Abstract
Chronic hyperglycemia in type 2 diabetes mellitus increases oxidative stress and inflammation which contributes to long-term diabetic kidney disease. Tocotrienol-rich vitamin E, as Tocovid, has been shown to reduce oxidative stress and inflammation to ameliorate diabetes in rat models and human subjects. In this prospective, multicenter, double-blinded, placebo-controlled clinical trial, 54 patients (duration = 18.4 years, HbA1c = 8.8%) with diabetic nephropathy were randomized to receive Tocovid 200 mg or placebo for 12 weeks. Fasting blood samples were taken to measure HbA1c, serum creatinine, estimate glomerular filtration rate (eGFR), urine albumin:creatinine ratio, malondialdehyde, tumor necrosis factor receptor-1, vascular cell adhesion molecule-1 (VCAM-1), and thromboxane-B2. Patients were reassessed 6–9 months post-washout. After 12 weeks of supplementation, Tocovid significantly decreased serum creatinine levels (mean difference: –3.3 ± 12.6 versus 5.4 ± 14.2, p = 0.027) and significantly increase eGFR (mean difference: 1.5 ± 7.6 versus –2.9 ± 8.0, p = 0.045) compared with placebo. There were no significant changes in HbA1c, blood pressure, and other parameters. Subgroup analysis revealed that in patients with low serum vitamin E concentrations at baseline, Tocovid reduced serum creatinine, eGFR, and VCAM-1 significantly. After 6–9 months of washout, persistent difference in serum creatinine remained between groups (mean difference: 0.82 ± 8.33 versus 11.26 ± 15.47, p = 0.031), but not eGFR. Tocovid at 400 mg/day significantly improved renal function in 12 weeks of supplementation, as assessed by serum creatinine and eGFR, which remained significant 6–9 months post-washout.
Keywords: anti-inflammatory, antioxidant, diabetes, diabetic nephropathy, vitamin E, tocotrienol
Introduction
Type 2 diabetes mellitus (T2DM) is a disease characterized by insufficient production of insulin and insulin resistance leading to chronic hyperglycemia. The prevalence of T2DM has increased steadily over the past few decades and has reached pandemic levels worldwide. The World Health Organization has reported that in 2012, 422 million people were afflicted with this disease, the prevalence has increased from 4.7% in 1980 to 8.5% in 2012 worldwide.1
Diabetic nephropathy (DN) is a complication of diabetes and is the leading cause of end-stage renal disease (ESRD), which requires long-term dialysis or renal transplant. The mainstay of DN management are strict glycemic, blood pressure, and cholesterol control.2 Recently, the CREDENCE study (2019) has shown that canagliflozin, an SGLT2-inhibitor, reduced the incidence of ESRD.3 Despite these advances in diabetic management, patients still developed albuminuria and renal failure, indicating further avenues for treatment advancement in DN.
The pathophysiology of DN is complex. The crux of the current theory points to chronic hyperglycemia, which accelerates the production of superoxide anions in the mitochondria of endothelial cells. These superoxide anions are then converted into a variety of reactive oxygen species (ROS), which cause increased production of advanced glycation endproducts (AGEs), elevated hexosamine pathway flux, increased polyol pathway flux (reducing NADPH and glutathione), and activation of protein kinase C. These pathways culminate in the net increase of inflammation resulting in the macrovascular and microvascular complications of diabetes.4,5 The interplay between oxidative stress and inflammation provides a pathway for a strong antioxidant and anti-inflammatory agent to act upon.
