Abstract
The development of induced pluripotent stem cell (iPSC) techniques has solved various limitations in cell culture including cellular proliferation and potency. Hence, the expectations on wider applications and the quality of manufactured iPSCs are rapidly increasing. To answer such growing expectations, enhancement of technologies to improve cell-manufacturing efficiency is now a challenge for the bioengineering field. Mechanization of conventional manual operations, aimed at automation of cell manufacturing, is quickly advancing. However, as more processes are being automated in cell manufacturing, it is need to be more critical about influential parameters that may not be as important in manual operations. As a model of such parameters, we focused on the effect of mechanical vibration, which transmits through the vessel to the cultured iPSCs. We designed 7 types of vertical vibration conditions in cell culture vessels using a vibration calibrator. These conditions cover a wide range of potential situations in cell culture, such as tapping or closing an incubator door, and examined their effects by continuous passaging (P3 to P5). Detailed evaluation of cells by time-course image analysis revealed that vibrations can enhance cell growth as an early effect but can negatively affect cell adhesion and growth profile after several passages as a delayed effect. Such unexpected reductions in cell quality are potentially critical issues in maintaining consistency in cell manufacturing. Therefore, this work reveals the importance of continuous examination across several passages with detailed, temporal, quantitative measurements obtained by non-invasive image analysis to examine when and how the unknown parameters will affect the cell culture processes.
Keywords: Induced pluripotent stem cell, Mechanical vibration stress, Image analysis, Cell quality, Colony tracking analysis
1. Introduction
Recent advances in cell engineering technology have allowed for the widespread use of human cells in life sciences research. Due to successful advancements in stem cell research, such as the generation of induced pluripotent stem cells (iPSCs) [1], [2], human cells are now recognized as biological material that can be manufactured and distributed globally at industrial scales. Triggered by high demands for such cellular products, both in drug discovery and therapeutic applications [3], [4], there has been a growth in technological development for cell manufacturing processes. Beyond techniques derived from cell biology, there are also technological advancements in engineering that are accelerating cell manufacturing [5], [6], [7], [8], [9], [10], [11], [12], [13], [14]. Because of these rapid advancements in cell culture technologies, it is now an industrial revolution era for cell manufacturing [15], [16], [17]. However, since new technology is being introduced into cell culture methods so quickly, there are critical issues that have not yet been fully investigated to optimize these processes for advancing manufacturing of cell cultures.
Advancement in larger scale cell manufacturing processes has historically been limited because they depend on manual handling. Experienced technicians can perform complex procedures in manual cell culture; however, it is difficult to standardize techniques for consistency and for large-scale manufacturing. To address this weakness in manual processes, robotic technology has been introduced for automated culture operations [9], [10], [11], [12], [13], [14]. To mimic or replace the conventional human manual operations by robotic technology, it is essential to quantitatively understand the parameters related to an operation, and its effects on cells. However, in most of the cell culture–related operations, such as pipetting, tapping, and movements of culture vessels, have rarely been quantitatively investigated.
In this report, we investigated the effect of mechanical vibration which transmits through culture vessels to the cells. We chose 7 different types of vertical vibrational movements (10 min/day for 7 days) and evaluated their effect on the quality of iPSCs. For these conditions, we mimicked common sources of vibrational in manual cell culture, such as repeated closing of incubator doors and tapping of plates during the cell collection process, as well as some less common, more extreme impacts. There is an increasing understanding of cellular responses to mechanical/physical stress in the field of mechanobiology [18], [19]. Previous studies have examined the effect of vibrational stresses on cellular potency [20], [21], [22], [23], [24]. However, their vibration conditions are not relevant to the vibration conditions that are important in cell manufacturing. Hence, this work is one of the first investigations that examined the effect of vibrations that practically involved in cell manufacturing.
To quantitatively monitor cellular responses to the vibrational stress throughout several passages during cell culture, we applied label-free iPSC colony image analysis, which our group has previously developed to evaluate iPSC quality based on their morphology [25], [26]. By detailed analysis of phenotypic changes over time together with conventional end-point marker staining, we profiled when and how the mechanical vibrations influenced iPSC quality (e.g. adhesion, proliferation, and undifferentiated status in this work). Since this work was designed to investigate vibrational effects in cell manufacturing which commonly culture cells continuously for expansion, we examined cell phenotypes not only after a single passage but also after three continuous passages. Our data reveal the existence of two types of critical effects from mechanical vibration stress, “the early” and “the delayed,” that can disturb consistent cell manufacturing. Moreover, by comparing image-based evaluation and conventional marker staining evaluation, our study highlights the importance of time-course monitoring of phenotypic responses from cultured cells to accurately detect unexpected effects from vibration. In the coming era, where more manual processes become mechanized, such detailed investigation of technology-derived stimuli will be important in the process design for cell manufacturing.
