Abstract
Aims
Sex differences in cardiac fibrosis point to the regulatory role of 17β-Estradiol (E2) in cardiac fibroblasts (CF). We, therefore, asked whether male and female CF in rodent and human models are differentially susceptible to E2, and whether this is related to sex-specific activation of estrogen receptor alpha (ERα) and beta (ERβ).
Methods and results
In female rat CF (rCF), 24 h E2-treatment (10−8 M) led to a significant down-regulation of collagen I and III expression, whereas both collagens were up-regulated in male rCF. E2-induced sex-specific collagen regulation was also detected in human CF, indicating that this regulation is conserved across species. Using specific ERα- and ERβ-agonists (10−7 M) for 24 h, we identified ERα as repressive and ERβ as inducing factor in female and male rCF, respectively. In addition, E2-induced ERα phosphorylation at Ser118 only in female rCF, whereas Ser105 phosphorylation of ERβ was exclusively found in male rCF. Further, in female rCF we found both ER bound to the collagen I and III promoters using chromatin immunoprecipitation assays. In contrast, in male rCF only ERβ bound to both promoters. In engineered connective tissues (ECT) from rCF, collagen I and III mRNA were down-regulated in female ECT and up-regulated in male ECT by E2. This was accompanied by an impaired condensation of female ECT, whereas male ECT showed an increased condensation and stiffness upon E2-treatment, analysed by rheological measurements. Finally, we confirmed the E2-effect on both collagens in an in vivo mouse model with ovariectomy for E2 depletion, E2 substitution, and pressure overload by transverse aortic constriction.
Conclusion
The mechanism underlying the sex-specific regulation of collagen I and III in the heart appears to involve E2-mediated differential ERα and ERβ signaling in CFs.
Keywords: Cardiac fibroblasts, Collagen I and III, Sex differences, 17β-Estradiol , Estrogen receptors
1. Introduction
Cardiac fibrosis is a major feature of the pathological response of the diseased heart. It leads to global heart dysfunction and thus contributes to heart failure (HF) progression. Pro-fibrotic mechanisms are characterized by excessive collagen deposition, including an increase in collagen I and III expression and synthesis.1 Cardiac fibroblasts of intra-cardiac origin are considered to be the major cellular mediators of these structural changes1 and are mainly responsible for cardiac fibrosis development.2,3
Sex differences in cardiac fibrosis in patients with aortic stenosis (AS), hypertension, and arteriosclerosis have been reported.4–9 We showed previously that men with AS exhibit significant higher degree of cardiac fibrosis associated with stronger up-regulation of collagen I and III compared with women.5,6,10 In line with clinical data, a mouse model with pressure overload induced myocardial hypertrophy (MH) by transverse aortic constriction (TAC) showed that male mice exhibit significant more cardiac fibrosis and pro-fibrotic gene expression compared with females.11,12 The underlying molecular mechanisms of observed sex differences are multifactorial and not fully understood.13
Evidence points to the contribution of the sex steroid hormone 17β-Estradiol (E2). As for example, E2-supplemented ovariectomized (OVX) rodents showed less cardiac fibrosis after TAC and myocardial infarction compared with their respective controls without E2-treatment.14–16 In the clinic, large-scale clinical trials on hormone replacement therapies revealed conflicting data about the E2-effect on the heart in women.17,18 In men with HF, abnormally low and high E2-levels are a significant predictor of poor prognosis and higher mortality.19 This demonstrates the complexity of the cardiac E2-actions and points to the crucial importance to clarify the cardiac effects and mechanisms of E2 in more detail in both sexes.
E2 predominantly mediates its effects via estrogen receptor alpha (ERα) and beta (ERβ). Recent studies showed that ERα and ERβ impact the development of cardiac fibrosis in mice of both sexes.11,15,20,21 We recently demonstrated that the ERα-agonist 16α-LE2 inhibits cardiac fibrosis after TAC in OVX mice.15 In addition, ERβ deletion in female mice led to an increase in cardiac fibrosis compared with female WT mice, whereas male mice with an ERβ deficiency showed significantly less fibrosis compared with WT male mice after TAC.11 Thus, both ER contribute to the sex-specific development of cardiac fibrosis. In order to therapeutically mimic or block E2 and ER mediated effects on cardiac fibrosis in both sexes, studies which identify the involved cardiac cell type and clarify the underlying mechanisms of E2 and ER action are necessary.
In previous investigations, we analysed the effect of E2 on pro-fibrotic genes in rat cardiac fibroblasts (rCF) isolated from male and female animals. We showed that E2 inhibits matrix metalloproteinase-2 (MMP-2) expression22 and found that E2 significantly decreased collagen I and III in female rCF, but significantly increased collagen I and III expression in male cells.5 However, the underlying mechanisms of E2-mediated sex-specific gene transcription regulation have not been clarified in detail.
We, therefore, aimed to define the role of E2 and ER on sex-specific collagen I and III expression in primary rCF and human CF (hCF) from both sexes. We analysed whether E2-mediated sex-specific collagen I and III regulation is caused by sex-dimorphic ER-activation leading to a divergent ER down-stream signaling. The better understanding of sex-dimorphic E2-signaling may pave the way to develop novel sex-specific strategies to counter cardiac fibrosis.
2. Methods
2.1 Cell culture
CF isolation from 9 to 11-week-old female and male Wistar rats, cultivation and E2-treatment was carried out as previously described.22 hCF were obtained by the outgrowth from heart biopsies (left ventricular septum or atrium) from patients with AS. For more details on CF isolation, cultivation and E2-treatment, see Supplementary material online.
2.2 Engineered connective tissue
Engineered connective tissue (ECT) were derived from rCF of both sexes in the second passage as previously described.23 For more details on ECT preparation, measurement, re-isolation of rCF from ECT, cell count, and FACS, see Supplementary material online.
2.3 Quantitative real-time polymerase chain reaction
Total RNA extraction and quantitative real-time polymerase chain reaction (qPCR) were performed as previously described.22 mRNA content of target genes was normalized to the expression of hypoxanthine-guanine phosphoribosyltransferase (HPRT) in cardiac fibroblasts and ribosomal protein L10 (RPL0) in mouse heart tissue. Primer sequences are listed in the Supplementary material online, Table S1.
2.4 Western blot
Cell extract was isolated using modified RIPA buffer and separated by SDS-polyacrylamide gel electrophoresis as recently described.22 Antibodies used are listed in the Supplementary material online.
2.5 Chromatin immunoprecipitation assays (ChIP)
Analyses were carried out to analyse ER-binding to putative ERE within the rat collagen I (Col1A1) and collagen III (Col3A1) gene promoter as revealed by database searches using MatInspector (www.genomatix.de) and TRANSFAC® (http://www.gene-regulation.com/pub/databases.html). For further details, see Supplementary material online.
2.6 Animal experiments
Experiments were performed in accordance with the guidelines of Charité-Universitaetsmedizin Berlin, approved by the Landesamt für Gesundheit und Soziales (LaGeSo, Berlin, Germany, G0027/11), and followed the ‘Principles of Laboratory Animal Care’ (NIH Publication No. 85-23, revised 2011). Mice were anaesthetized with Ketamine Hydrochloride (80 mg/ml)/Xylazine Hydrochloride (12 mg/mL) solution administered by intraperitoneal injection (1 mg/kg). The animals were treated with Rimadyl (5 mg/kg) for analgesia up to 7 days post-surgery. After 4 weeks of TAC, mice were anaesthetized with isoflurane (1.5%) and killed by cervical dislocation. For further details regarding OVX and TAC surgery, see Supplementary material online.
