Abstract
Aims
Efficacy of aspirin in primary prevention of cardiovascular disease (CVD) may be influenced by a common allele in guanylate cyclase GUCY1A3, which has been shown to modify platelet function and increase CVD risk.
Methods and results
We investigated whether homozygotes of the GUCY1A3 rs7692387 risk (G) allele benefited from aspirin in two long-term, randomized placebo-controlled trials of aspirin in primary CVD prevention: the Women’s Genome Health Study (WGHS, N = 23 294) and a myocardial infarction (MI, N = 550) and stroke (N = 382) case–control set from the Physician’s Health Study (PHS, N = 22 071). Bleeding risk was evaluated in the WGHS. In the placebo group of the WGHS, the GUCY1A3 risk (G) allele was confirmed to increase CVD risk [hazard ratio 1.38; 95% confidence interval (CI) 1.08–1.78; P = 0.01]. Random-effects meta-analysis of the WGHS and PHS revealed that aspirin reduced CVD events among risk allele homozygotes [G/G: odds ratio (OR) 0.79; 95% CI 0.65–0.97; P = 0.03] but increased CVD events among non-risk allele carriers (e.g. G/A: OR 1.39; 95% CI 1.03–1.87; P = 0.03) thus implying an interaction between genotype stratum and aspirin intake (Pinteraction = 0.01). Bleeding associated with aspirin increased in all genotype groups, with higher risks in heterozygotes.
Conclusion
In two randomized placebo-controlled trials in the setting of primary prevention, aspirin reduced the incidence of CVD events in individuals homozygous for the GUCY1A3 risk (G) allele, whereas heterozygote individuals had more events when taking aspirin.
Keywords: Coronary artery disease risk gene, GUCY1A3, Guanylate cyclase, rs7692387, Aspirin, Primary prevention
Introduction
Platelet inhibition with aspirin is a cornerstone for preventing recurrent ischaemic cardiovascular disease (CVD) events, but its use in primary prevention is controversial.1 Aspirin irreversibly acetylates platelet cyclooxygenase-1 (COX-1) which inhibits formation of thromboxane A2 and, thereby, blocks platelet activation and aggregation.2 Inherent to its pharmacological mechanism, aspirin also slightly increases the risk of extracranial bleeding events and haemorrhagic stroke.3 While the benefit-to-risk-ratio in patients who have experienced ischaemic stroke or myocardial infarction (MI) favours aspirin treatment,4 a meta-analysis of primary prevention trials in healthy individuals found the decrease of ischaemic events to be only twice the increase in bleeding events.5 Thus, because of uncertain net benefit, the US Preventive Task Force guidelines limited use of low-dose aspirin in primary prevention to adults, aged 50–59 years with a 10% or higher 10-year risk of CVD, who were not at increased risk for bleeding.6 Nonetheless, millions of Americans and Europeans use aspirin in this indication.7
GUCY1A3 (according to a newly introduced nomenclature GUCY1A1)—together with GUCY1B3 (GUCY1B1)—forms soluble guanylyl cyclase (sGC), an enzyme which, upon stimulation with nitric oxide, inhibits platelet aggregation.8 Recent genome-wide association studies identified common variants in GUCY1A3 that increased risk for coronary artery disease (CAD)/MI and impaired platelet inhibition upon nitric oxide stimulation.8–15
It is not known whether the GUCY1A3 risk allele also influences aspirin therapy outcomes. As homozygous carriers of the GUCY1A3 rs7692387 risk (G) allele, who represent ∼63% of a Western European population,16 are relatively less sensitive to platelet inhibition in response to the natural inhibitor nitric oxide,8,13 we hypothesized that these individuals might uniquely benefit from aspirin in primary prevention. Thus, we examined whether GUCY1A3 genotype rs7692387 interacts with randomized aspirin treatment in subsets from two large randomized aspirin trials, the Women’s Genome Health Study (WGHS)17 and an MI18 and stroke19 case–control set from Physician’s Health Study (PHS).20
Methods
Participants, clinical measurements, and outcomes
The WGHS17 is a large subset of the Women’s Health Study (WHS), including 23 294 women of verified European ancestry. Detailed study methods were previously published17,21 and are available in the Supplemental Material online. In brief, the WHS was a randomized, placebo-controlled trial which examined the effect of aspirin (100 mg) and/or vitamin E (600 IU) on primary CVD and cancer prevention over 10 years among 39 876 initially healthy female health-care professionals ≥45 years at baseline.
Two nested case–control studies including individuals of European ancestry from PHS were used to validate WGHS findings. In PHS, 22 071 male physicians aged 40–84 and free from known MI, stroke, transient ischaemic attack, or cancer were randomized to aspirin (50 mg) or beta-carotene (325 mg) using a 2 × 2 factorial design to test CVD and cancer primary prevention.22 As randomization was terminated in 1988 and study participants were allowed to continue regular aspirin use irrespective of treatment allocation, we focused on the randomization period, i.e. until 1988. Detailed study methods were previously published.18–20
Genotyping
Genotyping in WGHS was performed twice using DNA extracted from baseline blood samples. The original genotyping used Human-Hap300 Duo ‘+’ (Illumina, San Diego, CA, USA) with Infinium II protocol.17 This dataset included 23 294 WGHS participants of self-reported European ancestry confirmed by PLINK multi-dimensional scaling.23 This genotyping array did not include rs7692387 which was imputed as maximum likelihood allele dose from HumanHap300 experimental genotype using minimac324 and Haplotype Reference Consortium reference panel.25 Independently, 22 618 (97.1%) WGHS samples were genotyped using exome array (v.1.1) (Illumina, San Diego, CA, USA) which included rs7692387.26,27 For rs7692387, correspondence between exome array and maximum likelihood allele dose was high (R2 = 99.8%). Experimentally determined genotypes were used for primary analysis while imputed data provided technical replication. For further corroboration, imputed GUCY1A3 variants rs13139571 and rs7688323 in linkage disequilibrium with rs7692387 were also examined. Baseline demographics and CVD risk factors did not differ between exome array and whole WGHS European ancestry datasets (all P > 0.1).
Of the 595 PHS cases reported in the original trial,20 our analysis was limited to those whose samples were available for genotyping, were successfully genotyped for the lead single nucleotide polymorphism (SNP) rs7692387, and self-reported their ancestry as European, N = 409. For PHS, rs7692387, rs13139571, and rs7688323 TaqMan SNP genotyping assays from Applied Biosystems (Foster City, CA, USA) were carried out on Applied Biosystems 7900HT instrument, using SDS version 2.4 software. Genotyping was successful in 95%, 95%, and 92% of all samples for rs7692387, rs7688323, and rs13139571, respectively. All three SNPs were consistent with Hardy–Weinberg Equilibrium among controls (all P > 0.05).28
Statistical analysis
GUCY1A3 CAD lead SNP rs7692387 was the primary SNP in all analyses. For estimates of aspirin effects in WGHS and PHS, the two arms with aspirin were combined and labelled ‘Aspirin’ and compared to the two arms without aspirin, labelled ‘Placebo’.
