Prisms 7.04 (GraphPad, California, US). Statistical significance between each dataset was determined by one way ANOVA methods with Tukey's multiple comparison test.
3. Results
3.1. Morphological pattern and proliferation rate of hiPSCs spheroids during hepatic differentiation
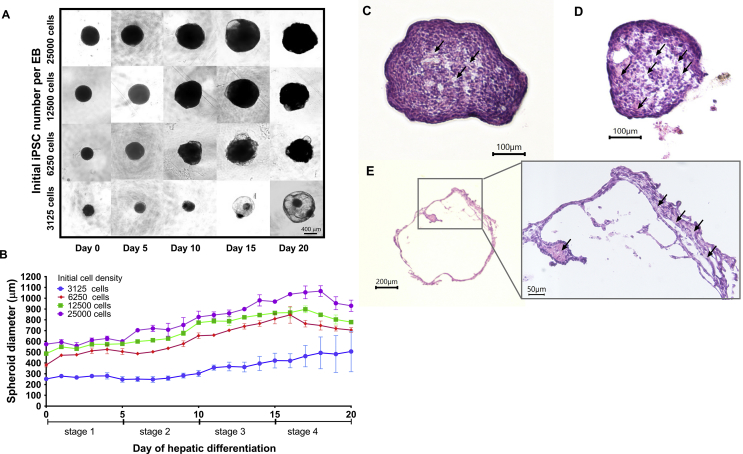
During definitive endoderm differentiation, several amounts of the hiPSCs were detached from the outer layer of spheroid. This condition was related to activin A-induced apoptosis [14]. However, most of the spheroid maintained their size by keep proliferating up to day 5. During day 10–15 of differentiation, most of the larger spheroid size (6250–25,000 initial cell number) were increased in size, and gradually forming the smaller dense compact spheroid at hepatic maturation stage (Fig. 2A and 2B). The partial swelling was started to occur at day 10–15 in several amounts of all size of the spheroid, which gradually reduced in hepatic maturation stage. However, in smaller spheroid (3125 initial cell number), the swelling area become increase from day 10 and altered into the cystic-like structure which mostly tends to keep swelling at the various size. This smaller spheroid also persistently maintain their structure until the end of differentiation. The higher error bar in small Spheroid showed the variability between the cyst-like structure (Fig. 2A and B).
Fig. 2.
The different morphological change of the hiPSC spheroid was occurred during hepatic differentiation (A). The hiPSCs spheroid morphology in each differentiation stage (B). The differences of spheroid growth in size during hepatic differentiation. The three types of spheroid resulted from the hepatic differentiation (C). The dense spheroid (D). Partially swelled spheroid; and (E). Cystic-like spheroid. The arrows showed non viable cells.
The hiPSCs spheroid differentiation resulted in three different structure of iHeps, consist of the dense iHeps, the partially swelled iHeps, and the cystic-like iHeps. Based on histological examination of cross-sectioned spheroid, it is was revealed that the dense spheroid exhibit higher cell viability which exhibits the small central necrotic area was appeared in the dense spheroid (Fig. 2C). Apparently, the increase of swelling area were affecting the cell viability inside the spheroid. This phenomenon can be observed in partially swelled spheroid (Fig. 2D). Moreover, the larger amount of the dead cells were observed in the cystic spheroid (Fig. 2E).