2.7 Data analysis and statistics
Results are expressed as mean ± standard error of the mean (SEM) from at least three independent experiments carried out in independent duplicates or triplicates. Significant differences were assessed by unpaired Mann–Whitney U-test, one-way ANOVA followed by Dunn’s or Tukey’s multiple comparison test as appropriate, using GraphPad Prism 7.01 software. P-values of ≤0.05 were considered statistically significant.
3. Results
3.1 E2 mediates sex-specific collagen I and III regulation in hCF
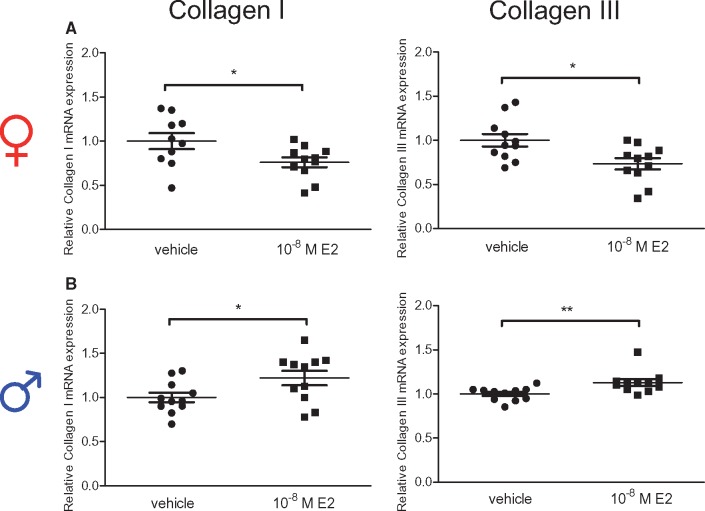
We recently demonstrated on RNA level, that collagen I and III expression underlies a sex-dimorphic regulation in response to E2-treatment in rCF.5 In line with these data, we showed that E2-treatment significantly down-regulated collagen I and III mRNA in hCF isolated from women (Figure 1A), whereas both collagen mRNA levels were up-regulated in response to E2 in male hCF (Figure 1B).
Figure 1.
Collagen I and III mRNA level are regulated in a sex-specific manner by E2 in human cardiac fibroblasts. (A) CF isolated from women and (B) men with AS after 12 h vehicle or 10−8 M E2-treatment. Collagen I and III mRNA level were quantified by qPCR and normalized to HPRT. Collagen I and III expression of E2-treated cells are given as fold induction of vehicle-treated cells. Mean ± SEM from 10 to 11 independent samples derived from four independently repeated experiments; **P ≤ 0.01, *P ≤ 0.05, comparison was performed using Mann–Whitney U-test.
3.2 Collagen I and III protein levels are regulated in a sex-specific manner by 17β-Estradiol in rCF
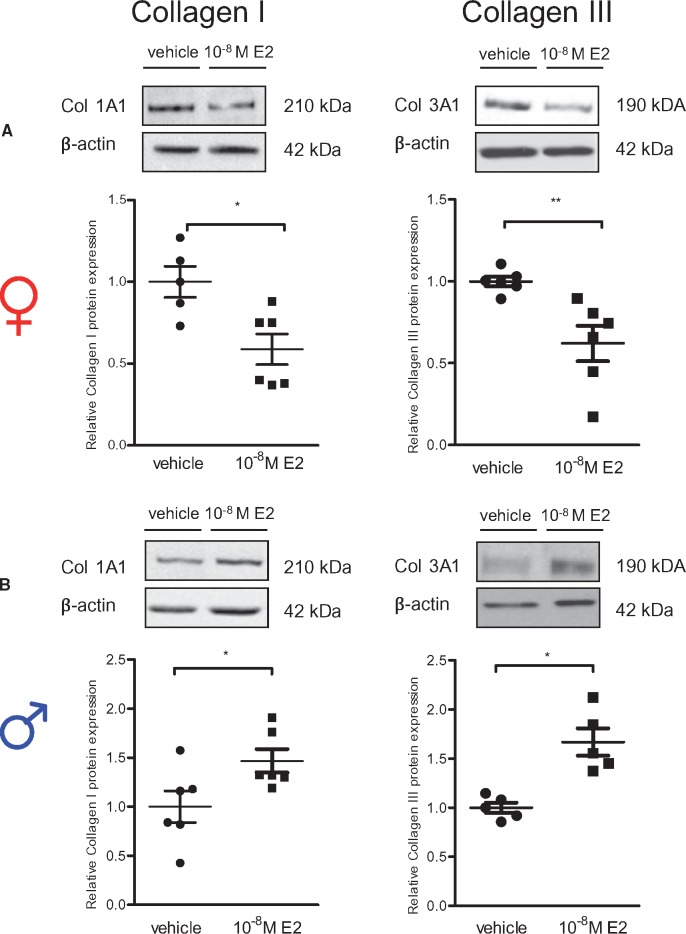
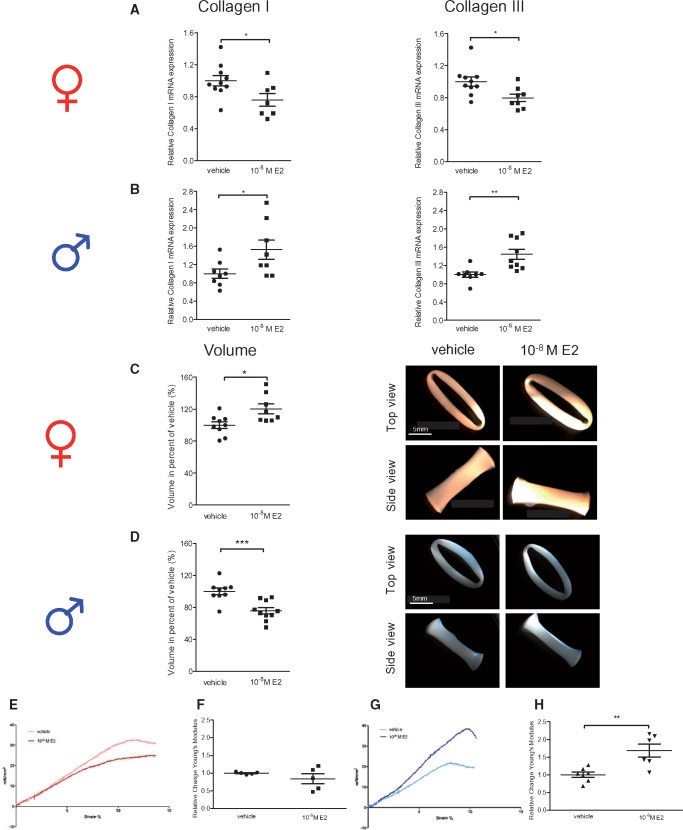
Further, we confirmed that the observed E2-mediated sex-specific collagen I and III mRNA regulation5 led to sex differences in collagen type I and III protein abundance in rCF (Figure 2). After 24 h E2-treatment, collagen I and III protein levels were significantly down-regulated in female rCF (Figure 2A), whereas collagen I and III protein was increased in male rCF (Figure 2B). To ensure that the differences in collagen I and III expression are not simply a result of a difference in cell number, we performed a proliferation analysis. Female rCF proliferated slightly faster; however, neither in female nor in male rCF did E2-treatment have any influence on cell number after 24 h compared with vehicle groups (Supplementary material online, Figure S1).
Figure 2.
Collagen I and III protein level are regulated in a sex-specific manner by E2 in primary rat cardiac fibroblasts. (A) Female and (B) male rCF were analysed after 24 h vehicle or 10−8 M E2-treatment by western blot. Representative immunoblots of collagen I, III, and β-actin are shown. Expression of collagen I and III were quantified and normalized to β-actin. Collagen I and III levels of E2-treated cells are given as fold induction of vehicle-treated cells. Mean ± SEM from five to six independent samples derived from three independently repeated experiments; **P ≤ 0.01, *P ≤ 0.05, comparison was performed using Mann–Whitney U-test.
