Abstract
A rapid, sensitive, and specific colorimetric biosensor based on the use of magnetic nanoparticles (MNPs) was designed for the detection of Pseudomonas aeruginosa in clinical samples. The biosensing platform was based on the measurement of P. aeruginosa proteolytic activity using a specific protease substrate. At the N-terminus, this substrate was covalently bound to MNPs and was linked to a gold sensor surface via cystine at the C-terminus of the substrates. The golden sensor appears black to naked eyes because of the coverage of the MNPs. However, upon proteolysis, the cleaved peptide–MNP moieties will be attracted by an external magnet, revealing the golden color of the sensor surface, which can be observed by the naked eye. In vitro, the biosensor was able to detect specifically and quantitatively the presence of P. aeruginosa with a detection limit of 102 cfu/mL in less than 1 min. The colorimetric biosensor was used to test its ability to detect in situ P. aeruginosa in clinical isolates from patients. This biochip is anticipated to be useful as a rapid point-of-care device for the diagnosis of P. aeruginosa-related infections.
1. Introduction
Pseudomonas aeruginosa is an opportunistic pathogen1 which is involved in various nosocomial diseases such as respiratory tract infections,2,3 urinary tract infections,4 wound infections,5 and bacteremia.6P. aeruginosa was identified as the second infectious pathogen isolated from patients with hospital-associated pneumonia (HAP).7 Therefore, rapid and proper diagnosis is essential to enable timely treatment in order to reduce the risk of mortality. Accordingly, the American Thoracic Society (ATS) and the Infectious Diseases Society of America (IDSA) issued guidelines for the management of HAP and emphasized on the importance of “quantitative cultures” for specific HAP diagnosis without deleterious consequences.8
Conventional diagnostic methods are based on culturing and require at least 24 h to report the results, reducing the chance of appropriate and successful treatment.3,8 Alternatively, rapid quantitative detection methods based on real-time polymerase chain reaction (PCR)9−11 and enzyme-linked immunosorbent assays12 were developed to detect P. aeruginosa in HAP clinical specimens. In these methods, results were obtained within a few hours with high specificity and sensitivity. However, these methods are costly and laborious and require handling by highly skilled personnel. Bacterial enzymes, such as proteases, are ideally suited as biomarkers for quick and sensitive identification of micro-organisms in clinical samples.13 Many of these enzymes are released into the surrounding microenvironment and are accessible for detection based on sensitive fluorogenic and/or colorimetric substrates.14−17 Recently, a specific peptide substrate was identified to detect the activity of the P. aeruginosa specific LasA protease, of which the expression appears to be mediated by the Las and Rhl quorum sensing (QS) systems.13,18,19 In this study, this P. aeruginosa specific peptide substrate was coupled to magnetic nanoparticles (MNPs) to be utilized in a rapid and specific colorimetric biosensor.
2. Results and Discussion
P. aeruginosa is considered the second most prevalent nosocomial bacterium in hospital environments and can contaminate medical equipment.1 Its infection is challenging due to its resistance to a large number of antibiotics.20 Therefore, there is a high-demand for the development of rapid and early detection method in clinical samples to guide therapeutic treatment.
Kaman et al.13 designed and evaluated a fluorogenic substrate as a potential tool to detect the virulence of P. aeruginosa. This P. aeruginosa specific protease substrate was utilized in the development of the paper-based colorimetric assay. In brief, hexanoic acid (Ahx) linkers were attached to both terminals of the peptide sequence (Gly-Gly-Gly) to enhance the protease accessibility to the peptide substrate near the sensor surface. Then, a cysteine amino acid was linked to the C-terminal, allowing the gold–thiol interaction and resulting in the formation of a self-assembled monolayer (SAM) of P. aeruginosa peptide–MNPs onto the gold sensor surface. The N-terminal of the peptide was attached to the MNPs.
