Abstract
Background
Few studies have investigated the effects of riociguat on pulmonary hemodynamics in Asian patients with chronic thromboembolic pulmonary hypertension (CTEPH). In this study, we evaluated the effects of riociguat on pulmonary hemodynamics in inoperable CTEPH patients.
Methods
We retrospectively collected the clinical data of 11 inoperable CTEPH patients. Pulmonary hemodynamic parameters of right heart catheterization, echocardiography, 6-minute walk distance and World Health Organization (WHO) functional class were assessed at baseline and after riociguat treatment.
Results
The median duration of riociguat treatment was 12 months, and all 11 patients tolerated riociguat 7.5 mg/day well after titration. With regards to pulmonary hemodynamic data, both mean pulmonary artery pressure and pulmonary vascular resistance significantly decreased from 41 ± 8 mmHg to 38 ± 9 mmHg (p = 0.045) and 787 ± 417 dyn·s·cm-5 to 478 ± 267 dyn·s·cm-5 (p = 0.007), respectively. With regards to clinical symptoms, WHO functional class significantly improved in nine of the 11 patients, and there was no change in the other two patients (p = 0.004). In addition, the median level of N-terminal pro-brain natriuretic peptide also significantly decreased from 281 (117-5943) pg/ml to 226 (48-1276) pg/ml (p = 0.021).
Conclusions
Riociguat treatment improved both clinical symptoms and pulmonary hemodynamics in the inoperative CTEPH patients in this study.
Keywords: Chronic thromboembolic pulmonary hypertension, Pulmonary hemodynamics, Riociguat
INTRODUCTION
Chronic thromboembolic pulmonary hypertension (CTEPH) is characterized by obliteration of the pulmonary vasculature caused by unresolved thrombi undergoing fibrotic transformation.1,2 It is an under-recognized but serious complication of pulmonary embolism. The cumulative incidence of CTEPH has been reported to be approximately 3.8% at 2 years after the initial episode of pulmonary embolism.3 However, the true prevalence of CTEPH is difficult to assess and has probably been underestimated. Gall et al. reported a prevalence of CTEPH of 2-5 patients per 100000 population, of whom more than 80% had New York Heart Association functional class III/IV at diagnosis.4 These results highlight the urgent need to increase awareness of CTEPH and provide these patients with adequate management. Unlike well-known pulmonary arterial hypertension, the pathophysiology of CTEPH is the progressive remodeling and obstruction of pulmonary arteries leading to increased pulmonary artery pressure (PAP), pulmonary vascular resistance (PVR), and progressive right ventricle dysfunction.1,5-7
The treatment of CTEPH depends on identifying surgically accessible thromboemboli. Pulmonary endarterectomy (PEA) is the gold standard treatment for operable CTEPH patients, and it can potentially cure the disease.8-12 However, only around 60% of patients are surgical candidates, of whom 5% to 35% have been reported to have residual pulmonary hypertension.13 For inoperable CTEPH patients with distal pulmonary artery obstruction and those with recurrent or residual pulmonary hypertension after PEA, medical treatment is currently the standard treatment.
Riociguat, a soluble guanylate cyclase stimulator, is the first U. S. Food and Drug Administration (FDA)-approved CTEPH medication.14-16 It was evaluated in the CHEST-1 and CHEST-2 trials, and was shown to significantly improve exercise capacity and PVR in patients with inoperable or recurrent CTEPH.17,18 However, real-world data on the effects of riociguat on pulmonary hemodynamics are limited, especially in Asian patients. Therefore, we conducted this study to investigate the effects of riociguat on pulmonary hemodynamics in inoperable CTEPH patients.
MATERIALS AND METHODS
Patients
We retrospectively reviewed the cardiac catheterization laboratory database and National Taiwan University Hospital patient database and selected CTEPH patients with right heart catherization data from January 2010 to November 2018. The inclusion criteria were: 1) CTEPH patients with right heart catheterization data pre- and post-riociguat therapy; and 2) CTEPH patients who used riociguat for at least 16 weeks, including at least 8 weeks of the maximum tolerated dose (usually 7.5 mg/day) before follow-up right heart catherization. Patients who received balloon pulmonary angioplasty (BPA) before the post-riociguat pulmonary hemodynamic studies were excluded from this study. The diagnostic criteria for CTEPH were based on the following findings after 3 months of effective anticoagulation therapy: 1) at least one segmental pulmonary perfusion defect on lung scans; 2) specific pulmonary angiography signs such as ring-like stenoses, webs, slits and chronic total occlusions; 3) elevated mean PAP ≥ 25 mmHg; and 4) pulmonary arterial wedge pressure ≤ 15 mmHg.9 The obstruction level of CTEPH was evaluated according to the University of California, San Diego (UCSD) classification.19 Patients were also enrolled if they met criteria 1-3, had a high PVR (> 3 woods) after review by two experts in our research team (C-K Wu and Y-H Lin), and successfully applied for riociguat from the Taiwan National Health Insurance system. Operability was assessed by an experience surgeon (H-H Hsu).
