Abstract
Biological rhythms exist in organisms at all levels of complexity, in most organs and at myriad time scales. Our own biological rhythms are driven by energy emitted by the sun, interacting via our retinas with brain stem centres, which then send out complex messages designed to synchronize the behaviour of peripheral non-light sensing organs, to ensure optimal physiological responsiveness and performance of the organism based on the time of day. Peripheral organs themselves have autonomous rhythmic behaviours that can act independently from central nervous system control but is entrainable. Dysregulation of biological rhythms either through environment or disease has far-reaching consequences on health that we are only now beginning to appreciate. In this review, we focus on cardiovascular rhythms in health, with ageing and under disease conditions.
Keywords: Circadian rhythm, Cardiovascular, Heart rate variability, Sinus node
Graphical Abstract
Graphical Abstract.
And the rhythm of life is a powerful beat
Puts a tingle in your fingers and a tingle in your feet
Rhythm in your bedroom, rhythm in the street
Yes, the rhythm of life is a powerful beat
—Sammy Davis Jr, Rhythm of Life 1969
1. Introduction
Physiological rhythms are intrinsic to all forms of life, from microscopic bacteria to large mammals. These persist even when the organism is withdrawn from environmental cues that might otherwise be held responsible for their existence. Such rhythms occur across broad time scales, from once daily (light-dark, or ‘circadian’, from the Latin circa diem, meaning ‘for about a day’),1 to less frequent than once daily (‘infradian’),2 to more frequent than once daily (‘ultradian’).3 Life on our planet is subject to a day–night cycle lasting 24 h, and the circadian period of organisms on earth matches this. Rhythmicity is likely to have persisted through evolution because it allows the body to adapt to optimal and appropriate functioning during the day and at night, conferring the ‘selective advantage of anticipation’,4 allowing organisms to tailor their physiology to light/dark, activity/rest, and sleeping/wakefulness. Circadian rhythms are believed to have originally evolved in aerobic organisms due to solar-cycle driven fluctuations in environmental oxygen from photosynthetic bacteria.5
It was once thought that an organism’s biochemistry and physiology were continuous, compartmentalized processes. It is now known that circadian, infradian, and ultradian rhythms exist in all organs of the body, and at all levels of biological organization, from organ systems, to tissues, to single cells and even molecules.6–9 These rhythms are not unique to animals, indeed the origins of circadian biology were based upon observations made in plants.10 The heart and cardiovascular system as a whole exhibit many co-ordinated rhythmical behaviours, and modification or loss of rhythmicity may be both a predictor and detector of morbidity and mortality across a wide gamut of both cardiac and non-cardiac conditions. In this article, we will review the rhythms that govern a wide variety of cardiovascular parameters. We will discuss what is considered normal, and how this changes with both disease and with ageing.
2. Physiological rhythms in cardiovascular biology
Rhythmicity in the heart exists because of the complex output from a number of intrinsic and extrinsic oscillating factors. Intrinsic rhythmicity begins at the subcellular level, with rhythmical output of proteins from genes, and the readout of this occurs in the form of measurable cardiovascular parameters; each of these is considered in detail below:
2.1 Cardiac genes
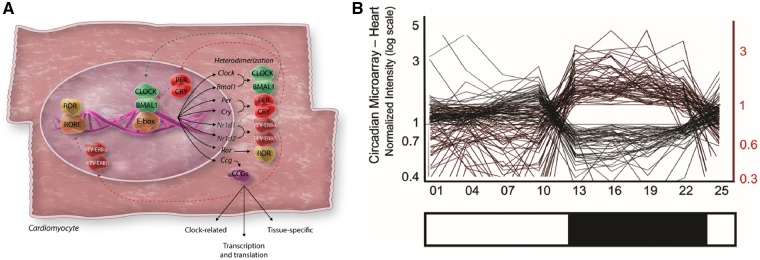
Before considering rhythms within the heart itself, one must recognize that there is a ‘central clock’ or ‘master pacemaker’ that generates circadian rhythms in mammals, located in the suprachiasmatic nuclei (SCN) of the hypothalamus. Light sensed in the eye is transmitted via the retinohypothalamic tract to the SCN, where a molecular clock exists, consisting of multiple sets of transcription factors engaged in autoregulatory transcription-translation feedback loops.5 This conversion of photic energy into neuronal and hormonal signals is the essence of the master or central circadian clock. The current dogma (see Figure 1A) is that production of protein from the core clock genes BMAL1 (also known as ARNTL) and CLOCK, or BMAL1 and NPAS2, leads to their heterodimerization in the cytoplasm. Translocation of this complex to the nucleus is followed by binding to canonical enhancer-box (E-box) sequences of clock-controlled genes (CCGs), and further expression of the genes CLOCK, BMAL1, PER, CRY, NR1D1, NR1D2, ROR, and CCG. Expression of BMAL1 and CLOCK proteins simultaneously induces their own repression through expression of PER and CRY, which also heterodimerize, translocate to the nucleus and inhibit transcription. The cycle completes when levels of PER and CRY fall because of the falling levels of BMAL1/CLOCK that they themselves have caused. Multiple other modulators of this process exist, for example casein kinase that phosphorylates PER and CRY, leading to inhibition of their transcription. Comprehensive reviews of this process are given elsewhere (see Ref.11). Critically, one complete transcription-translation loop of this cycle takes 24 h. The central clock of the body interacts with and synchronizes multiple peripheral light-insensitive clocks that themselves are capable of oscillating autonomously.
Figure 1.
(A) Adapted from Scheiermann et al.5 Transcription of the core clock genes CLOCK and BMAL1 results in their heterodimerization within the cytoplasm of the cell. This transcription factor heterodimer is then translocated to the nucleus, where it binds to E-box sequences, leading to further self-transcription, along with transcription of core clock genes (CCGs) that are largely tissue specific and drive circadian processes. In addition, the CLOCK/BMAL1 heterodimer leads to transcription of PER and CRY, which also form a heterodimer that acts as a negative regulator of the cycle by interfering with the binding of CLOCK/BMAL1 to the E-box sequences. Other positive regulators of the process are also produced, including ROR, which binds to ROR response elements (ROREs) in the BMAL1 promoter zone to induce further expression of BMAL1. This is antagonized by another product of CLOCK/BMAL1 binding to E-box sequences that is the products of the Nr1d1 and Nr1d2 genes, REV-ERBα and REV-ERBβ. These form a complex that inhibit the expression of BMAL1 by interfering with ROR binding to ROREs. The period of the ebbing and flowing of this transcription-translation loop is 24 h on earth. (B) (from Martino et al.75) illustration of the circadian variation of expression of a subset of cardiac genes in the mouse, demonstrating remarkably abrupt changes in expression, especially at the transition point between light and dark phases of the cycle. Black lines reflect light activated genes and red lines reflect dark activated genes.
The heart’s specific peripheral clock uses essentially the same molecular components as the central clock described above (see Figure 1). In general, there are tissue specific differences in the actual clock proteins produced, which are necessary for each peripheral circadian oscillator to have effects tailored to the individual organ/tissue. The first description of the peripheral clock in the human heart came in 2009.12 Like in the other central and peripheral clocks, cardiac clock genes are ‘autonomous’, i.e. intrinsically maintained and self-sustained, as proven by the presence of rhythmical changes in single cardiac cells, and cultured cardiomyocytes. In the heart, the molecular clock plays a critical role in all physiological processes, for example in the diurnal variation in myocardial oxidative and non-oxidative metabolism of carbohydrates and lipids (described in more detail below),13 and the dysregulation of clock gene-controlled diurnal rhythms has been shown to lead to dysfunctional cardiac metabolism and function.12 Core clock genes in the heart include BMAL1, CLOCK, CRY1, CRY2, PER1, PER2, PER3, DBP, HLF, and TEF. The current dogma of a transcription-translation loop of 24 h duration in cardiac cells is the same as that already described in the central oscillator of the SCN.14
Components of the core clock in the heart also regulate genes outside of the clock mechanism, designated as CCGs, which encode other transcription factors or proteins controlling rate-limiting steps in cardiac cell physiology.15 Studies in mice using high-density oligonucleotide microarrays to study the 3-hourly myocardial expression of virtually all of the genes of the heart (almost 12 500) found statistically significant variation in 13% of these (1634/12 488) over a 24 h period, with some showing a smoothly rhythmic temporal profile change, with others demonstrating abrupt day–night/night–day transitions (see Figure 1B).16 Those that did demonstrate significant cyclical change were mapped to key biological pathways, including growth, remodelling, transcription, translation, mitochondrial respiration, and signalling pathways.16 Undoubtedly, ‘the heart is transcriptionally a different organ in the day vs the night’.17 These findings were confirmed in a later study comparing rhythmical gene expression in heart and liver—here >8–10% of 12 488 genes exhibited circadian regulation, but only 37 genes were regulated similarly in the two different organs, confirming the organ-specific heterogenous nature of circadian gene expression.18
In terms of location, expression of CCGs has been specifically shown to exhibit significant circadian variation in the atria of mice, in the hearts of rats,19,20 and in human hearts.12 The expression of clock genes in humans is in antiphase to that seen in rodents, reflecting the nocturnal waking life of the rodent vs. the daytime waking life of humans.12 Destruction of the suprachiasmatic nucleus eliminates, while autonomic blockade only dampens, this circadian variation in expression of clock genes in mice,19 suggesting that central control of clock genes in the heart is exerted in additional ways to autonomic nervous system (ANS) signalling.
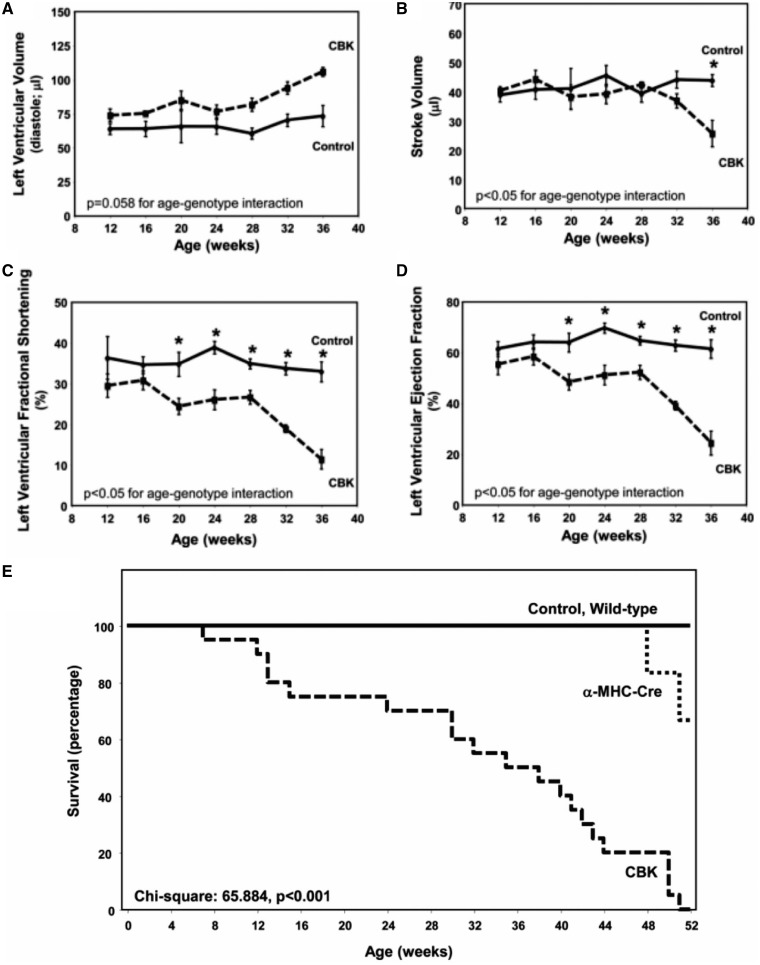
Targeted disruption of clock genes disrupts circadian rhythms, and has far-reaching implications for the organism involved, including a shortened life span.21 Specific disruption of core clocks genes, for example by overexpression of mutant CLOCK protein (the so-called ‘CCM mouse’),22 or through targeted cardiac-specific deletion of Bmal1 (the so-called ‘CBK mouse’),23 has profound yet distinct effects on the molecular clock in cardiomyocytes, leading to significant reductions in heart rate (HR) through the day, altered substrate metabolism, and deficient contractile function. The importance of these genes is emphasized by the fact that the cardiomyocyte specific Bmal1 KO (CBK) mouse begins to exhibit echocardiographic features of heart failure by around 30 weeks of age, and uniformly dies by the age of 1 year (see Figure 2);4 subsequent studies on the same mouse demonstrated evidence of progressive abnormal diastolic septal annular wall motion and reduced pulmonary venous flow at 28 weeks of age, progressive worsening of fibrosis in the interstitial and endocardial regions from 8 to 28 weeks, increased expression of collagen and matrix metalloproteinases at 28 weeks, increased transcript levels of neutrophil chemotaxis and leucocyte migration genes and decreased levels of enzymes indicating impaired resolution of inflammation.24
Figure 2.
Adapted from Young et al.’s4 seminal paper studying CBK mice. Age-dependent depression of cardiac function in CBK, but not littermate controls, including left ventricular volume during diastole (A), stroke volume (B), left ventricular fractional shortening (C), and left ventricular ejection fraction (D). Serial echocardiography was performed at 4 week intervals at the same time in the light:dark cycle. (E) It shows markedly decreased survival in CBK mice compared with littermate controls.
