Abstract
Treatment of cancer has evolved in the last decade with the introduction of new therapies. Despite these successes, the lingering cardiotoxic side-effects from chemotherapy remain a major cause of morbidity and mortality in cancer survivors. These effects can develop acutely during treatment, or even years later. Although many risk factors can be identified prior to beginning therapy, unexpected toxicity still occurs, often with lasting consequences. Specifically, cardiotoxicity results in cardiac cell death, eventually leading to cardiomyopathy and heart failure. Certain risk factors may predispose an individual to experiencing adverse cardiovascular effects, and when unexpected cardiotoxicity occurs, it is generally managed with supportive care. Animal models of chemotherapy-induced cardiotoxicity have provided some mechanistic insights, but the precise mechanisms by which these drugs affect the heart remains unknown. Moreover, the genetic rationale as to why some patients are more susceptible to developing cardiotoxicity has yet to be determined. Many genome-wide association studies have identified genomic variants that could be associated with chemotherapy-induced cardiotoxicity, but the lack of validation has made these studies more speculative rather than definitive. With the advent of human induced pluripotent stem cell (iPSC) technology, researchers not only have the opportunity to model human diseases, but also to screen drugs for their efficacy and toxicity using human cell models. Furthermore, it allows us to conduct validation studies to confirm the role of genomic variants in human diseases. In this review, we discuss the role of iPSCs in modelling chemotherapy-induced cardiotoxicity.
Keywords: Cardio-oncology, Chemotherapy, Induced pluripotent stem cells, Pharmacogenomics, Precision medicine
This article is part of the Spotlight Issue on Cardio-oncology.
1. Introduction
Cancer remains one of the leading causes of morbidity and mortality in the United States,1 and the annual number of new cancer cases is projected to rise to about 24 million by 2030. Notwithstanding the upward trend of cancer cases, substantial improvements in the diagnosis and management of cancer patients have been made in recent decades to significantly reduce the overall cancer death rate and boost the 5-year survival rate. The progress has been attributed mostly to newer and more targeted therapies for cancer, including immunotherapies,2 nanomaterials,3 and the ongoing development of new drugs. Despite these efforts, significant barriers remain for entry of new therapies. With average clinical approval rates of 13.4% and costs of $1.4 billion per successful drug,4 the development of new chemotherapeutics has become an unsustainable endeavour, with an overall negative return on investments. While efforts are underway to decrease costs and improve the success rate of drug development, it is clear that most chemotherapy regimens will require the use of our existing array of approved drugs.
While many of the approved drugs are effective as primary or adjunctive cancer treatments, subsets of patients experience severe, deleterious side effects. Perhaps some of the most debilitating side effects occur from cardiovascular toxicity, which can be classified in two ways: traditional and targeted chemotherapeutics.5 Traditional chemotherapeutics cause irreversible damage to the myocardium, including apoptosis or necrosis of cardiomyocytes, whereas targeted chemotherapeutics cause reversible damage to cellular function and physiology. Toxicity has been observed in multiple classes of drugs, including anthracyclines,6 anti-microtubule agents,7 tyrosine kinase inhibitors (TKIs),8 and antibody-based drugs such as trastuzumab.9 The question as to why certain patients are impacted severely by these drugs, while others tolerate them well has yet to be answered. Furthermore, the mechanisms of toxicity of these drugs are likely multifactorial.
A wealth of clinical information is available on chemotherapy-induced adverse drug events, including both acute and chronic cardiotoxicity.10 However, most clinical information is collected during the terminal stages of cardiotoxicity, when the patients have already developed irreversible myocardial injury. Similarly, several preclinical models have been developed to exhibit chemotherapy-induced cardiomyopathy11; however, these animal models are disadvantaged for not being suitable for comparative studies with respect to cardiac function measurements. Moreover, as animals possess different cardiovascular physiology compared with that of humans, it is difficult to reliably identify suitable biomarkers that can detect or predict the onset of cardiotoxicity.11,12 Thus, there is a compelling need to establish a unique human-based platform that can evaluate the cardiac safety profile of patients receiving chemotherapy.
The advent of induced pluripotent stem cell (iPSC) technology13,14 has finally allowed researchers to circumvent these issues, and to model human cardiovascular disease.15,16 Importantly, it has allowed us to screen drugs for efficacy and toxicity studies in vitro.17 In this review, we discuss the state-of-the-art knowledge in chemotherapy-induced cardiotoxicity and discuss how human iPSC-derived cardiomyocytes (iPSC-CMs) can be leveraged as an ideal platform to conduct cardiotoxicity testing. We also highlight the importance of iPSC-CMs in the pharmacogenomics of chemotherapy-induced cardiotoxicity.
2. Traditional chemotherapeutics: anthracyclines
Anthracyclines are one of the most effective classes of chemotherapeutics. Daunorubicin, the first identified member of the class, was isolated in the 1960s from the soil microbe Streptomyces peucetius and was found to have extensive tumour activity against lymphopblastic and myeloblastic leukaemia in mice; however, marked cardiotoxicity was observed.18 A short time later, the related compound doxorubicin (DOX) was isolated from a separate strain of S. peucetius19 with an even broader effectiveness against tumours. By the 1970s, DOX entered clinical use and has remained a chemotherapy staple for over 40 years.
Though effective, DOX comes with harsh side effects, notably cardiotoxicity that has both acute and chronic presentations. Acute toxicity occurs either during or immediately following the infusion of an anthracycline and is characterized by cardiac rhythm disturbances and hypotension.20 These effects are typically temporary and will resolve once the infusion is completed. New dosing guidelines have made acute toxicity a rare occurrence.21 The most clinically significant toxicities are sub-chronic and chronic toxicities, which have similar clinical features and are distinguished mainly by the time of onset. Early chronic toxicity is observed within weeks to months following anthracycline treatment. Chronic toxicity is observed years or even decades later, which has significant implications for survivors of childhood cancers who received anthracyclines as part of their chemotherapy regimen.22 A recent prospective study suggested that improved surveillance might detect an early insult from anthracycline therapy in approximately 98% of toxicity cases, perhaps demonstrating that chronic toxicity cases are simply the outcome of progressive dysfunction over months to years.23
The mechanisms behind anthracycline toxicity, such as the mechanisms behind its anti-neoplastic effects, are complex. Anthracyclines intercalate into DNA, forming adducts that interfere with its replication. Similarly, they are also known to generate reactive oxygen species (ROS), resulting in DNA and mitochondrial damage. Initially, much of the toxic effects were believed to be related to the abundant ROS generation.24 However, the role of ROS in cardiotoxicity of anthracyclines is controversial, as other studies have found that it does not contribute significantly to cardiomyocyte death.25 Therapeutics towards ROS-mediated cardiotoxicity have also failed to be translated clinically, as free-radical scavenging compounds such as vitamin E, N-acetylcysteine, and others have failed to confer protection.26 Further research identified topoisomerase IIβ (TOP2β) as a major player in the cardiotoxic effects of anthracyclines.27 The investigators showed that deletion of TOP2β in mice failed to recapitulate the effects of DOX-induced cardiomyopathy, including the formation of ROS and DNA damage. This mechanism was further supported by the observation that dexrazoxane, a TOP2β19 inhibitor, conferred protection against anthracycline toxicity.28
3. Other traditional chemotherapeutic agents
Cardiotoxicity has also been observed with other traditional chemotherapeutic agents. These include antimetabolites, alkylating agents, platinum agents, and anti-microtubule agents. The antimetabolite drug 5-fluorouracil (5-FU) has a common incidence of cardiotoxic effects, primarily rhythm disturbances and angina. More serious effects such as congestive heart failure (CHF) occur in less than 2% of patients. Proposed mechanisms of 5-FU toxicity include ROS-mediated damage, accumulation of intracellular citrate,29 toxic degradation products from improper storage,30 and myocardial damage secondary to vascular toxicity and vasospasm.31 The remaining classes of traditional chemotherapeutics have rare cardiotoxic effects, and their mechanisms are not well studied. Documented effects of cyclophosphamide, an alkylating agent, include tachyarrhythmias, CHF, pericarditis, and haemorrhagic myocarditis.32 Taxanes, which target microtubules, have been associated with rhythm disturbances, but clinically significant cardiotoxicity is rarely observed.
4. Targeted chemotherapeutics: kinase inhibitors
Kinase inhibitors are a class of drugs comprised of small molecule inhibitors and monoclonal antibodies. Generally, these drugs target tyrosine kinases, although some may directly or indirectly target kinases in other families, such as serine/threonine kinases. The two classes of kinase inhibitors that are widely used in clinics include trastuzumab (Herceptin), a monoclonal antibody, and TKIs. These new drugs differ from older agents in that they are often developed through ‘rational drug design’, a method that attempts to target specific proteins and receptors vital for malignant survival, proliferation, and metastases, while attempting to minimize off-target effects.33 Trastuzumab works by targeting human epidermal growth factor receptor 2 (HER2), a receptor tyrosine kinase and oncogene present in approximately 30% of breast cancers patients.34 TKIs were developed against vascular endothelial growth factor receptor 2, epidermal growth factor receptor, and serine/threonine protein kinase B-raf.35
4.1 HER2 inhibitors
Although kinase inhibitors tend to be tolerated better than other classes, adverse effects including cardiovascular toxicity continue to be documented. Patients with trastuzumab cardiotoxicity usually show mild reduction in their left ventricular ejection fraction (LVEF) and an increase in their serum cardiac troponin I (cTnI) concentration, but patients may occasionally develop CHF.36 Alone, trastuzumab causes overt cardiotoxicity in about 4% of treated patients; however, when it is combined with an anthracycline or taxane, the incidence rises to 27% and 12%, respectively.37 For the majority of these treated patients, the effects are reversible upon discontinuation, but there is lasting damage for others.36 Trastuzumab cardiotoxicity appears to be due to blocked cardioprotective effects of the HER2/4 signalling pathway. Although HER2 is an orphan receptor, the inhibition of HER2 by trastuzumab can lead to impaired HER2/4 signalling, which interferes with neuregulin-1 (NRG-1) signalling and eventually results in ROS production and mitochondrial dysfunction.38 Although the clinical effects appear to be largely reversible, trastuzumab seems to cause lasting ultrastructural damage and changes in the expression of genes associated with DNA repair in cultured rat ventricular cardiomyocytes. The significance of these chronic changes is unknown.