2. Materials and methods
2.1. Cell culture
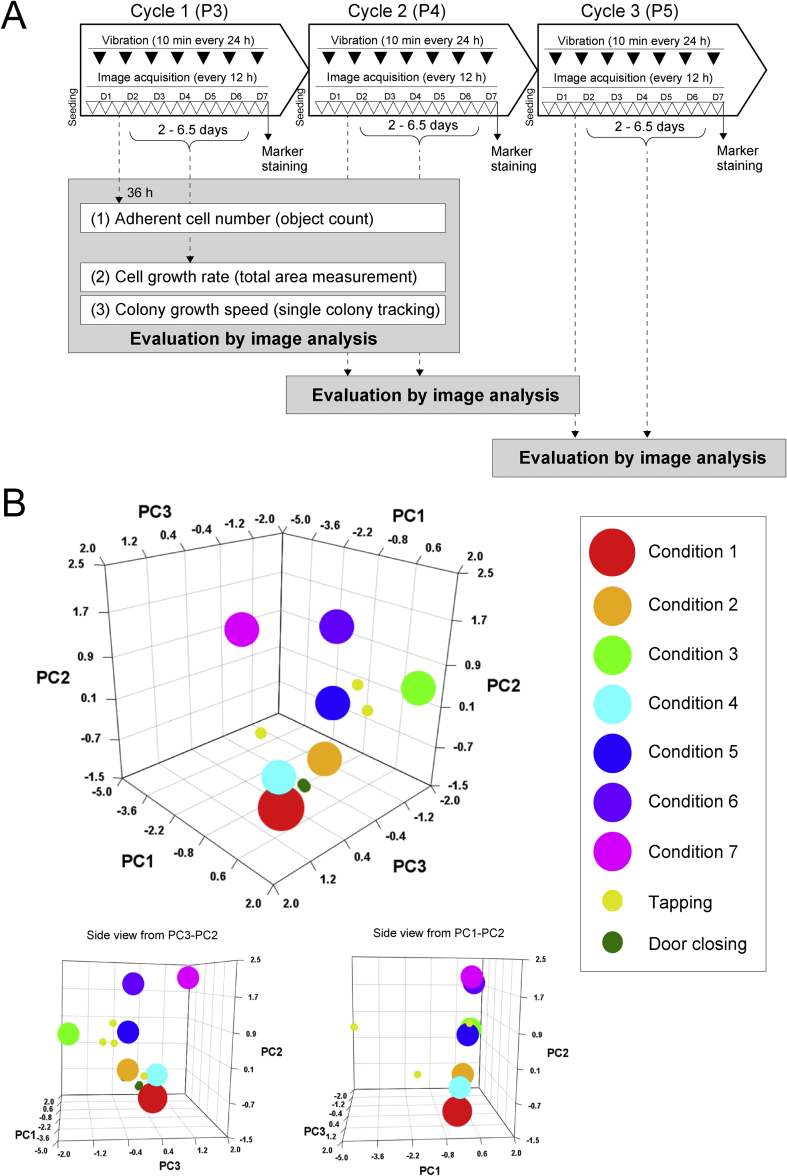
Human iPSC line 201B7-1A, a sub-clone of their parental clone 201B7, was provided by the Center for iPS Cell Research and Application (CiRA), Kyoto University. We chose 201B7-1A cells because they are prone to chromosomal abnormalities and morphological changes, making them ideal for observing phenotypic changes in response to external stimuli. Cells were maintained in StemFit AK02N (Ajinomoto, Tokyo, Japan) on iMatrix-511 coated plates (Nippi, Tokyo, Japan). ROCK inhibitor was only added to the media the first day after seeding (Wako Pure Chemical Industries, Ltd., Osaka, Japan). Media were changed every day. Cells were dissociated into single cells with TrypLE Select (Life Technologies, Carlsbad, CA, USA) and gentle pipetting. Cells dissociated into single cells were seeded at the concentration of 5000 cells/well in 6-well plates (Corning Life Sciences, Corning, NY, USA). To examine vibrational effects on the early passages of iPSCs, cells were examined after passage three (P3), which we labeled as cycle 1. All sample cells were continuously passaged from the 6-well plate on day 7 of each cycle to a new 6-well plate and continued for three cycles to passage 5 (P5, cycle 3). A schematic of the culturing cycles is illustrated in Fig. 1A.
Fig. 1.
(A) Schematic illustration of mechanical vibration experiment in this work. Vertical mechanical vibration was applied to the 6-well plate for 10 min every 24 h after the medium change. Phase-contrast microscopic images were acquired every 12 h. Cells were passaged continuously from P3 (Cycle 1) to P5 (Cycle 3). On the 7th day, the samples treated with vibration were collected, and seeded into a new 6-well plate. At the same time, partial samples were stained to evaluate the undifferentiation marker (B) Visualization of relative distances between designed conditions in this work. Four related parameters (acceleration, frequency, amplitude, energy) are visualized by principal component analysis. The proportion of variance for each principal component (PC) is PC1 (0.56), PC2 (0.27), and PC3 (0.16). The loadings are: acceleration: frequency: amplitude: energy = PC1 (−0.04, 0.42, −0.65, −0.64), PC2 (0.89, −0.43, 0.09, 0.13), PC3 (0.46, −0.80, −0.25, −0.30). The color representation is visualized in the legend. Representative four points from tapping and closing of incubator door condition measured in the preliminary examination is visualized with smaller dots to indicate the range of vibration.
2.2. Mechanical vibration conditions
To simulate various mechanical vibrations in the culture vessel, plate holders were attached to a 9100D portable Shaker Vibration Calibrator (The Modal Shop, Inc., Cincinnati, OH, USA) which guarantees accuracy and reliability of test with integrated reference accelerometer traceable to NIST (National Institute of Standards and Technology). On the vibration calibrator, the 6-well plate with adhered iPSCs was attached, held horizontally, and vibrated perpendicularly with two controllable parameters: acceleration [G] and frequency [Hz]. In all the conditions (Condition 1 to 7), sample vessel was once attached to the plate holders on 9100D, and the vibration conditions were set by the two controllable switches of acceleration and frequency according to the values on Table 1. The control condition was a static culture without any vibration (condition 1). We designed 6-types of vibration conditions (conditions 2–7) to include plausible sources of mechanical stress during cell culture, such as tapping (similar to condition 5) and closing of incubator doors (between condition 1 and 2), which were measured before the experiment (data not shown). We excluded the conditions that resulted in culture medium splashing outside of the wells. The plates were vibrated with the different conditions for 10 min every day for 7 days in each cycle (cycle 1 to 3) right after the daily media change. The schedule is illustrated in Fig. 1A.
Table 1.