In the WGHS, Cox proportional-hazard models were used to evaluate rs7692387 effects on rates of major CVD, stroke, and MI assuming a standard additive (on log scale) genetic model; results are presented here in terms of risk alleles. Major CVD, the primary WGHS outcome, was a composite of MI, stroke, or death from cardiovascular causes. Because the PHS cases used to replicate our findings in WGHS consisted of only stroke and MI cases, we also examined a composite endpoint of WGHS stroke and MI events. This outcome had 96 fewer events than the primary outcome major CVD. Models were adjusted for age and smoking or fully adjusted for cardiovascular risk factors which included age, systolic blood pressure, diastolic pressure, LDL-cholesterol, HDL-cholesterol, body mass index (BMI), family history of MI, family history of diabetes, and smoking. The proportionality assumption was verified for each model. The interaction of SNP with aspirin was tested by inclusion of a term corresponding to the product of SNP genotype (0, 1, or 2) and an indicator variable for aspirin use (0 = placebo/1 = aspirin). P-values for interaction were verified empirically by permutation procedure. For 10 000 iterations, the regressions were performed using genotypes that were resampled at random without replacement. An empirical two-sided P-value was computed as the fraction of the beta-coefficients of the interaction term from the permutations with absolute value greater than the absolute value of the beta-coefficient from the unpermuted data. The effect of randomized aspirin allocation was also examined within genotype strata. Kaplan–Meier curves were generated stratified by genotype determined using exome array. The rs7688323 and rs13139571 were examined in parallel analyses. The threshold for statistical significance for both main and interaction effects was P-value <0.05.
In PHS, conditional logistic regression models based on matching for age and smoking were evaluated to estimate odds ratios (ORs) of cardiovascular events and interaction with aspirin for each genotype assuming additive allele encoding (on the log scale). Associations with major CVD for PHS were examined by combining MI and stroke case/controls sets. Models were adjusted for randomized treatment assignment and either adjusted for age and smoking alone or with cardiovascular risk factors (hypertension history—140/90 mmHg or on antihypertensive medication, cholesterol, diabetes, and BMI). Primary analyses were performed among matched case/control pairs of European ancestry from randomization period (before 25 January 1988). Secondary analyses included additional case/control pairs occurring after this date when participants were allowed to cross-over. Logistic regression models were used to determine ORs of CVD outcomes within separate genotype strata of randomized aspirin or placebo allocation. Because there were so few participants homozygous for the non-risk A-allele, we also conducted secondary analyses combining G/A with A/A participants (A-carriers). The results of these analyses are presented in the Supplementary Material online. Number needed to treat for aspirin was estimated for women in the WGHS where we had over 10 years of follow-up in a population randomized to aspirin treatment. Analyses were conducted using R.
Given that the WGHS and PHS were conducted in populations of uniformly different sex (women vs. men) and a decade apart, we used a random-effects models to estimate the mean effect size and confidence interval (CI) across the populations. Meta-analyses of GUCY1A3 effect estimates from logistic regression age/smoking and fully adjusted models for WGHS and PHS were meta-analysed using Comprehensive Meta-Analysis (Englewood, NJ, USA). Authors K.T.H. and D.I.C. had full access to all the data in the study and take responsibility for its integrity and the data analysis.
Study oversight
Women’s Health Study, WGHS, and PHS analyses were approved by the Institutional Review Board of Brigham and Women’s Hospital, Boston, and performed in accordance with the Declaration of Helsinki.
Results
Baseline and demographics of participants
Demographics and baseline characteristics of the WGHS women and PHS men are presented in Supplementary material online, Tables S1 and S2, respectively. The GUCY1A3 rs7692387 risk (G) allele frequency was 81% in the WGHS and 84% in PHS.
Replication of GUCY1A3 as a cardiovascular disease locus
Consistent with the original studies that identified GUCY1A3 as a CVD risk locus,10,12 but did not include the WGHS (or PHS), in a gene dosage model, the rs7692387 risk (G) allele was associated with higher incidence of major CVD events in the WGHS placebo arm [hazard ratio 1.38; 95% CI 1.08–1.78; P = 0.01, Table 1]. Similar effects were observed with independently imputed rs7692387 genotype and two other GUCY1A3 variants in linkage disequilibrium with rs7692387, rs13139571, and rs7688323 (Supplementary material online, Table S3). Adjustment for a panel of CVD risk factors did not modify these effects (Table 1).
Table 1.
Gene dosage model effects of GUCY1A3 rs7692387 risk (G) allelea on incident cardiovascular disease among Women’s Genome Health Study women randomized to aspirin or placebo
| Aspirin (N = 11 288) |
Placebo (N = 11 329) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Modelb | Outcome | Events | HR (95% CI) | P-value | Events | HR (95% CI) | P-value | P interaction |
| Age-adjusted | Major CVDc | 251 | 0.90 (0.72–1.11) | 0.31 | 253 | 1.38 (1.08–1.78) | 0.01 | 0.01 |
| MI | 104 | 0.84 (0.61–1.16) | 0.29 | 104 | 1.28 (0.87–1.87) | 0.20 | 0.10 | |
| Stroke | 96 | 1.01 (0.71–1.44) | 0.96 | 113 | 1.41 (0.97–2.06) | 0.07 | 0.21 | |
| Fully adjusted | Major CVD | 208 | 0.87 (0.69–1.10) | 0.25 | 210 | 1.39 (1.05–1.84) | 0.02 | 0.02 |
| MI | 89 | 0.82 (0.57–1.18) | 0.28 | 86 | 1.22 (0.80–1.87) | 0.36 | 0.17 | |
| Stroke | 77 | 0.91 (0.62–1.34) | 0.62 | 95 | 1.54 (1.00–2.36) | 0.05 | 0.08 | |
BMI, body mass index; HR, hazard ratio; MI, myocardial infarction.
Allele key: rs7692387 coded (risk allele) = G, reference = A. Genotype data from the exome array, N = 22 617 (see Methods section).
Cox gene dosage models were adjusted for age or fully adjusted for standard cardiovascular risk factors: age, systolic blood pressure, diastolic pressure, LDL-cholesterol, HDL-cholesterol, BMI, family history of MI, family history of diabetes and smoking. Observations with incomplete data were not included in the analyses.
Major CVD, the primary WGHS outcome is a composite of MI, stroke, or death from cardiovascular causes.
Benefit from aspirin in homozygous GUCY1A3 risk (G) allele carriers
In the WGHS, there was a significant interaction effect between rs7692387 and randomized aspirin allocation for incidence of major CVD, such that benefit of aspirin varied depending on the number of rs7692387 risk (G) alleles, Pinteraction = 0.01 (Table 1 and Take home figure). The interaction P-value was verified by empirical permutation procedure (empirical Pinteraction = 0.009). Interaction effects with randomized aspirin allocation were similar for rs13139571 (Pinteraction = 0.04) and rs7688323 (Pinteraction = 0.008) (Supplementary material online, Table S3).