4.2 Tyrosine kinase inhibitors
Concerns for cardiotoxicity with small molecule inhibitors were raised in 2006 when 10 patients who were treated with imatinib, a TKI, reportedly developed CHF after beginning therapy.39 However, this finding was controversial; a retrospective review of reported cases of cardiotoxicity found only 0.6% were considered as a possible consequence of drug treatment.40 Currently, six TKIs carry FDA warnings about cardiotoxicity: sunitinib, vandetanib, trametinib, vemurafenib, ponatinib, and nilotinib. Nilotinib, vendetanib, and vemurafenib are associated with QT prolongation and carry a risk of sudden death, whereas sunitinib is associated with decreased LVEF41 and QT interval prolongation.8 Ponatinib and trametinib are associated with CHF. Cardiac dysfunction has been noted in other TKIs that target the vascular endothelial growth factor pathway, including sorafenib,8 pazopinib,42 and axitinib.43 In addition, TKIs can have diverse cardiovascular sequelae that extend beyond cardiomyopathy. Depending on the specific kinases being targeted, cardiovascular toxicities may include vascular effects, QT interval prolongation, pericarditis/pericardial effusion, and arrhythmias such as atrial fibrillation.44
The toxicity of other TKIs has been less obvious. In 2006, Kerkelä et al.39 performed the first in-depth assessment of imatinib-induced cardiotoxicity using a mouse model. After 3–4 weeks of treatment in healthy mice, left ventricular (LV) dilation, and contractile dysfunction were observed. Imatinib was shown to induce the endoplasmic reticulum (ER) stress response, leading to the downstream collapse of the mitochondrial membrane potential, caspase inhibition, and ATP-depletion. The primary mechanism of cell death was through necrosis, supporting findings of decreased LV mass in the absence of apoptosis. A study in cultured rat ventricular cardiomyocytes found an inverse correlation between the target selectivity of the TKI and cardiotoxicity, implicating off-target effects as the primary driver of toxicity.45 Inhibition of colony stimulating factor 1 receptor (CSF1R), in particular, was identified as being involved in cardiotoxicity.
Other groups have studied TKIs with similar target profiles. A study of the multi-kinase inhibitors pazopanib, sorafenib, and sunitinib failed to demonstrate gross toxicity in the absence of cardiac stress; however, conduction abnormalities were noted in the sorafenib-treated group when stressed with dobutamine.46 Mitochondrial degeneration was observed in the sunitinib-treated rats as well. Despite the similarities in targeted kinases, the drugs appear to have different toxic effects, suggesting off-target effects are important to the pathophysiology. Arrhythmogenic and QT interval prolongation effects of other TKIs have also been investigated, and the mechanism appears to involve interference with phosphinositide-3 kinase signalling, which has downstream effects on multiple ion channels,47 although the clinical significance of QT prolongation is unknown.
5. Proteasome inhibitors and immunomodulators
Proteasome inhibitors (PIs) and immunomodulators are both commonly used for the treatment of multiple myeloma (MM), and patients taking either class of drugs have shown cardiovascular toxicity.48 Indeed, many clinical trials have shown that all the three approved PIs (bortezomib, carfilzomib, and ixazomib) induce cardiotoxicity in patients to varying degrees.49 PIs act by targeting malignant plasma cells and inhibiting their proteasome activity, leading to apoptosis of the cancer cells.50 Consequently, the PIs also inhibit proteasome activity and sarcomeric protein turnover in cardiomyocytes, leading to cardiac cell death via caspase-3/7 signalling.51 As these results do not reflect the clinical experience, it seems likely that the toxicity might be due to some other mechanism. Moreover, as MM more commonly occurs in older patients who often have concurrent cardiovascular complications, a better platform for toxicity screening is warranted.
Immunomodulators, including thalidomide, pomalidomide, and lenalidomide, function by inducing the selective ubiquitination and degradation of essential lymphoid transcription factors.52,53 These drugs have been associated with a dramatic increase in thromboembolic events when used in combination with cytotoxic therapies or dexamethasone. The mechanisms behind these events are unclear, but increased cytokine levels, tissue factors, and genetic factors have been hypothesized to play a role.48
6. Immunotherapies
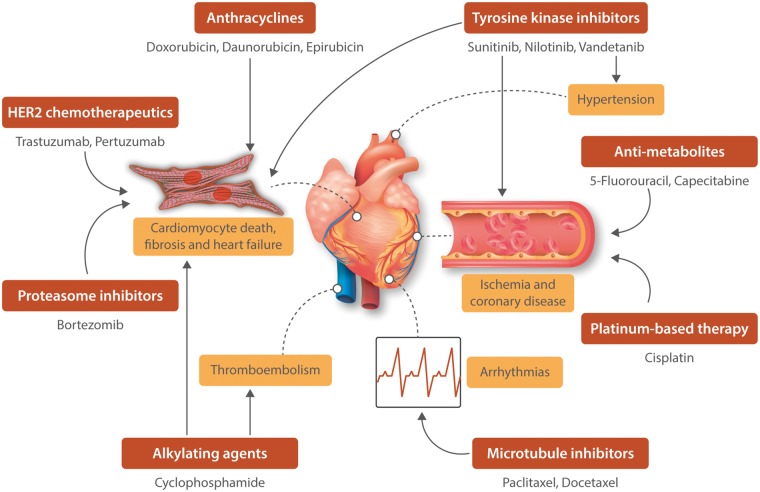
Immunotherapies, often referred to as biologic therapies, are cancer treatments that target or use the patient’s own immune system to kill cancer cells. In recent years, the field of oncology has seen an explosion of these therapies that have been particularly effective against certain types of cancers. There are several forms of immunotherapies, each assisting the immune system in their own unique way; however, the two most commonly used ones include immune checkpoint inhibitors and adoptive cellular therapy (ACT). While immune checkpoint inhibitors help immune cells such as T cells to mount an attack on the cancer cells,54 ACT provides additional power to these immune cells by increasing the number of T cells.55 Despite these advancements, the increased use of cancer immunotherapies has started to highlight adverse cardiotoxic events in patients. Indeed, immune checkpoint inhibitors targeting CTLA-4 and PD-1 have been shown to be associated with cardiotoxic events including acute myocarditis, conduction abnormalities, and ventricular dysfunction.56 Cardiotoxic reactions have also been observed in patients subjected to ACT, with one report showing multi-organ damage, lymphocytic myocarditis, and fatal cardiac arrest in a patient 6 days following ACT that targeted melanoma antigen recognized by T-cells (MART-1).57 Similarly, another case report showed that ACT targeting melanoma-associated antigen 3 (MAGE-3) on T cells led to the development of cardiogenic shock and myocardial damage in patients.58 While the incidence of cardiotoxicity remains low with immunotherapies, the appearance of immune-mediated myocarditis in patients raises the need to carefully surveil patients treated with cancer immunotherapy. Figure 1 summarizes the cardiotoxic side effects of the different chemotherapeutic agents.
Figure 1.
Schematic of the cardiotoxic side-effects of the different chemotherapeutic agents.
7. Current management of cardiotoxicity
Current management of chemotherapy toxicity begins with the identification of known risk factors such as age, smoking, previous cardiovascular history, and comorbidities such as diabetes mellitus and hypertension, combined with screening of patients through echocardiography, electrocardiogram, angiography, and serum biomarkers such as cardiac troponin T and brain natriuretic peptide59,60 (Figure 2A). In the case of anthracyclines, dexrazoxane is used as a clinical cardioprotective agent, but it has serious limitations. After findings showed an association between dexrazoxane use in children and later development of secondary malignancies such as acute myeloid leukaemia,61 the FDA released a statement emphasizing that dexrazoxane use is only approved for adults who have already received 300 mg/m2 of DOX or greater, and any other use is considered ‘off-label’. Additional medications have been found to have some protective benefit, including beta blockers, statins, and angiotensin II receptor blockers.62 Other strategies and techniques have been developed to minimize anthracycline toxicity, including limiting the cumulative life-time dose and using liposomal formulations63 to lessen accumulation inside cardiomyocytes. These approaches have reduced the frequency of cardiotoxicity, but some patients continue to experience debilitating effects.