2.1. Testing the P. aeruginosa Protease Biosensor
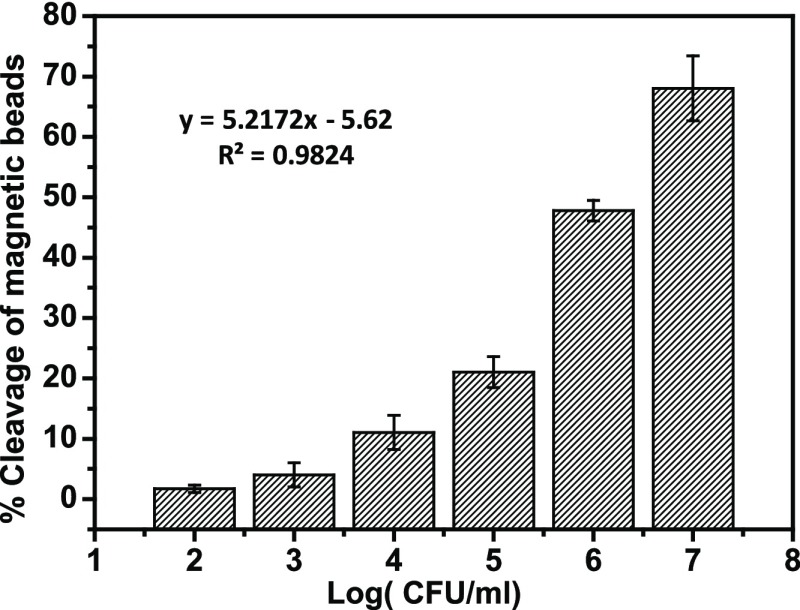
Initially, the fabricated sensor was examined to detect the proteolytic activity of P. aeruginosa protease by incubating 107 cfu/mL over the functionalized gold sensor surface. Upon proteolysis, the peptide segment–MNP moiety was released and collected by a circle shaped magnet placed at the back of the sensor strip. This results in revealing the golden color of the sensor surface, which is visible to the naked eye. Then, this biosensing method was applied for quantitative detection of P. aeruginosa. Accordingly, different concentrations of P. aeruginosa 4.5 × 107, 4.5 × 106, 4.5 × 105, 4.5 × 104, 4.5 × 103, 4.5 × 102, and 4.5 × 10 cfu/mL were added over the functionalized gold sensor. Results in Figures 1 and 2 show the gradual increase in the visible bare gold area with increasing bacteria concentration. This is explained by the ability of the higher protease enzyme concentration to dissociate the peptide–MNP moiety faster than the lower concentrations. Moreover, to validate the colorimetric biosensor, a negative blank [brain heart infusion (BHI) broth only] with no protease was incubated with the sensor and showed no cleavage (Figure 2). Results confirmed the ability of the fabricated biosensor to detect P. aeruginosa.
Figure 1.

Colorimetric P. aeruginosa proteases sensor probe tested with different concentrations of P. aeruginosa ranging from 4.5 × 107 to 4.5 × 10 cfu/mL.
Figure 2.
Dose response of the sensor under the effect of various concentrations of P. aeruginosa.
The developed colorimetric biosensor exhibited a limit of detection of 102 cfu/mL within one min time. This detection limit was determined by identifying the lowest protease concentration, capable of cleaving the covalently attached peptide black MNP moiety, which in turn revealed the sensors’ golden surface area. The negative blank (BHI broth only) showed no change in colors, as the sensor demonstrated no disruption of the SAM layer (Figure 1).
This colorimetric detection method provided better detection limit in a shorter time than the previously reported fluorescent dye including the lipid vesicle method, which was reported by Thet et al.21 Although, their method managed to correctly discriminate 40 clinical isolates of two pathogens, P. aeruginosa and Staphylococcus aureus (S. aureus), their method was used only for qualitative measurements. Another method developed to provide quantitative detection of P. aeruginosa was reported by Tang et al.22 This method is based on the use of magnetic enrichment and magnetic separation methodology and managed to detect as low as 10 cfu/mL. Dong et al.1 managed to develop a ten times more potent polymerase spiral reaction method with a lower detection limit of 2.3 pg/μL1 within 60 min. Tang et al.23 shortened the procedure time for DNA extraction to detection and retained a lower detection limit of 10 cfu/mL based on magnetic enrichment and nested PCR. All above mentioned methods are complex and require the use of centralized labs, instrumentation, and trained personnel. In addition, the PCR techniques are not suitable for bed-side routine testing, unlike the colorimetric assay described in this study which is approved to be cheap, simple, rapid, and sensitive. Furthermore, it does not require expensive equipment and trained personnel. It is to be mentioned that sensor stability in addition to the amount of a substrate–MNP composite was optimized in our previous work to achieve optimal monolayer performance.16,17,24−26
2.2. Specificity of the Sensor
The biosensor specificity was examined in the presence of two other pathogenic microbes: Listeria monocytogenes (L. monocytogenes), and S. aureus. Figure 3 shows the results of the P. aeruginosa sensors with L. monocytogenes and S. aureus, respectively. The sensor showed no disruption of the SAM layer and no significant change in the sensor surface golden color upon incubation with L. monocytogenes and S. aureus, showing sufficient specificity to detect P. aeruginosa.