2.2 Protein production within cardiomyocytes
Although instructive to study gene expression levels in terms of mRNA, proteins underlie most biological processes, and levels of the two do not always correspond. Studying rhythmical or circadian variation in proteins themselves is necessary to ensure that mRNA data are not misleading.25 The diurnal proteome has been studied in the mouse heart,17 demonstrating considerable daily variation (7.8%, 90/1147 proteins studied), similar to that seen in mRNAs. The cardiomyocyte-specific clock mutant (CCM) mouse introduced above has a significantly altered proteome, with myofilaments exhibiting a loss of time-of-day-dependent maximal calcium-dependent adenosine triphosphate (ATP) consumption and altered phosphorylation rhythms, with effects on enzymes regulating vital metabolic pathways, with highly detrimental effects on cardiac metabolism.17
2.3 Cardiac contractility and cardiac output
Cardiac output is known to show diurnal variation, in humans decreasing by 24–29% at night compared with daytime averages, while stroke volume decreases by 7%.26,27 Atrial pressure in humans also exhibits a circadian pattern that is correlated with HR.28 These observations are traditionally ascribed to fluctuations in neurohormonal influences in the intact organism.29 However, such rhythmicity is also intrinsic to the heart. The ex vivo working rat heart, for example, has been shown to exhibit circadian rhythmicity in contractile function, with greatest contractile performance in the middle of the night,30 a feature lost in hypertrophied hearts. Similarly, cardiac contractility in Langendorff-perfused murine hearts is greater when they are studied 3 h into the dark phase vs. 3 h into the light phase of a 12:12 h light:dark regime. Disruption of this circadian rhythm, by putting the mouse into a 10:10 h light:dark regime, causes normal diurnal variation in cardiac contractility to disappear.17 Contractile reserve of ex vivo rodent hearts is greater in the dark phase of the circadian cycle, consistent with anticipation of workload demand during waking hours.22 These changes in cardiac contractility may be related to recent observations suggesting molecular circadian control of the sarcomere, including circadian regulation of the titin-cap protein,31 rhythmic mRNA expression of cAMP-dependent protein kinase A (PKA),22 observations that disruption of diurnal pattern alters myofilament protein phosphorylation in a murine model of myocardial infarction (MI),32 and observed daily oscillations of cardiac myofilament composition and function,17,31 calcineurin activity, protein phosphorylation,33 and myocardial excitation-contraction coupling.34 Time-of-day-dependent oscillations in expression of the two isoforms of myosin heavy chain, a critical contractile protein in the heart, have also been demonstrated in rodent hearts.20,35
2.4 Cardiac metabolism
The circadian fluctuation in contractile function of the heart described in the prior section is inextricably linked to cardiac metabolism. Carbohydrate oxidation and oxygen consumption exhibit marked circadian variation.36 In the working rat and mouse heart, this has been shown to be timed to coincide with periods of increased workload (e.g. exercise), with a peak in the middle of the night.30,37,38 Glycogen content in the rat heart peaks in the dark-to-light phase transition, consistent with increased rates of glycogen synthesis during the awake-dark period.37
Unlike glucose utilization, fatty acid oxidation does not exhibit circadian variation in isolated rat or mouse heart preparations, suggesting that fatty acids form the consistent foundation for the energetic demands of the heart. However, triglyceride synthesis does oscillate in mice, peaking near the end of the dark/active phase,30,39 while lipolysis is elevated during the light/sleep phase.39
Far less is known about circadian variation in protein and amino acid metabolism. Some evidence exists that net protein synthesis appears to be increased in the rat myocardium during the light/sleep phase in vivo.40 Indirect evidence also points to diurnal amino acid metabolism, through the levels of certain amino acids fluctuating in a time-dependent manner,40,41 and expression of genes for amino acid metabolism enzymes show diurnal variation.16,18,22
It has been similarly challenging to investigate the relationship between the circadian clock and cardiac mitochondrial metabolism. Cardiac-specific ablation of the Bmal1 gene results in cardiomyocyte mitochondrial morphological and functional abnormalities, including reduced respiratory complex enzyme activity, and decreased expression of genes involved in fatty acid oxidation, the tricarboxylic acid cycle and the mitochondrial respiratory chain.42 These mice also demonstrate impaired ketone body metabolism, impaired glucose utilization in the fed state, and abnormal metabolic responsiveness to acute fasting.4 Furthermore, they develop severe progressive heart failure with age.42 Similar features were present in normal (C57BL/6J) mice exposed to chronic reversal of the normal light:dark cycle, confirming that the circadian clock is important for maintaining healthy mitochondrial dynamics and bioenergetics in the heart. The circadian component REV-ERBα regulates mitochondrial content and oxidative function in skeletal muscle, in part by repressing genes that trigger mitophagy.43 A similar role in cardiac muscle might be predicted, and several papers have also pointed to the importance of effective mitochondrial autophagy for the prevention of contractile dysfunction and heart failure.44–47
The rhythms described above in cardiac metabolism may be affected by rhythmicity in sympathetic activity,48 circulating insulin,49 thyroid hormone,50 and corticosteroid levels,49 as well as circadian variation in circulating fuel availability such as glucose, fatty acids, and ketone bodies.51,52
Similar to changes seen in the CBK mouse noted above, CCM mice demonstrate abnormalities in metabolism, including increased rates of myocardial oxygen consumption and fatty acid oxidation, and decreased cardiac efficiency relative to wild-type animals.22 CCM mice also exhibit lack of oscillatory lactate release and modest mitochondrial dysfunction, but no alterations in mitochondrial content or structure.22
The question of what links the cardiomyocyte’s circadian clock genes with cellular metabolism is slowly being addressed, with circadian control of several key modulators of myocardial cell metabolism now established, including AMPK and NAMPT.38,39,53 The latter of these contributes to NAD levels in the heart, and normal oscillation of NAD in wild-type hearts is lost in the hearts of CCM.39,54
2.5 Heart rate
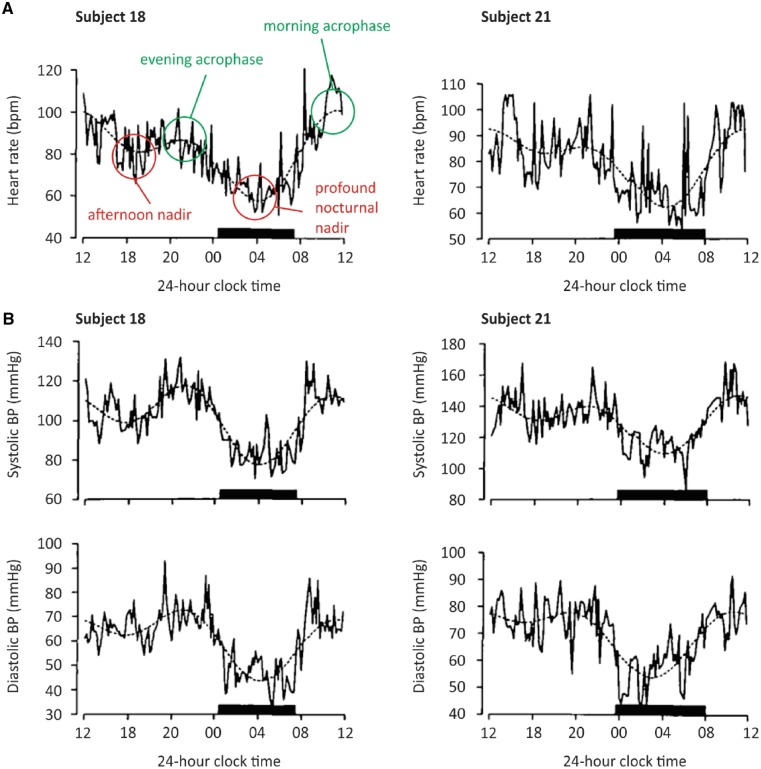
HR in humans exhibits a clear circadian pattern,55–60 with a morning ‘acrophase’ (a term used to denote a cycle’s peak, occurring at 10 am to midday), a small afternoon nadir (at around 3 pm), an evening acrophase (at around 8 pm), and a profound nocturnal nadir (at around 3–5 am) (see Figure 3A).55,61 CCM mice demonstrated decreased diurnal variation in HR.22
Figure 3.
Typical nadirs and acrophases of both HR (A) and BP (B) in two young and healthy subjects. Reproduced and modified with permission from Ref.55
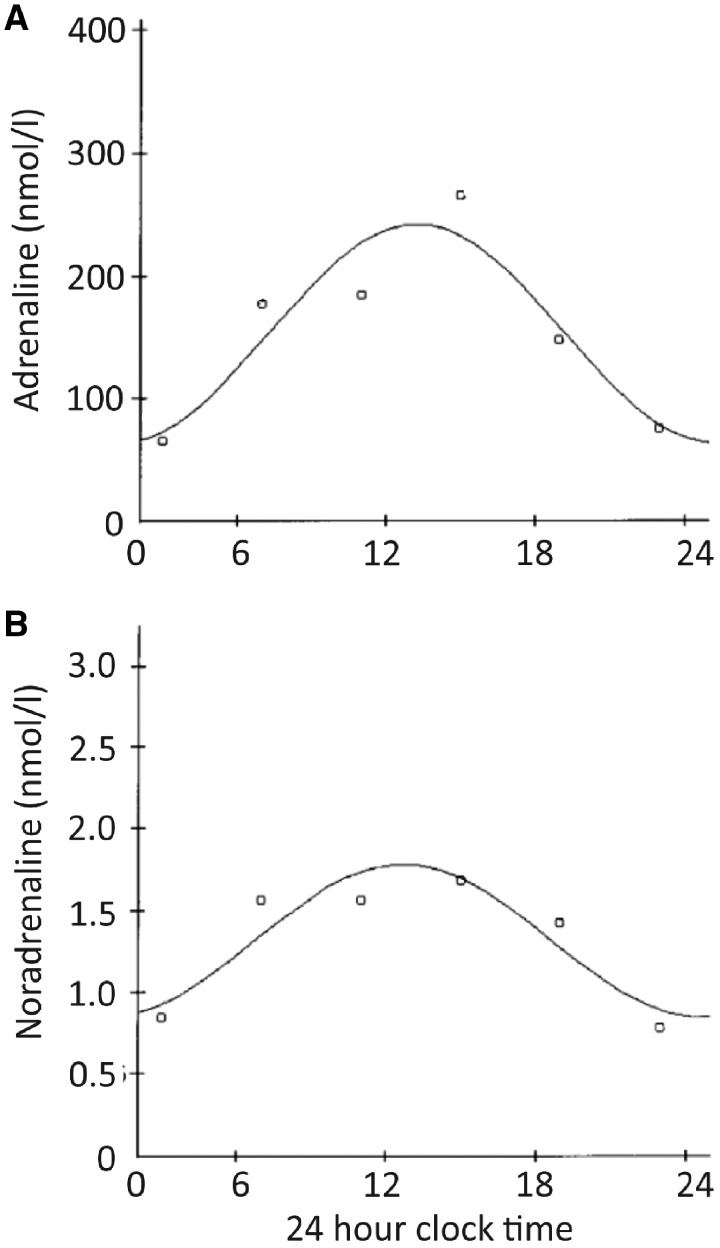
Increasing or decreasing HR in vivo occurs largely via the ANS’s two opposing arms—the catecholaminergic, sympathetic nervous system, and the acetylcholinergic, parasympathetic nervous system. Circulating catecholamines epinephrine (indicator of adrenal medullary activity) and norepinephrine (indicator of sympathetic nervous system activity) exhibit clear circadian periodicity, with a rapid elevation on waking, and a peak around midday, with a gradual tailing-off thereafter (see Figure 4).61,62 Plasma norepinephrine levels have been shown to be increased by upright posture and wakefulness, while levels of epinephrine are not so, suggesting that the latter is controlled by a central circadian oscillator, while the former is influenced by environmental conditions.62 Detailed review of circadian epinephrine and norepinephrine rhythms is available elsewhere.63
Figure 4.
Adrenaline (A) and noradrenaline (B) plasma levels (in nmol/L) during a 24-h observation period in control subjects. Curves are periodic regression curves for circadian variation. Reproduced and modified with permission from Kondo et al.223
Both arms of the ANS have direct effects on the cells of the sinoatrial node and their intrinsic clocks (described in detail later), leading to a so-called ‘brain hierarchical clock system’ (Figure 5A). The effects of the ANS are mediated by post-translational modification of critical intrinsic regulatory mechanisms of pacemaker cell automaticity via cAMP/PKA/CaMKII signalling. The nadirs in circadian HR have previously been ascribed to times when the parasympathetic nervous system becomes ‘dominant’ over the sympathetic.64,65 Similarly, acrophases in circadian HR are ascribed to vice versa shifts in the parasympathetic-sympathetic balance. These conclusions rely on assumptions made not from direct measures of ANS functioning, but instead from heart rate variability (HRV) data, which is recognized to be limited by a dependence on HR, an often overlooked but critically important correction.66–69 Although circadian variation in post-translational protein phosphorylating mechanisms has been demonstrated in other organs (e.g. CaMKII in chick retinal cells70), it is yet to be demonstrated in the cells of the heart, and further experiments are required to clarify whether circadian variation in phosphorylation is the ‘end effector’ of circadian variations in autonomic tone to the heart.
Figure 5.
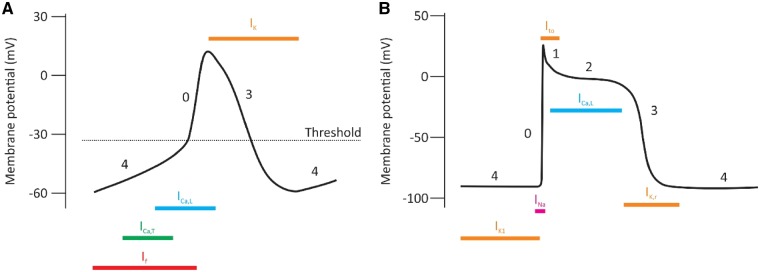
(A) Schematic figure of the brain-hierarchical clock system controlling SAN function. Special senses (vision, smell, and hearing) and chemical and baroreceptors feed information from the environment into the brainstem centres responsible for the central control of HR. Via largely opposing actions, the sympathetic and parasympathetic arms of the autonomic nervous system emerge from these brainstem centres, innervating the sinoatrial node directly, and having direct effects on levels of cAMP and phosphorylation of coupled clock proteins via their interaction with adrenoceptors and muscarinic receptors, respectively. The SAN is enlarged in yellow, and within is shown a schematic of the coupled clock system dictating automaticity. (B) Shift in leading PM site occurs in response to a variety of changing environmental conditions and drugs. Non-standard abbreviations: SVC, superior vena cava; SEP, septum; RA, right atrium; CT, crista terminalis; IVC, inferior vena cava; Ach, acetylcholine; Nif, nifedipine. Reproduced with permission from Ref.121. (C) Phases of the action potential in pacemaker type cells (i), which typically display an unstable resting membrane potential also known as phase 4 diastolic depolarization, and in working cardiomyocytes of the atria and ventricles (ii), which have a stable phase 4 resting membrane potential. The rapid upstroke of the action potential is referred to as phase 0, while repolarization is referred to as phase 3. In working cardiomyocytes, there are two further phases of the action potential—phase 1, which is early repolarization, and phase 2, which is referred to as the plateau potential. (D) the actual morphology of the action potential changes discretely but definitively as one moves down the cardiac conduction system through the working myocytes of the atria and ventricles.
Despite their substantial contribution, the brain and its autonomic nervous signalling are not essential for the presence of circadian rhythms in HR, and a variety of rhythms (circadian, infradian, and ultradian) persist in so-called ‘denervated’ cardiac preparations, including transplanted hearts,71 isolated Langendorff-perfused hearts,22,30,66 monolayers of cultured cardiomyocytes,72 and isolated sinoatrial nodal cells.66 Though rhythms persist in these denervated preparations, they are distinct from the rhythms that exist in the intact organism with a functioning ANS, regardless of whether one focusses on time-, frequency-, or non-linear domain measures of HRV.66 The best example of a ‘denervated’ SAN ‘in vivo’ is the transplanted heart. It has been shown that without intact autonomic innervation, such hearts beat faster and with less circadian variability than does the normally innervated healthy heart.73 The reasons behind this are unclear, but will be related to the lack of an intricate interaction between SAN autonomic innervation and the rhythmic processes that are occurring at a tissue, cellular and subcellular level, explored in more detail in Section 3.
2.6 Blood pressure
Circadian changes in blood pressure (BP) have been shown in humans,26,74 with a pattern mirroring HR in both systolic and diastolic pressures (see Figure 3B), for example, night-time BP is decreased by around 10% compared with daytime values,75 with the daily increase in BP starting between 4 and 7 am.26 This is true of both normotensive and hypertensive patients.76 Such a morning ‘surge’ and the associated increase in cardiac output have implications for the risk of atherosclerotic plaque rupture and likely plays a role in diurnal variation in incidence of MI, stroke, sudden death, and ventricular arrhythmia discussed elsewhere in this review. Hypertensive patients can be split into those who exhibit nocturnal dipping in BP and those who do not. Nocturnal dipping is believed to be a healthier state, and clinical studies have shown that loss of this behaviour (in patients referred to as ‘non-dippers’) is associated with left ventricular hypertrophy, increased risk of MI, renal failure, and an increase in cerebrovascular disease.77–79
The occurrence of vasovagal syncope in human patients also exhibits a circadian pattern, with more events occurring in the morning (0600 h to noon), thought to be related to differences in vagal tone or responsiveness to vagal outflow that occur through the course of the day.80
Excessive dietary Na+ intake is recognized to adversely affect circadian rhythms in BP and nocturnal BP dipping in humans, while Na+ restriction and treatment with diuretic medications can restore circadian dipping behaviour to non-dippers.81 Counter-intuitively, total peripheral resistance of the systemic arterial circulation behaves in almost the complete opposite manner to BP (and HR and cardiac output), with a nadir at around 10 am, being highest through the night.26 One might therefore predict that BP would be lowest around 10 am, but clearly this is not the case, and other factors than total peripheral resistance must contribute to circadian BP variation. In vitro, pressure induced hypertrophy in rats replicating that seen in response to long-standing hypertension attenuates circadian BP rhythms through attenuation of transcription of clock genes.20
Behavioural circadian clock disruption can have deleterious effects on the progression of hypertensive heart disease: in normal C57BL/6 mice exposed to pressure overload causing left ventricular hypertrophy, shifting them from a 24 h light:dark cycle to a 22 h light:dark cycle made cardiac remodelling more marked and the progression to heart failure more rapid.82 Disrupting the circadian clock genetically by knocking out BMAL1,83,84 or by mutating CLOCK83 or PER285,86 leads to aortic endothelial dysfunction that is likely to contribute to hypertension, confirming the importance of the symbiotic relationship of circadian rhythms, cardiac contractility with systemic vascular resistance in the genesis of hypertension.
