Abstract
To investigate the potential role of immunotherapies in the cellular and molecular mechanisms associated with ovarian cancer (OC), we applied a comparative proteomic toll using protein identification combined with mass spectrometry. Herein, the effects of the protein aggregate magnesium-ammonium phospholinoleate-palmitoleate anhydride, known as P-MAPA, and the human recombinant interleukin-12 (hrIL-12) were tested alone or in combination in human SKOV-3 cells. The doses and period were defined based on a previous study, which showed that 25 μg/mL P-MAPA and 1 ng/mL IL-12 are sufficient to reduce cell metabolism after 48 h. Indeed, among 2,881 proteins modulated by the treatments, 532 of them were strictly concordant and common. P-MAPA therapy upregulated proteins involved in tight junction, focal adhesion, ribosome constitution, GTP hydrolysis, semaphorin interactions, and expression of SLIT and ROBO, whereas it downregulated ERBB4 signaling, toll-like receptor signaling, regulation of NOTCH 4, and the ubiquitin proteasome pathway. In addition, IL-12 therapy led to upregulation of leukocyte migration, tight junction, and cell signaling, while cell communication, cell metabolism, and Wnt signaling were significantly downregulated in OC cells. A clear majority of proteins that were overexpressed by the combination of P-MAPA with IL-12 are involved in tight junction, focal adhesion, DNA methylation, metabolism of RNA, and ribosomal function; only a small number of downregulated proteins were involved in cell signaling, energy and mitochondrial processes, cell oxidation and senescence, and Wnt signaling. These findings suggest that P-MAPA and IL-12 efficiently regulated important proteins associated with OC progression; these altered proteins may represent potential targets for OC treatment in addition to its immunoadjuvant effects.
1. Introduction
Ovarian cancer (OC) represents the most lethal of gynecological malignancies being often diagnosed at a late stage.1,2 The majority of patients experience difficulties over the course of treatment, with a 5 years survival rate of 35% when diagnosed late.3 Because no evident symptom is present at an early-stage OC, the disease can rapidly progress to an incurable form.4−11 Importantly, patients are generally responsive to the treatments; however, through several mechanisms, they develop resistance to chemotherapy and OC grows wildly.12 New promising strategies to overcome chemoresistance and increase chemosensitivity have been developed for OC treatment, including immunotherapies.
We evaluated the effect of two immunotherapeutic agents from the proteomic standpoint. The immunomodulatory agent termed protein aggregate magnesium-ammonium phospholinoleate-palmitoleate anhydride (P-MAPA) is a natural biopolymer extracted from the Aspergillus oryzae. Experimentally, P-MAPA exhibits a number of antitumor responses in different in vivo models of cancer,11,13,14 and specifically in OC, P-MAPA potentiated the effects of cisplatin, thereby enhancing TLR signaling in immune cells and attenuating tumor growth.11 Another tested agent was interleukin-12 (IL-12), a cytokine secreted by antigen-presenting cells (APCs), which is involved in the differentiation of naïve T cells into a polarized Th1 immune response.15,16 Treatment with IL-12 promotes tumor regression and reduces the potential of tumor outgrowth in patients with recurrent OC;16,17 although IL-12 therapy results in an OC refractory state, the major challenge is related to its high cellular toxicity.18 Although these immunotherapies have demonstrated to be quite effective in the treatment of OC with regard to the tumor microenvironment, their exact role on OC cells in terms of protein synthesis and secretion is far from being understood; certainly, there must be other particular functions in addition to those related to the immune system.
Identifying successfully signaling pathways that coordinate tumor cell metabolism, protein–protein interactions, secretion of molecules and factors, and activity of specific enzymes is needed to discover new chemical agents and further modify the OC aggressiveness. Thus, proteomic analysis using mass spectrometry (MS) may provide a wide body of information considering proper candidates for OC development and treatment.10,19−21 Large or small molecules are definitely recognized to be involved in intra- or intercellular processes, in which one molecule can possibly be sharing different signaling mechanisms in the same tumor cell.
Although there are important studies reporting the use of mass spectrometry in the diagnosis of cancers, including the monitoring strategies for OC,10,22,23 none has shown the concurrent role of immunotherapies in predicting clinical outcomes from the “omics” standpoint. To further strengthen this issue, we studied the effects of immunotherapeutic agents, P-MAPA and IL-12, directly on human ovarian cancer SKOV-3 cells through proteomic profiling.
2. Results and Discussion
2.1. Influence of Low-Dose Administration of P-MAPA and IL-12 in SKOV-3 Cells’ Viability
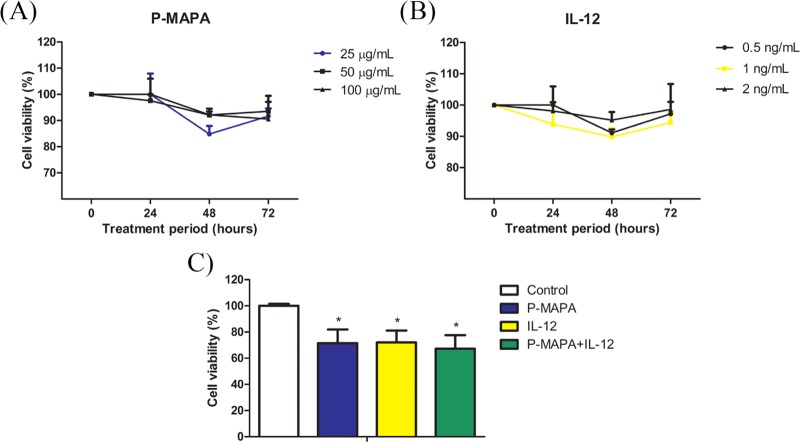
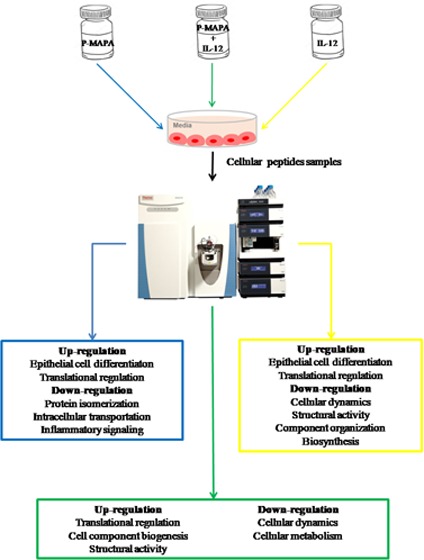
The global behavior of OC cells in response to different conditions of treatment is crucial for designing future strategies. We previously reported an immunostimulatory effect of P-MAPA in an in vivo OC model,11 while IL-12 is a well-known inductor of polarized Th1 immune response.15,16 Despite the consistent effects on the tumor microenvironment, the direct effect of these compounds in cancer cells is uncertain, and understanding how they act in OC in a safe dose regimen may be greatly beneficial for patients. Although several proteomic approaches in animal models with OC have recently been tested, little is known about the effects of P-MAPA and IL-12 on this disease. The effects of P-MAPA and IL-12 were first tested using three different doses and four exposure times (Figure 1A). After an 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, cell viability was reduced by P-MAPA at a dose of 25 μg/mL (∼ 25% reduction), by IL-12 at a dose of 1 ng/mL (∼ 25% reduction), and by the association of P-MAPA with IL-12 (∼ 30% reduction) after 48 h (Figure 1B) (see details in Experimental Section, Section 4.3). We then performed a global proteomic analysis in OC cells treated with P-MAPA, IL-12, and P-MAPA associated with IL-12, reporting an extensive catalogue of differentially expressed proteins that are affected by these immunotherapies. The majority of proteins described herein are involved in key cellular processes and signaling pathways, such as metabolic processes; catalytic functions; structural activity; protein, DNA, and RNA binding; and transcription/translation regulatory activity. The variety of biological functions impacted by these agents makes them interesting candidates as potential alternatives to OC treatment.
Figure 1.
Defining the treatments with P-MAPA and IL-12. (A) Cell viability (%) after exposure to three different doses of P-MAPA (25, 50, and 100 μg/mL) in four different periods (0, 24, 48, and 72 h). (B) Cell viability (%) after exposure to three different doses of IL-12 (0.5, 1, and 2 ng/mL) in four different periods (0, 24, 48, and 72 h). Colored line of the selected dose is highlighted. (C) Cell viability (%) was assessed by MTT (1 × 103 cells) after exposure to standardized doses of treatments (25 μg/mL P-MAPA, 1 ng/mL IL-12, or both) at 48 h. The results are expressed as the mean ± standard deviation (SD). *p < 0.05 vs control.
2.2. Label-Free Proteomic Analysis of SKOV-3 Cells Treated with P-MAPA and IL-12
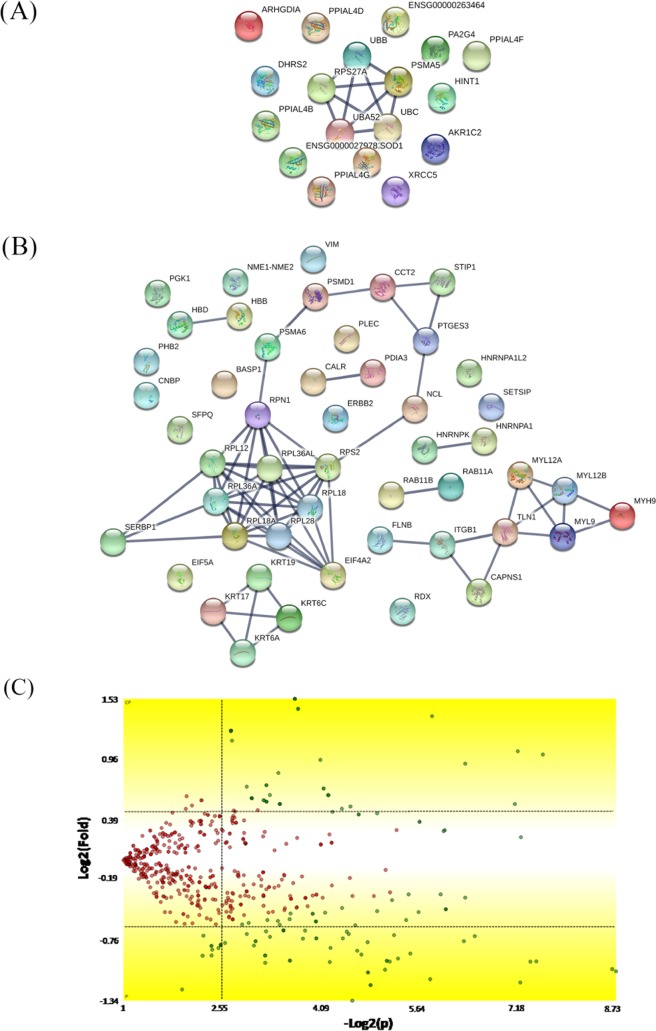
Samples were fractionated with reverse-phase columns to separate protein mixtures, and label-free analysis was performed on peptides by their charges and hydrophobicity. This analysis identified a total of 896 proteins among control, P-MAPA, IL-12, and P-MAPA + IL-12 groups. We reported a total of 636 molecules in the control, 719 in the P-MAPA group, 709 in the IL-12 group, and 740 proteins in the P-MAPA + IL-12 group, while 532 were commonly found in all tested groups (Figure 2). General intersections showing the proteins that are present in one or more groups were represented and varied with the treatment. Figure 3 shows the interaction between up- and downregulated proteins with the highest confidence (0.900) after P-MAPA therapy compared with the control. We found a total of 770 proteins in these groups, while 585 were coexpressed. In the P-MAPA group, there were 50 downregulated and 21 upregulated proteins (Table 1) compared with the control, considering the differential expression by at least ±1.5-fold change. The great majority of upregulated molecules are proteins related to protein localization to the membrane, epithelial cell differentiation, SRP-dependent cotranslational protein targeting the membrane, and translational initiation. Otherwise, the downregulated proteins are involved in protein peptidyl-prolyl isomerization, intracellular transport, error-free and -prone translesion synthesis, and the TRIF-dependent toll-like receptor signaling pathway.
Figure 2.
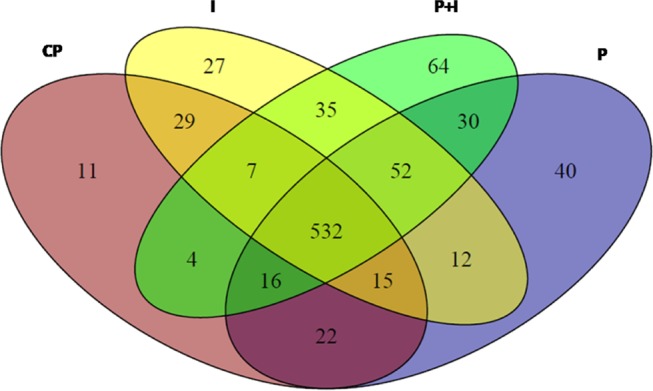
Cartoon displaying the intersection of proteins between the experimental groups; 532 proteins coexisted among the control, P-MAPA, IL-12, and P-MAPA + IL-12 groups. CP, control; I, IL-12; P, P-MAPA; P + I, P-MAPA + IL-12.
Figure 3.
Mapping of the protein association network in P-MAPA-treated SKOV-3 cells. Source of the protein–protein interaction is based on cellular processing and its related molecular systems. Thicker lines show the highest confidence score (0.900) for upregulated (A) and downregulated (B) molecules of a functional association in response to P-MAPA therapy in SKOV-3 cells. (C) Volcano plot indicates large magnitude fold-changes (y-axis) and statistical significance (p-value, x-axis); this plot indicates log 2 fold change vs −log 2 false discovery rate (FDR)-corrected p-value. Differentially expressed proteins are shown in the upper and lower right areas. See legends in Table 1.