All patients received baseline biochemistry, echocardiography, 6-minute walk test, and right heart catherization studies. Follow-up echocardiography, 6-minute walk test and right heart catherization data were also collected after at least 8 weeks of full-dose riociguat treatment for further analysis. None of the patients received combination therapy including endothelin receptor antagonists or prostanoids.
This study was approved by the Institutional Review Board of National Taiwan University Hospital and was performed in accordance with relevant guidelines and regulations.
Outcome measures
The primary outcome measurement was changes in pulmonary hemodynamic parameters after a median 12 months of riociguat therapy. N-terminal pro-brain natriuretic peptide (NT-proBNP) level, World Health Organization (WHO) functional class and the distance walked in 6 minutes were also analyzed after riociguat treatment. Adverse events including mortality and unplanned hospitalization were assessed throughout the study and during the follow-up period.
Statistical analysis
Statistical analysis was performed using SPSS version 25 for Windows (SPSS Inc., IL, USA). A two-sided p value less than 0.05 was considered to be statistically significant. Data were expressed as number (%) for categorical data, mean ± standard deviation for normally distributed numerical data, and median (25th-75th interquartile range) for non-normally distributed numerical data. Differences between proportions were calculated using the chi-square test or Fisher’s exact test. Comparisons of data between baseline and after riociguat treatment were performed using the paired sample T test (normally distributed data) and Wilcoxon test (non-normally distributed data).
RESULTS
Patients
The baseline characteristics and results of biochemical tests of the 11 patients (three men) enrolled in this study are listed in Table 1. The detailed clinical information, levels of pulmonary thromboembolism and reasons for treatment preferences are listed in Table 2. Only 55% of the patients had a history of venous thromboembolism. UCSD classification19 showed that nine (82%) patients were level III and two (18%) were level IV. All of the patients except one met all four diagnostic criteria [one patient had pulmonary capillary wedge pressure (PCWP) > 15 mmHg], and all 11 patients tolerated riociguat 7.5 mg daily well. The median time from first prescription of riociguat to follow-up right heart catherization was 12 (range: 4-17) months. In addition, there were no unplanned hospitalizations and none of the patients died during the follow-up period.
Table 1. Baseline clinical characteristics.
| Baseline clinical data | |
| Age (years) | 58 ± 11 |
| Body-mass index | 25 ± 3.8 |
| Male, n (%) | 3 (27%) |
| CAD, n (%) | 0 (0%) |
| DM, n (%) | 2 (18%) |
| HTN, n (%) | 5 (45%) |
| History of VTE | 6 (55%) |
| Dyslipidemia, n (%) | 3 (27%) |
| Hemoglobulin, g/dL | 14 ± 2.9 |
| Creatinine, mg/dL | 0.9 ± 0.3 |
| ALT, U/L | 23 ± 15 |
| AST, U/L | 27 ± 15 |
| T-bil, mg/dL | 1.7 ± 0.26 |
| TG, mg/dL | 101 ± 45 |
| T-Chol, mg/dL | 170 ± 34 |
| LDL, mg/dL | 95 ± 23 |
| HDL, mg/dL | 54 ± 15 |
Data were presented as mean ± standard deviation or number (percentage).
ALT, alanine transaminase; AST, aspartate transaminase; CAD, coronary artery disease; DM, diabetes mellitus; HDL, high density lipoprotein; HTN, hypertension; LDL, low-density lipoprotein; T-bil, total bilirubin; T-Chol, total cholesterol; TG, triglyceride; VTE, venous thromboembolism.