2.7 Endocrine rhythmicity relevant to the heart
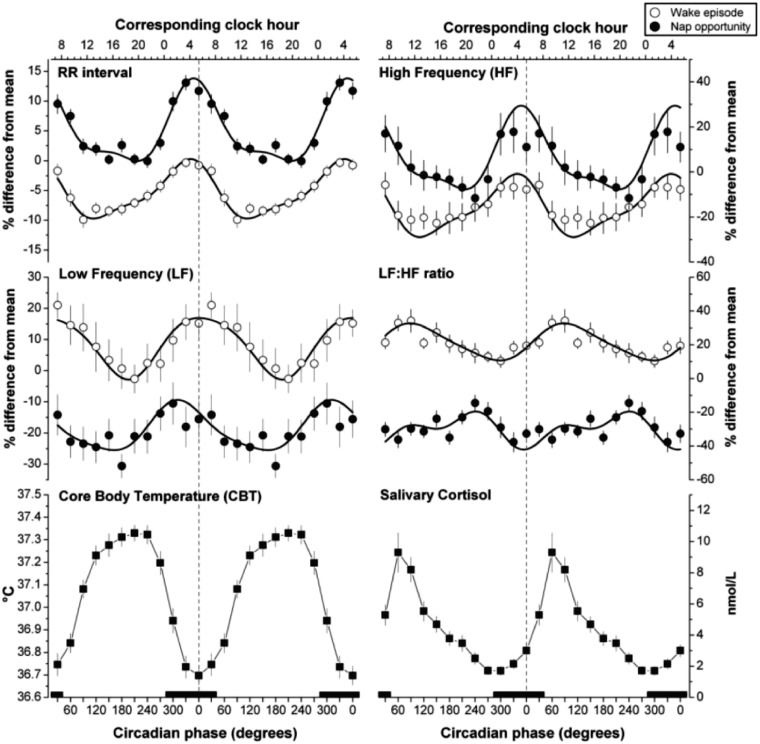
Circadian oscillation is present in the release characteristics of nearly every hormone. Cortisol secretion from the adrenal cortex, co-ordinated by the hypothalamic-pituitary-adrenal axis, exhibits clear diurnal variation, the peak of which occurs just after awakening, at a time coincidental with the maximal observed increase in plasma catecholamine levels and HRV parameters suggesting sympathetic dominance (see Figure 6).87–89 Some have proposed that cortisol is utilized by the central clock as a critical intermediary in the entrainment of peripheral clocks.90 Cortisol appears likely to contribute to the circadian rhythm of HRV, although exogenous administration of hydrocortisone only affects chronic but not acute markers of HRV.91 It is possible to completely reverse the daily cortisol rhythm by deliberate circadian misalignment in humans, wherein study subjects are made to eat and sleep 12 h out of phase from their habitual times.89
Figure 6.
Marked circadian variability in frequency domain HRV parameters, core body temperature and salivary cortisol. Reproduced with permission from Ref.87
Melatonin is ‘the most pronounced hormonal rhythm driven by the circadian system’.92 It is a highly lipophilic molecule synthesized and secreted by the pineal gland during darkness—in humans its secretion starts soon after sundown and peaks in the middle of the night around 2–4 am. During the day, serum concentrations are very low. It acutely inhibits neuronal firing in the mammalian SCN. Despite this, acute effects of melatonin on clock gene expression in the hypothalamus are not seen immediately following its administration. Rather, it takes around 48 h to see changes in clock gene mRNA in response to melatonin injection, suggesting that its effect is post-translational.93 It is considered to be an endogenous synchronizer of peripheral clocks to central ‘time’, with some viewing it as ‘the master clock output’.94 Melatonin administration in rats has been shown to effect the levels of cardiac clock gene expression,95 and lead to favourable effects on BP in humans.96 It also has marked antioxidant properties97 and has been shown to protect against ischaemia-reperfusion myocardial damage and normalize lipid profiles through its ability to directly scavenge free radicals and indirectly through its antioxidant and anti-inflammatory properties.98 This effect of melatonin is relevant since it is known that loss of function of BMAL1 leads to expression of genes related to oxidative stress, cardiac remodelling and inflammation,42 and some studies have demonstrated circadian rhythms in the expression of pro-oxidation enzymes like glutathione peroxidase.99
The renin-angiotensin-aldosterone system has circadian periodicity that coincides with that of the HR and BP.61,100,101 This particular circadian rhythm is likely to have profound effects on diurnal BP rhythms because of the powerful BP regulating effects of angiotensin II and aldosterone.
Brain natriuretic peptide (BNP) mRNA levels demonstrate circadian variability in the ventricles of mice, with a nadir at between 1200 and 1600 h (circadian time) and an acrophase at around midnight.102 Since natriuretic peptides are important in the regulation of fluid homeostasis and vascular tone, and protecting against accelerated local fibrosis in the myocardium, this study suggests that in the ventricles, circadian patterns in BNP may partially account for variation in outcome from MI based on the time of day the infarct occurs.103–106
2.8 Coagulation
The observed excess of thrombotic cardiovascular events, including MI and stroke, at certain times of day107–109 has generated interest in circadian coagulation rhythms. Circadian rhythms exist in both human vascular endothelial function110,111 and in platelet function.88,112 Regarding the former, flow-mediated vasodilatation of systemic vessels as an index of endothelial function is poorest in the morning,110 and coronary segments with dysfunctional endothelium exhibit exaggeration in early morning vasomotor activity,111 making coronary occlusion more likely at that time of day. Platelet aggregability demonstrates complex circadian rhythmicity, with some evidence that it peaks bimodally through the course of the day, at noon and 9 pm.88 Other evidence demonstrates circadian rhythms in platelet surface activated glycoprotein IIb-IIIa, Ib, and P-selectin,112 peaking at around 8–9 am, right when the excess of thrombotic cardiovascular events occurs. This article also demonstrated circadian peaks in other indices of platelet behaviour including count, aggregability and ATP release, peaking later in the day, between 3 and 8 pm,112 and further that circadian influences on these platelet behaviours was significantly greater than any environmental stressors. It is interesting to consider what might be the cause of circadian rhythms observed in platelet biology, since they are anucleate. It has been suggested112 that humoural factors control rhythms in platelet biology, including the previously discussed circadian rhythms in endocrine hormones (epinephrine, norepinephrine, melatonin, and cortisol), endothelial derived factors (nitric oxide and prostaglandin), and platelet-derived factors (ATP). Alternatively, circadian variation in organ sequestration and release of platelets by spleen/liver/lungs/bone marrow may be the critical factor. Without a nucleus, the clock genes and oscillating transcription-translation feedback described in the initial section cannot be the cause of rhythmical variation in platelet biology. Some insight has been given into the possibility of innate rhythmicity in platelets by studies of circadian rhythmicity in zero-DNA containing, anucleate human red blood cells.113 These studies have shown that non-transcriptional mechanisms involving peroxiredoxins (highly conserved antioxidant proteins) undergo reduction-oxidation cycles with a period of around 24 h, which persist for many days under constant conditions in the absence of external cues.113 These rhythms in red blood cells were entrainable to environmental cues and were also temperature compensated (did not change in response to fluctuation in ambient temperature),113 both of which represent key features of circadian rhythms. This work suggested for the first time that having a nucleus was not a fundamental requirement for the presence of circadian rhythms in mammalian cells. This backed several other observations that suggested transcription-translation independent circadian rhythmicity, including in the simplest organism known to exhibit circadian rhythmicity (the cyanobacterium Synechococcus elongatus), which demonstrates biochemical oscillations in the absence of transcription and translation.114,115
Indices of coagulation also show diurnal variation, with morning hypercoagulability and hypofibrinolysis being related to a broad variety of circadian changes in plasma levels of activators and inhibitors of coagulation and fibrinolysis.116 For example, plasminogen activator inhibitor-1 (PAI-1) inhibits fibrinolysis and is a key circulating prothrombotic factor that is known to rise in the morning in humans, peaking around 06:30 h.117 Data exist that show a direct effect of core clock genes (including BMAL1, CLOCK, and PER2) on the activity of haemostatic factors such as von Willebrand factor and megakaryocytes.118–120 Further, disruption of the so-called ‘positive’ limb of the transcription-translation cycle (CLOCK118 and BMAL1119) favours a thrombotic phenotype, while disruption of the so-called ‘negative’ limb (PER2120) favours a haemorrhagic phenotype.
3. The heart beat itself as a system of ultradian rhythms
The heart beat is comprised of discrete yet intricately woven ‘clocks’ that have been unpicked over the last 15–20 years. A small number of cells within the SAN dictate the heart’s beating rate—the ‘leading pacemaker site’—the location of which can vary depending on environmental conditions (see Figure 5A, B)121 due to marked electrophysiological heterogeneity in SAN cells.122 The cells leading pacemaking exhibit the fastest rate of phase IV diastolic depolarization (see Figure 5C-i, ii), and are the first to reach the threshold for the firing of an ‘all-or-nothing’ action potential. As these cells fire an action potential, they entrain and electrically capture the cells around them, causing almost simultaneous firing of action potentials in a ripple effect, to the rest of the heart (see Figure 5D). At the cellular level, SAN automaticity is governed by complex and dynamic interaction between two oscillatory clocks (Figures 5A and 7).123
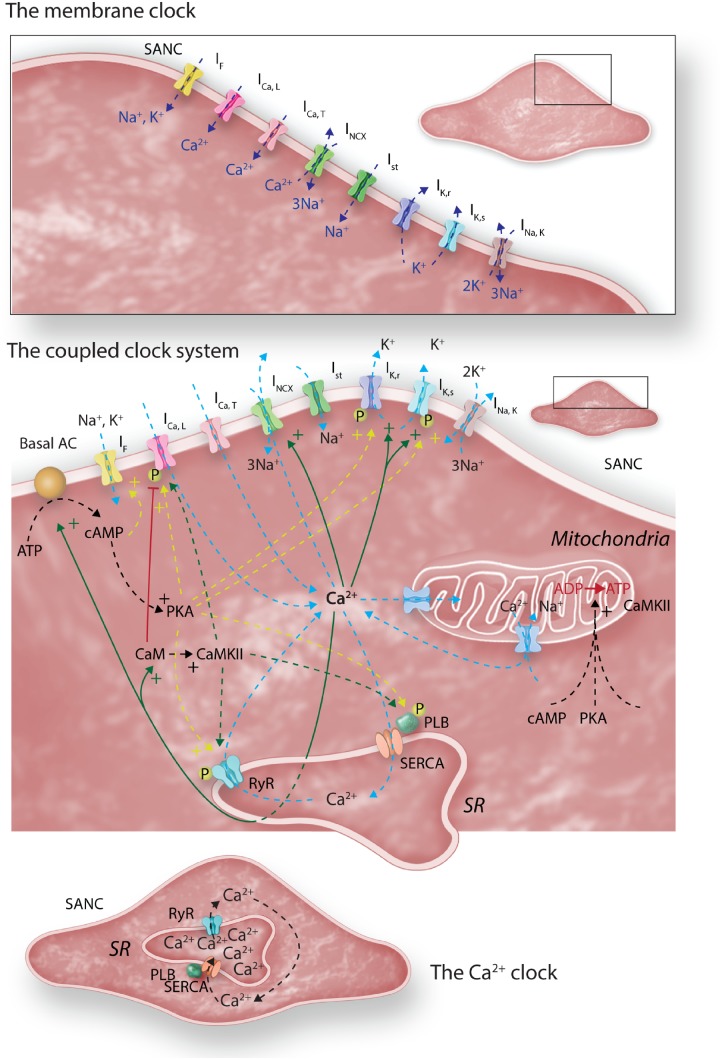
Figure 7.
Adapted from Ref.222; schematic figure of the coupled clock system. The upper inset shows the membrane clock of sarcolemmal ion channels in isolation. The lower inset shows the intracellular Ca2+ clock in isolation. When put together, as in life, and as shown in the main central panel of the figure, it is clear that there are multiple areas of crossover between the two clocks, which are facilitated by the so-called ‘nodes’ of Ca2+ and phosphorylation. A full description of the coupled clock system is given in the text. The role played by mitochondria in the couple clock system remains poorly understood, though it is known that they are involved in intracellular Na+ and Ca2+ homeostasis. Whether fluctuations in mitochondrial uptake and release of these crucial ions influence the coupled clock system is presently unclear.
3.1 The membrane clock
This rhythmic ‘clock’, distinct from the circadian clock, was the first described mechanism that could plausibly account for automaticity in sinoatrial node cells (Figure 7, top panel). Charged species (‘ions’) pass through membrane-bound ion channels, taking advantage of the differences in ionic concentrations between the cytoplasm and extracellular milieu of sinoatrial node cells, in a time- and voltage-dependent manner. The opening of ion channels is a fleeting event producing electrical currents that contribute to both diastolic depolarization and the action potential itself. Because of their different characteristics, different ion channels are important at different times in the action potential (see Figure 8). The fact that these ion channels exhibit time-dependence means that they have fundamental rhythmicity that contributes to ultradian rhythms in the heart. Circadian variation exists in important members of the membrane clock, as follows:
Figure 8.
Ionic current density from ion channels varies at different times during the action potential in both the sinoatrial node (A) and the working ventricular myocyte (B) due to voltage- and time-dependence.
3.1.1 Sodium channels
Knockout of Bmal1 has been shown to cause loss of circadian expression of the Scn5a gene, normally encoding the principle cardiac voltage gated Na+ channel, Nav1.5,14 with a decrease in Nav1.5 protein expression, and in the levels of INa in ventricular myocytes.14 It is likely that this contributes to HR slowing, loss of circadian HR variation, QRS prolongation and episodes of arrhythmia seen in these animals.
3.1.2 Repolarizing potassium channels
In rat heart, mRNA from two potassium channel genes, Kcna5 and Kcnd2 [the pore-forming subunit for the fast component of the transient outward K+ current (Ito, f)], exhibits significant circadian variation, which can be modulated by pharmacological blockade of the ANS.124 Similarly, in mice, there is also significant circadian variation in Kchip2 and Kcnk3 in both the atria and the ventricles.19 Other studies in mice have confirmed the circadian variation in KChIP2 (the regulatory beta-subunit for the transient outward K+ current, Ito), and also in the expression of the alpha subunit for Ito, Kv4.2, brought about through the clock genes Clock and Bmal1, via the transcriptional activator Klf15.125 Abnormalities in Klf15 led to loss of diurnal QT variation, abnormal repolarization and a higher rate of potentially life threatening ventricular arrhythmias. Inducible cardiac-specific knockout of Bmal1 in murine hearts disrupted the previously robust circadian pattern of expression of Kcnh2, responsible for the hERG channel that underlies the rapidly activating delayed-rectifier K+ current,126 which is 50% smaller in ventricular myocytes from knockout animals, leading to prolonged QTc on the electrocardiogram (ECG).126 Studies in humans have shown that high levels of circadian variation in ECG parameters of repolarization (so-called QT diurnality) are associated with ventricular arrhythmias in patients with a history of MI and a reduced ejection fraction, and that this seemed to be associated with hERG channel dysfunction.127
3.1.3 Funny current (If)
There is some early experimental evidence from our laboratory that circadian rhythms exist in the expression of If in murine sinus node cells, with corresponding circadianicity in HCN4 mRNA and protein expression (Lakatta, unpublished data).
3.2 The calcium clock
Rhythmical cycling of Ca2+ in and out of its major intracellular store, the sarcoplasmic reticulum (SR), occurs in two phases via its release channel, the ryanodine receptor (RyR) (Figure 7, bottom panel). The first involves rhythmical spontaneous, stochastic, locally propagating releases that occur in diastole known as ‘local Ca2+ releases’, ‘LCRs’. These emerge as the SR refills, courtesy of the sarco-endoplasmic reticulum ATPase (SERCA). Following the production of an LCR, the RyR exhibits a delay before a subsequent LCR can be produced, giving them an inherent rhythmicity. LCRs grow as diastole proceeds, in number, size, and signal mass.128 The Ca2+ that is produced in the form of these LCRs interacts critically with the membrane-bound electrogenic Na+-Ca2+ exchanger (NCX, Figure 7 middle panel), to generate inward current that increases diastolic depolarization rate, length of diastole, and therefore beating rate of the heart. The second phase of SR-derived Ca2+ release, involving en masse release of Ca2+ from the SR, known as the ‘Ca2+ transient’, is triggered by the action potential occurring when the diastolic membrane potential reaches a critical threshold. This resets the Ca2+ clock—the SR is completely depleted, and the Ca2+ clock starts to tick once more as the SR begins to refill.
3.2.1 Circadian rhythmicity, Ca2+ cycling, and the Ca2+ clock
There is little evidence linking the Ca2+ clock to circadian variation in cardiovascular parameters, with inferences being made from neuronal research. For example, cytosolic Ca2+,129 and L-type Ca2+ current density130 exhibit diurnal fluctuations in mammalian SCN neurons in the hypothalamus that, in the case of cytosolic Ca2+ fluctuations at least, are not sensitive to the presence or absence of an action potential.131 As far as the heart is concerned, circadian type fluctuations have been demonstrated in the hearts of chicks132 with respect to Ca2+ channel subunit and Ca2+ current densities. Further, time of day of isolation in adult rat left ventricular cardiomyocytes plays a role in their Ca2+ transient characteristics, related to differences in intracellular Ca2+ and SR Ca2+ load during the so-called ‘resting’ and ‘active’ periods (higher diastolic and systolic intracellular calcium, elevated peak Ca2+ release and more rapid relaxation in cells isolated during the resting period).34 There was also a significant difference in L-type Ca2+ current measured in response to isoprenaline depending on the time of day of isolation (higher L-type Ca2+ current during the active period), despite evidence from elsewhere documenting higher gene and protein expression of the voltage gated Ca2+ channel subunit α1D.133 Ventricular myocytes isolated during the active period were less sensitive to isoprenaline induced ‘arrhythmia’.34 This article was also the first to demonstrate diurnal variation in excitation: contraction coupling in ventricular myocytes.
