Abstract
Introduction
We describe the characteristics of patients with moderate/advanced chronic kidney disease (CKD) according to receipt of lipid-lowering therapy (LLT), and whether they achieved low-density lipoprotein cholesterol (LDL-C) targets for high- and very high-risk patients.
Methods
CKD-REIN (NCT03381950), a prospective cohort study conducted in 40 nephrology clinics in France, enrolled 3033 patients with moderate (stage G3) or advanced (stage G4/G5) CKD (2013−2016) who had not been on chronic dialysis or undergone kidney transplantation. Data were collected from patients’ interviews and medical records. Patients were followed up at 1 year.
Results
Among 2542 patients (mean [SD] age 67 [13] years, 34% women) with LDL-C measurements at baseline (mean [SD] LDL-C 2.7 [1.1] mmol/l; cholesterol 4.8 [1.3] mmol/l), 63% were on LLT; 24% were at high (CKD stage G3, no cardiovascular disease [CVD] or diabetes) and 74% at very high (CKD stage G3 with diabetes or CVD, or CKD stage G4/5) cardiovascular risk. Among high-risk patients, 45% of those on statin and/or ezetimibe achieved the LDL-C treatment target (<2.6 mmol/l). Among very high-risk patients, the percentage at goal (<1.8 mmol/l) was 38% for CKD stage G3 and 29% for stage G4/5. There was a trend toward higher achievement of LDL-C targets with increasing LLT intensity (adjusted odds ratios for moderate vs. low intensity 1.20; 95% confidence interval 0.92–1.56; high vs. low intensity 1.46; 1.02–2.09; Ptrend = 0.036).
Conclusion
Many patients with CKD stage G3−G5 who are eligible for LLT are not treated, and those on LLT rarely achieve LDL-C targets.
Keywords: cardiovascular disease, chronic kidney diseases, lipid-regulating agents, lipids, low-density lipoprotein cholesterol
The global prevalence of CKD has increased substantially, driven primarily by population growth and ageing.1 Declining kidney function is associated with an increased risk of CVD, with CVD being the leading cause of death in people with CKD.2 Patients with stage G3 and G4 disease, respectively, are at twofold and threefold higher risk of cardiovascular death than those with normal kidney function3 and are considered to be at high and very high cardiovascular risk.4 Furthermore, as the lipid profile worsens with declining kidney function, most patients with moderate to advanced CKD have mixed dyslipidemia and a highly atherogenic lipid profile.4 The Kidney Disease: Improving Global Outcomes Lipid Work Group5 recommends treatment with a statin or statin plus ezetimibe for adults aged ≥50 years with CKD and estimated glomerular filtration rate <60 ml/min per 1.73 m2 who are not being treated with dialysis or kidney transplantation, or statin alone in those with estimated glomerular filtration rate ≥60 ml/min per 1.73 m2; despite this recommendation, many people with CKD are underdiagnosed and undertreated with LLT.6 Compared with end-stage kidney disease, little is known about lipid management in moderate to advanced CKD. The French Chronic Kidney Disease-Renal Epidemiology and Information Network (CKD-REIN) cohort study was designed to provide insights into the factors associated with progression of CKD to end-stage kidney disease or to its associated complications, and to evaluate clinical practices in patients with moderate to advanced CKD.7 The study highlighted the very high-risk profiles of patients with CKD.8 It also revealed the high prevalence of atheromatous and nonatheromatous CVD in these patients.9 Using the CKD-REIN study,7 we describe the characteristics of patients with moderate to advanced CKD according to whether they were receiving, and continued to receive at 1 year, LLT, and whether they achieved the European4 LDL-C goals for patients with high and very high cardiovascular risk.
Methods
CKD-REIN (NCT03381950) is an ongoing prospective French cohort study that enrolled patients with CKD who attended a routine nephrology clinic between July 2013 and April 2016.7, 8 The study was carried out in 40 nationally representative health facilities (public, private, nonprofit, and private for profit) that provided outpatient nephrology care. The protocol was approved by the French National Institute of Health and Medical Research (Inserm) institutional review board (IRB00003888). All patients provided informed consent to participate.
During the census phase of the study (2013−2015), we screened all patients aged ≥18 years with a proven diagnosis of moderate (stage G3) or advanced (stage G4) CKD based on 2 measures of estimated glomerular filtration rate ≥15 and <60 ml/min per 1.73 m2 (done ≥1 month apart) who had not previously been on chronic dialysis or undergone kidney transplantation, and had no plans to relocate. During the inclusion phase (mid-2013 to April 2016), these eligible patients were invited to participate in the study when they attended their routine nephrology visit. Eligible patients who had moved to stage G2 or G5 (without being on dialysis or transplanted) between the census and inclusion phases were included, assuming that this would reduce selection bias resulting from variability in kidney function.
