Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is a hereditary disease that causes a decline of kidney function that progresses in relation to the enlargement of kidney cysts.1 Tolvaptan, a selective vasopressin V2-receptor antagonist, suppresses the production of cAMP, which is expected to be beneficial for suppressing the growth of renal cysts in patients with ADPKD.2 The effectiveness of tolvaptan was demonstrated in a phase III international clinical trial (TEMPO 3:4 trial)3 and its use is recommended in the guidelines.4
Elevation of liver enzymes was reported in 4.9% of patients who received tolvaptan in the TEMPO 3:4 trial.3 Eight of 135 Japanese patients in the TEMPO 3:4 trial had a more than 3-fold increase above the upper limit of normal (ULN) in aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels; however, these levels recovered after the discontinuation of tolvaptan.5 Thus far, tolvaptan-associated liver enzyme elevation has recovered after cessation or dose reduction, and no cases of tolvaptan-associated severe liver failure have been reported.
We herein report a patient with ADPKD who underwent liver transplantation for tolvaptan-associated acute liver failure.
Case Presentation
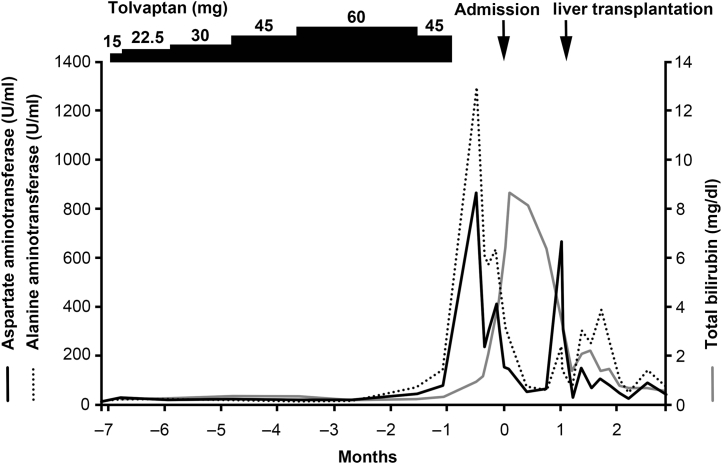
A 36-year-old Japanese woman with ADPKD presented to our hospital for a routine monitoring visit in October 2015. She had had pyelonephritis at 30 years of age. Her mother had also been diagnosed with ADPKD and was currently receiving hemodialysis. She had been receiving tolvaptan at a dosage of 15 mg per day since March 2017. Because her body weight and blood pressure were 51.6 kg and 125/77 mm Hg before the initiation of tolvaptan and there was no sign of dehydration, the dosage was gradually increased to 60 mg per day. Her total kidney volume and the rate of increase in kidney volume at the time of the initiation of tolvaptan were 1635 ml and 29.0%, respectively. Because her AST and ALT levels were elevated to 45 and 69 U/l, respectively, at 5 months after the initiation of tolvaptan treatment, the dose was reduced to 45 mg and was subsequently discontinued after 14 days because her AST and ALT levels were elevated to 82 and 142 U/l, respectively (Figure 1). Her body weight and blood pressure were 50.4 kg and 133/82 mm Hg at the time of the dose reduction and 48.8 kg and 149/89 mm Hg at the time of the discontinuation of tolvaptan. Despite the dose reduction and discontinuation of the drug, she experienced prolonged liver dysfunction and was admitted for a further workup.
Figure 1.
The clinical course after the initiation of tolvaptan.
A physical examination revealed height, 162 cm; body weight, 50 kg; temperature, 36.3 °C; blood pressure, 127/83 mm Hg; pulse rate, 77 beats per minute; and regular and pitting edema over the lower extremities. Her other physical findings were normal. She was taking domperidone (10 mg per day) and lactulose (30 ml per day). She was not using any over-the-counter supplements. She was not a habitual drinker.
