Abstract
This scientific commentary refers to ‘Persistent neuropathological effects 14 years following amyloid-β immunization in Alzheimer’s disease’ by Nicoll et al. (doi:10.1093/brain/awz142).
This scientific commentary refers to ‘Persistent neuropathological effects 14 years following amyloid-β immunization in Alzheimer’s disease’ by Nicoll et al. (doi:10.1093/brain/awz142).
Immunotherapy targeting amyloid-β has been at the forefront of experimental therapies for Alzheimer’s disease since it was independently proposed in the late 1990s by Schenk et al. (1999). Since then, this approach has been tested by targeting scores of other aggregating proteins in neurodegenerative disorders, including tau, α-synuclein and TDP-43 (Valera et al., 2016). Both active vaccination and immunotherapy with monoclonal antibodies targeting diverse regions of the amyloid-β protein have been tested. However, both forms of immunotherapy have come under considerable criticism following the negative results of several phase III clinical trials (Panza et al., 2019). AN1792 is an active vaccine in which the immunogen triggers the production of high antibody titres against the N-terminus of amyloid-β protein. In this issue of Brain, Nicoll and co-workers extend previous neuropathological studies of participants from the first clinical trial of immunization with AN1792 (Bayer et al., 2005). In a 15-year post-mortem neuropathology follow-up study of 22 of the 80 participants, they present new evidence indicating that the effects of the anti-amyloid-β protein vaccine on amyloid plaques persisted for over a decade (Nicoll et al., 2019).
Previous post-mortem studies of cases analysed a few years after vaccination with AN1792 showed a consistent and striking removal of amyloid plaques primarily in the neocortex, with the clearance of fibrillar amyloid-β protein material primarily driven by microglia/macrophage-type cells (Masliah et al., 2005; Boche et al., 2010; Serrano-Pozo et al., 2010). However, neurofibrillary tau pathology and cerebrovascular disease persisted, in spite of the widespread removal of amyloid. Furthermore, cognitive impairment in these patients continued to progress to severe dementia. This is consistent with other studies showing that although AN1792 reduces the accumulation of aggregated amyloid-β protein, this does not in itself result in clinical improvements (Gilman et al., 2005).
Given the negative results with respect to primary end-points in this very first immunotherapy trial, subsequent studies have instead targeted soluble amyloid-β protein, various fibrillar and oligomeric species, or post-translationally modified amyloid-β protein isoforms. However, even though some of these studies showed promise in phase II, the phase III studies have so far not met their primary end-points either (Panza et al., 2019). Taken together, these studies indicate that removal of fibrillar amyloid once Alzheimer’s plaque pathology has been established is not sufficient. Possible explanations for the negative results are that too little antibody reached the target, the treatment was started too late or that amyloid-β protein aggregate is the wrong target. It is worth noting that participants in the AN1792 trial all had cognitive impairment ranging from mild to moderate dementia (Fig. 1). The latest immunotherapy trial with a monoclonal antibody targeting amyloid-β protein oligomers tried to address several of these issues by including participants with mild cognitive impairment (MCI), ensuring that higher levels of antibody entered the brain, establishing target engagement and showing a dose-response effect on functional and imaging biomarkers in a phase II clinical trial (Sevigny et al., 2016) (Fig. 1). Unfortunately, phase III of this study was terminated due to futility, leading to the conclusion that amyloid-β protein immunotherapy might be best suited as a preventive strategy.
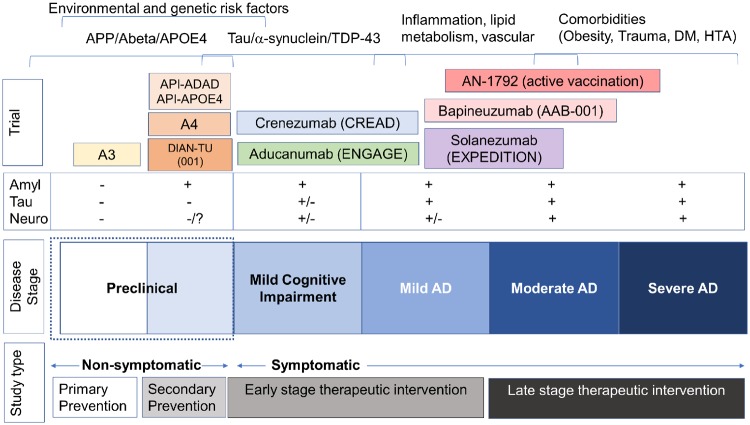
Figure 1.