List of mechanical vibration conditions.
| Condition name | Raw value |
Normalized value |
PC1 | PC2 | PC3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Acceleration [G] | Frequency [Hz] | Amplitude [mm] | Energy [J] | Acceleration [G] | Frequency [Hz] | Amplitude [mm] | Energy [J] | ||||
| Condition 1 | 0 | 0 | 0 | 0.0.E+00 | −0.849 | −1.138 | −0.357 | −0.319 | 0.00 | −1.32 | 0.71 |
| Condition 2 | 0.5 | 100 | 0.02 | 8.1.E−06 | −0.532 | −0.031 | −0.357 | −0.319 | 0.45 | −0.56 | −0.03 |
| Condition 3 | 0.5 | 300 | 0.00 | 9.0.E−07 | −0.532 | 2.183 | −0.357 | −0.319 | 1.37 | 0.40 | −1.80 |
| Condition 4 | 1 | 20 | 1.24 | 8.1.E−04 | −0.215 | −0.916 | −0.353 | −0.317 | 0.06 | −0.66 | 0.82 |
| Condition 5 | 2 | 150 | 0.04 | 5.8.E−05 | 0.418 | 0.522 | −0.357 | −0.319 | 0.63 | 0.52 | −0.04 |
| Condition 6 | 4 | 200 | 0.05 | 1.3.E−04 | 1.684 | 1.076 | −0.357 | −0.319 | 0.81 | 1.89 | 0.09 |
| Condition 7 | 5.5 | 70 | 0.56 | 2.0.E−03 | 2.634 | −0.363 | −0.355 | −0.313 | 0.16 | 2.11 | 1.67 |
| Tapping 1 | 2 | 200 | 0.02 | 3.2.E−05 | 0.418 | 1.076 | −0.357 | −0.319 | 0.86 | 0.76 | −0.48 |
| Tapping 2 | 2 | 1 | 992.95 | 1.3.E+00 | 0.418 | −1.127 | 3.211 | 3.503 | −4.80 | 0.62 | −0.76 |
| Tapping 3 | 1 | 200 | 0.01 | 8.1.E−06 | −0.215 | 1.076 | −0.357 | −0.319 | 0.89 | 0.20 | −0.77 |
| Tapping 4 | 1 | 1 | 496.47 | 3.2.E−01 | −0.215 | −1.127 | 1.427 | 0.638 | −1.79 | −0.47 | 0.25 |
| Door close 1 | 0.2 | 100 | 0.01 | 2.0.E−05 | −0.722 | −0.031 | −0.357 | −0.319 | 0.45 | −0.73 | −0.12 |
| Door close 2 | 0.2 | 50 | 0.04 | 7.8.E−05 | −0.722 | −0.584 | −0.357 | −0.319 | 0.22 | −0.97 | 0.32 |
| Door close 3 | 0.1 | 100 | 0.00 | 4.9.E−06 | −0.785 | −0.031 | −0.357 | −0.319 | 0.46 | −0.78 | −0.15 |
| Door close 4 | 0.1 | 50 | 0.02 | 2.0.E−05 | −0.785 | −0.584 | −0.357 | −0.319 | 0.23 | −1.02 | 0.29 |
2.3. Immunofluorescence
At day 7, the last day prior to passaging, rBC2LCN-635 (100-fold diluted; Wako Pure Chemical Industries, Ltd.) [27], [28] was added to new medium and incubated for 2 h at 37 °C with 5% CO2 to stain undifferentiated cells. Then, nuclei were stained with SYTOX Blue (10000-fold diluted; Thermo Fisher Scientific Inc., Waltham, MA, USA).
2.4. Image acquisition
To monitor the changes over time, phase-contrast images were acquired with BioStation CT (Nikon Corp., Tokyo, Japan) at 4 × magnification covering 1.6 cm2 in the center position of each well by 8 × 8 tiling with automated focus. Images were acquired every 12 h from the seeding of cells to the 7th day. Additionally, on the 7th day, fluorescent images of stained cells were acquired with BioStation CT at the same location as the phase-contrast images.
2.5. Image processing and analysis
All microscopic images were processed and quantified using CL-Quant software version 3.20 (Nikon Corp.). The iPSC colonies in the images were recognized in 5 steps (Fig. S1): (1) background adjustment, (2) recognition of colony, (3) removal of mis-identified noise (objects with diameter < 124 μm), (4) filling empty areas in the recognition mask and removing colonies that overlap with the edge of the image, and (5) quantification of colony morphology (area). For the measurement of colony staining rate, fluorescent images were binarized to identify the fluorescently positive pixels in the image. Then, their positive staining ratio per colony area were then measured in each colony, using the colony area recognition mask made from the phase contrast images (Fig. S2).
To quantify the number of adherent cells after 1.5 days, all objects in the phase-contrast images were identified. The time-point of 1.5 days for measuring adherent cells were decided because before this timepoint was confirmed to have stable cell adhesion of our iPSC culture condition which none of the cells will move even with tapping the vessel. Another reason is that too early timepoint can include more noise on cellular images by the condensation. These images were acquired after the medium change to remove detached cells from the field of view. Since iPSC culture is done with single cells, the number of recognized objects was regarded as the number of adherent cells, which also represents the number of seeded single cells.
To measure the cellular growth rate, the total sum of areas of all the colonies recognized in each phase-contrast image in the time course was first measured, and then normalized with the adherent cell number. Since growth rate and the colony recognition accuracy were most stable between 2 days till 6.5 days, we then calculated the specific growth rates per 12 h from 2 days to 6.5 days. The calculated specific growth rates were finally normalized to the first date for their comparison. There were very few biases of seeding between wells/plates (Fig. S3), indicating that our seeding was homogeneous enough between the samples, therefore we can discuss the data of adherent cells.
Nearly 250 to 2500 colonies were measured per sample to quantify the area of the colonies. The size of individual colonies in all of the experimental conditions were confirmed to be highly correlated to the area of stained nuclei (correlation coefficient = 0.91) (Fig. S4). Therefore, in this study, we quantified the changes in colony size, which we obtained from live imaging, to represent the growth rate of iPSC cells.