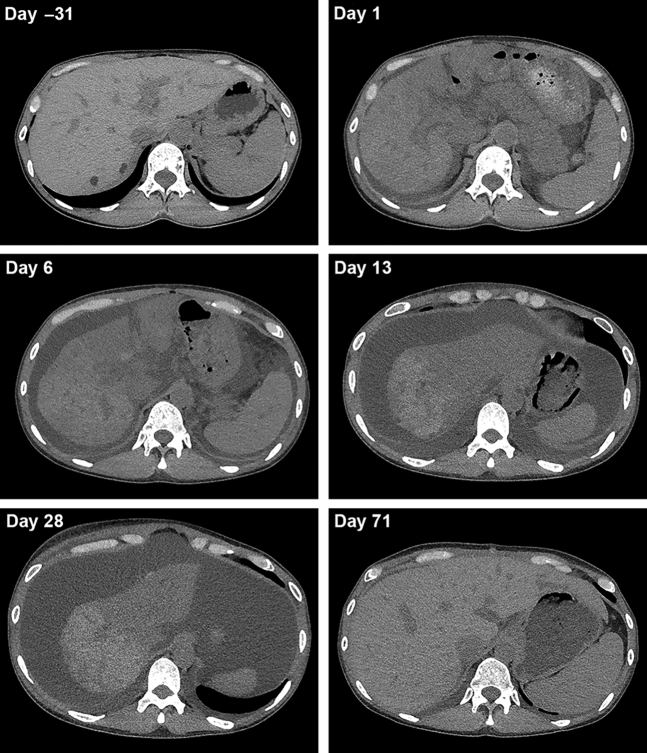
Her laboratory data on admission are summarized in Table 1. A urinalysis revealed no specific findings. Anemia and thrombocytopenia were observed. Her serum levels of aminotransferases and total bilirubin were increased, and her prothrombin time was prolonged. Her serum ammonia level was normal. Serological tests were negative, with the exception of an elevated Epstein-Barr virus IgG level, which suggested previous Epstein-Barr virus infection. Abdominal computed tomography (CT) showed hepatic atrophy with mild ascites (Figure 2, upper right) in comparison with a CT image from 1 month before admission (Figure 2, upper left) and repeated CT after 1 week showed the progression of the hepatic atrophy and an increased amount of ascites (Figure 2, middle left).
Table 1.
The laboratory data on admission
| Parameter | Patient value | Reference |
|---|---|---|
| Urinalysis | ||
| pH | 6 | 4.5–8.0 |
| Protein (g/gCr) | 0.12 | <0.15 |
| Occult blood | − | − |
| Hematology | ||
| White blood cells (/μl) | 5500 | 4000–9000 |
| Neutrophils (%) | 68.0 | 28–68 |
| Lymphocytes (%) | 21.0 | 20–60 |
| Eosinophils (%) | 1.0 | 0–8 |
| Red blood cells (× 104/μl) | 339 | 380–480 |
| Hemoglobin (g/dl) | 10.2 | 12.0–16.0 |
| Hematocrit (%) | 31.3 | 34.0–42.0 |
| Platelets (× 104/μl) | 11.4 | 12.0–40.0 |
| Coagulation | ||
| Activated partial thromboplastin time (s) | 39 | 21–36 |
| Prothrombin time (s) | 22 | 80–120 |
| Prothrombin time international normalized ratio | 2.82 | 0.88–1.08 |
| Fibrinogen (mg/dl) | 170 | 200–400 |
| Blood chemistry | ||
| Total protein (g/dl) | 5.1 | 6.5–8.5 |
| Albumin (g/dl) | 3.4 | 4.1–5.3 |
| Blood urea nitrogen (mg/dl) | 8.9 | 9.0–20.0 |
| Creatinine (mg/dl) | 0.64 | 0.50–1.10 |
| Aspartate aminotransferase (U/l) | 232 | 5–40 |
| Alanine aminotransferase (U/l) | 426 | 4–44 |
| Lactic dehydrogenation enzyme (U/l) | 269 | 110–225 |
| Alkaline phosphatase (U/l) | 305 | 120–340 |
| γ-Glutamyl transpeptidase (U/l) | 160 | 0–60 |
| Total bilirubin (mg/dl) | 5.2 | 0.2–1.3 |
| Direct bilirubin (mg/dl) | 3.5 | 0.0–0.5 |
| Uric acid (mg/dl) | 4.1 | 2.5–7.0 |
| Natrium (mmol/l) | 140 | 136–148 |
| Potassium (mmol/l) | 3.1 | 3.5–5.0 |
| Chlorine (mmol/l) | 107 | 98–110 |
| Calcium (mg/dl) | 8 | 8.5–10.5 |
| Bicarbonate ion (mmol/l) | 22.8 | 22–26 |
| C-reactive protein (mg/dl) | 0.19 | <0.30 |
| Ammonia (μg/dl) | 83 | 36–86 |
| Total cholesterol (mg/dl) | 96 | 150–219 |
| Triglyceride (mg/dl) | 35 | 50–150 |
| IgG (mg/dl) | 1168 | 870–1700 |
| IgA (mg/dl) | 89 | 110–410 |
| IgM (mg/dl) | 63 | 35–220 |
| Antinuclear antibody | <40 | <40 |
| Anti-mitochondrial antibody | − | − |