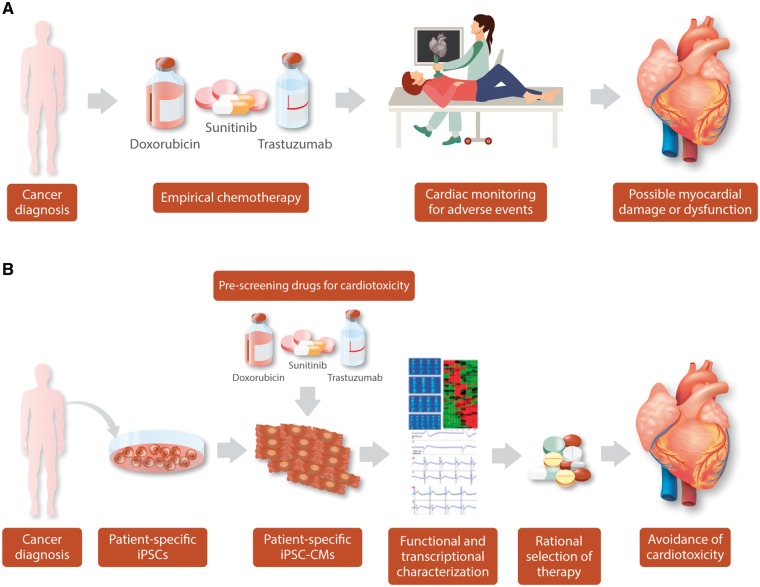
Figure 2.
Conventional vs. iPSC-based management of chemotherapy-induced cardiotoxicity. (A) Current approach to chemotherapy. A patient is diagnosed and receives established protocols. The patient may then undergo monitoring for cardiotoxicity, which may appear acutely or, in the case of doxorubicin, may appear years later, thus requiring life-long monitoring. Early detection of potential toxicity allows early intervention, though lasting damage to the heart may still occur. (B) Personalized Medicine approach: After a patient receives a cancer diagnosis, iPSC-CMs can be generated and screened against potential therapies. iPSC-CMs can then be assessed by a number of functional and transcriptional parameters for evidence of toxicity. Based on this, safer drugs can be selected for the patient much earlier, minimizing the risk of cardiotoxicity.
For other agents, there are few specific treatments for cardiotoxicity once it manifests and clinical management is largely symptomatic. Depending on the severity of the reaction, the chemotherapeutic drug may be discontinued, and approaches to manage arrhythmias, CHF, and other issues are employed as the need arises. Although the use of prophylactic agents such as calcium channel blockers and beta blockers has been explored to reduce 5-FU toxicity, there is limited evidence supporting their use.64 Fortunately, in the case of 5-FU and the newer targeted therapies, removal of the offending drug will typically result in resolution of cardiac dysfunction. However, as the adage ‘an ounce of prevention is worth a pound of cure’ tells us, avoiding cardiotoxicity in the first place would be preferable to management of active toxicity.
8. Modelling of cardiotoxicity with iPSC-CMs
Until now, modelling diseases and drug toxicity has largely depended on animal models. While useful, these models have serious limitations. Differences in physiology, drug metabolism, and gene expression limit the translational interpretation of animal experimental results. In contrast, human iPSCs allow researchers to have an unlimited supply of human cells that are patient-specific without any dependency on cell lines or animal models. Cardiovascular research has greatly benefited from the use of iPSCs as we can now obtain iPSC-CMs that are genomically identical to the patients and to some extent recapitulate the biology of in vivo cardiomyocytes.16 Specifically, these iPSC-CMs express most of the cardiac-specific ion channels, have a versatile contractile apparatus, and possess calcium-handling properties. Based on this, iPSC-CMs have been demonstrated to be a highly useful platform for pharmacologic studies and for modelling familial cardiac disease. An ever-growing list of diseases encompassing channelopathies and cardiomyopathies is being generated by using iPSC-CMs, including long QT syndrome,65 Brugada syndrome,66 LV non-compaction,67 dilated cardiomyopathies,68,69 and hypertrophic cardiomyopathies.70,71 Furthermore, iPSC technology provides a perfect platform to conduct ‘toxicity screens’ for each individual, and iPSC-CMs can be subjected to the chemotherapeutic agents to determine which individual has an increased propensity to develop cardiotoxicity. Based on this, an oncologist can make an informed decision on whether to use a different chemotherapeutic or titer the dose for each individual patient (Figure 2B). These ‘toxicity trials’ in-a-dish have gained momentum with many pharmaceutical companies now using the iPSC-CM model to screen drugs to determine their arrhythmogenic potential.72,73
8.1 Modelling anthracycline-induced cardiotoxicity
In addition to modelling cardiac diseases, iPSC-CMs are being used as a platform for modelling drug toxicity, specifically chemotherapy-mediated cardiotoxicity. One recent study found that iPSC-CMs derived from breast-cancer patients with clinical DOX cardiotoxicity recapitulated DOX sensitivity in vitro compared with iPSC-CMs derived from healthy controls and from patients not exhibiting cardiotoxicity after treatment.74 Markers of sensitivity to DOX included sarcomeric disarray, increased caspase 3/7 activity and ROS production, mitochondrial and metabolic dysfunction, and changes in calcium handling. The evaluation of cardioprotective compounds in the presence of DOX, however, diverges from the clinical experience and gives credence to the multifactorial nature of anthracycline toxicity. In vitro evaluation of dexrazoxane in combination with DOX showed an increase of toxicity across all lines, whereas cells treated with N-acetylcysteine showed a decreased sensitivity to DOX, suggesting that ROS may play an important role in this model.
Development of iPSC-CMs as a platform for modelling drug toxicity is ongoing. iPSC-CMs have been used to show decreased contractility, contraction velocity, and beating rates in response to DOX. They have also been used to document stress release biomarkers such as N-terminal pro-brain natriuretic peptide (NT-proBNP), cTnI, and heart-type fatty acid binding protein (hFABP),75 as well as to identify novel biomarkers such as growth differentiation factor 15 (GDF15).76 Tissue engineering technologies are also taking the platform beyond the traditional two-dimensional monolayer culture methods towards more physiologic three-dimensional (3D) approaches, allowing the integration of other cardiac cell types into the model.77 Indeed, this 3D model was used to test DOX-induced toxicity and was found to mimic the in vivo environment better than the 2D cultures,78 suggesting that combination of iPSC-CM technology and tissue engineering might provide better understanding of drug toxicity.
Beyond toxicity studies, iPSC-CMs are also being used for the investigation of novel drug targets, compounds, and therapies that may be cardioprotective when used in conjunction with DOX. As mentioned above, activation of the HER2 signalling pathway in iPSC-CMs has been demonstrated to attenuate DOX toxicity, whereas inhibition of HER2 signalling exacerbates toxicity.79 This is consistent with clinical data from patients treated simultaneously with DOX and trastuzamab, an anti-HER2 antibody, who experienced even greater cardiovascular dysfunction than with DOX alone.80 Investigators have found that HER2-activators such as NRG have a protective effect in iPSC-CMs. NRG itself is a pro-neoplastic agent, and its promise as a cardioprotective agent has led to the creation of a bivalent form of the molecule that confers similar protections on iPSC-CMs, while minimizing the pro-oncogenic effects.81 The search for new cardioprotective therapies even includes iPSCs themselves. For example, iPSC-derived mesenchymal stem cells (iPSC-MSCs) appear to have a protective function against DOX toxicity in mouse cardiomyocytes through paracrine functions mediated by GDF15 and macrophage migratory inhibitory factor,82 as well as through direct mitochondrial transfer to damaged cardiomyocytes mediated by Rho GTPase 1 and TNF-α signalling.83 Further research using direct injection or engraftment of iPSC-MSCs into the hearts of patients undergoing chemotherapy may lead to a better understanding of the pathways and factors involved in cardio-protection and novel therapies. Although there is significant morbidity with other traditional chemotherapeutic agents,84 thus far little research has focused on the potential use of iPSC-CMs as a platform for toxicity modelling of these agents. A summary of the available iPSC-CM studies on traditional chemotherapeutic agents is presented in Table 1.
Table 1.
iPSC-modelling of traditional chemotherapeutic agents
| Class | Representative drugs | Clinical cardiotoxic effects | iPSC-CM modelling | References |
|---|---|---|---|---|
| Anthracyclines | Doxorubicin | Arrhythmias, myocardial oedema, decreased LVEF, congestive heart failure, myopericarditis, and myocardial infarction | Sarcomeric disarray, increased caspase 3/7 activity, ROS production, mitochondrial and metabolic dysfunction, and changes in calcium handling | 74–76,78 |
| Anti-metabolites | 5-Fluorouracil | Angina, QT prolongation, arrhythmias, myocardial infarction, coronary vasospasm, and pericarditis | Cytotoxicity and induction of arrhythmic beats | 84 |
| Alkylating agents | Cyclophosphamide | Congestive heart failure, myopericarditis, arrhythmias, and haemorrhagic myocarditis | Cytotoxicity and induction of arrhythmic beats | 84 |
| Anti-microtubule | Paclitaxel | Bradycardia, VPCs, and ventricular tachycardia | Undetermined | NA |
NA, not applicable.
8.2 Modelling tyrosine kinase inhibitor-induced cardiotoxicity
With more clinical use of TKIs and clinical studies suggesting TKI-associated cardiovascular dysfunction such as CHF, vascular dysfunction, and arrhythmias,44 there is a compelling need to model TKI-induced cardiotoxicity using iPSC-CMs. TKIs have been used in a number of iPSC-CM-based toxicity studies, including mechanistic studies on toxicity exploring the utility of iPSC-CMs as a platform for toxicity screening in large numbers of drugs. The early mechanistic studies of cardiotoxicity revealed a broad range of effects of TKIs on cells, despite their targeted approach. A study of ponatinib cardiotoxicity, for example, revealed the generation of ROS, lipid accumulation, and the inhibition of ABL, AKT, and ERK survival pathways. Additionally, ponatinib disrupted the normal actin cytoskeleton, causing decreased expression of F-actin and slowing the cardiomyocyte beating rate in a dose-dependent manner.85 Further studies of ponatinib and other TKIs could ascertain which pathways are the key players in cardiotoxicity and may assist in the development of therapies engineered not only for specific targets, but also engineered to avoid specific kinases.