Vitamin E is a fat-soluble antioxidant with anti-inflammatory properties, however the role of vitamin E in diabetes and DN remains controversial. The MICRO-HOPE study (2000) showed that daily administration of tocopherol for 4.5 years did not reduce cardiovascular or nephropathy outcomes.6 Suksomboon et al. performed a systematic review and found that vitamin E supplementation did not improve glycemic control.7 This finding was supported by a meta-analysis performed by Xu et al., which found insufficient evidence to support the role of tocopherol supplementation, as it did not yield a significant reduction in HbA1c.8 These studies, however, focus only on tocopherol, an isoform of vitamin E. In contrast, our study focuses on tocotrienol, which is thought to be the superior isoform of vitamin E.9
Studies have shown that tocotrienol can neutralize peroxyl radicals and abate lipid peroxidation better than tocopherol.10,11 Tocotrienols are better antioxidants because the isoforms are distributed more homogenously in the cellular bilayer than tocopherols and disrupts membrane lipids which improves its efficiency in interacting with lipid radicals.12 Multiple preclinical studies have shown promise in this area. Tocotrienol-rich fraction (TRF) has been shown to attenuate DN in rat by downregulating inflammatory and profibrotic cytokines as such transforming growth factor beta-1 (TGF-β1), tumor necrosis factor-α (TNF-α), and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB).13,14 Furthermore, Cheng et al. have shown that in diabetic rats, TRF reduced HbA1c, blood pressure, cholesterol, AGE, and receptor of AGE (RAGE).15 Tan et al. found that 2 months of supplementation of tocotrienol-rich vitamin E as Tocovid significantly reduced serum creatinine in patients with DN, but not HbA1c, urine albumin:creatinine ratio (UACR), AGE, RAGE, Nε-CML, or Cystatin C.16
The meta-analyses by Suksomboon et al. and Xu et al. were on HbA1c, which is not a reliable marker to predict the progression and development of diabetic complications. The 10-year follow up to the UKPDS trial showed that patients in the original intervention group, despite having similar HbA1c in the control group after the trial, had persistently lower risk of developing microvascular (Relative risk, RR = 0.76) and macrovascular (RR = 0.85) complications.17 HbA1c is a short-term marker and does not necessarily reflect long-term exposure to glycemic damage. Chronic hyperglycemia is the main driving force of diabetic complications, as it drives the oxidative stress and inflammatory pathway leading to cellular and tissue damage.18,19 As such, management of diabetic complications should not only target HbA1c but also address these downstream pathways and the resulting complications.
The bulk of research regarding the role of vitamin E in diabetes pertains to tocopherol and not tocotrienol, which is the superior isoform of vitamin E. Studies regarding the role of tocotrienol-rich vitamin E in diabetes only consisted 3–4% of all research studies on vitamin E.20
This primary aim of the study was to investigate the effect of high-dose tocotrienol-rich vitamin E on DN, as assessed by UACR, serum creatinine, and estimated glomerular filtration rate (eGFR). The second aim was to investigate the effect of tocotrienol-rich vitamin E on biomarkers of inflammation and oxidative stress, namely malondialdehyde (MDA), tumor necrosis factor receptor-1 (TNFR-1), and vascular cell adhesion molecule-1 (VCAM-1). Third, we determine whether the effects of tocotrienol-rich vitamin E on the kidneys persist after 6–9 months of washout.
Materials and methods
Study design
Our study was a prospective, multicenter, double-blinded, placebo-controlled, randomized controlled trial to assess the effect of tocotrienol-rich vitamin E versus placebo on DN. Patients were treated in two different clinical sites within one country. Our team previously reported in our interim analysis, that 2 months of high-dose supplementation resulted in a reduction in serum creatinine compared with placebo.16
All patients from the study were followed up 6–9 months after the trial period to check on their progress. These patients’ blood was collected and compared with their stored sera from 2018. These samples were used to measure any changes at 6–9 months after stopping vitamin E supplementation.
Participants
Inclusion criteria
Participants aged between 18 and 80 years with T2DM who have stable HbA1c control (no more than 10% change over the previous 2 months) were eligible for our study. Patients with hypertension must have stable blood pressure control and should be less than 150/90 mmHg.
Patients were eligible for the study if they had chronic kidney disease, defined by (i) reduced eGFR (30–60 ml/min/1.73 m2) or (ii) microalbuminuria (UACR > 10 mg/mmol]. This is the benchmark of moderate severity kidney disease as defined by the KDOQI guidelines.2 Importantly, it is potentially reversible by tocotrienol supplementation. Patients with severe renal impairment (eGF < 15 ml/min/1.73 m2) were excluded.
Exclusion criteria
Kidney disease must be attributed to DN alone, hence patients with poor blood pressure control (>160/100 mmHg) or a nondiabetic kidney disease were excluded from the study. These illnesses include, but are not limited to, analgesic abuse, kidney stones, minimal change disease, multiple myeloma, glomerulopathies, and untreated urinary tract infection. Patients with such illnesses may result in falsely high urine protein or reduced eGFR irrespective of diabetes.
