Abstract
Background
Non-vitamin K oral antagonist anticoagulants (NOACs) have been widely used in stroke prevention in atrial fibrillation (SPAF). The aim of this study was to compare the pharmacoeconomic impact of oral anticoagulants (OACs) including warfarin, dabigatran, rivaroxaban, apixaban, and edoxaban in SPAF in Taiwan.
Methods
A decision tree, Markov model, and multiple sensitivity analyses were used to project the lifetime costs and quality-adjusted life years (QALYs) of OACs. Transitional probabilities were derived from a systematic review and network meta-analysis for Asian populations. Utilities and costs were obtained from published studies and the Taiwan National Health Insurance Research Database. Threshold of the willingness to pay (WTP) at USD 20,000 was applied to evaluate the results.
Results
In base-case analysis, warfarin had the lowest cost at $13,363 ± 4,036, and edoxaban 60 mg produced the most QALYs at 11.92 ± 1.98. The incremental cost-effectiveness ratios of dabigatran 150 and 110 mg, rivaroxaban 20 and 15 mg, apixaban 5 mg, and edoxaban 60 mg versus warfarin were $6,415, $4,225, $4,115 and $5,458 per QALY gained, respectively. Monte Carlo analysis revealed that dabigatran 150 and 110 mg, rivaroxaban 20 and 15 mg, apixaban 5 mg and edoxaban 60 mg were most cost-effective at 21.9%, 27.1%, 23.6%, and 27.4% of $20,000 compared to warfarin.
Conclusions
From a Taiwan national payer perspective, all NOACs are cost-effective substitutes for warfarin in SPAF. However, the likelihood of cost-effective iterations for NOACs is highly driven by their market prices at the time and different WTP thresholds of policymakers.
Keywords: Anticoagulant therapy, Atrial fibrillation, Cost-effectiveness analysis
INTRODUCTION
Atrial fibrillation (AF) is the most common arrhythmic disorder, and is associated with a 2- to 7-fold increased risk of stroke and 5-fold increased risk of death compared with a normal heart rhythm.1 Approximately 15 million people suffer from stroke annually worldwide, of whom one-third die and another third suffer from a permanent disability.2 Yearly expenditures on stroke treatment and care are close to $34 billion in the US and €64 billion in Europe.3,4 AF may cause more than 15% of strokes,5 and the costs of AF-related stroke on average are higher than costs of non-AF-related stroke.6 Warfarin, a vitamin-K antagonist, has been widely used as an oral anticoagulant (OAC) in stroke prevention in AF (SPAF).7 However, it needs to be tailored to each patient depending on their diet and co-existing medical conditions because it easily interacts with certain food and drugs.8 In addition, patients receiving warfarin treatment need regular blood monitoring to ensure the level of anticoagulation reaches the optimal level as specified by the international normalized ratio (INR). As a result, only two-thirds of these patients achieve the required INR therapeutic range,9 and only 10% of them attain a stable INR therapeutic range for one year.10 This means that although warfarin has clinical efficacy, it is still underused in the real-world due to its limitations, inconvenience and side effects.
Non-vitamin K antagonist oral coagulants (NOACs), including dabigatran, rivaroxaban, apixaban, and edoxaban, have been approved for SPAF. They have been shown to be able to overcome some of the limitations of warfarin, and to be at least as efficacious as warfarin without incurring a significant risk of bleeding in four large-scale international trials (ROCKET AF, RE-LY, ARISTOTLE, and ENGAGE AF-TIMI 48).11-14 However, the prices of NOACs are much higher than warfarin, which might result in less willingness to use NOACs. Despite their higher costs, studies performed in the United States and Europe have concluded that NOAC treatment is more cost-effective than warfarin in SPAF at the thresholds implemented in these countries.15-18
In Taiwan, the prevalence rates of AF are around 1.4% in men and 0.7% in women, with incidence rates of 1.68 and 0.76 per thousand person-years for men and women respectively.19 The incidence of ischemic stroke (IS) has been reported to be 46.2% among AF patients in the three years post-diagnosis, and 86.3% of strokes have been reported to occur in the first year.20 Most of these cases used warfarin for SPAF, however the prescription rate was only 10.8%.20,21 This low rate may be due to concerns of the risk of bleeding in Taiwanese patients and the drawbacks of warfarin.22 NOACs are now available as an alternative to warfarin in Taiwan, and they are expected to be able to overcome these problems.
Nevertheless, the monthly cost of NOACs is on average 10 times higher than warfarin in Taiwan, and this is likely to be the main restriction of their use. In addition, the results of cost-effectiveness analyses from other countries may not be applicable in Taiwan because of different disease progression, treatment patterns, healthcare systems, and healthcare financing structures. Under the Taiwan National Health Insurance (NHI) program, for instance, the cost of INR monitoring is cheap, and the costs of long-term care of stroke patients are also lower than in other developed countries. As such, it is uncertain whether or not NOACs would be a cost-effective alternative to warfarin treatment in Taiwan. Therefore, the aim of this study was to develop a decision and Markov model and perform cost-utility analysis to compare dabigatran, rivaroxaban, apixaban, edoxaban and warfarin in stroke prevention among AF patients from a Taiwan national payer perspective.
METHODS
This economic evaluation was a cost-utility analysis with cost-effectiveness assessed by incremental cost per quality-adjusted life year (QALY) gained. Since dabigatran 150 mg and 110 mg, rivaroxaban 20 mg and 15 mg, apixaban 5 mg, and edoxaban 60 mg are the approved doses for SPAF according to the Taiwan Food and Drug Administration, we constructed a decision model to evaluate the pharmacoeconomic benefits of dabigatran 150 and 110 mg, rivaroxaban 20 and 15 mg, apixaban 5 mg, edoxaban 60 mg, and dose-adjusted warfarin in preventing AF-related stroke in Taiwan. The Markov model was adopted from that used in previous studies (Figure 1).15,16,23,24 We then performed a systematic review to identify relevant trials, and performed a network meta-analysis to obtain the relevant input parameters (details in the supplement).
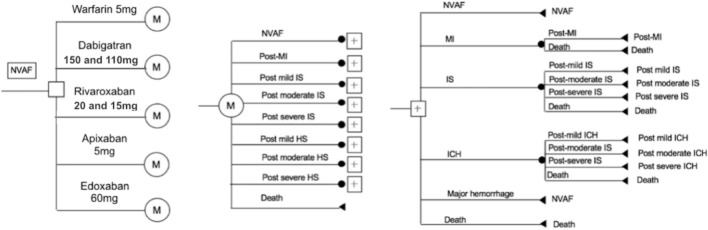
Figure 1.
Schematic representation of the decision tree and Markov model. It illustrates that all patients start at 65 years old with non-valvular atrial fibrillation (NVAF) with CHADS2 (congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, stroke [double weight]) ≥ 1, normal renal function, and no contraindication to anticoagulation therapy. Patients cycle between health statuses until death occurs or the 30-year model time-horizon is reached. The length of each cycle is one month. Depicted in the diagram are the decision node (square), Markov nodes (circles with ‘M’), chance nodes (black circles) directed by transition probabilities, and terminal nodes (triangles). Markov branches for the other 4 anticoagulants are identical to the warfarin branch shown. HS, hemorrhagic stroke; ICH, intracerebral hemorrhage; IS, ischemic stroke; MI, myocardial infarction.