Figure 3.

P. aeruginosa sensor specificity (A) L. monocytogenes before and after application and (B) S. aureus before and after application.
2.3. Detection of P. aeruginosa in Clinical Isolates
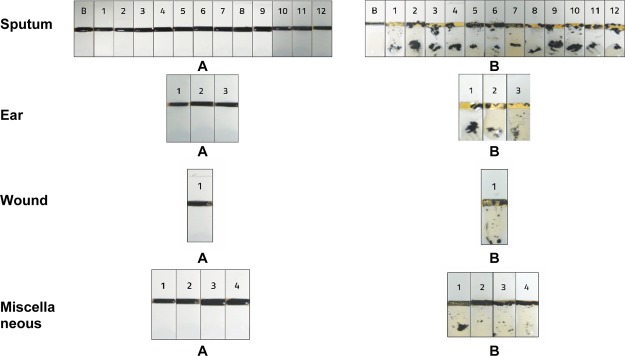
The clinical applicability of the developed biosensors was tested using 20 P. aeruginosa clinical isolates (among which sputum, ear, and wound). These samples were previously analyzed by conventional culture and PCR methods at King Faisal Specialist Hospital microbiology laboratory. These samples were incubated with the fabricated biosensor and all showed positive results, with a clear cleavage of the peptide–MNPs moiety, with a consequent appearance of the sensor golden surface color (Figure 4). Notably, the differences in cleavage intensity between tested samples were attributed to the difference in the number of colonies of P. aeruginosa. A negative control proved no cleavage of the peptide–MNPs moiety without any disruption of the SAM layer. The experiments were conducted in triplicate.
Figure 4.
Biosensing of P. aeruginosa in clinical samples from King Faisal Specialist Hospital microbiology laboratory. Sensor before (A) and after (B) clinical sample application.
3. Conclusions
This study demonstrated the ability of the designed colorimetric biosensor to detect P. aeruginosa protease in clinical samples. The assay was simple, rapid, sensitive, and specific and does not require any labeling or amplification steps. Furthermore, it does not require sample pretreatment or preconcentration and so can be applicable for onsite use by clinicians. This low-cost colorimetric biosensor was based on the use of specific substrate–MNPs, which were covalently attached to the gold sensor surface. This biosensing configuration is amenable for a qualitative and semi quantitative detection of P. aeruginosa proteases. The limit of detection was as low as 102 cfu/mL within one min. In conclusion, this biosensor presented a valuable onsite diagnostic tool to improve the control of potential risk infections caused by P. aeruginosa.
4. Materials and Methods
4.1. Materials and Reagents
Carboxyl-terminated beads (50 nm diameter), N-hydroxysuccinimide (NHS), 1-(3-dimethylaminopropyl)-3-ethyl-carbodiimide (EDC), and the plastic pH indicator strip were purchased from Sigma-Aldrich (Dorset, UK). Self-adhesive magnet sheets were purchased from Polarity Magnets Company (Wickford, Essex, UK). The P. aeruginosa peptide substrate NH2-Ahx-Gly-Gly-Gly-Ahx-Cys was synthesized by Pepmic Co. Ltd (Suzhou, China). BHI broth and agar were purchased from SDA, Oxoid Ltd (Basingstoke, UK). Sterile filters (0.22 μm) were obtained from Millipore (Watford, UK). The wash/storage buffer (10 mM Tris base, 150 mM sodium chloride, 0.1% (w/v) bovine serum albumin, 1 mM ethylenediaminetetraacetic acid, 0.1% sodium azide, pH 7.5) and the coupling buffer (10 mM potassium phosphate, 0.15 M sodium chloride, pH 5.5) were prepared from chemicals of analytical grade.