To measure the rate and its starting time of colony formation from single cells, colony-tracking analysis was performed with CL-Quant software (Nikon Corp.) according to the manufacturer's protocol. This measurement quantifies the trend of colony forming speed and timing in the culture vessel. Even if the final result after some period of time-window seems similar with similar number of colonies, such detailed trend can describe the difference of conditions by describing when and how each single colony started their growth. In the phase-contrast images acquired throughout the time-course, as colonies grew larger than a size threshold (diameter = 124 μm, which was the smallest colony), they were recognized as single colonies. Throughout the time-course images, the recognized single colony data were linked together individually by their centroid positions. In addition, we only used the tracking data that can continuously link the colonies for 4 repeated time-points. In other words, we only tracked the colonies which grew above the threshold size, and if they can be tracked over 2 days (4 × 12 h interval). Although there were many tracks that can be extracted, we examined all the tracks manually, and selected the perfect tracks for further analysis. In practical, if a colony remained beneath the threshold size in the intermediate time-points during their tracking, such track was excluded from the analysis because it consists of blank data. When colonies were fused together with the neighboring colonies and their tracks included such merged colonies, such data was excluded. Tracking data from different time-points were separated to illustrate rate of colony growth over time. To maintain accuracy of tracking, only the data from days 2 to days 6.5 were used.
To compare the colony staining rates between different vibration conditions (which resulted in different colony growth profiles), we randomly extracted the 50 colonies within a defined range of size (diameter > 300 μm). This threshold was defined to eliminate two effects from the analysis. One is the proliferation effect, which can bias the statistic comparison of average marker staining rates between conditions. The other is the effect of colony size, because rBC2LCN staining is inconsistent with small colonies, even if all of the cells have the same differentiating status. All the data processing and analysis was programmed with R (version 3.4.1) (R Development Core Team, https://www.r-project.org/). The statistical comparisons of the above measurement data between the control (condition 1) versus other conditions were evaluated by unpaired T-test with Bonferroni correction.
3. Results
3.1. Designing and mapping vibration conditions
To investigate the effect of mechanical vibrations on iPSCs, we first measured actual vibrations that appear in practical situations during cell culture. By measuring the vibration frequency and their acceleration amplitudes of culture vessel, we found that there were several representative categories in the vibrations found during cell culture (Table S1, Fig. S5).
We classified “periodic” types and “one-shot” types of vibration. Periodic vibrations were commonly caused by motor vibrations, which transmitted along metallic components that touch the culture vessels. Since these vibrations originate from motors, they impact the vessel periodically within short periods of time (seconds). One-shot vibrations were caused by direct impact on the culture vessel (e.g. tapping or transfer or dropping of vessels), or by indirect impacts from the surrounding equipment (e.g. from the incubator). The criteria of these two categories is not definite, because when cell culture requires repeated procedures, the one-shot type of vibration can be provided periodically over long periods of time, such as across hours or days.
Another aspect of mechanical vibrations in cell culture was whether it was caused unintentionally or intentionally. Most of the time, mechanical vibrations found in cell culture were unintentional since cells are commonly treated with extreme care. However, tapping of cell culture vessels is a common technique used to detach adherent cells after enzymatic treatment, which causes severe mechanical vibrations to the cells. Although it is a conventional technique, tapping has not been quantitatively standardized. Therefore, similar to other manual operations in the cell culture, tapping methods are widely varied even with the same protocol. Moreover, the degree of tapping needed is dependent on how well the cells are dissociating from the plate, therefore the procedure is dependent on a complex combination of physical movements as well as the amount of time. We categorized these mechanical vibrations from tapping as a mixture of periodic and one-shot types.
To understand the effect of such practical mechanical vibrations on cells during cell culture, we focused on some simplified conditions. In this work, we decided to focus on the vibrations vertical to the cell culture surface, which can be controlled by two parameters with a standardized calibrator: acceleration [G] and frequency [Hz]. However, to understand the total effect of vibrations, we decided to include the effect of two more parameters, amplitude [mm] (Formula 1) and energy [J] (Formula 2), which increasingly involve the effect of the two former parameters.
| (1) |
(A: amplitude [mm], Accel: acceleration [G: m/s2]. : frequency [Hz: times/s])
| (2) |
(E: Energy [J: kg·m2·s−2], m: weight [kg], f: frequency [Hz: times/s], A: amplitude [mm]).
Principal component analysis was used to visualize how different the 7 types of vibration conditions are based on these four parameters (Fig. 1B). For the chosen conditions, we tried to cover vibration types that range from more common sources (e.g. tapping and incubator door closing) to more extreme conditions.
3.2. Effect of mechanical vibration on iPSCs during early stages of cell culture
To investigate the effect of vibration conditions, we first focused on cells at lower passage number in the early stages of cell culture, soon after thawing from cryogenically frozen stocks.
Many cells require several passages, known as acclimatization, after thawing cryogenically frozen stocks to consistently proliferate. However, a satisfactory acclimatization period has never been quantitatively defined and is more of a “wait and see” period judged from experience. In cell manufacturing, this period is an ambiguous black-box period which is time consuming and expensive. As mechanized cell manufacturing is becoming more advanced, understanding this period is important for enhancing manufacturing efficiency. Therefore, we investigated the effect of mechanical vibration on iPSC quality at lower passage numbers. We began our investigation at P3, designated as cycle 1, which was the earliest passage number where we managed to prepare sufficient cell numbers for the assay after thawing from stocks.