The target population was a hypothetical cohort of 1000 Taiwanese patients aged 65 years with non-valvular AF (NVAF). For the base case analysis, typical patient profiles and treatment strategies were obtained from the trials and the rules of the indicated dose prescriptions according to the Taiwan NHI program.11-14 The health statuses and outcomes in the model included: NVAF-only; mild, moderate, severe and fatal IS; mild, moderate, severe, and fatal hemorrhagic stroke (HS); myocardial infarction (MI); major hemorrhage; and death (Figure 1).11-18 The severity of IS and HS was classified as independence, moderate disability, total dependence, and fatal status, and was based on the study by Chang et al. calculating the proportions from the NHI Research Database.25 The time horizon in this model was 30 years (up to 95 years of age), and one month was set as each cycle length. The yearly discount rate was set at 3% for costs and utilities of the base cases according to the guidelines of methodological standards for pharmacoeconomic evaluations.
In this model, all eligible patients receiving anticoagulant therapy were assumed to start from a status of NVAF-only, and to move or remain according to the transition probabilities after one cycle length. Temporary status included the health status IS, HS, MI, and major hemorrhage, and one-off treatment costs were calculated only for the acute stage. None of the patients remained at the acute stage for more than one cycle; that is, all of them moved to the next status after the acute stage. None of the patients were assumed to have discontinued anticoagulant therapy, and the treatment effect remained constant over time. Namely, the dose of anticoagulants was assumed not to need adjustment by age. If the patients experienced an HS and major hemorrhage, it was assumed that anticoagulants would be stopped for one-month and restarted the next month. It was also assumed that the patients who received interrupted anticoagulant therapy would not receive any other clinical benefit.
All-cause mortality was calculated from network analysis, and it was assumed that there were no differences in age. In addition, all parameters in the model were assumed to be independent of each health status. Model outcomes included the number of clinical events, QALYs, total costs including drugs, clinical events, follow-up fees and long-term care costs, and incremental cost per QALY gained. QALYs were weighted by their quality of life in different health status, and ranged from 1 (perfect health) to 0 (death). Costs were presented in US dollars (USD). This study was granted exemption from review by the Ethics Committee of the London School of Hygiene and Tropical Medicine (Ref. 10955).
Transition probability
Transition probabilities in the model were divided into three groups: clinical outcome events, mortality rates of acute MI and stroke, and severity of the stroke. For the clinical outcome events, the systematic review identified six studies targeting Asian population, and the indicated doses of NOACs in Taiwan were dabigatran 150 and 110 mg twice daily, rivaroxaban 20 and 15 mg once daily, apixaban 5 mg twice daily, and edoxaban 60 mg once daily according to the trials and the Taiwan NHI program.26-31 The probabilities of the clinical events for warfarin were obtained by pooling the identified trials.26-31 Subsequently, odds ratios with 95% confidence intervals for NOACs versus warfarin, including IS, HS, MI, major bleeding, and all-cause mortality were obtained from the network meta-analysis (Table 1 and Supplementary Material). For mortality due to MI and stroke and severity of stroke progress, this model assumed that the estimates were only divided into NOACs and warfarin, and the estimates were adopted from Chang et al.25
Table 1. Estimates, standard errors and distributions of the variables used in the model.
| Variables | Base case | 95% CI. | Distribution | References |
| Efficacy of NOACs | ||||
| Odds ratio for ischemic stroke, NOACs versus warfarin* | ||||
| Dabigatran | 0.80 | 0.52-1.25 | Lognormal | 26-31 |
| Rivaroxaban | 0.67 | 0.39-1.10 | Lognormal | 26-31 |
| Apixaban | 1.19 | 0.73-1.85 | Lognormal | 26-31 |
| Edoxaban | 0.61 | 0.29-1.26 | Lognormal | 26-31 |
| Odds ratio for hemorrhagic stroke, NOACs versus warfarin# | ||||
| Dabigatran | 0.18 | 0.06-0.49 | Lognormal | 26-31 |
| Rivaroxaban | 0.47 | 0.19-1.08 | Lognormal | 26-31 |
| Apixaban | 0.24 | 0.09-0.56 | Lognormal | 26-31 |
| Edoxaban | 0.37 | 0.14-0.86 | Lognormal | 26-31 |
| Odds ratio for myocardial infarction, NOACs versus warfarin† | ||||
| Dabigatran | 0.91 | 0.42-2.10 | Lognormal | 26-31 |
| Rivaroxaban | 1.22 | 0.49-3.03 | Lognormal | 26-31 |
| Apixaban | 1.18 | 0.40-3.45 | Lognormal | 26-31 |
| Edoxaban | 0.88 | 0.31-2.42 | Lognormal | 26-31 |
| Odds ratio for major bleeding, NOACs versus warfarin‡ | ||||
| Dabigatran | 0.57 | 0.41-0.81 | Lognormal | 26-31 |
| Rivaroxaban | 0.82 | 0.61-1.11 | Lognormal | 26-31 |
| Apixaban | 0.51 | 0.33-0.78 | Lognormal | 26-31 |
| Edoxaban | 0.59 | 0.39-0.86 | Lognormal | 26-31 |
| Odds ratio for all-cause death, NOACs versus warfarin§ | ||||
| Dabigatran | 0.90 | 0.69-1.18 | Lognormal | 26-31 |
| Rivaroxaban | 0.82 | 0.48-1.34 | Lognormal | 26-31 |
| Apixaban | 1.03 | 0.68-1.52 | Lognormal | 26-31 |
| Edoxaban | 0.61 | 0.39-0.99 | Lognormal | 26-31 |
CI., confidence interval; NOACs, non-vitamin K oral antagonist anticoagulants. Dose for dabigatran includes 150 and 110 mg, rivaroxaban includes 20 and 15 mg; apixaban is 5 mg, and edoxaban is 60 mg.
* Annual risk of ischemic stroke for warfarin: 0.033. # Annual risk of hemorrhagic stroke for warfarin: 0.0184. † Annual risk of myocardial infarction for warfarin: 0.0092. ‡ Annual risk of major bleeding for warfarin: 0.0775. § Annual risk of all-cause death for warfarin: 0.0609.
Utility and cost
Utility scores were derived from four studies which identified the sources of the utility by considering the sample size, the quality of methodology, and the reporting of the statistics analysis (with standard error, standard deviation or confidence interval) (Table 2).32-35 QALYs were calculated by multiplying the utility value associated with each health status by the proportion of the year living in that status. Drug costs, event costs, and status costs were obtained from the official drug price, the diagnosis-related groups system, Taiwan NHI claims system, and the study by Chang et al. (Table 3).25 All costs in this model were converted into USD in 2016.