Patients were excluded if they were taking any water-soluble antioxidants such as vitamin C, glutathione, or polyphenols in the past month, or if they were taking any fat-soluble antioxidants such as vitamin E in the past 2 months. Patients were also excluded if they have any recent severe or acute illnesses such as active cancer, acute coronary syndrome, and liver diseases.
Screening visit
Participants were recruited from a pool of existing patients who come for regular diabetic reviews at the Clinical Research Centre (CRC) in Monash University, Sunway Campus, and the CRC of Clinical School Johor Bahru. Some patients were referred by consultant endocrinologists or family doctors if they deemed the patient fit for study. The patients were selected based on their past medical records to determine their eligibility status.
Informed consent was obtained from all participants before the screening began. A thorough history and physical examination was conducted, followed by anthropometric measurements. The patient’s blood pressure, fasting blood glucose, and HbA1c were measured. Safety tests such as liver function tests, lipid profile, and electrocardiogram (ECG) were also conducted. Suitable participants were then invited to come back for randomization 2–4 weeks after screening.
Randomization and blinding
All participants were matched according to their gender, duration of diabetes, and HbA1c. Subsequently, randomization was conducted in a 1:1 ratio using a computer-generated random sequence and stratified according to gender (male or female), the HbA1c level at screening (<8.0% or ⩾8.0%), and duration of diabetes (<15 years or ⩾15 years). The intervention group was given high-dose tocotrienol-rich vitamin E (Tocovid SupraBioTM) 200 mg twice daily and the control group was given placebo.
The ingredient of the Tocovid SupraBioTM, called EVNol SupraBio, was manufactured by ExcelVite, Malaysia. This dosage was selected based on previous studies which showed that Tocovid at low doses did not yield significant results.21 The dose is the maximum dose approved by the US Food and Drug Administration (FDA).
The identity of investigational products was kept confidential by the manufacturers from the researchers and participants involved to avoid selection or performance bias. A drug code was assigned by an independent party for all patients to conceal the allocation from both participants and researchers.
Follow-up visits
Participants were followed up for monthly visits to monitor for compliance and any adverse events while taking the study drug. Compliance to the study drug was assessed by doing a pill count. Anthropometric measurements, blood pressure, and finger-prick fasting glucose test were carried out at every follow-up visit as per standard diabetic care. Fasting bloods were taken during every visit to test for renal profile, biomarkers, and safety tests.
Patients were followed up by their regular family doctor or endocrinologist to receive standard of care as per local guidelines. Any changes in medication were recorded. Investigators were encouraged to advise patients to control risk factors: these were guided by best practice in line with local guidelines.
Sample size
The power calculations were based on the ability to detect a 30% reduction in UACR in the primary analysis of Tocovid compared with placebo, assuming a 5% standard deviation (SD) of effect (α = 0.05 and 1 – β = 0.8) and an anticipated dropout rate of 4%. Since there are 3 variables used as primary outcomes, Bonferroni correction was used to correct the power analysis, α = 0.05/3 was used. To fulfill these specifications, 88 subjects were required.
Assessment of outcomes
The primary outcome variables of this study were serum creatinine, UACR, and eGFR. The secondary outcome variables of this study were HbA1c, serum uric acid, urea, MDA, TNFR-1, VCAM-1, and thromboxane B2.
We collected 18 ml of fasting blood from each patient and stored it in serum-separating tubes (SSTs). The samples were centrifuged (Eppendorf Centrifuge 5702R, Hamburg, Germany) at 3600 rpm for 15 min. The serum was then extracted and aliquoted into separate 1 ml Eppendorf tubes. One Eppendorf tube was sent to a national certified pathology lab on the same day for analysis of serum creatinine, eGFR, lipid profile, and liver function tests (ARCHITECT, Abbott diagnostic, Abbott Park, IL). eGFR was calculated by the lab using the CKD-EPI formula and presented as milliliters per minute per 1.73 m2. The coefficient variances for the tests were below 6%.