3.3 ERα mediates collagen I and III down-regulation in female rCF and ERβ up-regulates collagen I and III in male rCF
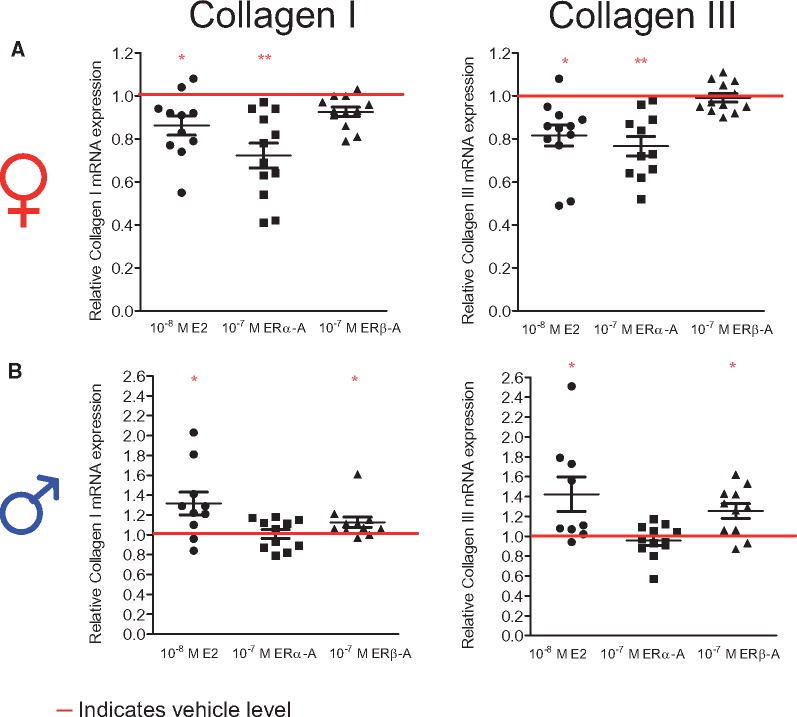
To investigate the underlying mechanisms of the E2-induced sex-specific collagen I and III regulation in female and male rCF in more detail, we first addressed the question of which ER subtype is involved. Therefore, cells of both sexes were treated with specific ERα- and ERβ-agonists and compared with cells treated with E2 and vehicle for 24 h (Figure 3). In female rCF, similar to E2, ERα-agonist treatment led to a significant down-regulation of collagen I and III mRNA levels (Figure 3A), whereas the ERβ-agonist had no effect. In contrast, similar to E2-treatment, the ERβ-agonist significantly increased collagen I and III mRNA level expression in male rCF (Figure 3B). The ERα-agonist showed no effect in male rCF.
Figure 3.
Collagen I and III mRNA level are regulated in a sex-specific manner by sex-dimorphic ERα- and ERβ-activation. (A) Female and (B) male rCF were analysed after 24 h vehicle, 10−8 M E2, 10−7 M ERα- and ERβ-agonist treatment. Collagen I and III mRNA levels were quantified by qPCR and normalized to HPRT. Collagen I and III expression of E2-, ERα-, and ERβ-agonist-treated cells are given as fold induction of vehicle-treated cells (indicated by the horizontal red line). Mean ± SEM from 9 to 12 independent samples derived from four independently repeated experiments; **P ≤ 0.01, *P ≤ 0.05, comparison between treated cells and respective control was performed using Mann–Whitney U-test.
3.4 ERα and ERβ expression differ in female and male rCF
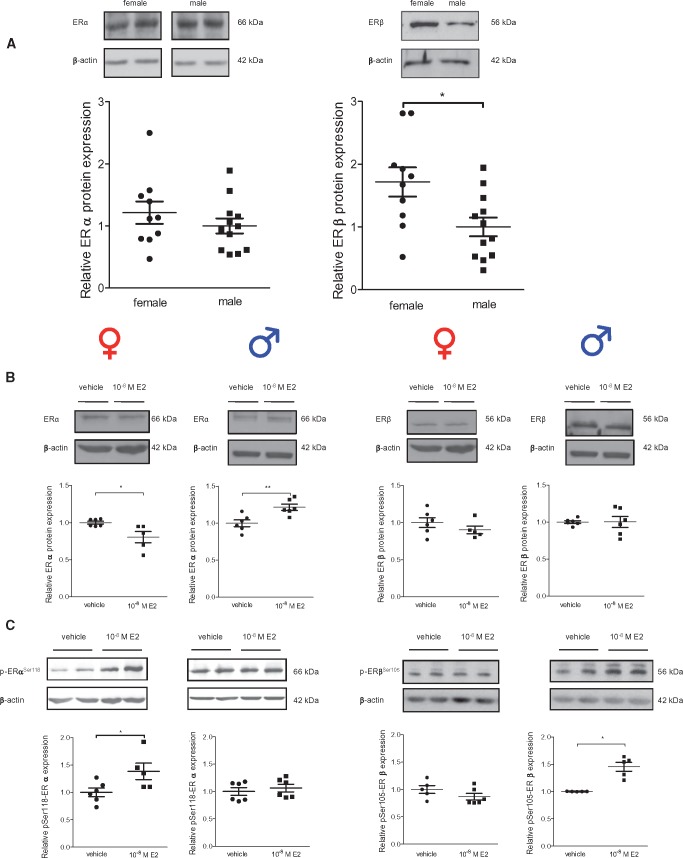
Differences in ER protein levels between female and male rCF could be one possible explanation for sex-specific ER activation by ER agonists. Therefore, we measured ERα and ERβ protein levels in rCF (Figure 4A). Under basal conditions, we found no sex differences for the ERα level, whereas the ERβ level was significantly higher in female compared with male rCF. A 24 h E2-treatment led to a significant down-regulation of ERα in female rCF and an up-regulation in male rCF (Figure 4B). In contrast, E2 had no effect on ERβ expression.
Figure 4.
ERα and ERβ protein and phosphorylation of ERα at serine 118 and ERβ at serine 105 differ between cardiac fibroblasts from male and female rats. (A) ERα and ERβ protein were investigated in non-treated female and male rCF. (B) ERα and ERβ protein was analysed in female and male rCF after 24 h of vehicle or 10−8 M E2-treatment. (C) Phosphorylation level of Ser118 of ERα and (D) of Ser105 of ERβ was analysed in female and male after 24 h of vehicle or 10−8 M E2-treatment. Probes were analysed by western blot and representative immunoblots of ERα ERβ, P-Ser118-ERα, P-Ser105-ERβ, and β-actin are shown. The expression of ER and P-ER were quantified and normalized to β-actin. In non-treated cells (A), relative protein levels of ERα and ERβ in female rCF are given as fold induction of male rCF. Protein and phosphorylation levels of ER of E2-treated cells (B–D) are given as fold induction of vehicle-treated cells. Mean ± SEM; ER comparison between sexes in non-treated rCF: female: n = 10 and male: n = 12; E2-treatment: from five to six independent samples derived from three independently repeated experiments; **P ≤ 0.01 and *P ≤ 0.05 comparison was performed using Mann–Whitney U-test.
3.5 ERα and ERβ phosphorylation in response to E2 differ in female and male rCF
The activities of ERα and ERβ are not only dependent on their expression levels, but also on post-translational modifications by phosphorylation. Serine 118 phosphorylation of ERα (P-Ser118-ERα) and serine 105 phosphorylation of ERβ (P-Ser105-ERβ) had been demonstrated to be essential for nuclear translocation and transcriptional activation upon E2-treatment.24,25 Interestingly, our data show that E2 significantly increased P-Ser118-ERα exclusively in female rCF and P-Ser105-ERβ only in male rCF (Figure 4C), suggesting that E2-treatment leads to a sex-specific activation of ER signaling in rCF.