A separate study tested erlotinib, sunitinib, imatinib, nilotinib, sorafenib, and crizotinib, in addition to 17 other non-TKI compounds, to determine if iPSC-CMs could accurately identify the compounds with known cardiotoxicity.86 Electrophysiology studies using iPSC-CMs revealed that crizotinib, sunitinib, and nilotinib potently blocked the hERG potassium channel, with erlotinib only weakly inhibiting it. Additionally, crizotinib and sunitinib also inhibited the Nav1.5 tonic current and Cav1.2 current.87 Using an improved cardiac differentiation protocol and high-throughput screening of iPSC-CMs,88 a safety index for 21 TKIs was recently developed with which seven TKIs with significant cardio-toxicity in vitro were identified.89 Out of the seven, two (nilotinib and vandetanib) have FDA black box warnings associated with cardiotoxicity.
Trastuzumab toxicity has also been modelled using iPSC-CMs, as three of the HER receptors (HER1, 2, and 4) are expressed by iPSC-CMs. Cardiotoxicity testing using iPSC-CMs confirmed previous data that trastuzumab blocking of HER2 interferes with NRG1 signalling, thereby leading to cardiotoxicity.79 Moreover, in vitro data from the iPSC-CM platform also reflected the clinical experience observed when trastuzumab is given in combination with anthracyclines.90 Another group studying the effects of trastuzumab on iPSC-CM metabolism found a decrease in oxidative phosphorylation and glucose utilization, with an upregulation of genes involved in glycolysis. While the iPSC-CMs utilized less glucose, interestingly there was no increase in lactate production.91 Similarly, a recent study showed mitochondrial dysfunction and altered cardiac metabolism as the leading causes of trastuzumab-induced cardiotoxicity.92 Here, the authors found that patient-specific iPSC-CMs, when exposed to clinical doses of trastuzumab, exhibited signicantly impaired contractile function without inducing cell death. Moreover, metabolic modulation of these iPSC-CMs by small molecules reversed the adverse effects induced by trastuzumab. These findings may implicate metabolic impairment as part of the toxic effects, however the foetal-like metabolism of iPSC-CMs makes these studies difficult to interpret. A summary of the available iPSC-CM studies on targeted chemotherapeutic agents is presented in Table 2.
Table 2.
iPSC-modelling of targeted chemotherapeutic agents
| Class | Representative drugs | Clinical cardiotoxic effects | iPSC-CM modelling | References |
|---|---|---|---|---|
| Monoclonal antibodies | Trastuzmab | Decreased LVEF, increased serum cardiac troponin I, congestive heart failure, and hypertension | Decrease in oxidative phosphorylation and glucose utilization, and metabolic impairment | 79,90,91 |
| Tyrosine kinase i nhibitors | Nilotinib Vemurafenib | QT prolongation, vascular events, hyperglycaemia, and risk of sudden death | Cytotoxicity, ROS production, lipid accumulation, and inhibition of ABL, AKT, and ERK survival pathways, disruption of actin cytoskeleton | 86,89 |
| Ponatinib | Vascular events | 89 | ||
| Trametinib | Congestive heart failure | 85,89 | ||
| Sunitinib | Hypertension, venous or arterial thromboembolic events, decreased LVEF, and congestive heart failure | 86,89 | ||
| Vendetanib | ||||
| Sorafenib Pazopinib | ||||
| Axitinib | ||||
| Dasatinib | Pulmonary hypertension, QT prolongation, peripheral oedema, pericardial effusion, and vascular events | 89 | ||
| Proteasome inhibitors | Bortezomib Carfilzomib | Hypertension, thromboembolic events, arrhythmias, and congestive heart failure | Undetermined | NA |
| Immuno-modulators | Thalidomide | Thromboembolic events | Undetermined | NA |
| Immune checkpoint inhibitors | Pembrolizumab Nivolumab | Myocarditis | Undetermined | NA |
| Ipilimumab |
NA, not applicable.
9. Toxicity trials in a dish: towards personalized medicine
Beyond the potential use of iPSC-CMs for high-throughput drug screening purposes, there is perhaps even greater excitement about studying the mechanisms behind cardiotoxicity.93 Chemotherapy-induced cardiotoxicity can have structural or functional consequences that could include loss of electrophysiological or mechanical properties and/or cardiac cell death. However, every person responds uniquely to chemotherapeutic agents with respect to clinical cardiotoxicity, which could range from 8% to 26% for DOX, 7% to 28% for trastuzumab, or 5% to 30% for paclitaxel.94 As preclinical testing that includes both in vitro and in vivo assays has already been adopted to determine the efficacy of a drug,95 it is possible to extend the same principles towards predicting chemotherapy-induced cardiotoxicity. Indeed iPSC-based drug toxicity screening could lead the way by allowing a personalized approach to pre-screen a patient’s own cardiomyocytes before being subjected to chemotherapy. For example, iPSC-CMs generated from cancer patients could undergo comprehensive in vitro characterization to determine which patient’s iPSC-CMs are more prone to developing chemotherapy-induced cardiotoxicity. Once assessed, a safety score can be assigned to each patient to inform the treating oncologist who has a higher propensity to develop clinical cardiotoxicity, thereby allowing informed decisions to be made regarding the choice and dose of chemotherapeutic agents.
However, there are some limitations to the use of iPSC-CMs for drug toxicity screening. For instance, these iPSC-CMs are immature and closely resemble a foetal phenotype rather than an adult with regards to structural and electrophysiological properties. This limitation makes it difficult to interpret the response of the chemotherapeutic agents and predict the translational impact in vivo. In addition, the generation of iPSC-CMs is costly and time-intensive and it may be several months before drug toxicity trials could be conducted on the iPSC-CMs of cancer patients. This in turn could delay the assignment of a safety score to the patient, thereby delaying the start of cancer treatment, which could be detrimental as early treatment can save lives and keep treatment costs to a minimum. To overcome this, it is imperative that protocols are developed that can generate iPSCs and differentiate them to mature iPSC-CMs in a more time-sensitive manner for drug toxicity screening. Similarly, advancements in tissue engineering technology should be implemented to take the platform beyond the two-dimensional (2D) monolayer culture methods towards more physiologic three-dimensional (3D) models. Furthermore, preemptively identifying and biobanking iPSC-CMs from high-risk individuals that are susceptible to developing cancer, such as patients with known family history or those exposed to cancer-causing environmental or occupational hazards, could eliminate the delay or ‘waiting time’ for the patients, in case they are subjected to chemotherapy. Combined with the ever-improving cardiac differentiation protocols,88,96 it is possible that preclinical toxicity testing could be offered as a commercial application from biobanked iPSCs (Figure 3).
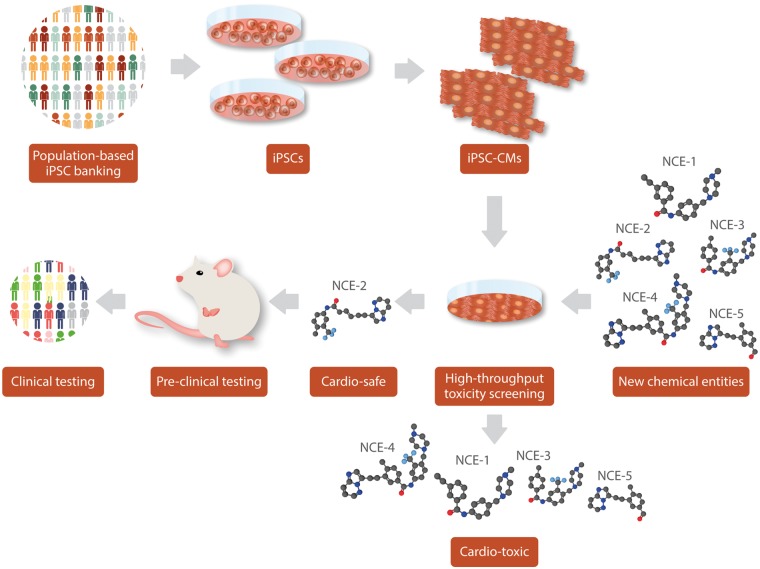
Figure 3.
Scalability of iPSCs for drug toxicity-screening. By using iPSC-derived tissues from diverse populations, screening for uncommon or rare toxic reactions can be performed on New Chemical Entities (NCE) before beginning preclinical or clinical testing. Cardiotoxic candidates can be removed from the testing pool instead of moving on to more expensive clinical trials, thereby boosting success rates.
10. Pharmacogenomics: towards precision medicine
It is very clear that the response of each patient to chemotherapy is different. Variability in response could be attributed to patient risk factors such as age, prior cardiovascular incidents, or prior chemotherapy, but could also be due to each patient’s genetic predisposition towards developing chemotherapy-induced cardiotoxicity. For example, polymorphisms in key molecular components responsible for the pharmacokinetics and pharmacodynamics of chemotherapeutic agents might influence the individual patient’s responses, varying their susceptibility towards cardiotoxicity. Moreover, these genetic variations might be responsible for tipping the balance for a given chemotherapeutic agent from being efficacious to cardiotoxic. Thus, there is a compelling need to conduct pharmacogenetic testing on cancer patients being subjected to chemotherapy to better understand their genetic variability, predict cardiotoxicity, and develop tailor-made treatment strategies for each patient. With the National Institute of Health’s Precision Medicine Initiative,97 efforts are underway to understand how the genetic background of a patient can affect their response to drugs.