Table 2. Utilities, standard errors and distributions of the variables used in the model.
| Annual utility | Estimates | Standard error | Distribution | Reference |
| Atrial fibrillation | 0.81 | 0.067 | Beta | 32 |
| Decrement for age | -0.00029 | 0.00002 | Beta | 32 |
| Decrement for IS | -0.1385 | 0.010 | Beta | 32 |
| Decrement for HS | -0.1385 | 0.010 | Beta | 32 |
| Decrement for MI | -0.1247 | 0.009 | Beta | 32 |
| Decrement for major bleeding | -0.1814 | 0.013 | Beta | 32 |
| Neurological deficit | ||||
| Mild | 0.75 | 0.040 | Beta | 34 |
| Moderate | 0.39 | 0.036 | Beta | 34 |
| Severe | 0.11 | 0.024 | Beta | 34 |
HS, hemorrhagic stroke; IS, ischemic stroke; MI, myocardial infarction.
Table 3. All costs, standard errors and distributions of the variables used in the model.
| Costs in USD ($) | Estimates | Range | Distribution | References |
| Monthly cost of the drug | ||||
| Warfarin | 5 | N/A | Fixed | NHI |
| Dabigatran 150 or 110 mg | 89 | N/A | Fixed | NHI |
| Rivaroxaban 20 or 15 mg | 63 | N/A | Fixed | NHI |
| Apixaban 5 mg | 64 | N/A | Fixed | NHI |
| Edoxaban 60 mg | 80 | N/A | Fixed | NHI |
| Monitor costs per year | ||||
| Warfarin monitor | 39 | 20 | Gamma | NHI |
| One time event costs | ||||
| Ischemic stroke | ||||
| Minor | 2,420 | 1,210 | Gamma | NHI, 25 |
| Moderate | 12,635 | 6,318 | Gamma | NHI, 25 |
| Major | 17,495 | 8,748 | Gamma | NHI, 25 |
| Fatal | 8,967 | 4,484 | Gamma | NHI, 25 |
| Hemorrhagic stroke | ||||
| Minor | 6,448 | 3,224 | Gamma | NHI, 25 |
| Moderate | 11,607 | 5,804 | Gamma | NHI, 25 |
| Major | 15,476 | 7,738 | Gamma | NHI, 25 |
| Fatal | 15,054 | 7,527 | Gamma | NHI, 25 |
| Myocardial infarction | ||||
| Non-fatal | 10,737 | 5,369 | Gamma | NHI, 25 |
| Fatal | 15,032 | 7,516 | Gamma | NHI, 25 |
| Major bleeding | 7,494 | 3,747 | Gamma | NHI, 25 |
| Long-term event costs of the ischemic strokes | ||||
| Mild | 1,116 | 558 | Gamma | NHI, 25 |
| Moderate | 1,825 | 913 | Gamma | NHI, 25 |
| Severe | 2,050 | 1,025 | Gamma | NHI, 25 |
| Long-term event costs of the hemorrhagic strokes | ||||
| Mild | 858 | 429 | Gamma | NHI, 25 |
| Moderate | 1,785 | 893 | Gamma | NHI, 25 |
| Severe | 2,419 | 1,210 | Gamma | NHI, 25 |
Reporting of results
The model was run for 360 cycles (30 years). The costs and QALYs for each treatment option were calculated by multiplying the number of patients in each health status and the utility (QALYs) or the costs in the corresponding status. Incremental cost-effectiveness ratios (ICERs) were obtained by dividing the incremental cost by the incremental QALYs for the NOAC therapy groups versus the warfarin treatment groups. We used USD 20,000 as the threshold of willingness to pay (WTP) to assess the results, because Taiwan gross domestic product (GDP) per capita in 2016 was reported to be USD 22,540 by the International Monetary Fund. The ICER per QALY gained was calculated as:
ICER per QALY = (total costs ($,NOACs) – total costs ($,warfarin)) ÷ ((total QALYs (NOACs) – total QALYs (warfarin))
Sensitivity analysis
Multiple sensitivity analyses were performed. First, deterministic sensitivity analysis (DSA) with one-way sensitivity analysis was performed to evaluate the impact of each input parameter of the probabilities, costs, and utilities on the results of the model. A tornado diagram was used to present the influence of each model parameter and the model assumptions (Figure 2). Second, scenario analysis took the different drug prices and discount rates into account. We assumed that the monthly costs of all NOACs were the same at $63, (i.e., the lowest drug price of the NOACs at that time), and then compared the ICERs per QALY gained versus warfarin. In addition, we used 0% and 10% for the different discount rates to evaluate the impact. Third, we conducted probabilistic sensitivity analysis (PSA) using Monte Carlo Simulation (MCS) to evaluate intraindividual and parameter uncertainty. The lognormal distribution for transitional probabilities, beta distribution for utilities, and standard error for range were used (i.e., probabilities and utilities must range between 0 and 1) (Table 1 and 2).24,36 Furthermore, ±50% of the costs were considered as the upper and lower range, and the gamma distributions for costs were used because the costs could not be less than 0 (Table 3).24,36 The MCS used random sampling and was repeated 1000 times to simulate the outcomes. The results of the PSA were depicted as cost-effectiveness acceptability curve (Figure 3).
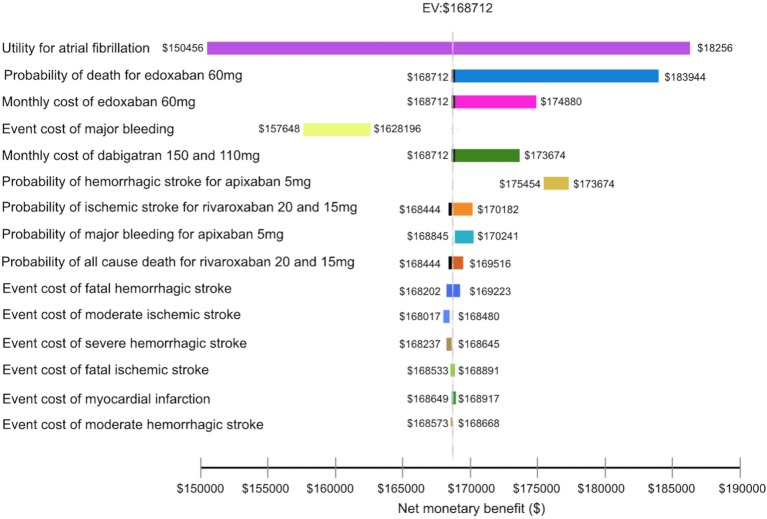
Figure 2.
The tornado diagram depicts the impact of inputs on the net monetary benefit. The vertical black dot line represents an expected cost from the preferred treatment for all input variables being analyzed. Each horizontal bar represents net monetary benefit value expected from a range of the variable we assessed.