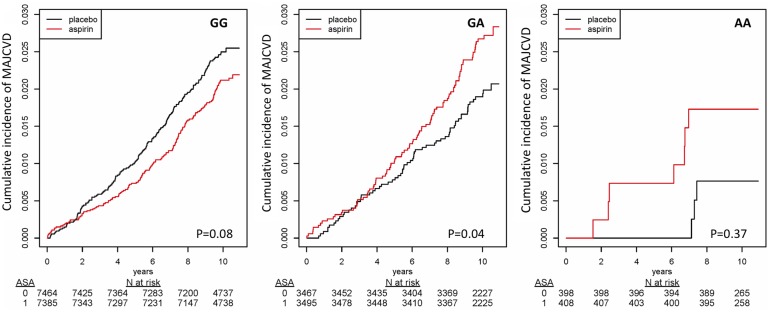
Take home figure.
Kaplan–Meier curves showing cumulative incidence of major cardiovascular disease (MAJCVD) events across the 10 years of the Women’s Genome Health Study (N = 22 617) stratified by randomized aspirin (ASA, red) or placebo (black) allocation and GUCY1A3 rs7692387 genotype: (A) GG stratum; (B) GA stratum; and (C) AA stratum.
In WGHS analyses of rs7692387 genotype strata, among the 65% women with the rs7692387 homozygous risk allele (G/G) genotype, risk of major CVD was lower but non-significant with randomized assignment to aspirin compared to placebo (OR 0.83; 95% CI 0.66–1.03; P = 0.09, Table 3 and Take home figure). In contrast, CVD rates were higher among the 31% of women with the G/A (OR 1.38; 95% CI 1.00–1.91; P = 0.048) or the 4% with the A/A (OR 1.91; 95% CI 0.48–7.55; P = 0.36) genotypes, and A-allele carriers combined (OR 1.39; 95% CI 1.03–1.89; P = 0.03, Supplementary material online, Table S4). Results were similar for a composite endpoint consisting of just stroke and MI events. The direction of the effects for the individual MI and stroke endpoints were similar to that of major CVD but were non-significant (Table 3). Adjustment for a panel of CVD risk factors did not modify these effects.
Table 3.
Combined effect of aspirin compared to placebo on risk of cardiovascular disease, myocardial infarction, and stroke by GUCY1A3 rs7692387 genotype strata in Women’s Genome Health Study and Physician’s Health Studya
| Outcome | Trial | rs7692387 | Events/N cases/controls | OR (95% CI)b | P-value | I2 |
|---|---|---|---|---|---|---|
| CVD | WGHS | G/G | 335/14 849 | 0.83 (0.66–1.03) | 0.09 | |
| PHS | G/G | 154/195 | 0.63 (0.38–1.03) | 0.07 | ||
| Overallc | G/G | 0.79 (0.65–0.97) | 0.03 | 0 | ||
| WGHS | G/A | 99/6962 | 1.38 (1.00–1.91) | 0.05 | ||
| PHS | G/A | 68/81 | 1.42 (0.66–3.05) | 0.37 | ||
| Overall | G/A | 1.39 (1.03–1.87) | 0.03 | 0 | ||
| MI | WGHS | G/G | 137/14 849 | 0.85 (0.61–1.20) | 0.36 | |
| PHS | G/G | 110/136 | 0.58 (0.31–1.05) | 0.07 | ||
| Overall | G/G | 0.77 (0.55–1.07 | 0.12 | 13 | ||
| WGHS | G/A | 64/6962 | 1.28 (0.78–2.11) | 0.33 | ||
| PHS | G/A | 48/53 | 2.24 (0.90–5.59) | 0.08 | ||
| Overall | G/A | 1.48 (0.92–2.38) | 0.11 | 10 | ||
| Stroke | WGHS | G/G | 138/14 849 | 0.74 (0.53–1.04) | 0.08 | |
| PHS | G/G | 44/59 | 0.76 (0.30–1.91) | 0.56 | ||
| Overall | G/G | 0.74 (0.54–1.02) | 0.07 | 0 | ||
| WGHS | G/A | 67/6962 | 1.09 (0.67–1.77) | 0.72 | ||
| PHS | G/A | 20/28 | 0.32 (0.06–1.82) | 0.20 | ||
| Overall | G/A | 0.99 (0.62–1.59) | 0.98 | 46 |
White cases and controls before 25 January 1988.
Odd ratios were estimated from logistic models. Models were adjusted for randomized treatment assignment, age, and smoking.
Random effects meta-analysis.
In PHS, gene dosage response models based on case–control replication sets matched on age and smoking status, and controlled for CVD risk factors, the association of the rs7692387 risk (G) allele with the combined endpoint of MI and stroke (major CVD), was directionally concordant with previously observed increased risk (OR 1.31; 95% CI 0.66–2.61; P = 0.44, Table 2). The interaction between randomized aspirin allocation and rs7692387 genotype was significant for MI (Pinteraction = 0.02), but not significant for major CVD (Pinteraction = 0.06), or stroke (Pinteraction = 0.23). Furthermore, the significant interaction for MI persisted through the post-randomization observational period from 1988 to 1990 (Pinteraction = 0.02).
Table 2.
Gene dosage effects of GUCY1A3 rs7692387 risk (G) allele on incident cardiovascular disease among Physician’s Health Study men randomized to aspirin or placeboa
| Modela | Outcome | Cases/controls | Aspirin OR (95% CI) | P-value | Placebo OR (95% CI) | P-value | P interaction |
|---|---|---|---|---|---|---|---|
| Before 1988b | Major CVD | 228/260 | 0.43 (0.17–1.04) | 0.06 | 1.31 (0.66–2.61) | 0.44 | 0.06 |
| MI | 163/192 | 0.29 (0.09–1.00) | 0.05 | 2.04 (0.91–4.54) | 0.08 | 0.02 | |
| Stroke | 65/68 | 0.46 (0.10–2.23) | 0.34 | 0.08 (0.00–1.26) | 0.07 | 0.23 | |
| Through 1990 | Major CVD | 409/523 | 0.51 (0.28–0.92) | 0.03 | 0.88 (0.52–1.51) | 0.65 | 0.19 |
| MI | 250/300 | 0.36 (0.15–0.88) | 0.02 | 1.42 (0.72–2.77) | 0.31 | 0.02 | |
| Stroke | 159/223 | 0.67 (0.28–1.59) | 0.36 | 0.40 (0.14–1.12) | 0.08 | 0.43 |
Estimates from conditional logistic regression gene dosage models including terms for additive genetic effect (on the log scale) of the ‘G’ risk allele, randomized drug allocation, and interaction between the genetic effect and drug allocation. The genetic effect in aspirin or placebo strata, respectively, was estimated by encoding drug allocation as 0 = placebo/1 = aspirin or 1 = placebo/0 = aspirin. Controls were matched on age and smoking history were used only once.
Models were adjusted for CVD risk factors: history of diabetes, cholesterol, BMI, systolic and diastolic blood pressure. Models: Before 1988 = white cases before 25 January 1988 when randomization to aspirin was terminated; through 1990 = all white cases and controls through 1990 when the trial ended.