Left ventricular myocytes from guinea pigs demonstrate circadian rhythmicity in expression of the alpha subunit of L-type Ca2+ channels, CACNA1C, associated with increases in action potential duration during periods of highest expression.134 Overexpression of CLOCK:BMAL1 in these cells significantly reduced the levels of CACNA1C, suggesting that the molecular clock has a fundamental role to play in the expression of the L-type Ca2+ current, and disrupting it may affect action potential duration in a manner that is likely to be pro-arrhythmic.
3.3 HRV reflects rhythmical complexity in the heart beat
It is possible to dissect out rhythms in the beating of the heart to reveal complex variability existing on a beat-to-beat basis—‘HRV’ (see Figure 9). Historically, HRV is almost entirely ascribed to behaviour of the ANS, while the modern view is that it is likely significantly contributed to by the ultradian rhythms in the behaviour of components of the membrane- and Ca2+-clocks described above.
Figure 9.
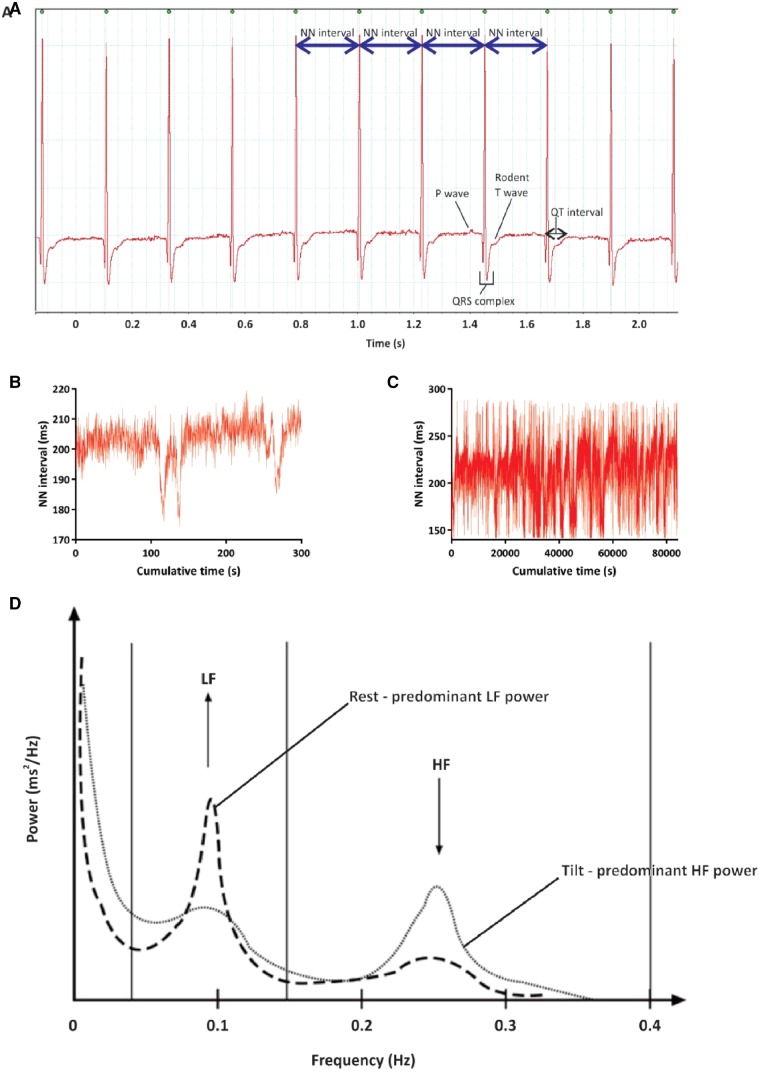
HRV in recordings of spontaneous ECG data from in vivo rats. (A) HRV data are calculated from consecutive normal-to-normal (NN) intervals, usually being measured at the peak of the R-wave of the ECG. Although not obvious to the naked eye, when consecutive NN intervals are plotted on a graph of cumulative time (a so-called tachogram, shown in B and C) there is marked variation over both the short and long term. (B) Tachogram of spontaneous NN intervals over 5 min in a rat. (C) Tachogram of spontaneous NN intervals over 24 h in the same rat as shown in panel (B). (D) Reproduced and modified with permission from Gunther et al.224: typical power spectrum from frequency domain analysis of human short-term HRV. Note especially the peaks localized to the low and high frequency bands, and how they vary between rest and in this case tilt testing. The frequency bands are dependent on the mean HR of the organism being investigated and should be altered based on the mean HR of the organism being studied.
Many parameters for objectively quantifying HRV exist (see Table 1).65 The need for so many descriptors emphasizes the complexity of variability contained within the HR signal as a whole, and also the fact that no single parameter is capable of comprehensively quantifying HRV. Certain parameters of HRV selectively reflect sympathetic and parasympathetic components of innervation to the heart, and sympathovagal balance.65 Although this is debated,69 phosphorylation changes effected by sympathetic and parasympathetic signalling contribute substantially to ultradian rhythmical variation in HR. For example, sympathetic signalling not only quickens HR, but also decreases indices of ultradian variability.135,136 Contrastingly, parasympathetic stimulation slows HR and increases ultradian measures of HRV.137,138 Parameters of HRV are capable of predicting and detecting morbidity and mortality in a wide variety of cardiovascular and non-cardiovascular conditions, and even in healthy populations.65 HRV initially increases from birth to early adulthood,139,140 but from early adulthood it decreases,140–150 as does the complexity of cardiovascular dynamics,150–152 possibly related to the recognized age-associated decline in autonomic agonist sensitivity.153–156 HRV parameters vary in a circadian fashion (Figure 6).87,157 Across most parameters, HRV increases during the night and decreases during the day.61,157 Indirect HRV-based measures of sympathovagal balance have been legitimized by direct measures of plasma catecholamine levels (see Figure 4).158 The effect of vigilance state transitions on HRV parameters also varies dependent on the time of day,87 with sleep-to-wake transitions that occur in the morning being most associated with increased sympathetic nervous activity compared with similar transitions at other times of the day. This suggests that both behaviour and circadian processes interact to affect the autonomic modulation of the heart in a complex way to produce optimal cardiovascular responses to vigilance state transitions. The presence and severity of cardiovascular disease also has an effect on the circadian periodicity of HRV—for example, the presence and severity of angina destabilizes circadian rhythms in HRV,159 causing phase shifts in the nadirs and acrophases of several HRV parameters.160 Similarly, ischaemic stroke reversibly abolishes circadian fluctuations in HRV, with loss of the vagal nocturnal dominance of HRV, reverting to normal after around 6 months of recovery.161
Table 1.
Different parameters used to objectively assess HRV across multiple domains
| Time domain | Frequency domain | Non-linear domain |
|---|---|---|
| Standard deviation (SDNN) | Total power (TP) | Entropy (approximate, multi-scale, etc.) |
| Standard deviation of segment averages (SDANN) | High frequency (HF) domain power | α1, α2 (short and long-term fractal exponents) |
| Root mean square of successive differences (RMSSD) | Low frequency (LF) domain power | Beta slope |
| Coefficient of variation (CoV) | Very low frequency (VLF) domain power | SD1 and SD2 of Poincare plot |
| Standard error (STENN) | Ultra low frequency (ULF) domain power | Detrended fluctuation analysis (DFA) |
| SDNN:SDSD | LF:HF ratio | Hurst exponent |
| Range NN | ||
| Number of intervals differing by a given number (NNx) | ||
| Proportion of all intervals differing by a given number (pNNx) |
This list is not exhaustive, and, for example, the frequency domain parameters can be expressed in a variety of ways, including raw units, or normalized to the total power, or normalized to the total power minus the VLF/ULF band power.
4. The clinical importance of rhythmicity
Evidence of the importance of maintaining healthy ultra-, infra-, and circadian rhythms is apparent throughout cardiovascular research. Starting with the most easily measurable ultradian cardiovascular rhythm, the beating rate of the heart, it is known across a variety of organisms that low resting HR equates to longer life span.162 Disruption of normal circadian rhythms increases cardiovascular disease risk. Such disruption is a modern day epidemic—night shifts, travelling across time zones, ubiquitous electrical lighting, and sleep disorders163 are all increasingly prevalent with time as our lifestyle changes, and all uncouple our lives from our circadian clock, at a substantial cost. For example, humans working night shifts are more prone to heart disease, having a 40% increase in coronary heart disease risk and risk of cardiac events compared with non-night shift workers.164–167 This has led to the suggestion of prophylactic anti-platelet medication use in night shift workers.112 This increase in risk has significant public health ramifications, given that around 28% of the Western workforce operates outside of the conventional working day.168 Deliberate circadian misalignment, such as that which occurs when study subjects are made to eat and sleep 12 h out of phase, leads not only to complete inversion of the cortisol profile described above, but also increases postprandial glucose (to pre-diabetic levels in some previously healthy individuals) despite increasing insulin levels, increases mean arterial pressure, and decreases plasma leptin and sleep efficiency.89
Alterations of the circadian rhythm in HR also predict cardiovascular morbidity and mortality. For example, with respect to peripheral and coronary atherosclerosis, higher levels of diurnal HR variation predict greater atherosclerosis.169 Significant circadian variations in the incidence of cardiac arrhythmias and sudden cardiac death exist in almost all acquired and hereditary forms of heart disease.109,170 This is likely to be at least in part due to circadian variation in conduction and refractoriness throughout the heart. In general, circadian rhythms in conduction are attenuated at night (manifest by significant prolongation of effective refractoriness), while conduction is enhanced during daylight hours (with associated shortening of the effective refractory period).61,171–173 Sinus node recovery time (the length of time taken for the sinus node to recommence spontaneous beating following a period of fast pacing) in patients with sick sinus syndrome also exhibits a circadian rhythm, with an acrophase between midnight and 7 am.174 ECG manifestations of repolarization, specifically the QT interval (shown in Figure 9), demonstrate diurnal variation in a variety of species, including mice.19,175 Destruction of the hypothalamic SCN, or use of ANS blockers leads to a loss of this circadian QT variation in mice. Such loss of circadian QT rhythm leads to an enhanced susceptibility to ventricular arrhythmias.125 Similarly, QT dispersion, an infrequently used ECG parameter that describes beat-to-beat heterogeneity in ventricular repolarization (high QT dispersion being associated with greater risk of sudden cardiac death)176 shows circadian variation, being greatest in the morning hours, both in normal humans and those with coronary heart disease. Ventricular late potentials are considered to be indicators of risk for malignant arrhythmias of the heart,177 and in patients post-MI with a history of ventricular fibrillation, late potentials exhibit circadian rhythmicity,178 being localized to the morning hours between 6 and 12 am. Late potentials do not exhibit such rhythmicity in people post-MI with a history of less severe arrhythmia or with no history of arrhythmia at all. Such a phenomenon may plausibly be used to risk stratify post-MI patients. Like QT dispersion and ventricular late potentials, T-wave alternans is an ECG marker of malignant arrhythmia risk, describing beat-to-beat variability in amplitude/morphology/polarity of the T wave. It too exhibits circadian periodicity, with a higher density during morning hours.179 Circadian and ultradian rhythms also exist in the occurrence of premature atrial and ventricular ectopic beats, which can be associated with the development of arrhythmia and cardiomyopathy.60 It is possible that underlying this are circadian variations in the expression of ion channels (described above), or in the physiological responsiveness of heart cells to autonomic signalling via the multi-synaptic pathways leading from the brain (cells in the SCN of the hypothalamus) to the end effectors (the cells of the SAN and working cardiomyocytes of the atria and ventricles).
Circadian rhythms also exist in the incidence of other cardiovascular events, not only arrhythmias, including the onset of heart failure and MI,180–184 with morning hours being the time of greatest risk.87 For example, the hours between 6 am and midday impart a 40% increased risk of MI and a 29% increased risk of sudden cardiac death in diurnally active individuals.184 The reasons underlying this remain unclear, yet time-of-day variation in the incidence and severity of MI, sudden cardiac death, ventricular arrhythmia and stroke have been shown repeatedly, and are concisely summarized in Ref.185 Basic science reinforces this message, with the molecular pathways that are activated in mice in response to coronary artery ligation differing depending on the time of day that this occurs,186 and such differences are likely to affect the organ response to this insult, including favourable or unfavourable remodelling, and survivorship. Furthermore, disturbing circadian rhythms and sleep in the days following MI in a mouse interferes with the orderly cascade of humoural and cellular healing that is necessary, and leads to a worse long-term outcome.32 This is especially worrying given the known detrimental effect on sleep and circadian rhythm that is known to occur in human patients when they are hospitalized.187,188
While the above evidence suggests that the disease incidence/prevalence can be affected by the hearts clock, vice versa the hearts clock can be affected by a variety of disease states, including pressure overload,20,23 hypertension,23,189 diabetes mellitus,190,191 obesity,190 coronary artery ligation,192 simulated shift work,193 and ageing.
Deliberate circadian misalignment and its consequences is an active field of study—for example, it is possible to mutate one allele of the protein tau (casein kinase 1ε) in hamsters to cause their circadian clock to shorten from 24 to 22 h.194 When these hamsters are housed in a 12:12 h light:dark environment (dyssynchronization between circadian clock and environment) they demonstrate an excess of age-dependent cardiovascular disease, developing a marked cardiomyopathy, extensive myocardial fibrosis, and severely impaired contractility. When the environment is switched to an 11:11 h light:dark environment (resynchronization of circadian clock and environment) the excess cardiovascular disease disappears.36
5. Ageing and cardiovascular circadian rhythms
Ageing influences cardiovascular circadian rhythms. For example, it decreases the body’s ability to respond to perturbation in the circadian clock, decreasing the speed of adaptation to night working,195,196 which even in the young only occurs at a rate of 1 h per day.197 Ageing significantly reduces the amplitude and phase of circadian rhythms in both HR and BP,198,199 possibly via age-dependent changes in responsiveness to cardiac autonomic signalling, although circadian variations in plasma catecholamine levels do continue to occur, irrespective of age.158 While epinephrine levels are not different with ageing, norepinephrine levels are, being 28% higher during the day and 75% higher at night in older individuals, possibly contributing to the insomnia of ageing.158 It may be that age-associated changes in intrinsic clock mechanisms within SAN cells are responsible for the age-blunted circadian rhythms in HR—evidence to support this includes age-associated decrease of beating interval variability in mice, suggesting both imbalance in autonomic neural input to the heart and altered SAN cell responses to neural input.200
It would appear that, unlike the situation with HR, circadian variation of HRV is maintained in older people, although the extremes of day–night differences are smaller than in young individuals,144 and the effect of ageing on circadian changes in HRV is different in the two sexes.201,202 Elsewhere, however, it has been shown that there is a decrease in the total power of the power spectrum of 24 h circadian rhythm in very elderly patients, with a shift in the circadian acrophase to a later time point.203
Ageing is one of the major risk factors for cardiovascular disease, and it is recognized to decrease the amplitude of many circadian rhythms, and increase the tendency towards ‘internal desynchronization’,165 which can manifest clinically as heart failure. Perturbing the circadian system, for example by mutating the Clock gene in mice (specifically ClockΔ19/Δ19 that results in the production of a protein incapable of transcriptional activation), leads to the age-dependent development of a heart failure phenotype, with cardiac dilatation, cardiomyocyte hypertrophy, interstitial fibrosis, and maladaptive remodelling, with eventual development of systolic dysfunction (decreased fractional shortening and decreased ejection fraction).185 This suggests that maintaining circadian ‘health’ is critical in the avoidance of cardiovascular morbidity as we age—in the case of Clock, this occurs through an effect on the PTEN-AKT signalling pathway, known to be crucial for cardiac growth and renewal,185 and such work raises the intriguing possibility of specifically targeting the circadian mechanism as a novel therapeutic approach to the management of cardiovascular disease. Notably, female mice with the same ClockΔ19/Δ19 mutation did not develop the heart failure phenotype unless they were ovariectomized, suggesting that female hormones protect the heart in this model of accelerated cardiac ageing due to clock gene mutation.204
6. Conclusions and future directions
Rhythmical variations in HR and other cardiovascular parameters represent a basic quality of human physiology. The molecular mechanisms that control these rhythms exist at multiple levels of both chronicity and location (from single molecules, all the way up to cells, tissues, and organs). Variation in gene transcription for critical molecules involved in the coupled clock system is rhythmical at the level of hours and days, whereas endocrine modulation of HR, especially that involving the renin-angiotensin-aldosterone system, occurs over the order of minutes to hours. Similarly, autonomic sympathetic rhythmical control of HR occurs over minutes, whereas autonomic parasympathetic control of HR occurs over seconds. Finally, rhythms involving the behaviour of molecules involved in SR Ca2+ cycling, or in ion channel activation and inactivation, or in mitochondrial ATP production occur over the order of milliseconds or faster. The rhythms are caused by a variety of factors that are both extrinsic and intrinsic to the heart/SAN cell itself. Extrinsic autonomic rhythmicity has traditionally been believed to be the major player responsible for this rhythmicity, but an increasing body of evidence suggests that this extrinsic rhythmicity has to interact with highly complex intrinsic rhythmicity detailed painstakingly above exhibited by the cells of the different areas of the heart on multiple levels, which is further complicated because it changes in the presence of ageing and disease.