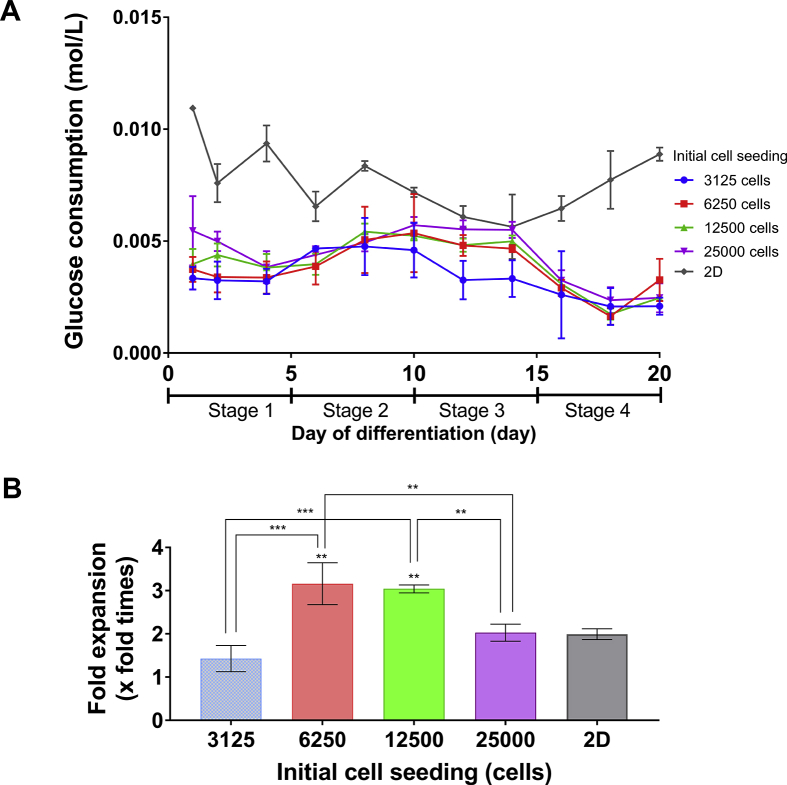
To evaluate the cellular kinetic index during hepatic differentiation of hiPSCs, the glucose consumption were determined by its concentration in differentiation medium. At definitive endoderm differentiation stage, the glucose consumption were relatively decreased more in larger spheroid. This condition mostly related to the larger surface area which exposed by activin A priming during endodermal differentiation. In foregut endoderm and immature hepatocyte differentiation stage, the glucose consumption of all spheroid size were increased altogether with enhanced proliferation rate, and begin to decrease during hepatic maturation at last stage of differentiation (Fig. 3A). In terms of proliferation level, the spheroid formed from 6250 to 12,500 cells show higher fold increase compared to the other group of initial cell seeding density (Fig. 3B).
Fig. 3.
The growth of hiPSCs during hepatic differentiation which reflected by metabolism and cell number (A). The glucose consumption during differentiation period (B). Fold expansion from initial seeding at day 20 per spheroid during hepatic differentiation. Values presented as means ± standard deviation. Statistical significance: *p < 0.05; **p < 0.01; and ***p < 0.001.
3.2. Role of WNT/β-catenin signaling in foregut endoderm and hepatoblast
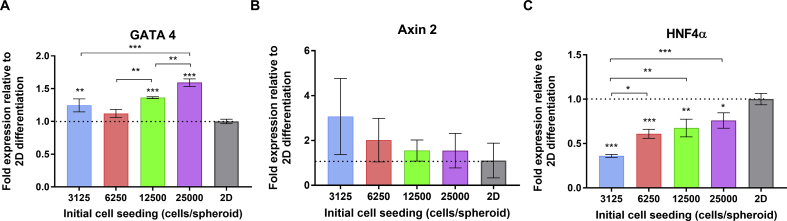
The expression of endoderm gene marker GATA4 were slightly increased in larger spheroid size, indicated that the progress of endodermal differentiation at this step was not yet affected by mass transfer limitation of nutrition, oxygen, and growth factors (Fig. 4A). WNT/β-catenin signaling pathway plays the important role of hepatic differentiation in stage-specific manner [15]. During in vivo liver organogenesis, the WNT/β-catenin were enhanced in endoderm development. However, suppression of this pathway is required to promote foregut endoderm into hepatoblast [16], [17]. Axin 2 is the negative regulator of WNT/β-catenin signaling which induced by the feedback mechanisms of WNT/β-catenin signaling [18]. The increasing level of Axin 2 can be used as an adequate representative indicator of WNT/β-catenin upregulation.
Fig. 4.
The efficiency of endodermal differentiation and effect of Wnt/β-catenin signaling to hepatoblast development transcription factor (A). The gene expression level of GATA4 after endoderm differentiation stage (B). Wnt/β-catenin signaling marker Axin 2 and (C). Early hepatic development marker HNF4α at the end of hepatoblast differentiation stage. Dotted line indicated the gene expression level in conventional 2D differentiation. Values presented as means ± standard deviation. Statistical significance: *p < 0.05; **p < 0.01, and ***p < 0.001.