Data were extracted by trained clinical research associates from patients’ medical records and hospitalization reports. Patient interviews, self-administered questionnaires, and routine biological measurements were collected at baseline and annually. The study database included information on patient characteristics (age, sex, educational level, smoking status, body mass index); medical history (CKD history, CVD history, and risk factors); laboratory measurements (CKD-Epidemiology Collaboration10–based estimated glomerular filtration rate, LDL-C, high-density lipoprotein cholesterol, triglycerides, glucose, and glycated hemoglobin), using the value taken closest to the time of inclusion, up to 6 months before the date of inclusion; and medication use (including type of LLT) and adherence to prescribed medications. Patients were classified as having hypertension, diabetes, albuminuria, and proteinuria as defined in Supplementary Text S1. Only treatments taken in the 3 months preceding inclusion were considered; treatments that were interrupted before this period and those started on the day of inclusion were not considered. Adherence to medication was determined using a validated score11; this score is based on 6 questions about patient behavior, regarding their actual use of prescribed treatment; for example, whether they tend to miss medications, or to forget to take them, or whether they think they have too many medications to take. A score of 6 indicates adherence to prescribed medication; 4 to 5 indicates poor adherence; and ≤3 indicates nonadherence. Patients reported the occurrence of muscle pains and cramps using the validated Kidney Disease Quality of Life instrument and were classified as being moderately to extremely bothered versus not at all or somewhat bothered.12
Cardiovascular history included atheromatous and nonatheromatous cardiovascular disease. Atheromatous cardiovascular disease included coronary artery disease (i.e., angina pectoris, myocardial infarction, coronary artery bypass graft, percutaneous coronary intervention); ischemic stroke or transient ischemic attack; and peripheral artery disease (i.e., history of amputation, angioplasty, or lower limb bypass due to atheromatous distal ischemic lesions). Nonatheromatous CVD encompassed heart failure in the absence of coronary artery disease, atrial fibrillation, other cardiac rhythm disorders, and valvular heart disease.
Low-, moderate-, and high-intensity statin treatments were defined as detailed in Supplementary Text S2. Cardiovascular risk level was defined according to the 2016 European Society of Cardiology guidelines.4 High cardiovascular risk was defined as moderate CKD (glomerular filtration rate 30−59 ml/min per 1.73 m2) with no history of atheromatous CVD or diabetes; very high cardiovascular risk was defined as moderate CKD with history of atherosclerotic CVD or diabetes or severe CKD (glomerular filtration rate <30 ml/min per 1.73 m2) regardless of the presence of atherosclerotic CVD or diabetes. The LDL-C goals were defined as <2.6 mmol/l (100 mg/dl) for patients at high cardiovascular risk and <1.8 mmol/l (70 mg/dl) for patients at very high cardiovascular risk.4
Data on adverse drug reactions were collected prospectively over the first year of follow-up by trained clinical research associates, using patients’ medical records. A pharmacologist evaluated and coded all adverse drug events, using the MedDRA international classification.
Data from patients on LLT versus not on LLT were compared using the Wilcoxon test or χ2 test. As patients with both CKD and type 2 diabetes have the highest cardiovascular risk, a complementary analysis in this subgroup was undertaken. LLT use by CKD stage was compared using the Cochran-Armitage trend P value for binary variables. LDL-C target attainment, by cardiovascular risk category, was assessed, overall and by treatment modality (no LLT, statin and/or ezetimibe, or other LLT). Among patients treated with a statin and/or ezetimibe, the odds ratios (95% confidence intervals) of reaching the LDL-C target associated with the intensity of treatment were estimated using logistic regression adjusted for age, sex, cardiovascular risk, treatment adherence, educational level, body mass index, and albuminuria. An analysis of patient characteristics at baseline according to changes in LLT over the first year of follow-up was also performed.
Results
Between July 2013 and April 2016, 3033 patients were enrolled in the French CKD-REIN study, of whom 2542 had data on medications and LDL-C values at inclusion. After excluding 15 patients with missing treatment data at 1 year, 63 who died before starting renal replacement therapy, 81 who started renal replacement therapy, and 12 who withdrew from the study, the study population for the 1-year analysis comprised 2371 patients (Supplementary Figure S1).
The mean (SD) age of the population was 67 (13) years, 34% were women, and 36% were obese (Table 1). More than half (53%) of the patients had a history of CVD (atherosclerotic in 37%), 90% had hypertension, and 42% had diabetes. Overall, 602 patients (24%) were classified at high cardiovascular risk, and 1886 (74%) at very high risk: 762 (30%) with CKD stage G3 and a history of atherosclerotic CVD or diabetes, and 1124 (44%) with CKD stage G4/5. The data required to determine level of cardiovascular risk were not available in 54 patients. Overall, 62% had <12 years of education and 63% were moderately adherent or nonadherent (score <6) with prescription medications. The mean (SD) LDL-C level was 2.7 (1.1) mmol/l; 51% had an LDL-C level <2.6 mmol/l and 21% <1.8 mmol/l.
Table 1.