Table 1. Differentially Expressed Proteins in the P-MAPA Group versus the Control Group.
| protein ID | description | % coverage | protein score | fold change |
|---|---|---|---|---|
| P62979 | ubiquitin-40S ribosomal protein S27a | 21.8 | 15.5 | 2.90 |
| P62987 | ubiquitin-60S ribosomal protein L40 | 26.6 | 15.5 | 2.90 |
| P0CG47 | polyubiquitin-B | 14.9 | 15.5 | 2.90 |
| P28066 | proteasome subunit α type-5 | 12.0 | 8.5 | 2.58 |
| P0CG48 | polyubiquitin-C | 14.9 | 15.5 | 2.35 |
| P49773 | histidine triad nucleotide-binding protein 1 | 7.1 | 2.9 | 2.34 |
| Q13268 | SDR family member 2, mitochondrial | 3.6 | 4.6 | 2.05 |
| Q9UQ80 | proliferation-associated protein 2G4 | 5.1 | 5.6 | 1.89 |
| B2RPK0 | putative high mobility group protein B1-like 1 | 10.9 | 7.6 | 1.78 |
| P52895 | aldo-keto reductase family 1 member C2 | 10.2 | 8.8 | 1.62 |
| Q6DRA6 | putative histone H2B type 2-D | 10.4 | 12.9 | 1.60 |
| Q6DN03 | putative histone H2B type 2-C | 8.8 | 12.9 | 1.60 |
| P52565 | Rho GDP-dissociation inhibitor 1 | 8.3 | 7.3 | 1.54 |
| P13010 | X-ray repair cross-complementing protein 5 | 3.1 | 4.4 | 1.54 |
| P00441 | superoxide dismutase [Cu–Zn] | 10.4 | 3.0 | 1.52 |
| P0DN37 | peptidyl-prolyl cis–trans isomerase A-like 4G | 7.9 | 4.4 | 1.51 |
| A0A0B4J2A2 | peptidyl-prolyl cis–trans isomerase A-like 4C | 7.9 | 4.4 | 1.51 |
| A0A075B759 | peptidyl-prolyl cis–trans isomerase A-like 4E | 7.9 | 4.4 | 1.51 |
| F5H284 | peptidyl-prolyl cis–trans isomerase A-like 4D | 7.9 | 4.4 | 1.51 |
| P0DN26 | peptidyl-prolyl cis–trans isomerase A-like 4F | 7.9 | 4.4 | 1.51 |
| Q9Y536 | peptidyl-prolyl cis–trans isomerase A-like 4A | 7.9 | 4.4 | 1.51 |
| P63241 | eukaryotic translation initiation factor 5A-1 | 18.2 | 6.6 | –1.50 |
| P46779 | 60S ribosomal protein L28 | 25.6 | 10.1 | –1.51 |
| P00558 | phosphoglycerate kinase 1 | 18.2 | 24.0 | –1.51 |
| P80723 | brain acid soluble protein 1 | 37.9 | 19.0 | –1.53 |
| P15880 | 40S ribosomal protein S2 | 13.7 | 15.3 | –1.53 |
| P83881 | 60S ribosomal protein L36a | 8.5 | 3.8 | –1.59 |
| Q969Q0 | 60S ribosomal protein L36a-like | 8.5 | 3.8 | –1.59 |
| O14950 | myosin regulatory light chain 12B | 11.6 | 4.6 | –1.59 |
| P24844 | myosin regulatory light polypeptide 9 | 11.6 | 4.6 | –1.59 |
| P19105 | myosin regulatory light chain 12A | 11.7 | 4.6 | –1.59 |
| P27797 | calreticulin | 20.4 | 39.1 | –1.60 |
| P04626 | receptor tyrosine-protein kinase erbB-2 | 4.3 | 9.4 | –1.63 |
| Q99460 | 26S proteasome non-ATPase regulatory subunit 1 | 1.0 | 3.1 | –1.63 |
| P31948 | stress-induced-phosphoprotein 1 | 10.3 | 15.8 | –1.64 |
| P08670 | vimentin | 24.5 | 46.7 | –1.64 |
| O75369 | filamin-B | 6.8 | 41.9 | –1.65 |
| P30101 | protein disulfide-isomerase A3 | 19.2 | 36.4 | –1.66 |
| Q9Y490 | Talin-1 | 6.9 | 49.0 | –1.67 |
| P61978 | heterogeneous nuclear ribonucleoprotein K | 23.5 | 44.7 | –1.67 |
| P30050 | 60S ribosomal protein L12 | 24.2 | 18.5 | –1.68 |
| P08727 | keratin, type I cytoskeletal 19 | 33.5 | 51.3 | –1.71 |
| P0DME0 | protein SETSIP | 4.3 | 7.9 | –1.72 |
| Q15149 | plectin | 3.5 | 37.4 | –1.72 |
| P02042 | hemoglobin subunit delta | 12.9 | 8.9 | –1.75 |
| P68871 | hemoglobin subunit β | 12.9 | 8.9 | –1.75 |
| Q04695 | keratin, type I cytoskeletal 17 | 21.5 | 54.7 | –1.76 |
| P35241 | radixin | 10.6 | 23.9 | –1.78 |
| P02538 | keratin, type II cytoskeletal 6A | 21.6 | 41.7 | –1.80 |
| P60900 | proteasome subunit α type-6 | 9.4 | 11.1 | –1.82 |
| P22392 | nucleoside diphosphate kinase B | 36.2 | 25.8 | –1.85 |
| Q8NC51 | plasminogen activator inhibitor 1 RNA-binding protein | 12.5 | 17.6 | –1.86 |
| Q02543 | 60S ribosomal protein L18a | 11.9 | 6.0 | –1.87 |
| Q32P51 | heterogeneous nuclear ribonucleoprotein A1-like 2 | 10.0 | 10.3 | –1.87 |
| P48668 | keratin, type II cytoskeletal 6C | 21.6 | 44.2 | –1.87 |
| P35579 | myosin-9 | 9.7 | 49.0 | –1.91 |
| P62633 | cellular nucleic acid-binding protein | 11.9 | 5.8 | –1.94 |
| P62491 | ras-related protein Rab-11A | 14.8 | 6.7 | –1.94 |
| Q15907 | ras-related protein Rab-11B | 14.7 | 6.7 | –1.94 |
| P04632 | calpain small subunit 1 | 9.3 | 5.3 | –1.95 |
| Q14240 | eukaryotic initiation factor 4A-II | 9.1 | 13.9 | –1.95 |
| P05556 | integrin β-1 | 7.6 | 15.6 | –1.96 |
| P04843 | dolichyl-diphosphooligosaccharide-protein glycosyltransferase subunit 1 | 4.5 | 6.7 | –1.97 |
| O60361 | putative nucleoside diphosphate kinase | 31.4 | 18.4 | –2.05 |
| P23246 | splicing factor, proline- and glutamine-rich | 5.9 | 12.4 | –2.06 |
| P78371 | T-complex protein 1 subunit β | 11.0 | 15.6 | –2.08 |
| P09651 | heterogeneous nuclear ribonucleoprotein A1 | 15.9 | 18.9 | –2.10 |
| P19338 | nucleolin | 6.2 | 13.9 | –2.19 |
| Q99623 | prohibitin-2 | 4.0 | 5.0 | –2.24 |
| Q15185 | prostaglandin E synthase 3 | 14.4 | 7.2 | –2.28 |
| Q07020 | 60S ribosomal protein L18 | 11.7 | 8.2 | –2.52 |
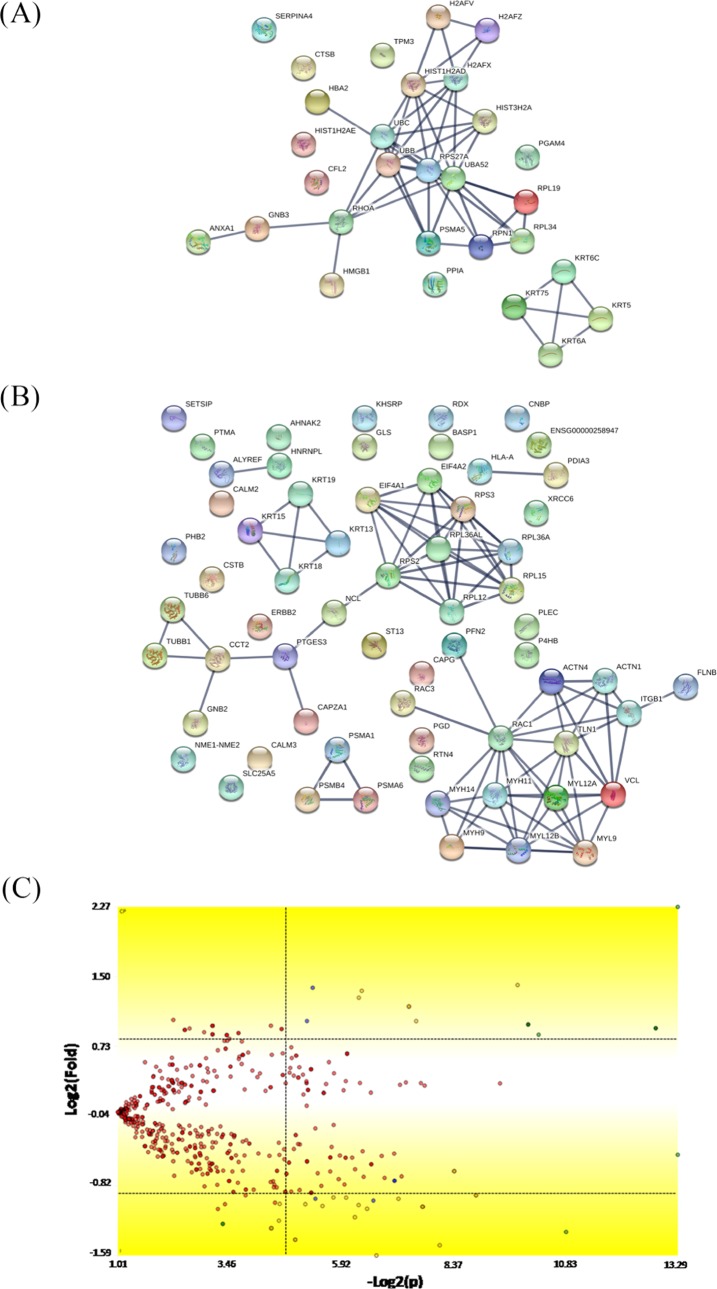
Figure 4 shows the up- and downregulated protein networks with clusters of strong confidence (highest confidence: 0.900) between the proteins produced by the SKOV-3 cells after IL-12 treatment versus the control group. A total of 762 proteins were identified, and 583 of them were coexpressed in the two groups. Based on differential expression by at least a factor of ±1.5 in the IL-12 group relative to the corresponding control group, we reported 85 downregulated proteins (Table 2), which are involved in different biological processes and molecular function: cytoskeleton organization, cellular component organization or biogenesis, structural molecule activity, protein-containing complex binding, and actin filament binding. Conversely, from the 30 upregulated proteins, we found some of them involved in a number of cellular events, such as intracellular transport, chromosome and organelle organization, chromatin silencing, structural molecule activity, and protein heterodimerization.
Figure 4.
Mapping of the protein association network in IL-12-treated SKOV-3 cells. Source of the protein–protein interaction is based on cellular processing and its related molecular systems. Thicker lines show the highest confidence score (0.900) for upregulated (A) and downregulated (B) molecules of a functional association in response to IL-12 therapy in SKOV-3 cells. (C) Volcano plot indicates large magnitude fold-changes (y-axis) and statistical significance (p-value, x-axis); this plot indicates log 2 fold change vs −log 2 FDR-corrected p-value. Differentially expressed proteins are shown in the upper and lower right areas. See legends in Table 2.