In PHS, the direction of the randomized aspirin effects in the genotype strata was directionally consistent with that observed in WGHS (Table 3), with lower risk of major CVD among individuals homozygous for the risk allele (G/G) in fully adjusted analysis (OR 0.49; 95% CI 0.27–0.89; P = 0.02, Supplementary material online, Table S4). A significant association with MI but not with ischaemic stroke appeared to drive the association such that in fully adjusted models, men homozygous for the risk allele (G/G) had lower risk of MI (OR 0.41; 95% CI 0.19–0.87; P = 0.02) with aspirin allocation, while heterozygous individuals (G/A) displayed higher risk (OR 3.28; 95% CI 1.08–9.95; P = 0.04) (data not shown), but the combined effect among A-allele carriers was not significant (OR 2.36; 95% CI 0.85–6.57; P = 0.10). Meaningful effects for MI in carriers of the A/A genotype could not be estimated due to a very small number of cases (n = 13), but the direction was concordant with heterozygous individuals in WGHS. Similar results were observed for the two other GUCY1A3 variants in linkage disequilibrium (Supplementary material online, Table S5).
To evaluate the overall effect of aspirin compared to placebo in both sexes and to increase the statistical power, random-effects meta-analyses of the WGHS and PHS effect estimates in genotype strata were performed and both resulted in a significant major CVD risk reduction in individuals homozygous for the rs7692387 risk allele (OR 0.79; 95% CI 0.65–0.97; P = 0.03; I2 = 0) in age-adjusted models (Table 3). In contrast, in heterozygous individuals, the effect was reversed resulting in significantly increased risk of major CVD with aspirin treatment (OR 1.39; 95% CI 1.03–1.87; P = 0.03; I2 = 0). Results were essentially unchanged when A-allele carriers were combined (OR 1.39; 95% CI 1.03–1.87; P = 0.03; I2 = 0, Supplementary material online, Table S4).
GUCY1A3 genotype and bleeding risk
In the WGHS, rates of a composite endpoint of all bleeding events increased significantly with aspirin use in all rs7692387 genotype groups (‘all bleeds’, Supplementary material online, Table S6). However, with increasing numbers of the CVD risk (G) alleles, ‘all bleeds’ showed weak trends toward decreased bleeding within the aspirin stratum and increased bleeding within the placebo stratum with higher rates of transfusion, gastrointestinal bleeds, and peptic ulcers observed among G/A heterozygotes compared to G/G homozygotes. These divergent trends, although individually non-significant, nevertheless were significantly different for the ‘all bleeds’ (Pinteraction = 0.04) and transfusion (Pinteraction = 0.03) endpoints. Omitting the A-allele carriers yielded similar results with significant interactions for gastrointestinal bleeds (Pinteraction = 0.03) and peptic ulcers (Pinteraction = 0.02).
Discussion
Here, we demonstrate an interaction between the outcome of aspirin therapy and a genetic variant in the GUCY1A3 gene. In two major primary CVD prevention trials, aspirin was found to confer a significant (21%) reduction in risk of major CVD amongst the 66% of participants who were homozygous for the rs7692387 risk (G) allele. Conversely, among the 31% of participants who were heterozygous (G/A), rates of major CVD significantly increased by 39% with randomization to aspirin. The aspirin effects in non-risk allele homozygotes were similar to the heterozygotes, but there were too few participants to estimate the overall effect in this group. Hence, these results suggest a significant benefit of aspirin in primary prevention among homozygotes of the GUCY1A3 CVD risk allele (G), but potential increased risk among carriers of the non-risk allele (A).
Loss-of-function alleles in GUCY1A3 had been found to enhance platelet aggregation and to co-segregate with MI in families.11,29 Our study focused on a common GUCY1A3 rs7692387 risk allele because—in addition to its broadly replicated effects on coronary risk and blood pressure14,15—functional assessment revealed that this variant results in reduced expression of the alpha1-subunit of sGC,13 an enzyme that counterbalances pro-thrombotic stimuli in platelets.30 Due to reduced cGMP-mediated signalling, platelet aggregation in homozygotes of the risk allele is less inhibited by nitric oxide which could lead to greater benefit from antiplatelet treatment in primary prevention.13 Consistent with our hypothesis, in the combined analysis of both studies, homozygous GUCY1A3 risk allele carriers experienced a marked reduction in CVD risk with randomization to aspirin. Unexpectedly, in both studies, individuals who were not homozygous for the GUCY1A3 risk allele displayed increased CVD event rates when treated with aspirin. The biological mechanisms underlying the increase in risk observed in non-risk allele carriers are not known. One hypothesis is that non-risk allele carriers taking aspirin display more pronounced platelet inhibition, and this may in turn increase their risk of bleeding. Indeed, in a recent study we found a lower on-aspirin platelet reactivity in non-risk allele carriers which translated into a lower incidence of cardiovascular death/stent thrombosis within 30 days after coronary stenting.31 Whereas lower on-aspirin platelet reactivity might reduce the risk for ischaemic events in such patients, it could increase risk of bleeding in primary prevention. Our findings of higher rates of transfusion, gastrointestinal bleeding, and peptic ulcers among G/A heterozygotes are consistent with this hypothesis. Bleeding, in particular gastrointestinal bleeding can induce anaemia,32 hypotension, and reduce oxygen-carrying capacity, which are in turn associated with myocardial injury33 and subsequent ischaemic events including MI and stroke.34 Nevertheless, these hypotheses remain untested and further studies should address the exact mechanism and magnitude of aspirin counterbalancing sGC activity depending on the number of GUCY1A3 risk alleles.35,36
In addition to contributing to CVD risk assessment, knowledge of GUCY1A3 genotype might help discriminate low- and intermediate-risk individuals who may—or may not—benefit from aspirin. In the WGHS, where women were free from any CVD at entry, the number of homozygous risk allele carriers to be treated to avoid one major CVD event was 121. Likewise, in the PHS, aspirin completely neutralized the risk increase otherwise seen in subjects homozygous for the risk allele. While benefit of aspirin was accompanied by increased rates in bleeding in WGHS, the rates of bleeding were relatively lower for risk allele homozygotes (Pinteraction = 0.04).
Our study has several limitations. First, this is a post hoc analysis of two randomized clinical trials. Besides the inherent limitations of this approach, both studies were conducted several years ago and may not reflect current prevalence of risk factors and treatments. Nevertheless, both studies are unique and have made major contributions to current guidelines on aspirin use in primary prevention. Second, measures of sGC availability and activity or platelet function were not available in these studies making it impossible to correlate genotype and outcome to intermediate phenotypes. Third, while effects were similar for both MI and ischaemic stroke in the WGHS, a comparable effect among men in PHS was observed only for MI (and overall CVD) but not stroke, which cannot be explained using the available data and warrants further exploration. Interestingly, the original publication of PHS reported a reduction of incidence of MI but a trend towards increased incidence of stroke with aspirin.20 Fourth, our data apply only to subjects of Western European descent in whom the risk allele was identified and functionally characterized. Fifth, these results have to be regarded as preliminary until validated by prospective trials and, importantly, this study does not question the role of aspirin in secondary prevention of CAD. Finally, whereas the direction of effect was the same, the effect size of the risk allele in the placebo stratum was larger in WGHS than in the published data. This may reflect the specific aspects or homogeneous nature of the WGHS or its prospective design as compared to the clinical case–control samples that dominate the CARDIoGRAMplusC4D meta-analysis.10
Interestingly, overall neutral results of aspirin in primary prevention were recently reported for the ASPREE,37 and ASCEND35 trials, which support reducing use in primary prevention at this time. This is in agreement with the current European guidelines on CVD prevention, in which aspirin is recommended in individuals with established CVD (IA) but not without CVD (IIIA).38 Despite recommendations for limiting aspirin in US and European guidelines, it remains amongst the most extensively used medications for primary prevention of CVD, and is regarded as widely overused in this indication.7 US surveys report that ∼40% of men over 40 years old take aspirin for CVD prevention.39 About 37% of these individuals will carry a GUCY1A3 non-risk allele, suggesting millions of Americans taking aspirin for primary CVD prevention may have a genotype that was found to have increased risk in both the WGHS and PHS. However, individuals taking aspirin for primary prevention should consult their physician if they consider stopping aspirin as this might increase CVD risk.40
In conclusion, our findings present an example of the potential for genetics in precision medicine to differentiate between potential benefit and harm. Prospective, randomized, placebo-controlled trials studies of aspirin stratified by GUCY1A3 genotype will be needed to further evaluate the extent to which aspirin may be useful for reducing incidence of CVD in primary prevention.