At the end of hepatoblast differentiation, we found that the Axin 2 were significantly reduced in larger Spheroid size (Fig. 4B). Increasing activity of WNT/β-catenin signaling had previously known as an inhibiting factor of HNF4α [19]. To examine the influence of WNT/β-catenin signaling in hepatoblast development, we measure the expression level of the nuclear hormone receptor HNF4α as one of the important transcription factors to initiate various hepatic gene expression in the spheroid. In contrast, the result showed that HNF4α were significantly increased gradually in larger spheroid size (Fig. 4C).
3.3. Characterization of resulted iHeps
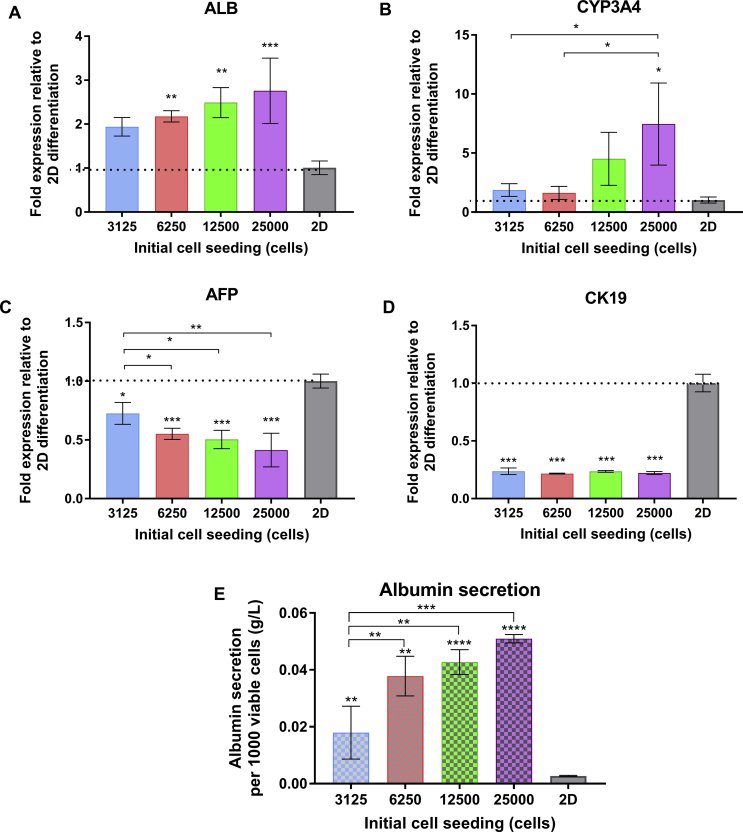
The higher albumin gene expression of iHeps has resulted from larger initial spheroid size (Fig. 5A). To confirm these results, the amount of albumin production remaining in differentiation medium also measured by enzyme-linked immunoabsorbent assay (ELISA) in every 5 days. The results showed consistent data with previous gene expression analysis. The albumin was initially detected after hepatic maturation stage and the average albumin production was increased in iHeps obtained from larger spheroid (Fig. 5E). Importantly, the iHeps produced from larger Spheroid possessed higher drug metabolism capability by expressing CYP3A4 in a significant manner compared to the smaller size spheroid (Fig. 5B). In addition, the larger spheroid exhibits lower expression level of AFP, the protein which responsible for the development of immature hepatocyte (Fig. 5C). All of the 3D iHeps spheroid also showing lower expression of cholangiocyte marker CK19 compared to 2D iHeps which indicate higher specification rate of resulted iHeps (Fig. 5D).
Fig. 5.
The gene expression analysis showing that the better hepatic maturation can be better achieved in larger spheroid, indicated by higher expression of hepatic marker ALB (A) and CYP3A4 (B) with lower fetal hepatic marker AFP (C). In addition, the lower cholangiocyte marker CK19 was resulted in all of 3D iHeps spheroids compared to 2D iHeps (E). The secreted albumin per viable cell in culture medium also detected at a higher concentration in larger spheroid. Dotted line indicated the gene expression level in conventional 2D differentiation. Values presented as means ± standard deviation. Statistical significance: *p < 0.05; **p < 0.01, ***p < 0.001 and ****p < 0.0001.