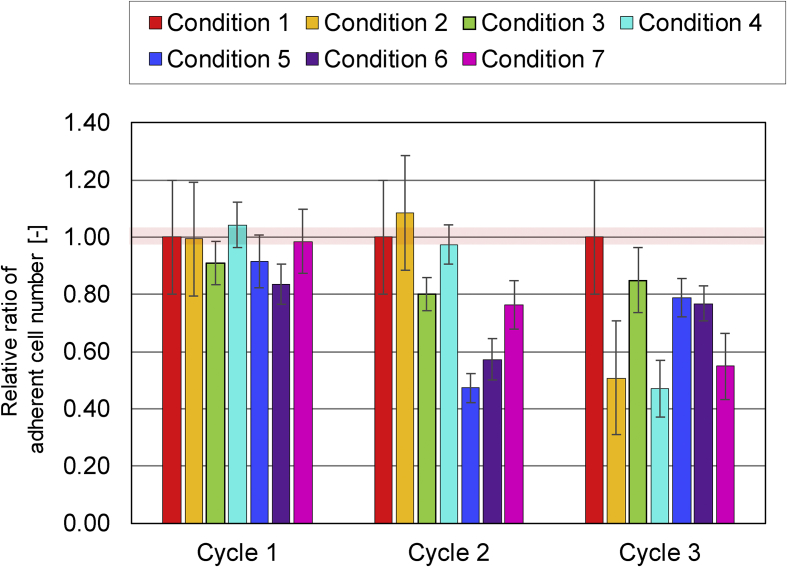
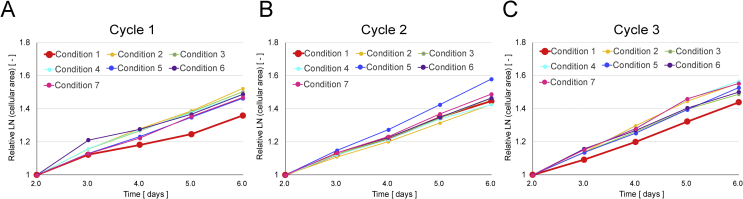
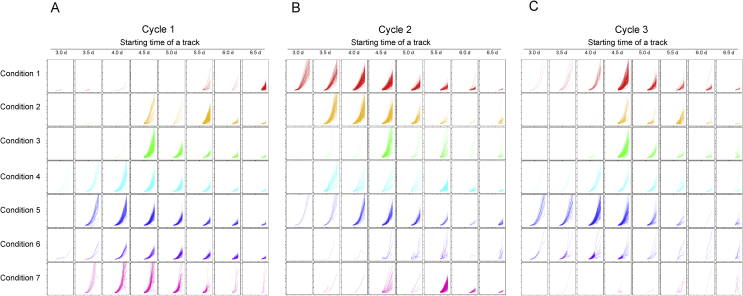
When the primary adherent cell numbers (1.5 days after seeding) were measured, there were no significant differences between the 7 types of conditions (Fig. 2A). However, when their growth rates were compared, most of all the vibration conditions showed increased growth rates compared to the non-vibrated control sample (Condition 1) (Fig. 3A). When the rates of colony formation were compared by colony tracking analysis, the seeded cells started to form larger colonies ewearlier when cells were vibrated (Fig. 4A). Faster colony formation rates correlated with more intense vibration conditions.
Fig. 2.
Effect of mechanical vibration on adherent cell number in Cycles 1 to 3. The adherent cell number is normalized in each cycle to condition 1 (the control without vibration). The color representation of bars is shown in the legend.
Fig. 3.
Effect of mechanical vibration on cell growth rate in Cycles 1 to 3. Each logarithm of total colony area in each time point (day 2 to day 6.5) was normalized to the adherent cell number to calculate specific growth rate every 12 h. Then each specific growth rate was normalized to day 2 in each cycle (A) Cycle 1 (B) Cycle 2 (C) Cycle 3.
Fig. 4.
Effect of mechanical vibration on the trend of individual colony growth rate and its growth starting times. Colonies which exceeded a diameter 124 μm was recognized and tracked by colony tracking analysis. The logarithm of tracked colony area transition is visualized separately by the timing when their tracking was started. The rise of tracks indicates that starting time and rate of single colonies in the adherent cell population. If the tracks appear in early times, it indicates that there was early-starting colony growth. If the gradient of tracks is higher, it indicates that those colonies achieved higher colony growth rate.
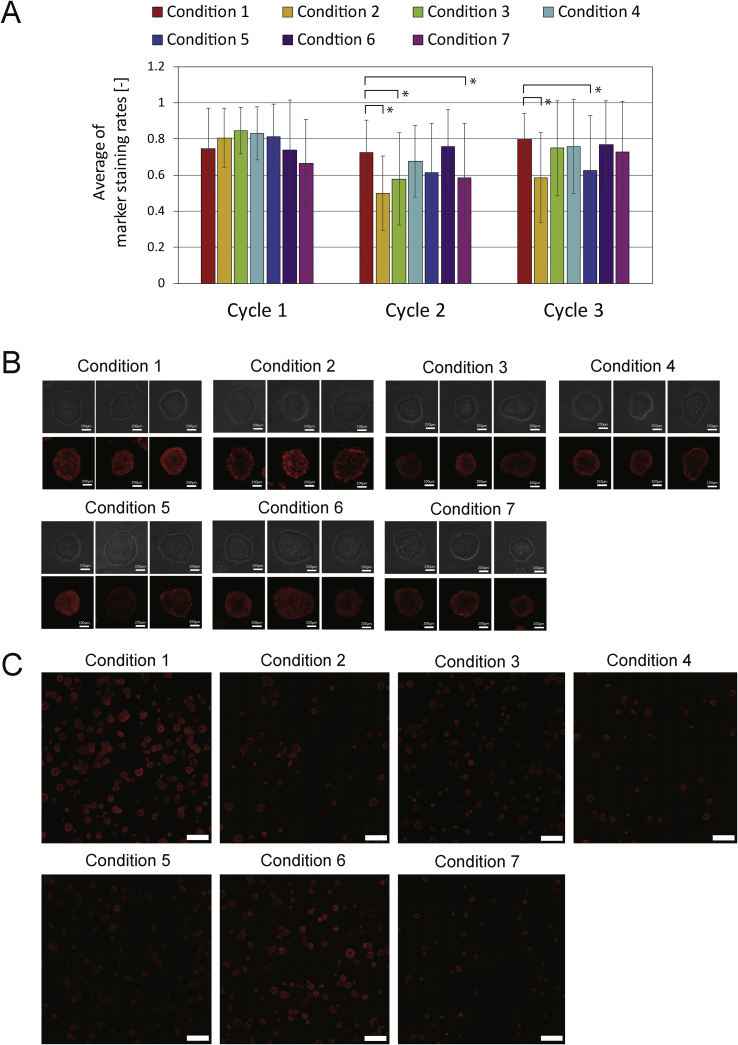
Cells in each condition were then stained with rBC2LCN, a marker of undifferentiated cells, at the end of P3 to compare their undifferentiation profile (Fig. 5). When 50 out of 250–2500 randomly collected colonies with a diameter >300 μm were compared, some differences between the conditions were found statistically, however it was difficult to find comprehensive rule between the marker staining rates and the strength of vibration conditions.