The remaining Eppendorf tubes with serum samples were stored in −80°C on the same day. These biomarkers were measured by using enzyme-linked immunosorbent assay (ELISA) and their respective ELISA kits. Processing of the specimens could only be done at the end of the study on a batch-to-batch basis to reduce inter-assay variation.
ELISA was performed in duplicates and quantified by calorimetric method. An ELISA plate reader (TECAN Infinite 200 PRO, Switzerland) was used. The ELISA kit for MDA (Elabscience E-EL-0060, United States), TNFR-1 (Elabscience E-EL-H0217), VCAM-1 (Elabscience E-EL-H5587), and thromboxane (Elabscience E-EL-H2191) have intra-assay coefficient variances of <4% and inter-assay coefficient variances of <8%.
One remaining Eppendorf tube above was used to analyze serum tocopherol concentration. The participants’ tocopherol levels were measured using high-performance liquid chromatography (HPLC; HPLC 1200, Agilent, Santa Clara, CA) with fluorescence detector. The serum sample was prepared using specified methods by Liu et al.22 and tocopherol was extracted for measurement.
Urine samples were sent to the same pathology lab on the same day they were collected to assess for UACR. The UACR kit (ARCHITECT) has coefficient variances of less than 6% for microalbuminuria and less than 5% for urine creatinine. UACR measurement was performed twice on two different days to obtain an average for the baseline reading.
Statistical analysis
All statistical analyses were performed using SPSS version 24 (IBM SPSS Inc., Chicago, IL).
To determine changes seen between each visit, the mean difference of each parameter between the first and final visits were taken as a continuous variable. These variables were then evaluated for normality using the Shapiro–Wilk test. If the data was parametric, independent t test was then performed on these variables to determine the average change seen between intervention and control group. For nonparametric data, Mann–Whitney test was conducted. Effect size was then calculated between groups using the sample means of both groups post-intervention (12 weeks) and the pooled standard deviation.
To demonstrate the persistent changes seen, the same was done between the final visit and post-washout. Per protocol analysis was done as large amount of attrition made imputation unfeasible.
Post hoc subgroup analysis was done based on serum tocopherol levels at baseline. As previous animal studies largely relied on vitamin E deficient models, our team aimed to determine whether effects are seen globally or whether the effects are constrained only to the subgroup with lower tocopherol concentration.
Ethics
The study was carried out in accordance with the Declaration of Helsinki, and the study protocol was approved by the Monash University Human Research Ethics Committee (project number: 12090). The patient explanatory statement and consent forms in English and Bahasa Malaysia were reviewed and approved by MUHREC for the recruitment of participants. The clinical trial was carried out in accordance with the terms and conditions set by the Australian Code for the Responsible Conduct of Research.
Results
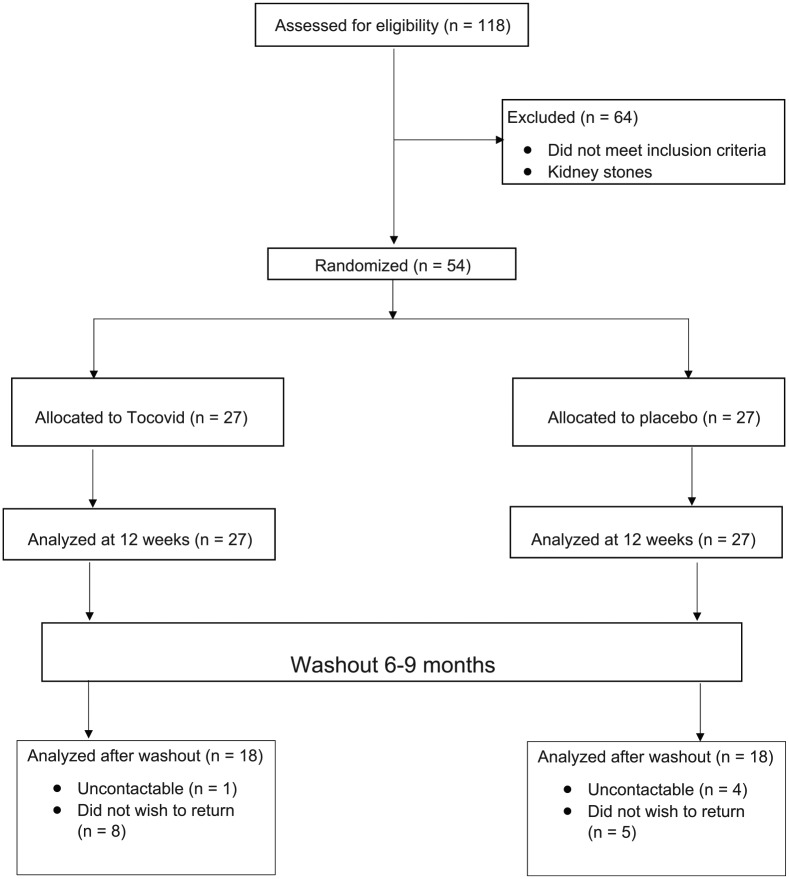
A total of 118 T2DM patients were screened over 2 months. Of these, 61 subjects did not meet the inclusion criteria and were excluded. Another three male subjects were excluded because of kidney stones that caused hematuria. A total of 54 patients were included in the study; 27 in the intervention group and 27 in the placebo group. All 54 patients were analyzed at 12 weeks post-supplementation.