Table 2. Levels of pulmonary thromboembolism, comorbidity and reasons of treatment preferences.
| UCSD classification | Comorbidity | Reasons of treatment preferences | ||
| Case 1 | 71F | III | Rectal cancer | Old age, comorbidity, difficult surgical accessibility |
| Case 2 | 53M | III | Protein S deficiency, HTN | Difficult surgical accessibility |
| Case 3 | 54M | III | CAD, HTN, DM | Difficult surgical accessibility |
| Case 4 | 53F | IV | ESRD, HTN, dyslipidemia | Comorbidities, difficult surgical accessibility |
| Case 5 | 76F | III | APS, HTN | Old age, comorbidities, difficult surgical accessibility |
| Case 6 | 44F | III | HTN, DM, dyslipidemia | Patient refused PEA, difficult surgical accessibility |
| Case 7 | 44F | III | APS | Comorbidity, difficult surgical accessibility |
| Case 8 | 55F | III | - | Patient refused PEA, difficult surgical accessibility |
| Case 9 | 54F | III | AIHA | Patient refused PEA, difficult surgical accessibility |
| Case 10 | 71F | III | HTN | Old age, difficult surgical accessibility |
| Case 11 | 66M | IV | - | Patient refused PEA, difficult surgical accessibility |
The definition of UCSD classification: level 0: no evidence for CTEPH; level I: disease involved one of the main pulmonary arteries; level II: disease involved the lobar branches or past the takeoff of the upper lobe artery; level III: disease is distal and started from the segmental branches; level IV disease: disease only involved the subsegmental branches.19
AIHA, autoimmune hemolytic anemia; APS, antiphospholipid syndrome; CAD, coronary artery disease; DM, diabetes mellitus; ESRD, end stage renal disease; HTN, hypertension; PEA, pulmonary endarterectomy; UCSD, University of California, San Diego.
Pulmonary hemodynamic data
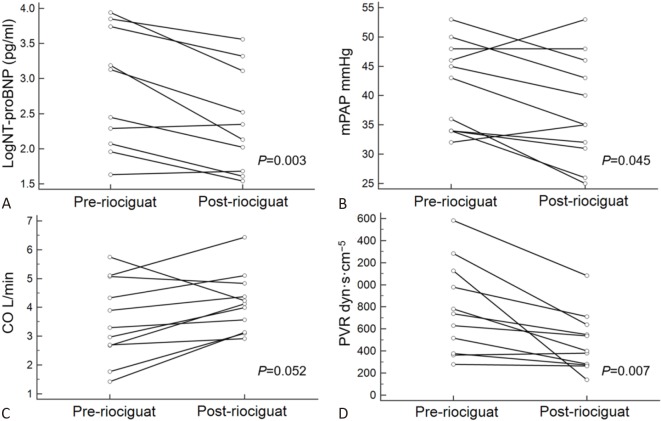
The pulmonary hemodynamic data are listed in Table 3 and Figure 1. PAP and PVR significantly decreased from 41 ± 8 to 38 ± 9 mmHg (p = 0.045) and from 787 ± 417 to 478 ± 267 dyn·s·cm-5 (p = 0.007), respectively. There was a borderline increase in cardiac output from 3.6 ± 1.4 to 4.2 ± 1.0 L/min (p = 0.052).
Table 3. Hemodynamics data, exercise capacity, biochemistry and echocardiogram data before and after riociguat treatment.