Table 2. Differentially Expressed Proteins in the IL-12 Group versus the Control Group.
| protein ID | description | % coverage | protein score | fold change |
|---|---|---|---|---|
| O95678 | keratin, type II cytoskeletal 75 | 4.9 | 9.2 | 4.84 |
| Q9Y281 | cofilin-2 | 16.9 | 7.7 | 2.41 |
| P07858 | cathepsin B | 6.2 | 4.7 | 2.03 |
| Q8N0Y7 | probable phosphoglycerate mutase 4 | 3.9 | 3.9 | 2.01 |
| P62979 | ubiquitin-40S ribosomal protein S27a | 21.8 | 15.5 | 1.94 |
| P62987 | ubiquitin-60S ribosomal protein L40 | 26.6 | 15.5 | 1.94 |
| P0CG48 | polyubiquitin-C | 5.0 | 15.5 | 1.94 |
| P0CG47 | polyubiquitin-B | 14.9 | 15.5 | 1.94 |
| P49207 | 60S ribosomal protein L34 | 15.4 | 7.8 | 1.94 |
| P16520 | guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit β-3 | 2.9 | 3.7 | 1.91 |
| P84098 | 60S ribosomal protein L19 | 4.6 | 3.0 | 1.90 |
| P02538 | keratin, type II cytoskeletal 6A | 6.6 | 15.8 | 1.89 |
| P69905 | hemoglobin subunit α | 15.5 | 6.1 | 1.88 |
| P29622 | kallistatin | 1.9 | 5.7 | 1.84 |
| P13647 | keratin, type II cytoskeletal 5 | 7.8 | 21.7 | 1.81 |
| P48668 | keratin, type II cytoskeletal 6C | 6.6 | 15.8 | 1.72 |
| P28066 | proteasome subunit α type-5 | 12.0 | 8.5 | 1.65 |
| B2RPK0 | putative high mobility group protein B1-like 1 | 10.9 | 7.6 | 1.61 |
| P62937 | peptidyl-prolyl cis–trans isomerase A | 18.8 | 18.9 | 1.58 |
| P0C0S5 | histone H2A.Z | 12.5 | 17.8 | 1.57 |
| Q71UI9 | histone H2A.V | 12.5 | 17.8 | 1.57 |
| Q7L7L0 | histone H2A type 3 | 12.5 | 17.8 | 1.57 |
| P20671 | histone H2A type 1-D | 12.5 | 17.8 | 1.57 |
| P04908 | histone H2A type 1-B/E | 12.5 | 17.8 | 1.57 |
| P16104 | histone H2AX | 12.5 | 17.8 | 1.57 |
| P06753 | tropomyosin α-3 chain | 10.9 | 15.6 | 1.57 |
| P61586 | transforming protein RhoA | 10.9 | 7.8 | 1.55 |
| P04083 | annexin A1 | 17.3 | 32.9 | 1.52 |
| P04843 | dolichyl-diphosphooligosaccharide-protein glycosyltransferase subunit 1 | 1.7 | 3.8 | 1.52 |
| P09429 | high mobility group protein B1 | 17.2 | 16.2 | 1.51 |
| O94925 | glutaminase kidney isoform, mitochondrial | 7.9 | 19.7 | –1.51 |
| P04080 | cystatin-B | 39.8 | 8.6 | –1.53 |
| P04439 | HLA class I histocompatibility antigen, A-3 α chain | 9.0 | 8.5 | –1.55 |
| P16188 | HLA class I histocompatibility antigen, A-30 α chain | 9.0 | 8.5 | –1.55 |
| P13746 | HLA class I histocompatibility antigen, A-11 α chain | 9.0 | 8.5 | –1.55 |
| P30512 | HLA class I histocompatibility antigen, A-29 α chain | 9.0 | 8.5 | –1.55 |
| P16189 | HLA class I histocompatibility antigen, A-31 α chain | 9.0 | 8.5 | –1.55 |
| P30453 | HLA class I histocompatibility antigen, A-34 α chain | 9.0 | 8.5 | –1.55 |
| P30459 | HLA class I histocompatibility antigen, A-74 α chain | 9.0 | 8.5 | –1.55 |
| P10316 | HLA class I histocompatibility antigen, A-69 α chain | 9.0 | 8.5 | –1.55 |
| P05534 | HLA class I histocompatibility antigen, A-24 α chain | 9.0 | 8.5 | –1.55 |
| Q09160 | HLA class I histocompatibility antigen, A-80 α chain | 9.0 | 8.5 | –1.55 |
| P30450 | HLA class I histocompatibility antigen, A-26 α chain | 9.0 | 8.5 | –1.55 |
| P10314 | HLA class I histocompatibility antigen, A-32 α chain | 9.0 | 8.5 | –1.55 |
| P16190 | HLA class I histocompatibility antigen, A-33 α chain | 9.0 | 8.5 | –1.55 |
| P01891 | HLA class I histocompatibility antigen, A-68 α chain | 9.0 | 8.5 | –1.55 |
| P30456 | HLA class I histocompatibility antigen, A-43 α chain | 9.0 | 8.5 | –1.55 |
| P30447 | HLA class I histocompatibility antigen, A-23 α chain | 9.0 | 8.5 | –1.55 |
| P01892 | HLA class I histocompatibility antigen, A-2 α chain | 9.0 | 8.5 | –1.55 |
| P30457 | HLA class I histocompatibility antigen, A-66 α chain | 9.0 | 8.5 | –1.55 |
| P18462 | HLA class I histocompatibility antigen, A-25 α chain | 9.0 | 8.5 | –1.55 |
| P35080 | profilin-2 | 10.0 | 8.8 | –1.55 |
| P60763 | ras-related C3 botulinum toxin substrate 3 | 15.6 | 7.2 | –1.55 |
| P14866 | heterogeneous nuclear ribonucleoprotein L | 4.4 | 10.1 | –1.55 |
| P60900 | proteasome subunit α type-6 | 9.4 | 7.3 | –1.57 |
| P62879 | guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit β-2 | 9.4 | 6.5 | –1.57 |
| P06454 | prothymosin α | 23.4 | 23.0 | –1.57 |
| P0DME0 | protein SETSIP | 6.3 | 5.9 | –1.57 |
| P30050 | 60S ribosomal protein L12 | 24.2 | 17.1 | –1.57 |
| P19012 | keratin, type I cytoskeletal 15 | 7.5 | 27.6 | –1.58 |
| Q9NQC3 | reticulon-4 | 2.3 | 9.9 | –1.58 |
| P22392 | nucleoside diphosphate kinase B | 31.6 | 18.1 | –1.58 |
| P04626 | receptor tyrosine-protein kinase erbB-2 | 1.9 | 8.5 | –1.59 |
| Q7Z406 | myosin-14 | 1.8 | 16.5 | –1.60 |
| P35749 | myosin-11 | 1.8 | 16.5 | –1.60 |
| P18206 | vinculin | 12.8 | 50.7 | –1.60 |
| P12956 | X-ray repair cross-complementing protein 6 | 3.9 | 4.6 | –1.61 |
| P05783 | keratin, type I cytoskeletal 18 | 10.0 | 17.8 | –1.61 |
| P07237 | protein disulfide-isomerase | 12.8 | 12.8 | –1.64 |
| O43707 | α-actinin-4 | 15.2 | 49.8 | –1.64 |
| O75369 | filamin-B | 5.4 | 37.0 | –1.66 |
| P25786 | proteasome subunit α type-1 | 15.2 | 10.2 | –1.67 |
| P05141 | ADP/ATP translocase 2 | 14.4 | 16.7 | –1.69 |
| P12814 | α-actinin-1 | 9.9 | 43.6 | –1.70 |
| P83881 | 60S ribosomal protein L36a | 8.5 | 3.5 | –1.70 |
| Q969Q0 | 60S ribosomal protein L36a-like | 8.5 | 3.5 | –1.70 |
| P13646 | keratin, type I cytoskeletal 13 | 10.3 | 24.1 | –1.72 |
| P35241 | radixin | 9.4 | 21.8 | –1.74 |
| Q92945 | far upstream element-binding protein 2 | 4.8 | 7.3 | –1.75 |
| P40121 | macrophage-capping protein | 6.3 | 4.2 | –1.77 |
| Q9H4B7 | tubulin β-1 chain | 6.2 | 11.5 | –1.78 |
| P52907 | F-actin-capping protein subunit α-1 | 8.0 | 5.5 | –1.81 |
| P60842 | eukaryotic initiation factor 4A-I | 10.6 | 18.3 | –1.81 |
| Q9Y490 | talin-1 | 8.2 | 52.2 | –1.82 |
| P08727 | keratin, type I cytoskeletal 19 | 26.3 | 46.6 | –1.84 |
| P15880 | 40S ribosomal protein S2 | 16.0 | 19.5 | –1.84 |
| P0DP25 | calmodulin-3 | 6.0 | 8.1 | –1.86 |
| P0DP24 | calmodulin-2 | 6.0 | 8.1 | –1.86 |
| P0DP23 | calmodulin-1 | 6.0 | 8.1 | –1.86 |
| P30101 | protein disulfide-isomerase A3 | 21.2 | 31.3 | –1.89 |
| Q13509 | tubulin β-3 chain | 13.8 | 15.8 | –1.89 |
| Q14240 | eukaryotic initiation factor 4A-II | 5.4 | 11.6 | –1.93 |
| O60361 | putative nucleoside diphosphate kinase | 31.4 | 18.1 | –1.94 |
| Q8IVF2 | protein AHNAK2 | 1.0 | 12.8 | –1.95 |
| P23396 | 40S ribosomal protein S3 | 21.4 | 19.5 | –1.97 |
| P80723 | brain acid soluble protein 1 | 45.8 | 24.8 | –2.00 |
| P19338 | nucleolin | 8.3 | 13.7 | –2.02 |
| P35579 | myosin-9 | 8.3 | 52.2 | –2.03 |
| P63000 | ras-related C3 botulinum toxin substrate 1 | 22.9 | 10.1 | –2.03 |
| Q15149 | plectin | 4.0 | 47.9 | –2.04 |
| P61313 | 60S ribosomal protein L15 | 4.4 | 5.4 | –2.05 |
| P28070 | proteasome subunit β type-4 | 3.8 | 4.0 | –2.07 |
| Q99623 | prohibitin-2 | 4.0 | 3.9 | –2.07 |
| Q86V81 | THO complex subunit 4 | 4.3 | 4.8 | –2.07 |
| P78371 | T-complex protein 1 subunit β | 9.2 | 16.0 | –2.24 |
| P62633 | cellular nucleic acid-binding protein | 11.9 | 7.7 | –2.30 |
| Q15185 | prostaglandin E synthase 3 | 11.3 | 5.7 | –2.36 |
| Q8NFI4 | putative protein FAM10A5 | 5.2 | 5.2 | –2.44 |
| P50502 | Hsc70-interacting protein | 5.2 | 5.2 | –2.44 |
| Q8IZP2 | putative protein FAM10A4 | 7.9 | 5.2 | –2.44 |
| P52209 | 6-phosphogluconate dehydrogenase, decarboxylating | 6.6 | 15.4 | –2.51 |
| P24844 | myosin regulatory light polypeptide 9 | 17.4 | 8.2 | –2.66 |
| O14950 | myosin regulatory light chain 12B | 17.4 | 8.2 | –2.66 |
| P19105 | myosin regulatory light chain 12A | 17.5 | 8.2 | –2.66 |
| P05556 | integrin β-1 | 6.3 | 19.3 | –2.78 |
| Q9BUF5 | tubulin β-6 chain | 12.3 | 14.4 | –3.01 |
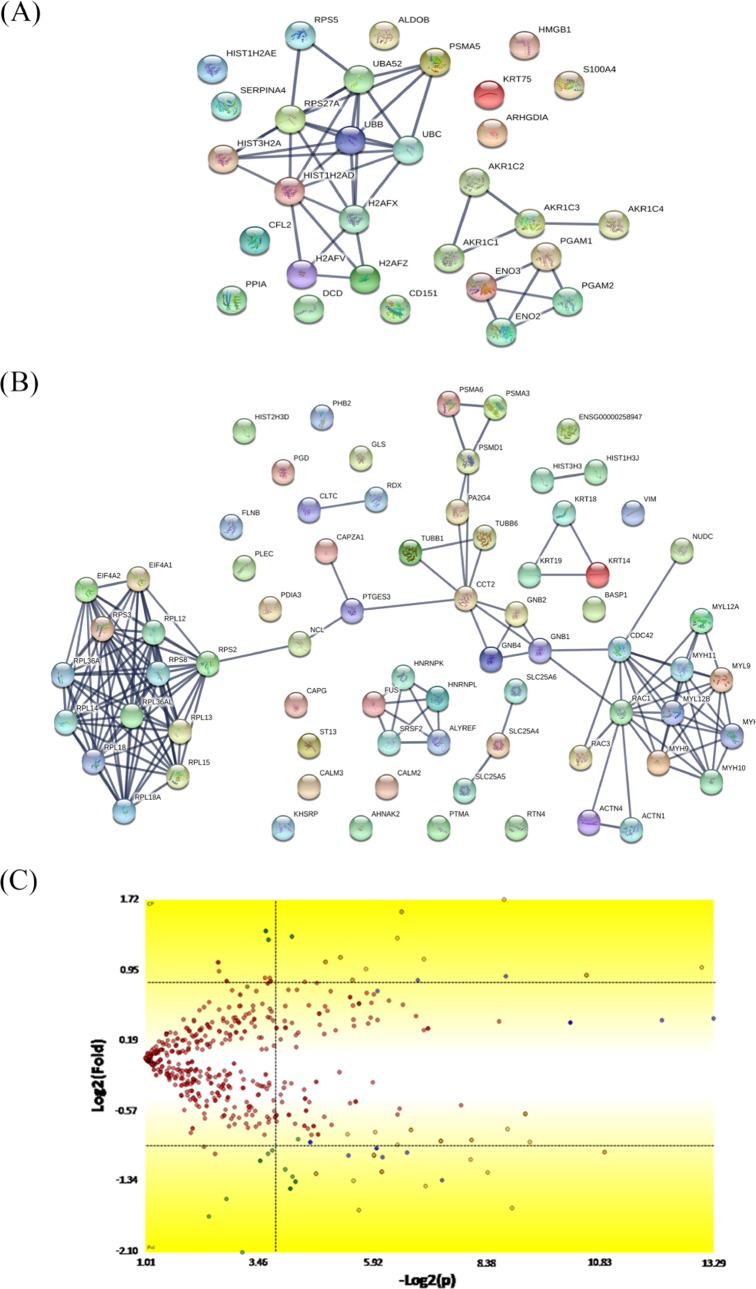
The combinatory treatment of P-MAPA and IL-12 versus the control group showed the up- and downregulated protein network with a very strong confidence relationship (0.900) (Figure 5). We found a total of 817 proteins, with 559 of them being differentially expressed by at least a factor of ±1.5 in the P-MAPA + IL-12 group relative to the corresponding untreated group. Among the 31 upregulated proteins, most of them were involved in different biological processes and molecular functions including canonical glycolysis, doxorubicin and daunorubicin metabolic processes, trans-1,2-dihydrobenzene-1,2-diol dehydrogenase activity, ketosteroid monooxygenase activity, androsterone dehydrogenase activity, phenanthrene 9,10-monooxygenase activity, and aldo-keto reductase (NADP) activity (Table 3). We found 76 downregulated proteins, with proteins involved in translational initiation, SRP-dependent cotranslational protein targeting the membrane, nuclear-transcribed mRNA catabolic process, nonsense-mediated decay, mRNA catabolic process, cellular component biogenesis, nucleosomal DNA binding, structural molecule activity, protein-containing complex binding, cytoskeletal protein binding, and structural constituent of ribosome.