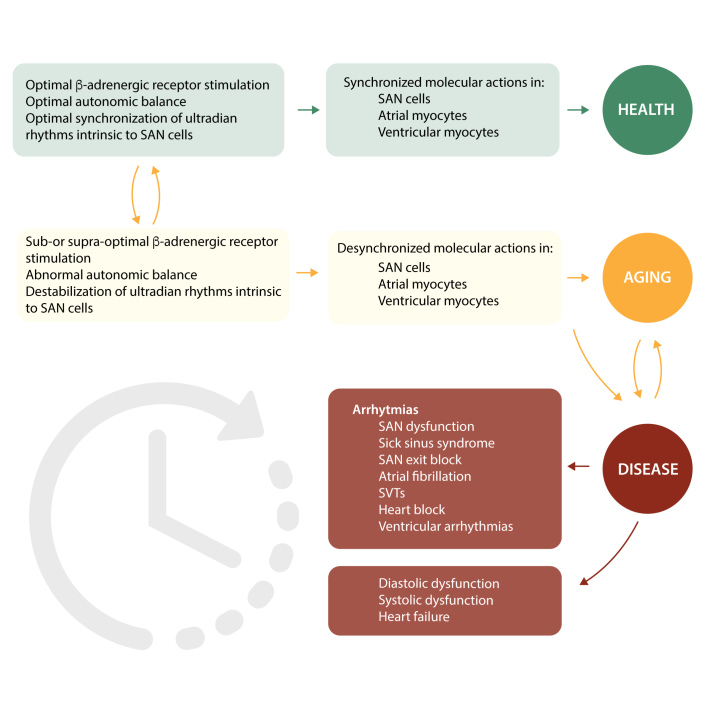
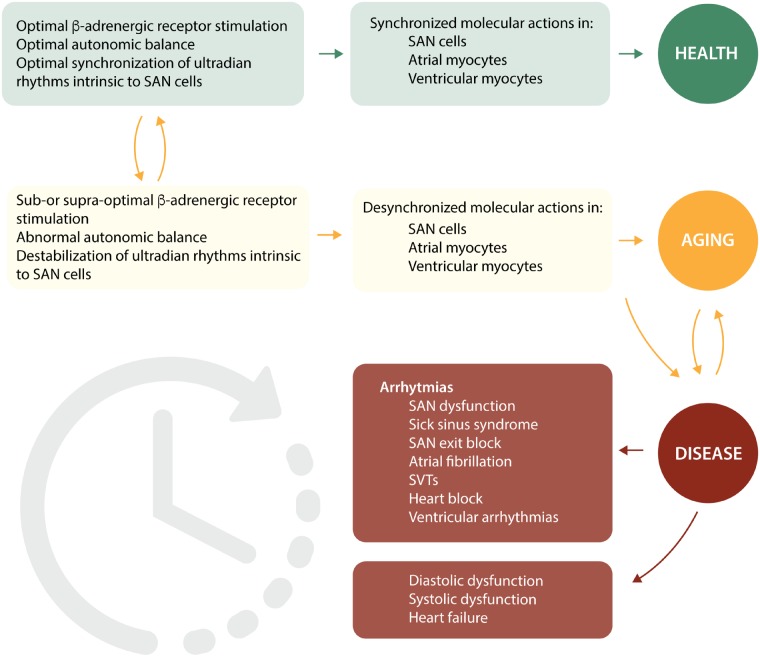
Synchronization of both extrinsic and intrinsic rhythms is crucial for optimal functioning of the heart,205 and represents one important way in which the full chronotropic repertoire of the heart is achieved. Desynchronization of rhythms leads to pathological phenotypes, including cardiac arrhythmias, and also because a similar set of molecules are involved in atrial and ventricular myocytes as in SAN cells, desynchronization here can lead to the heart failure phenotype (see Figure 10).205
Figure 10.
A schema depicting potential links between the extent of synchronization of molecular actions. RyR activation and Ca2+ release, to cardiac function and structure in health and disease’. Synchronization within and between cells of the heart is crucial for optimal chronotropism and inotropism of the heart. If this becomes suboptimal or disordered, then the clinical outcome is of arrhythmias and heart failure. ECG panel shows the irregularly irregular rhythm typical of atrial fibrillation, while the echocardiogram snapshot shows an enlarged dilated left ventricle with thinned walls typical of severe heart failure. Reproduced and modified with permission from Ref.205
Future progress is required to turn what we have learned about circadian rhythms affecting the heart into therapeutic applications, focusing on restoring or maintaining these rhythms, and the effect of doing so on restoring and maintaining health. Most interventions, predominantly pharmacological, are presently given to have an effect throughout any given 24 h period, effectively neglecting the evolutionary significance of circadian rhythmicity. There is however some reasonable suggestion that targeting interventions to certain times of the day would maximize their benefit,206–208 while leaving other periods in the day ‘free’ from substantial intervention, where the likely beneficial effect of the intervention is less substantial.185 Examples include preferential timing of angiotensin-converting enzyme (ACE) inhibitor therapy (sleep-time dosing of ACE inhibitors in mice with pressure overload hypertrophy preferentially mitigated against cardiac remodelling, whereas dosing this medicine during wake time had no discernibly different effect than placebo, a difference reflecting the diurnal rhythmicity of the renin-angiotensin-aldosterone system),209 evening administration of anti-hypertensives for non-dippers,210 nocturnal aspirin to prevent morning thrombo-occlusive events in the cardio- and cerebrovascular systems,211 nocturnal haemodialysis to cause regression of left ventricular hypertrophy,212 and nocturnal continuous positive airways pressure for patients with obstructive sleep apnoea.213 The design of true ‘chronotherapeutic oral drug absorption systems’ has been reported (e.g. Ref.214) and their use has shown some promise in preferentially targeting high risk times of day, although uptake into clinical practice has been limited, and the field of ‘chronomics’ is underexplored.215,216 Working to simply maintain normal circadian rhythms by preventing sleep disturbance and excessive exposure to electric lighting during admission to hospital would seem to be one relatively easy way to prevent harm from circadian dysregulation, and work is ongoing in this field.217–219
Certainly, more detailed studies are required into chronomic therapy, and into the exciting field of cardiovascular rhythms in general, including the important differences that are beginning to be uncovered between males and females,220 so that novel approaches to therapy may be developed to tackle the epidemic of cardiovascular diseases worldwide.221
Conflict of interest: none declared.
References
- 1. Chen L, Yang G.. Recent advances in circadian rhythms in cardiovascular system. Front Pharmacol 2015;6:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Halberg F, Cornelissen G, Sothern RB, Hillman D, Watanabe Y, Haus E, Schwartzkopff O, Wr B.. Decadal cycles in the human cardiovascular system. World Heart J 2012;4:263–287. [PMC free article] [PubMed] [Google Scholar]
- 3. Yaniv Y, Lakatta EG.. The end effector of circadian heart rate variation: the sinoatrial node pacemaker cell. BMB Rep 2015;48:677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Young ME, Brewer RA, Peliciari-Garcia RA, Collins HE, He L, Birky TL, Peden BW, Thompson EG, Ammons BJ, Bray MS, Chatham JC, Wende AR, Yang Q, Chow CW, Martino TA, Gamble KL.. Cardiomyocyte-specific BMAL1 plays critical roles in metabolism, signaling, and maintenance of contractile function of the heart. J Biol Rhythms 2014;29:257–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scheiermann C, Kunisaki Y, Frenette PS.. Circadian control of the immune system. Nat Rev Immunol 2013;13:190–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dibner C, Schibler U, Albrecht U.. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol 2010;72:517–549. [DOI] [PubMed] [Google Scholar]
- 7. Lowrey PL, Takahashi JS.. Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu Rev Genom Hum Genet 2004;5:407–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lowrey PL, Takahashi JS.. Genetics of circadian rhythms in mammalian model organisms. Adv Genet 2011;74:175–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Menaker M, Takahashi JS, Eskin A.. The physiology of circadian pacemakers. Annu Rev Physiol 1978;40:501–526. [DOI] [PubMed] [Google Scholar]
- 10. Young ME, Reddy AB, Pollock DM.. Introduction to special issue: circadian regulation of metabolism, redox signaling and function in health and disease. Free Radic Biol Med 2018;119:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dardente H, Dardente H, Cermakian N.. Molecular circadian rhythms in central and peripheral clocks in mammals. Chronobiol Int 2007;24:195–213. [DOI] [PubMed] [Google Scholar]
- 12. Leibetseder V, Humpeler S, Svoboda M, Schmid D, Thalhammer T, Zuckermann A, Marktl W, Ekmekcioglu C.. Clock genes display rhythmic expression in human hearts. Chronobiol Int 2009;26:621–636. [DOI] [PubMed] [Google Scholar]
- 13. Bray MS, Young ME.. Diurnal variations in myocardial metabolism. Cardiovasc Res 2008;79:228–237. [DOI] [PubMed] [Google Scholar]
- 14. Schroder EA, Lefta M, Zhang X, Bartos DC, Feng HZ, Zhao Y, Patwardhan A, Jin JP, Esser KA, Delisle BP.. The cardiomyocyte molecular clock, regulation of Scn5a, and arrhythmia susceptibility. Am J Physiol Cell Physiol 2013;304:C954–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB.. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 2002;109:307–320. [DOI] [PubMed] [Google Scholar]
- 16. Martino T, Arab S, Straume M, Belsham DD, Tata N, Cai F, Liu P, Trivieri M, Ralph M, Sole MJ.. Day/night rhythms in gene expression of the normal murine heart. J Mol Med 2004;82:256–264. [DOI] [PubMed] [Google Scholar]
- 17. Podobed P, Pyle WG, Ackloo S, Alibhai FJ, Tsimakouridze EV, Ratcliffe WF, Mackay A, Simpson J, Wright DC, Kirby GM, Young ME, Martino TA.. The day/night proteome in the murine heart. Am J Physiol Regul Integr Comp Physiol 2014;307:R121–R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ.. Extensive and divergent circadian gene expression in liver and heart. Nature 2002;417:78–83. [DOI] [PubMed] [Google Scholar]
- 19. Tong M, Watanabe E, Yamamoto N, Nagahata-Ishiguro M, Maemura K, Takeda N, Nagai R, Ozaki Y.. Circadian expressions of cardiac ion channel genes in mouse might be associated with the central clock in the SCN but not the peripheral clock in the heart. Biol Rhythm Res 2013;44:519–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Young ME, Razeghi P, Taegtmeyer H.. Clock genes in the heart: characterization and attenuation with hypertrophy. Circ Res 2001;88:1142–1150. [DOI] [PubMed] [Google Scholar]
- 21. Yu EA, Weaver DR.. Disrupting the circadian clock: gene-specific effects on aging, cancer, and other phenotypes. Aging 2011;3:479–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bray MS, Shaw CA, Moore MW, Garcia RA, Zanquetta MM, Durgan DJ, Jeong WJ, Tsai JY, Bugger H, Zhang D, Rohrwasser A, Rennison JH, Dyck JR, Litwin SE, Hardin PE, Chow CW, Chandler MP, Abel ED, Young ME.. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am J Physiol Heart Circ Physiol 2008;294:H1036–H1047. [DOI] [PubMed] [Google Scholar]
- 23. Durgan DJ, Tsai JY, Grenett MH, Pat BM, Ratcliffe WF, Villegas-Montoya C, Garvey ME, Nagendran J, Dyck JR, Bray MS, Gamble KL, Gimble JM, Young ME.. Evidence suggesting that the cardiomyocyte circadian clock modulates responsiveness of the heart to hypertrophic stimuli in mice. Chronobiol Int 2011;28:187–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ingle KA, Kain V, Goel M, Prabhu SD, Young ME, Halade GV.. Cardiomyocyte-specific Bmal1 deletion in mice triggers diastolic dysfunction, extracellular matrix response, and impaired resolution of inflammation. Am J Physiol Heart Circ Physiol 2015;309:H1827–H1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Martino TA, Tata N, Bjarnason GA, Straume M, Sole MJ.. Diurnal protein expression in blood revealed by high throughput mass spectrometry proteomics and implications for translational medicine and body time of day. Am J Physiol Regul Integr Comp Physiol 2007;293:R1430–R1437. [DOI] [PubMed] [Google Scholar]
- 26. Veerman DP, Imholz BP, Wieling W, Wesseling KH, van Montfrans GA.. Circadian profile of systemic hemodynamics. Hypertension 1995;26:55–59. [DOI] [PubMed] [Google Scholar]
- 27. Idema RN, van den Meiracker AH, Balk AH, Bos E, Schalekamp MA, Man In't Veld AJ.. Abnormal diurnal variation of blood pressure, cardiac output, and vascular resistance in cardiac transplant recipients. Circulation 1994;90:2797–2803. [DOI] [PubMed] [Google Scholar]
- 28. Sato R, Mizuno M, Miura T, Kato Y, Watanabe S, Fuwa D, Ogiyama Y, Tomonari T, Ota K, Ichikawa T, Shirasawa Y, Ito A, Yoshida A, Fukuda M, Kimura G.. Angiotensin receptor blockers regulate the synchronization of circadian rhythms in heart rate and blood pressure. J Hypertens 2013;31:1233–1238. [DOI] [PubMed] [Google Scholar]
- 29. Delp MD, Manning RO, Bruckner JV, Armstrong RB.. Distribution of cardiac output during diurnal changes of activity in rats. Am J Physiol 1991;261:H1487–H1493. [DOI] [PubMed] [Google Scholar]
- 30. Young ME, Razeghi P, Cedars AM, Guthrie PH, Taegtmeyer H.. Intrinsic diurnal variations in cardiac metabolism and contractile function. Circ Res 2001;89:1199–1208. [DOI] [PubMed] [Google Scholar]
- 31. Podobed PS, Alibhai FJ, Chow CW, Martino TA.. Circadian regulation of myocardial sarcomeric Titin-cap (Tcap, telethonin): identification of cardiac clock-controlled genes using open access bioinformatics data. PLoS One 2014;9:e104907.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alibhai FJ, Tsimakouridze EV, Chinnappareddy N, Wright DC, Billia F, O’Sullivan ML, Pyle WG, Sole MJ, Martino TA.. Short-term disruption of diurnal rhythms after murine myocardial infarction adversely affects long-term myocardial structure and function. Circ Res 2014;114:1713–1722. [DOI] [PubMed] [Google Scholar]
- 33. Sachan N, Dey A, Rotter D, Grinsfelder DB, Battiprolu PK, Sikder D, Copeland V, Oh M, Bush E, Shelton JM, Bibb JA, Hill JA, Rothermel BA.. Sustained hemodynamic stress disrupts normal circadian rhythms in calcineurin-dependent signaling and protein phosphorylation in the heart. Circ Res 2011;108:437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Collins HE, Rodrigo GC.. Inotropic response of cardiac ventricular myocytes to beta-adrenergic stimulation with isoproterenol exhibits diurnal variation: involvement of nitric oxide. Circ Res 2010;106:1244–1252. [DOI] [PubMed] [Google Scholar]
- 35. Wang ZR, Wang L, Wan CM, Cornelissen G, Anand I, Halberg F.. Circadian rhythm of gene expression of myocardial contractile protein, left ventricular pressure and contractility. Space Med Med Eng (Beijing )1999;12:391–396. [PubMed] [Google Scholar]
- 36. Chatham JC, Young ME.. Regulation of myocardial metabolism by the cardiomyocyte circadian clock. J Mol Cell Cardiol 2013;55:139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Durgan DJ, Moore MW, Ha NP, Egbejimi O, Fields A, Mbawuike U, Egbejimi A, Shaw CA, Bray MS, Nannegari V, Hickson-Bick DL, Heird WC, Dyck JR, Chandler MP, Young ME.. Circadian rhythms in myocardial metabolism and contractile function: influence of workload and oleate. Am J Physiol Heart Circ Physiol 2007;293:H2385–H2393. [DOI] [PubMed] [Google Scholar]
- 38. Durgan DJ, Pat BM, Laczy B, Bradley JA, Tsai JY, Grenett MH, Ratcliffe WF, Brewer RA, Nagendran J, Villegas-Montoya C, Zou C, Zou L, Johnson RL Jr, Dyck JR, Bray MS, Gamble KL, Chatham JC, Young MO.. GlcNAcylation, novel post-translational modification linking myocardial metabolism and cardiomyocyte circadian clock. J Biol Chem 2011;286:44606–44619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tsai JY, Kienesberger PC, Pulinilkunnil T, Sailors MH, Durgan DJ, Villegas-Montoya C, Jahoor A, Gonzalez R, Garvey ME, Boland B, Blasier Z, McElfresh TA, Nannegari V, Chow CW, Heird WC, Chandler MP, Dyck JR, Bray MS, Young ME.. Direct regulation of myocardial triglyceride metabolism by the cardiomyocyte circadian clock. J Biol Chem 2010;285:2918–2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rau E, Meyer DK.. A diurnal rhythm of incorporation of L-[3H] leucine in myocardium of the rat. Recent Adv Stud Cardiac Struct Metab 1975;7:105–110. [PubMed] [Google Scholar]
- 41. Tsai JY, Young ME.. Diurnal variations in myocardial metabolism. Heart Metab 2009;44:5–9. [Google Scholar]