Supplementary Material
Acknowledgements
NHLBI-K01HL130625; Harvard DICP CATALYST faculty fellowship; Deutsche Forschungsgemeinschaft, CRC 1123(B02); Corona-Stiftung [S199/10070/2017]; Fondation Leducq [CADgenomics, 12CVD02]; German Federal Ministry of Education and Research-ERA-NET [ERA-CVD: JTC2017_21-040], target validation [BlockCAD: 16GW0198K], e: Med [AbCD-Net: 01ZX1706C, e: AtheroSysMed: 01ZX1313A-2014]; and European Union Seventh Framework Programme FP7/2007–2013 [CVgenes-at-target: HEALTH-F2-2013-601456].
Conflict of interest: none declared.
See page 3393 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz456)
References
- 1. Patrono C. The multifaceted clinical readouts of platelet inhibition by low-dose aspirin. J Am Coll Cardiol 2015;66:74–85. [DOI] [PubMed] [Google Scholar]
- 2. Davi G, Patrono C.. Platelet activation and atherothrombosis. N Engl J Med 2007;357:2482–2494. [DOI] [PubMed] [Google Scholar]
- 3. Guirguis-Blake JM, Evans CV, Senger CA, Rowland MG, O'Connor EA, Whitlock EP. Aspirin for the Primary Prevention of Cardiovascular Events: A Systematic Evidence Review for the US Preventive Services Task Force. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews. Rockville, MD; 2015; Report No.: 13-05195-EF-1.
- 4. Patrono C, Garcia Rodriguez LA, Landolfi R, Baigent C.. Low-dose aspirin for the prevention of atherothrombosis. N Engl J Med 2005;353:2373–2383. [DOI] [PubMed] [Google Scholar]
- 5.Antithrombotic Trialists' (ATT) Collaboration, Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, Buring J, Hennekens C, Kearney P, Meade T, Patrono C, Roncaglioni MC, Zanchetti A.. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet 2009;373:1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bibbins-Domingo K; U.S. Preventive Services Task Force. Aspirin use for the primary prevention of cardiovascular disease and colorectal cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2016;164:836–845. [DOI] [PubMed] [Google Scholar]
- 7. Whitlock EP, Burda BU, Williams SB, Guirguis-Blake JM, Evans CV.. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the U.S. Preventive Services Task Force. Ann Intern Med 2016;164:826–835. [DOI] [PubMed] [Google Scholar]
- 8. Feil R, Kemp-Harper B.. cGMP signalling: from bench to bedside. Conference on cGMP generators, effectors and therapeutic implications. EMBO Rep 2006;7:149–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dichgans M, Malik R, König IR, Rosand J, Clarke R, Gretarsdottir S, Thorleifsson G, Mitchell BD, Assimes TL, Levi C, O'Donnell CJ, Fornage M, Thorsteinsdottir U, Psaty BM, Hengstenberg C, Seshadri S, Erdmann J, Bis JC, Peters A, Boncoraglio GB, März W, Meschia JF, Kathiresan S, Ikram MA, McPherson R, Stefansson K, Sudlow C, Reilly MP, Thompson JR, Sharma P, Hopewell JC, Chambers JC, Watkins H, Rothwell PM, Roberts R, Markus HS, Samani NJ, Farrall M, Schunkert H; METASTROKE Consortium; CARDIoGRAM Consortium; C4D Consortium; International Stroke Genetics Consortium. Shared genetic susceptibility to ischemic stroke and coronary artery disease: a genome-wide analysis of common variants. Stroke 2014;45:24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. CARDIoGRAMplusC4D ConsortiumDeloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR, Ingelsson E, Saleheen D, Erdmann J, Goldstein BA, Stirrups K, König IR, Cazier JB, Johansson A, Hall AS, Lee JY, Willer CJ, Chambers JC, Esko T, Folkersen L, Goel A, Grundberg E, Havulinna AS, Ho WK, Hopewell JC, Eriksson N, Kleber ME, Kristiansson K, Lundmark P, Lyytikäinen LP, Rafelt S, Shungin D, Strawbridge RJ, Thorleifsson G, Tikkanen E, Van Zuydam N, Voight BF, Waite LL, Zhang W, Ziegler A, Absher D, Altshuler D, Balmforth AJ, Barroso I, Braund PS, Burgdorf C, Claudi-Boehm S, Cox D, Dimitriou M, Do RDIAGRAM Consortium; CARDIOGENICS ConsortiumDoney AS, El Mokhtari N, Eriksson P, Fischer K, Fontanillas P, Franco-Cereceda A, Gigante B, Groop L, Gustafsson S, Hager J, Hallmans G, Han BG, Hunt SE, Kang HM, Illig T, Kessler T, Knowles JW, Kolovou G, Kuusisto J, Langenberg C, Langford C, Leander K, Lokki ML, Lundmark A, McCarthy MI, Meisinger C, Melander O, Mihailov E, Maouche S, Morris AD, Müller-Nurasyid MMuTHER ConsortiumNikus K, Peden JF, Rayner NW, Rasheed A, Rosinger S, Rubin D, Rumpf MP, Schäfer A, Sivananthan M, Song C, Stewart AF, Tan ST, Thorgeirsson G, van der Schoot CE, Wagner PJWellcome Trust Case Control ConsortiumWells GA, Wild PS, Yang TP, Amouyel P, Arveiler D, Basart H, Boehnke M, Boerwinkle E, Brambilla P, Cambien F, Cupples AL, de Faire U, Dehghan A, Diemert P, Epstein SE, Evans A, Ferrario MM, Ferrières J, Gauguier D, Go AS, Goodall AH, Gudnason V, Hazen SL, Holm H, Iribarren C, Jang Y, Kähönen M, Kee F, Kim HS, Klopp N, Koenig W, Kratzer W, Kuulasmaa K, Laakso M, Laaksonen R, Lee JY, Lind L, Ouwehand WH, Parish S, Park JE, Pedersen NL, Peters A, Quertermous T, Rader DJ, Salomaa V, Schadt E, Shah SH, Sinisalo J, Stark K, Stefansson K, Trégouët DA, Virtamo J, Wallentin L, Wareham N, Zimmermann ME, Nieminen MS, Hengstenberg C, Sandhu MS, Pastinen T, Syvänen AC, Hovingh GK, Dedoussis G, Franks PW, Lehtimäki T, Metspalu A, Zalloua PA, Siegbahn A, Schreiber S, Ripatti S, Blankenberg SS, Perola M, Clarke R, Boehm BO, O'Donnell C, Reilly MP, März W, Collins R, Kathiresan S, Hamsten A, Kooner JS, Thorsteinsdottir U, Danesh J, Palmer CN, Roberts R, Watkins H, Schunkert H, Samani NJ.. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet 2013;45:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Erdmann J, Stark K, Esslinger UB, Rumpf PM, Koesling D, de Wit C, Kaiser FJ, Braunholz D, Medack A, Fischer M, Zimmermann ME, Tennstedt S, Graf E, Eck S, Aherrahrou Z, Nahrstaedt J, Willenborg C, Bruse P, Brænne I, Nöthen MM, Hofmann P, Braund PS, Mergia E, Reinhard W, Burgdorf C, Schreiber S, Balmforth AJ, Hall AS, Bertram L, Steinhagen-Thiessen E, Li SC, März W, Reilly M, Kathiresan S, McPherson R, Walter UCARDIoGRAMOtt J, Samani NJ, Strom TM, Meitinger T, Hengstenberg C, Schunkert H.. Dysfunctional nitric oxide signalling increases risk of myocardial infarction. Nature 2013;504:432–436. [DOI] [PubMed] [Google Scholar]