Figure 5.
Mapping of the protein association network in P-MAPA + IL-12-treated SKOV-3 cells. Source of the protein–protein interaction is based on cellular processing and its related molecular systems. Thicker lines show the highest confidence score (0.900) for upregulated (A) and downregulated (B) molecules of a functional association in response to P-MAPA + IL-12 therapy in SKOV-3 cells. (C) Volcano plot indicates large magnitude fold-changes (y-axis) and statistical significance (p-value, x-axis); this plot indicates log 2 fold change vs −log 2 FDR-corrected p-value. Differentially expressed proteins are shown in the upper and lower right areas. See legends in Table 3.
Table 3. Differentially Expressed Proteins in the P-MAPA + IL-12 Group versus the Control Group.
| protein ID | description | % coverage | protein score | fold change |
|---|---|---|---|---|
| P62987 | ubiquitin-60S ribosomal protein L40 | 26.6 | 15.5 | 2.60 |
| P62979 | ubiquitin-40S ribosomal protein S27a | 21.8 | 15.5 | 2.60 |
| P0CG47 | polyubiquitin-B | 14.9 | 15.5 | 2.60 |
| Q9Y281 | cofilin-2 | 16.9 | 7.7 | 2.46 |
| O95678 | keratin, type II cytoskeletal 75 | 4.9 | 9.2 | 2.10 |
| Q04828 | aldo-keto reductase family 1 member C1 | 8.1 | 8.8 | 2.06 |
| P42330 | aldo-keto reductase family 1 member C3 | 7.7 | 7.6 | 2.06 |
| P52895 | aldo-keto reductase family 1 member C2 | 8.1 | 8.8 | 2.06 |
| P0CG48 | polyubiquitin-C | 5.0 | 15.5 | 2.05 |
| P28066 | proteasome subunit α type-5 | 12.0 | 7.7 | 1.98 |
| P48509 | CD151 antigen | 6.7 | 8.3 | 1.95 |
| P52565 | Rho GDP-dissociation inhibitor 1 | 8.3 | 6.3 | 1.86 |
| P81605 | dermcidin | 10.0 | 5.3 | 1.83 |
| P15259 | phosphoglycerate mutase 2 | 7.9 | 5.5 | 1.80 |
| P18669 | phosphoglycerate mutase 1 | 7.9 | 5.5 | 1.80 |
| P09429 | high mobility group protein B1 | 13.5 | 16.2 | 1.78 |
| P09104 | γ-enolase | 10.4 | 18.8 | 1.78 |
| P13929 | β-enolase | 10.4 | 18.8 | 1.78 |
| P26447 | protein S100-A4 | 26.7 | 7.6 | 1.77 |
| P17516 | aldo-keto reductase family 1 member C4 | 2.8 | 5.6 | 1.76 |
| P46782 | 40S ribosomal protein S5 | 8.3 | 7.3 | 1.75 |
| P05062 | fructose-bisphosphate aldolase B | 3.9 | 9.1 | 1.67 |
| P62937 | peptidyl-prolyl cis–trans isomerase A | 18.8 | 18.9 | 1.67 |
| B2RPK0 | putative high mobility group protein B1-like 1 | 7.1 | 7.6 | 1.65 |
| P29622 | kallistatin | 1.9 | 5.7 | 1.61 |
| Q71UI9 | histone H2A.V | 12.5 | 17.8 | 1.58 |
| P0C0S5 | histone H2A.Z | 12.5 | 17.8 | 1.58 |
| P16104 | histone H2AX | 16.1 | 17.8 | 1.51 |
| P20671 | histone H2A type 1-D | 17.7 | 17.8 | 1.51 |
| Q7L7L0 | histone H2A type 3 | 17.7 | 17.8 | 1.51 |
| P04908 | histone H2A type 1-B/E | 17.7 | 17.8 | 1.51 |
| P60763 | ras-related C3 botulinum toxin substrate 3 | 10.4 | 5.6 | –1.52 |
| P02533 | keratin, type I cytoskeletal 14 | 27.1 | 47.6 | –1.52 |
| Q9H4B7 | tubulin β-1 chain | 6.2 | 11.5 | –1.53 |
| Q71DI3 | histone H3.2 | 14.0 | 7.1 | –1.53 |
| Q16695 | histone H3.1t | 14.0 | 7.1 | –1.53 |
| P68431 | histone H3.1 | 14.0 | 7.1 | –1.53 |
| O94925 | glutaminase kidney isoform, mitochondrial | 7.9 | 21.8 | –1.53 |
| O43707 | α-actinin-4 | 15.4 | 46.5 | –1.53 |
| P19338 | nucleolin | 4.7 | 9.7 | –1.54 |
| P62873 | guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit β-1 | 9.4 | 11.9 | –1.54 |
| P60953 | cell division control protein 42 homolog | 11.5 | 7.8 | –1.54 |
| O60361 | putative nucleoside diphosphate kinase | 31.4 | 13.5 | –1.55 |
| Q9HAV0 | guanine nucleotide-binding protein subunit β-4 | 5.9 | 7.8 | –1.55 |
| P30050 | 60S ribosomal protein L12 | 24.2 | 15.6 | –1.56 |
| P06454 | prothymosin α | 23.4 | 23.5 | –1.56 |
| P40121 | macrophage-capping protein | 6.3 | 4.0 | –1.57 |
| Q9UQ80 | proliferation-associated protein 2G4 | 12.2 | 12.0 | –1.58 |
| P52907 | F-actin-capping protein subunit α-1 | 8.0 | 5.3 | –1.58 |
| P12814 | α-actinin-1 | 10.3 | 40.8 | –1.61 |
| Q00610 | clathrin heavy chain 1 | 4.8 | 21.3 | –1.62 |
| P60842 | eukaryotic initiation factor 4A-I | 10.6 | 15.9 | –1.63 |
| P30101 | protein disulfide-isomerase A3 | 19.4 | 30.0 | –1.64 |
| O75369 | filamin-B | 5.5 | 34.6 | –1.65 |
| P12235 | ADP/ATP translocase 1 | 11.1 | 16.5 | –1.66 |
| P12236 | ADP/ATP translocase 3 | 11.1 | 16.5 | –1.66 |
| P61978 | heterogeneous nuclear ribonucleoprotein K | 17.3 | 30.1 | –1.66 |
| Q01130 | serine-/arginine-rich splicing factor 2 | 10.0 | 7.4 | –1.67 |
| Q99460 | 26S proteasome non-ATPase regulatory subunit 1 | 1.0 | 3.3 | –1.68 |
| P63000 | ras-related C3 botulinum toxin substrate 1 | 10.4 | 7.9 | –1.69 |
| Q15185 | prostaglandin E synthase 3 | 19.4 | 5.5 | –1.69 |
| P62879 | guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit β-2 | 6.5 | 7.8 | –1.71 |
| P62241 | 40S ribosomal protein S8 | 13.0 | 9.8 | –1.75 |
| P05141 | ADP/ATP translocase 2 | 14.4 | 20.8 | –1.77 |
| P35241 | radixin | 10.3 | 25.2 | –1.77 |
| Q14240 | eukaryotic initiation factor 4A-II | 5.4 | 11.3 | –1.78 |
| Q9Y266 | nuclear migration protein nudC | 8.8 | 9.5 | –1.78 |
| P50914 | 60S ribosomal protein L14 | 10.7 | 11.4 | –1.84 |
| Q13509 | tubulin β-3 chain | 13.8 | 14.8 | –1.85 |
| P0DP24 | calmodulin-2 | 6.0 | 6.6 | –1.86 |
| P0DP25 | calmodulin-3 | 6.0 | 6.6 | –1.86 |
| P0DP23 | calmodulin-1 | 6.0 | 6.6 | –1.86 |
| Q9NQC3 | reticulon-4 | 2.3 | 10.2 | –1.87 |
| P08727 | keratin, type I cytoskeletal 19 | 33.3 | 54.1 | –1.88 |
| P14866 | heterogeneous nuclear ribonucleoprotein L | 4.4 | 10.8 | –1.91 |
| P35637 | RNA-binding protein FUS | 3.0 | 7.3 | –1.94 |
| P05783 | keratin, type I cytoskeletal 18 | 11.9 | 22.4 | –1.97 |
| P61313 | 60S ribosomal protein L15 | 4.4 | 6.6 | –2.00 |
| Q86V81 | THO complex subunit 4 | 4.3 | 5.1 | –2.02 |
| P23396 | 40S ribosomal protein S3 | 21.0 | 18.2 | –2.03 |
| P25788 | proteasome subunit α type-3 | 11.0 | 6.8 | –2.05 |
| Q92945 | far upstream element-binding protein 2 | 4.8 | 9.1 | –2.05 |
| P60900 | proteasome subunit α type-6 | 12.6 | 11.0 | –2.06 |
| P80723 | brain acid soluble protein 1 | 45.8 | 20.6 | –2.10 |
| Q8IVF2 | protein AHNAK2 | 1.0 | 15.5 | –2.14 |
| P35749 | myosin-11 | 2.2 | 22.4 | –2.16 |
| Q7Z406 | myosin-14 | 2.2 | 22.4 | –2.16 |
| P35580 | myosin-10 | 2.6 | 22.2 | –2.30 |
| Q99623 | prohibitin-2 | 7.0 | 5.7 | –2.34 |
| O14950 | myosin regulatory light chain 12B | 6.4 | 6.9 | –2.34 |
| P24844 | myosin regulatory light polypeptide 9 | 6.4 | 6.9 | –2.34 |
| P19105 | myosin regulatory light chain 12A | 6.4 | 6.9 | –2.34 |
| P52209 | 6-phosphogluconate dehydrogenase, decarboxylating | 6.6 | 13.4 | –2.35 |
| Q8NFI4 | putative protein FAM10A5 | 5.2 | 5.3 | –2.38 |
| P50502 | Hsc70-interacting protein | 5.2 | 5.3 | –2.38 |
| Q8IZP2 | putative protein FAM10A4 | 7.9 | 5.3 | –2.38 |
| P15880 | 40S ribosomal protein S2 | 16.0 | 26.8 | –2.50 |
| P35579 | myosin-9 | 9.7 | 62.2 | –2.51 |
| P08670 | vimentin | 31.3 | 88.0 | –2.52 |
| Q15149 | plectin | 6.4 | 71.7 | –2.61 |
| P83881 | 60S ribosomal protein L36a | 16.0 | 7.8 | –2.66 |
| Q969Q0 | 60S ribosomal protein L36a-like | 16.0 | 7.8 | –2.66 |
| P26373 | 60S ribosomal protein L13 | 14.2 | 17.2 | –2.87 |
| P78371 | T-complex protein 1 subunit β | 11.0 | 19.5 | –3.08 |
| Q9BUF5 | tubulin β-6 chain | 9.6 | 15.1 | –3.12 |
| Q02543 | 60S ribosomal protein L18a | 16.5 | 14.2 | –3.27 |
| Q07020 | 60S ribosomal protein L18 | 30.9 | 20.8 | –4.29 |
2.3. P-MAPA and IL-12 Differentially Alter Cellular Function-Related Proteins in SKOV-3 Cells
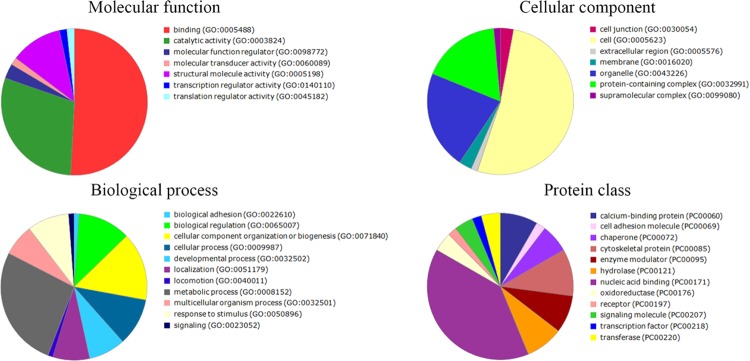
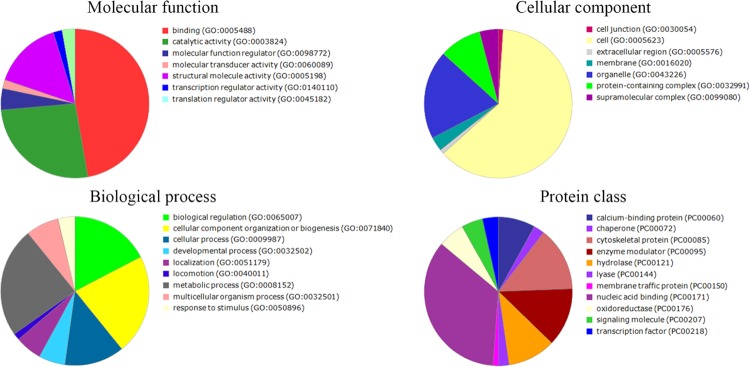
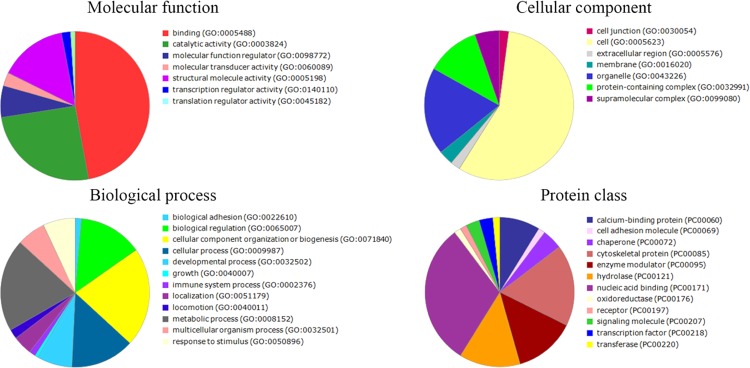
To provide another view of which categories the differentially expressed proteins belong to, gene ontology (GO) annotation was carried out. We used PANTHER classification to demonstrate the molecular function, cellular component, biological processes, and protein class whereby specific treatment was able to alter. The result was a large and complex molecule network for each treatment. The main protein functions that differentially varied after treatments with P-MAPA, IL-12, and P-MAPA + IL-12 are summarized in Figures 6–8, respectively. The proteins differentially regulated by P-MAPA, IL-12, and P-MAPA + IL-12 treatments were the most closely related to molecular functions such as binding, catalytic activity, and structural molecule activity. With regard to biological processes, the majority of these proteins were involved in a metabolic process, biological regulation, and cellular component organization or biogenesis, being represented mostly by chaperones, cytoskeletal proteins, enzyme modulators, and hydrolases. Importantly, modulation of key processes that allow cancer cells to have a metabolic advantage and increased capacity to regulate cell binding with other tissues might be an important feature of all treatments.