At 6–9-month follow-up post-washout, 18 patients out of the original cohort did not return (33.3% of all patients), of which 9 were from the intervention group and 9 were from the control group. Five patients were not contactable, the remaining 13 patients expressed that they did not wish to return. Overall, 18 patients from the intervention group and 18 patients from the placebo group were included in the analysis at 6–9 months post-washout.
The summary of the patient flow diagram is shown in Figure 1.
Figure 1.
Patient flow diagram.
Baseline characteristics
At baseline, the mean age of all patients was 61.3 with an average duration of diabetes of 18.4 years, 35.2% of all patients were female and had a mean HbA1c of 8.8%. Overall, most patients have stage 3A chronic kidney disease assessed by eGFR of 45–59 ml/min/1.73 m2 and moderate to severe albuminuria (>30 mg/mmol). There were no significant differences in all parameters measured at baseline. The baseline characteristics of all 54 patients were similar between both groups as shown in Table 1.
Table 1.
Baseline characteristics before Tocovid supplementation.
| Characteristic | Intervention
group (n = 27) |
Control
group (n = 27) |
p valuea |
|---|---|---|---|
| Sociodemographic | |||
| Age, years | 59 ± 10 | 62.8 ± 11.6 | 0.321 |
| Female (%) | 9 (33.3) | 10 (37.0) | 0.776b |
| Duration of DM, years | 20.7 ± 9.9 | 16.2 ± 8.1 | 0.072 |
| General | |||
| HbA1c, % | 9.0 ± 2.0 | 8.7 ± 1.4 | 0.423 |
| SBP, mmHg | 136 ± 18 | 139 ± 15 | 0.601 |
| DBP, mmHg | 77 ± 10 | 79 ± 9 | 0.617 |
| BMI, kg/m2 | 29.4 ± 5.4 | 29.3 ± 4.7 | 0.978 |
| Renal parameters | |||
| eGFR, ml/min/1.73 m2 | 61.0 ± 23.2 | 59.5 ± 26.0 | 0.617 |
| Serum creatinine, µmol/l | 119.9 ± 53 | 122.4 ± 57 | 0.915 |
| UACR, mg/mmolc | 37.5 (65.0) | 54.6 (99.7) | 0.424c |
| Urea, mmol/l | 5.79 ± 1.8 | 6.95 ± 2.8 | 0.346 |
| Uric acid, mmol/l | 401 ± 100 | 395 ± 120 | 0.843 |
| Biomarkers | |||
| Tocopherol. µmol/l | 42.8 ± 20.0 | 40.8 ± 13.0 | 0.726 |
| TNFR1, pg/ml | 57.8 ± 37 | 40.6 ± 27.3 | 0.099 |
| MDA, ng/ml | 1137 ± 454 | 1133 ± 234 | 0.976 |
| VCAM-1, ng/ml | 77.7 ± 47.9 | 55.7 ± 32.0 | 0.105 |
| Thromboxane-B2, pg/ml | 122.0 ± 61.4 | 114.4 ± 67.3 | 0.711 |
All data presented as mean ± standard deviation.