| Pre-medication (N = 11) | Post-medication (N = 11) | Change | p value | |
| Right heart hemodynamic studies | ||||
| RAP, mmHg | 8 ± 5 | 8 ± 4 | 0.4 ± 2.4 | 1.000 |
| Systolic PAP, mmHg | 73 ± 18 | 68 ± 21 | 5 ± 9 | 0.078 |
| Diastolic PAP, mmHg | 25 ± 6 | 22 ± 6 | 2 ± 5 | 0.164 |
| Mean PAP, mmHg | 41 ± 8 | 38 ± 9 | 4 ± 5 | 0.045 |
| PCWP, mmHg | 13 ± 6 | 13 ± 4 | 0.1 ± 4.1 | 0.942 |
| Cardiac output, liters/min | 3.6 ± 1.4 | 4.2 ± 1.0 | 0.6 ± 0.9 | 0.052 |
| PVR, dyn·s·cm-5 | 787 ± 417 | 478 ± 267 | 268 ± 222 | 0.007 |
| Exercise capacity | ||||
| WHO Fc | 0.004 | |||
| I | 0 | 5 | Improved (9/11) | |
| II | 6 | 5 | Stationary (2/11) | |
| III | 4 | 1 | Worse (0/11) | |
| IV | 1 | 0 | ||
| 6MWD | 375 ± 68 | 388 ± 77 | 21 ± 79 | 0.635 |
| NT-proBNP | ||||
| NT-proBNP, pg/mL | 281 (117-5943) | 226 (48-1276) | 177 (-6.1-3450) | 0.021 |
| Log NT-proBNP | 2.8 ± 0.8 | 2.4 ± 0.7 | 0.4 ± 0.4 | 0.007 |
| Echocardiogram* | ||||
| LVEF, mm | 69 ± 9 | 73 ± 5 | 4 ± 9 | 0.227 |
| LVEDD, mm | 45 ± 6 | 45 ± 6 | 1 ± 2 | 0.074 |
| LA diameter, mm | 36 ± 4 | 39 ± 4 | 2 ± 5 | 0.227 |
| TR grading | 0.480 | |||
| Moderate | 5 | 6 | Improved (3/10) | |
| Moderate to severe | 4 | 4 | Stationary (5/10) | |
| Severe | 1 | 0 | Worse (2/10) | |
| TRPG, mmHg | 78 ± 19 | 74 ± 24 | 4 ± 23 | 0.558 |
* Data was collected from 10 patients.
Data were presented as mean ± standard deviation or median (25th-75th percentile).
LA, left atrium; LVEDD, left ventricular end diastolic diameter; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-brain natriuretic peptide; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; TR, tricuspid regurgitation; TRPG, tricuspid regurgitation peak gradient; WHO Fc, World Health Organization functional class; 6MWD, 6-minute walk distance.
Figure 1.
Hemodynamic data and NT-proBNP level before and after riociguat treatment. (A) NT-proBNP, (B) mPAP, (C) CO and (D) PVR before and after riociguat treatment. CO, cardiac output; MPAP, mean pulmonary artery pressure; NT-proBNP, N-terminal probrain natriuretic peptide; PVR, pulmonary vascular resistance.
Clinical, biochemical and echocardiographic data
The clinically relevant parameters and echocardiographic data before and after riociguat treatment are listed in Table 3. After riociguat treatment, the level of NT-proBNP significantly decreased (p = 0021), and WHO functional class significantly improved (p = 0.004). Among the 11 patients, nine had an improvement in WHO functional class and two remained unchanged. No patient deteriorated after riociguat treatment. The 6-minute walk distance increased by 21 ± 79 m but did not reach statistical significance. Echocardiographic studies revealed both non-significantly increased left ventricular ejection fraction and decreased tricuspid regurgitation peak gradient, and the tricuspid regurgitation grading did not improve after riociguat use.
DISCUSSION
In this study, riociguat significantly improved hemodynamic parameters including mean PAP and PVR in symptomatic and inoperable CTEPH patients. Significant improvements were also observed in clinically relevant parameters including NT-proBNP level and WHO functional class.
The treatment of CTEPH depends on the obstructive location and the patients’ general condition. Current guidelines emphasize that operability is determined by multiple factors that cannot easily be standardized.11,20 Thromboembolic disease located proximally in the main, lobar or segmental arteries has been reported to be suitable for surgery, however distal-type CTEPH from mid-segmental and subsegmental branches has been reported to be more challenging for the surgeon.21 In operable CTEPH patients, PEA is the recommended potentially curative treatment.11 However, only about 60% of CTEPH patents are operable,8 and inoperable patients face worse outcomes. Delcroix et al. reported survival rates of approximately 70% for inoperable patients and 89% for operated patients after 3 years of follow-up.22 Furthermore, even after successful PEA, around 35% of patients still have residual or recurrent pulmonary hypertension.13 For inoperable patients and those with residual or recurrent pulmonary hypertension after PEA, medical treatment is currently the standard treatment.