Baseline characteristics, overall and according to LLT at baseline
| Characteristic | Overall populationa(N = 2542) | On LLTa(n = 1602) | No LLTa(n = 940) | Pvalueb |
|---|---|---|---|---|
| Age, yr, mean (SD) | 67 (13) | 68 (11) | 63 (15) | <0.001 |
| Women, n (%) | 875 (34) | 502 (31) | 373 (40) | <0.001 |
| BMI, kg/m2, mean (SD) | 29 (6) | 30 (6) | 27 (6) | <0.001 |
| Obese (BMI ≥30 kg/m2) | 889 (36) | 672 (43) | 217 (24) | <0.001 |
| Education <12 yr, n (%) | 1586 (62) | 1064 (66) | 522 (56) | <0.001 |
| Adherent to medications (score of 6), n (%) | 938 (37) | 539 (34) | 399 (43) | <0.001 |
| CKD stage, n (%) | 0.1 | |||
| ≤3A (≥45 ml/min per 1.73 m2) | 464 (18) | 270 (17) | 194 (21) | |
| 3B (30−44 ml/min per 1.73 m2) | 954 (38) | 608 (38) | 346 (37) | |
| 4 (15−29 ml/min per 1.73 m2) | 1025 (40) | 661 (41) | 364 (39) | |
| 5 (<15 ml/min per 1.73 m2) (not on dialysis) | 99 (4) | 63 (4) | 36 (4) | |
| Cardiovascular riskc | <0.001 | |||
| Normal | 54 (2) | 30 (2) | 24 (3) | |
| High | 602 (24) | 274 (17) | 328 (35) | |
| Very high | 1886 (74) | 1298 (81) | 588 (63) | |
| Albuminuria categories, n (%) (n = 2343) | <0.001 | |||
| Normal or minimal increase (A1) | 651 (28) | 382 (26) | 269 (31) | |
| Moderate increase (A2) | 732 (31) | 434 (29) | 298 (34) | |
| Severe increase (A3) | 960 (41) | 663 (45) | 297 (34) | |
| Smoker (current), n (%) | 298 (12) | 185 (12) | 113 (12) | 0.7 |
| History of CVD, n (%) | 1335 (53) | 977 (61) | 358 (39) | <0.001 |
| Atherosclerotic CVD, n (%) | 923 (37) | 742 (47) | 181 (20) | <0.001 |
| Blood pressure >130/80 mm Hg, n (%) | 2018 (81) | 1268 (81) | 750 (81) | 0.8 |
| Hypertension,dn (%) | 2292 (90) | 1503 (94) | 789 (84) | <0.001 |
| Diabetes, n (%) | 1071 (42) | 809 (51) | 262 (28) | <0.001 |
| Treatment for diabetes, n (%) | 895 (35) | 700 (44) | 195 (21) | <0.001 |
| Time since diagnosis of diabetes, yr, mean (SD) | 18 (12) | 18 (12) | 18 (12) | 0.1 |
| Moderately to extremely bothered by muscle pains, n (%) | 1145 (51) | 751 (53) | 394 (47) | 0.008 |
| Moderately to extremely bothered by cramps, n (%) | 890 (40) | 552 (39) | 338 (40) | 0.5 |
| Total cholesterol, mmol/l, mean (SD) | 4.8 (1.3) | 4.5 (1.2) | 5.4 (1.2) | <0.001 |
| LDL-C, mmol/l, mean (SD) | 2.7 (1.1) | 2.4 (1.0) | 3.2 (1.1) | <0.001 |
| LDL-C, mg/dl, mean (SD) | 104 (42) | 93 (38) | 123 (41) | <0.001 |
| LDL-C category, n (%) | ||||
| <2.6 mmol/l (100 mg/dl) | 1294 (51) | 1032 (64) | 262 (28) | <0.001 |
| <1.8 mmol/l (70 mg/dl) | 543 (21) | 456 (28) | 87 (9) | <0.001 |
| HDL-C, mmol/l, mean (SD) | 1.3 (0.5) | 1.3 (0.4) | 1.4 (0.5) | <0.001 |
| Triglycerides, mmol/l, mean (SD) | 1.8 (1.0) | 1.9 (1.1) | 1.7 (1.0) | <0.001 |
| Glucose, mmol/l, mean (SD) | 6.3 (2.2) | 6.6 (2.3) | 5.9 (1.8) | <0.001 |
| Glycated hemoglobin, %, mean (SD) | 6.4 (1.2) | 6.6 (1.2) | 6.0 (1.0) | <0.001 |
BMI, body mass index; CKD, chronic kidney disease; CVD, cardiovascular disease; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LLT, lipid-lowering therapy.
Percentages calculated based on available data. Percentages may not add to 100 due to rounding.
Wilcoxon test or χ2 test, comparing patients on LLT with those not on LLT.
Cardiovascular risk: normal (CKD stage G2), high (CKD stage G3, no CVD or diabetes), and very high (CKD stage G3 with diabetes or CVD, or CKD stage G4/5).
According to patient medical records or the use of antihypertensive medication.
At baseline, 63% of patients were on LLT. Compared with patients who were not on LLT, those on LLT were older, less likely to be women, and less likely to be adherent to prescription medications; but more likely to be obese, have <12 years of education, and to have a history of CVD, diabetes, or hypertension, and to report muscle pains (all P < 0.01; Table 1). In addition, they had lower LDL-C and high-density lipoprotein cholesterol levels, and higher triglyceride, glucose, and glycated hemoglobin levels (all P < 0.001).
More than half (55%) of the patients were receiving monotherapy (50% with a statin) and 8% were receiving ≥2 LLTs, most frequently a statin plus ezetimibe (7%) (Table 2). Use of statin LLT increased with progressing CKD stage (P = 0.004). Among 1481 patients on statin and/or ezetimibe LLT and LLT intensity data, 226 (15%), 883 (60%), and 372 (25%) were on high-, moderate-, and low-intensity doses, respectively.
Table 2.
Baseline treatment, overall and according to CKD stage
| Treatment | Overall(N = 2542) |
CKD stage |
Pvaluea | |||
|---|---|---|---|---|---|---|
| ≤3A(n = 464) | 3B(n = 954) | 4(n = 1025) | 5(n = 99) | |||
| LLT, n (%) | ||||||
| Statin | 1476 (58) | 237 (51) | 564 (59) | 615 (60) | 60 (61) | 0.004 |
| Fibrate | 77 (3) | 23 (5) | 23 (2) | 29 (3) | 2 (2) | 0.06 |
| Bile acid sequestrants | 3 (0.1) | 0 | 3 (0.3) | 0 | 0 | 0.5 |
| Ezetimibe | 231 (9) | 47 (10) | 80 (8) | 96 (9) | 8 (8) | 0.7 |
| Omega 3 fatty acids | 30 (1) | 7 (2) | 12 (1) | 11 (1) | 0 | 0.3 |
| Classes of LLT, n (%) | ||||||
| None | 940 (37) | 194 (42) | 346 (36) | 364 (36) | 36 (36) | |
| Monotherapy | 1393 (55) | 227 (49) | 537 (56) | 573 (56) | 56 (57) | |
| Statin | 1272 (50) | 196 (42) | 495 (52) | 528 (52) | 53 (54) | |
| Ezetimibe | 44 (2) | 9 (2) | 17 (2) | 17 (2) | 1 (1) | |
| Fibrate | 70 (3) | 21 (5) | 21 (2) | 26 (3) | 2 (2) | |
| Bile acid sequestrant | 2 (0.1) | 0 | 2 (0.2) | 0 | 0 | |
| Omega 3 fatty acid | 5 (0.2) | 1 (0.2) | 2 (0.2) | 2 (0.2) | 0 | |
| Double therapy | 203 (8) | 42 (9) | 68 (7) | 86 (8) | 7 (7) | |
| Statin + ezetimibe | 180 (7) | 36 (8) | 60 (6) | 77 (8) | 7 (7) | |
CKD, chronic kidney disease; LLT, lipid-lowering treatment.