Figure 6.
Pie charts of the proteins that were differentially altered in SKOV-3 cells following P-MAPA treatment. PANTHER classification indicates functionally distinct proteins according to their molecular functions, biological processes, cellular components, and class.
Figure 8.
Pie charts of the proteins that were differentially altered in SKOV-3 cells following P-MAPA + IL-12 treatment. PANTHER classification indicates functionally distinct proteins according to their molecular functions, biological processes, cellular components, and class.
Figure 7.
Pie charts of the proteins that were differentially altered in SKOV-3 cells following IL-12 treatment. PANTHER classification indicates functionally distinct proteins according to their molecular functions, biological processes, cellular components, and class.
We observed increased expressions of hybrid ubiquitin-ribosomal protein L40 (RPL40) and ubiquitin-ribosomal protein s27a (RPS27a) after the treatment with P-MAPA (2.9-fold increase), IL-12 (1.94-fold increase), and P-MAPA + IL-12 (2.6-fold increase). It has long been documented that ubiquitin targets proteins for proteasome degradation or nondegradation signaling.24 Anticancer drugs, such as 5-fluorouracil, trichostatin A, and paclitaxel, often lead to overexpression of ubiquitin, which causes increased susceptibility to apoptotic cell death in a number of tumor cell lines.25 In this process, the aggregation of ubiquitylated proteins in the nucleus is an important event, and this transport can be facilitated by ubiquitin binding to either RPS27a or RPL40.25 In colorectal cancer cells, upregulation of the UBA52 gene, which encodes the ubiquitin-RPL40 hybrid protein, is related to cell-cycle arrest and apoptosis induction via activation of the RPL40-MDM2-p53 pathway, resulting in increased expression of the tumor-suppressor p53.26 Additionally, all treatments were able to upregulate polyubiquitin-B and -C, in which the chains are highly related with caspase-8 activation mediated by tumor necrosis factor receptor-associated factor 2 (TRAF2) and, consequently, sensibilization of TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis.27 Since chemoresistance and tumor aggressiveness are common challenges in the treatment of OC, the combined use of traditional chemotherapeutics and adjuvant components capable of modulating ubiquitination and promoting cell death might improve the therapy efficacy.
Among proteins that were downregulated by P-MAPA, IL-12, and P-MAPA + IL-12 treatments were myosin-9 (1.9-, 2.03-, and 2.5-fold decrease vs the control group, respectively), T-complex protein 1 (2.08-, 2.24-, and 3.08-fold decrease), and prohibitin-2 (2.24-, 2.07-, and 2.34-fold decrease). Myosin-9 is a heavy chain of myosin IIA and is involved in cytokinesis, maintenance of cell shape, and cell motility.28 It has been suggested as a possible target for anti-invasive treatment in the breast cancer cell line MCF-729 and in gastric cancer.30 Its overexpression has been related to both decreased overall survival and disease-free survival in esophageal squamous cell carcinoma (ESCC)30 and also in lung adenocarcinoma.31 T-complex 1 protein is involved in a number of biological processes, particularly in cytoskeletal organization and cell-cycle progression,32 and many studies found these proteins to be strictly related to tumor progression.33,34 Particularly, in ESCC, a T-complex protein 1 subunit expression increased cell migration and invasion via regulation of α-actin and β-tubulin, and its silencing has been suggested as a possible treatment alternative.35 Notably, prohibitins have been shown to be involved in cancer cell growth and survival, resistance to chemotherapy, immune response, and metastasis through a number of mechanisms, including p53-related transcriptional regulation, TGF-β signaling pathway modulation, and Ras/ERK pathway activation.36,37 In ovarian epithelial tumors, prohibitin-2 is thought to induce cancer cell growth and increase the susceptibility of malignant transformation,38 while prohibitin-1 promotes survival of cancer cells, acting as an antiapoptotic factor.39 Furthermore, cytoplasmic expression of prohibitin-2 is involved in hormone-related growth of breast cancer cells because it upregulates the estrogen-signaling pathway.40 Prohibitin-1 and prohibitin-2 are overexpressed in colorectal cancer, and serum concentration of these proteins, also elevated in colorectal cancer patients, was suggested as a potential biomarker for this disease.41 Prohibitin-2 clustering with glucose-related protein 75 (GPR75)/mortalin is related to increased cell proliferation and tumorigenesis in metastatic cells.42 To determine whether P-MAPA and IL-12 are capable of slowing down the tumor cell motility (as they were already proved to reduce migratory/proliferation capacity of cells), novel approaches based on timing and tumor features (subtype, grading, and staging) should be taken into consideration during OC treatment.
Among proteins that were simultaneously and significantly altered after treatments with IL-12 or the association P-MAPA + IL-12, we observed upregulation of cofilin-2 (2.41- and 2.46-fold increase vs the control group, respectively). Cofilin-2, which is known as the actin depolymerization factor, is a member of the cofilins family, being crucial modulators of actin dynamics.43 The role of human cofilin-2 (protein and gene) is controversial in cancer. Important data suggest that cofilin-2 is downregulated in pancreatic tumor tissues44 and that the ability of actin depolymerization by cofilin is associated with mitochondrial damage, cytochrome c release, and apoptosis induction in cancer cells.45 Additionally, cofilin-2 translocation to mitochondria and interaction with Drp1 constitute key events for mitochondrial fission and apoptosis induced by erucin in breast cancer cells.46 On the other hand, studies have suggested that cofilin-2 expression may play a role in malignant progression, modulating cancer cell proliferation, invasion, and metastasis.47−49 Since we previously reported a reduction in cell viability after exposure to all treatments, we believe that increased cofilin-2 levels might hamper the ovarian tumor growth by disrupting the cell metabolism, thus inducing cell death and diminishing metabolic activity.
The most positive effects of treatments with IL-12 and P-MAPA + IL-12 seem to be associated with downregulated proteins: 6-phosphogluconate dehydrogenase (6PGD) (2.51- and 2.35-fold decrease vs the control group, respectively), Hsc70-interacting protein (2.44- and 2.38-fold decrease), plectin (2.04- and 2.61-fold decrease), and myosin light chains (2.66- and 2.34-fold decrease). 6PGD is an enzyme of the oxidative pentose phosphate pathway, which is involved in anabolic biosynthesis, glycolysis, and redox homeostasis in cancer cells, thus providing metabolic advantages to cellular survival and proliferation.50,51 The activity of this enzyme is upregulated in a number of cancers and has been implicated in chemoresistance of ovarian and other tumors.52 Inhibition of 6PGD selectively targeted breast cancer cells, sparing normal breast cells53 and increasing the efficacy of chemotherapy in cervical cancer through AMPK-independent inhibition of RhoA and Rac1 activities.54 In addition, suppression of 6PGD sensitized cisplatin-resistant ovarian cancer cells to cisplatin treatment via the AMPK-dependent pathway, thus restoring its therapeutic efficacy.52 In the highly aggressive anaplastic thyroid carcinoma, 6PGD inhibition is associated with resensitization to doxorubicin treatment by decreasing levels of NADPH, NADH, and enzymatic activity of sirtuin-1.55 Briefly, the disruption of cancer cell metabolism provided by downregulation of 6PGD might be a highly positive effect of treatment with IL-12 and association of P-MAPA and IL-12, including chemosensitivity restoration.
Modulating the tumor ability for signaling and interacting with normal cells is an important aim of alternative therapy. In this context, treatment of OC cells with IL-12 and P-MAPA + IL-12 significantly reduced the levels of the carboxyl terminus Hsc70-interacting protein (CHIP). Notably, CHIP seems to have dual functions in cancer, with some studies reporting its role as a tumor suppressor and others as an oncogene.56 Decreased CHIP expression in ER-positive breast cancer and pancreatic cancer tissues is related with poor prognostic and short survival of patients.56,57 Additionally, CHIP levels are inversely correlated with tumor malignancy in gastric cancer.58 Conversely, CHIP promotes the downregulation of Profilin-1 in breast cancer cells, thus possibly playing a role in cell migration and metastasis.59 A number of studies have been implicating CHIP in the modulation of the apoptosis-inducing factor (AIF), tumor-suppressor p53, and interferon regulatory factor 1 (IRF-1).60−62 In esophageal squamous cell carcinoma, high CHIP expression is correlated with increased number of metastatic lymph nodes.63 Xu and colleagues64 reported CHIP activation of MAPK and AKT signaling activities and upregulation of E-cadherin, which led to epithelial–mesenchymal transition and enhanced migration and invasion potential of cells.
Cellular dynamics is a fundamental characteristic for cellular proliferation and, consequently, for tumor growth. Decreased levels of myosin regulatory light chain (MLC) and plectin after IL-12 and the combination of P-MAPA and IL-12 may be able to impair cancer cell dynamics, directly by avoiding cell proliferation and reducing their aggressiveness. The MLC phosphorylation seems to be an important marker for cancer cell functions, including cell growth, adhesion, and migration.65,66 Leiomyosarcomas with high proliferative activity present increased MLC phosphorylation,67,68 which is majorly measured by MLC kinase (MLCK) expression. In fact, the blockage of MLCK activities by a pharmacological inhibitor decreases breast cancer cell growth.69,70 The expression of phosphomimetic MLC is able to increase the proliferative rate calculated by Ki-67 expression.69 Another important protein to control cellular dynamics is plectin, playing key roles in cytoskeleton anchoring, signal transduction, and even in apoptosis induction.71−73 Katada and colleagues74 observed that plectin expression is significantly higher in tumor tissues compared with nontumor tissues in head and neck squamous cell carcinoma (HNSCC). Overexpression of this protein has also been reported in colorectal cancer, pancreatic cancer, and prostatic cancer.75−77 Additionally, its upregulation is directly associated with poor prognosis and increased frequency of recurrence. Plectin also activates ERK 1/2 kinases to promote migration and invasion of HNSCC cells.74 The depletion of plectin is able to impair cellular proliferation and migration in the MCF-7 breast cancer cell line78 and in PC3 prostate cancer cells.79
Finally, treatment with P-MAPA and the association of P-MAPA and IL-12 significantly reduced the levels of the key protein vimentin (1.64- and 2.52-fold decrease vs the control group, respectively). Vimentin is an intermediate filament found in various mesenchymal cells in a wide variety of tissues, responsible for the maintenance of cell and tissue integrity.80 In platinum-resistant ovarian cancer cell lineages, the reduction of vimentin levels by miRNA let-7g overexpression resulted in reduced epithelial-to-mesenchymal transition (EMT), decreased their migratory potential, and restored sensitivity to platinum-based chemotherapy.81 High expression of vimentin has also been implicated in poor prognosis in a number of other cancer types, such as breast cancer, prostate cancer, colorectal cancer, and nonsmall-cell lung cancer.82−85 Its upregulation is further associated with decreased disease-free survival and increased lymph node metastasis in colorectal cancer.85 Vimentin is a key factor for epithelial plasticity and cytoplasm architecture maintenance during the epithelial–mesenchymal transition, and its overexpression confers elevated cancer cell motility, directional migration, and metastasis.82,86,87 In the MDA-MB231 breast cancer cell line, the depletion of vimentin promoted reorganization of the cytoskeleton and decreased cell proliferation and motility.87 We believe that the association of P-MAPA with IL-12 is important to disassemble part of the cytoskeleton that regulates tumor cell aggressiveness.
In summary, our present findings reveal important mechanisms by which immunotherapy with P-MAPA and IL-12 may impact OC progression and aggressiveness. The wide variety of targets exhibited herein shows that these treatments are able to attack OC cell malignant functions through different regulatory mechanisms. Future studies associating these therapies with OC standard chemotherapy (e.g., taxanes and platinum derivatives) could also bring an important approach regarding the use of these compounds as adjuvant therapeutics.