4. Discussion
HiPSCs cultured and differentiated in the form of spheroid has the capability to form their own microenvironment similar to original organ development in vivo [20], [21]. This advantage can be applied in directing the hiPSCs toward hepatocytes in large scale suspension culture to meet the necessity of a large amount of the cells for several application. Although most of the hepatic differentiation study by using 3D spheroid were focusing on large scale differentiation, the optimal individual spheroid size were not well investigated as an consideration. To observe the effect of the spheroid size in the hepatic differentiation, we formed the individual hiPSCs spheroid which consisting identical size to perform hepatic differentiation in suspension culture. By using this method, the spheroid can be analyzed in a more synchronous manner compared to mass-formed spheroid grown in suspension culture which mostly tends to produce spheroid population with heterogeneous size. Our current study was showing that the optimum spheroid size for hepatic differentiation can be achieved from around 500–600 μm diameter spheroid which formed from 12,500–25,000 hiPSCs which can be used as consideration when initiating the hepatic differentiation using 3D spheroid form.
Based on the fold increase of viable cells, the middle range diameter of our experimental set (spheroid formed from 6250–12,500 cells) resulted in higher expansion rate. We hypothesized that this condition was related to the cystic structure resulted in most of the smallest spheroid population and increasing necrotic area at the center of dense spheroid. In order to confirm this situation, the histological analyses were performed by using cross-sectioned spheroid. The result showing the necrotic area which observed at the center of dense iHeps spheroids (Fig. 2C). This phenomenon possibly related with the limitation of the mass transfer limitation of oxygen, nutrient, and cellular waste product at the center of spheroid altogether with increased spheroid size [22], [23], [24], [25]. In contrast, the cystic like-iHeps spheroid showing distinct histological feature indicated an increasing amount of necrotic area in swelling or cystic spheroid which reduced the viable cells in cystic spheroid which mostly form the ephitelial like-cells surrounding the cystic wall (Fig. 2E). Although the partially swelling structure was found at several numbers of the larger size spheroid (Fig. 2D), this condition was found more severe in smaller spheroids which mostly formed cystic-like structure at the end of differentiation.
Glucose consumption is the important factor which reflects cellular growth index and energy metabolism of hiPSCs during differentiation. Therefore, the change of their concentration in culture medium was measured to observe the metabolic process during differentiation. In most of the spheroids, after the cell number were tend to decrease because of Activin A-induced apoptosis, the glucose consumption rate significantly increased in consistence with increasing of spheroid growth at foregut endoderm and hepatoblast differentiation stage (Fig. 3A). However, their rate was decreased at approximately early of hepatic maturation stage which related to decreased spheroid diameters or shifting its metabolic signature to oxidative phosphorylation in order to obtain their energy source in late differentiation process [26].
The activity of the stage-specific WNT/β-catenin signaling taking an important role in hepatic differentiation. Some study was showed the involvement of WNT/β-catenin signaling both in 2D or 3D hepatic differentiation. However, the involvement of WNT/β-catenin signaling related to size dependent-spheroid in hepatic differentiation was still few to be reported. Following definitive endoderm differentiation, the hiPSCs differentiated toward hepatoblast by hepatic specification stage. In this stage, repression of WNT/β-catenin signaling is necessary to directing the definitive endoderm into hepatoblast. This signaling also has previously identified as the inhibitor of early functional hepatic regulatory genes, such as HNF4α [16], [27]. In our study, the inhibition of HNF4α by this signaling activity in the hepatoblast differentiation stage were occurred and increase the reduction of maturation capability, mainly in smaller spheroid (Fig. 4C). As the result, the downregulation of several hepatic markers such as ALB and CYP3A4 was occurred altogether with upregulated immature hepatic marker AFP (Fig. 5A, B, and C). This condition also confirmed by the decreasing of albumin secretion in lower spheroid size (Fig. 5E). Other factors which also influence the downregulation of hepatic functionality is increased portion of cystic-like forming which consisting a large amount of necrotic cells (2E). Apparently, the loose cell–cell interaction in the cystic structure formed from smaller spheroid reduce the capability to regulate the normal hepatic differentiation mechanism by WNT/β-catenin signaling inhibition and causing the apoptosis at the same time.