LLT use by CKD stage was compared using the Cochran-Armitage trend P value for binary variables.
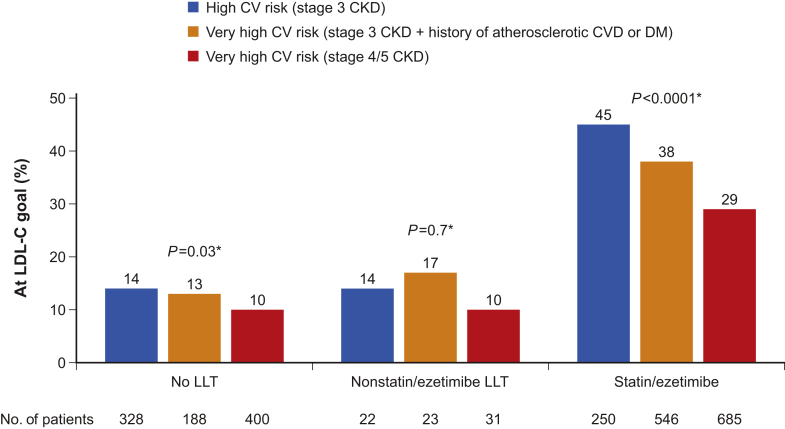
Among patients at high cardiovascular risk, 112 of 250 (45%) of those on statin and/or ezetimibe treatment achieved the LDL-C treatment target (<2.6 mmol/l), whereas among patients at very high cardiovascular risk, the percentage at goal (<1.8 mmol/l) was 38% for patients with CKD stage G3 (206 of 546) and 29% for patients with CKD stage G4/5 (199 of 685) (P < 0.0001; Figure 1). A minority (10%−17%) of patients who were not on any LLT or who were on LLT other than statins had LDL-C values at goal.
Figure 1.
Achievement of low-density lipoprotein cholesterol (LDL-C) goals by cardiovascular (CV) risk level and type of treatment. *P values comparing the 3 risk groups. CKD, chronic kidney disease; DM, diabetes mellitus; LLT, lipid-lowering therapy. Numbers below columns indicate the population size.
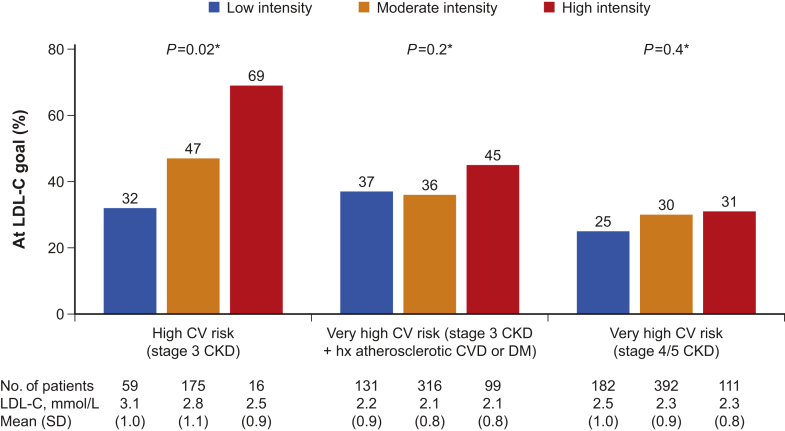
Achievement of LDL-C targets increased with increasing intensity of LLT among high-risk patients, and was lower with worsening risk profiles (Figure 2). There was no significant interaction with cardiovascular risk in the relation between LLT intensity and achievement of LDL-C targets (Pinteraction = 0.20). There was a trend toward higher achievement of LDL-C targets with increasing treatment intensity, before and after adjusting for potential confounders. Adjusted odds ratios for the achievement of LDL-C goals for moderate- and high- versus low-intensity LLT were 1.20 (95% confidence interval 0.92–1.56) and 1.46 (95% confidence interval 1.02–2.09), respectively (Ptrend = 0.036).
Figure 2.
Achievement of low-density lipoprotein cholesterol (LDL-C) goals according to treatment intensity (among patients on statin and/or ezetimibe) by cardiovascular (CV) risk level. *P values comparing the 3 treatment intensity levels within each CV risk group. CKD, chronic kidney disease; DM, diabetes mellitus; hx, history. Numbers below columns indicate the population size.
Among the subgroup of 930 patients with CKD and type 2 diabetes, LDL-C levels were significantly lower in those on LLT, and more had LDL-C levels <2.6 or <1.8 mmol/l compared with those not on LLT (Supplementary Table S1).
At 1 year, 1347 patients (57%) were on the same LLT as at baseline, 801 (34%) were still not on any LLT, and 223 (9%) had modified their LLT: 78 started LLT, 98 stopped LLT, and 47 changed the type of LLT.
Compared with patients who remained on the same LLT at 1 year, patients who stopped LLT were less likely to be adherent with medications, more likely to have reported cramps at baseline, and had a worse CKD stage (Table 3). No difference was apparent in total cholesterol or LDL-C level, or in the percentage of patients with LDL-C <1.8 mmol/l. During the first year of follow-up, no serious adverse drug reactions related to LLT were reported. Of the patients who discontinued LLT, 10% reported adverse drug reactions compared with <1% of those who continued LLT (Table 3).