3.6 ERα and ERβ binding to collagen I and III promoters is sex-specifically regulated
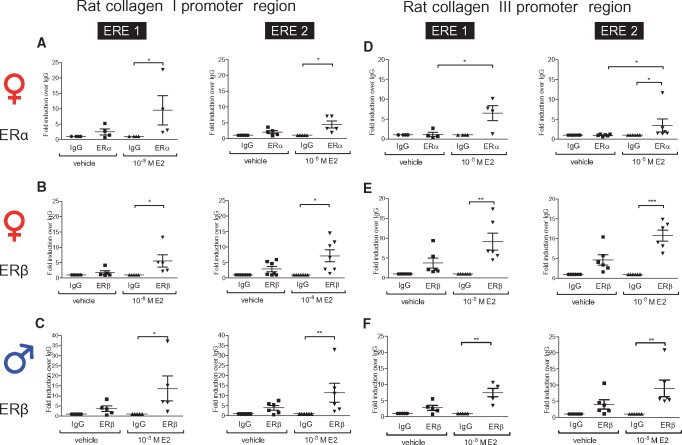
We further tested whether sex-specific E2-mediated ER activation leads to sex-dimorphic binding of ERα and ERβ to the rat collagen I and III gene promoters. In female rCF, E2 significantly induced the binding of ERα and ERβ to two putative ERE (Col1A1-ERE1: −957 bp to −952 bp and Col1A1-ERE2: −415 bp to −400 bp; relative to the translational start site) within the rat collagen I gene promoter (Figure 5A and B). In male cells, only ERβ bound to both putative ERE after E2-treatment (Figure 5C). Similarly, analysis of the collagen III gene promoter showed significant binding of ERα and ERβ to two putative ERE (Col3A1-ERE1: −648 bp to −644 bp and Col3A1-ERE2: −369 bp to −355 bp; relative to the translational start site) after E2-treatment in female rCF (Figure 5D and E). In contrast, only ERβ bound to both ERE upon E2-treatment in male cells (Figure 5F).
Figure 5.
ER bind in a sex-specific manner within the collagen I and III promoter in rat cardiac fibroblasts in the presence of E2. (A–F) ERα and ERβ binding to the rat collagen I and III promoter upon 1 h 10−8 M E2-treatment in rCF was analysed using ChIP assays. (A) Upon E2-treatment, ERα and (B) ERβ bind to the collagen I promoter in female rCF (C), whereas only ERβ binds within the collagen I promoter in male rCF. (D) In female rCF, ERα and (E) ERβ bind within the collagen III promoter, and (F) in male rCF only ERβ binds collagen III promoter upon E2-treatment. Mean ± SEM from four to seven independently repeated experiments per sex. Comparisons between all four groups was performed by one-way ANOVA followed by Dunn's multiple comparison test; *P ≤ 0.05, **P ≤ 0.01, and ***P ≤ 0.001.
3.7 E2 mediates sex differences in three-dimensional engineered connective tissue properties
We next analysed whether E2-mediated sex-specific collagen I and III regulation results in sexually dimorphic tissue properties in a physiological tissue-like environment. Therefore, ECT derived from female and male rCF were generated and exposed to E2 or vehicle (Figure 6).
Figure 6.
E2 modulates collagen I and III mRNA and tissue properties of ECT derived from female and male rat cardiac fibroblasts in a sex-specific manner. (A and C) ECT derived from female and (B and D) male rCF were analysed after 6 days of vehicle or 10−8 M E2-treatment. Collagen I and III mRNA level were quantified by qPCR and normalized to HPRT. Collagen I and III expression and ECT volumes of E2-treated ECT are given as fold induction of vehicle-treated ECT. Mean ± SEM from seven to ten individual ECT derived from four independently repeated experiments; *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, comparison between treated ECT and related vehicle control was performed using Mann–Whitney U-test. (E–H) Rheological destructive tensile strength measurements of ECT derived from female and male rCF after 6 days of vehicle or 10−8 M E2-treatment were performed in an organ bath. Representative stress-strain curves of vehicle and 10−8 M E2-treated (E) female (slope of vehicle treated ECT: 3.27 ± 0.01; E2-treated: 2.65 ± 0.01) and (G) male ECT are shown (slope of vehicle treated ECT: 3.02 ± 0.01; E2-treated ECT: 4.86 ± 0.01). Changes of the Young’s modulus of (F) female and (H) male ECT are shown. Changes in the Young’s modulus of E2-treated ECT are given as fold induction of vehicle-treated ECT. Mean ± SEM from five to seven individual ECT derived from three independently repeated experiments; **P ≤ 0.01, comparison between E2-treated cells and related vehicle control was performed using Mann–Whitney U-test.
In accordance with the 2D cell culture data, E2-treatment significantly inhibited collagen I and III mRNA levels in female ECT (Figure 6A) whereas E2 up-regulated collagen I and III mRNA levels in male ECT (Figure 6B). Additionally, we found a significant increase in female ECT volume upon E2-treatment when compared with the vehicle control (Figure 6C). In contrast, E2 significantly reduced male ECT volume compared with the control (Figure 6D). Further, E2-treatment of female ECT led to no significant change in tissue properties (Figure 6E and F). In contrast, E2-treatment of male ECT increased tissue stiffness (Figure 6G), which was reflected by a higher Young’s modulus (Figure 6H). These data suggest that the sex-specific collagen I and III regulation by E2 in ECT is associated with differences in tissue dimensions and biomechanical properties between female and male ECT. This was not due to changes in collagen I to III ratio. In fact, in all models examined, the ratios between both collagens were found to be constant (Supplementary material online, Figure S2). To further ensure that E2-induced differences in ECT are not due to changes in cell number or proliferation capacity, we performed total cell count and cell cycle analyses of rCF re-isolated from ECT. Neither in female nor in male ECT a significant E2-effect on cell number or cell cycle could be detected compared with vehicle groups (Supplementary material online, Figure S3).
3.8 E2 attenuates collagen I and III mRNA in female mouse hearts
Previous studies reported that E2 attenuates collagen deposition in the diseased female mouse heart.14,15 However, so far it has not been shown whether this is associated with E2-mediated modulation of collagen I and III mRNA level. Therefore, we tested the E2-effect on collagen I and III mRNA in vivo using intact female and OVX mice with and without E2-supplementation that were subjected to 4 weeks of TAC (Figure 7).
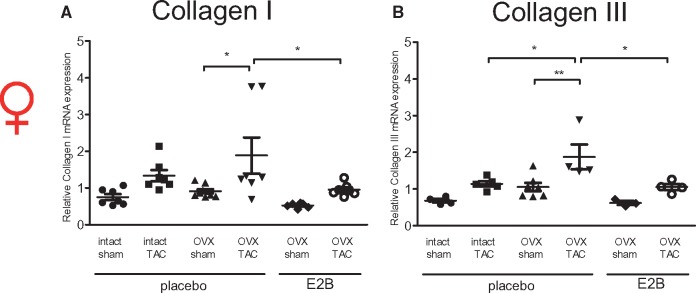
Figure 7.
E2 attenuates collagen I and III mRNA level in murine hearts subjected to TAC. (A) Collagen I and (B) III mRNA level were determined in hearts from female mice 4 weeks after TAC. Collagen I and III mRNA level were quantified by qPCR and normalized to RPL0. Mean ± SEM from six to nine mice per group. *P ≤ 0.05, **P ≤ 0.01, one-way ANOVA followed by Tukey's multiple comparison test. Sham, sham operated mice; TAC, transverse aortic constriction; OVX, ovariectomy; placebo, phytoestrogen-free chow and E2-supplementation: 17β-estradiolbenzoate (E2B) in phytoestrogen-free chow.