Pharmacogenomics aims at understanding the role of inter-individual variability in drug efficacy and toxicity and has the potential to significantly impact adverse effects in cancer and precision medicine.98 Drugs are defined by their therapeutic ratios (efficacy/toxicity), and the last decade has seen significant efforts to improve the risk-benefit ratios of anti-cancer drugs. The focus has largely been on maximizing the efficacy by identifying tumour biomarkers, with more limited effort on identifying markers that can predict chemotherapeutic side-effects. Advancements in next-generation sequencing (NGS) methods, along with reduced cost and turnaround time for these technologies, have also significantly boosted the field of pharmacogenomics, with an exponential growth in the number of genome-wide association studies (GWAS) and quantitative trait locus (QTL) studies being conducted in the last decade.99 GWAS and QTL studies aim to identify common single nucleotide polymorphisms (SNPs) or other genetic variants associated with complex diseases, providing a powerful tool to investigate the impact of genetic variation on individual drug response. Indeed, recent GWAS studies have identified a number of adverse drug reaction (ADR) risk loci,100 but there is still limited understanding as to how these variants predispose people to ADR.
Millions of SNPs across the entire genome can be assayed by GWAS, and pharmacogenomic studies in drug responses have found many genetic variations that can determine whether a patient is high-risk or low-risk. For example, a recent study identified a nonsynonymous coding variant, rs2229774 in the RARG (retinoic acid receptor) gene101 that was significantly associated with paediatric DOX-induced cardiotoxicity. Similarly, a number of SNPs have been identified within genes that are associated with DOX treatment and toxicity.94 Importantly, genotype–phenotype correlations revealed that the majority of SNPs associated with DOX response were located in genes that encode drug transporters or enzymes, suggesting that polymorphisms in patient pharmacodynamics might influence DOX response and toxicity. In contrast to DOX pharmacogenomics, where a number of variants have been correlated to DOX-induced cardiotoxicity, little work has been done to identify SNPs that could be culpable. This is despite the fact that a majority of TKIs come with a black box warning for cardiac side-effects.
SNPs are capable of not only altering the gene with which they are associated, but they can also alter expression levels of many other genes.102 Loci responsible for this genetic control are known as expression quantitative trait loci (eQTLs), which have been postulated to be an important determinant of disease susceptibility for each patient. However, it remains difficult to run validation studies for these GWAS analysis to determine the causal relationship between genetic variants and disease progression. Validation studies thus far have relied on human cardiac biopsies that are difficult to acquire or animal models that are genomically different from humans. Thus, a better platform to conduct GWAS validation studies is needed. As human iPSCs can be cultured in a dish for an extended period of time and still retain the genetic variance present in the patients, they can serve as an ideal model to validate the GWAS studies. Indeed, iPSCs and iPSC-derived cells have been utilized by many groups to validate eQTL studies.103,104 Moreover, with the advent of genome engineering such as the clustered regularly interspaced short palindromic repeat (CRISPR) technology, researchers have been able to correct these variants in a dish to further validate the importance of these genetic variants in disease progression.105–107
In addition, human iPSCs and their derivatives have emerged as a powerful in vitro model system that uses multi-omic analysis to investigate the genotype–phenotype relationship of ADR. For instance, iPSC-CMs derived from DOX-induced cardiotoxicity patients were recently shown capable of recapitulating patient-specific clinical susceptibility to DOX.74,108 Our data showed that iPSC-CMs from DOX-induced cardiotoxicity patients were more sensitive to DOX when compared with iPSC-CMs from patients not exhibiting cardiotoxicity. Taken together, these new findings indicate that iPSC-derivatives can be used to accurately validate genetic variants that make individual patients susceptible to drug toxicity.
11. Conclusion
The last decade has seen a substantial improvement in the diagnosis and management of cancer, mostly due to advanced technologies and newer drugs, which has significantly improved the mean 5-year survival rate and other metrics. Despite these efforts, cancer patients continue to suffer from unpredictable ADR after chemotherapy. In particular, cardiotoxicity remains the most predominant side-effect for several of these chemotherapeutic agents, including anthracyclines and kinase inhibitors. Nevertheless, the question as to why certain patients are more adversely affected by these drugs than others remains unanswered. Identification of those patients who are at a higher risk of developing cardiotoxicity is an important strategy to reduce the associated morbidity and mortality. The iPSC platform offers promise to advance the development of personalized medicine approaches based on the response of a patient’s own cells and tissues to chemotherapy. The iPSC technology may finally allow the all-important functional validation of the various pharmacogenomic studies instrumental in identifying crucial genetic polymorphisms associated with chemotherapy-induced cardiotoxicity.
Acknowledgements
The authors gratefully acknowledge Amanda Chase and Blake Wu for critical reading of the manuscript.
Conflict of interest: J.C.W. is a co-founder of Khloris Biosciences. All remaining authors have declared no conflicts of interest.
Funding
This work was supported in part by research grants from the National Institutes of Health (NIH) [R01 HL141851, R01 HL123968, and R01 HL132875], and Burroughs Wellcome Foundation [1015009 to J.C.W.], and NIH K01 HL135455 and Stanford Translational Research and Applied Medicine (TRAM) scholar award to N.S. Because of space constraints, the authors apologize in advance for not including all relevant citations on the subject matter.
References
- 1. Heron M. Deaths: leading causes for 2016. Natl Vital Stat Rep 2018;67:1–77. [PubMed] [Google Scholar]
- 2. Palucka K, Banchereau J.. Cancer immunotherapy via dendritic cells. Nat Rev Cancer 2012;12:265.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fern Ndez-Medarde A, PÈrez-Herrero E.. Advanced targeted therapies in cancer: drug nanocarriers, the future of chemotherapy. Eur J Pharm Biopharm 2015;79:52–79. [DOI] [PubMed] [Google Scholar]
- 4. DiMasi JA, Reichert JM, Feldman L, Malins A.. Clinical approval success rates for investigational cancer drugs. Clin Pharmacol Ther 2013;94:329–335. [DOI] [PubMed] [Google Scholar]
- 5. Ewer MS, Lippman SM.. Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J Clin Oncol 2005;23:2900–2902. [DOI] [PubMed] [Google Scholar]
- 6. Rinehart JJ, Lewis RP, Balcerzak SP.. Adriamycin cardiotoxicity in man. Ann Intern Med 1974;81:475–478. [DOI] [PubMed] [Google Scholar]
- 7. Shek TW, Luk IS, Ma L, Cheung KL.. Paclitaxel-induced cardiotoxicity. An ultrastructural study. Arch Pathol Lab Med 1996;120:89–91. [PubMed] [Google Scholar]
- 8. Schmidinger M, Zielinski CC, Vogl UM, Bojic A, Bojic M, Schukro C, Ruhsam M, Hejna M, Schmidinger H.. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J Clin Oncol 2008;26:5204–5212. [DOI] [PubMed] [Google Scholar]
- 9. Jerian S, Keegan P.. Cardiotoxicity associated with paclitaxel/trastuzumab combination therapy. J Clin Oncol 1999;17:1647–1648. [PubMed] [Google Scholar]
- 10. Florescu M, Cinteza M, Vinereanu D.. Chemotherapy-induced cardiotoxicity. Maedica 2013;8:59–67. [PMC free article] [PubMed] [Google Scholar]
- 11. Herman EH, Ferrans VJ.. Animal models of anthracycline cardiotoxicity: basic mechanisms and cardioprotective activity. Prog Pediatr Cardiol 1997;8:49–58. [Google Scholar]
- 12. Herman EH, Ferrans VJ.. Preclinical animal models of cardiac protection from anthracycline-induced cardiotoxicity. Semin Oncol 1998;25:15–21. [PubMed] [Google Scholar]
- 13. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S.. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861–872. [DOI] [PubMed] [Google Scholar]
- 14. Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA.. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007;318:1917–1920. [DOI] [PubMed] [Google Scholar]