Table 3.
Patient characteristics at baseline according to change in LLT at 1 year
| Characteristic | On LLTa(n = 1347) | Stopped LLTa(n = 98) | Pvalueb |
|---|---|---|---|
| Age, yr, mean (SD) | 68 (11) | 69 (12) | 0.6 |
| Women, n (%) | 424 (31) | 27 (28) | 0.4 |
| BMI, kg/m2, mean (SD) | 30 (6) | 29 (6) | 0.6 |
| BMI ≥30 kg/m2, n (%) | 559 (42) | 40 (41) | 0.8 |
| Adherent to medications (score of 6), n (%) | 470 (35) | 21 (21) | 0.008 |
| Moderately to extremely bothered by muscle pains, n (%) | 614 (52) | 52 (58) | 0.2 |
| Moderately to extremely bothered by cramps, n (%) | 444 (37) | 47 (53) | 0.004 |
| LLT-related adverse drug events, n (%) | 4 (0.3) | 10 (10) | <0.001 |
| CKD stage, n (%) | 0.01 | ||
| ≤3A (45−59 ml/min per 1.73 m2) | 243 (18) | 10 (10) | |
| 3B (30−44 ml/min per 1.73 m2) | 526 (39) | 37 (38) | |
| 4 (15−29 ml/min per 1.73 m2) | 548 (41) | 43 (44) | |
| 5 (<15 ml/min per 1.73 m2) (not on dialysis) | 30 (2) | 8 (8) | |
| Total cholesterol, mmol/l, mean (SD) | 4.5 (1.1) | 4.7 (1.3) | 0.07 |
| LDL-C, mmol/l, mean (SD); median (range) | 2.4 (0.9); 2.5 (0.2–7.2) | 2.5 (1.2); 2.4 (0.6–5.3) | 0.4 |
| LDL-C category, n (%) | |||
| <2.6 mmol/l (100 mg/dl) | 879 (65) | 53 (54) | 0.03 |
| <1.8 mmol/l (70 mg/dl) | 377 (28) | 32 (33) | 0.3 |
| HDL-C, mmol/l, mean (SD) | 1.3 (0.4) | 1.3 (0.4) | 0.4 |
| Triglycerides, mmol/l, mean (SD) | 1.9 (1.0) | 1.8 (1.1) | 0.9 |
BMI, body mass index; CKD, chronic kidney disease; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LLT, lipid-lowering therapy.
Percentages calculated based on available data.
Wilcoxon test or χ2 test.
Among the patients who were receiving statin monotherapy at baseline, 10 had changed to taking more than 1 LLT and 34 to more intensive LLT treatment at 1 year. Compared with those who did not change treatment intensity, these 44 patients had similar ages, sex, body mass index, CKD stages, and high-density lipoprotein cholesterol and triglyceride levels at baseline (data not shown), but had higher total cholesterol (4.9 ± 1.3 vs. 4.4 ± 1.0 mmol/l, respectively; P = 0.007) and LDL-C (2.8 ± 1.0 mmol/l vs. 2.3 ± 1.0 mmol/l; P < 0.001) levels. Finally, compared with their counterparts who remained untreated, patients who started LLT had similar ages, body mass index, CKD stages, and total and LDL-C levels (data not shown), but were more likely to be men (72% vs. 60%; P = 0.05) and to have lower high-density lipoprotein cholesterol (1.3 ± 0.5 mmol/l vs. 1.6 ± 0.5 mmol/l; P = 0.02) and higher triglyceride (2.0 ± 1.2 vs. 1.6 ± 0.9 mmol/l; P < 0.001) levels at baseline.
Discussion
This prospective French cohort study provides insights into the characteristics, lipid profile, and management of patients with moderate to advanced CKD who were not on dialysis or undergoing renal transplantation and were being treated by nephrologists. At inclusion in the study, 74% of patients were categorized as at very high cardiovascular risk and 24% at high cardiovascular risk. Sixty-three percent of the patients were on LLT at inclusion, most commonly statin monotherapy, with the percentage on treatment increasing with advancing CKD stage. Half of the patients had an LDL-C value that exceeded the recommended target for high-risk patients (<2.6 mmol/l) and only 21% achieved the target for very high-risk patients (<1.8 mmol/l).
Palmer et al. conducted a systematic review13 involving 50 studies (45,285 participants) that compared the benefits and harms of statins versus placebo, or no treatment, or the standard care, or another statin in adults with CKD who were not on dialysis. The authors found that statins consistently reduced the risk of death by 21% and major cardiovascular events by 28%, including in primary prevention, but they also highlighted a lack of evidence on the toxicity profile of statins in this population. These findings were consistent with a meta-analysis of 11 studies (21,295 patients with CKD, of whom 6857 were on dialysis) in which statin treatment reduced the risk of all-cause death by 34%, cardiovascular death by 31%, cardiovascular events by 45%, and stroke by 34% among patients who were not on dialysis.14 The Study of Heart And Renal Protection (SHARP) showed that lowering LDL-C with simvastatin 20 mg/d plus ezetimibe 10 mg/d versus placebo in 9270 patients with moderate to severe CKD (6247 of whom were not on dialysis) with no known history of myocardial infarction or coronary revascularization reduced the risk of major atherosclerotic events by 17% (risk ratio 0.83; 95% confidence interval 0.74–0.94).15 In light of this evidence, the Kidney Disease: Improving Global Outcomes committee recommends statin treatment in people aged ≥50 years with CKD who are not on dialysis, and combination therapy with a statin plus ezetimibe in those with stage G3 to G5 CKD.5 A German study, involving 5217 adults with moderately severe CKD under nephrological care, indicated that a substantial change in prescription practice would be necessary to meet these new recommendations.16 Consequently, it is perhaps not surprising that, despite these recommendations, a sizable percentage (37%) of the patients in the current study, all of whom were eligible for statin treatment, were not on LLT, only 9% were treated by ezetimibe, and 49% had LDL-C values that exceeded the recommended level for high-risk patients (<2.6 mmol/l).