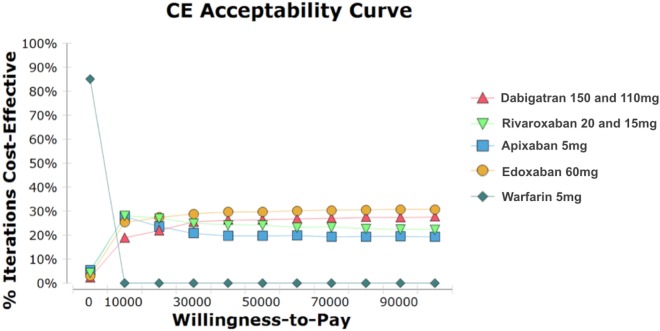
Figure 3.
The cost-effectiveness acceptability curve illustrates the probability of a treatment becoming cost-effective at varying willingness-to-pay thresholds for a patient. Y-axis: percentage of iterations for which the treatment is cost-effective; X-axis: the amount, in US dollars, that a decision maker is willing to pay to achieve an additional quality-adjusted life-year.
RESULTS
Base case analysis
In the base-case analysis, dabigatran 150 and 110 mg ($35,686 ± 15,138) was the costliest treatment, followed by edoxaban 60 mg ($33,232 ± 14,091), rivaroxaban 20 and 15 mg ($27,645 ± 11,821) and apixaban 5 mg ($26,656 ± 10,516), while dose-adjusted warfarin was the least expensive ($13,363 ± 4,036) (Table 4). The total number of QALYs gained with edoxaban 60 mg was the highest among all oral anticoagulants (OACs) (11.92 ± 1.98). Dabigatran 150 and 110 mg (11.76 ± 2.1), rivaroxaban 20 and 15 mg (11.66 ± 1.82) and apixaban 5 mg (11.51 ± 1.84) all produced more QALYs than warfarin (8.28 ± 1.58) (Table 4). All of the NOACs had ICERs less than $20,000. Apixaban 5 mg versus warfarin had the lowest ICER at $4,115/ QALY gained, while dabigatran 150 and 110 mg versus warfarin had the highest ICER at $6,415/QALY gained. The ICERs for edoxaban 60 mg and rivaroxaban 20 and 15 mg were $5,458/ QALY gained and $4,225/QALY gained, respectively.
Table 4. Total costs, QALYs and ICER of base case analysis, scenario analysis, and probabilistic sensitivity analysis.
| Strategy | Base case analysis | Scenario analysis* | Probabilistic sensitivity analysis | ||||||
| Total cost ± S | QALY ± SD | ICER | Total cost | QALY | ICER | Total cost ± SD | QALY ± SD | ICER | |
| Warfarin | $13,363 ± 4,036 | 8.28 ± 1.58 | N/A | $13,363 | 8.28 | N/A | $13,076 ± 12,187 | 8.27 ± 5.66 | N/A |
| Dabigatran | $35,686 ± 15,138 | 11.76 ± 2.1 | $6,415 | $27,156 | 11.76 | $3,964 | $34,123 ± 19,018 | 10.78 ± 6.01 | $8,385 |
| Rivaroxaban | $27,645 ± 11,821 | 11.66 ± 1.82 | $4,225 | $27,645 | 11.66 | $4,225 | $27,260 ± 15,196 | 11.08 ± 5.98 | $5,047 |
| Apixaban | $26,656 ± 10,517 | 11.51 ± 1.84 | $4,115 | $26,284 | 11.51 | $4,000 | $27,848 ± 15,172 | 11.20 ± 5.97 | $5,041 |
| Edoxaban | $33,232 ± 14,091 | 11.92 ± 1.98 | $5,458 | $27,684 | 11.92 | $3,934 | $32,599 ± 17,627 | 11.17 ± 5.98 | $6,732 |
ICER, incremental cost-effectiveness ratio ($/QALY gained); QALY, quality-adjusted life year; SD, standard deviation.
Dose for dabigatran includes 150 and 110 mg, rivaroxaban includes 20 and 15 mg; apixaban is 5 mg, and edoxaban is 60 mg.
* Dabigatran 150 and 110 mg, apixaban 5 mg and edoxaban 60 mg were assumed to have the same monthly cost as rivaroxaban 20 and 15 mg at $63.
Sensitivity analyses
Utility for AF was the most influential input in the model (Figure 2). Probabilities contributing the most significant impact on the model were the probabilities of death for edoxaban 60 mg, HS and major bleeding for apixaban 5 mg, and IS and all-cause death for rivaroxaban 20 and 15 mg. The costs that significantly affected the results included the monthly costs of edoxaban 60 mg and dabigatran 150 and 110 mg, and also the costs of major bleeding, HS, IS and MI. The scenario test using discount rates at 0 and 10% showed that the ICER of each NOAC was still lower than $20,000. Given that all of the NOACs had the same monthly cost at $63, edoxaban 60 mg had the lowest ICER at $3,934 per QALY gained, followed by dabigatran 150 and 110 mg ($3,963), apixaban 5 mg ($4,000), and rivaroxaban 20 and 15 mg ($4,225) (Table 4).
The mean QALYs and costs derived from PSA are presented in Table 4, and Figure 3 depicts the likelihood of the cost-effectiveness of all OACs in SPAF according to WTP threshold. With the WTP threshold at $20,000/ QALY gained, edoxaban 60 mg, rivaroxaban 20 and 15 mg, apixaban 5 mg, dabigatran 150 and 110 mg, and warfarin were cost-effective at 27.4%, 27.1%, 23.6%, 21.9%, and 0, respectively (Figure 3).
DISCUSSION
This economic study used data of Asian populations from a systematic review and network meta-analysis, and constructed a decision tree and Markov model to compare the pharmacoeconomic benefits of OACs available in Taiwan for stroke prevention among NVAF patients aged > 65 years. Although using NOACs was more expensive, they produced more QALYs than warfarin. In comparisons of the ICER per QALY gained, all of the NOACs were highly cost-effective substitutes for warfarin in SPAF in Taiwan. In PSA, all of the NOACs were economically attractive at a WTP threshold of $20,000, and the iteration of cost-effectiveness differed according to the threshold. For example, apixaban 5 mg and rivaroxaban 20 and 15 mg appeared to be more economically attractive when the WTP threshold was lower than $10,000. However, edoxaban 60 mg and dabigatran 150 and 110 mg were more attractive with a higher threshold.