| Serological tests | ||
| IgM anti-HA antibody | − | − |
| IgM anti-HBc antibody | − | − |
| Anti-HBs antibody | − | − |
| HBs antigen | − | − |
| HBV-DNA (log U/ml) | <1.0 | <1.0 |
| Anti-HCV antibody | − | − |
| HCV-RNA (log U/ml) | <1.2 | <1.2 |
| Anti-HEV antibody | − | − |
| CMV-antigen | − | − |
| EBV-EA IgG | 0.9 | <0.4 |
| EBV-VCA IgM | − | <0.4 |
| EBV-VCA IgG | 1.3 | <0.4 |
| EBV-EBNA IgG | 3.4 | <0.4 |
CMV, cytomegalovirus; EA, early antigen; EBNA, Epstein-Barr nuclear antigen; EBV, Epstein-Barr virus; HA, hemagglutinin; HBc, hepatitis B core; HBV, hepatitis B virus; HBs, hepatitis B surface; HCV, hepatitis C virus; HEV, hepatitis E virus; VCA, viral capsid antigen.
Figure 2.
Abdominal computed tomography. The progression of the hepatic atrophy and increase of ascites was observed temporally between day −31 and day 28 and recovered after liver transplantation (day 71).
Although tolvaptan was stopped, the liver dysfunction persisted with conservative treatment. The patient was transferred to a tertiary-care center for consideration of liver transplantation. Follow-up CT showed the progression of hepatic atrophy, edematous change around the portal vein, and a large amount of ascites (Figure 2, middle right). Because the CT image on day 28 showed the further progression of hepatic atrophy (Figure 2, lower left), she was registered for deceased donor liver transplantation for drug-induced acute liver failure on day 25 and underwent deceased donor liver transplantation on day 31. The surgical findings showed markedly atrophied liver (19.5 × 13.0 × 7.0 cm) with an irregular surface and reddish brownish protuberance (Figure 3a). The cut surface of the liver showed massive brownish changes that indicated necrotic liver tissue macroscopically and a yellowish area that was thought to be relatively preserved liver tissue macroscopically, which was limited to the center of the right lobe (Figure 3b). Microscopic findings showed hemorrhaging, marked biliary ductular reaction, and total necrosis in the brownish area (Figure 3c). In the yellowish area, the hepatic structure was preserved; however, perivenular zonal necrosis with lymphoplasmacytic infiltration with macrophages was observed (Figure 3d). Eosinophilic infiltration also was seen in other necrotic brownish areas (Figure 3e). These changes were compatible with drug-induced liver injury (DILI). During the postoperative course, she suffered from several episodes of acute rejection, including antibody-mediated rejection, which were successfully treated with i.v. Ig and plasma exchange. The patient recovered and was discharged on the 86th day.
Figure 3.