- 12. Lu X, Wang L, Chen S, He L, Yang X, Shi Y, Cheng J, Zhang L, Gu CC, Huang J, Wu T, Ma Y, Li J, Cao J, Chen J, Ge D, Fan Z, Li Y, Zhao L, Li H, Zhou X, Chen L, Liu D, Chen J, Duan X, Hao Y, Wang L, Lu F, Liu Z, Yao C, Shen C, Pu X, Yu L, Fang X, Xu L, Mu J, Wu X, Zheng R, Wu N, Zhao Q, Li Y, Liu X, Wang M, Yu D, Hu D, Ji X, Guo D, Sun D, Wang Q, Yang Y, Liu F, Mao Q, Liang X, Ji J, Chen P, Mo X, Li D, Chai G, Tang Y, Li X, Du Z, Liu X, Dou C, Yang Z, Meng Q, Wang D, Wang R, Yang J, Schunkert H, Samani NJ, Kathiresan S, Reilly MP, Erdmann JCoronary ARtery DIsease Genome-Wide Replication And Meta-Analysis (CARDIoGRAM) ConsortiumPeng X, Wu X, Liu D, Yang Y, Chen R, Qiang B, Gu D.. Genome-wide association study in Han Chinese identifies four new susceptibility loci for coronary artery disease. Nat Genet 2012;44:890–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kessler T, Wobst J, Wolf B, Eckhold J, Vilne B, Hollstein R, von Ameln S, Dang TA, Sager HB, Moritz Rumpf P, Aherrahrou R, Kastrati A, Björkegren JLM, Erdmann J, Lusis AJ, Civelek M, Kaiser FJ, Schunkert H.. Functional characterization of the GUCY1A3 coronary artery disease risk locus. Circulation 2017;136:476–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ehret GB, Ferreira T, Chasman DI, Jackson AU, Schmidt EM, Johnson T, Thorleifsson G, Luan J, Donnelly LA, Kanoni S, Petersen AK, Pihur V, Strawbridge RJ, Shungin D, Hughes MF, Meirelles O, Kaakinen M, Bouatia-Naji N, Kristiansson K, Shah S, Kleber ME, Guo X, Lyytikäinen LP, Fava C, Eriksson N, Nolte IM, Magnusson PK, Salfati EL, Rallidis LS, Theusch E, Smith AJP, Folkersen L, Witkowska K, Pers TH, Joehanes R, Kim SK, Lataniotis L, Jansen R, Johnson AD, Warren H, Kim YJ, Zhao W, Wu Y, Tayo BO, Bochud MCHARGE-EchoGen consortium; CHARGE-HF Consortium; Wellcome Trust Case Control ConsortiumAbsher D, Adair LS, Amin N, Arking DE, Axelsson T, Baldassarre D, Balkau B, Bandinelli S, Barnes MR, Barroso I, Bevan S, Bis JC, Bjornsdottir G, Boehnke M, Boerwinkle E, Bonnycastle LL, Boomsma DI, Bornstein SR, Brown MJ, Burnier M, Cabrera CP, Chambers JC, Chang IS, Cheng CY, Chines PS, Chung RH, Collins FS, Connell JM, Döring A, Dallongeville J, Danesh J, de Faire U, Delgado G, Dominiczak AF, Doney ASF, Drenos F, Edkins S, Eicher JD, Elosua R, Enroth S, Erdmann J, Eriksson P, Esko T, Evangelou E, Evans A, Fall T, Farrall M, Felix JF, Ferrières J, Ferrucci L, Fornage M, Forrester T, Franceschini N, Duran OHF, Franco-Cereceda A, Fraser RM, Ganesh SK, Gao H, Gertow K, Gianfagna F, Gigante B, Giulianini F, Goel A, Goodall AH, Goodarzi MO, Gorski M, Gräßler J, Groves C, Gudnason V, Gyllensten U, Hallmans G, Hartikainen AL, Hassinen M, Havulinna AS, Hayward C, Hercberg S, Herzig KH, Hicks AA, Hingorani AD, Hirschhorn JN, Hofman A, Holmen J, Holmen OL, Hottenga JJ, Howard P, Hsiung CA, Hunt SC, Ikram MA, Illig T, Iribarren C, Jensen RA, Kähönen M, Kang H, Kathiresan S, Keating BJ, Khaw KT, Kim YK, Kim E, Kivimaki M, Klopp N, Kolovou G, Komulainen P, Kooner JS, Kosova G, Krauss RM, Kuh D, Kutalik Z, Kuusisto J, Kvaløy K, Lakka TA, Lee NR, Lee IT, Lee WJ, Levy D, Li X, Liang KW, Lin H, Lin L, Lindström J, Lobbens S, Männistö S, Müller G, Müller-Nurasyid M, Mach F, Markus HS, Marouli E, McCarthy MI, McKenzie CA, Meneton P, Menni C, Metspalu A, Mijatovic V, Moilanen L, Montasser ME, Morris AD, Morrison AC, Mulas A, Nagaraja R, Narisu N, Nikus K, O'Donnell CJ, O'Reilly PF, Ong KK, Paccaud F, Palmer CD, Parsa A, Pedersen NL, Penninx BW, Perola M, Peters A, Poulter N, Pramstaller PP, Psaty BM, Quertermous T, Rao DC, Rasheed A, Rayner NWNWR, Renström F, Rettig R, Rice KM, Roberts R, Rose LM, Rossouw J, Samani NJ, Sanna S, Saramies J, Schunkert H, Sebert S, Sheu WH, Shin YA, Sim X, Smit JH, Smith AV, Sosa MX, Spector TD, Stančáková A, Stanton A, Stirrups KE, Stringham HM, Sundstrom J, Swift AJ, Syvänen AC, Tai ES, Tanaka T, Tarasov KV, Teumer A, Thorsteinsdottir U, Tobin MD, Tremoli E, Uitterlinden AG, Uusitupa M, Vaez A, Vaidya D, van Duijn CM, van Iperen EPA, Vasan RS, Verwoert GC, Virtamo J, Vitart V, Voight BF, Vollenweider P, Wagner A, Wain LV, Wareham NJ, Watkins H, Weder AB, Westra HJ, Wilks R, Wilsgaard T, Wilson JF, Wong TY, Yang TP, Yao J, Yengo L, Zhang W, Zhao JH, Zhu X, Bovet P, Cooper RS, Mohlke KL, Saleheen D, Lee JY, Elliott P, Gierman HJ, Willer CJ, Franke L, Hovingh GK, Taylor KD, Dedoussis G, Sever P, Wong A, Lind L, Assimes TL, Njølstad I, Schwarz PE, Langenberg C, Snieder H, Caulfield MJ, Melander O, Laakso M, Saltevo J, Rauramaa R, Tuomilehto J, Ingelsson E, Lehtimäki T, Hveem K, Palmas W, März W, Kumari M, Salomaa V, Chen