Obtained from independent t test.
Obtained from chi-squared test.
Urine albumin:creatinine ratio (UACR) presented as mean (interquartile range), p value obtained using Mann–Whitney test.
MDA, malondialdehyde; TNFR1, tumor necrosis factor receptor-1; VCAM-1, vascular cell adhesion molecule-1.
Twelve weeks post-Tocovid supplementation
Table 2 shows the treatment changes between the intervention and control groups at the end of 12 weeks of supplementation. An increase in eGFR denotes an improvement in renal function, whereas for all other parameters a decrease denotes improvement.
Table 2.
Comparison between intervention and control groups after 12 weeks of supplementation.
| Parameters | Intervention group (n = 27) |
Control group (n = 27) |
p valuea | Effect size | ||||
|---|---|---|---|---|---|---|---|---|
| Baseline | At 12 weeks of supplementation | Change | Baseline | At 12 weeks of supplementation | Change | |||
| General | ||||||||
| HbA1c, % | 9.0 ± 2.0 | 8.4 ± 1.5 | −0.60 ± 0.95 | 8.7 ± 1.4 | 8.3 ± 1.2 | −0.38 ± 0.89 | 0.397 | 0.19 |
| SBP, mmHg | 136.2 ± 18.4 | 130.5 ± 14.9 | −5.7 ± 14 | 138.8 ± 15 | 137.1 ± 16.4 | −1.7 ± 14 | 0.299 | 0.42 |
| DBP, mmHg | 77.0 ± 10.2 | 77.9 ± 11.2 | 0.9 ± 8 | 78.5 ± 9.4 | 77.6 ± 10.5 | −0.9 ± 6 | 0.383 | 0.03 |
| Renal parameters | ||||||||
| eGFR, ml/min/1.73 m2 | 61.0 ± 23.2 | 62.7 ± 24.7 | 1.5 ± 7.6 | 59.5 ± 26.0 | 56.7 ± 25.2 | –2.9 ± 8.0 | 0.045 * | 0.29 |
| Serum creatinine, µmol/l | 119.9 ± 53 | 116.6 ± 54 | –3.3 ± 12.6 | 122.4 ± 57 | 127.7 ± 57 | 5.4 ± 14.2 | 0.027 * | 0.23 |
| UACR, mg/mmolb | 37.5 (65.0) | 24.5 (53.9) | −7.5 ± 22.4 | 54.6 (99.7) | 26.8 (74.3) | −21.5 ± 22.3 | 0.047*b | 0.05 |
| Urea, mmol/l | 5.79 ± 1.8 | 6.11 ± 1.8 | 0.32 ± 1.36 | 6.95 ± 2.8 | 8.16 ± 3.7 | 1.22 ± 1.63 | 0.044 * | 0.70 |
| Uric acid, mmol/l | 401 ± 100 | 395 ± 93 | −6.2 ± 55.8 | 395 ± 120 | 421 ± 126 | 25.9 ± 55.2 | 0.056 | 0.24 |
| Biomarkers | ||||||||
| Tocopherol, µmol/l | 47.1 ± 23.1 | 70.5 ± 25.4 | 23.4 ± 28.1 | 41.5 ± 12.8 | 47.9 ± 25.3 | 6.5 ± 23.0 | 0.033 * | 0.89 |
| TNFR1, pg/ml | 57.8 ± 37 | 146.3 ± 69.0 | 79.9 ± 55.0 | 40.6 ± 27.3 | 100.7 ± 58.1 | 60.1 ± 49.9 | 0.228 | 0.83 |
| MDA, ng/ml | 1137 ± 454 | 1089 ± 330 | −102 ± 597 | 1133 ± 234 | 901 ± 303 | −232 ± 333 | 0.418 | 0.52 |
| VCAM-1, ng/ml | 77.7 ± 47.9 | 81.8 ± 42.7 | 4.1 ± 26.0 | 55.7 ± 32.0 | 85.6 ± 53.0 | 30.0 ± 44.6 | 0.043 * | 0.08 |
| Thromboxane-B2, pg/ml | 122.0 ± 61.4 | 108.0 ± 49.3 | 38.3 ± 50.0 | 114.4 ± 7.3 | 146.6 ± 75.5 | 34.6 ± 47.5 | 0.818 | 0.00 |
All data presented as mean ± standard deviation.