Fig. 5.
Effect of mechanical vibration on staining of a marker of differentiation status (A) Average of marker staining rates per 50 colonies (>diameter 300 μm) through cycle 1 to 3 (B) Representative images of colonies in each vibration conditions. White bars: 200 μm. Staining: rBC2LCN-635 (C) Representative images of colony population in each vibration conditions. White bars: 200 μm. Staining: rBC2LCN-635. White bars: 2000 μm. Staining: rBC2LCN-635.
Therefore, mechanical vibrational stress on iPSCs in early passages increased proliferation, but had an unclear effect on differentiation status (which can be detected by rBC2LCN).
3.3. Delayed effect of mechanical vibrations on iPSCs after continuous culturing
One of the difficulties in cell manufacturing is the unpredictable delay in time from when the cells are affected to when those effects are revealed. This makes it extremely difficult to identify critical parameters events throughout the long and complex cell culture process. Sudden changes in cell growth are common in cell culture over several passages. These unexpected changes in cell quality can hinder production consistency in cell manufacturing processes. To investigate the effect of repeated mechanical vibrations throughout extended culturing of iPSCs, we continuously evaluated cellular phenotypic responses over two or more continuous passage cycles.
When the numbers of primary adherent cells were compared throughout the passages (Fig. 2), the relative number of adherent cells after seeding gradually decreased in most of the vibration conditions. However, when the cell growth rates were compared, there were no significant changes between the conditions (Fig. 3). Therefore, as a relative comparison, the effect of vibration seemed to affect adhesion of cells to the plate, rather than decreasing cellular proliferation speed. Compared to an early effect where cell number was increased in cycle 1, chronic vibration actually decreased cell number by changing the adherent properties of the cells. In other words, our work indicated the vibration effect on cell yield by separating the problem into “growth rate problem” and “adhesion problem”.
Although cell growth rates were similar between vibration conditions, the rates and its staring times of individual colony growth, were different between vibration conditions as indicated (Fig. 4). Cells in condition 1 (control) showed recovered early-starting colony growth in the second cycle, as the effect of acclimatization. On the contrary, there was a sudden decrease in early-starting colony growth in the cycle 3 for conditions 4 and 7 (Fig. 1B), even if there was increased early-starting colony growth in the 1st and 2nd cycles. Cells in condition 5 and 6, which are relatively far from the control (condition 1) as a vibration condition, showed enhanced early-starting colony growth even at later passage numbers, but did not result in a sudden decrease in early-starting colony growth. Cells in condition 2 and 3, which are relatively close to the control condition, showed similar early-starting colony growth, however, there was a loss in the number of primary adherent cells (Fig. 3).
When a marker of undifferentiated cells was compared throughout the three cycles, there were some conditions which decreased staining, however, most of the vibrational effects did not affect differentiation status greatly (Fig. 5).
4. Discussion
Advancements in cell engineering technology have triggered a gradual transition from conventional cell culture with manual processes to more mechanized, and automated processes. However, for these newly developed methods, there are many parameters that must be considered that may not have been as important in manual processes. To effectively and safely utilize this new technology in cell manufacturing processes, especially for therapeutic uses, it is important to identify and understand these parameters.
We evaluated a potentially influencing factor, mechanical vibration, which exists in cell culture processes but is rarely considered or quantitatively profiled. As manual operations are being replaced by mechanized technology, the stress from such mechanical vibrations in the culture should be considered to design the cell manufacturing process.
In this study, we investigated the effect of vibrational stress on cells by designing simplified, accelerated tests, combined with real-time image analysis. In this work, we examined the effect of 7 conditions of vertical vibrations on iPSCs. Although we have carefully designed the 7 conditions to cover a wide range of potential vibrational effects, our conditions are still limited and sparse compared to the numerous potential examples that can happen during cell culture. More detailed data are needed to understand the detailed mechanisms behind how such vibration affects cellular profiles. However, it should be noted that the design of vibration conditions was not simple. If the vibration condition is too intense, then the physical stress can simply lead to cell death. If the vibration condition is too weak, it cannot be stably or precisely measured as investigation data. Although our examined conditions are not sufficient, our designed conditions provide a starting point for quantitative evaluation since there are no previous studies reporting such a variety of vibrations on iPSCs with regard to manufacturing processes.
Throughout our investigation, we found that the vibration stress has a major effect on cellular production efficiency. However, interestingly, there were two paradoxical effects depending on the stage of cells. One is a positive effect, which enhances the rate of cell proliferation in their early passages, which we designated “early effect.” However, the other is a negative effect, which decreases the number of cells that adhere to the plate and rate of colony formation when cells were passaged continuously, which we designated “delayed effect”. We concluded that for cell manufacturing purposes, which essentially involve several continuous passages to obtain a certain yield of cells, the delayed effect on cells is more important to evaluate when the effect of parameter is unknown; therefore, evaluation of a single passage cycle is not enough.