3. Conclusions
We described a number of important changes in several protein signatures that are modulated by P-MAPA and IL-12 therapies in ovarian cancer SKOV-3 cells. While P-MAPA and IL-12 alone were efficient to upregulate structural proteins (e.g., related to tight junctions), they downregulated molecules involved in cell signaling that are associated with cancer progression. Combinatory therapy with P-MAPA and IL-12 was the most efficient to distinctively regulate proteins involved in metabolic processes, such as energy and mitochondrial processes, cell oxidation, and senescence; inhibition of these activities may render cancer cells more vulnerable to structural instability, apoptosis, and metabolical dysfunctions. This approach provides reliable insights into the cellular regulation associated with OC progression and, alternatively, suggests the therapeutic use of P-MAPA and IL-12 as a complementary strategy for OC treatment.
4. Experimental Section
4.1. Cell Line and Culture
Human OC cell line, SKOV-3, was purchased from the American Type Culture Collection (ATCC, Rockville, MD). During the experiment, SKOV-3 cells were incubated with RPMI 1640 (Life Technologies, Grand Island, NY) supplemented with 10% fetal bovine serum and 1% anti-anti solution (100 mg/mL penicillin G, and 100 μg/mL streptomycin (Merck, Darmstadt, Germany)). Cells were maintained at 37 °C in a humidified atmosphere of 5% CO2, and the culture medium was changed every 2–3 days.
4.2. P-MAPA and IL-12 Treatments
To determine the better dose–response effect, three different doses of P-MAPA (25, 50, and 100 μg/mL) were used in accordance with Fávaro.13 Initially, 5 mg of P-MAPA was diluted in 1 mL of saline to achieve a stock solution of 5 mg/mL; this solution was then diluted in the cell culture medium to obtain the proper concentrations. For the treatment with recombinant (rh)IL-12, doses of 0.5, 1, and 2 ng/mL were used in the culture medium in accordance the method of Su and colleagues.88 To combine P-MAPA with IL-12, the most representative dose and incubation time were chosen after performing the MTT assay. The saline solution was used as a solvent vehicle control and administered in the same procedures for treatments.
4.3. MTT Assay
SKOV-3 cells were seeded in a plate at a density of 1 × 103 cells/well. Cellular activity or toxicity was first evaluated using the three concentrations of both treatments, P-MAPA and IL-12, after 0, 24, 48, and 72 h exposure. All analyses were performed in three technical and biological replicates. MTT solution was added to the wells for 4 h, and the crystals were diluted with dimethyl sulfoxide (DMSO) under agitation. The concentration was determined by an Epoch microplate reader (BioTek Instruments, Highland Park, PO) at 540 nm, with the reference curve fixed at 650 nm. The percentage (%) of crystal formation was calculated based on the control group as the reference. After the testing assay, we determined the doses of 25 μg/mL P-MAPA and 1 ng/mL IL-12 and set the period of treatment at 48 h. Since the study proposed to globally identify proteins involved in potential signaling pathways that are modulated by the treatments, we defined doses and periods capable of decreasing 10–30% cell viability; higher doses could bias the results because the increased cell death might result in loss of important proteins.
4.4. Protein Quantification
After treatments, the proteins were extracted from SKOV-3 cells in biological triplicate from each experiment and were quantified in triplicate using a colorimetric method described by Bradford (BioRad Protein Assay Kit; Cod. 500-0001). Bovine serum albumin (0.1% BSA) was used as the reference protein. After quantification, individual samples were transferred to Eppendorf LoBind tubes and diluted with 0.9% (m/v) saline solution until reaching the concentration of 50 μg/40 μL for each sample.
4.5. In-Solution Trypsin Digestion
All samples were subjected to the protocol for trypsin digestion. First, 50 μg of lyophilized proteins was diluted in 50 mM ammonium bicarbonate, and 25 μL of RapiGest SF surfactant (code 186001861, Waters Corporation) was added to the samples for 60 min at 37 °C. Then, samples were reduced and alkylated with 10 mM dithiothreitol (DTT) and 45 mM iodoacetamide (IAA) at room temperature (RT) for 20 min. Enzymatic digestion was performed using trypsin (1:100; enzyme/sample) solubilized in 50 mM ammonium bicarbonate buffer (pH 7.8) for 18 h. Hydrolysis was stopped by adding 1% (v/v) formic acid to the samples for 1 h. Samples were incubated for 90 min at RT and then centrifuged at 14 000g for 30 min at 6 °C. Supernatants were removed into a new tube and subjected to desalting columns Peptide Cleanup C18 Spin (code 5188-2750 Agilent Technologies). After peptides were dried in vacuum (SpeedVac; Thermo Scientific), they were dissolved in 3% acetonitrile (ACN) with 0.1% formic acid solution before analysis.
4.6. Peptide Sequencing for Mass Spectrometry
Label-free analysis was performed using a liquid nanocromatograph (Ultimate 3000 LC Dionex, Germering, Germany) coupled to a quadrupole-orbitrap model mass spectrometer Q-Exactive (ThermoFisher Scientific, Bremen, Germany). The chromatograph was equipped with a binary system of pumps and an automatic sample applicator. The mobile phase consisted of 0.1% (v/v) formic acid in water LCMS (solvent A) and 0.1% (v/v) formic acid in 80% (v/v) ACN (solvent B). The peptides were loaded in a precolumn C18, 30 μm × 5 mm (code 164649, ThermoFisher Scientific), and desalting was carried out in an isocratic gradient of 4% B for 3 min at a flow rate of 300 nL/min. Then, peptides were fractioned using the analytical column Reprosil-Pur C18-AQ, 3 μm, 120 Å, 105 mm (code 1PCH7515-105H354-NV, PICOCHIP) using a linear gradient of 4–55% B for 30 min, 55–90% B for 1 min, maintained at 90% B for 5 min, and recalibrated at 4% B for 20 min (flow rate of 300 nL/min). Positive ionization was obtained in a Nanospray ion source (PICOCHIP) with the DDA method. Mass spectra were acquired in the mass range of m/z 200–2000, resolution of 70.000, and 100 ms for injection time. The fragmentation chamber was conditioned with collision energy between 29 and 35%, with resolution of 17.500, 50 ms of injection time, 4.0 m/z of MS/MS isolation window, and dynamic exclusion of 10 s. All samples were quantified in biological and technical triplicate. Spectrometry data were acquired using Thermo Xcalibur software (version 4.0.27.19, ThermoFisher Scientific Inc.).
4.7. Data Analysis
For proteomic analyses, the raw data.RAW was subjected to software PatternLab (version 4.0.0.84; Carvalho and colleagues)89 to identify and determine which proteins were differentially expressed. The parameters used were the Swiss-Prot database (Homo sapiens taxonomy), trypsin as the proteolytic enzyme, permission of two lost cleavages, fixed modifications of cysteine carbamidomethylation and methionine oxidation as variable modification, and tolerance errors of MS 40 ppm and MS/MS 0.0200 ppm. The false discovery rate (FDR) was set at ≤1%. We used only proteins obtained in all three runs, and the spectral counts for each protein were normalized by the weighted average of replicates of each sample. Missing data were analyzed by multiple imputation according to Royston,90 and the standard errors were computed according to the “Rubin rules”.91 Only proteins that showed statistical significance at p < 0.05 and a protein ratio less than 1.5-fold change or greater than 1.5-fold change were used. Results were compared using Student’s t test to set the differences between the groups (p < 0.05). Additional analyses of cellular components, molecular function, and biological processes were determined through Protein Annotation Through Evolutionary Relationship (PANTHER) (http://pantherdb.org/) classification, and network interactions between proteins were obtained using STRING software (http://string-db.org/) under the basic parameters of cutoff score of 0.900 (highest confidence), evidence as network edges, and PPI enrichment p-value of <1.0 × 10–16.
Acknowledgments
We are grateful to Mister Bruno Rossini for his excellent technical support, and special thanks to the Center for the Study of Venoms and Venomous Animals (CEVAP) from UNESP, Botucatu, São Paulo, Brazil.
Author Contributions
L.G.d.A.C., L.A.L.J.: collected and analyzed the data, drafted the manuscript, and conceived the main idea of the study. L.D.d.S., R.F.D., H.S.S., W.J.F., M.M., F.E.M., I.d.S.N., and M.S.C.: participated in the acquisition of the data and in the design and intellectual conception of the study. All authors approved the final version of the manuscript.
We would like to extend special thanks to Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Grant numbers: 2016/03993-9; 2019/00906-6), CAPES, and PROPG/PROPE 12/2019 for providing financial support.
The authors declare no competing financial interest.
References
- Cannistra S. A. Cancer of the ovary. N. Engl. J. Med. 2004, 35, 2519–2565. 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- Siegel R. L.; Miller K. D.; Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- Tessitore A.; Gaggiano A.; Cicciarelli G.; Verzella D.; Capece D.; Fischietti M.; Zazzeroni F.; Alesse E. Serum biomarkers identification by mass spectrometry in high-mortality tumors. Int. J. Proteomics 2013, 2013, 125858 10.1155/2013/125858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessmon P.; Boulanger T.; Zhou W.; Patwardhan P. Epidemiology and treatment patterns of epithelial ovarian cancer. Expert. Rev. Anticancer Ther. 2017, 17, 427–437. 10.1080/14737140.2017.1299575. [DOI] [PubMed] [Google Scholar]
- Chuffa L. G. A.; Seiva F. R.; Fávaro W. J.; Teixeira G. R.; Amorim J. P.; Mendes L. O.; Fioruci B. A.; Pinheiro P. F.; Fernandes A. A.; Franci J. A.; Delella F. K.; Martinez M.; Martinez F. E. Melatonin reduces LH, 17 beta-estradiol and induces differential regulation of sex steroid receptors in reproductive tissues during rat ovulation. Reprod. Biol. Endocrinol. 2011a, 9, 108. 10.1186/1477-7827-9-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuffa L. G.; Amorim J. P.; Teixeira G. R.; Mendes L. O.; Fioruci B. A.; Pinheiro P. F.; Seiva F. R.; Novelli E. L.; Mello Júnior W.; Martinez M.; Martinez F. E. Long-term melatonin treatment reduces ovarian mass and enhances tissue antioxidant defenses during ovulation in the rat. Braz. J. Med. Biol. Res. 2011b, 44, 217–223. 10.1590/S0100-879X2011007500018. [DOI] [PubMed] [Google Scholar]
- Chuffa L. G.; Fioruci-Fontanelli B. A.; Mendes L. O.; Fávaro W. J.; Pinheiro P. F.; Martinez M.; Martinez F. E. Characterization of chemically induced ovarian carcinomas in an ethanol-preferring rat model: influence of long-term melatonin treatment. PLoS One 2013, 8, e81676 10.1371/journal.pone.0081676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuffa L. G.; Fioruci-Fontanelli B. A.; Mendes L. O.; Ferreira Seiva F. R.; Martinez M.; Fávaro W. J.; Domeniconi R. F.; Pinheiro P. F.; Delazari Dos Santos L.; Martinez F. E. Melatonin attenuates the TLR4-mediated inflammatory response through MyD88- and TRIF-dependent signaling pathways in an in vivo model of ovarian cancer. BMC Cancer 2015, 6, 15–34. 10.1186/s12885-015-1032-4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Chuffa L. G. A.; Alves M. S.; Martinez M.; Camargo I. C.; Pinheiro P. F.; Domeniconi R. F.; Júnior L. A.; Martinez F. E. Apoptosis is triggered by melatonin in an in vivo model of ovarian carcinoma. Endocr. Relat. Cancer 2016, 23, 65–76. 10.1530/ERC-15-0463. [DOI] [PubMed] [Google Scholar]
- Chuffa L. G.; Lupi Júnior L. A.; Seiva F. R.; Martinez M.; Domeniconi R. F.; Pinheiro P. F.; Dos Santos L. D.; Martinez F. E. Quantitative proteomic profiling reveals that diverse metabolic pathways are influenced by melatonin in an in vivo model of ovarian carcinoma. J. Proteome Res. 2016, 15, 3872–3882. 10.1021/acs.jproteome.6b00713. [DOI] [PubMed] [Google Scholar]