4.2. Bacteria Culture and Protease Preparation
P. aeruginosa (ATCC 15692), S. aureus (ATCC 25923), and L. monocytogenes (ATCC 19115) were individually cultured on BHI agar plates for 24 h at 37 °C. Subsequently, a single colony from each bacterium was grown in 5 mL BHI medium and incubated at 37 °C for 16 h to provide the primary bacterial culture (PBC) stock. Then, each bacterial concentration PBC was pelted by centrifugation at 3000g for 10 min, and the culture supernatant was filtered to obtain P. aeruginosa crude protease solution to be used later in sensitivity and specificity studies. Also, the bacterial count was analyzed via a spread-plate technique by plating 10-fold serial dilutions from each bacterial concentration on BHI plates and then incubating at 37 °C overnight.
4.3. Clinical Isolates and Protease Preparation
Twenty clinical isolates of P. aeruginosa were collected from King Faisal Specialist Hospital bacterial biobank in Riyadh kingdom at Saudi Arabia. The bibliographic data of the sources were not reviled to us. The samples were as follows: 12 from sputum, 3 from ear, 1 from wound, and 4 from different (unregistered) sites. The specimens were examined in King Faisal Specialist Hospital microbiology lab for complete identification and antibiotic susceptibility testing. Clinical isolates were then stored at −70 °C. After which, samples were thawed and recultured, and a single colony from each clinical isolate was grown in 5 mL BHI medium and incubated at 37 °C for 16 h to provide the PBC stock which was then centrifuged to pellet bacteria. Consequently, culture supernatant containing secreted proteases was added dropwise over the constructed biosensor to examine its applicability.
4.4. Preparation of the Substrate–MNP Composite
The carboxylated MNP suspension (1 mL) was mixed with the peptide substrate (1.0 mg/mL), EDC (0.57 mg/mL) and NHS (12 μg/mL). The mixture was shaken gently on a rotary shaker at room temperature for 24 h. The substrate–MNP composites were isolated using a magnet separator and washed three times, using a washing buffer to remove uncoupled components (Scheme 1A). Finally, the conjugate was dispersed in a storage buffer and stored at 4 °C until further use.24,27
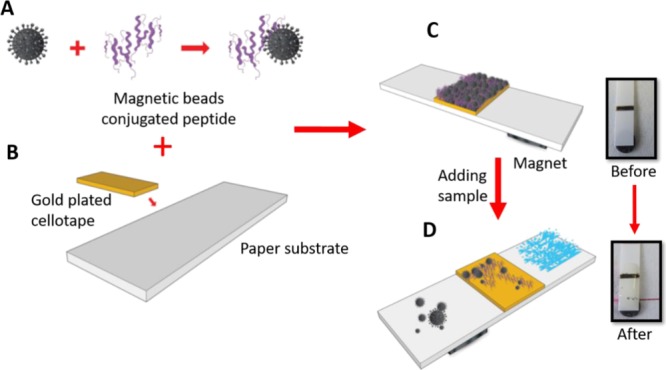
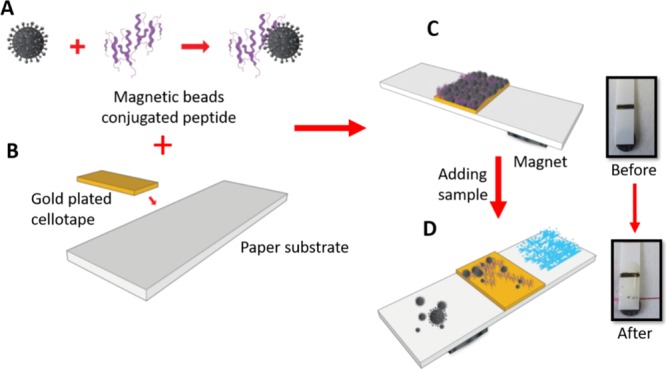
Scheme 1. Fabrication of the P. aeruginosa Functional Sensor; (A) Conjugation of the Peptide Substrate to the Magnetic Beads; (B) Sticking the Gold Plated Cellotape on the Paper Solid Support; (C) Self-Assembly of the Magnetic Beads Conjugated Peptides to the Sensor Surface; and (D) Application of the Test Sample and Color Change Observation.