YI, Rotter JI, Froguel P, Jarvelin MR, Lakatta EG, Kuulasmaa K, Franks PW, Hamsten A, Wichmann HE, Palmer CNA, Stefansson K, Ridker PM, Loos RJF, Chakravarti A, Deloukas P, Morris AP, Newton-Cheh C, Munroe PB.. The genetics of blood pressure regulation and its target organs from association studies in 342,415 individuals. Nat Genet 2016;48:1171–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Emdin CA, Khera AV, Klarin D, Natarajan P, Zekavat SM, Nomura A, Haas M, Aragam K, Ardissino D, Wilson JG, Schunkert H, McPherson R, Watkins H, Elosua R, Bown MJ, Samani NJ, Baber U, Erdmann J, Gormley P, Palotie A, Stitziel NO, Gupta N, Danesh J, Saleheen D, Gabriel S, Kathiresan S.. Phenotypic consequences of a genetic predisposition to enhanced nitric oxide signaling. Circulation 2018;137:222–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yates A, Akanni W, Amode MR, Barrell D, Billis K, Carvalho-Silva D, Cummins C, Clapham P, Fitzgerald S, Gil L, Girón CG, Gordon L, Hourlier T, Hunt SE, Janacek SH, Johnson N, Juettemann T, Keenan S, Lavidas I, Martin FJ, Maurel T, McLaren W, Murphy DN, Nag R, Nuhn M, Parker A, Patricio M, Pignatelli M, Rahtz M, Riat HS, Sheppard D, Taylor K, Thormann A, Vullo A, Wilder SP, Zadissa A, Birney E, Harrow J, Muffato M, Perry E, Ruffier M, Spudich G, Trevanion SJ, Cunningham F, Aken BL, Zerbino DR, Flicek P.. Ensembl 2016. Nucleic Acids Res 2016;44:D710–D716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ridker PM, Chasman DI, Zee RY, Parker A, Rose L, Cook NR, Buring JE; Women's Genome Health Study Working Group. Rationale, design, and methodology of the Women's Genome Health Study: a genome-wide association study of more than 25,000 initially healthy American women. Clin Chem 2008;54:249–255. [DOI] [PubMed] [Google Scholar]
- 18. Zee RY, Lindpaintner K, Struk B, Hennekens CH, Ridker PM.. A prospective evaluation of the CD14 C(-260)T gene polymorphism and the risk of myocardial infarction. Atherosclerosis 2001;154:699–702. [DOI] [PubMed] [Google Scholar]
- 19. Zee RY, Cheng S, Hegener HH, Erlich HA, Ridker PM.. Genetic variants of arachidonate 5-lipoxygenase-activating protein, and risk of incident myocardial infarction and ischemic stroke: a nested case-control approach. Stroke 2006;37:2007–2011. [DOI] [PubMed] [Google Scholar]
- 20.Steering Committee of the Physicians' Health Study Research Group. Final report on the aspirin component of the ongoing Physicians' Health Study. N Engl J Med 1989;321:129–135. [DOI] [PubMed] [Google Scholar]
- 21. Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE.. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005;352:1293–1304. [DOI] [PubMed] [Google Scholar]
- 22. Stampfer MJ, Buring JE, Willett W, Rosner B, Eberlein K, Hennekens CH.. The 2 x 2 factorial design: its application to a randomized trial of aspirin and carotene in U.S. physicians. Stat Med 1985;4:111–116. [DOI] [PubMed] [Google Scholar]
- 23. Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC.. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 2007;81:559–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Das S, Forer L, Schönherr S, Sidore C, Locke AE, Kwong A, Vrieze SI, Chew EY, Levy S, McGue M, Schlessinger D, Stambolian D, Loh PR, Iacono WG, Swaroop A, Scott LJ, Cucca F, Kronenberg F, Boehnke M, Abecasis GR, Fuchsberger C.. Next-generation genotype imputation service and methods. Nat Genet 2016;48:1284–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McCarthy S, Das S, Kretzschmar W, Delaneau O, Wood AR, Teumer A, Kang HM, Fuchsberger C, Danecek P, Sharp K, Luo Y, Sidore C, Kwong A, Timpson N, Koskinen S, Vrieze S, Scott LJ, Zhang H, Mahajan A, Veldink J, Peters U, Pato C, van Duijn CM, Gillies CE, Gandin I, Mezzavilla M, Gilly A, Cocca M, Traglia M, Angius A, Barrett JC, Boomsma D, Branham K, Breen G, Brummett CM, Busonero F, Campbell H, Chan A, Chen S, Chew E, Collins FS, Corbin LJ, Smith GD, Dedoussis G, Dorr M, Farmaki AE, Ferrucci L, Forer L, Fraser RM, Gabriel S, Levy S, Groop L, Harrison T, Hattersley A, Holmen OL, Hveem K, Kretzler M, Lee JC, McGue M, Meitinger T, Melzer D, Min JL, Mohlke KL, Vincent JB, Nauck M, Nickerson D, Palotie A, Pato M, Pirastu N, McInnis M, Richards JB, Sala C, Salomaa V, Schlessinger D, Schoenherr S, Slagboom PE, Small K, Spector T, Stambolian D, Tuke M, Tuomilehto J, Van den Berg LH, Van Rheenen W, Volker U, Wijmenga C, Toniolo D, Zeggini E, Gasparini P, Sampson MG, Wilson JF, Frayling T, de Bakker PI, Swertz MA, McCarroll S, Kooperberg C, Dekker A, Altshuler D, Willer C, Iacono W, Ripatti S, Soranzo N, Walter K, Swaroop A, Cucca F, Anderson CA, Myers RM, Boehnke M, McCarthy MI, Durbin R; Haplotype Reference Consortium. A reference panel of 64,976 haplotypes for genotype imputation. Nat Genet 2016;48:1279–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chu AY, Workalemahu T, Paynter NP, Rose LM, Giulianini F, Tanaka T, Ngwa JSCHARGE Nutrition Working GroupQi Q, Curhan GC, Rimm EB, Hunter DJ, Pasquale LR, Ridker PM, Hu FB, Chasman DI, Qi L; DietGen Consortium. Novel locus including FGF21 is associated with dietary macronutrient intake. Hum Mol Genet 2013;22:1895–1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu C, Kraja AT, Smith JA, Brody JA, Franceschini N, Bis JC, Rice K, Morrison AC, Lu Y, Weiss S, Guo X, Palmas W, Martin LW, Chen YD, Surendran P, Drenos F, Cook JP, Auer PL, Chu AY, Giri