Our present study showed that despite of the existence of necrotic area in larger dense spheroid, the larger iHeps spheroid showed better physiological maturation. The albumin secretion were only detected at the end of final stage of differentiation in all experimental group. In addition, the higher albumin gene expression which confirmed by its production in the medium also highly detected in larger spheroids (Fig. 5E). This result were corresponded with the previous study showing the increasing hypoxic area inside the larger spheroids resulting better endodermal differentiation capability which exhibits higher potential of early hepatic progenitor marker AFP [28]. Other previous report showed that the low oxygen concentration was able to increasing the differentiation capability of stem cells into hepatic progenitor cell in 2D culture system [29]. Moreover, the higher cell–cell interaction in larger 3D spheroid was necessary for directing the hiPSCs cellular fate into the hepatocyte. This effect of increasing cellular interaction in hepatic differentiation has not been largely considered. The accumulation of their own endogenous factor apparently contributes to maintaining the microenvironment inside the spheroid by increasing the cell–cell interaction which possibly occurred in larger spheroid size which stimulates the hepatic maturation [20].
The hepatic differentiation was often resulting the heterogeneous population consisted of cells expressed hepatic and cholangiocyte marker which related with the bipotential differentiation capability of hepatoblast [5], [30], [31], [32]. Moreover, the previous study was shown that the three-dimensional structure hepatoblast promotes the epithelial polarity contributed to the forming of differentiated cholangiocyte which characterized by cystic like structure [33], [34], [35]. Therefore, OSM was administrated at the last stage of hepatic differentiation to blocking the cholangiocyte differentiation and directing differentiation into hepatocyte lineage [33], [36]. To identify the status of the cyst-like spheroid, we analyzed the level of cholangiocyte gene expression marker CK19. Interestingly, the gene expression analyses revealed that the expression level of cholangiocyte marker CK19 was almost similar in all size of the spheroid. In contrast, the differentiated hiPSCs population in 2D culture were demonstrated higher tendencies to differentiate into cholangiocyte by expressing higher CK19 (Fig. 5D). This result demonstrated the importance of three-dimensional structure support to decrease the tendency of the bipotent hepatoblast to differentiated into cholangiocyte.
This study showing the importance to determine the optimum spheroid size for hepatic differentiation. The balance between increasing of self auto-paracrine interaction in larger spheroid density and the existence of necrotic area at the center of large spheroid were necessarily considered. The suggested spheroid size were specifically applicable for hepatic differentiation in larger scalable culture systems. In addition, enhancement of the self paracrine stimulation between spheroids may be maintained in high density spheroid number to increase the differentiation efficiency. However, some other culture environmental challenge such as nutrition transfer, mechanical stress, and excess aggregation were still remain and need to be addressed in the future. The utilization of some biomaterial such as microcarrier also can be applied to avoid the mass transfer limitation of nutrition, oxygen, and waste product which appeared at the center of larger spheroid. In addition, modulation of WNT/β-catenin signaling were possibly administrated between specific differentiation stage to enhanced the hepatic differentiation efficiency [15], [27].
5. Conclusion
In this study, we suggest that the higher size of initial hiPSCs Spheroid at some extent can be able to increase the differentiation efficiency. The use of 500–600 μm diameter initial spheroid size formed from up to 12,500–25,000 cells seems to be a potential size to increase the efficiency of hepatic differentiation which also can be applied into several types of larger scale suspension culture systems.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Si-tayeb K., Noto F.K., Nagaoka M., Li J., Battle M.A., Duris C. Highly efficient generation of human hepatocyte–like cells from induced pluripotent stem cells. Hepatology. 2010;9:297–305. doi: 10.1002/hep.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amimoto N., Mizumoto H., Nakazawa K., Ijima H., Funatsu K., Kajiwara T. Hepatic differentiation of mouse embryonic stem cells and induced pluripotent stem cells during organoid formation in hollow fibers. Tissue Eng. 2011;17(15–16):2071–2078. doi: 10.1089/ten.TEA.2010.0689. [DOI] [PubMed] [Google Scholar]