Riociguat is a guanylate cyclase stimulator that targets the NO-soluble guanylate cyclase-cyclic GMP pathway,14-16 and it is the first medication approved by the FDA to treat CTEPH. In the landmark CHEST-1 trial, riociguat was shown to significantly improve 6-minute walk distance, mean PAP, cardiac output, PVR, and NT-proBNP after 16 weeks of treatment.17 The open-label CHEST-2 trial extended the follow-up period of riociguat treatment to 1 year, and the results showed sustained benefits in 6-minute walk distance and functional capacity.18 Despite the different follow-up times, the degrees of mean PAP, cardiac output and PVR improvements in our study were similar to the results of the CHEST-1 trial.17 The decreases in mean PAP and PVR in the CHEST-1 trial were 4 ± 7 mmHg and 226 ± 248 dyn·s·cm-5, respectively, comparted to 4 ± 5 mmHg and 268 ± 222 dyn· s·cm-5, respectively, in the present study. The CHEST-1 trial showed short-term pulmonary hemodynamic benefits with riociguat treatment in CTEPH patients. In addition, the CHEST-2 trial reported the long-term clinical outcomes of riociguat treatment, although it did not provide hemodynamic data. Moreover, only a very limited number of studies have reported hemodynamic data after long-term riociguat treatment. Therefore, the current study adds to the knowledge by providing the hemodynamic data of a long duration of riociguat therapy.
Hemodynamic studies can provide an objective and rigorous measurement of the pulmonary circulation status and serve as predictors of mortality in CTEPH patients.23,24 Baseline PVR has been associated with the clinical outcomes of patients with pulmonary arterial hypertension, and a reduction in PVR may improve their surival.25 In addition, the severity of pulmonary hypertension before PEA and residual pulmonary hypertension after PEA have been strongly associated with mortality.26,27 Taken together, these findings indicate the importance of pulmonary hemodynamics in pulmonary hypertension.
Available hemodynamic data on the effect of riociguat on the pulmonary circulation system in Asian patients are still very limited. Yamamoto et al. reported hemodynamic changes in a prospective cohort of Japanese patients with CTEPH after riociguat treatment.28 Among 15 CTEPH patients, 10 received riociguat without previous medications as in our study, and five patients who were already on endothelin receptor antagonists (ERAs) or oral prostacyclin received riociguat as add-on therapy. The results showed that mean PAP, PVR and cardiac index improved, but the improvements did not reach statistical significance. Another Chinese subgroup analysis of the CHEST-1 trial found that 21 CTEPH patients who received 7.5 mg riociguat per day had an improvement in 6-minute walk distance of approximately 55 m, a decrease in PVR of 379 dyn·s·cm-5, a decrease in NT-proBNP of 538 pg/ml, and improvement in WHO functional class in 38% of the patients.29 These improvements in pulmonary hemodynamics were greater than in our real-world data. A possible explanation is that the average age of the Chinese subgroup in the CHEST-1 trial was younger than in our study (47 vs. 58 years), and the baseline PVR and NT-proBNP levels were also higher compared with our study (1075 vs. 787 dyn·s·cm-5 and 1601 vs. 281 pg/mL, respectively). These differences in baseline characteristics and disease severity may have led to the differences between these two studies.
In addition to pulmonary hemodynamic parameters, the WHO functional class also significantly improved after riociguat treatment in this study, however the increase in 6-minute walk distance was insignificant. Yamamoto et al. reported similar findings in CTEPH patients who changed medication from phosphodiesterase 5 inhibitors to riociguat.28 Unlike hemodynamic measurements, the 6-minute walk test can be affected by the patients’ physical condition, such as obesity, peripheral artery occlusive disease-related claudication or degenerative osteoarthritis.30,31 Although the 6-minute walk test remains the gold standard functional study to assess pulmonary hypertension, it must be carefully interpreted, especially in studies with a small number of cases.
Although riociguat significantly decreased the mean PAP by approximately 4 mmHg and PVR by 268 dyn·s·cm-5 in the current study, a few patients remained symptomatic and the PVR and mean PAP were still elevated even under riociguat treatment. In addition, the mean PAP and PVR remained about 38 mmHg and 478 dyn·s·cm-5, respectively, under riociguat treatment. Further interventions would therefore be needed to improve pulmonary hemodynamics and clinical outcomes. BPA has recently been shown to provide promising clinical outcomes in inoperable CTEPH patients.32 In the past, patients with distal-type CTEPH who could not receive PEA faced grave outcomes. Sugimura et al. reported that 12 distal-type CTEPH patients who received BPA had significantly better outcomes.33 In addition, Wiedenroth et al. reported that sequential treatment with riociguat and BPA could improve exercise capacity and hemodynamics.34 Combining riociguat and BPA therefore appears to be a reasonable therapeutic strategy for inoperable CTEPH patients and those with residual or recurrent pulmonary hypertension after PEA.