Patients with CKD are at higher risk than those without CKD of side effects from LLT due to reduced renal excretion, polypharmacy, and the presence of comorbid conditions. However, a quantitative meta-analysis, based on data from 23 trials (39,419 participants), found that statins reduced the rates of microalbuminuria, proteinuria, and deaths, but did not slow the clinical progression of non–end-stage CKD.17 The Kidney Disease: Improving Global Outcomes guidelines recommend the use of moderate-intensity statins (e.g., atorvastatin 20 mg, rosuvastatin 10 mg, simvastatin 40 mg, pravastatin 40 mg, fluvastatin 80 mg, or pitavastatin 2 mg) in this population,5 which may explain the low rate of use of high-intensity statins in our study. Of note, a small percentage of the patients with CKD stage G4/5 in the present study (28 patients; 3%) received fibrate treatment, which is not recommended with an estimated glomerular filtration rate <30 ml/min per 1.73 m2.18
In a subanalysis of 904 French patients with an acute coronary syndrome or with stable coronary heart disease enrolled in the DYSIS II study19 (<5% of whom had CKD), statins were used in 97% of acute coronary syndrome patients at discharge for the index event and in 95% at 120-day follow-up, at which time 51% of the patients achieved the LDL-C goal. Among patients with stable CHD, 97% were on LLT (57% on high-intensity therapy), only 29% of whom met the LDL-C treatment target of <1.8 mmol/l. Fewer of the patients in the current study were on LLT than in DYSIS II, and only 21% had an LDL-C value <1.8 mmol/l.
In our study, 98 of the patients on LLT at inclusion had stopped taking it at 1 year. This change may reflect adherence issues or intolerance to LLT. Low social status, health literacy, presence of comorbid conditions, polypharmacy, negative perceptions about the treatments, high costs, and side effects can influence adherence to cardiovascular medications.20, 21, 22 In our study, older age, male sex, obesity, a shorter education, moderate adherence to prescription medications, history of CVD or diabetes, and hypertension medication were all more frequent among patients on LLT. Of interest, however, 65% of patients who remained on LLT achieved an LDL-C value of <2.6 mmol/l (and 28% <1.8 mmol/l) compared with 54% and 33%, respectively, who stopped LLT. Moreover, patients who discontinued LLT more often reported cramps at baseline and LLT-related adverse drug events over the 1-year period than their counterparts who continued LLT.
Our study has several strengths, including the representativeness of participating nephrology practice patterns and outpatients regarding the use of LLT in CKD; the large sample size of patients with detailed phenotyping; and the accuracy of the type and timing of LLT prescription. However, several limitations also must be acknowledged. Our findings are only generalizable to patients with CKD under nephrology care. We used a conservative approach to the evaluation of risk, limited to history of symptomatic CVD. However, most patients without a history of atherosclerotic CVD have imaging evidence of disease and should be categorized as at very high risk.4
In conclusion, people with moderate to advanced CKD who are not on dialysis or undergoing renal transplantation are at substantially increased risk of CVD, and the evaluation and management of their lipid profile forms an important part of their overall care. At least half of the patients in our study had LDL-C values that exceeded the recommended target and a substantial percentage were not taking any LLT or were on monotherapy. Greater use of evidence-based LLT, including combination LLT, would be likely to increase the achievement of LDL-C treatment targets and reduce the risk of morbidity and mortality due to coronary heart disease and stroke in patients with CKD. The impact of LLT on cardiovascular outcomes will be further evaluated.
Appendix
CKD-REIN Study Group
Carole Ayav (Clinical Epidemiology, Inserm CIC-EC, CHU de Nancy, Vandoeuvre-lès-Nancy, France); Christian Combe (Service de Néphrologie Transplantation Dialyse Aphérèse, Centre Hospitalier Universitaire de Bordeaux, Bordeaux, France; INSERM, U1026, Univ. Bordeaux Segalen, Bordeaux, France); Denis Fouque (Department of Nephrology, Centre Hospitalier Lyon Sud, Univ. Lyon, UCBL, Carmen, F-69495 Pierre-Bénite, France); Luc Frimat (Clinical Epidemiology, Inserm CIC-EC, CHU de Nancy, Vandoeuvre-lès-Nancy, France; Nephrology Department, CHU de Nancy, Vandoeuvre-lès-Nancy, France); Yves-Edouard Herpe (Picardie Biobank, CHU Amiens, Amiens, France); Maurice Laville (Department of Nephrology, Centre Hospitalier Lyon Sud, Univ. Lyon, UCBL, Carmen, F-69495 Pierre-Bénite, France); Ziad Massy (Centre for Research in Epidemiology and Population Health [CESP], Inserm UMRS 1018; Univ. Versailles-Saint Quentin, Univ. Paris-Saclay, Villejuif, France; Department of Nephrology, CHU Ambroise Paré, APHP, Boulogne, France); Bénédicte Stengel (CESP Centre for Research in Epidemiology and Population Health, Univ. Paris-Saclay, Univ. Paris Sud, UVSQ, UMRS 1018, F-94807 Villejuif, France); Céline Lange (Agence de Biomédecine, La Plaine-Saint Denis, France); Karine Legrand (Clinical Epidemiology, Inserm CIC1433, CHRU de Nancy, Vandoeuvre-lès-Nancy, France); Sophie Liabeuf (Pharmacology Department, Amiens University Hospital, Amiens, France; INSERM U1088, Jules Vernes University, Amiens, France); Marie Metzger (CESP Centre for Research in Epidemiology and Population Health, Univ. Paris-Saclay, Univ. Paris Sud, UVSQ, UMRS 1018, F-94807 Villejuif, France); and Elodie Speyer (CESP Centre for Research in Epidemiology and Population Health, Univ. Paris-Saclay, Univ. Paris Sud, UVSQ, UMRS 1018, F-94807 Villejuif, France).