- 15. Sayed N, Liu C, Wu JC.. Translation of human-induced pluripotent stem cells: from clinical trial in a dish to precision medicine. J Am Coll Cardiol 2016;67:2161–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Matsa E, Ahrens JH, Wu JC.. Human induced pluripotent stem cells as a platform for personalized and precision cardiovascular medicine. Physiol Rev 2016;96:1093–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Magdy T, Schuldt AJT, Wu JC, Bernstein D, Burridge PW.. Human induced pluripotent stem cell (hiPSC)-derived cells to assess drug cardiotoxicity: opportunities and problems. Annu Rev Pharmacol Toxicol 2018;58:83–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dimarco A, Gaetani M, Dorigotti L, Soldati M, Bellini O.. Daunomycin: a new antibiotic with antitumor activity. Cancer Chemother Rep 1964;38:31–38. [PubMed] [Google Scholar]
- 19. Arcamone F, Cassinelli G, Fantini G, Grein A, Orezzi P, Pol C, Spalla C.. Adriamycin, 14-hydroxydaimomycin, a new antitumor antibiotic from S. Peucetius var. caesius. Biotechnol Bioeng 1969;11:1101–1110. [DOI] [PubMed] [Google Scholar]
- 20. Bristow MR, Thompson PD, Martin RP, Mason JW, Billingham ME, Harrison DC.. Early anthracycline cardiotoxicity. Am J Med 1978;65:823–832. [DOI] [PubMed] [Google Scholar]
- 21. Swain SM, Whaley FS, Ewer MS.. Congestive heart failure in patients treated with doxorubicin. Cancer 2003;97:2869–2879. [DOI] [PubMed] [Google Scholar]
- 22. Von Hoff DD, Rozencweig M, Layard M, Slavik M, Muggia FM.. Daunomycin-induced cardiotoxicity in children and adults: a review of 110 cases. Am J Med 1977;62:200–208. [DOI] [PubMed] [Google Scholar]
- 23. Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, Fiorentini C, Cipolla CM.. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015;131:1981–1988. [DOI] [PubMed] [Google Scholar]
- 24. Hahn VS, Lenihan DJ, B K.. Cancer therapy-induced cardiotoxicity: basic mechanisms and potential cardioprotective therapies. J Am Heart Assoc 2014;3:e000665.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rharass T, Gbankoto A, Canal C, Kurşunluoğlu G, Bijoux A, Panáková D, Ribou A-C.. Oxidative stress does not play a primary role in the toxicity induced with clinical doses of doxorubicin in myocardial H9c2 cells. Mol Cell Biochem 2016;413:199–215. [DOI] [PubMed] [Google Scholar]
- 26. van Dalen EC, Caron HN, Dickinson HO, Kremer LCM.. Cardioprotective interventions for cancer patients receiving anthracyclines. Cochrane Database Syst Rev 2008;2:CD003917. [DOI] [PubMed] [Google Scholar]
- 27. Zhang S, Liu X, Bawa-Khalfe T, Lu L-S, Lyu YL, Liu LF, Yeh ETH.. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med 2012;18:1639.. [DOI] [PubMed] [Google Scholar]
- 28. Hasinoff BB, Chee GL, Thampatty P, Allan WP, Yalowich JC.. The cardioprotective and DNA topoisomerase II inhibitory agent dexrazoxane (ICRF-187) antagonizes camptothecin-mediated growth inhibition of Chinese hamster ovary cells by inhibition of DNA synthesis. Anticancer Drugs 1999;10:47–54. [DOI] [PubMed] [Google Scholar]
- 29. Lischke J, Lang C, Sawodny O, Feuer R. Impairment of energy metabolism in cardiomyocytes caused by 5-FU catabolites can be compensated by administration of amino acids. In 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) Milan, Italy: IEEE; 2015. pp. 5363–5366. [DOI] [PubMed]
- 30. Layoun ME, Wickramasinghe CD, Peralta MV, Yang EH.. Fluoropyrimidine-induced cardiotoxicity: manifestations, mechanisms, and management. Curr Oncol Rep 2016;18:35.. [DOI] [PubMed] [Google Scholar]
- 31. Mosseri M, Fingert HJ, Varticovski L, Chokshi S, Isner JM.. In vitro evidence that myocardial ischemia resulting from 5-fluorouracil chemotherapy is due to protein kinase c-mediated vasoconstriction of vascular smooth muscle. Cancer Res 1993;53:3028–3033. [PubMed] [Google Scholar]
- 32. Dhesi S, Chu MP, Blevins G, Paterson I, Larratt L, Oudit GY, Kim DH.. Cyclophosphamide-induced cardiomyopathy: a case report, review, and recommendations for management. J Investig Med High Impact Case Rep 2013;1:2324709613480346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Traxler P, Bold G, Buchdunger E, Caravatti G, Furet P, Manley P, O'Reilly T, Wood J, Zimmermann J.. Tyrosine kinase inhibitors: from rational design to clinical trials. Med Res Rev 2001;21:499–512. [DOI] [PubMed] [Google Scholar]
- 34. Slamon D, Clark G, Wong S, Levin W, Ullrich A, McGuire W.. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177–182. [DOI] [PubMed] [Google Scholar]
- 35. Levitzki A. Tyrosine kinase inhibitors: views of selectivity, sensitivity, and clinical performance. Annu Rev Pharmacol Toxicol 2013;53:161–185. [DOI] [PubMed] [Google Scholar]
- 36. Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L.. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783–792. [DOI] [PubMed] [Google Scholar]
- 37. Baselga J. Safety profile of Herceptin (R) as a single agent and in combination with chemotherapy. Eur J Cancer 1999;35:S324. [Google Scholar]
- 38. Jie B, Zhang X, Wu X, Xin Y, Liu Y, Guo Y.. Neuregulin-1 suppresses cardiomyocyte apoptosis by activating PI3K/Akt and inhibiting mitochondrial permeability transition pore. Mol Cell Biochem 2012;370:35–43. [DOI] [PubMed] [Google Scholar]
- 39. Kerkelä R, Grazette L, Yacobi R, Iliescu C, Patten R, Beahm C, Walters B, Shevtsov S, Pesant S, Clubb FJ, Rosenzweig A, Salomon RN, Van Etten RA, Alroy J, Durand JB, Force T.. Cardiotoxicity of the cancer therapeutic agent imatinib mesylate. Nat Med 2006;12:908.. [DOI] [PubMed] [Google Scholar]
- 40. Atallah E, Durand J-B, Kantarjian H, Cortes J.. Congestive heart failure is a rare event in patients receiving imatinib therapy. Blood 2007;110:1233–1237. [DOI] [PubMed] [Google Scholar]
- 41. Chu TF, Rupnick MA, Kerkela R, Dallabrida SM, Zurakowski D, Nguyen L, Woulfe K, Pravda E, Cassiola F, Desai J, George S, Harris DM, Ismail NS, Chen J-H, Schoen FJ, Van den Abbeele AD, Demetri GD, Force T, Chen MH, Morgan JA.. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet 2007;370:2011–2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, Nathan P, Staehler M, de Souza P, Merchan JR, Boleti E, Fife K, Jin J, Jones R, Uemura H, De Giorgi U, Harmenberg U, Wang J, Sternberg CN, Deen K, McCann L, Hackshaw MD, Crescenzo R, Pandite LN, Choueiri TK.. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013;369:722–731. [DOI] [PubMed] [Google Scholar]
- 43. Rini BI, Wilding G, Hudes G, Stadler WM, Kim S, Tarazi J, Rosbrook B, Trask PC, Wood L, Dutcher JP.. Phase II study of axitinib in sorafenib-refractory metastatic renal cell carcinoma. J Clin Oncol 2009;27:4462–4468. [DOI] [PubMed] [Google Scholar]
- 44. Moslehi JJ. Cardiovascular toxic effects of targeted cancer therapies. N Engl J Med 2016;375:1457–1467. [DOI] [PubMed] [Google Scholar]
- 45. Hasinoff BB. The cardiotoxicity and myocyte damage caused by small molecule anticancer tyrosine kinase inhibitors is correlated with lack of target specificity. Toxicol Appl Pharmacol 2010;244:190–195. [DOI] [PubMed] [Google Scholar]
- 46. French KJ, Coatney RW, Renninger JP, Hu CX, Gales TL, Zhao S, Storck LM, Davis CB, McSurdy-Freed J, Chen E, Frazier KS.. Differences in effects on myocardium and mitochondria by angiogenic inhibitors suggest separate mechanisms of cardiotoxicity. Toxicol Pathol 2010;38:691–702. [DOI] [PubMed] [Google Scholar]
- 47. Lu Z, Wu CY, Jiang YP, Ballou LM, Clausen C, Cohen IS, Lin RZ.. Suppression of phosphoinositide 3-kinase signaling and alteration of multiple ion currents in drug-induced long QT syndrome. Sci Transl Med 2012;4:131ra50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li W, Croce K, Steensma DP, McDermott DF, Ben-Yehuda O, Moslehi J.. Vascular and metabolic implications of novel targeted cancer therapies: focus on kinase inhibitors. J Am Coll Cardiol 2015;66:1160–1178. [DOI] [PubMed] [Google Scholar]
- 49. Dimopoulos MA, Moreau P, Palumbo A, Joshua D, Pour L, Hájek R, Facon T, Ludwig H, Oriol A, Goldschmidt H, Rosiñol L, Straub J, Suvorov A, Araujo C, Rimashevskaya E, Pika T, Gaidano G, Weisel K, Goranova-Marinova V, Schwarer A, Minuk L, Masszi T, Karamanesht I, Offidani M, Hungria V, Spencer A, Orlowski RZ, Gillenwater HH, Mohamed N, Feng S, Chng W-J.. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol 2016;17:27–38. [DOI] [PubMed] [Google Scholar]
- 50. Groll M, Berkers CR, Ploegh HL, Ovaa H.. Crystal structure of the boronic acid-based proteasome inhibitor bortezomib in complex with the yeast 20s proteasome. Structure 2006;14:451–456. [DOI] [PubMed] [Google Scholar]