There are several limitations to this study. First, this was a small retrospective study with a limited number of cases. Further large prospective studies are needed to verify our findings. However, we provided valuable hemodynamic data of CTEPH patients after riociguat treatment. Second, we only provided TRPG and tricuspid regurgitation grading without comprehensive right heart echocardiography assessments in this study. Further prospective studies should include more parameters. Third, the follow-up time varied among cases in our study, and this may have affected the data of responses to the medication. The data in this study should therefore be interpreted with caution.
CONCLUSIONS
In this study, riociguat treatment significantly improved the mean pulmonary artery pressure, pulmonary vascular resistance, and other clinical outcomes including NT-proBNP level and WHO functional class in the patients with inoperable CTEPH.
CONFLICT OF INTEREST
All the authors declare no conflict of interest.
REFERENCES
- 1.Delcroix M, Vonk Noordegraaf A, Fadel E, et al. Vascular and right ventricular remodelling in chronic thromboembolic pulmonary hypertension. Eur Respir J. 2013;41:224–232. doi: 10.1183/09031936.00047712. [DOI] [PubMed] [Google Scholar]
- 2.Pepke-Zaba J, Delcroix M, Lang I, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation. 2011;124:1973–1981. doi: 10.1161/CIRCULATIONAHA.110.015008. [DOI] [PubMed] [Google Scholar]
- 3.Pengo V, Lensing AW, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350:2257–2264. doi: 10.1056/NEJMoa032274. [DOI] [PubMed] [Google Scholar]
- 4.Gall H, Hoeper MM, Richter MJ, et al. An epidemiological analysis of the burden of chronic thromboembolic pulmonary hypertension in the USA, Europe and Japan. Eur Respir Rev. 2017;26 doi: 10.1183/16000617.0121-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lang I. Chronic thromboembolic pulmonary hypertension: a distinct disease entity. Eur Respir Rev. 2015;24:246–252. doi: 10.1183/16000617.00001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Wang K. The changing landscape of pulmonary arterial hypertension in 21(st) century. Acta Cardiol Sin. 2017;33:510–513. doi: 10.6515/ACS20170810A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang LY, Lee KT, Lin CP, et al. Long-term survival of patients with pulmonary arterial hypertension at a single center in Taiwan. Acta Cardiol Sin. 2017;33:498–509. doi: 10.6515/ACS20170612A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayer E, Jenkins D, Lindner J, et al. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg. 2011;141:702–710. doi: 10.1016/j.jtcvs.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 9.Kim NH, Delcroix M, Jenkins DP, et al. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol. 2013;62:D92–D99. doi: 10.1016/j.jacc.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 10.Luo WC, Huang SC, Lin YH, et al. Pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension--a single-center experience in Taiwan. J Formos Med Assoc. 2015;114:1197–1203. doi: 10.1016/j.jfma.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 11.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 12.Chen YJ, Ho CT, Tsai FC, et al. Outcomes of pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension at a single center in Taiwan. Acta Cardiol Sin. 2019;35:153–164. doi: 10.6515/ACS.201903_35(2).20180904A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freed DH, Thomson BM, Berman M, et al. Survival after pulmonary thromboendarterectomy: effect of residual pulmonary hypertension. J Thorac Cardiovasc Surg. 2011;141:383–387. doi: 10.1016/j.jtcvs.2009.12.056. [DOI] [PubMed] [Google Scholar]
- 14.Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation. 2011;123:2263–2273. doi: 10.1161/CIRCULATIONAHA.110.981738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Follmann M, Griebenow N, Hahn MG, et al. The chemistry and biology of soluble guanylate cyclase stimulators and activators. Angew Chem Int Ed Engl. 2013;52:9442–9462. doi: 10.1002/anie.201302588. [DOI] [PubMed] [Google Scholar]
- 16.Klinger JR. The nitric oxide/cGMP signaling pathway in pulmonary hypertension. Clin Chest Med. 2007;28:143–167, ix. doi: 10.1016/j.ccm.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 17.Ghofrani HA, D’Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369:319–329. doi: 10.1056/NEJMoa1209657. [DOI] [PubMed] [Google Scholar]