In our network meta-analysis, we found that none of the NOACs had statistically significant differences in odds for IS and MI. However, dabigatran 150 and 110 mg, apixaban 5 mg, and edoxaban 60 mg had reduced significantly odds compared to warfarin and rivaroxaban 20 and 15 mg for HS and major bleeding, while only edoxaban 60 mg significantly reduced the odds of all-cause mortality among all NOACs. The clinical efficacies and adverse event rates of the OACs critically influenced their pharmacoeconomic impact. In one-way DSA, api-xaban 5 mg might not have been an optimal replacement for warfarin among the NOACs. For example, when the clinical efficacy and safety of rivaroxaban 20 and 15 mg increased and the efficacy of apixaban 5 mg decreased, rivaroxaban 20 and 15 mg was the most economically attractive. Likewise, the impact of apixaban 5 mg and rivaroxaban 20 and 15 mg was also sensitive to the efficacy and safety of dabigatran 150 and 110 mg and edoxaban 60 mg. Therefore, when more clinical data are available, the preferred substitutes for warfarin may differ according to the small differences in QALY between the NOACs. In addition, apart from the clinical efficacy and risk of adverse events, monthly drug costs were likely to greatly influence the pharmacoeconomic consideration of using NOACs. In our scenario analysis, given that all of the NOACs had the same monthly cost of $63, edoxaban 60 mg had the lowest ICER, meaning that edoxaban had the best pharmacoeconomic benefit. It is important to note that one of the reasons why apixaban 5 mg and rivaroxaban 20 and 15 mg had lower ICERs may have been due to their lower monthly market costs, even though edoxaban 60 mg and dabigatran 150 and 110 mg produced more QALYs. This result strengthens the finding that the pharmacoeconomic benefit of NOACs was quite sensitive to monthly drug costs. Therefore, our model may provide a reference for policymakers when negotiating for discounts and concessions for NOACs.
This study is the first to evaluate the pharmacoeconomics of all OACs available on the market from a Taiwan payer’s perspective. Chang et al. and Vilain et al. evaluated the cost-effectiveness of a single NOAC versus warfarin, i.e., dabigatran versus warfarin and edoxaban versus warfarin.25,38 Likewise, by directly extracting estimates from trials, including all races, Liu et al. demonstrated that dabigatran, rivaroxaban, and apixaban were cost-effective substitutes for warfarin.37 Our results are consistent with the previous studies in that all of the NOACs were cost-effective alternatives to warfarin in SPAF in Taiwan, and also improved some weaknesses of the previous studies, i.e., obtaining estimates from observational studies or extracting input parameters with mixed races may cause greater uncertainty. First, although direct comparisons of cost-effectiveness between NOACs were not available due to a lack of head-to-head trials, our network meta-analysis allowed for comparisons of each OAC. Second, in order to lower the uncertainty caused by different ethnicities, we extracted the transitional probability of clinical events by targeting Asian populations instead of extracting the data from the clinical trials directly. Third, the severity of stroke and mortality of MI and stroke were adopted from Chang et al., who analyzed data from the NHI Research Database.25 This method could decrease the uncertainty compared to estimates assumed or extracted from foreign databases in the previous studies. Therefore, compared to the previous studies, the discrepancy in ICER values mainly resulted from the different model design and the sources of estimates.
In addition, our study showed that the ICER of all NOACs versus warfarin was much lower than in studies from the US.23,24 NOACs were a cost-effective replacement for warfarin at a WTP threshold of $20,000/QALY gained and even at $10,000/QALY gained in Taiwan, but they were rational choices at a WTP of up to $100,000/QALY gained in the US. Moreover, PSA in the previous studies showed that apixaban was the preferred therapy, whereas rivaroxaban and edoxaban were the optimal choices in our study. This may be due to the fact that NOACs are more cost-effective in patients with higher risks, and our model focused on those with a higher stroke risk, such as elderly patients. On the other hand, NOACs have been shown to have better effectiveness and safety in Asian than in non-Asian populations.39,40 Furthermore, the medical costs in the US are much higher than the costs in Taiwan. For example, the monthly costs of NOACs in the US are approximately four times higher than the monthly costs in Taiwan.
The results of cost-effectiveness iterations for NOACs may change at different WTP thresholds. To date, there is no consensus in Taiwan for a WTP threshold for cost-effectiveness analysis. In this study, we used $20,000 as the threshold because it is close to the GDP per capita in Taiwan at $22,540 [around New Taiwan Dollars (NTD) $698,740] in 2016. The ICER values of each NOAC versus warfarin were $4,115 (NTD $127,565)/QALY gained for apixaban 5 mg, $4,225 (NTD $130,975)/QALY gained for rivaroxaban 20 and 15 mg, $5,458 (NTD $169,198)/QALY gained for edoxaban 60 mg, and $6,415 (NTD $198,865)/QALY gained for dabigatran 150 and 110 mg, respectively. Although all of the ICER values were much lower than $20,000 (NTD $698,740), national payers may need to take into account of the social context and relative consensus of the WTP threshold, as well as opportunity costs and equity issues.
A notable limitation of this study is the need for parameters from multiple sources, which may have led to uncertainty, although we performed a systematic review, network analysis, and sensitivity analysis. For example, pooling dabigatran 150 mg and 110 mg, and rivaroxaban 20 mg and 15 mg would increase the uncertainty of the ICERs. Nevertheless, the sample size of individual doses for each NOAC among Asians meant that we could not perform network meta-analysis. This may have resulted in a lack of comparisons of each NOAC in the cost-effectiveness model, given the traditional meta-analysis. Although a recent retrospective study using Taiwan NHI database showed that rivaroxaban 15 mg might had a higher risk of all-cause death than dabigatran 110 mg,41 all doses of dabigatran and rivaroxaban were the indicated dose for SAPF for Taiwanese. As the daily drug price for both doses were the same, pooling both doses of dabigatran and rivaroxaban may have reflected real-world practice when only comparing the different type of NOACs. The uncertainty may have been reduced if local head-to-head randomized controlled trials or more real-world data were available. Another limitation is the simplification of disease complexity, which may have contributed to different results. In our model, the hypothetic cohort of patients aged 65 years was assumed to receive the indicated doses of NOACs throughout their lifetime. Nevertheless, in Taiwan, reduced doses of NOACs are also prescribed for older patients and those with more comorbidities, and the article by Chan et al. showed the effectiveness and safety.42 Given that the estimates from Chan et al. were input to the model, the ICER per QALY gained for reduced-dose NOAC versus warfarin was still lower than the WTP threshold, because the estimates for the clinical events were within or lower than the range of our input parameters. Therefore, reduced-dose NOACs may still have pharmacoeconomic benefits for SPAF in Taiwan. Moreover, we did not consider minor bleeding and gastrointestinal bleeding in our model. Relevant clinical trials have shown that NOACs have better safety profile than warfarin in Asian populations.39,40 Taking the safety data into account, NOACs would be much more cost-effective due to their smaller ICERs than warfarin. Finally, our model used all-cause mortality rather than age-adjusted mortality as the outcome input because of a lack of the associated mortality rate among the AF patients who received OACs in the NHI Research Database. This may have resulted in uncertainty around the model. A multivariate statistical analysis of age-adjusted mortality for each NOAC from the NHI Research Database may help to provide a more accurate estimation of this model.
CONCLUSIONS
In the current study, all NOACs were cost-effective alternatives for warfarin in SPAF in Taiwan from a national payer’s perspective. The difference in cost-effectiveness of the NOACs was highly dependent on their market prices at the time and the threshold of WTP set by policymakers. The findings of our study may provide an estimation for clinical decision making and healthcare policy.
Acknowledgments
None.