A, Zhao W, Jakobsdottir J, Lin LA, Stafford JM, Amin N, Mei H, Yao J, Voorman ACHD Exome+ Consortium; ExomeBP Consortium; GoT2DGenes Consortium; T2D-GENES Consortium Larson MG, Grove ML, Smith AV, Hwang SJ, Chen H, Huan T, Kosova G, Stitziel NO, Kathiresan S, Samani N, Schunkert H, Deloukas PMyocardial Infarction Genetics and CARDIoGRAM Exome ConsortiaLi M, Fuchsberger C, Pattaro C, Gorski MCKDGen ConsortiumKooperberg C, Papanicolaou GJ, Rossouw JE, Faul JD, Kardia SL, Bouchard C, Raffel LJ, Uitterlinden AG, Franco OH, Vasan RS, O'Donnell CJ, Taylor KD, Liu K, Bottinger EP, Gottesman O, Daw EW, Giulianini F, Ganesh S, Salfati E, Harris TB, Launer LJ, Dörr M, Felix SB, Rettig R, Völzke H, Kim E, Lee WJ, Lee IT, Sheu WH, Tsosie KS, Edwards DR, Liu Y, Correa A, Weir DR, Völker U, Ridker PM, Boerwinkle E, Gudnason V, Reiner AP, van Duijn CM, Borecki IB, Edwards TL, Chakravarti A, Rotter JI, Psaty BM, Loos RJ, Fornage M, Ehret GB, Newton-Cheh C, Levy D, Chasman DI.. Meta-analysis identifies common and rare variants influencing blood pressure and overlapping with metabolic trait loci. Nat Genet 2016;48:1162–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rodriguez S, Gaunt TR, Day IN.. Hardy-Weinberg equilibrium testing of biological ascertainment for Mendelian randomization studies. Am J Epidemiol 2009;169:505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wobst J, von Ameln S, Wolf B, Wierer M, Dang TA, Sager HB, Tennstedt S, Hengstenberg C, Koesling D, Friebe A, Braun SL, Erdmann J, Schunkert H, Kessler T.. Stimulators of the soluble guanylyl cyclase: promising functional insights from rare coding atherosclerosis-related GUCY1A3 variants. Basic Res Cardiol 2016;111:51. [DOI] [PubMed] [Google Scholar]
- 30. Moro MA, Russel RJ, Cellek S, Lizasoain I, Su Y, Darley-Usmar VM, Radomski MW, Moncada S.. cGMP mediates the vascular and platelet actions of nitric oxide: confirmation using an inhibitor of the soluble guanylyl cyclase. Proc Natl Acad Sci USA 1996;93:1480–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kessler T, Wolf B, Eriksson N, Kofink D, Mahmoodi BK, Rai H, Tragante V, Åkerblom A, Becker RC, Bernlochner I, Bopp R, James S, Katus HA, Mayer K, Munz M, Nordio F, O’Donoghue ML, Sager HB, Sibbing D, Solakov L, Storey RF, Wobst J, Asselbergs FW, Byrne RA, Erdmann J, Koenig W, Laugwitz KL, ten Berg JM, Wallentin L, Kastrati A, Schunkert H.. Association of the coronary artery disease risk gene GUCY1A3 with ischemic events after coronary intervention. Cardiovasc Res 2019. [DOI] [PubMed] [Google Scholar]
- 32. Westenbrink BD, Alings M, Connolly SJ, Eikelboom J, Ezekowitz MD, Oldgren J, Yang S, Pongue J, Yusuf S, Wallentin L, van Gilst WH.. Anemia predicts thromboembolic events, bleeding complications and mortality in patients with atrial fibrillation: insights from the RE-LY trial. J Thromb Haemost 2015;13:699–707. [DOI] [PubMed] [Google Scholar]
- 33. Bellotto F, Fagiuoli S, Pavei A, Gregory SA, Cati A, Silverj E, Plebani M, Zaninotto M, Mancuso T, Iliceto S.. Anemia and ischemia: myocardial injury in patients with gastrointestinal bleeding. Am J Med 2005;118:548–551. [DOI] [PubMed] [Google Scholar]
- 34. Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S.. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation 2006;114:774–782. [DOI] [PubMed] [Google Scholar]
- 35. ASCEND Study Collaborative GroupBowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, Murphy K, Aung T, Haynes R, Cox J, Murawska A, Young A, Lay M, Chen F, Sammons E, Waters E, Adler A, Bodansky J, Farmer A, McPherson R, Neil A, Simpson D, Peto R, Baigent C, Collins R, Parish S, Armitage J.. Effects of aspirin for primary prevention in persons with diabetes mellitus. N Engl J Med. 2018;379:1529–1539. [DOI] [PubMed] [Google Scholar]
- 36. Gaziano JM, Brotons C, Coppolecchia R, Cricelli C, Darius H, Gorelick PB, Howard G, Pearson TA, Rothwell PM, Ruilope LM, Tendera M, Tognoni G; ARRIVE Executive Committee. Use of aspirin to reduce risk of initial vascular events in patients at moderate risk of cardiovascular disease (ARRIVE): a randomised, double-blind, placebo-controlled trial. Lancet 2018;392:1036–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McNeil JJ, Wolfe R, Woods RL, Tonkin AM, Donnan GA, Nelson MR, Reid CM, Lockery JE, Kirpach B, Storey E, Shah RC, Williamson JD, Margolis KL, Ernst ME, Abhayaratna WP, Stocks N, Fitzgerald SM, Orchard SG, Trevaks RE, Beilin LJ, Johnston CI, Ryan J, Radziszewska B, Jelinek M, Malik M, Eaton CB, Brauer D, Cloud G, Wood EM, Mahady SE, Satterfield S, Grimm R, Murray AM; ASPREE Investigator Group. Effect of aspirin on cardiovascular events and bleeding in the healthy elderly. N Engl J Med 2018;379:1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Løchen ML, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM.. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pignone M, Anderson GK, Binns K, Tilson HH, Weisman SM.. Aspirin use among adults aged 40 and older in the United States: results of a national survey. Am J Prev Med 2007;32:403–407. [DOI] [PubMed] [Google Scholar]
- 40. Sundstrom J, Hedberg J, Thuresson M, Aarskog P, Johannesen KM, Oldgren J.. Low-dose aspirin discontinuation and risk of cardiovascular events: a Swedish nationwide, population-based cohort study. Circulation 2017;136:1183–1192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.