CKD-REIN Collaborators
Bruno Moulin (CHU, Strasbourg, France); Gaétan Lebrun (CH, Aix-en-Provence, France); Éric Magnant (Polyclinique du Parc Rambot, Aix-en-Provence, France); Gabriel Choukroun (CHU, Amiens, France); Jean Philippe Bourdenx (Clinique St. Augustin, Bordeaux, France); Marie Essig (CHU Ambroise Paré, APHP, Boulogne, France); Raymond Azar (CH, Dunkerque, France); Mustafa Smati (CH, Epinal, France); Mohamed Jamali (Clinique Louis Pasteur, Essey-les-Nancy, France); Alexandre Klein (CH Colmar, France); Michel Delahousse (Hôpital Foch, Suresnes, France); Christian Combe (CHU, Bordeaux, France); Séverine Martin (CH, Libourne, France); Eric Thervet (CHU HEGP, APHP, Paris, France); Ziad Massy (CHU Ambroise Paré, APHP, Boulogne, France); Xavier Belenfant (CH, Montreuil, France); Pablo Urena (AURA, St. Ouen, France); Carlos Vela (CH, Perpignan, France); Luc Frimat (CHU de Nancy, Vandoeuvre-lès-Nancy, France); Dominique Chauveau (CHU − Hôpital Rangueil, Toulouse, France); Viktor Panescu (Polyclinique de Gentilly, Nancy, France); François Glowacki (CHU, Lille, France); Maxime Hoffmann (Hôpital privé La Louvière, Lille, France); Maryvonne Hourmant (CHU, Nantes, France); Dominique Besnier (CH, St. Nazaire, France); Angelo Testa (Centre de dialyse, Rezé, France); Philippe Zaoui (CHU, Grenoble, France); Charles Chazot (NephroCare Tassin-Charcot, Ste. Foy-les-Lyon, France); Laurent Juillard (CHU Edouard Herriot, Lyon, France); Stéphane Burtey (CHU, Hôpital La Conception, Marseille, France); Adrien Keller (CH, Libourne, France); Nassim Kamar (CHU − Hôpital Rangueil, Toulouse, France); Denis Fouque (CHU Lyon Sud, Lyon, France); and Maurice Laville (CHU Lyon Sud, Lyon, France).
Disclosure
CKD-REIN is supported by a public-private partnership with funding from 9 pharmaceutical companies (Merck Sharp & Dohme-Chibret [MSD France], Amgen, Fresenius Medical Care, GlaxoSmithKline [GSK], Baxter, Lilly France, Otsuka Pharmaceutical, Vifor Fresenius, and Sanofi-Genzyme). ZAM reports grants for CKD-REIN and other research projects from Amgen, Baxter, Fresenius Medical Care, GlaxoSmithKline, Merck Sharp and Dohme-Chibret, Sanofi-Genzyme, Lilly, Otsuka, and the French government, as well as fees and grants to charities from Amgen, Daichii, and Sanofi-Genzyme. These sources of funding are not necessarily related to the content of the present manuscript. JF has received travel expenses, payment for speaking at meetings and funding for research from Amgen, Akcea, AstraZeneca, MSD, Sanofi and Servier. MV-R is an employee of MSD France. BS reports grants for CKD-REIN from Amgen, Baxter, Fresenius Medical Care, GlaxoSmithKline, Merck Sharp and Dohme-Chibret, Sanofi-Genzyme, Lilly, Otsuka, and Vifor Fresenius, as well as speaker honoraria at the French Society of Diabetology from Lilly, and at the French-speaking Society of Nephrology, Dialysis and Transplantation from MSD. All the other authors declared no competing interests.
Acknowledgments
We thank the patients, study staff (Sophie Renault, Marie Metzger, Elodie Speyer, Celine Lange, Reine Ketchemin) and clinical research associates. Sophie Rushton-Smith, PhD (MedLink Healthcare Communications) provided medical writing assistance, under the direction of the authors, and was funded by MSD France. The CKD-REIN cohort study is funded by the Agence Nationale de la Recherche through the 2010 “Cohortes-Investissements d’Avenir” program and by the 2010 national Programme Hospitalier de Recherche Clinique. CKD-REIN is also supported through a public–private partnership with Amgen, Fresenius Medical Care, and GlaxoSmithKline (GSK) since 2012; Lilly France since 2013; Otsuka Pharmaceutical since 2015; Baxter and Merck Sharp & Dohme-Chibret (MSD France) from 2012 to 2017; Sanofi-Genzyme from 2012 to 2015, and Vifor Fresenius and AstraZeneca since 2018.
Footnotes
Text S1. Classifications for hypertension, diabetes, albuminuria, and proteinuria.
Text S2. Low-, moderate-, and high-intensity statins.
Table S1. Patient baseline characteristics according to LLT in patients with type 2 diabetes.
Figure S1. STROBE study flow diagram.
Contributor Information
Bénédicte Stengel, Email: benedicte.stengel@inserm.fr.