4.5. Biosensing Platform Preparation and Functionalization
Self-adhesive sheets were purchased from Whatman (London, U.K.) and coated with gold using a sputtering machine in the clean room at KAUST-KSA. The gold-coated sheet was cut into rectangular pieces (4 mm × 2 mm) and stacked over the plastic strip at a specified distance 3 mm. This plastic strip was used as a physical support for the whole biofunctionalization process as well as the P. aeruginosa protease detection and quantification sensor (Scheme 1B).
The biosensing gold surface was functionalized with a layer of the black color substrate–MNPs composite. At the beginning, the substrate–MNPs composite suspension was mounted over the gold sensor surface and allowed to stand at room temperature for 30 min for dryness (Scheme 1C). Subsequently, an external magnet (12.5 × 12.5 × 5 mm) with a field strength of 3360 and 573 G at 1 and 10 mm distance, respectively, was passed over the functionalized strip to remove any unattached substrate–MNPs conjugates. At this stage, the sensor surface golden color is masked and turned black (Scheme 1C). After that, a round paper magnet was fixed on the strip back, 2–3 mm distance below the sensor platform.
4.6. Biosensing of P. aeruginosa Proteases
Culture medium supernatant solution containing P. aeruginosa crude proteases was added dropwise on the functionalized black color sensor surface. During the enzymatic cleavage reaction, the paper magnet attracted the cleaved peptide segment–MNPs, prompting a visual observation of the sensor golden color for a qualitative evaluation of the tested samples (Scheme 1D). Moreover, a quantitative evaluation was performed by using different counts of P. aeruginosa 4.5 × 107, 4.5 × 106, 4.5 × 105, 4.5 × 104, 4.5 × 103, 4.5 × 102, and 4.5 × 10 cfu/mL.
4.7. Quantitative Measurements
The images of the sensors were taken and saved as JPEG format and processed using the ImageJ software, which was developed by the National Institute of Health28 to calculate the quantitative data. The concentration was calculated by dividing the cleaved area (yellow color) to the total black sensor area. The quantitation was tested using different bacteria concentrations. Experiments were conducted in triplicate.
Acknowledgments
M.M.Z. would like to acknowledge the financial support from King Abdulaziz City for Science and Technology (KACST) under project number MN23786.
The authors declare no competing financial interest.
References
- Dong D.; Zou D.; Liu H.; Yang Z.; Huang S.; Liu N.; He X.; Liu W.; Huang L. Rapid detection of Pseudomonas aeruginosa targeting the toxA gene in intensive care unit patients from Beijing, China. Front. Microbiol. 2015, 6, 1100. 10.3389/fmicb.2015.01100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira S. G.; Cardoso O. Mobile genetic elements of Pseudomonas aeruginosa isolates from hydrotherapy facility and respiratory infections. Clin. Microbiol. Infect. 2014, 20, O203–O206. 10.1111/1469-0691.12359. [DOI] [PubMed] [Google Scholar]