TAC induced collagen I mRNA level in all three groups (intact, OVX, and OVX mice with E2-supplementation) compared with sham operated controls, reaching statistical significance in the OVX mice. Overall, collagen I transcription was the highest in OVX TAC mice, followed by intact TAC (not significant vs. OVX TAC) and OVX E2-supplemented TAC mice (P ≤ 0.05 vs. OVX TAC). In the sham operated animals, a similar trend was observed (Figure 7A). The up-regulation of collagen III after TAC compared with sham mice showed a similar pattern as observed for collagen I, reaching statistical significance in OVX mice. Collagen III mRNA level was highest in OVX TAC mice, followed by intact (P < 0.05 vs. OVX TAC) and OVX E2-suplemented TAC mice (P < 0.05 vs. OVX TAC). The sham groups showed a similar trend (Figure 7B). These data indicate that E2 attenuates cardiac collagen I and III mRNA in female mice under physiological and pathological conditions.
4. Discussion
Sex differences in cardiac fibrosis development4–8,10 and the regulatory role of E2 and ER within this process have been reported.11,14–16,20,21 However, these studies did not elucidate the underlying molecular mechanisms. We, therefore, aimed to clarify the role of E2 and ER on collagen I and III expression in CF from females and males in more detail. Here, we found that collagen I and III are regulated in a sex-specific manner by E2 in rat and human CF, indicating that this is conserved across species. Data from ECT confirm the sex-dimorphic collagen regulation by E2 in a tissue-like environment. Translational relevance of our results is underscored by the findings in mice, showing that the E2-mediated inhibition of collagen I and III in females also holds true in vivo under physiological and pathological conditions after pressure overload. Further, we identified sex-dimorphic ER-activation and collagen I and III promoter binding as possible underlying mechanisms for the observed distinct transcriptional regulation in female and male CF. Thus, we show that sex differences in cardiac fibrosis development possibly arise from the sex-specific activation of ER by E2, leading to sex differences in collagen I and III gene expression regulation in CF. Altogether, we present novel mechanisms, which help to better understand observed sex differences in cardiac fibrosis, and in the future, this might serve to design pharmacological interventions according to the sex of patients.
4.1 E2-mediated sex differences in collagen I and III expression in cardiac fibroblasts
Although the E2-dependent regulation of collagen I and III26–28 and the E2-induced sex-dimorphic expression of other genes and proteins have been described in different cellular contexts,5,29–32 the underlying mechanisms are still obscure. Previously, we found that in cardiac cells from both sexes E2 has either similar (MMP-222) or divergent effects on gene expression (myosin regulatory light chain interacting protein29 and micro RNAs32). We recently showed that activated ERβ plays a role in sex-specific regulation of miR-106a and -106b in rCF.32 However, in the present study we show that both ER are involved in sex-specific regulation of collagen I and III. Activated ERα is responsible for collagen I and III mRNA down-regulation in female rCF, whereas activated ERβ up-regulates collagen I and III levels only in male rCF. Thus, we report for the first time an exclusively divergent E2-mediated regulation of collagen I and III mediated by a sex-specific activation of both ER in CF.
4.2 Sex differences in ER protein expression levels in cardiac fibroblasts
One possible explanation for sex-dimorphic ER activation by E2 could be that both receptors show significant differences in protein levels between male and female rCF. Different expression levels of ER in hepatocytes from male and females have been shown to underlie E2-mediated sex-specific transcription regulation of the vitellogenein gene.30 We found no sex-differences in ERα protein level, but higher ERβ protein level in female compared with male rCF. E2 significantly down-regulated ERα protein level in female rCF, but up-regulated ERα in male rCF, compared with the vehicle control. There were no changes in ERβ protein expression upon E2-treatment in rCF from both sexes. These data suggest that differences in ER protein levels at basal conditions and after E2-treatment between females and males are no possible explanation for the shown sex-specific ER activation in rCF.
4.3 Sex differences in ER phosphorylation in cardiac fibroblasts
We considered ER phosphorylation upon E2-treatment as another possible underlying mechanism for sex-specific receptor activation. Post-translational modifications, such as phosphorylation, are crucial events in ER-activation.24,25,33 Several studies of ERα and ERβ have shown that phosphorylation mediates E2-initiated non-genomic signaling and time-delayed genomic actions of these receptors. In particular, phosphorylation at serine 118 and 105 in ERα and ERβ respectively, has been reported to alter E2-binding capacity, protein stability, receptor dimerization, subcellular localization, DNA-binding, and interaction with co-regulators of both ER.24,25 The consequence is an altered gene-specific transcription. In our study, we found that ERα phosphorylation at serine 118 is exclusively activated in female rCF, whereas an increase in ERβ phosphorylation at serine 105 only occurred in male rCF in response to E2-treatment. Therefore, we speculate that sex-specific ER phosphorylation underlies E2-mediated sex differences in collagen I and III gene transcription regulation.
The underlying pathways of ER phosphorylation have been shown to be interwoven and to include the activation of several kinases, such as MAPK, PI3k/AKT, Cdk7, and GSK3β.24,33 Based on that, we speculate that both ER are targeted by sex-dimorphic kinase signalling in rCF. Interestingly, a much more pronounced E2-dependent increase in MAPK phosphorylation could be detected in female compared with male lung adenocarcinoma cells.31 Moreover, similar to our study, E2-treatment resulted in an increased phosphorylation of ERα at serine 118 only in female cells, which led to increased cyclin D1 gene transcription only in female lung adenocarcinoma cells. In contrast, as a possible result of E2-mediated ERα phosphorylation, we report a decline in collagen I and III transcription in female rCF. In HeLa cells it was demonstrated that phosphorylation of Ser118-ERα influences transcription in a gene-specific manner,34 thereby causing an increase, decrease, or no change in transcription. While ERα has been studied well, there is only limited information on E2-mediated phosphorylation of ERβ. Data from Lam et al.33 suggest that E2-mediated phosphorylation at Ser105-ERβ is mediated via ERK1/2 and p38-MAPK in human breast cancer cells. How E2-mediated P-Ser105-ERβ influences gene expression is not yet clear.
4.4 Sex-specific ER binding to collagen I and III promoters in cardiac fibroblasts
Based on the sex-specific activation and E2-mediated differences of ERα- and ERβ-phosphorylation in male and female rCF, we speculated that sex-dimorphic binding of ER to the collagen I and III promoters in rCF occurs. Indeed, we showed that upon E2-treatment, ERα binds together with ERβ to the collagen I and III promoter in female rCF. In male rCF, E2-treatment only induced ERβ binding to the collagen I and III promoter. In line with our data, Powell and Xu35 showed that selectively activated ERα stimulates the formation of ERα homodimers or ERα-ERβ heterodimers, whereas selectively activated ERβ promote the formation of ERβ homodimers only.