- 18.Simonneau G, D'Armini AM, Ghofrani HA, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension: a long-term extension study (CHEST-2). Eur Respir J. 2015;45:1293–1302. doi: 10.1183/09031936.00087114. [DOI] [PubMed] [Google Scholar]
- 19.Madani MM. Surgical treatment of chronic thromboembolic pulmonary hypertension: pulmonary thromboendarterectomy. Methodist Debakey Cardiovasc J. 2016;12:213–218. doi: 10.14797/mdcj-12-4-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jenkins DP, Biederman A, D'Armini AM, et al. Operability assessment in CTEPH: lessons from the CHEST-1 study. J Thorac Cardiovasc Surg. 2016;152:669–674 e3. doi: 10.1016/j.jtcvs.2016.02.062. [DOI] [PubMed] [Google Scholar]
- 21.Jenkins D. Pulmonary endarterectomy: the potentially curative treatment for patients with chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2015;24:263–271. doi: 10.1183/16000617.00000815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Delcroix M, Lang I, Pepke-Zaba J, et al. Long-term outcome of patients with chronic thromboembolic pulmonary hypertension: results from an International Prospective Registry. Circulation. 2016;133:859–871. doi: 10.1161/CIRCULATIONAHA.115.016522. [DOI] [PubMed] [Google Scholar]
- 23.Condliffe R, Kiely DG, Gibbs JS, et al. Prognostic and aetiological factors in chronic thromboembolic pulmonary hypertension. Eur Respir J. 2009;33:332–338. doi: 10.1183/09031936.00092008. [DOI] [PubMed] [Google Scholar]
- 24.Saouti N, de Man F, Westerhof N, et al. Predictors of mortality in inoperable chronic thromboembolic pulmonary hypertension. Respir Med. 2009;103:1013–1019. doi: 10.1016/j.rmed.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 25.Sitbon O, Humbert M, Nunes H, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol. 2002;40:780–788. doi: 10.1016/s0735-1097(02)02012-0. [DOI] [PubMed] [Google Scholar]
- 26.Jamieson SW, Kapelanski DP, Sakakibara N, et al. Pulmonary endarterectomy: experience and lessons learned in 1,500 cases. Ann Thorac Surg. 2003;76:1457–1462; discussion 62-4. doi: 10.1016/s0003-4975(03)00828-2. [DOI] [PubMed] [Google Scholar]
- 27.Ishida K, Masuda M, Tanabe N, et al. Long-term outcome after pulmonary endarterectomy for chronic thromboembolic pulmonary hypertension. J Thorac Cardiovasc Surg. 2012;144:321–326. doi: 10.1016/j.jtcvs.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Yamamoto K, Tanabe N, Suda R, et al. Riociguat for patients with chronic thromboembolic pulmonary hypertension: usefulness of transitioning from phosphodiesterase type 5 inhibitor. Respir Investig. 2017;55:270–275. doi: 10.1016/j.resinv.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Wang C, Jing ZC, Huang YG, et al. Riociguat for the treatment of pulmonary hypertension: Chinese subgroup analyses and comparison. Heart Asia. 2016;8:74–82. doi: 10.1136/heartasia-2015-010712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montgomery PS, Gardner AW. The clinical utility of a six-minute walk test in peripheral arterial occlusive disease patients. J Am Geriatr Soc. 1998;46:706–711. doi: 10.1111/j.1532-5415.1998.tb03804.x. [DOI] [PubMed] [Google Scholar]
- 31.Hulens M, Vansant G, Claessens AL, et al. Predictors of 6-minute walk test results in lean, obese and morbidly obese women. Scand J Med Sci Sports. 2003;13:98–105. doi: 10.1034/j.1600-0838.2003.10273.x. [DOI] [PubMed] [Google Scholar]
- 32.Lang I, Meyer BC, Ogo T, et al. Balloon pulmonary angioplasty in chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;26 doi: 10.1183/16000617.0119-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sugimura K, Fukumoto Y, Satoh K, et al. Percutaneous transluminal pulmonary angioplasty markedly improves pulmonary hemodynamics and long-term prognosis in patients with chronic thromboembolic pulmonary hypertension. Circ J. 2012;76:485–488. doi: 10.1253/circj.cj-11-1217. [DOI] [PubMed] [Google Scholar]
- 34.Wiedenroth CB, Ghofrani HA, Adameit MSD, et al. Sequential treatment with riociguat and balloon pulmonary angioplasty for patients with inoperable chronic thromboembolic pulmonary hypertension. Pulm Circ. 2018;8:2045894018783996. doi: 10.1177/2045894018783996. [DOI] [PMC free article] [PubMed] [Google Scholar]