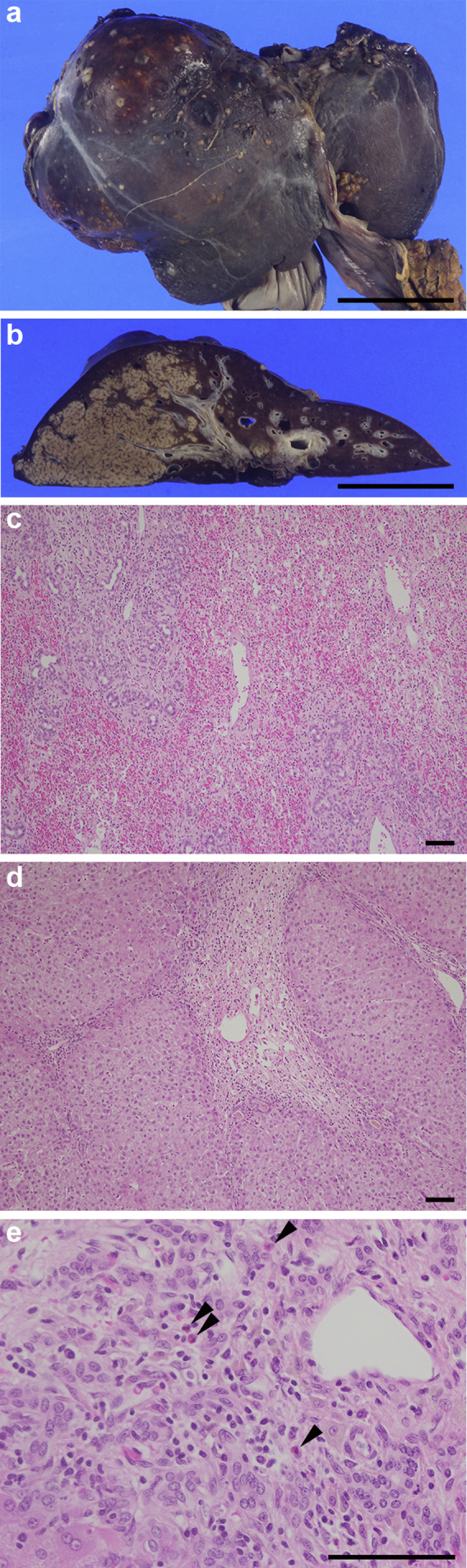
(a) The liver was markedly atrophied with an irregular surface and a reddish brownish protuberance. Bar = 5 cm. (b) The cut surface of the liver showed massive brownish changes. A yellowish area, which was limited to the center of the right lobe, also was seen. Bar = 5 cm. (c) Hemorrhaging, marked biliary ductular reaction, and total necrosis were observed in the brownish area. Hematoxylin-eosin stain was used. Bar = 100 μm. (d) Perivenular zonal necrosis with lymphoplasmacytic infiltration with macrophages was observed in the yellowish area. Bar = 100 μm. (e) Eosinophilic infiltration was observed in other necrotic brownish areas (arrowheads). Bar = 100 μm.
Discussion
We herein report a case of ADPKD in which liver transplantation was required because of the development of acute liver failure during treatment with tolvaptan. Tolvaptan-associated hepatocellular injury has been previously reported to occur between 3 and 18 months after the initiation of the drug and resolved within 4 months after the cessation of therapy.6 Although liver dysfunction was observed at 5 months after the initiation of tolvaptan treatment in the present case, it did not resolve, despite prompt dose reduction and the cessation of tolvaptan, leading to the eventual development of acute liver failure.
Tolvaptan was discontinued because the levels of AST and ALT were progressively elevated despite a dose reduction, with ALT increasing to more than 3 times the ULN after the dose reduction, as advised by the Food and Drug Administration.7 According to Food and Drug Administration guidance, AST or ALT levels exceeding 3 times the ULN signal a risk of severe DILI, although the details were not specified.7 Therefore, repeating liver enzyme tests approximately 2 or 3 times per week is recommended7; however, the follow-up laboratory examination was performed 2 weeks later in the present case because discontinuation of tolvaptan was thought to be the only effective therapy.
Hy's law can be applied to predict the prognosis of liver dysfunction. The risk of fatal liver dysfunction increases in cases in which AST or ALT levels are 3 times higher than the ULN and in which the serum total bilirubin is 2 times the ULN.8 Although the AST and ALT levels were 3 times the ULN in the present case, the elevation of the serum total bilirubin level was not recognized at the time of the diagnosis of DILI. Nevertheless, the patient developed liver failure that required liver transplantation.
The isolated liver showed brownish and yellowish areas. The brownish area indicated necrotic tissue macroscopically, and the findings of a microscopic analysis were compatible with necrosis. In contrast, the yellowish area might have indicated the surviving area macroscopically, although a microscopic analysis showed perivenular zonal necrosis. Regarding the patient’s clinical course, there was no sign of dehydration or any histologic changes suggesting dehydration-related ischemia. Eosinophilic infiltration in the brownish area might imply an allergic reaction to the drug. There was marked biliary ductular reaction, which prevented the assessment of biliary excretory disorder. Because etiologies such as acute viral hepatitis, alcoholic and autoimmune hepatitis, hepatobiliary disorders, nonalcoholic steatohepatitis, and cardiovascular disorders were interpreted as negative, the present case was diagnosed with tolvaptan-associated DILI.