SUPPLEMENTARY MATERIAL
Method of systematic review and network meta-analysis
Search strategy and study selection
This study systematically searched the relevant studies describing the outcome of the NOACs in SPAF among Asians until July 2017 in Medline/PubMed, EMBASE, and Cochrane databases. The citations were also included if they involved in the outcome of NOACs utilization in SPAF among Asian-based population. The following Medical Subject Headings terms were used as: "new oral anticoagulant" or "new oral anticoagulants" or "NOAC" or "NOACs" or "novel oral anticoagulant" or "novel oral anticoagulants" or "non-warfarin oral anticoagulants" or "non-warfarin oral anticoagulant" or "non-vitamin k antagonist oral anticoagulant" or "non-vitamin k antagonist oral anticoagulants" or "dabigatran" or "rivaroxaban" or "edoxaban" or "apixaban", and "Asian" or "China" or "Japan" or "Taiwan" or "Korea" or "Hong Kong" or "Indian" or "Malaysia" or "Philippines" or "Singapore" or "Thailand", and "atrial" or "fibrillation" or "atrial fibrillation". In addition, we identified other studies by using the reference sections of relevant papers and by corresponding with subject experts. Finally, unpublished studies were collected from the ClinicalTrials. gov registry (http://clinicaltrials.gov/).
Figure S1.
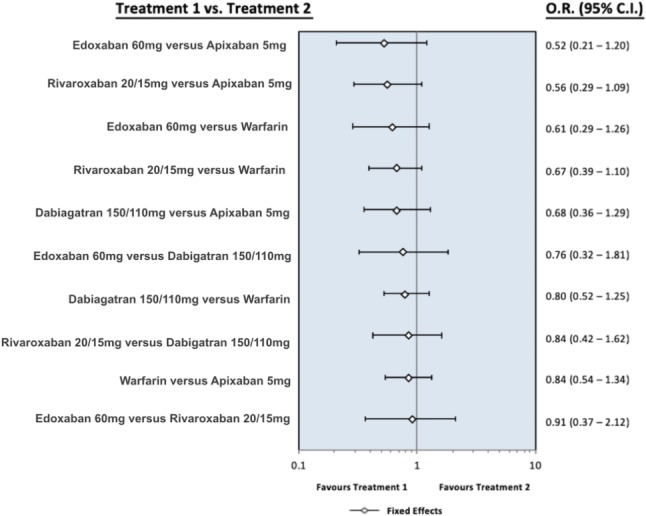
The forest plot presents the odds ratio for ischemic stroke between the different oral anticoagulants. Dabigatran means pooling dabigatran 150 mg and 110 mg, rivaroxaban means pooling rivaroxaban 20 mg and 15 mg, apixaban means apixaban 5 mg, and edoxaban means edoxaban 60 mg. CI, confidence interval; OR, odds ratio.
Figure S2.
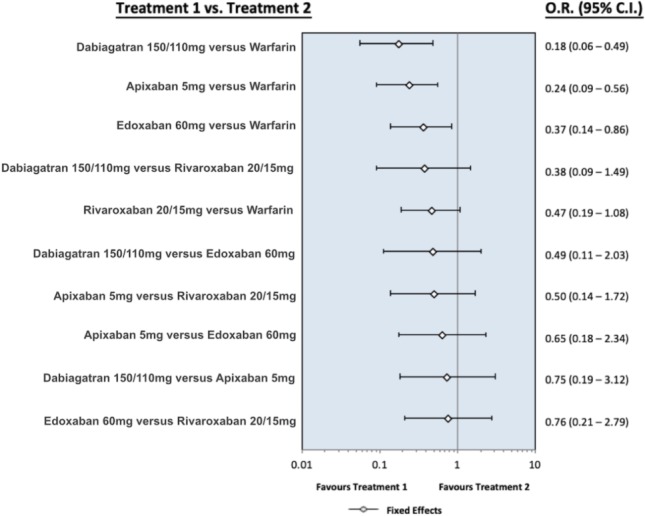
The forest plot presents the odds ratio for hemorrhagic stroke between the different oral anticoagulants. Dabigatran means pooling dabigatran 150 mg and 110 mg, rivaroxaban means pooling rivaroxaban 20 mg and 15 mg, apixaban means apixaban 5 mg, and edoxaban means edoxaban 60 mg. Abbreviations are in Figure S1.
Figure S3.
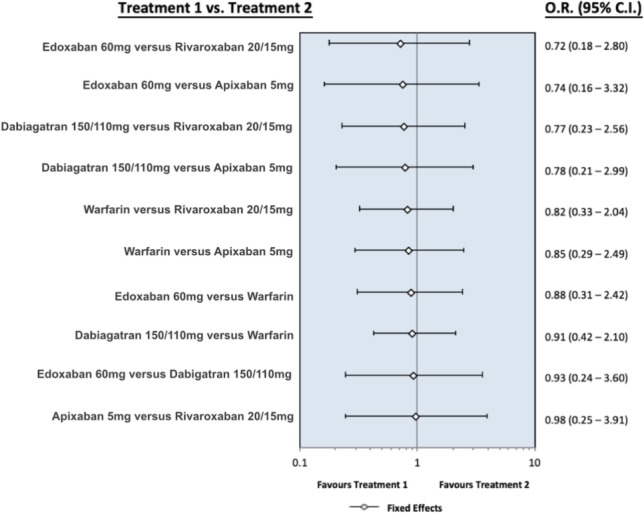
The forest plot presents the odds ratio for myocardial infarction between the different oral anticoagulants. Dabigatran means pooling dabigatran 150 mg and 110 mg, rivaroxaban means pooling rivaroxaban 20 mg and 15 mg, apixaban means apixaban 5 mg, and edoxaban means edoxaban 60 mg. Abbreviations are in Figure S1.
Figure S4.
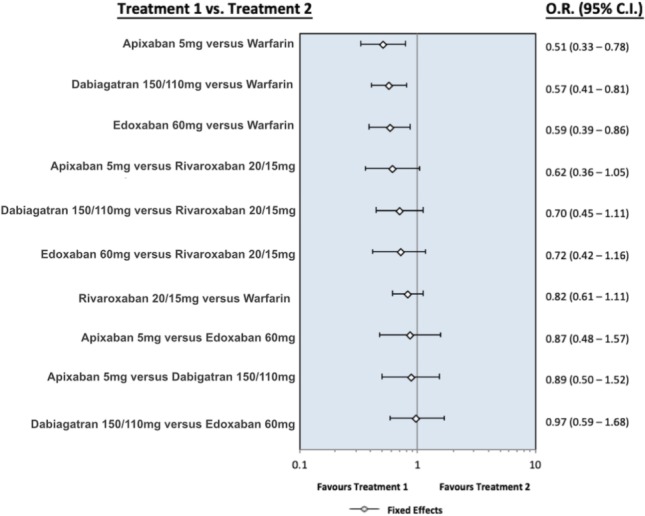
The forest plot presents the odds ratio for major bleeding between the different oral anticoagulants. Dabigatran means pooling dabigatran 150 mg and 110 mg, rivaroxaban means pooling rivaroxaban 20 mg and 15 mg, apixaban means apixaban 5 mg, and edoxaban means edoxaban 60 mg. Abbreviations are in Figure S1.