CKD-REIN Collaborators:
Carole Ayav, Christian Combe, Denis Fouque, Luc Frimat, Yves-Edouard Herpe, Maurice Laville, Ziad Massy, Bénédicte Stengel, Céline Lange, Karine Legrand, Sophie Liabeuf, Marie Metzger, Elodie Speyer, Bruno Moulin, Gaétan Lebrun, Éric Magnant, Gabriel Choukroun, Jean Philippe Bourdenx, Marie Essig, Raymond Azar, Mustafa Smati, Mohamed Jamali, Alexandre Klein, Michel Delahousse, Christian Combe, Séverine Martin, Eric Thervet, Ziad Massy, Xavier Belenfant, Pablo Urena, Carlos Vela, Luc Frimat, Dominique Chauveau, Viktor Panescu, François Glowacki, Maxime Hoffmann, Maryvonne Hourmant, Dominique Besnier, Angelo Testa, Philippe Zaoui, Charles Chazot, Laurent Juillard, Stéphane Burtey, Adrien Keller, Nassim Kamar, Denis Fouque, and Maurice Laville
Supplementary Material
References
- 1.Xie Y., Bowe B., Mokdad A.H. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018;94:567–581. doi: 10.1016/j.kint.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Thompson S., James M., Wiebe N. Cause of death in patients with reduced kidney function. J Am Soc Nephrol. 2015;26:2504–2511. doi: 10.1681/ASN.2014070714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chronic Kidney Disease Prognosis Consortium. Matsushita K., van der Velde M. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375:2073–2081. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catapano A.L., Graham I., De Backer G. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999–3058. doi: 10.1093/eurheartj/ehw272. [DOI] [PubMed] [Google Scholar]
- 5.Kidney Disease: Improving Global Outcomes (KDIGO) Lipid Work Group KDIGO clinical practice guideline for lipid management in chronic kidney disease. Kidney Int Suppl. 2013;3:259–305. [Google Scholar]
- 6.Colantonio L.D., Baber U., Banach M. Contrasting cholesterol management guidelines for adults with CKD. J Am Soc Nephrol. 2015;26:1173–1180. doi: 10.1681/ASN.2014040400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stengel B., Combe C., Jacquelinet C. The French Chronic Kidney Disease-Renal Epidemiology and Information Network (CKD-REIN) cohort study. Nephrol Dial Transplant. 2014;29:1500–1507. doi: 10.1093/ndt/gft388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stengel B., Metzger M., Combe C. Risk profile, quality of life and care of patients with moderate and advanced CKD: The French CKD-REIN Cohort Study. Nephrol Dial Transplant. 2019;34:277–286. doi: 10.1093/ndt/gfy058. [DOI] [PubMed] [Google Scholar]
- 9.Villain C, Metzger M, Combe C, et al. Prevalence of atheromatous and non-atheromatous cardiovascular disease by age in chronic kidney disease [e-pub ahead of print]. Nephrol Dial Transplant. 10.1093/ndt/gfy277. [DOI] [PubMed]
- 10.Couchoud C., Stengel B., Landais P. The renal epidemiology and information network (REIN): a new registry for end-stage renal disease in France. Nephrol Dial Transplant. 2006;21:411–418. doi: 10.1093/ndt/gfi198. [DOI] [PubMed] [Google Scholar]
- 11.Girerd X., Radauceanu A., Achard J.M. [Evaluation of patient compliance among hypertensive patients treated by specialists] Arch Mal Coeur Vaiss. 2001;94:839–842. [PubMed] [Google Scholar]
- 12.Hays R.D., Kallich J.D., Mapes D.L. Development of the kidney disease quality of life (KDQOL) instrument. Qual Life Res. 1994;3:329–338. doi: 10.1007/BF00451725. [DOI] [PubMed] [Google Scholar]
- 13.Palmer S.C., Navaneethan S.D., Craig J.C. HMG CoA reductase inhibitors (statins) for people with chronic kidney disease not requiring dialysis. Cochrane Database Syst Rev. 2014;5 doi: 10.1002/14651858.CD007784.pub2. [DOI] [PubMed] [Google Scholar]
- 14.Barylski M., Nikfar S., Mikhailidis D.P. Statins decrease all-cause mortality only in CKD patients not requiring dialysis therapy--a meta-analysis of 11 randomized controlled trials involving 21,295 participants. Pharmacol Res. 2013;72:35–44. doi: 10.1016/j.phrs.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Baigent C., Landray M.J., Reith C. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schneider M.P., Hubner S., Titze S.I. Implementation of the KDIGO guideline on lipid management requires a substantial increase in statin prescription rates. Kidney Int. 2015;88:1411–1418. doi: 10.1038/ki.2015.246. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Z., Wu P., Zhang J. The effect of statins on microalbuminuria, proteinuria, progression of kidney function, and all-cause mortality in patients with non-end stage chronic kidney disease: a meta-analysis. Pharmacol Res. 2016;105:74–83. doi: 10.1016/j.phrs.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Zentiva. Fenofibrate 267mg Capsules. Available at: https://www.medicines.org.uk/emc/product/4468/smpc
- 19.Ferrieres J., Rouyer M.V., Lautsch D. Improvement in achievement of lipid targets in France: Comparison of data from coronary patients in the DYSIS and DYSIS II studies. Int J Cardiol. 2016;222:793–794. doi: 10.1016/j.ijcard.2016.08.084. [DOI] [PubMed] [Google Scholar]
- 20.Chowdhury R., Khan H., Heydon E. Adherence to cardiovascular therapy: a meta-analysis of prevalence and clinical consequences. Eur Heart J. 2013;34:2940–2948. doi: 10.1093/eurheartj/eht295. [DOI] [PubMed] [Google Scholar]
- 21.Bosworth H.B., Granger B.B., Mendys P. Medication adherence: a call for action. Am Heart J. 2011;162:412–424. doi: 10.1016/j.ahj.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bowry A.D., Shrank W.H., Lee J.L. A systematic review of adherence to cardiovascular medications in resource-limited settings. J Gen Intern Med. 2011;26:1479–1491. doi: 10.1007/s11606-011-1825-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.