Although both ER have similar affinities to E236 and recognize similar ERE motives,37 they can display different trans-regulatory capacities. E2-bound ER homo- and heterodimers interact with a palindromic DNA sequence separated by three nonspecific nucleotides (GGTCAnnnTGACC), the consensus ERE. This is accompanied by the ordered recruitment of a series of coregulators leading to a positively or negatively regulated E2-target gene transcription.38 Specific ERα and ERβ coregulators are known and it has been suggested that they play a significant role in mediating transcriptional differences.38 Based on these data, we hypothesize that sex-dimorphic ER-binding to the collagen I and III promoter leads to sex-specific recruitment of coregulators (Figure 8). We assume that in female rCF, upon E2-treatment, activated/phosphorylated ERα heterodimerizes with ERβ and bind to the collagen I and III promoter. Since it has been shown that ERα is the functional dominant partner in the ER heterodimer39 this would suggest that ERα dictates regulatory properties in female rCF; that is by co-repressor recruitment. In contrast, phosphorylation of ERβ in male rCF results in ERβ homodimerization and collagen promoter binding, followed by co-activator recruitment. In addition, epigenetic modifications at the collagen I and III promoter could be a possible explanation for observed E2 mediated sex differences. It has been reported that upon E2-treatment, ER are involved in epigenetic modifications at the promoter of several ER-target genes.40,41 Thereby, E2-activated ER induces gene expression by interacting with a variety of methylases leading to the formation of active modifications (H3K4), by interacting with demethylases leading to removal of repressive histone modifications (H3K9), and by the association with co-activators with histone acetyltransferase activity. E2/ER-mediated gene inhibition includes the ER induced removal of H3K4 and formation of H3K9 modifications, recruitment of histone deacetylases and of co-repressors, acting as histone deacetylases.42,43 Future studies will clarify which coregulators are recruited by E2-activated ER and whether epigenetic changes at the collagen I and III promoter are responsible for sexually dimorphic regulation of collagen I and III in CF.
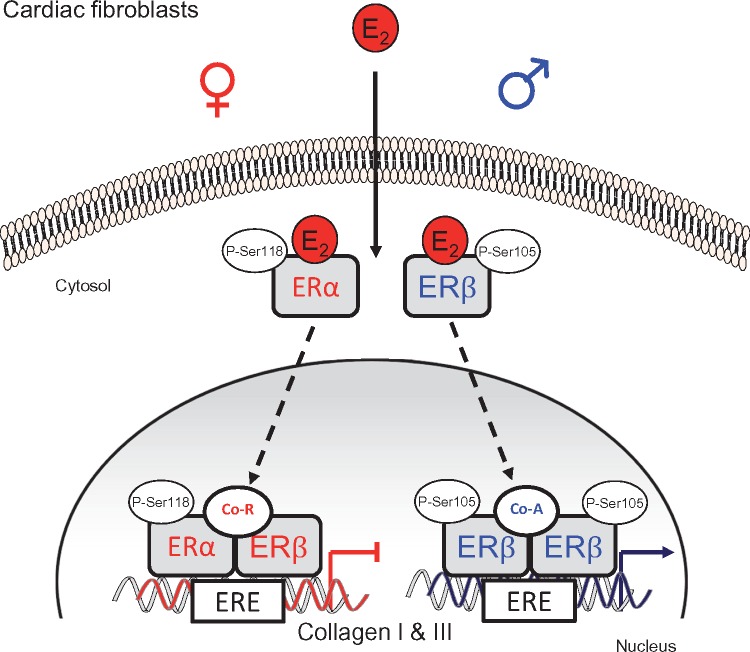
Figure 8.
Schematic presentation on how E2 and ER modulate collagen I and III in a sex-specific manner in cardiac fibroblasts. In female CF, E2 phosphorylates ERα at serine 118 (P-Ser118), which binds to ERβ. Both ER translocate into the nucleus and bind to the identified ERE within the collagen I and III promoter. Recruitment of co-repressor(s) (Co-R) results in down-regulation of collagen I and III gene expression in female cells. In male CF, E2-treatment phosphorylates ERβ at serine 105 (P-Ser105), leading to ERβ homodimer formation, which then translocates into the nucleus and binds to the identified ERE within the collagen I and III promoter. This is accompanied by co-activator (Co-A) recruitment leading to up-regulation of collagen gene expression in male cells.
4.5 Sex-specific role of ER in collagen I and III regulation
The impact of ERα and ERβ on cardiac fibrosis development has been reported in mouse models with induced pathological MH.11,15,20,21,44 In mice with pressure overload induced MH, ERβ deletion attenuated cardiac fibrosis in males compared with WT-controls.11 This indicates that ERβ promotes cardiac fibrosis in males. Our data suggest that this is possibly mediated by E2-induced up-regulation of collagen I and III via ERβ in male CF. In contrast, specific activation of ERα15 and ERβ44 abolished cardiac fibrosis development, while ERβ-deletion11,20,21 increased cardiac fibrosis development in female mice after TAC. These data suggest that ERα probably acts in conjunction with ERβ to attenuate cardiac fibrosis development in female pressure overloaded hearts. Our data support these findings and suggest that this is mediated via E2-activated ERα. How E2 and ER inhibit cardiac fibrosis development in female sex is not specified in detail so far. Recent studies from Pedram et al.44 suggest that E2 via ERβ activates protein kinase A and AMP kinase and inhibits therefore stimulation of Rho kinase and prevents cardiac fibrosis. Lee et al.16 showed that E2-mediated activation of membrane ERα attenuates cardiac fibrosis via RhoA/ROCK-mediated actin remodeling. However, by modulating these signaling pathways in a therapeutically manner in whole heart tissue, interfering side effects in other cardiac cells or organs cannot be excluded. We show here that E2-activated ER regulate directly collagen I and III gene transcription, acting as transcription factors, in CF. This offers the advantage to modulate collagen I and III expression and therefore cardiac fibrosis development in a more precise manner.
5. Translational aspect
Under different pathological conditions, men show significantly higher collagen deposition and higher increase in collagen I and III mRNA compared with women.4–8,10 Collagen accumulation adversely influences tissue stiffness, ventricular function and leads to HF progression. Sex differences in the onset and progression of HF have been reported, with males usually showing an earlier transition to HF than females.13 Postmenopausal women still have significant circulating E2-levels that are approximately the same levels as those of elderly men, synthesized mainly in adipose tissue.45 This suggests that E2 can act in the hearts of both sexes, even at increased age. Based on that, we speculate that the E2-mediated sex-specific collagen I and III regulation in CF might be one possible explanation for the observed sex differences in cardiac fibrosis and therefore of clinical relevance. Of note, it has been shown that the upstream region of the Col1A1 gene promoter shows a high degree of homology in rats, mice, and humans.46–48 This leads to the speculation that the demonstrated binding of ERα and ERβ to the ERE within the rat Col1A1 promoter region in this study might be also conserved across these species.
Further, ECT data showed E2-mediated sex-specific collagen I and III regulation in a more tissue-like environment. E2-treated female ECT displayed higher volumes, while E2-treated male ECT displayed lower volumes compared with their respective controls. As CF attach to the collagen fibrils within a collagen matrix, spread and migrate, they exert a tractional force leading to the condensation of the tissue.49 Based on that, we speculate that a reduced collagen synthesis within the female ECT upon E2 leads to a lower tissue condensation. On the other hand, higher collagen secretion by male CF upon E2 leads to higher condensation and more compact male ECT. However, extracellular matrix (ECM) remodelling is a complex process. Other ECM components are also regulated in a sex-specific manner under pathological conditions5,6,10,12 and can be modulated by E2.16,21,22,32 Therefore, the regulation of other ECM factors by E2 and their impact on observed sex-dimorphic ECT condensation cannot be excluded and has to be studied in future analysis.
6. Conclusions
Sex-specific regulation of collagen I and III expression is mediated via sex-dimorphic ER activation involving differential phosphorylation and promoter binding of ERα and ERβ. This regulation is conserved across species and could serve as future target to treat cardiac fibrosis by receptor-specific modulation of collagen expression in CF.
Supplementary Material
Acknowledgements
We thank Karo Bio AB (Sweden) for providing ERβ-agonist. We also thank A. Kühne, B. Fielitz, and J. Thomas for technical assistance. We further thank the patients and surgeons at the German Heart Institute Berlin, especially Professor Knossala and Léa Gaignebet for providing us with human cardiac tissue.