Figure S5.
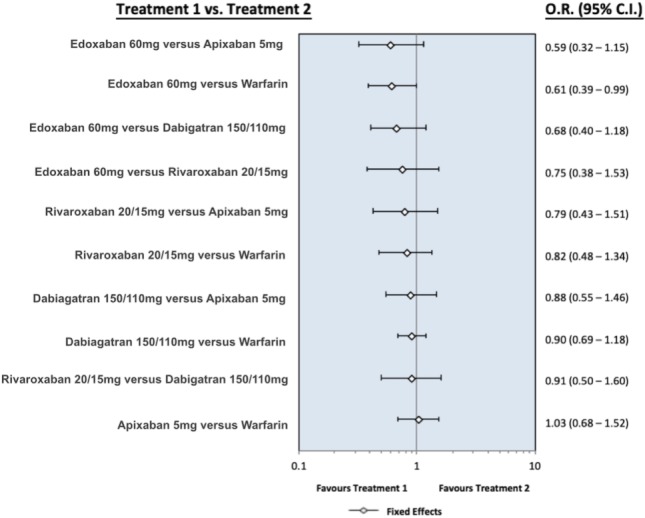
The forest plot presents the odds ratio for all-cause death between the different oral anticoagulants. Dabigatran means pooling dabigatran 150 mg and 110 mg, rivaroxaban means pooling rivaroxaban 20 mg and 15 mg, apixaban means apixaban 5 mg, and edoxaban means edoxaban 60 mg. Abbreviations are in Figure S1.
Inclusion and exclusion criteria
We selected the randomized controlled trials (RCTs) reporting the inclusion and exclusion criteria for SPAF, including warfarin, dabigatran, rivaroxaban, apixaban, and edoxaban. Besides, we excluded the trials with at least one of the following criteria: (1) population under 18 years old, (2) follow-up period below two years, (3) lack of the Asian people data, and (4) the duplicate cohorts.
Data extraction
The baseline data and outcomes were independently abstracted by two reviewers. The study designs, study population characteristics, inclusion and exclusion criteria, drug administration strategies and doses, time to intervention, follow up time and complications were extracted. Decisions individually recorded by the reviewers were compared, and disagreements were resolved by a third reviewer. There were six RCTs identified, including Hori M, et al., Wong KSL, et al, Hori M, et al., Mao L, et al.,Goto S, et al., and Yamashita T, et al.27-31
Methodological quality appraisal
Two reviewers independently assessed the methodological quality of each study with utilization the risk of bias method recommended by the Cochrane Collaboration. Several domains were assessed, including the adequacy of randomization, concealment of allocation, blinding of the patients and outcome assessors, length of follow-up, information provided to the patients regarding study withdrawals, whether intention-to-treat analysis was performed, and freedom from other biases.
Statistical analysis
The data extracted were entered and analyzed by Winbugs. The network meta-analysis was performed in concordance with the PRISMA guidelines. Standard deviations (SD) were estimated from the provided confidence interval (CI) limits or standard error (SE). Furthermore, dichotomous outcomes were analyzed, and the odds ratios (ORs) were presented as the summary statistics. The precision levels of the effect sizes were reported as 95% CIs.
CONFLICT OF INTEREST
All the authors declare no conflict of interest.
REFERENCES
- 1.Lin HJ, Wolf PA, Kelly-Hayes M, et al. Stroke severity in atrial fibrillation. The Framingham Study. Stroke. 1996;27:1760–1764. doi: 10.1161/01.str.27.10.1760. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Global burden of stroke. In: Mackay J, Mensah G, Eds. The Atlas of Heart Disease and Stroke. Geneva, Switzerland: World Health Organization; 2004. pp. 50–51. [Google Scholar]
- 3.Gustavsson A, Svensson M, Jacobi F, et al. Cost of disorders of the brain in Europe 2010. Eur Neuropsychopharmacol. 2011;21:718–779. doi: 10.1016/j.euroneuro.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;1991:983–988. doi: 10.1161/01.str.22.8.983. [DOI] [PubMed] [Google Scholar]
- 6.Brüggenjürgen B, Rossnagel K, Roll S, et al. The impact of atrial fibrillation on the cost of stroke: the Berlin acute stroke study. Value Health. 2007;10:137–143. doi: 10.1111/j.1524-4733.2006.00160.x. [DOI] [PubMed] [Google Scholar]
- 7.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 8.Lin PJ. Reviewing the reality: why we need to change. Eur Heart J. 2005;Suppl 2005(7 Suppl E):E15–E20. [Google Scholar]
- 9.Ansell J, Hollowell J, Pengo V, et al. Descriptive analysis of the process and quality of oral anticoagulation management in real-life practice in patients with chronic non-valvular atrial fibrillation: the international study of anticoagulation management (ISAM). J Thromb Thrombolysis. 2007;23:83–91. doi: 10.1007/s11239-006-9022-7. [DOI] [PubMed] [Google Scholar]
- 10.Witt DM, Delate T, Clark NP, et al. Twelve-month outcomes and predictors of very stable INR control in prevalent warfarin users. J Thromb Haemost. 2010;8:744–749. doi: 10.1111/j.1538-7836.2010.03756.x. [DOI] [PubMed] [Google Scholar]
- 11.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 12.Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 13.Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 14.Giugliano RP, Ruff CT, Braunwald E, et al. Edoxaban versus war-farin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 15.Coyle D, Coyle K, Cameron C, et al. Cost-effectiveness of new oral anticoagulants compared with warfarin in preventing stroke and other cardiovascular events in patients with atrial fibrillation. Value Health. 2013;16:498–506. doi: 10.1016/j.jval.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 16.Harrington AR, Armstrong EP, Nolan PE, Jr., Malone DC. Cost-effectiveness of apixaban, dabigatran, rivaroxaban, and warfarin for stroke prevention in atrial fibrillation. Stroke. 2013;44:1676–1681. doi: 10.1161/STROKEAHA.111.000402. [DOI] [PubMed] [Google Scholar]
- 17.Krejczy M, Harenberg J, Marx S, et al. Comparison of cost-effectiveness of anticoagulation with dabigatran, rivaroxaban and apixaban in patients with non-valvular atrial fibrillation across countries. J Thromb Thrombolysis. 2014;37:507–523. doi: 10.1007/s11239-013-0989-6. [DOI] [PubMed] [Google Scholar]
- 18.Rognoni C, Marchetti M, Quaglini S, Liberato NL. Apixaban, dabigatran, and rivaroxaban versus warfarin for stroke prevention in non-valvular atrial fibrillation: a cost-effectiveness analysis. Clin Drug Investig. 2014;34:9–17. doi: 10.1007/s40261-013-0144-3. [DOI] [PubMed] [Google Scholar]
- 19.Chien KL, Su TC, Hsu HC, et al. Atrial fibrillation prevalence, incidence and risk of stroke and all-cause death among Chinese. Int J Cardiol. 2010;139:173–180. doi: 10.1016/j.ijcard.2008.10.045. [DOI] [PubMed] [Google Scholar]