- de Almeida Chuffa L. G.; de Moura Ferreira G.; Lupi L. A.; da Silva Nunes I.; Fávaro W. J. P-MAPA immunotherapy potentiates the effect of cisplatin on serous ovarian carcinoma through targeting TLR4 signaling. J. Ovarian Res. 2018, 11, 8 10.1186/s13048-018-0380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M. G.; Alvero A. B.; Chen R.; Silasi D. A.; Abrahams V. M.; Chan S.; Visintin I.; Rutherford T.; Mor G. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res. 2006, 66, 3859–3868. 10.1158/0008-5472.CAN-05-3948. [DOI] [PubMed] [Google Scholar]
- Fávaro W. J.; Nunes O. S.; Seiva F. R.; Nunes I. S.; Woolhiser L. K.; Durán N.; Lenaerts A. J. Effects of P-MAPA immunomodulator on toll-like receptors and p53: potential therapeutic strategies for infectious diseases and cancer. Infect. Agent. Cancer 2012, 7, 14. 10.1186/1750-9378-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia P. V.; Seiva F. R.; Carniato A. P.; de Mello Júnior W.; Duran N.; Macedo A. M.; de Oliveira A. G.; Romih R.; da Silva Nunes I.; da Silva Nunes O.; Fávaro W. J. Increased toll-like receptors and p53 levels regulate apoptosis and angiogenesis in non-muscle invasive bladder cancer: mechanism of action of P-MAPA biological response modifier. BMC Cancer 2016, 16, 422. 10.1186/s12885-016-2474-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003, 3, 133–146. 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- Cohen C. A.; Shea A. A.; Heffron C. L.; Schmelz E. M.; Roberts P. C. Interleukin-12 immunomodulation delays the onset of lethal peritoneal disease of ovarian cancer. J. Interferon Cytokine Res. 2016, 36, 62–73. 10.1089/jir.2015.0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurteau J. A.; Blessing J. A.; De Cesare S. L.; Creasman W. T. Evaluation of recombinant human interleukin-12 in patients with recurrent or refractory ovarian cancer: a gynecologic oncology group study. Gynecol. Oncol. 2001, 82, 7–10. 10.1006/gyno.2001.6255. [DOI] [PubMed] [Google Scholar]
- Lenzi R.; Edwards R.; June C.; Seiden M. V.; Garcia M. E.; Rosenblum M.; Freedman R. S. Phase II study of intraperitoneal recombinant interleukin-12 (rhIL-12) in patients with peritoneal carcinomatosis (residual disease <1 cm) associated with ovarian cancer or primary peritoneal carcinoma. J. Transl. Med. 2007, 5, 66. 10.1186/1479-5876-5-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odunsi K.; Wollman R. M.; Ambrosone C. B.; Hutson A.; McCann S. E.; Tammela J.; Geisler J. P.; Miller G.; Sellers T.; Cliby W.; Qian F.; Keitzm B.; Intengan M.; Lele S.; Alderfer J. L. Detection of epithelial ovarian cancer using 1H-NMR-based metabonomics. Int. J. Cancer 2005, 113, 782–788. 10.1002/ijc.20651. [DOI] [PubMed] [Google Scholar]
- Zhang T.; Wu X.; Ke C.; Yin M.; Li Z.; Fan L.; Zhang W.; Zhang H.; Zhao F.; Zhou X.; Lou G.; Li K. Identification of potential biomarkers for ovarian cancer by urinary metabolomic profiling. J. Proteome Res. 2013, 12, 505–512. 10.1021/pr3009572. [DOI] [PubMed] [Google Scholar]
- Shender V. O.; Pavlyukov M. S.; Ziganshin R. H.; Arapidiz G. P.; Kovalchukz S. I.; Anikanov N. A.; Altukhov I. A.; Alexeev D. G.; Butenko I. O.; Shavarda A. L.; Khomyakova E. B.; Evtushenko E.; Ashrafyan L. A.; Antonova I. B.; Kuznetcov I. N.; Gorbachev A. Y.; Shakhparonov M. I.; Govorun V. M. Proteome-metabolome profiling of ovarian cancer ascites reveals novel components involved in intercellular communication. Mol. Cell. Proteomics 2014, 13, 3558–3571. 10.1074/mcp.M114.041194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak P.; Pils D.; Kaider A.; Pinter A.; Elandt K.; Sax C.; Zielinski C. C.; Horvat R.; Zeillinger R.; Reinthaller A.; Krainer M. Perturbation of the tumor necrosis factor-related apoptosis-inducing ligand cascade in ovarian cancer: overexpression of FLIPL and deregulation of the functional receptors DR4 and DR5. Clin. Cancer Res. 2005, 11, 8585–8591. 10.1158/1078-0432.CCR-05-1276. [DOI] [PubMed] [Google Scholar]
- Le Moguen K.; Lincet H.; Deslandes E.; Hubert-Roux M.; Lange C.; Poulain L.; Gauduchon P.; Baudin B. Comparative proteomic analysis of cisplatin sensitive IGROV1 ovarian carcinoma cell line and its resistant counterpart IGROV1–R10. Proteomics 2006, 6, 5183–5192. 10.1002/pmic.200500925. [DOI] [PubMed] [Google Scholar]
- Hershko A. Ubiquitin: roles in protein modification and breakdown. Cell 1983, 34, 11–12. 10.1016/0092-8674(83)90131-9. [DOI] [PubMed] [Google Scholar]
- Han X. J.; Lee M. J.; Yu G. R.; Lee Z. W.; Bae J. Y.; Bae Y. C.; Kang S. H.; Kim D. G. Altered dynamics of ubiquitin hybrid proteins during tumor cell apoptosis. Cell. Death Dis. 2012, 3, e255 10.1038/cddis.2011.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q.; Hou Z.; Zuo S.; Zhou X.; Feng Y.; Sun Y.; Yuan X. LUCAT1 promotes colorectal cancer tumorigenesis by targeting the ribosomal protein L40-MDM2-p53 pathway through binding with UBA52. Cancer Sci. 2019, 110, 1194–1207. 10.1111/cas.13951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L.; Zhang Y.; Qu X.; Che X.; Guo T.; Li C.; Ma R.; Fan Y.; Ma Y.; Hou K.; Li D.; Hu X.; Liu B.; Yu R.; Yan H.; Gong J.; Liu Y. DR5-Cbl-b/c-Cbl-TRAF2 complex inhibits TRAIL-induced apoptosis by promoting TRAF2-mediated polyubiquitination of caspase-8 in gastric cancer cells. Mol Oncol. 2017, 11, 1733–1751. 10.1002/1878-0261.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H. H.; Zhang S. Y.; Shen J. H.; Wu Z. Y.; Wu J. Y.; Wang S. H.; Li E. M.; Xu L. Y. A three-protein signature and clinical outcome in esophageal squamous cell carcinoma. Oncotarget 2015, 6, 5435–5448. 10.18632/oncotarget.3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derycke L.; Stove C.; Vercoutter-Edouart A. S.; De Wever O.; Dollé L.; Colpaert N.; Depypere H.; Michalski J. C.; Bracke M. The role of non-muscle myosin IIA in aggregation and 5448 invasion of human MCF-7 breast cancer cells. Int. J. Dev. Biol. 2011, 55, 835–840. 10.1387/ijdb.113336ld. [DOI] [PubMed] [Google Scholar]
- Liang S.; He L.; Zhao X.; Miao Y.; Gu Y.; Guo C.; Xue Z.; Dou W.; Hu F.; Wu K.; Nie Y.; Fan D. MicroRNA let-7f inhibits tumor invasion and metastasis by targeting MYH9 in human gastric cancer. PLoS One 2011, 6, e18409 10.1371/journal.pone.0018409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z. K.; Yuan Y. C.; Yin N.; Yin B. L.; Tan Z. P.; Hu Y. R. Nonmuscle myosin IIA is associated with poor prognosis of esophageal squamous cancer. Dis. Esophagus 2012, 25, 427–436. 10.1111/j.1442-2050.2011.01261.x. [DOI] [PubMed] [Google Scholar]
- Brackley K. I.; Grantham J. Subunits of the chaperonin CCT interact with F-actin and influence cell shape and cytoskeletal assembly. Exp. Cell Res. 2010, 316, 543–553. 10.1016/j.yexcr.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Huang X.; Wang X.; Cheng C.; Cai J.; He S.; Wang H.; Liu F.; Zhu C.; Ding Z.; Huang X.; Zhang T.; Zhang Y. Chaperonin containing TCP1, subunit 8 (CCT8) is upregulated in hepatocellular carcinoma and promotes HCC proliferation. APMIS 2014, 122, 1070–1079. 10.1111/apm.12258. [DOI] [PubMed] [Google Scholar]
- Qiu X.; He X.; Huang Q.; Liu X.; Sun G.; Guo J.; Yuan D.; Yang L.; Ban N.; Fan S.; Tao T.; Wang D. Overexpression of CCT8 and its significance for tumor cell proliferation, migration and invasion in glioma. Pathol. Res. Pract. 2015, 211, 717–725. 10.1016/j.prp.2015.04.012. [DOI] [PubMed] [Google Scholar]
- Yang X.; Ren H.; Shao Y.; Sun Y.; Zhang L.; Li H.; Zhang X.; Yang X.; Yu W.; Fu J. Chaperonin-containing T-complex protein 1 subunit 8 promotes cell migration and invasion in human esophageal squamous cell carcinoma by regulating α-actin and β-tubulin expression. Int. J. Oncol. 2018, 52, 2021–2030. 10.3892/ijo.2018.4335. [DOI] [PubMed] [Google Scholar]
- Fusaro G.; Dasgupta P.; Rastogi S.; Joshi B.; Chellappan S. Prohibitin induces the transcriptional activity of p53 and is exported from the nucleus upon apoptotic signaling. J. Biol. Chem. 2003, 278, 47853–47861. 10.1074/jbc.M305171200. [DOI] [PubMed] [Google Scholar]
- Thuaud F.; Ribeiro N.; Nebigil C. G.; Désaubry L. Prohibitin ligands in cell death and survival: mode of action and therapeutic potential. Chem. Biol. 2013, 20, 316–331. 10.1016/j.chembiol.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L.; Yi X. F.; Zhang Z. B.; Zhuang Z. P.; Li J.; Chambers S. K.; Kong B. H.; Zheng W. Prohibitin as a novel target protein of luteinizing hormone in ovarian epithelial carcinogenesis. Neoplasma 2011, 58, 104–109. 10.4149/neo_2011_02_104. [DOI] [PubMed] [Google Scholar]
- Gregory-Bass R. C.; Olatinwo M.; Xu W.; Matthews R.; Stiles J. K.; Thomas K.; Liu D.; Tsang B.; Thompson W. E. Prohibitin silencing reverses stabilization of mitochondrial integrity and chemoresistance in ovarian cancer cells by increasing their sensitivity to apoptosis. Int. J. Cancer 2008, 122, 1923–1930. 10.1002/ijc.23351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.-W.; Akiyama M.; Park J.-H.; Lin M.-L.; Shimo A.; Ueki T.; Daigo Y.; Tsunoda T.; Nishidate T.; Nakamura Y.; Katagiri T. Activation of an estrogen/estrogen receptor signaling by BIG3 through its inhibitory effect on nuclear transport of PHB2/REA in breast cancer. Cancer Sci. 2009, 100, 1468–1478. 10.1111/j.1349-7006.2009.01209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengwasser J.; Piau A.; Schlag P.; Sleeman J. P. Differential immunization identifies PHB1/PHB2 as blood-borne tumor antigens. Oncogene 2004, 23, 7430–7435. 10.1038/sj.onc.1207987. [DOI] [PubMed] [Google Scholar]
- Martín B.; Sanz R.; Aragüés R.; Oliva B.; Sierra A. Functional clustering of metastasis proteins describes plastic adaptation resources of breast-cancer cells to new microenvironments. J. Proteome Res. 2008, 7, 3242–3253. 10.1021/pr800137w. [DOI] [PubMed] [Google Scholar]
- Maciver S. K.; Hussey P. J. The ADF/cofilin family: Actin remodeling proteins. Genome Biol. 2002, 3, reviews3007.1 10.1186/gb-2002-3-5-reviews3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Kuramitsu Y.; Ueno T.; Suzuki N.; Yoshino S.; Iizuka N.; Zhang X.; Oka M.; Nakamura K. Differential expression of up-regulated cofilin-1 and down-regulated cofilin-2 characteristic of pancreatic cancer tissues. Oncol. Rep. 2011, 26, 1595–1599. 10.3892/or.2011.1447. [DOI] [PubMed] [Google Scholar]