Diagrammatic representation of the alignment between stages of Alzheimer’s disease, immunotherapy clinical trials targeting amyloid-β protein and the potential pathogenic mechanisms involved. Anti-amyloid immunotherapy trials such as the AN1792 active immunization study from which the neuropathological study by Nicoll et al. (2019) originated, were conducted in symptomatic participants with mild to moderate Alzheimer’s disease. At this stage there is extensive amyloid-β protein deposition, and tau, α-synuclein and TDP-43 also begin to accumulate. Further complicating the picture is the contribution by other processes such as inflammation, alterations in lipid metabolism and vasculature and the development of co-morbidities such as obesity, diabetes mellitus (DM), arterial hypertension (HTA) and trauma. A number of passive immunotherapy trials that showed amyloid removal but no clinical improvement were conducted at the mild to moderate stage, including studies with bapineuzumab (AAB-001) and solanezumab (EXPEDITION I, II, III). More recent trials conducted at earlier stages of the Alzheimer’s disease continuum with monoclonal antibodies against higher order multimers (crenezumab and aducanumab) failed to show clinical improvement in spite of demonstrable effects on amyloid biomarkers. Even at the MCI stage, tau pathology and neurodegenerative pathology characterized by synapse loss are already developing. Current trials with anti-amyloid antibodies are being performed in asymptomatic participants who are positive for amyloid. Examples of such secondary prevention trials include A4 (solanezumab) and API-APOE4 [active vaccination with CD106 versus a BACE inhibitor (CNP520)] in sporadic preclinical Alzheimer’s disease and API-ADAD (crenezumab) and DIAN-TU (solanezumab and gantenerumab) in familial Alzheimer’s disease. The antibody to be used in the A3 trial in asymptomatic cases has yet to be decided. Overall, this diagram suggests that anti-amyloid immunotherapy might be best suited for preclinical stages, while in symptomatic stages other targets including tau, α-synuclein, TDP-43, inflammation, lipid metabolism, vascular and ageing-related comorbidities might need to be included in the context of combinatorial therapy. Amyl = amyloid; Neuro = neurodegeneration.
Late-stage trials of amyloid-β protein immunotherapy supported by the NIH National Institute on Aging (NIA) are currently underway to test whether starting the treatment at earlier stages of the disease is effective (Fig. 1). For example, in the A4 trial, asymptomatic individuals selected for positive amyloid-β protein biomarkers have been treated with a higher dose of an antibody against soluble amyloid-β, solanezumab (Sperling et al., 2014). The objective of this trial in participants who are biomarker positive (PET imaging of fibrillar amyloid-β protein) and cognitively asymptomatic is to determine whether immunotherapy can delay or prevent the onset of Alzheimer’s disease. Similarly, the NIA is also sponsoring secondary prevention trials in individuals with dominantly inherited Alzheimer’s disease (Fig. 1). These participants, who have been extensively characterized using biomarkers, begin immunotherapy years before their predicted onset of cognitive impairment. The dominantly inherited Alzheimer’s disease network (DIAN) clinical trial includes multiple sites in the US and Europe in which individuals with APP and PSEN1 mutations are being treated with solanezumab and gantenerumab (Bateman et al., 2017), while the Alzheimer’s Prevention Initiative is testing crenezumab in the Colombia kindred with the ‘Paisa’ mutation in PSEN1 (van Dyck, 2018). Results of these studies are expected in the next couple of years (Fig. 1).
There are a number of other potential reasons why amyloid-β protein immunotherapy might have failed in patients with MCI and dementia, including the fact that Alzheimer’s disease pathology is not limited to amyloid-β deposition. Tau oligomer accumulation and neurofibrillary pathology might play even more important roles. In addition, recent neuropathology studies have shown that pure Alzheimer’s disease pathology (plaques and tangles) is not the rule; most cases of dementia over the age of 80 years have combined vascular, Lewy body and TDP-43 pathology. Nicoll et al. (2019) show that amyloid-β protein vaccination removed not only fibrillar amyloid but also tau neuritic pathology, although not tau neurofibrillary pathology in neuronal cell bodies. However, monotherapy targeting amyloid-β protein may be insufficient to produce clinical improvement. We may need to combine immunotherapy targets including at a minimum amyloid-β protein, tau, α-synuclein and TDP-43. This can be achieved by mixing monoclonal antibodies or immunogens for active vaccination or designing multivalent single chain antibodies. To date, vaccinations against tau (Novak et al., 2018) and α-synuclein have advanced to phase II (Valera et al., 2016), showing safety in their use, but there is still a long road until these antibodies are tested in later stage clinical trials and in combination with anti-amyloid-β protein immunotherapy.