- Feizabadi M. M.; Majnooni A.; Nomanpour B.; Fatolahzadeh B.; Raji N.; Delfani S.; Habibi M.; Asadi S.; Parvin M. Direct detection of Pseudomonas aeruginosa from patients with healthcare associated pneumonia by real time PCR. Infect. Genet. Evol. 2010, 10, 1247–1251. 10.1016/j.meegid.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Mittal R.; Aggarwal S.; Sharma S.; Chhibber S.; Harjai K. Urinary tract infections caused by Pseudomonas aeruginosa: a minireview. J. Infect. Public Health 2009, 2, 101–111. 10.1016/j.jiph.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Altoparlak U.; Aktas F.; Celebi D.; Ozkurt Z.; Akcay M. N. Prevalence of metallo-beta-lactamase among Pseudomonas aeruginosa and Acinetobacter baumannii isolated from burn wounds and in vitro activities of antibiotic combinations against these isolates. Burns 2005, 31, 707–710. 10.1016/j.burns.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Kang C.-I.; Sung H.; kim H.; kim S.; Park S.; Choe Y.; Oh M.; Kim E.; Choe K. Pseudomonas aeruginosa Bacteremia: Risk Factors for Mortality and Influence of Delayed Receipt of Effective Antimicrobial Therapy on Clinical Outcome. Clin. Infect. Dis. 2003, 15, 745–751. 10.1086/377200. [DOI] [PubMed] [Google Scholar]
- Anuj S. N.; Whiley D. M.; Kidd T. J.; Bell S. C.; Wainwright C. E.; Nissen M. D.; Sloots T. P. Identification of Pseudomonas aeruginosa by a duplex real-time polymerase chain reaction assay targeting the ecfX and the gyrB genes. Diagn. Microbiol. Infect. Dis. 2009, 63, 127–131. 10.1016/j.diagmicrobio.2008.09.018. [DOI] [PubMed] [Google Scholar]
- Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 388–416. 10.1164/rccm.200405-644st. [DOI] [PubMed] [Google Scholar]
- Mackay I. M. Real-time PCR in the microbiology laboratory. Clin. Microbiol. Infect. 2004, 10, 190–212. 10.1111/j.1198-743x.2004.00722.x. [DOI] [PubMed] [Google Scholar]
- Huang Q.; Hu Q.; Li Q. Identification of 8 foodborne pathogens by multicolor combinational probe coding technology in a single real-time PCR. Clin. Chem. 2007, 53, 1741–1748. 10.1373/clinchem.2007.087502. [DOI] [PubMed] [Google Scholar]
- Aghamollaei H.; Moghaddam M. M.; Kooshki H.; Heiat M.; Mirnejad R.; Barzi N. S. Detection of Pseudomonas aeruginosa by a triplex polymerase chain reaction assay based on lasI/R and gyrB genes. J. Infect. Public Health 2015, 8, 314–322. 10.1016/j.jiph.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Mauch R. M.; Rossi C.; Ribeiro A.; Nolasco da Silva M.; Levy C. Assessment of IgG antibodies to Pseudomonas aeruginosa in patients with cystic fibrosis by an enzyme-linked immunosorbent assay (ELISA). Diagn. Pathol. 2014, 9, 158. 10.1186/s13000-014-0158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaman W. E.; Arkoubi-El Arkoubi N. E.; Roffel S.; Endtz H. P.; van Belkum A.; Bikker F. J.; Hays J. P. Evaluation of a FRET-Peptide Substrate to Predict Virulence in Pseudomonas aeruginosa. PLoS One 2013, 8, e81428 10.1371/journal.pone.0081428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhogail S.; Suaifan G. A. R. Y.; Zourob M. Rapid colorimetric sensing platform for the detection of Listeria monocytogenes foodborne pathogen. Biosens. Bioelectron. 2016, 86, 1061–1066. 10.1016/j.bios.2016.07.043. [DOI] [PubMed] [Google Scholar]
- Alhogail S.; Suaifan G.; Bizzarro S.; Kaman W.; Bikker F.; Weber K.; Cialla-May D.; Popp J.; Zourob M. On site visual detection of Porphyromonas gingivalis related periodontitis by using a magnetic-nanobead based assay for gingipains protease biomarker. Mikrochim. Acta 2018, 185, 149. 10.1007/s00604-018-2677-x. [DOI] [PubMed] [Google Scholar]
- Suaifan G. A. R. Y.; Alhogail S.; Zourob M. Rapid and low-cost biosensor for the detection of Staphylococcus aureus. Biosens. Bioelectron. 2017, 90, 230–237. 10.1016/j.bios.2016.11.047. [DOI] [PubMed] [Google Scholar]