- 42. Kohsaka A, Das P, Hashimoto I, Nakao T, Deguchi Y, Gouraud SS, Waki H, Muragaki Y, Maeda M.. The circadian clock maintains cardiac function by regulating mitochondrial metabolism in mice. PLoS One 2014;9:e112811.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Woldt E, Sebti Y, Solt LA, Duhem C, Lancel S, Eeckhoute J, Hesselink MK, Paquet C, Delhaye S, Shin Y, Kamenecka TM, Schaart G, Lefebvre P, Neviere R, Burris TP, Schrauwen P, Staels B, Duez H.. Rev-erb-alpha modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat Med 2013;19:1039–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bhandari P, Song M, Chen Y, Burelle Y, Dorn GW 2nd. Mitochondrial contagion induced by Parkin deficiency in Drosophila hearts and its containment by suppressing mitofusin. Circ Res 2014;114:257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dhingra R, Margulets V, Chowdhury SR, Thliveris J, Jassal D, Fernyhough P, Dorn GW 2nd, Kirshenbaum LA.. Bnip3 mediates doxorubicin-induced cardiac myocyte necrosis and mortality through changes in mitochondrial signaling. Proc Natl Acad Sci USA 2014;111:E5537–E5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sadoshima J. The role of autophagy during ischemia/reperfusion. Autophagy 2008;4:402–403. [DOI] [PubMed] [Google Scholar]
- 47. Rothermel BA, Hill JA.. Autophagy in load-induced heart disease. Circ Res 2008;103:1363–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell'Orto S, Piccaluga E.. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res 1986;59:178–193. [DOI] [PubMed] [Google Scholar]
- 49. Ahlersova E, Ahlers I, Smajda B, Kassayova M.. The effect of various photoperiods on daily oscillations of serum corticosterone and insulin in rats. Physiol Res 1992;41:315–321. [PubMed] [Google Scholar]
- 50. Jordan D, Rousset B, Perrin F, Fournier M, Orgiazzi J.. Evidence for circadian variations in serum thyrotropin, 3,5,3'-triiodothyronine, and thyroxine in the rat. Endocrinology 1980;107:1245–1248. [DOI] [PubMed] [Google Scholar]
- 51. Fuller RW, Diller ER.. Diurnal variation of liver glycogen and plasma free fatty acids in rats fed ad libitum or single daily meal. Metab Clin Exp 1970;19:226–229. [DOI] [PubMed] [Google Scholar]
- 52. Benavides A, Siches M, Llobera M.. Circadian rhythms of lipoprotein lipase and hepatic lipase activities in intermediate metabolism of adult rat. Am J Physiol 1998;275:R811–817. [DOI] [PubMed] [Google Scholar]
- 53. Durgan DJ, Pulinilkunnil T, Villegas-Montoya C, Garvey ME, Frangogiannis NG, Michael LH, Chow CW, Dyck JR, Young ME.. Short communication: ischemia/reperfusion tolerance is time-of-day-dependent: mediation by the cardiomyocyte circadian clock. Circ Res 2010;106:546–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Powanda MC, Wannemacher RW Jr.. Evidence for a linear correlation between the level of dietary tryptophan and hepatic NAD concentration and for a systematic variation in tissue NAD concentration in the mouse and the rat. J Nutr 1970;100:1471–1478. [DOI] [PubMed] [Google Scholar]
- 55. Degaute JP, van de Borne P, Linkowski P, Van Cauter E.. Quantitative analysis of the 24-hour blood pressure and heart rate patterns in young men. Hypertension 1991;18:199–210. [DOI] [PubMed] [Google Scholar]
- 56. Burgess HJ, Trinder J, Kim Y, Luke D.. Sleep and circadian influences on cardiac autonomic nervous system activity. Am J Physiol 1997;273:H1761–H1768. [DOI] [PubMed] [Google Scholar]
- 57. Hu K, Ivanov P, Hilton MF, Chen Z, Ayers RT, Stanley HE, Shea SA.. Endogenous circadian rhythm in an index of cardiac vulnerability independent of changes in behavior. Proc Natl Acad Sci USA 2004;101:18223–18227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vandewalle G, Middleton B, Rajaratnam SM, Stone BM, Thorleifsdottir B, Arendt J, Dijk DJ.. Robust circadian rhythm in heart rate and its variability: influence of exogenous melatonin and photoperiod. J Sleep Res 2007;16:148–155. [DOI] [PubMed] [Google Scholar]
- 59. Viola AU, Simon C, Ehrhart J, Geny B, Piquard F, Muzet A, Brandenberger G.. Sleep processes exert a predominant influence on the 24-h profile of heart rate variability. J Biol Rhythms 2002;17:539–547. [DOI] [PubMed] [Google Scholar]
- 60. De Scalzi M, De Leonardis V, Calzolari F, Barchielli M, Cinelli P, Chiodi L, Fabiano FS, Vergassola R.. Heart rate and premature beats: a chronobiologic study. G Ital Cardiol 1984;14:465–470. [PubMed] [Google Scholar]
- 61. Guo YF, Stein PK.. Circadian rhythm in the cardiovascular system: chronocardiology. Am Heart J 2003;145:779–786. [DOI] [PubMed] [Google Scholar]
- 62. Linsell CR, Lightman SL, Mullen PE, Brown MJ, Causon RC.. Circadian rhythms of epinephrine and norepinephrine in man. J Clin Endocrinol Metab 1985;60:1210–1215. [DOI] [PubMed] [Google Scholar]
- 63. Dodt C, Breckling U, Derad I, Fehm HL, Born J.. Plasma epinephrine and norepinephrine concentrations of healthy humans associated with nighttime sleep and morning arousal. Hypertension 1997;30:71–76. [DOI] [PubMed] [Google Scholar]
- 64. Somers VK, Dyken ME, Mark AL, Abboud FM.. Sympathetic-nerve activity during sleep in normal subjects. N Engl J Med 1993;328:303–307. [DOI] [PubMed] [Google Scholar]
- 65. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J 1996;17:354–381. [PubMed] [Google Scholar]
- 66. Monfredi O, Lyashkov AE, Johnsen AB, Inada S, Schneider H, Wang R, Nirmalan M, Wisloff U, Maltsev VA, Lakatta EG, Zhang H, Boyett MR.. Biophysical characterization of the underappreciated and important relationship between heart rate variability and heart rate. Hypertension 2014;64:1334–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Boyett MR, D’Souza A, Zhang H, Morris GM, Dobrzynski H, Monfredi O.. Viewpoint: is the resting bradycardia in athletes the result of remodeling of the sinoatrial node rather than high vagal tone? J Appl Physiol (1985) 2013;114:1351–1355. [DOI] [PubMed] [Google Scholar]
- 68. Monfredi O, Nirmalan M, Zhang H, Boyett M. Heart rate variability as a measure of autonomic nerve activity is fundamentally flawed. Heart Rhythm 2013;10:S181. [Google Scholar]
- 69. Billman GE. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front Physiol 2013;4:26.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ko GY, Ko ML, Dryer SE.. Circadian regulation of cGMP-gated cationic channels of chick retinal cones. Erk MAP Kinase and Ca2+/calmodulin-dependent protein kinase II. Neuron 2001;29:255–266. [DOI] [PubMed] [Google Scholar]
- 71. Bigger JT Jr, Steinman RC, Rolnitzky LM, Fleiss JL, Albrecht P, Cohen RJ.. Power law behavior of RR-interval variability in healthy middle-aged persons, patients with recent acute myocardial infarction, and patients with heart transplants. Circulation 1996;93:2142–2151. [DOI] [PubMed] [Google Scholar]
- 72. Mandel Y, Weissman A, Schick R, Barad L, Novak A, Meiry G, Goldberg S, Lorber A, Rosen MR, Itskovitz-Eldor J, Binah O.. Human embryonic and induced pluripotent stem cell-derived cardiomyocytes exhibit beat rate variability and power-law behavior. Circulation 2012;125:883–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kotsis VT, Stabouli SV, Pitiriga V, Lekakis JP, Nanas IN, Toumanidis ST, Zakopoulos NA.. Impact of cardiac transplantation in 24 hours circadian blood pressure and heart rate profile. Transplant Proc 2005;37:2244–2246. [DOI] [PubMed] [Google Scholar]
- 74. Shea SA, Hilton MF, Hu K, Scheer FA.. Existence of an endogenous circadian blood pressure rhythm in humans that peaks in the evening. Circ Res 2011;108:980–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Martino TA, Sole MJ.. Molecular time: an often overlooked dimension to cardiovascular disease. Circ Res 2009;105:1047–1061. [DOI] [PubMed] [Google Scholar]
- 76. Millar-Craig MW, Bishop CN, Raftery EB.. Circadian variation of blood-pressure. Lancet 1978;1:795–797. [DOI] [PubMed] [Google Scholar]
- 77. Harshfield GA, Barbeau P, Richey PA, Alpert BS.. Racial differences in the influence of body size on ambulatory blood pressure in youths. Blood Press Monit 2000;5:59–63. [PubMed] [Google Scholar]
- 78. Kario K, Matsuo T, Kobayashi H, Imiya M, Matsuo M, Shimada K.. Nocturnal fall of blood pressure and silent cerebrovascular damage in elderly hypertensive patients. Advanced silent cerebrovascular damage in extreme dippers. Hypertension 1996;27:130–135. [DOI] [PubMed] [Google Scholar]
- 79. de la Sierra A, Redon J, Banegas JR, Segura J, Parati G, Gorostidi M, de la Cruz JJ, Sobrino J, Llisterri JL, Alonso J, Vinyoles E, Pallarés V, Sarría A, Aranda P, Ruilope LM; Spanish Society of Hypertension Ambulatory Blood Pressure Monitoring Registry Investigators. Prevalence and factors associated with circadian blood pressure patterns in hypertensive patients. Hypertension 2009;53:466–472. [DOI] [PubMed] [Google Scholar]
- 80. Hu K, Scheer FA, Laker M, Smales C, Shea SA.. Endogenous circadian rhythm in vasovagal response to head-up tilt. Circulation 2011;123:961–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Uzu T, Kimura G.. Diuretics shift circadian rhythm of blood pressure from nondipper to dipper in essential hypertension. Circulation 1999;100:1635–1638. [DOI] [PubMed] [Google Scholar]
- 82. Martino TA, Tata N, Belsham DD, Chalmers J, Straume M, Lee P, Pribiag H, Khaper N, Liu PP, Dawood F, Backx PH, Ralph MR, Sole MJ.. Disturbed diurnal rhythm alters gene expression and exacerbates cardiovascular disease with rescue by resynchronization. Hypertension 2007;49:1104–1113. [DOI] [PubMed] [Google Scholar]
- 83. Anea CB, Zhang M, Stepp DW, Simkins GB, Reed G, Fulton DJ, Rudic RD.. Vascular disease in mice with a dysfunctional circadian clock. Circulation 2009;119:1510–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Shang X, Pati P, Anea CB, Fulton DJ, Rudic RD.. Differential regulation of BMAL1, CLOCK, and endothelial signaling in the aortic arch and ligated common carotid artery. J Vasc Res 2016;53:269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Viswambharan H, Carvas JM, Antic V, Marecic A, Jud C, Zaugg CE, Ming XF, Montani JP, Albrecht U, Yang Z.. Mutation of the circadian clock gene Per2 alters vascular endothelial function. Circulation 2007;115:2188–2195. [DOI] [PubMed] [Google Scholar]
- 86. Wang CY, Wen MS, Wang HW, Hsieh IC, Li Y, Liu PY, Lin FC, Liao JK.. Increased vascular senescence and impaired endothelial progenitor cell function mediated by mutation of circadian gene Per2. Circulation 2008;118:2166–2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Boudreau P, Yeh WH, Dumont GA, Boivin DB.. A circadian rhythm in heart rate variability contributes to the increased cardiac sympathovagal response to awakening in the morning. Chronobiol Int 2012;29:757–768. [DOI] [PubMed] [Google Scholar]
- 88. Scheer FA, Hu K, Evoniuk H, Kelly EE, Malhotra A, Hilton MF, Shea SA.. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc Natl Acad Sci USA 2010;107:20541–20546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Scheer FA, Hilton MF, Mantzoros CS, Shea SA.. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA 2009;106:4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Mavroudis PD, Scheff JD, Calvano SE, Lowry SF, Androulakis IP.. Entrainment of peripheral clock genes by cortisol. Physiol Genomics 2012;44:607–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nonell A, Bodenseh S, Lederbogen F, Kopf D, Hamann B, Gilles M, Deuschle M.. Chronic but not acute hydrocortisone treatment shifts the response to an orthostatic challenge towards parasympathetic activity. Neuroendocrinology 2005;81:63–68. [DOI] [PubMed] [Google Scholar]
- 92. Zeman M, Herichova I.. Melatonin and clock genes expression in the cardiovascular system. Front Biosci 2013;5:743–753. [DOI] [PubMed] [Google Scholar]
- 93. Poirel VJ, Boggio V, Dardente H, Pevet P, Masson-Pevet M, Gauer F.. Contrary to other non-photic cues, acute melatonin injection does not induce immediate changes of clock gene mRNA expression in the rat suprachiasmatic nuclei. Neuroscience 2003;120:745–755. [DOI] [PubMed] [Google Scholar]
- 94. Pevet P, Challet E.. Melatonin: both master clock output and internal time-giver in the circadian clocks network. J Physiol Paris 2011;105:170–182. [DOI] [PubMed] [Google Scholar]
- 95. Zeman M, Szantoova K, Stebelova K, Mravec B, Herichova I.. Effect of rhythmic melatonin administration on clock gene expression in the suprachiasmatic nucleus and the heart of hypertensive TGR(mRen2)27 rats. J Hypertens Suppl 2009;27:S21–S26. [DOI] [PubMed] [Google Scholar]
- 96. Scheer FA, Van Montfrans GA, van Someren EJ, Mairuhu G, Buijs RM.. Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension 2004;43:192–197. [DOI] [PubMed] [Google Scholar]
- 97. Khaper N, Bailey CDC, Ghugre NR, Reitz C, Awosanmi Z, Waines R, Martino TA.. Implications of disturbances in circadian rhythms for cardiovascular health: a new frontier in free radical biology. Free Radic Biol Med 2018;119:85–92. [DOI] [PubMed] [Google Scholar]
- 98. Dominguez-Rodriguez A, Abreu GP, Avanzas P.. The role of melatonin in acute myocardial infarction. Front Biosci 2012;17:2433–2441. [DOI] [PubMed] [Google Scholar]
- 99. Lapenna D, De Gioia S, Mezzetti A, Porreca E, Ciofani G, Marzio L, Capani F, Di Ilio C, Cuccurullo F.. Circadian variations in antioxidant defences and lipid peroxidation in the rat heart. Free Radic Res Commun 1992;17:187–194. [DOI] [PubMed] [Google Scholar]
- 100. Kawasaki T, Uezono K, Ueno M, Omae T, Matsuoka M, Haus E, Halberg F.. Comparison of circadian rhythms of the renin-angiotensin-aldosterone system and electrolytes in clinically healthy young women in Fukuoka (Japan) and Minnesota (USA). Acta Endocrinol (Copenh) 1983;102:246–251. [DOI] [PubMed] [Google Scholar]
- 101. Cugini P, Halberg F, Sothern RB, Centanni M, Salandi E, Scavo D.. Sodium restriction amplifies and propranolol loading inhibits circadian rhythm of plasma renin-angiotensin and aldosterone. Chronobiologia 1985;12:155–165. [PubMed] [Google Scholar]
- 102. Goetze JP, Georg B, Jorgensen HL, Fahrenkrug J.. Chamber-dependent circadian expression of cardiac natriuretic peptides. Regul Pept 2010;160:140–145. [DOI] [PubMed] [Google Scholar]
- 103. Cannon CP, McCabe CH, Stone PH, Schactman M, Thompson B, Theroux P, Gibson RS, Feldman T, Kleiman NS, Tofler GH, Muller JE, Chaitman BR, Braunwald E.. Circadian variation in the onset of unstable angina and non-Q-wave acute myocardial infarction (the TIMI III Registry and TIMI IIIB). Am J Cardiol 1997;79:253–258. [DOI] [PubMed] [Google Scholar]