- 20.Yu HC, Tsai YF, Chen MC, Yeh CH. Underuse of antithrombotic therapy caused high incidence of ischemic stroke in patients with atrial fibrillation. Int J Stroke. 2012;7:112–117. doi: 10.1111/j.1747-4949.2011.00667.x. [DOI] [PubMed] [Google Scholar]
- 21.Lin LJ, Cheng MH, Lee CH, et al. Compliance with antithrombotic prescribing guidelines for patients with atrial fibrillation--a nationwide descriptive study in Taiwan. Clin Ther. 2008;30:1726–1736. doi: 10.1016/j.clinthera.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Yang YN, Yin WH, Feng AN, et al. Low-intensity warfarin therapy for the prevention of stroke in patients with high-risk nonvalvular atrial fibrillation. Acta Cardiol Sin. 2011;27:158–165. [Google Scholar]
- 23.Janzic A, Kos M. Cost-effectiveness of novel oral anticoagulants for stroke prevention in atrial fibrillation depending on the quality of warfarin anticoagulation control. Pharmacoeconomics. 2015;33:395–408. doi: 10.1007/s40273-014-0246-7. [DOI] [PubMed] [Google Scholar]
- 24.Shah A, Shewale A, Hayes CJ, Martin BC. Cost effectiveness of oral anticoagulants for ischemic stroke prophylaxis among nonvalvular atrial fibrillation patients. Stroke. 2016;47:1555–1561. doi: 10.1161/STROKEAHA.115.012325. [DOI] [PubMed] [Google Scholar]
- 25.Chang CH, Yang YH, Chen JH, Lin LJ. Cost-effectiveness of dabigatran etexilate for the prevention of stroke and systemic embolism in atrial fibrillation in Taiwan. Thromb Res. 2014;133:782–789. doi: 10.1016/j.thromres.2014.02.024. [DOI] [PubMed] [Google Scholar]
- 26.Hori M, Fukaya T, Kleine E, et al. Efficacy and safety of dabigatran etexilate vs. warfarin in Asian RE-LY patients according to baseline renal function or CHADS2 score. Circ J. 2015;79:2138–2147. doi: 10.1253/circj.CJ-15-0594. [DOI] [PubMed] [Google Scholar]
- 27.Wong KSL, Hu DY, Oomman A, et al. Rivaroxaban for stroke prevention in East Asian patients from the ROCKET AF trial. Stroke. 2014;45:1739–1747. doi: 10.1161/STROKEAHA.113.002968. [DOI] [PubMed] [Google Scholar]
- 28.Hori M, Matsumoto M, Tanahashi N, et al. Rivaroxaban vs. warfarin in Japanese patients with atrial fibrillation. Circ J. 2012;76:2104–2111. doi: 10.1253/circj.cj-12-0454. [DOI] [PubMed] [Google Scholar]
- 29.Mao L, Li C, Li T, Yuan K. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in Chinese patients with atrial fibrillation. Vascular. 2014;22:252–258. doi: 10.1177/1708538113490423. [DOI] [PubMed] [Google Scholar]
- 30.Goto S, Zhu J, Liu L, et al. Efficacy and safety of apixaban compared with warfarin for stroke prevention in patients with atrial fibrillation from East Asia: a subanalysis of the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) Trial. Am Heart J. 2014;168:303–309. doi: 10.1016/j.ahj.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 31.Yamashita T, Koretsune Y, Yang Y, et al. Edoxaban vs. Warfarin in East Asian patients with atrial fibrillation–an ENGAGE AF-TIMI 48 subanalysis–. Circ J. 2016;80:860–869. doi: 10.1253/circj.CJ-15-1082. [DOI] [PubMed] [Google Scholar]
- 32.Sullivan PW, Ghushchyan V. Preference-based EQ-5D index scores for chronic conditions in the United States. Med Decis Making. 2006;26:410–420. doi: 10.1177/0272989X06290495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sullivan PW, Lawrence WF, Ghushchyan V. A national catalog of preference-based scores for chronic conditions in the United States. Med Care. 2005;43:736–749. doi: 10.1097/01.mlr.0000172050.67085.4f. [DOI] [PubMed] [Google Scholar]
- 34.Gage BF, Cardinalli AB, Owens DK. The effect of stroke and stroke prophylaxis with aspirin or warfarin on quality of life. Arch Intern Med. 1996;156:1829–1836. [PubMed] [Google Scholar]
- 35.Earnshaw SR, Scheiman J, Fendrick AM, et al. Cost-utility of aspirin and proton pump inhibitors for primary prevention. Arch Intern Med. 2011;171:218–225. doi: 10.1001/archinternmed.2010.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Briggs AH. Handling uncertainty in cost-effectiveness models. Pharmacoeconomics. 2000;17:479–500. doi: 10.2165/00019053-200017050-00006. [DOI] [PubMed] [Google Scholar]
- 37.Liu CY, Chen HC. Cost-effectiveness analysis of apixaban, dabigatran, rivaroxaban, and warfarin for stroke prevention in atrial fibrillation in Taiwan. Clin Drug Investig. 2017;37:285–293. doi: 10.1007/s40261-016-0487-7. [DOI] [PubMed] [Google Scholar]
- 38.Vilain KA, Yang MC, Tan ECH, et al. Cost-effectiveness of edoxaban vs. warfarin in patients with atrial fibrillation based on results of the ENGAGE AF-TIMI 48 trial: Taiwanese perspective. Value in Health Regional Issues. 2017;12:74–83. doi: 10.1016/j.vhri.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 39.Lip GY, Wang KL, Chiang CE. Non-vitamin K antagonist oral anticoagulants (NOACs) for stroke prevention in Asian patients with atrial fibrillation: time for a reappraisal. Int J Cardiol. 2015;180:246–254. doi: 10.1016/j.ijcard.2014.11.182. [DOI] [PubMed] [Google Scholar]
- 40.Wang KL, Lip GY, Lin SJ, Chiang CE. Non–vitamin K antagonist oral anticoagulants for stroke prevention in Asian patients with nonvalvular atrial fibrillation: meta-analysis. Stroke. 2015;46:2555–2561. doi: 10.1161/STROKEAHA.115.009947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meng SW, Lin TT, Liao MT, et al. Direct comparison of low-dose dabigatran and rivaroxaban for effectiveness and safety in patients with non-valvular atrial fibrillation. Acta Cardiol Sin. 2019;35:42–54. doi: 10.6515/ACS.201901_35(1).20180817A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chan YH, See LC, Tu HT, et al. Efficacy and safety of apixaban, dabigatran, rivaroxaban, and warfarin in Asians with nonvalvular atrial fibrillation. J Am Heart Assoc. 2018;7:e008150. doi: 10.1161/JAHA.117.008150. [DOI] [PMC free article] [PubMed] [Google Scholar]