- 51. Hasinoff BB, Patel D, Wu X.. Molecular mechanisms of the cardiotoxicity of the proteasomal-targeted drugs bortezomib and carfilzomib. Cardiovasc Toxicol 2017;17:237–250. [DOI] [PubMed] [Google Scholar]
- 52. Krönke J, Udeshi ND, Narla A, Grauman P, Hurst SN, McConkey M, Svinkina T, Heckl D, Comer E, Li X, Ciarlo C, Hartman E, Munshi N, Schenone M, Schreiber SL, Carr SA, Ebert BL.. Lenalidomide causes selective degradation of ikzf1 and ikzf3 in multiple myeloma cells. Science 2014;343:301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lu G, Middleton RE, Sun H, Naniong M, Ott CJ, Mitsiades CS, Wong K-K, Bradner JE, Kaelin WG.. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of ikaros proteins. Science 2014;343:305–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Postow MA, Callahan MK, Wolchok JD.. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015;33:1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rosenberg SA, Restifo NP.. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015;348:62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, Becker JR, Slosky DA, Phillips EJ, Pilkinton MA, Craig-Owens L, Kola N, Plautz G, Reshef DS, Deutsch JS, Deering RP, Olenchock BA, Lichtman AH, Roden DM, Seidman CE, Koralnik IJ, Seidman JG, Hoffman RD, Taube JM, Diaz LA, Anders RA, Sosman JA, Moslehi JJ.. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016;375:1749–1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. van den Berg JH, Gomez-Eerland R, van de Wiel B, Hulshoff L, van den Broek D, Bins A, Tan HL, Harper JV, Hassan NJ, Jakobsen BK, Jorritsma A, Blank CU, Schumacher TNM, Haanen JBAG.. Case report of a fatal serious adverse event upon administration of t cells transduced with a MART-1-specific T-cell receptor. Mol Ther 2015;23:1541–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Linette GP, Stadtmauer EA, Maus MV, Rapoport AP, Levine BL, Emery L, Litzky L, Bagg A, Carreno BM, Cimino PJ, Binder-Scholl GK, Smethurst DP, Gerry AB, Pumphrey NJ, Bennett AD, Brewer JE, Dukes J, Harper J, Tayton-Martin HK, Jakobsen BK, Hassan NJ, Kalos M, June CH.. Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma. Blood 2013;122:863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Morandi P, Ruffini PA, Benvenuto GM, La Vecchia L, Mezzena G, Raimondi R.. Serum cardiac troponin I levels and ECG/Echo monitoring in breast cancer patients undergoing high-dose (7 g/m2) cyclophosphamide. Bone Marrow Transplant 2001;28:277.. [DOI] [PubMed] [Google Scholar]
- 60. Nakamae H, Hino M, Akahori M, Terada Y, Yamane T, Ohta K, Hayashi T, Tsumura K.. Predictive value of QT dispersion for acute heart failure after autologous and allogeneic hematopoietic stem cell transplantation. Am J Hematol 2004;76:1–7. [DOI] [PubMed] [Google Scholar]
- 61. Tebbi CK, London WB, Friedman D, Villaluna D, Alarcon PAD, Constine LS, Mendenhall NP, Sposto R, Chauvenet A, Schwartz CL.. Dexrazoxane-associated risk for acute myeloid leukemia/myelodysplastic syndrome and other secondary malignancies in pediatric Hodgkin's disease. J Clin Oncol 2007;25:493–500. [DOI] [PubMed] [Google Scholar]
- 62. Colombo A, Meroni CA, Cipolla CM, Cardinale D.. Managing cardiotoxicity of chemotherapy. Curr Treat Options Cardiovasc Med 2013;15:410–424. [DOI] [PubMed] [Google Scholar]
- 63. Safra T. Cardiac safety of liposomal anthracyclines. Oncologist 2003;8:17–24. [DOI] [PubMed] [Google Scholar]
- 64. Jensen SA, Sørensen JB.. Risk factors and prevention of cardiotoxicity induced by 5-fluorouracil or capecitabine. Cancer Chemother Pharmacol 2006;58:487–493. [DOI] [PubMed] [Google Scholar]
- 65. Wang Y, Liang P, Lan F, Wu H, Lisowski L, Gu M, Hu S, Kay MA, Urnov FD, Shinnawi R, Gold JD, Gepstein L, Wu JC.. Genome editing of isogenic human induced pluripotent stem cells recapitulates long QT phenotype for drug testing. J Am Coll Cardiol 2014;64:451–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Liang P, Sallam K, Wu H, Li Y, Itzhaki I, Garg P, Zhang Y, Termglichan V, Lan F, Gu M, Gong T, Zhuge Y, He C, Ebert AD, Sanchez-Freire V, Churko J, Hu S, Sharma A, Lam CK, Scheinman MM, Bers DM, Wu JC.. Patient-specific and genome-edited induced pluripotent stem cell-derived cardiomyocytes elucidate single-cell phenotype of Brugada syndrome. J Am Coll Cardiol 2016;68:2086–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kodo K, Ong SG, Jahanbani F, Termglinchan V, Hirono K, InanlooRahatloo K, Ebert AD, Shukla P, Abilez OJ, Churko JM, Karakikes I, Jung G, Ichida F, Wu SM, Snyder MP, Bernstein D, Wu JC.. iPSC-derived cardiomyocytes reveal abnormal TGF-β signalling in left ventricular non-compaction cardiomyopathy. Nat Cell Biol 2016;18:1031.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sun N, Yazawa M, Liu J, Han L, Sanchez-Freire V, Abilez OJ, Navarrete EG, Hu S, Wang L, Lee A, Pavlovic A, Lin S, Chen R, Hajjar RJ, Snyder MP, Dolmetsch RE, Butte MJ, Ashley EA, Longaker MT, Robbins RC, Wu JC.. Patient-specific induced pluripotent stem cells as a model for familial dilated cardiomyopathy. Sci Transl Med 2012;4:130ra47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wu H, Lee J, Vincent LG, Wang Q, Gu M, Lan F, Churko JM, Sallam KI, Matsa E, Sharma A, Gold JD, Engler AJ, Xiang YK, Bers DM, Wu JC.. Epigenetic regulation of phosphodiesterases 2A and 3A underlies compromised β-adrenergic signaling in an iPSC model of dilated cardiomyopathy. Cell Stem Cell 2015;17:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lan F, Lee AS, Liang P, Sanchez-Freire V, Nguyen PK, Wang L, Han L, Yen M, Wang Y, Sun N, Abilez OJ, Hu S, Ebert AD, Navarrete EG, Simmons CS, Wheeler M, Pruitt B, Lewis R, Yamaguchi Y, Ashley EA, Bers DM, Robbins RC, Longaker MT, Wu JC.. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell 2013;12:101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Karakikes I, Termglinchan V, Cepeda DA, Lee J, Diecke S, Hendel A, Itzhaki I, Ameen M, Shrestha R, Wu H, Ma N, Shao N-Y, Seeger T, Woo N, Wilson KD, Matsa E, Porteus MH, Sebastiano V, Wu JC.. A comprehensive TALEN-based knockout library for generating human-induced pluripotent stem cell-based models for cardiovascular diseases. Circ Res 2017;120:1561–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gintant G, Fermini B, Stockbridge N, Strauss D.. The evolving roles of human iPSC-derived cardiomyocytes in drug safety and discovery. Cell Stem Cell 2017;21:14–17. [DOI] [PubMed] [Google Scholar]
- 73. Fermini B, Hancox JC, Abi-Gerges N, Bridgland-Taylor M, Chaudhary KW, Colatsky T, Correll K, Crumb W, Damiano B, Erdemli G, Gintant G, Imredy J, Koerner J, Kramer J, Levesque P, Li Z, Lindqvist A, Obejero-Paz CA, Rampe D, Sawada K, Strauss DG, Vandenberg JI.. A new perspective in the field of cardiac safety testing through the comprehensive in vitro proarrhythmia assay paradigm. J Biomol Screen 2016;21:1–11. [DOI] [PubMed] [Google Scholar]
- 74. Burridge PW, Li YF, Matsa E, Wu H, Ong S-G, Sharma A, Holmström A, Chang AC, Coronado MJ, Ebert AD, Knowles JW, Telli ML, Witteles RM, Blau HM, Bernstein D, Altman RB, Wu JC.. Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat Med 2016;22:547.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kopljar I, De Bondt A, Vinken P, Teisman A, Damiano B, Goeminne N, Van den Wyngaert I, Gallacher DJ, Lu HR.. Chronic drug-induced effects on contractile motion properties and cardiac biomarkers in human induced pluripotent stem cell-derived cardiomyocytes. Br J Pharmacol 2017;174:3766–3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Holmgren G, Synnergren J, Bogestål Y, Améen C, Åkesson K, Holmgren S, Lindahl A, Sartipy P.. Identification of novel biomarkers for doxorubicin-induced toxicity in human cardiomyocytes derived from pluripotent stem cells. Toxicology 2015;328:102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Liu C, Oikonomopoulos A, Sayed N, Wu JC.. Modeling human diseases with induced pluripotent stem cells: from 2D to 3D and beyond. Development 2018;145:dev156166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Amano Y, Nishiguchi A, Matsusaki M, Iseoka H, Miyagawa S, Sawa Y, Seo M, Yamaguchi T, Akashi M.. Development of vascularized iPSC derived 3D-cardiomyocyte tissues by filtration layer-by-layer technique and their application for pharmaceutical assays. Acta Biomat 2016;33:110–121. [DOI] [PubMed] [Google Scholar]
- 79. Eldridge S, Guo L, Mussio J, Furniss M, Hamre J, Davis M.. Examining the protective role of ErbB2 modulation in human-induced pluripotent stem cell-derived cardiomyocytes. Toxicol Sci 2014;141:547–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Gianni L, Salvatorelli E, Minotti G.. Anthracycline cardiotoxicity in breast cancer patients: synergism with trastuzumab and taxanes. Cardiovasc Toxicol 2007;7:67–71. [DOI] [PubMed] [Google Scholar]