DILI is divided into intrinsic DILI and idiosyncratic DILI.9 Although intrinsic DILI develops dose-dependently, idiosyncratic DILI develops dose-independently. Although the mechanism of idiosyncratic DILI is thought to be related to host reactions, drug metabolites, and environmental factors, it is not well documented. The outcomes of idiosyncratic DILI are generally good; however, ≤10% patients develop acute liver failure.S1 On the other hand, there are cases in which liver dysfunction progresses even if the causative drug is discontinued. The present case resulted in acute liver failure despite the discontinuation of tolvaptan, suggesting the possibility that tolvaptan caused idiosyncratic DILI. Tolvaptan is reported to inhibit hepatic bile acid transporters.S2 Mouse experiments demonstrated that the change in bile acid homeostasis might lead to susceptibility to DILI.S3 Currently, there are no definitive therapies for idiosyncratic DILI, and patients with advanced idiosyncratic DILI require transplantation.S1 Antihistamine, ursodeoxycholic acid, and corticosteroids were administered before liver transplantation in the present case.
In conclusion, we experienced a case of tolvaptan-associated acute liver failure that required liver transplantation (Table 2).
Table 2.
Teaching points
| Liver enzyme elevation, which is thought to be reversible after cessation or dose reduction, has been reported in approximately 5% of patients undergoing tolvaptan treatment. |
| To our knowledge, this is the first report of a case of tolvaptan-associated acute liver failure that required liver transplantation. |
| Although acute live failure is rare, caution might be needed if liver enzymes increase to more than 3 times the upper limit of normal. |
Disclosure
All the authors declared no competing interests.
Author Contributions
ME, KK, HM, SH, TH, RS, and AT participated in the acquisition of clinical data. ME, KK, SN, AH, and EI carried out the analysis of the patient’s clinical course and data interpretation. ME and KK wrote a draft of the manuscript, and HM, SH, SN, AH, EI, TH, RS, AT, KS, SI, and MI revised it critically. All authors read and approved the final manuscript. SUPPLEMENTARY MATERIAL Supplementary File (Word) Supplementary References.
Footnotes
Supplementary References.
Supplementary Material
References
- 1.Chapman A.B., Devuyst O., Eckardt K.U. Autosomal-dominant polycystic kidney disease (ADPKD): executive summary from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2015;88:17–27. doi: 10.1038/ki.2015.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miyazaki T., Fujiki H., Yamamura Y. Tolvaptan, an orally active vasopressin V(2)-receptor antagonist - pharmacology and clinical trials. Cardiovasc Drug Rev. 2007;25:1–13. doi: 10.1111/j.1527-3466.2007.00001.x. [DOI] [PubMed] [Google Scholar]
- 3.Torres V.E., Chapman A.B., Devuyst O. Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med. 2012;367:2407–2418. doi: 10.1056/NEJMoa1205511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horie S., Mochizuki T., Muto S. Evidence-based clinical practice guidelines for polycystic kidney disease 2014. Clin Exp Nephrol. 2016;20:493–509. doi: 10.1007/s10157-015-1219-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muto S., Okada T., Yasuda M. Long-term safety profile of tolvaptan in autosomal dominant polycystic kidney disease patients: TEMPO Extension Japan Trial. Drug Healthc Patient Saf. 2017;9:93–104. doi: 10.2147/DHPS.S142825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watkins P.B., Lewis J.H., Kaplowitz N. Clinical pattern of tolvaptan-associated liver injury in subjects with autosomal dominant polycystic kidney disease: analysis of clinical trials database. Drug Saf. 2015;38:1103–1113. doi: 10.1007/s40264-015-0327-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) Guidance for industry drug-induced liver injury: premarketing clinical evaluation. Available at: https://www.fda.gov/media/116737/download
- 8.Temple R. Hy's law: predicting serious hepatotoxicity. Pharmacoepidemiol Drug Saf. 2006;15:241–243. doi: 10.1002/pds.1211. [DOI] [PubMed] [Google Scholar]
- 9.Mosedale M., Watkins P.B. Drug-induced liver injury: advances in mechanistic understanding that will inform risk management. Clin Pharmacol Ther. 2017;101:469–480. doi: 10.1002/cpt.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.