- 3.Badylak S.F., Taylor D., Uygun K. Whole organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu Rev Biomed Eng. 2010;13 doi: 10.1146/annurev-bioeng-071910-124743. 110301095218061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lei Y., Schaffer D.V. A fully defined and scalable 3D culture system for human pluripotent stem cell expansion and differentiation. Proc Natl Acad Sci Unit States Am. 2013;110(52):E5039–E5048. doi: 10.1073/pnas.1309408110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meier F., Freyer N., Brzeszczynska J., Knospel F., Armsrong L., Lako M. Hepatic differentiation of human iPSCs in different 3D models: a comparative study. Int J Mol Med. 2017;40(6):1759–1771. doi: 10.3892/ijmm.2017.3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Godoy P., Schmidt-Heck W., Natarajan K., Lucendo-Villarin B., Szkolnicka D., Asplund A. Gene networks and transcription factor motifs defining the differentiation of stem cells into hepatocyte-like cells. J Hepatol. 2015;63(4):934–942. doi: 10.1016/j.jhep.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baxter M., Withey S., Harrison S., Segeritz C., Zhang F., Atkinson-Dell R. Phenotypic and functional analyses show stem cell-derived hepatocyte-like cells better mimic fetal rather than adult hepatocytes. J Hepatol. 2015;62(3):581–589. doi: 10.1016/j.jhep.2014.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomizawa M., Shinozaki F., Motoyoshi Y., Sugiyama T., Yamamoto S., Ishige N. Induction of hepatocyte differentiation in human pluripotent. J Gastroenterol Hepatol. 2015;4(6):1627–1639. [Google Scholar]
- 9.Vosough M. Generation of functional hepatocyte-like cells from human pluripotent stem cells in a scalable suspension culture. Stem Cell Dev. 2013;22(20):1–13. doi: 10.1089/scd.2013.0088. [DOI] [PubMed] [Google Scholar]
- 10.Yin C., Chen W., Hsiao C., Kuo C., Chen C., Wu W. Production of mouse embryoid bodies with hepatic differentiation potential by stirred Tank Bioreactor. BioSci Biotechno Biochem. 2007;71(3):728–734. doi: 10.1271/bbb.60568. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto K., Mizumoto H., Nakazawa K., Ijima H., Funatsu K., Kajiwara T. Hepatic differentiation of mouse embryonic stem cells in a bioreactor using polyurethane/spheroid culture. Transplant Proc. 2008;616:614–616. doi: 10.1016/j.transproceed.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 12.Gabriel E., Schievenbusch S., Kolossov E., Hengstler J.G., Rotshteyn T., Drobinskaya I. Differentiation and selection of hepatocyte precursors in suspension spheroid culture of transgenic murine embryonic stem cells. PLoS One. 2012;7(9) doi: 10.1371/journal.pone.0044912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takayama N., Nishimura S., Nakamura S., Shimizu T., Ohnishi R., Endo H. Transient activation of C-myc expression is critical for efficient platelet generation from human induced pluripotent stem cells. J Exp Med. 2010;207(13) doi: 10.1084/jem.20100844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishihara T., Okahashi N., Ueda N. Activin A induces apoptotic cell death. Biochem Biophys Res Commun. 1993;197(2):985–991. doi: 10.1006/bbrc.1993.2576. [DOI] [PubMed] [Google Scholar]
- 15.Touboul T., Chen S., To C.C., Mora-Castilla S., Sabatini K., Tukey R.H. Stage-specific regulation of the WNT/β-catenin pathway enhances differentiation of hESCs into hepatocytes. J Hepatol. 2016;64(6):1315–1326. doi: 10.1016/j.jhep.2016.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lade A.G., Monga S.P.S. Beta-catenin signaling in hepatic development and progenitors: which way does the WNT blow? Dev Dynam. 2011;240(3):486–500. doi: 10.1002/dvdy.22522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hay D.C., Fletcher J., Payne C., Terrace J.D., Gallagher R.C.J., Snoeys J. Highly efficient differentiation of hESCs to functional hepatic endoderm requires ActivinA and Wnt3a signaling. Proc Natl Acad Sci Unit States Am. 2008;105(34) doi: 10.1073/pnas.0806522105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jho E., Zhang T., Domon C., Joo C., Freund J., Costantini F. Wnt/β -catenin/tcf signaling induces the transcription of Axin 2 , a negative regulator of the signaling pathway Wnt/ß-catenin/tcf signaling induces the transcription of Axin2 , a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22(4):1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang M., Li S., Anjum K.M., Gui L., Zhu S., Liu J. A double-negative feedback loop between Wnt–β-catenin signaling and HNF4α regulates epithelial–mesenchymal transition in hepatocellular carcinoma. J Cell Sci. 2013;126(24):5692–5703. doi: 10.1242/jcs.135053. [DOI] [PubMed] [Google Scholar]