Staining with a marker for undifferentiated cells showed that our designed vibration conditions did not critically disturb the potency of iPSCs. We also observed OCT 3/4 marker staining with several vibration conditions; however, there were no significant differences between the vibrated samples and non-vibrated samples (data not shown). However, it is possible that if we compare the quality of iPSCs with different markers, or after their differentiation process, we might find the differences even within the present conditions. Further examination is necessary, and it should be noted that the comparison of iPSC quality by immuno-staining is difficult when their adhesion/growth rates are affected. When we quantified the marker staining rates in all sizes of colonies, we found that there was size-dependent sensitivity to marker staining (Fig. S6). When colonies exceed a certain size, their marker staining rates were similar. However, staining in small sized colonies showed large variability, suggesting that both unstained and stained cells potentially exists even in the undifferentiation maintained culture. Therefore, when colony growth is different between two conditions, their size distribution will be different. In a population of cells that grow slowly, there are primarily small colonies, which result in inconsistent staining. In our investigation, mechanical vibrations affected cell growth profile, adhesion, and colony distribution. Therefore, in our analysis, only a randomly sampled number of colonies that exceeded a certain size was compared. However, such evaluation can neglect the details of total population differences. Therefore, if the effect of new parameters is necessary for evaluating cellular potency, these data should be carefully examined for whether it is reflecting a change in biology or simply reflecting a change in growth rate.
For evaluating the effects of vibrations on cells, we introduced an image analysis technique to quantitatively profile the responses of cells over time. By label-free image analysis, we measured unstained iPSC colonies in real-time in the culture vessel and quantified several parameters. First, the adherent cells were quantified, which reflected the adhesion potency of total cells. Compared to other parameters, adhesion was the most independent criteria, as it directly reflected the condition of total cells and was not influenced by the time-course effect. Second, the cell growth rate was quantified by measuring the total colony area in the vessel. These data reflected the average cellular proliferation rate of total cells (250–2500 colonies). By normalizing to their initial size, these data reflected whether cellular proliferation rate has changed. However, this second criteria masks the variety of information regarding cellular population. The rate of colonization, in contrast, reflects the variety of proliferation rates of individual cells and was quantified by colony tracking analysis. Images were also analyzed to quantitatively measure the staining rate of conventional marker of differentiation status. However, through our work, we found that a single end-point staining evaluation was insufficient for detecting and understanding the differences between the conditions we investigated. Moreover, we also found that commonly evaluated criteria, such as cell yield at the end of culture, was also not sufficient for understanding the differences between our conditions. This is because the “final cell yield” is a combined result of “numbers of adherent cells” and “rate of proliferation”, and is therefore not sensitive enough to determine the contributing effect on cells. Therefore, real-time quantification of cellular status by image analysis should be a powerful tool for understanding the total cell culture process, which is commonly a complex combination of processes.
In this study, the mechanisms behind the effects of mechanical vibration on cell growth were not investigated in detail. Therefore, our investigation is one of the few examples that show the both early and delayed effects of vibration on cells. Beyond physical vibrational stresses, we will be further expanding our investigation to observing the effect of shear stresses from the medium. For example, tapping is not only causing vibrational stress, but also shear stress to dissociate the cells. Such combined effects can make the situation more complex. Since there are many reports in the mechanobiology field studying the effect of shear stress on cells, there should be common factors that will help us understand the effect of vibration from a more biological view point. For further understanding of such combinational effect which triggered by “vibration of vessel”, we now consider that computational simulation work should be another effective approach to understand the effect of individual physical parameters.
In conclusion, in this work, we have demonstrated that there is a delayed effect of mechanical vibration in continuously passaged cells and concluded that vibration mainly disrupted cell adhesion. Although this work shows a limited example of mechanical vibration effects on cell manufacturing in the case of iPS cells, this work indicates the importance of such external stimuli which might be involved in technological advancement. To investigate the effects on other stem cells is one of our next investigations.