- Li G. B.; Cheng Q.; Liu L.; Zhou T.; Shan C. Y.; Hu X. Y.; Zhou J.; Liu E. H.; Li P.; Gao N. Mitochondrial translocation of cofilin is required for allyl isothiocyanate-mediated cell death via ROCK1/PTEN/PI3K signaling pathway. Cell Commun. Signal 2013, 11, 50. 10.1186/1478-811X-11-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G.; Zhou J.; Budhraja A.; Hu X.; Chen Y.; Cheng Q.; Liu L.; Zhou T.; Li P.; Liu E.; Gao N. Mitochondrial translocation and interaction of cofilin and Drp1 are required for erucin-induced mitochondrial fission and apoptosis. Oncotarget 2015, 6, 1834–1849. 10.18632/oncotarget.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensley P. J.; Zetter D.; Horbinski C. M.; Strup S. E.; Kyprianou N. Association of epithelial-mesenchymal transition and nuclear cofilin with advanced urothelial cancer. Hum. Pathol. 2016, 57, 68–77. 10.1016/j.humpath.2016.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maimaiti Y.; Tan J.; Liu Z.; Guo Y.; Yan Y.; Nie X.; Huang B.; Zhou J.; Huang T. Overexpression of cofilin correlates with poor survival in breast cancer: A tissue microarray analysis. Oncol. Lett. 2017, 14, 2288–2294. 10.3892/ol.2017.6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappa K. I.; Lygirou V.; Kontostathi G.; Zoidakis J.; Makridakis M.; Vougas K.; Daskalakis G.; Polyzos A.; Anagnou N. P. Proteomic analysis of normal and cancer cervical cell lines reveals deregulation of cytoskeleton-associated proteins. Cancer Genomics Proteomics 2017, 14, 253–266. 10.21873/cgp.20036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns R. A.; Harris I. S.; Mak T. W. Regulation of cancer cell metabolism. Nat. Rev. Cancer 2011, 11, 85–95. 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- Lin R.; Elf S.; Shan C.; Kang H. B.; Ji Q.; Zhou L.; Hitosugi T.; Zhang L.; Zhang S.; Seo J. H.; Xie J.; Tucker M.; Gu T. L.; Sudderth J.; Jiang L.; Mitsche M.; De Berardinis R. J.; Wu S.; Li Y.; Mao H.; Chen P. R.; Wang D.; Chen G. Z.; Hurwitz S. J.; Lonial S.; Arellano M. L.; Khoury H. J.; Khuri F. R.; Lee B. H.; Lei Q.; Brat D. J.; Ye K.; Boggon T. J.; He C.; Kang S.; Fan J.; Chen J. 6-Phosphogluconate dehydrogenase links oxidative PPP, lipogenesis and tumour growth by inhibiting LKB1-AMPK signalling. Nat. Cell Biol. 2015, 17, 1484–1496. 10.1038/ncb3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W.; Feng Q.; Liu J.; Guo Y.; Gao L.; Li R.; Xu M.; Yan G.; Yin Z.; Zhang S.; Liu S.; Shan C. Inhibition of 6-phosphogluconate dehydrogenase reverses cisplatin resistance in ovarian and lung cancer. Front. Pharmacol. 2017, 8, 421. 10.3389/fphar.2017.00421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.; Peng X.; Huang J. Inhibiting 6-phosphogluconate dehydrogenase selectively targets breast cancer through AMPK activation. Clin. Transl. Oncol. 2018, 20, 1145–1152. 10.1007/s12094-018-1833-4. [DOI] [PubMed] [Google Scholar]
- Guo H.; Xiang Z.; Zhang Y.; Sun D. Inhibiting 6-phosphogluconate dehydrogenase enhances chemotherapy efficacy in cervical cancer via AMPK-independent inhibition of RhoA and Rac1. Clin. Transl. Oncol. 2019, 21, 404–411. 10.1007/s12094-018-1937-x. [DOI] [PubMed] [Google Scholar]
- Ma L.; Cheng Q. Inhibiting 6-phosphogluconate dehydrogenase reverses doxorubicin resistance in anaplastic thyroid cancer via inhibiting NADPH-dependent metabolic reprogramming. Biochem. Biophys. Res. Commun. 2018, 498, 912–917. 10.1016/j.bbrc.2018.03.079. [DOI] [PubMed] [Google Scholar]
- Cao Z.; Li G.; Shao Q.; Yang G.; Zheng L.; Zhang T.; Zhao Y. CHIP: A new modulator of human malignant disorders. Oncotarget 2016, 7, 29864–29874. 10.18632/oncotarget.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurozumi S.; Yamaguchi Y.; Hayashi S.; Hiyoshi H.; Suda T.; Gohno T.; Matsumoto H.; Takei H.; Horiguchi J.; Takeyoshi I.; Oyama T.; Kurosumi M. Prognostic value of the ubiquitin ligase carboxyl terminus of the Hsc70-interacting protein in postmenopausal breast cancer. Cancer Med. 2016, 5, 1873–1882. 10.1002/cam4.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S.; Wu X.; Zhang J.; Chen Y.; Xu J.; Xia X.; He S.; Qiang F.; Li A.; Shu Y.; Røe O. D.; Li G.; Zhou J. W. CHIP functions as a novel suppressor of tumour angiogenesis with prognostic significance in human gastric cancer. Gut 2013, 62, 496–508. 10.1136/gutjnl-2011-301522. [DOI] [PubMed] [Google Scholar]
- Choi Y. N.; Lee S. K.; Seo T. W.; Lee J. S.; Yoo S. J. C-Terminus of Hsc70-interacting protein regulates profilin1 and breast cancer cell migration. Biochem. Biophys. Res. Commun. 2014, 446, 1060–1066. 10.1016/j.bbrc.2014.03.061. [DOI] [PubMed] [Google Scholar]
- Esser C.; Scheffner M.; Hohfeld J. The chaperone-associated ubiquitin ligase CHIP is able to target p53 for proteasomal degradation. J. Biol. Chem. 2005, 280, 27443–27448. 10.1074/jbc.M501574200. [DOI] [PubMed] [Google Scholar]
- Narayan V.; Pion E.; Landre V.; Muller P.; Ball K. L. Docking-dependent ubiquitination of the interferon regulatory factor-1 tumor suppressor protein by the ubiquitin ligase CHIP. J. Biol. Chem. 2011, 286, 607–619. 10.1074/jbc.M110.153122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh K. H.; Yang S. W.; Park J. M.; Seol J. H.; Iemura S.; Natsume T.; Murata S.; Tanaka K.; Jeon Y. J.; Chung C. H. Control of AIF-mediated cell death by antagonistic functions of CHIP ubiquitin E3 ligase and USP2 deubiquitinating enzyme. Cell Death Differ. 2011, 18, 1326–1336. 10.1038/cdd.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J.; Luo K. J.; Hu Y.; Yang H.; Fu J. H. Metastatic lymph node CHIP expression is a potential prognostic marker for resected esophageal squamous cell carcinoma patients. Ann. Surg. Oncol. 2013, 20, 1668–1675. 10.1245/s10434-012-2733-4. [DOI] [PubMed] [Google Scholar]
- Xu J.; Zhou J.; Dai H.; Liu F.; Li W.; Wang W.; Guo F. CHIP functions as an oncogene by promoting colorectal cancer metastasis via activation of MAPK and AKT signaling and suppression of E-cadherin. J. Transl. Med. 2018, 16, 169. 10.1186/s12967-018-1540-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia D.; Stull J. T.; Kamm K. E. Myosin phosphatase targeting subunit 1 affects cell migration by regulating myosin phosphorylation and actin assembly. Exp. Cell Res. 2005, 304, 506–517. 10.1016/j.yexcr.2004.11.025. [DOI] [PubMed] [Google Scholar]
- Su L.; Nalle S. C.; Shen L.; Turner E. S.; Singh G.; Breskin L. A.; Khramtsova E. A.; Khramtsova G.; Tsai P. Y.; Fu Y. X.; Abraham C.; Turner J. R. TNFR2 activates MLCK- dependent tight junction dysregulation to cause apoptosis-mediated barrier loss and experimental colitis. Gastroenterology 2013, 145, 407–415. 10.1053/j.gastro.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D.; Weinberg R. A. Hallmarks of cancer: the next generation. Cell 2011, 144, 646–674. 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Li H. S.; Lin Q.; Wu J.; Jiang Z. H.; Zhao J. B.; Pan J.; He W. Q.; Zha J. M. Myosin regulatory light chain phosphorylation is associated with leiomyosarcoma development. Biomed. Pharmacother. 2017, 92, 810–818. 10.1016/j.biopha.2017.05.139. [DOI] [PubMed] [Google Scholar]
- Yamashiro S.; Yamakita Y.; Totsukawa G.; Goto H.; Kaibuchi K.; Ito M.; Hartshorne D. J.; Matsumura F. Myosin phosphatase-targeting subunit 1 regulates mitosis by antagonizing polo-like kinase 1. Dev. Cell 2008, 14, 787–797. 10.1016/j.devcel.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X.; Liu Y.; You J.; Zhang H.; Zhang X.; Ye L. Myosin light-chain kinase contributes to the proliferation and migration of breast cancer cells through cross-talk with activated ERK1/2. Cancer Lett. 2008, 270, 312–327. 10.1016/j.canlet.2008.05.028. [DOI] [PubMed] [Google Scholar]
- Andra K.; Nikolic B.; Stocher M.; Drenckhahn D.; Wiche G. Not just scaffolding: plectin regulates actin dynamics in cultured cells. Genes Dev. 1998, 12, 3442–3451. 10.1101/gad.12.21.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiche G. Role of plectin in cytoskeleton organization and dynamics. J. Cell Sci. 1998, 111, 2477–2486. [DOI] [PubMed] [Google Scholar]
- Stegh A. H.; Herrmann H.; Lampel S.; Weisenberger D.; Andrä K.; Seper M.; Wiche G.; Krammer P. H.; Peter M. E. Identification of the cytolinker plectin as a major early in vivo substrate for caspase 8 during CD95- and tumor necrosis factor receptor-mediated apoptosis. Mol. Cell. Biol. 2000, 20, 5665–5679. 10.1128/MCB.20.15.5665-5679.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katada K.; Tomonaga T.; Satoh M.; Matsushita K.; Tonoike Y.; Kodera Y.; Hanazawa T.; Nomura F.; Okamoto Y. Plectin promotes migration and invasion of cancer cells and is a novel prognostic marker for head and neck squamous cell carcinoma. J. Proteomics 2012, 75, 1803–1815. 10.1016/j.jprot.2011.12.018. [DOI] [PubMed] [Google Scholar]
- Nagle R. B.; Hao J.; Knox J. D.; Dalkin B. L.; Clark V.; Cress A. E. Expression of hemidesmosomal and extracellular matrix proteins by normal and malignant human prostate tissue. Am. J. Pathol. 1995, 146, 1498–1507. [PMC free article] [PubMed] [Google Scholar]
- Lee K. Y.; Liu Y. H.; Ho C. C.; Pei R. J.; Yeh K. T.; Cheng C. C.; Lai Y. S. An early evaluation of malignant tendency with plectin expression in human colorectal adenoma and adenocarcinoma. J. Med. 2004, 35, 141–149. [PubMed] [Google Scholar]
- Kelly K. A.; Bardeesy N.; Anbazhagan R.; Gurumurthy S.; Berger J.; Alencar H.; Depinho R. A.; Mahmood U.; Weissleder R. Targeted nanoparticles for imaging incipient pancreatic ductal adenocarcinoma. PLoS Med. 2008, 5, e85 10.1371/journal.pmed.0050085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boczonadi V.; McInroy L.; Maatta A. Cytolinker cross-talk: periplakin N-terminus interacts with plectin to regulate keratin organisation and epithelial migration. Exp. Cell Res. 2007, 313, 3579–3591. 10.1016/j.yexcr.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Burch T. C.; Watson M. T.; Nyalwidhe J. O. Variable metastatic potentials correlate with differential plectin and vimentin expression in syngeneic androgen independent prostate cancer cells. PLoS One 2013, 8, e65005 10.1371/journal.pone.0065005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe P. A.; Wong P. Cytoplasmic intermediate filaments revealed as dynamic and multipurpose scaffolds. Nat. Cell Biol. 2004, 6, 699–706. 10.1038/ncb0804-699. [DOI] [PubMed] [Google Scholar]
- Biamonte F.; Santamaria G.; Sacco A.; Perrone F. M.; Di Cello A.; Battaglia A. M.; Salatino A.; Di Vito A.; Aversa I.; Venturella R.; Zullo F.; Costanzo F. MicroRNA let-7g acts as tumor suppressor and predictive biomarker for chemoresistance in human epithelial ovarian cancer. Sci. Rep. 2019, 9, 5668 10.1038/s41598-019-42221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satelli A.; Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol. Life Sci. 2011, 68, 3033–3046. 10.1007/s00018-011-0735-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtinen L.; Ketola K.; Makela R.; Mpindi J. P.; Viitala M.; Kallioniemi O.; Iljin K. High-throughput RNAi screening for novel modulators of vimentin expression identifies MTHFD2 as a regulator of breast cancer cell migration and invasion. Oncotarget 2013, 4, 48–63. 10.18632/oncotarget.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadokoro A.; Kanaji N.; Liu D.; Yokomise H.; Haba R.; Ishii T.; Takagi T.; Watanabe N.; Kita N.; Kadowaki N.; Bandoh S. Vimentin Regulates Invasiveness and Is a Poor Prognostic Marker in Non-small Cell Lung Cancer. Anticancer Res. 2016, 36, 1545–1551. [PubMed] [Google Scholar]
- Du L.; Li J.; Lei L.; He H.; Chen E.; Dong J.; Yang J. High vimentin expression predicts a poor prognosis and progression in colorectal cancer: a study with meta-analysis and TCGA database. Biomed. Res. Int. 2018, 2018, 6387810 10.1155/2018/6387810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W.; Grund C.; Kuhn C.; Jackson B. W.; Illmensee K. Formation of cytoskeletal elements during mouse embryogenesis. III. Primary mesenchymal cells and the first appearance of vimentin filaments. Differentiation 1982, 23, 43–59. 10.1111/j.1432-0436.1982.tb01266.x. [DOI] [PubMed] [Google Scholar]
- Liu C. Y.; Lin H. H.; Tang M. J.; Wang Y. K. Vimentin contributes to epithelial-mesenchymal transition cancer cell mechanics by mediating cytoskeletal organization and focal adhesion maturation. Oncotarget 2015, 6, 15966–15983. 10.18632/oncotarget.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W.; Ito T.; Oyama T.; Kitagawa T.; Yamori T.; Fujiwara H.; Matsuda H. The direct effect of IL-12 on tumor cells: IL-12 acts directly on tumor cells to activate NF-kappaB and enhance IFN-gamma-mediated STAT1 phosphorylation. Biochem. Biophys. Res. Commun. 2001, 280, 503–512. 10.1006/bbrc.2000.4150. [DOI] [PubMed] [Google Scholar]
- Carvalho P. C.; Lima D. B.; Leprevost F. V.; Santos M. D.; Fischer J. S.; Aquino P. F.; Moresco J. J.; Yates J. R. 3rd; Barbosa V. C. Integrated analysis of shotgun proteomic data with PatternLab for proteomics 4.0. Nat. Protoc. 2016, 11, 102–117. 10.1038/nprot.2015.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royston P. Multiple imputation of missing values. Stata J. 2004, 227–241. 10.1177/1536867X0400400301. [DOI] [Google Scholar]
- Rubin D. B.Multiple Imputation for Non-response in Surveys; John Wiley & Sons: New York, 1987. [Google Scholar]