- 20.Asai A., Aihara E., Watson C., Mourya R., Mizuochi T., Shivakumar P. Paracrine signals regulate human liver organoid maturation from iPSC. Development. 2017;144:1056–1064. doi: 10.1242/dev.142794. dev.142794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gieseck R.L., Hannan N.R.F., Bort R., Hanley N.A., Drake R.A.L., Cameron G.W.W. Maturation of induced pluripotent stem cell derived hepatocytes by 3D-culture. PLoS One. 2014;9(1) doi: 10.1371/journal.pone.0086372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winkle Van A.P., Gates D., Kallos M.S. Mass transfer limitations in embryoid bodies during human embryonic stem cell differentiation. Cells Tissues Organs. 2012;196:34–47. doi: 10.1159/000330691. [DOI] [PubMed] [Google Scholar]
- 23.Mckee C., Chaudhry G.R. Colloids and Surfaces B : biointerfaces Advances and challenges in stem cell culture. Colloids Surfaces B Biointerfaces. 2017;159:62–77. doi: 10.1016/j.colsurfb.2017.07.051. [DOI] [PubMed] [Google Scholar]
- 24.Cui X., Hartanto Y., Zhang H. Advances in multicellular spheroids formation. J R Soc Interface. 2017;22(147) doi: 10.1098/rsif.2016.0877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartosh T.J., Ylöstalo J.H., Mohammadipoor A., Bazhanov N., Coble K., Claypool K. Aggregation of human mesenchymal stromal cells ( MSCs ) into 3D spheroids enhances their antiin fl ammatory properties. Proc Natl Acad Sci Unit States Am. 2010;107(31):13724–13729. doi: 10.1073/pnas.1008117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varum S., Rodrigues A.S., Moura M.B., Momcilovic O., Easley C.A., Ramalho-Santos J. Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS One. 2011;6(6) doi: 10.1371/journal.pone.0020914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pettinato G., Ramanathan R., Fisher R.A., Mangino M.J., Zhang N., Wen X. Scalable differentiation of human iPSCs in a multicellular spheroid-based 3D culture into hepatocyte-like cells through direct wnt/β-catenin pathway inhibition. Sci Rep. 2016;6 doi: 10.1038/srep32888. September. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miyamoto D., Nakazawa K. Differentiation of mouse iPS cells is dependent on embryoid body size in microwell chip culture. J Biosci Bioeng. 2016;122(4) doi: 10.1016/j.jbiosc.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 29.Katsuda T., Teratani T., Mahfuz M. Hypoxia efficiently induces differentiation of mouse embryonic stem cells into endodermal and hepatic progenitor cells. Biochem Eng J. 2013;74:95–101. [Google Scholar]
- 30.Yanagida A., Ito K., Chikada H., Nakauchi H., Kamiya A. An in vitro expansion system for generation of human iPS cell-derived hepatic progenitor-like cells exhibiting a bipotent differentiation potential. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0067541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanaka M., Miyajima A. CPM is a useful cell surface marker to isolate expandable Bi-potential liver progenitor cells derived from human iPS cells. Stem Cell Rep. 2015;5(4):508–515. doi: 10.1016/j.stemcr.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu B., He Z., You P., Han Q., Xiang D., Chen F. Article reprogramming fibroblasts into bipotential hepatic stem cells by defined factors. Stem Cell. 2013;13(3):328–340. doi: 10.1016/j.stem.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Tanimizu N., Miyajima A., Mostov K.E. Liver progenitor cells develop cholangiocyte-type epithelial polarity in three-dimensional culture. Mol Biol Cell. 2007;18(April):1472–1479. doi: 10.1091/mbc.E06-09-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sampaziotis F., De Brito M.C., Geti I., Bertero A. Directed differentiation of human induced pluripotent stem cells into functional cholangiocyte-like cells. Nat Protoc. 2017;12(4):814–827. doi: 10.1038/nprot.2017.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogawa M., Ogawa S., Bear C.E., Ahmadi S., Chin S., Li B. Directed differentiation of cholangiocytes from human pluripotent stem cells. Nat Biotechnol. 2015;33(8):853–861. doi: 10.1038/nbt.3294. [DOI] [PubMed] [Google Scholar]
- 36.Zhao D., Chen S., Cai J., Guo Y., Song Z., Che J. Derivation and characterization of hepatic progenitor cells from human embryonic stem cells. PLoS One. 2009;4(7) doi: 10.1371/journal.pone.0006468. [DOI] [PMC free article] [PubMed] [Google Scholar]