Acknowledgments
This research was supported by the Development of Cell Production and Processing Systems for Commercialization of Regenerative Medicine program of the Japan Agency for Medical Research and Development (AMED), grants-in-aid from the New Energy and Industrial Technology Development Organization (NEDO) (09C46036a), and the Japan Science and Technology Agency (JST) Program for Creating STart-ups from Advanced Research and Technology (START Program) program. We also greatly thank Professor Masahiro Kino-oka, Dr. Mee-Hae Kim, for the experimental help in measuring vibrational stresses in Fig. S5 and assisting in conceptual framework which was the backbone of this study.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.reth.2019.05.002.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Yamanaka S., Takahashi K., Okita K., Nakagawa M. Induction of pluripotent stem cells from fibroblast cultures. Nat Protoc. 2007;2:3081–3089. doi: 10.1038/nprot.2007.418. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K. SUPP/Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Normile D. iPS cell therapy reported safe. Science. 2017;355:1109–1110. doi: 10.1126/science.355.6330.1109. (80 - ) [DOI] [PubMed] [Google Scholar]
- 4.Aoi T. 10th anniversary of iPS cells: the challenges that lie ahead. J Biochem. 2016;160:121–129. doi: 10.1093/jb/mvw044. [DOI] [PubMed] [Google Scholar]
- 5.Kempner M.E., Felder R.A. A review of cell culture automation. JALA – J Assoc Lab Autom. 2002 [Google Scholar]
- 6.Gómez-Sjöberg R., Leyrat A.A., Pirone D.M., Chen C.S., Quake S.R. Versatile, fully automated, microfluidic cell culture system. Anal Chem. 2007;79:8557–8563. doi: 10.1021/ac071311w. [DOI] [PubMed] [Google Scholar]
- 7.Meyvantsson I., Warrick J.W., Hayes S., Skoien A., Beebe D.J. Automated cell culture in high density tubeless microfluidic device arrays. Lab Chip. 2008;8:717–724. doi: 10.1039/b715375a. [DOI] [PubMed] [Google Scholar]
- 8.Kato R., Iejima D., Agata H., Asahina I., Okada K., Ueda M. A compact, automated cell culture system for clinical scale cell expansion from primary tissues. Tissue Eng C Methods. 2010;16:947–956. doi: 10.1089/ten.TEC.2009.0305. [DOI] [PubMed] [Google Scholar]
- 9.Rodrigues C.A.V., Fernandes T.G., Diogo M.M., da Silva C.L., Cabral J.M.S. Stem cell cultivation in bioreactors. Biotechnol Adv. 2011 doi: 10.1016/j.biotechadv.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Terstegge S., Laufenberg I., Pochert J., Schenk S., Itskovitz-Eldor J., Endl E. Automated maintenance of embryonic stem cell cultures. Biotechnol Bioeng. 2007;96:195–201. doi: 10.1002/bit.21061. [DOI] [PubMed] [Google Scholar]
- 11.Thomas R.J., Anderson D., Chandra A., Smith N.M., Young L.E., Williams D. Automated, scalable culture of human embryonic stem cells in feeder-free conditions. Biotechnol Bioeng. 2009 doi: 10.1002/bit.22187. [DOI] [PubMed] [Google Scholar]
- 12.Koike H., Kubota K., Sekine K., Takebe T., Ouchi R., Zheng Y.W. Establishment of automated culture system for murine induced pluripotent stem cells. BMC Biotechnol. 2012;12 doi: 10.1186/1472-6750-12-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marx U., Schenk F., Behrens J., Meyr U., Wanek P., Zang W. Automatic production of induced pluripotent stem cells. Procedia CIRP. 2013 [Google Scholar]
- 14.Maddah M., Shoukat-Mumtaz U., Nassirpour S., Loewke K. A system for automated, noninvasive, morphology-based evaluation of induced pluripotent stem cell cultures. J Lab Autom. 2014;19:454–460. doi: 10.1177/2211068214537258. [DOI] [PubMed] [Google Scholar]
- 15.Kirouac D.C., Zandstra P.W. The systematic production of cells for cell therapies. Cell Stem Cell. 2008 doi: 10.1016/j.stem.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Ährlund-Richter L., De Luca M., Marshak D.R., Munsie M., Veiga A., Rao M. Isolation and production of cells suitable for human therapy: challenges ahead. Cell Stem Cell. 2009 doi: 10.1016/j.stem.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 17.Ratcliffe E., Thomas R.J., Williams D.J. Current understanding and challenges in bioprocessing of stem cell-based therapies for regenerative medicine. Br Med Bull. 2011;100:137–155. doi: 10.1093/bmb/ldr037. [DOI] [PubMed] [Google Scholar]
- 18.Wang J.H.C., Thampatty B.P. Chapter 7 mechanobiology of adult and stem cells. Int Rev Cell Mol Biol. 2008 doi: 10.1016/S1937-6448(08)01207-0. [DOI] [PubMed] [Google Scholar]
- 19.Earls J.K., Jin S., Ye K. Mechanobiology of human pluripotent stem cells. Tissue Eng B Rev. 2013;19:420–430. doi: 10.1089/ten.teb.2012.0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beaupré G.S., Stevens S.S., Carter D.R. Mechanobiology in the development, maintenance, and degeneration of articular cartilage. J Rehabil Res Dev. 2000;37:145–151. [PubMed] [Google Scholar]
- 21.Pollock R.D., Woledge R.C., Mills K.R., Martin F.C., Newham D.J. Muscle activity and acceleration during whole body vibration: effect of frequency and amplitude. Clin Biomech. 2010;25:840–846. doi: 10.1016/j.clinbiomech.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Xie L., Jacobson J.M., Choi E.S., Busa B., Donahue L.R., Miller L.M. Low-level mechanical vibrations can influence bone resorption and bone formation in the growing skeleton. Bone. 2006;39:1059–1066. doi: 10.1016/j.bone.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Lau E., Al-Dujaili S., Guenther A., Liu D., Wang L., You L. Effect of low-magnitude, high-frequency vibration on osteocytes in the regulation of osteoclasts. Bone. 2010;46:1508–1515. doi: 10.1016/j.bone.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang L., Hsu H.Y., Li X., Xian C.J. Effects of frequency and acceleration amplitude on osteoblast mechanical vibration responses: a finite element study. BioMed Res Int. 2016:1–15. doi: 10.1155/2016/2735091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kato R., Matsumoto M., Sasaki H., Joto R., Okada M., Ikeda Y. Parametric analysis of colony morphology of non-labelled live human pluripotent stem cells for cell quality control. Sci Rep. 2016;6 doi: 10.1038/srep34009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagasaka R., Matsumoto M., Okada M., Sasaki H., Kanie K., Kii H. Visualization of morphological categories of colonies for monitoring of effect on induced pluripotent stem cell culture status. Regen Ther. 2017;6:41–51. doi: 10.1016/j.reth.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tateno H., Matsushima A., Hiemori K., Onuma Y., Ito Y., Hasehira K. Podocalyxin is a glycoprotein ligand of the human pluripotent stem cell-specific probe rBC2LCN. Stem Cells Transl Med. 2013;2:265–273. doi: 10.5966/sctm.2012-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onuma Y., Tateno H., Hirabayashi J., Ito Y., Asashima M. RBC2LCN, a new probe for live cell imaging of human pluripotent stem cells. Biochem Biophys Res Commun. 2013;431:524–529. doi: 10.1016/j.bbrc.2013.01.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.