Conflict of interest: none declared.
Funding
This work was supported by the German Research Foundation (DFG) (DW 70/1-1 to E.D., V.R.Z., FOR1045 to S.M., V.R.Z.); Deutsche Stiftung für Herzforschung (to D.F.); DZHK (German Centre for Cardiovascular Research; Berlin partner site to E.D., Göttingen partner site to S.L.), and by the BMBF (German Ministry of Education and Research). G.S. was supported by IRTG 1816. W.H.Z. was supported by DFG (ZI 708/10-1, SFB 937 TP18, SFB 1002 TPs C04, S1, IRTG 1618 RP12) and the Foundation Leducq. S.M.H. and Y.K.L. were supported by the US National Institutes of Health (R01 DK061084, R01 CA015776 to S.M.H., P30 ES006096 to S.M.H. and Y.K.L.).
Footnotes
Time for primary review: 23 days
This manuscript was handled by Consulting Editor, Ryozo Nagai.
References
- 1. Segura AM, Frazier OH, Buja LM.. Fibrosis and heart failure. Heart Fail Rev 2014;19:173–185. [DOI] [PubMed] [Google Scholar]
- 2. Moore-Morris T, Guimaraes-Camboa N, Banerjee I, Zambon AC, Kisseleva T, Velayoudon A, Stallcup WB, Gu Y, Dalton ND, Cedenilla M, Gomez-Amaro R, Zhou B, Brenner DA, Peterson KL, Chen J, Evans SM.. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Invest 2014;124:2921–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pichler M, Rainer PP, Schauer S, Hoefler G.. Cardiac fibrosis in human transplanted hearts is mainly driven by cells of intracardiac origin. J Am Coll Cardiol 2012;59:1008–1016. [DOI] [PubMed] [Google Scholar]
- 4. Villari B, Campbell SE, Schneider J, Vassalli G, Chiariello M, Hess OM.. Sex-dependent differences in left ventricular function and structure in chronic pressure overload. Eur Heart J 1995;16:1410–1419. [DOI] [PubMed] [Google Scholar]
- 5. Petrov G, Regitz-Zagrosek V, Lehmkuhl E, Krabatsch T, Dunkel A, Dandel M, Dworatzek E, Mahmoodzadeh S, Schubert C, Becher E, Hampl H, Hetzer R.. Regression of myocardial hypertrophy after aortic valve replacement: faster in women? Circulation 2010;122:S23–S28. [DOI] [PubMed] [Google Scholar]
- 6. Petrov G, Dworatzek E, Schulze TM, Dandel M, Kararigas G, Mahmoodzadeh S, Knosalla C, Hetzer R, Regitz-Zagrosek V.. Maladaptive remodeling is associated with impaired survival in women but not in men after aortic valve replacement. JACC Cardiovasc Imaging 2014;7:1073–1080. [DOI] [PubMed] [Google Scholar]
- 7. Campbell DJ, Somaratne JB, Jenkins AJ, Prior DL, Yii M, Kenny JF, Newcomb AE, Kelly DJ, Black MJ.. Differences in myocardial structure and coronary microvasculature between men and women with coronary artery disease. Hypertension 2011;57:186–192. [DOI] [PubMed] [Google Scholar]
- 8. Ambale Venkatesh B, Volpe GJ, Donekal S, Mewton N, Liu CY, Shea S, Liu K, Burke G, Wu C, Bluemke DA, Lima JA.. Association of longitudinal changes in left ventricular structure and function with myocardial fibrosis: the Multi-Ethnic Study of Atherosclerosis study. Hypertension 2014;64:508–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Treibel TA, Lopez B, Gonzalez A, Menacho K, Schofield RS, Ravassa S, Fontana M, White SK, DiSalvo C, Roberts N, Ashworth MT, Diez J, Moon JC.. Reappraising myocardial fibrosis in severe aortic stenosis: an invasive and non-invasive study in 133 patients. Eur Heart J 2018;39:699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kararigas G, Dworatzek E, Petrov G, Summer H, Schulze TM, Baczko I, Knosalla C, Golz S, Hetzer R, Regitz-Zagrosek V.. Sex-dependent regulation of fibrosis and inflammation in human left ventricular remodelling under pressure overload. Eur J Heart Fail 2014;16:1160–1167. [DOI] [PubMed] [Google Scholar]
- 11. Fliegner D, Schubert C, Penkalla A, Witt H, Kararigas G, Kararigas G, Dworatzek E, Staub E, Martus P, Ruiz Noppinger P, Kintscher U, Gustafsson J-A, Regitz-Zagrosek V.. Female sex and estrogen receptor-beta attenuate cardiac remodeling and apoptosis in pressure overload. Am J Physiol Regul Integr Comp Physiol 2010;298:R1597–1R1606. [DOI] [PubMed] [Google Scholar]
- 12. Witt H, Schubert C, Jaekel J, Fliegner D, Penkalla A, Tiemann K, Stypmann J, Roepcke S, Brokat S, Mahmoodzadeh S, Brozova E, Davidson MM, Ruiz Noppinger P, Grohe C, Regitz-Zagrosek V.. Sex-specific pathways in early cardiac response to pressure overload in mice. J Mol Med 2008;86:1013–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Regitz-Zagrosek V, Kararigas G.. Mechanistic pathways of sex differences in cardiovascular disease. Physiol Rev 2017;97:1–37. [DOI] [PubMed] [Google Scholar]
- 14. Zhan E, Keimig T, Xu J, Peterson E, Ding J, Wang F, Yang XP.. Dose-dependent cardiac effect of oestrogen replacement in mice post-myocardial infarction. Exp Physiol 2008;93:982–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Westphal C, Schubert C, Prelle K, Penkalla A, Fliegner D, Petrov G, Regitz-Zagrosek V.. Effects of estrogen, an ERalpha agonist and raloxifene on pressure overload induced cardiac hypertrophy. PLoS One 2012;7:e50802.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee TM, Lin SZ, Chang NC.. Membrane ERalpha attenuates myocardial fibrosis via RhoA/ROCK-mediated actin remodeling in ovariectomized female infarcted rats. J Mol Med 2014;92:43–51. [DOI] [PubMed] [Google Scholar]
- 17. Grodstein F, Stampfer MJ, Colditz GA, Willett WC, Manson JE, Joffe M, Rosner B, Fuchs C, Hankinson SE, Hunter DJ, Hennekens CH, Speizer FE.. Postmenopausal hormone therapy and mortality. N Engl J Med 1997;336:1769–1775. [DOI] [PubMed] [Google Scholar]
- 18. Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC, Kotchen JM, Ockene J.. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 2002;288:321–333. [DOI] [PubMed] [Google Scholar]
- 19. Jankowska EA, Rozentryt P, Ponikowska B, Hartmann O, Kustrzycka-Kratochwil D, Reczuch K, Nowak J, Borodulin-Nadzieja L, Polonski L, Banasiak W, Poole-Wilson PA, Anker SD, Ponikowski P.. Circulating estradiol and mortality in men with systolic chronic heart failure. JAMA 2009;301:1892–1901. [DOI] [PubMed] [Google Scholar]
- 20. Gurgen D, Hegner B, Kusch A, Catar R, Chaykovska L, Hoff U, Gross V, Slowinski T, da Costa Goncalves AC, Kintscher U, Gustafsson JA, Luft FC, Dragun D.. Estrogen receptor-beta signals left ventricular hypertrophy sex differences in normotensive deoxycorticosterone acetate-salt mice. Hypertension 2011;57:648–654. [DOI] [PubMed] [Google Scholar]