One last and important concept to keep in mind is that although protein accumulation might play a key role in Alzheimer’s disease and other neurodegenerative disorders, the importance of ageing as a risk factor suggests that other factors might be independently or co-dependently contributing to these disorders (Fig. 1). These include age-related alterations in proteostasis, inflammation, stem cell biogenesis, mitochondrial function, cell senescence and DNA damage/repair. Combinatorial therapeutics might therefore require targeting not only multiple proteins but also some of these pillars of geroscience. This multi-pronged approach is consistent with the idea of personalized medicine. The key issue, as illustrated by Fig. 1, is to identify the ideal window of opportunity for anti-amyloid immunotherapy and when to combine it with antibodies against other proteins as well as approaches targeting inflammatory, lipid metabolism, vascular and insulin signalling pathways, among others.
Competing interests
The authors report no competing interests.
References
- Bateman RJ, Benzinger TL, Berry S, Clifford DB, Duggan C, Fagan AM, et al. The DIAN-TU next generation Alzheimer’s prevention trial: adaptive design and disease progression model. Alzheimers Dement 2017; 13: 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer AJ, Bullock R, Jones RW, Wilkinson D, Paterson KR, Jenkins L, et al. Evaluation of the safety and immunogenicity of synthetic Abeta42 (AN1792) in patients with AD. Neurology 2005; 64: 94–101. [DOI] [PubMed] [Google Scholar]
- Boche D, Denham N, Holmes C, Nicoll JA. Neuropathology after active Abeta42 immunotherapy: implications for Alzheimer’s disease pathogenesis. Acta Neuropathol 2010; 120: 369–84. [DOI] [PubMed] [Google Scholar]
- Gilman S, Koller M, Black RS, Jenkins L, Griffith SG, Fox NC, et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology 2005; 64: 1553–62. [DOI] [PubMed] [Google Scholar]
- Masliah E, Hansen L, Adame A, Crews L, Bard F, Lee C, et al. Abeta vaccination effects on plaque pathology in the absence of encephalitis in Alzheimer disease. Neurology 2005; 64: 129–31. [DOI] [PubMed] [Google Scholar]
- Nicoll JAR, Buckland GR, Harrison CH, Page A, Harris S, Love S. et al. Persistent neuropathological effects 14 years following amyloid-β immunization in Alzheimer's disease. Brain 2019; 142: 2113–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak P, Kontsekova E, Zilka N, Novak M. Ten years of tau-targeted immunotherapy: the path walked and the roads ahead. Front Neurosci 2018; 12: 798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panza F, Lozupone M, Seripa D, Imbimbo BP. Amyloid-beta immunotherapy for Alzheimer disease: is it now a long shot? Ann Neurol 2019; 85: 303–15. [DOI] [PubMed] [Google Scholar]
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, et al. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 1999; 400: 173–7. [DOI] [PubMed] [Google Scholar]
- Serrano-Pozo A, William CM, Ferrer I, Uro-Coste E, Delisle MB, Maurage CA, et al. Beneficial effect of human anti-amyloid-beta active immunization on neurite morphology and tau pathology. Brain 2010; 133: 1312–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevigny J, Chiao P, Bussiere T, Weinreb PH, Williams L, Maier M, et al. The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature 2016; 537: 50–6. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Rentz DM, Johnson KA, Karlawish J, Donohue M, Salmon DP, et al. The A4 study: stopping AD before symptoms begin? Sci Transl Med 2014; 6: 228fs13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera E, Spencer B, Masliah E. Immunotherapeutic approaches targeting amyloid-beta, alpha-synuclein, and tau for the treatment of neurodegenerative disorders. Neurotherapeutics 2016; 13: 179–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dyck CH. Anti-amyloid-beta monoclonal antibodies for Alzheimer’s disease: pitfalls and promise. Biol Psychiatr 2018; 83: 311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]