- 104. Henriques JP, Haasdijk AP, Zijlstra F, Zwolle MISG.. Outcome of primary angioplasty for acute myocardial infarction during routine duty hours versus during off-hours. J Am Coll Cardiol 2003;41:2138–2142. [DOI] [PubMed] [Google Scholar]
- 105. Assali AR, Brosh D, Vaknin-Assa H, Fuchs S, Teplitsky I, Sela O, Kornowski R.. The impact of circadian variation on outcomes in emergency acute anterior myocardial infarction percutaneous coronary intervention. Cathet Cardiovasc Intervent 2006;67:221–226. [DOI] [PubMed] [Google Scholar]
- 106. Leiza JR, de Llano JM, Messa JB, Lopez CA, Fernandez JA, Group AS.. New insights into the circadian rhythm of acute myocardial infarction in subgroups. Chronobiol Int 2007;24:129–141. [DOI] [PubMed] [Google Scholar]
- 107. Muller JE, Stone PH, Turi ZG, Rutherford JD, Czeisler CA, Parker C, Poole WK, Passamani E, Roberts R, Robertson T.. Circadian variation in the frequency of onset of acute myocardial infarction. N Engl J Med 1985;313:1315–1322. [DOI] [PubMed] [Google Scholar]
- 108. Casetta I, Granieri E, Fallica E, la Cecilia O, Paolino E, Manfredini R.. Patient demographic and clinical features and circadian variation in onset of ischemic stroke. Arch Neurol 2002;59:48–53. [DOI] [PubMed] [Google Scholar]
- 109. Muller JE, Ludmer PL, Willich SN, Tofler GH, Aylmer G, Klangos I, Stone PH.. Circadian variation in the frequency of sudden cardiac death. Circulation 1987;75:131–138. [DOI] [PubMed] [Google Scholar]
- 110. Otto ME, Svatikova A, Barretto RB, Santos S, Hoffmann M, Khandheria B, Somers V.. Early morning attenuation of endothelial function in healthy humans. Circulation 2004;109:2507–2510. [DOI] [PubMed] [Google Scholar]
- 111. el-Tamimi H, Mansour M, Pepine CJ, Wargovich TJ, Chen H.. Circadian variation in coronary tone in patients with stable angina. Protective role of the endothelium. Circulation 1995;92:3201–3205. [DOI] [PubMed] [Google Scholar]
- 112. Scheer FA, Michelson AD, Frelinger AL 3rd, Evoniuk H, Kelly EE, McCarthy M, Doamekpor LA, Barnard MR, Shea SA.. The human endogenous circadian system causes greatest platelet activation during the biological morning independent of behaviors. PLoS One 2011;6:e24549.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. O’Neill JS, Reddy AB.. Circadian clocks in human red blood cells. Nature 2011;469:498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Tomita J, Nakajima M, Kondo T, Iwasaki H.. No transcription-translation feedback in circadian rhythm of KaiC phosphorylation. Science 2005;307:251–254. [DOI] [PubMed] [Google Scholar]
- 115. Lakin-Thomas PL. Transcriptional feedback oscillators: maybe, maybe not. J Biol Rhythms 2006;21:83–92. [DOI] [PubMed] [Google Scholar]
- 116. Kapiotis S, Jilma B, Quehenberger P, Ruzicka K, Handler S, Speiser W.. Morning hypercoagulability and hypofibrinolysis. Diurnal variations in circulating activated factor VII, prothrombin fragment F1 + 2, and plasmin-plasmin inhibitor complex. Circulation 1997;96:19–21. [DOI] [PubMed] [Google Scholar]
- 117. Scheer FA, Shea SA.. Human circadian system causes a morning peak in prothrombotic plasminogen activator inhibitor-1 (PAI-1) independent of the sleep/wake cycle. Blood 2014;123:590–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ohkura N, Oishi K, Sudo T, Hayashi H, Shikata K, Ishida N, Matsuda J, Horie S.. CLOCK regulates circadian platelet activity. Thromb Res 2009;123:523–527. [DOI] [PubMed] [Google Scholar]
- 119. Somanath PR, Podrez EA, Chen J, Ma Y, Marchant K, Antoch M, Byzova TV.. Deficiency in core circadian protein Bmal1 is associated with a prothrombotic and vascular phenotype. J Cell Physiol 2011;226:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Zhao Y, Zhang Y, Wang S, Hua Z, Zhang J.. The clock gene Per2 is required for normal platelet formation and function. Thromb Res 2011;127:122–130. [DOI] [PubMed] [Google Scholar]
- 121. Boyett MR, Honjo H, Kodama I.. The sinoatrial node, a heterogeneous pacemaker structure. Cardiovasc Res 2000;47:658–687. [DOI] [PubMed] [Google Scholar]
- 122. Monfredi O, Tsutsui K, Ziman B, Stern MD, Lakatta EG, Maltsev VA.. Electrophysiological heterogeneity of pacemaker cells in the rabbit intercaval region, including the SA node: insights from recording multiple ion currents in each cell. Am J Physiol Heart Circ Physiol 2018;314:H403–H414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Lakatta EG, Maltsev VA, Vinogradova TA.. Coupled system of intracellular Ca2+ clocks and surface membrane voltage clocks controls the timekeeping mechanism of the heart's pacemaker. Circ Res 2010;106:659–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Yamashita T, Sekiguchi A, Iwasaki YK, Sagara K, Iinuma H, Hatano S, Fu LT, Watanabe H.. Circadian variation of cardiac K+ channel gene expression. Circulation 2003;107:1917–1922. [DOI] [PubMed] [Google Scholar]
- 125. Jeyaraj D, Haldar SM, Wan X, McCauley MD, Ripperger JA, Hu K, Lu Y, Eapen BL, Sharma N, Ficker E, Cutler MJ, Gulick J, Sanbe A, Robbins J, Demolombe S, Kondratov RV, Shea SA, Albrecht U, Wehrens XH, Rosenbaum DS, Jain MK.. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature 2012;483:96–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Schroder EA, Burgess DE, Zhang X, Lefta M, Smith JL, Patwardhan A, Bartos DC, Elayi CS, Esser KA, Delisle BP.. The cardiomyocyte molecular clock regulates the circadian expression of Kcnh2 and contributes to ventricular repolarization. Heart Rhythm 2015;12:1306–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Du Pre BC, Van Laake LW, Meine M, Van der Heijden JF, Doevendans PA, Vos MA, Van Veen TAB.. Analysis of 24-h rhythm in ventricular repolarization identifies QT diurnality as a novel clinical parameter associated with previous ventricular arrhythmias in heart failure patients. Front Physiol 2017;8:590.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Monfredi O, Maltseva LA, Spurgeon HA, Boyett MR, Lakatta EG, Maltsev VA.. Beat-to-beat variation in periodicity of local calcium releases contributes to intrinsic variations of spontaneous cycle length in isolated single sinoatrial node cells. PLoS One 2013;8:e67247.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Colwell CS. Circadian modulation of calcium levels in cells in the suprachiasmatic nucleus. Eur J Neurosci 2000;12:571–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Pennartz CM, de Jeu MT, Bos NP, Schaap J, Geurtsen AM.. Diurnal modulation of pacemaker potentials and calcium current in the mammalian circadian clock. Nature 2002;416:286–290. [DOI] [PubMed] [Google Scholar]
- 131. Ikeda M. Calcium dynamics and circadian rhythms in suprachiasmatic nucleus neurons. Neuroscientist 2004;10:315–324. [DOI] [PubMed] [Google Scholar]
- 132. Ko ML, Shi L, Grushin K, Nigussie F, Ko GY.. Circadian profiles in the embryonic chick heart: L-type voltage-gated calcium channels and signaling pathways. Chronobiol Int 2010;27:1673–1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Ko ML, Shi L, Tsai JY, Young ME, Neuendorff N, Earnest DJ, Ko GY.. Cardiac-specific mutation of Clock alters the quantitative measurements of physical activities without changing behavioral circadian rhythms. J Biol Rhythms 2011;26:412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Chen Y, Zhu D, Yuan J, Han Z, Wang Y, Qian Z, Hou X, Wu T, Zou J.. CLOCK-BMAL1 regulate the cardiac L-type calcium channel subunit CACNA1C through PI3K-Akt signaling pathway. Can J Physiol Pharmacol 2016;94:1023–1032. [DOI] [PubMed] [Google Scholar]
- 135. Yaniv Y, Ahmet I, Liu J, Lyashkov AE, Guiriba TR, Okamoto Y, Ziman BD, Lakatta EG.. Synchronization of sinoatrial node pacemaker cell clocks and its autonomic modulation impart complexity to heart beating intervals. Heart Rhythm 2014;11:1210–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Zaza A, Robinson RB, DiFrancesco D.. Basal responses of the L-type Ca2+ and hyperpolarization-activated currents to autonomic agonists in the rabbit sino-atrial node. J Physiol 1996;491:347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Rocchetti M, Malfatto G, Lombardi F, Zaza A.. Role of the input/output relation of sinoatrial myocytes in cholinergic modulation of heart rate variability. J Cardiovasc Electrophysiol 2000;11:522–530. [DOI] [PubMed] [Google Scholar]
- 138. Zaza A, Lombardi F.. Autonomic indexes based on the analysis of heart rate variability: a view from the sinus node. Cardiovasc Res 2001;50:434–442. [DOI] [PubMed] [Google Scholar]
- 139. Finley JP, Nugent ST.. Heart rate variability in infants, children and young adults. J Auton Nerv Syst 1995;51:103–108. [DOI] [PubMed] [Google Scholar]
- 140. Korkushko OV, Shatilo VB, Plachinda Yu I, Shatilo TV.. Autonomic control of cardiac chronotropic function in man as a function of age: assessment by power spectral analysis of heart rate variability. J Auton Nerv Syst 1991;32:191–198. [DOI] [PubMed] [Google Scholar]
- 141. O’Brien IA, O’Hare P, Corrall RJ.. Heart rate variability in healthy subjects: effect of age and the derivation of normal ranges for tests of autonomic function. Br Heart J 1986;55:348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Shannon DC, Carley DW, Benson H.. Aging of modulation of heart rate. Am J Physiol 1987;253:H874–H877. [DOI] [PubMed] [Google Scholar]
- 143. Tsuji H, Venditti FJ Jr, Manders ES, Evans JC, Larson MG, Feldman CL, Levy D.. Determinants of heart rate variability. J Am Coll Cardiol 1996;28:1539–1546. [DOI] [PubMed] [Google Scholar]
- 144. Burger AJ, Charlamb M, Sherman HB.. Circadian patterns of heart rate variability in normals, chronic stable angina and diabetes mellitus. Int J Cardiol 1999;71:41–48. [DOI] [PubMed] [Google Scholar]
- 145. Hellman JB, Stacy RW.. Variation of respiratory sinus arrhythmia with age. J Appl Physiol 1976;41:734–738. [DOI] [PubMed] [Google Scholar]
- 146. Lipsitz LA, Mietus J, Moody GB, Goldberger AL.. Spectral characteristics of heart rate variability before and during postural tilt. Relations to aging and risk of syncope. Circulation 1990;81:1803–1810. [DOI] [PubMed] [Google Scholar]
- 147. Bigger JT Jr, Fleiss JL, Steinman RC, Rolnitzky LM, Schneider WJ, Stein PK.. RR variability in healthy, middle-aged persons compared with patients with chronic coronary heart disease or recent acute myocardial infarction. Circulation 1995;91:1936–1943. [DOI] [PubMed] [Google Scholar]
- 148. Iyengar N, Peng CK, Morin R, Goldberger AL, Lipsitz LA.. Age-related alterations in the fractal scaling of cardiac interbeat interval dynamics. Am J Physiol 1996;271:R1078–1084. [DOI] [PubMed] [Google Scholar]
- 149. Ryan SM, Goldberger AL, Pincus SM, Mietus J, Lipsitz LA.. Gender- and age-related differences in heart rate dynamics: are women more complex than men? J Am Coll Cardiol 1994;24:1700–1707. [DOI] [PubMed] [Google Scholar]
- 150. Parvaneh S, Howe CL, Toosizadeh N, Honarvar B, Slepian MJ, Fain M, Mohler J, Najafi B.. Regulation of cardiac autonomic nervous system control across frailty statuses: a systematic review. Gerontology 2015;62:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Kaplan DT, Furman MI, Pincus SM, Ryan SM, Lipsitz LA, Goldberger AL.. Aging and the complexity of cardiovascular dynamics. Biophys J 1991;59:945–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Lipsitz LA, Goldberger AL.. Loss of ‘complexity’ and aging. Potential applications of fractals and chaos theory to senescence. JAMA 1992;267:1806–1809. [PubMed] [Google Scholar]
- 153. Santulli G, Iaccarino G.. Pinpointing beta adrenergic receptor in ageing pathophysiology: victim or executioner? Evidence from crime scenes. Immun Ageing 2013;10:10.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. White M, Roden R, Minobe W, Khan MF, Larrabee P, Wollmering M, Port JD, Anderson F, Campbell D, Feldman AM.. Age-related changes in beta-adrenergic neuroeffector systems in the human heart. Circulation 1994;90:1225–1238. [DOI] [PubMed] [Google Scholar]
- 155. Ziegler MG, Lake CR, Kopin IJ.. Plasma noradrenaline increases with age. Nature 1976;261:333–335. [DOI] [PubMed] [Google Scholar]
- 156. Xiao RP, Lakatta EG.. Deterioration of beta-adrenergic modulation of cardiovascular function with aging. Ann N Y Acad Sci 1992;673:293–310. [DOI] [PubMed] [Google Scholar]
- 157. Sammito S, Sammito W, Bockelmann I.. The circadian rhythm of heart rate variability. Biol Rhythm Res 2016;47:717–730. [Google Scholar]
- 158. Prinz PN, Halter J, Benedetti C, Raskind M.. Circadian variation of plasma catecholamines in young and old men: relation to rapid eye movement and slow wave sleep. J Clin Endocrinol Metab 1979;49:300–304. [DOI] [PubMed] [Google Scholar]
- 159. Wennerblom B, Lurje L, Karlsson T, Tygesen H, Vahisalo R, Hjalmarson A.. Circadian variation of heart rate variability and the rate of autonomic change in the morning hours in healthy subjects and angina patients. Int J Cardiol 2001;79:61–69. [DOI] [PubMed] [Google Scholar]
- 160. D’Negri CE, Marelich L, Vigo D, Acunzo RS, Girotti LA, Cardinali DP, Siri LN.. Circadian periodicity of heart rate variability in hospitalized angor patients. Clin Auton Res 2005;15:223–232. [DOI] [PubMed] [Google Scholar]
- 161. Korpelainen JT, Sotaniemi KA, Huikuri HV, Myllyla VV.. Circadian rhythm of heart rate variability is reversibly abolished in ischemic stroke. Stroke 1997;28:2150–2154. [DOI] [PubMed] [Google Scholar]
- 162. Boudoulas KD, Borer JS, Boudoulas H.. Heart rate, life expectancy and the cardiovascular system: therapeutic considerations. Cardiology 2015;132:199–212. [DOI] [PubMed] [Google Scholar]
- 163. Reid KJ, Zee PC.. Circadian rhythm disorders. Semin Neurol 2009;29:393–405. [DOI] [PubMed] [Google Scholar]
- 164. Knutsson A, Akerstedt T, Jonsson BG, Orth-Gomer K.. Increased risk of ischaemic heart disease in shift workers. Lancet 1986;2:89–92. [DOI] [PubMed] [Google Scholar]