- 21. Pedram A, Razandi M, O'Mahony F, Lubahn D, Levin ER.. Estrogen receptor-beta prevents cardiac fibrosis. Mol Endocrinol 2010;24:2152–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mahmoodzadeh S, Dworatzek E, Fritschka S, Pham TH, Regitz-Zagrosek V.. 17beta-Estradiol inhibits matrix metalloproteinase-2 transcription via MAP kinase in fibroblasts. Cardiovasc Res 2010;85:719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ongherth A, Pasch S, Wuertz CM, Nowak K, Kittana N, Weis CA, Jatho A, Vettel C, Tiburcy M, Toischer K, Hasenfuss G, Zimmermann WH, Wieland T, Lutz S.. p63RhoGEF regulates auto- and paracrine signaling in cardiac fibroblasts. J Mol Cell Cardiol 2015;88:39–54. [DOI] [PubMed] [Google Scholar]
- 24. Lannigan DA. Estrogen receptor phosphorylation. Steroids 2003;68:1–9. [DOI] [PubMed] [Google Scholar]
- 25. Sanchez M, Picard N, Sauve K, Tremblay A.. Challenging estrogen receptor beta with phosphorylation. Trends Endocrinol Metab 2010;21:104–110. [DOI] [PubMed] [Google Scholar]
- 26. Silbiger S, Lei J, Neugarten J.. Estradiol suppresses type I collagen synthesis in mesangial cells via activation of activator protein-1. Kidney Int 1999;55:1268–1276. [DOI] [PubMed] [Google Scholar]
- 27. Ernst M, Schmid C, Froesch ER.. Enhanced osteoblast proliferation and collagen gene expression by estradiol. Proc Natl Acad Sci USA 1988;85:2307–2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Irie T, Takahata M, Majima T, Abe Y, Komatsu M, Iwasaki N, Minami A.. Effect of selective estrogen receptor modulator/raloxifene analogue on proliferation and collagen metabolism of tendon fibroblast. Connect Tissue Res 2010;51:179–187. [DOI] [PubMed] [Google Scholar]
- 29. Kararigas G, Bito V, Tinel H, Becher E, Baczko I, Knosalla C, Albrecht-Kupper B, Sipido KR, Regitz-Zagrosek V.. Transcriptome characterization of estrogen-treated human myocardium identifies myosin regulatory light chain interacting protein as a sex-specific element influencing contractile function. J Am Coll Cardiol 2012;59:410–417. [DOI] [PubMed] [Google Scholar]
- 30. Corthesy B, Claret FX, Wahli W.. Estrogen receptor level determines sex-specific in vitro transcription from the Xenopus vitellogenin promoter. Proc Natl Acad Sci USA 1990;87:7878–7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ivanova MM, Mazhawidza W, Dougherty SM, Klinge CM.. Sex differences in estrogen receptor subcellular location and activity in lung adenocarcinoma cells. Am J Respir Cell Mol Biol 2010;42:320–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Queiros AM, Eschen C, Fliegner D, Kararigas G, Dworatzek E, Westphal C, Sanchez Ruderisch H, Regitz-Zagrosek V.. Sex- and estrogen-dependent regulation of a miRNA network in the healthy and hypertrophied heart. Int J Cardiol 2013;169:331–338. [DOI] [PubMed] [Google Scholar]
- 33. Lam HM, Suresh Babu CV, Wang J, Yuan Y, Lam YW, Ho SM, Leung YK.. Phosphorylation of human estrogen receptor-beta at serine 105 inhibits breast cancer cell migration and invasion. Mol Cell Endocrinol 2012;358:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cheng J, Zhang C, Shapiro DJ.. A functional serine 118 phosphorylation site in estrogen receptor-alpha is required for down-regulation of gene expression by 17beta-estradiol and 4-hydroxytamoxifen. Endocrinology 2007;148:4634–4641. [DOI] [PubMed] [Google Scholar]
- 35. Powell E, Xu W.. Intermolecular interactions identify ligand-selective activity of estrogen receptor alpha/beta dimers. Proc Natl Acad Sci USA 2008;105:19012–19017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kuiper GG, Carlsson B, Grandien K, Enmark E, Ha Ggblad J, Nilsson S, Gustafsson JK.. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 1997;138:863–870. [DOI] [PubMed] [Google Scholar]
- 37. Loven MA, Wood JR, Nardulli AM.. Interaction of estrogen receptors alpha and beta with estrogen response elements. Mol Cell Endocrinol 2001;181:151–163. [DOI] [PubMed] [Google Scholar]
- 38. Nilsson S, Koehler KF, Gustafsson JA.. Development of subtype-selective oestrogen receptor-based therapeutics. Nat Rev Drug Discov 2011;10:778–792. [DOI] [PubMed] [Google Scholar]
- 39. Li X, Huang J, Yi P, Bambara RA, Hilf R, Muyan M.. Single-chain estrogen receptors (ERs) reveal that the ERalpha/beta heterodimer emulates functions of the ERalpha dimer in genomic estrogen signaling pathways. Mol Cell Biol 2004;24:7681–7694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Palijan A, Fernandes I, Verway M, Kourelis M, Bastien Y, Tavera-Mendoza LE, Sacheli A, Bourdeau V, Mader S, White JH.. Ligand-dependent corepressor LCoR is an attenuator of progesterone-regulated gene expression. J Biol Chem 2009;284:30275–30287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhu P, Baek SH, Bourk EM, Ohgi KA, Garcia-Bassets I, Sanjo H, Akira S, Kotol PF, Glass CK, Rosenfeld MG, Rose DW.. Macrophage/cancer cell interactions mediate hormone resistance by a nuclear receptor derepression pathway. Cell 2006;124:615–629. [DOI] [PubMed] [Google Scholar]
- 42. Mann M, Cortez V, Vadlamudi RK.. Epigenetics of estrogen receptor signaling: role in hormonal cancer progression and therapy. Cancers (Basel) 2011;3:1691–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Metivier R, Penot G, Hubner MR, Reid G, Brand H, Kos M, Gannon F.. Estrogen receptor-alpha directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 2003;115:751–763. [DOI] [PubMed] [Google Scholar]
- 44. Pedram A, Razandi M, Narayanan R, Levin ER.. Estrogen receptor beta signals to inhibition of cardiac fibrosis. Mol Cell Endocrinol 2016;434:57–68. [DOI] [PubMed] [Google Scholar]
- 45. Bjornerem A, Straume B, Midtby M, Fonnebo V, Sundsfjord J, Svartberg J, Acharya G, Oian P, Berntsen GK.. Endogenous sex hormones in relation to age, sex, lifestyle factors, and chronic diseases in a general population: the Tromso Study. J Clin Endocrinol Metab 2004;89:6039–6047. [DOI] [PubMed] [Google Scholar]
- 46. Ravazzolo R, Karsenty G, de Crombrugghe B.. A fibroblast-specific factor binds to an upstream negative control element in the promoter of the mouse alpha 1(I) collagen gene. J Biol Chem 1991;266:7382–7387. [PubMed] [Google Scholar]
- 47. Norman JT, Lindahl GE, Shakib K, En-Nia A, Yilmaz E, Mertens PR.. The Y-box binding protein YB-1 suppresses collagen alpha 1(I) gene transcription via an evolutionarily conserved regulatory element in the proximal promoter. J Biol Chem 2001;276:29880–29890. [DOI] [PubMed] [Google Scholar]
- 48. Roche P, Czubryt MP.. Transcriptional control of collagen I gene expression. Cardiovasc Hematol Disord Drug Targets 2014;14:107–120. [DOI] [PubMed] [Google Scholar]
- 49. Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA.. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 2002;3:349–363. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.