- Suaifan G. A. R. Y.; Alhogail S.; Zourob M. Paper-based magnetic nanoparticle-peptide probe for rapid and quantitative colorimetric detection of Escherichia coli O157: H7. Biosens. Bioelectron. 2017, 92, 702–708. 10.1016/j.bios.2016.10.023. [DOI] [PubMed] [Google Scholar]
- Girard G.; Bloemberg G. V. Central role of quorum sensing in regulating the production of pathogenicity factors in Pseudomonas aeruginosa. Future Microbiol. 2008, 3, 97–106. 10.2217/17460913.3.1.97. [DOI] [PubMed] [Google Scholar]
- Brint J. M.; Ohman D. E. Synthesis of multiple exoproducts in Pseudomonas aeruginosa is under the control of RhlR-RhlI, another set of regulators in strain PAO1 with homology to the autoinducer-responsive LuxR-LuxI family. J. Bacteriol. 1995, 177, 7155–7163. 10.1128/jb.177.24.7155-7163.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveday H. P.; Wilson J. A.; Kerr K.; Pitchers R.; Walker J. T.; Browne J. Association between healthcare water systems and Pseudomonas aeruginosa infections: a rapid systematic review. J. Hosp. Infect. 2014, 86, 7–15. 10.1016/j.jhin.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Thet N. T.; Hong S. H.; Marshall S.; Laabei M.; Toby A.; Jenkins A. Visible, colorimetric dissemination between pathogenic strains of Staphylococcus aureus and Pseudomonas aeruginosa using fluorescent dye containing lipid vesicles. Biosens. Bioelectron. 2013, 41, 538–543. 10.1016/j.bios.2012.09.019. [DOI] [PubMed] [Google Scholar]
- Tang Y.; Zou J.; Ma C.; Ali Z.; Li Z.; Li X.; Ma N.; Mou X.; Deng Y.; Zhang L.; Li K.; Lu G.; Yang H.; He N. Highly sensitive and rapid detection of Pseudomonas aeruginosa based on magnetic enrichment and magnetic separation. Theranostics 2013, 3, 85–92. 10.7150/thno.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y.; Ali Z.; Zou J.; Yang K.; Mou X.; Li Z.; Deng Y.; Lu Z.; Ma C.; Shah M. A. A.; Elingarami S.; Yang H.; He N. Detection of Pseudomonas aeruginosa based on magnetic enrichment and nested PCR. J. Nanosci. Nanotechnol. 2014, 14, 4886–4890. 10.1166/jnn.2014.8707. [DOI] [PubMed] [Google Scholar]
- Suaifan G. A. R. Y.; Esseghaier C.; Ng A.; Zourob M. Wash-less and highly sensitive assay for prostate specific antigen detection. Analyst 2012, 137, 5614–5619. 10.1039/c2an36243k. [DOI] [PubMed] [Google Scholar]
- Suaifan G. A. R. Y.; Esseghaier C.; Ng A.; Zourob M. Ultra-rapid colorimetric assay for protease detection using magnetic nanoparticle-based biosensors. Analyst 2013, 138, 3735–3739. 10.1039/c3an36881e. [DOI] [PubMed] [Google Scholar]
- Suaifan G. A. R. Y.; Zourob M. Portable paper-based colorimetric nanoprobe for the detection of Stachybotrys chartarum using peptide labeled magnetic nanoparticles. Microchim. Acta 2019, 186, 230. 10.1007/s00604-019-3313-0. [DOI] [PubMed] [Google Scholar]
- Suaifan G. A. R. Y.; Shehadeh M.; Al-Ijel H.; Ng A.; Zourob M. Recent progress in prostate-specific antigen and HIV proteases detection. Expert Rev. Mol. Diagn. 2013, 13, 707–718. 10.1586/14737159.2013.835576. [DOI] [PubMed] [Google Scholar]
- Rajwa B.; McNally H. A.; Varadharajan P.; Sturgis J.; Robinson J. P. AFM/CLSM data visualization and comparison using an open-source toolkit. Microsc. Res. Tech. 2004, 64, 176–184. 10.1002/jemt.20067. [DOI] [PubMed] [Google Scholar]