- 165. Harma MI, Ilmarinen JE.. Towards the 24-hour society—new approaches for aging shift workers? Scand J Work Environ Health 1999;25:610–615. [DOI] [PubMed] [Google Scholar]
- 166. Boggild H, Knutsson A.. Shift work, risk factors and cardiovascular disease. Scand J Work Environ Health 1999;25:85–99. [DOI] [PubMed] [Google Scholar]
- 167. Vyas MV, Garg AX, Iansavichus AV, Costella J, Donner A, Laugsand LE, Janszky I, Mrkobrada M, Parraga G, Hackam DG.. Shift work and vascular events: systematic review and meta-analysis. BMJ 2012;345:e4800.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Williams C. Work-life balance of shift workers. Perspectives 2008;5–16. [Google Scholar]
- 169. Bassiouny HS, Zarins CK, Lee DC, Skelly CL, Fortunato JE, Glagov S.. Diurnal heart rate reactivity: a predictor of severity of experimental coronary and carotid atherosclerosis. J Cardiovasc Risk 2002;9:331–338. [DOI] [PubMed] [Google Scholar]
- 170. Matsuo K, Kurita T, Inagaki M, Kakishita M, Aihara N, Shimizu W, Taguchi A, Suyama K, Kamakura S, Shimomura K.. The circadian pattern of the development of ventricular fibrillation in patients with Brugada syndrome. Eur Heart J 1999;20:465–470. [DOI] [PubMed] [Google Scholar]
- 171. Kong TQ Jr, Goldberger JJ, Parker M, Wang T, Kadish AH.. Circadian variation in human ventricular refractoriness. Circulation 1995;92:1507–1516. [DOI] [PubMed] [Google Scholar]
- 172. Cinca J, Moya A, Figueras J, Roma F, Rius J.. Circadian variations in the electrical properties of the human heart assessed by sequential bedside electrophysiologic testing. Am Heart J 1986;112:315–321. [DOI] [PubMed] [Google Scholar]
- 173. Hayano J, Sakata S, Okada A, Mukai S, Fujinami T.. Circadian rhythms of atrioventricular conduction properties in chronic atrial fibrillation with and without heart failure. J Am Coll Cardiol 1998;31:158–166. [DOI] [PubMed] [Google Scholar]
- 174. Mitsuoka T, Ueyama C, Matsumoto Y, Hashiba K.. Influences of autonomic changes on the sinus node recovery time in patients with sick sinus syndrome. Jpn Heart J 1990;31:645–660. [DOI] [PubMed] [Google Scholar]
- 175. Bexton RS, Vallin HO, Camm AJ.. Diurnal variation of the QT interval–influence of the autonomic nervous system. Br Heart J 1986;55:253–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Costantini O, Drabek C, Rosenbaum DS.. Can sudden cardiac death be predicted from the T wave of the ECG? A critical examination of T wave alternans and QT interval dispersion. Pacing Clin Electro 2000;23:1407–1416. [DOI] [PubMed] [Google Scholar]
- 177. Breithardt G, Cain ME, el-Sherif N, Flowers NC, Hombach V, Janse M, Simson MB, Steinbeck G.. Standards for analysis of ventricular late potentials using high-resolution or signal-averaged electrocardiography: a statement by a task force committee of the European Society of Cardiology, the American Heart Association, and the American College of Cardiology. J Am Coll Cardiol 1991;17:999–1006. [DOI] [PubMed] [Google Scholar]
- 178. Steinbigler P, Haberl R, Jilge G, Steinbeck G.. Circadian variability of late potential analysis in Holter electrocardiograms. Pacing Clin Electrophysiol 1999;22:1448–1456. [DOI] [PubMed] [Google Scholar]
- 179. Cruz Filho FE, Maia IG, Fagundes ML, Barbosa RC, Alves PA, Sa RM, Boghossian SH, Ribeiro JC.. Electrical behavior of T-wave polarity alternans in patients with congenital long QT syndrome. J Am Coll Cardiol 2000;36:167–173. [DOI] [PubMed] [Google Scholar]
- 180. Muller JE, Tofler GH, Stone PH.. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation 1989;79:733–743. [DOI] [PubMed] [Google Scholar]
- 181. Mukamal KJ, Muller JE, Maclure M, Sherwood JB, Mittleman MA.. Increased risk of congestive heart failure among infarctions with nighttime onset. Am Heart J 2000;140:438–442. [DOI] [PubMed] [Google Scholar]
- 182. Carson PA, O’Connor CM, Miller AB, Anderson S, Belkin R, Neuberg GW, Wertheimer JH, Frid D, Cropp A, Packer M.. Circadian rhythm and sudden death in heart failure: results from Prospective Randomized Amlodipine Survival Trial. J Am Coll Cardiol 2000;36:541–546. [DOI] [PubMed] [Google Scholar]
- 183. Krantz DS, Kop WJ, Gabbay FH, Rozanski A, Barnard M, Klein J, Pardo Y, Gottdiener JS.. Circadian variation of ambulatory myocardial ischemia. Triggering by daily activities and evidence for an endogenous circadian component. Circulation 1996;93:1364–1371. [DOI] [PubMed] [Google Scholar]
- 184. Cohen MC, Rohtla KM, Lavery CE, Muller JE, Mittleman MA.. Meta-analysis of the morning excess of acute myocardial infarction and sudden cardiac death. Am J Cardiol 1997;79:1512–1516. [DOI] [PubMed] [Google Scholar]
- 185. Mistry P, Duong A, Kirshenbaum L, Martino TA.. Cardiac clocks and preclinical translation. Heart Fail Clin 2017;13:657–672. [DOI] [PubMed] [Google Scholar]
- 186. Bennardo M, Alibhai F, Tsimakouridze E, Chinnappareddy N, Podobed P, Reitz C, Pyle WG, Simpson J, Martino TA.. Day-night dependence of gene expression and inflammatory responses in the remodeling murine heart post-myocardial infarction. Am J Physiol Regul Integr Comp Physiol 2016;311:R1243–R1254. [DOI] [PubMed] [Google Scholar]
- 187. Salas RE, Gamaldo CE.. Adverse effects of sleep deprivation in the ICU. Crit Care Clin 2008;24:461–476, v-vi. [DOI] [PubMed] [Google Scholar]
- 188. Hardin KA. Sleep in the ICU: potential mechanisms and clinical implications. Chest 2009;136:284–294. [DOI] [PubMed] [Google Scholar]
- 189. Mohri T, Emoto N, Nonaka H, Fukuya H, Yagita K, Okamura H, Yokoyama M.. Alterations of circadian expressions of clock genes in Dahl salt-sensitive rats fed a high-salt diet. Hypertension 2003;42:189–194. [DOI] [PubMed] [Google Scholar]
- 190. Durgan DJ, Trexler NA, Egbejimi O, McElfresh TA, Suk HY, Petterson LE, Shaw CA, Hardin PE, Bray MS, Chandler MP, Chow CW, Young ME.. The circadian clock within the cardiomyocyte is essential for responsiveness of the heart to fatty acids. J Biol Chem 2006;281:24254–24269. [DOI] [PubMed] [Google Scholar]
- 191. Young ME, Wilson CR, Razeghi P, Guthrie PH, Taegtmeyer H.. Alterations of the circadian clock in the heart by streptozotocin-induced diabetes. J Mol Cell Cardiol 2002;34:223–231. [DOI] [PubMed] [Google Scholar]
- 192. Kung TA, Egbejimi O, Cui J, Ha NP, Durgan DJ, Essop MF, Bray MS, Shaw CA, Hardin PE, Stanley WC, Young ME.. Rapid attenuation of circadian clock gene oscillations in the rat heart following ischemia-reperfusion. J Mol Cell Cardiol 2007;43:744–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Esquirol Y, Perret B, Ruidavets JB, Marquie JC, Dienne E, Niezborala M, Ferrieres J.. Shift work and cardiovascular risk factors: new knowledge from the past decade. Arch Cardiovasc Dis 2011;104:636–668. [DOI] [PubMed] [Google Scholar]
- 194. Martino TA, Oudit GY, Herzenberg AM, Tata N, Koletar MM, Kabir GM, Belsham DD, Backx PH, Ralph MR, Sole MJ.. Circadian rhythm disorganization produces profound cardiovascular and renal disease in hamsters. Am J Physiol Regul Integr Comp Physiol 2008;294:R1675–R1683. [DOI] [PubMed] [Google Scholar]
- 195. Harma M, Knauth P, Ilmarinen J, Ollila H.. The relation of age to the adjustment of the circadian rhythms of oral temperature and sleepiness to shift work. Chronobiol Int 1990;7:227–233. [DOI] [PubMed] [Google Scholar]
- 196. Harma MI, Hakola T, Akerstedt T, Laitinen JT.. Age and adjustment to night work. Occup Environ Med 1994;51:568–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Minors DS, Waterhouse JM.. Separating the endogenous and exogenous components of the circadian rhythm of body temperature during night work using some ‘purification’ models. Ergonomics 1993;36:497–507. [DOI] [PubMed] [Google Scholar]
- 198. Atkinson G, Witte K, Nold G, Sasse U, Lemmer B.. Effects of age on circadian blood pressure and heart rate rhythms in patients with primary hypertension. Chronobiol Int 1994;11:35–44. [DOI] [PubMed] [Google Scholar]
- 199. Acharya DU, Heber ME, Dore CJ, Raftery EB.. Ambulatory intraarterial blood pressure in essential hypertension. Effects of age, sex, race, and body mass—the Northwick Park Hospital Database Study. Am J Hypertens 1996;9:943–952. [DOI] [PubMed] [Google Scholar]
- 200. Yaniv Y, Ahmet I, Tsutsui K, Behar J, Moen JM, Okamoto Y, Guiriba TR, Liu J, Bychkov R, Lakatta EG.. Deterioration of autonomic neuronal receptor signaling and mechanisms intrinsic to heart pacemaker cells contribute to age-associated alterations in heart rate variability in vivo. Aging Cell 2016;15:716–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Stein PK, Kleiger RE, Rottman JN.. Differing effects of age on heart rate variability in men and women. Am J Cardiol 1997;80:302–305. [DOI] [PubMed] [Google Scholar]
- 202. Yamasaki Y, Kodama M, Matsuhisa M, Kishimoto M, Ozaki H, Tani A, Ueda N, Ishida Y, Kamada T.. Diurnal heart rate variability in healthy subjects: effects of aging and sex difference. Am J Physiol 1996;271:H303–H310. [DOI] [PubMed] [Google Scholar]
- 203. Shimizu K, Hirose N, Yonemoto T, Wakida Y.. Circadian heart rate rhythms in Japanese centenarians. J Am Geriatr Soc 1999;47:1094–1099. [DOI] [PubMed] [Google Scholar]
- 204. Alibhai FJ, Reitz CJ, Peppler WT, Basu P, Sheppard P, Choleris E, Bakovic M, Martino TA.. Female ClockDelta19/Delta19 mice are protected from the development of age-dependent cardiomyopathy. Cardiovasc Res 2018;114:259–271. [DOI] [PubMed] [Google Scholar]
- 205. Lakatta EG. Beyond Bowditch: the convergence of cardiac chronotropy and inotropy. Cell Calcium 2004;35:629–642. [DOI] [PubMed] [Google Scholar]
- 206. Reinberg A, Smolensky M, Levi F.. Aspects of clinical chronopharmacology. Cephalalgia 1983;3(Suppl. 1):69–78. [DOI] [PubMed] [Google Scholar]
- 207. Reinberg A, Gervais P, Chaussade M, Fraboulet G, Duburque B.. Circadian changes in effectiveness of corticosteroids in eight patients with allergic asthma. J Allergy Clin Immunol 1983;71:425–433. [DOI] [PubMed] [Google Scholar]
- 208. Tsimakouridze EV, Alibhai FJ, Martino TA.. Therapeutic applications of circadian rhythms for the cardiovascular system. Front Pharmacol 2015;6:77.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Martino TA, Tata N, Simpson JA, Vanderlaan R, Dawood F, Kabir MG, Khaper N, Cifelli C, Podobed P, Liu PP, Husain M, Heximer S, Backx PH, Sole MJ.. The primary benefits of angiotensin-converting enzyme inhibition on cardiac remodeling occur during sleep time in murine pressure overload hypertrophy. J Am Coll Cardiol 2011;57:2020–2028. [DOI] [PubMed] [Google Scholar]
- 210. Manfredini R, Fabbian F.. A pill at bedtime, and your heart is fine? Bedtime hypertension chronotherapy: an opportune and advantageous inexpensive treatment strategy. Sleep Med Rev 2017;33:1–3. [DOI] [PubMed] [Google Scholar]
- 211. Bonten TN, Saris A, van Oostrom MJ, Snoep JD, Rosendaal FR, Zwaginga J, Eikenboom J, van der Meer PF, van der Bom JG.. Effect of aspirin intake at bedtime versus on awakening on circadian rhythm of platelet reactivity. A randomised cross-over trial. Thromb Haemost 2014;112:1209–1218. [DOI] [PubMed] [Google Scholar]
- 212. Chan CT, Floras JS, Miller JA, Richardson RM, Pierratos A.. Regression of left ventricular hypertrophy after conversion to nocturnal hemodialysis. Kidney Int 2002;61:2235–2239. [DOI] [PubMed] [Google Scholar]
- 213. Ayas NT, Hirsch AA, Laher I, Bradley TD, Malhotra A, Polotsky VY, Tasali E.. New frontiers in obstructive sleep apnoea. Clin Sci 2014;127:209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Smith DH, Neutel JM, Weber MA.. A new chronotherapeutic oral drug absorption system for verapamil optimizes blood pressure control in the morning. Am J Hypertens 2001;14:14–19. [DOI] [PubMed] [Google Scholar]
- 215. Halberg F, Cornelissen G, Wang Z, Wan C, Ulmer W, Katinas G, Singh R, Singh RK, Singh RK, Gupta BD, Singh RB, Kumar A, Kanabrocki E, Sothern RB, Rao G, Bhatt ML, Srivastava M, Rai G, Singh S, Pati AK, Nath P, Halberg F, Halberg J, Schwartzkopff O, Bakken E; Governor Shri Vishnu Kant Shastri. Chronomics: circadian and circaseptan timing of radiotherapy, drugs, calories, perhaps nutriceuticals and beyond. J Exp Therapeutics 2003;3:223–260. [DOI] [PubMed] [Google Scholar]
- 216. Singh RB, Cornelissen G, Weydahl A, Schwartzkopff O, Katinas G, Otsuka K, Watanabe Y, Yano S, Mori H, Ichimaru Y, Mitsutake G, Pella D, Fanghong L, Zhao Z, Rao RS, Gvozdjakova A, Halberg F.. Circadian heart rate and blood pressure variability considered for research and patient care. Int J Cardiol 2003;87:9–28; discussion 29-30. [DOI] [PubMed] [Google Scholar]
- 217. Buxton OM, Ellenbogen JM, Wang W, Carballeira A, O’Connor S, Cooper D, Gordhandas AJ, McKinney SM, Solet JM.. Sleep disruption due to hospital noises. Ann Intern Med 2012;157:170–179. [DOI] [PubMed] [Google Scholar]
- 218. Drouot X, Cabello B, d’Ortho MP, Brochard L.. Sleep in the intensive care unit. Sleep Med Rev 2008;12:391–403. [DOI] [PubMed] [Google Scholar]
- 219. Reitz CJ, Martino TA.. Disruption of circadian rhythms and sleep on critical illness and the impact on cardiovascular events. Curr Pharm Des 2015;21:3505–3511. [DOI] [PubMed] [Google Scholar]
- 220. Crnko S, Ernens I, Van Laake LW.. New dimensions in circadian clock function: the role of biological sex. Cardiovasc Res 2018;114:203–204. [DOI] [PubMed] [Google Scholar]
- 221. Kuehn BM. The heart’s circadian rhythms point to potential treatment strategies. Circulation 2016;134:1907–1908. [DOI] [PubMed] [Google Scholar]
- 222. Monfredi O, Maltsev VA, Lakatta EG.. Modern concepts concerning the origin of the heartbeat. Physiology (Bethesda) 2013;28:74–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Kondo K, Matsubara T, Nakamura J, Hotta N.. Characteristic patterns of circadian variation in plasma catecholamine levels, blood pressure and heart rate variability in type 2 diabetic patients. Diabet Med 2002;19:359–365. [DOI] [PubMed] [Google Scholar]
- 224. Gunther A, Witte OW, Hoyer D.. Autonomic dysfunction and risk stratification assessed from heart rate pattern. Open Neurol J 2010;4:39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]