- 81. Jay SM, Murthy AC, Hawkins JF, Wortzel JR, Steinhauser ML, Alvarez LM, Gannon J, Macrae CA, Griffith LG, Lee RT.. An engineered bivalent neuregulin protects against doxorubicin-induced cardiotoxicity with reduced proneoplastic potential. Circulation 2013;128:152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Zhang Y, Liang X, Liao S, Wang W, Wang J, Li X, Ding Y, Liang Y, Gao F, Yang M, Fu Q, Xu A, Chai Y-H, He J, Tse H-F, Lian Q.. Potent paracrine effects of human induced pluripotent stem cell-derived mesenchymal stem cells attenuate doxorubicin-induced cardiomyopathy. Sci Rep 2015;5:11235.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Zhang Y, Yu Z, Jiang D, Liang X, Liao S, Zhang Z, Yue W, Li X, Chiu S-M, Chai Y-H, Liang Y, Chow Y, Han S, Xu A, Tse H-F, Lian Q.. iPSC-MSCs with high intrinsic MIRO1 and sensitivity to TNF-α yield efficacious mitochondrial transfer to rescue anthracycline-induced cardiomyopathy. Stem Cell Rep 2016;7:749–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Guo L, Eldridge S, Furniss M, Mussio J, Davis M.. Use of human induced pluripotent stem cell–derived cardiomyocytes (hiPSC-CMs) to monitor compound effects on cardiac myocyte signaling pathways. Curr Protoc Chem Biol 2017;7:141–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Talbert DR, Doherty KR, Trusk PB, Moran DM, Shell SA, Bacus S.. A multi-parameter in vitro screen in human stem cell-derived cardiomyocytes identifies ponatinib-induced structural and functional cardiac toxicity. Toxicol Sci 2015;143:147–155. [DOI] [PubMed] [Google Scholar]
- 86. Doherty KR, Talbert DR, Trusk PB, Moran DM, Shell SA, Bacus S.. Structural and functional screening in human induced-pluripotent stem cell-derived cardiomyocytes accurately identifies cardiotoxicity of multiple drug types. Toxicol Appl Pharmacol 2015;285:51–60. [DOI] [PubMed] [Google Scholar]
- 87. Doherty KR, Wappel RL, Talbert DR, Trusk PB, Moran DM, Kramer JW, Brown AM, Shell SA, Bacus S.. Multi-parameter in vitro toxicity testing of crizotinib, sunitinib, erlotinib, and nilotinib in human cardiomyocytes. Toxicol Appl Pharmacol 2013;272:245–255. [DOI] [PubMed] [Google Scholar]
- 88. Sharma A, McKeithan WL, Serrano R, Kitani T, Burridge PW, del Álamo JC, Mercola M, Wu JC.. Use of human induced pluripotent stem cell-derived cardiomyocytes to assess drug cardiotoxicity. Nat Protoc 2018;13:3018–3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Sharma A, Burridge PW, McKeithan WL, Serrano R, Shukla P, Sayed N, Churko JM, Kitani T, Wu H, Holmström A, Matsa E, Zhang Y, Kumar A, Fan AC, del Álamo JC, Wu SM, Moslehi JJ, Mercola M, Jc W.. High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells. Sci Transl Med 2017;9:eaaf2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Kurokawa YK, Shang MR, Yin RT, George SC.. Modeling trastuzumab-related cardiotoxicity in vitro using human stem cell-derived cardiomyocytes. Toxicol Lett 2018;285:74–80. [DOI] [PubMed] [Google Scholar]
- 91. Necela BM, Axenfeld BC, Serie DJ, Kachergus JM, Perez EA, Thompson EA, Norton N.. The antineoplastic drug, trastuzumab, dysregulates metabolism in iPSC-derived cardiomyocytes. Clin Transl Med 2017;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kitani T, Ong SG, Lam CK, Rhee JW, Zhang JZ, Oikonomopoulos A, Ma N, Lei T, Lee J, Telli ML, Witteles RM, Sharma A, Sayed N, Wu JC.. Human induced pluripotent stem cell model of trastuzumab-induced cardiac dysfunction in breast cancer patients. Circulation 2019;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Stack JP, Moslehi J, Sayed N, Wu JC.. Cancer therapy-induced cardiomyopathy: can human induced pluripotent stem cell modelling help prevent it? Eur Heart J 2018;doi:10.1093/eurheartj/ehx811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Magdy T, Burmeister BT, Burridge PW.. Validating the pharmacogenomics of chemotherapy-induced cardiotoxicity: what is missing? Pharmacol Ther 2016;168:113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Stillitano F, Hansen J, Kong CW, Karakikes I, Funck-Brentano C, Geng L, Scott S, Reynier S, Wu M, Valogne Y, Desseaux C, Salem JE, Jeziorowska D, Zahr N, Li R, Iyengar R, Hajjar RJ, Hulot JS.. Modeling susceptibility to drug-induced long QT with a panel of subject-specific induced pluripotent stem cells. eLife 2017;6:e19406.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Burridge PW, Matsa E, Shukla P, Lin ZC, Churko JM, Ebert AD, Lan F, Diecke S, Huber B, Mordwinkin NM, Plews JR, Abilez OJ, Cui B, Gold JD, Wu JC. Chemically defined generation of human cardiomyocytes. Nat Methods 2014;11:855–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lowy DR, Collins FS.. Aiming high—changing the trajectory for cancer. N Engl J Med 2016;374:1901–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Pereira NL, Weinshilboum RM.. Cardiovascular pharmacogenomics and individualized drug therapy. Nat Rev Cardiol 2009;6:632–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. MacArthur J, Bowler E, Cerezo M, Gil L, Hall P, Hastings E, Junkins H, McMahon A, Milano A, Morales J, Pendlington ZM, Welter D, Burdett T, Hindorff L, Flicek P, Cunningham F, Parkinson H.. The new NHGRI-EBI catalog of published genome-wide association studies (GWAS Catalog). Nucleic Acids Res 2017;45:D896–D901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Giacomini KM, Yee SW, Mushiroda T, Weinshilboum RM, Ratain MJ, Kubo M.. Genome-wide association studies of drug response and toxicity: an opportunity for genome medicine. Nat Rev Drug Discov 2017;16:1.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Aminkeng F, Bhavsar AP, Visscher H, Rassekh SR, Li Y, Lee JW, Brunham LR, Caron HN, van Dalen EC, Kremer LC, van der Pal HJ, Amstutz U, Rieder MJ, Bernstein D, Carleton BC, Hayden MR, Ross CJ; Canadian Pharmacogenomics Network For Drug Safety Consortium. A coding variant in RARG confers susceptibility to anthracycline-induced cardiotoxicity in childhood cancer. Nat Genet 2015;47:1079.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Shastry BS. SNPs: impact on gene function and phenotype In Komar AA (ed). Single Nucleotide Polymorphisms: Methods and Protocols. Humana Press; 2009. pp. 3–22. [DOI] [PubMed] [Google Scholar]
- 103. Carcamo-Orive I, Hoffman GE, Cundiff P, Beckmann ND, D’Souza SL, Knowles JW, Patel A, Papatsenko D, Abbasi F, Reaven GM, Whalen S, Lee P, Shahbazi M, Henrion MYR, Zhu K, Wang S, Roussos P, Schadt EE, Pandey G, Chang R, Quertermous T, Lemischka I.. Analysis of transcriptional variability in a large human iPSC library reveals genetic and non-genetic determinants of heterogeneity. Cell Stem Cell 2017;20:518–532.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Warren CR, O’Sullivan JF, Friesen M, Becker CE, Zhang X, Liu P, Wakabayashi Y, Morningstar JE, Shi X, Choi J, Xia F, Peters DT, Florido MHC, Tsankov AM, Duberow E, Comisar L, Shay J, Jiang X, Meissner A, Musunuru K, Kathiresan S, Daheron L, Zhu J, Gerszten RE, Deo RC, Vasan RS, O’Donnell CJ, Cowan CA.. Induced pluripotent stem cell differentiation enables functional validation of GWAS variants in metabolic disease. Cell Stem Cell 2017;20:547–557.e7. [DOI] [PubMed] [Google Scholar]
- 105. Ma N, Zhang J, Itzhaki I, Zhang SL, Chen H, Haddad F, Kitani T, Wilson KD, Tian L, Shrestha R, Wu H, Lam CK, Sayed N, Wu JC.. Determining the pathogenicity of a genomic variant of uncertain significance using CRISPR/Cas9 and human-induced pluripotent stem cells. Circulation 2018;138:2666–2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Garg P, Oikonomopoulos A, Chen H, Li Y, Lam CK, Sallam K, Perez M, Lux RL, Sanguinetti MC, Wu JC.. Genome editing of induced pluripotent stem cells to decipher cardiac channelopathy variant. J Am Coll Cardiol 2018;72:62–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Seeger T, Porteus M, Wu JC.. Genome editing in cardiovascular biology. Circ Res 2017;120:778–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Chaudhari U, Nemade H, Wagh V, Gaspar JA, Ellis JK, Srinivasan SP, Spitkovski D, Nguemo F, Louisse J, Bremer S, Hescheler J, Keun HC, Hengstler JG, Sachinidis A.. Identification of genomic biomarkers for anthracycline-induced cardiotoxicity in human iPSC-derived cardiomyocytes: an in vitro repeated exposure toxicity approach for safety assessment. Arch Toxicol 2016;90:2763–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]