Significant at p < 0.05.
Effect size between groups was calculated using sample means and pooled standard deviation at 12 weeks of supplementation.
p values obtained using independent t test comparing change in intervention group against change in placebo group.
UACR presented as median (interquartile range), p value obtained using Mann–Whitney test.
eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin, MDA, malondialdehyde, TNFR1, tumor necrosis factor receptor-1; UACR, urine albumin:creatinine ratio; VCAM-1, vascular cell adhesion molecule-1.
There were statistically significant differences in serum creatinine and eGFR between the intervention and control groups. Patients in the intervention group had a significant decrease of 3.3 µmol/l in serum creatinine resulting in an increase in eGFR of 1.48 ml/min/1.73 m2. This is contrasted in the control group where there was an increase in serum creatinine of 5.36 µmol/l leading to a reduction in eGFR of 2.89 ml/min/1.73 m2 in the control group. There was a statistically significant difference in urea. UACR in the control group decreased more than the intervention group.
After supplementation, there was a significant increase in serum vitamin E in the intervention group. There was no significant difference in TNFR-1, a marker of inflammation that correlates to the risk of end-stage renal failure, between intervention and placebo groups.
There was a significant difference in VCAM-1 after 12 weeks. VCAM-1 increased by 4.1 ng/ml in the intervention group while it increased by 30 ng/ml in the control group. However, there was no difference in sample means in VCAM-1. There was no significant difference in thromboxane B2, a marker of diabetic retinopathy. Nor was there a significant difference in MDA, a marker of oxidative stress, between the control group compared with the intervention group.
Subgroup analysis
Patients were dichotomized based on their serum tocopherol concentrations at baseline. Sample median was measured at 41.8 µmol/L. Based on that, 25 patients were classified as low Vitamin E and 26 patients were classified as high Vitamin E (Table 3).
Table 3.
Classification of patients into low vitamin E and high vitamin E.
| Low vitamin E | High vitamin E | Total | |
|---|---|---|---|
| Tocopherol concentration, µmol/l | <41.8 | ⩾41.8 | – |
| Tocovid, n | 13 | 12 | 25 |
| Placebo, n | 12 | 14 | 26 |
| Total | 25 | 26 | 51 |
Mean differences between each group were analyzed in a similar manner. Data in Table 4 is presented as mean difference between baseline and 12 weeks post-supplementation. In patients with low baseline tocopherol, there was a statistically significant improvement in renal function in the intervention group as assessed by serum creatinine and eGFR. Serum creatinine in the intervention group decreased by 2.97 µmol/l with a corresponding 3.85 ml/min/1.73 m2 increase in eGFR. Serum vitamin E concentrations increased by 48.9 µmol/l while VCAM-1 decreased by 11 ng/ml in the intervention group. In contrast, patients with high baseline tocopherol concentrations have no significant changes in all parameters measured.
Table 4.
Subgroup analysis for mean changes between groups.
| Parameters | High serum tocopherol at
baseline (n = 26) |
Low serum tocopherol at
baseline (n = 25) |
||||
|---|---|---|---|---|---|---|
| Tocovid (n = 12) |
Placebo (n = 14) |
p-valuea | Tocovid (n = 13) |
Placebo (n = 12) |
p-valuea | |
| General | ||||||
| HbA1c, % | −0.30 ± 0.73 | −0.59 ± 0.55 | 0.254 | −0.72 ± 1.07 | −0.03 ± 1.01 | 0.112 |
| Renal parameters | ||||||
| eGFR, ml/min/1.73 m2 | −1.41 ± 4.70 | −1.36 ± 9.48 | 0.984 | 3.85 ± 9.39 | –4.09 ± 5.61 | 0.019 * |
| Serum creatinine, µmol/l | 5.24 ± 9.66 | 0.15 ± 13.3 | 0.305 | –2.97 ± 15.1 | 11.1 ± 13.9 | 0.028 * |
| UACR, mg/mmolb | −12.5 ± 17.1 | −17.7 ± 19.9 | 0.518 | −5.96 ± 25.7 | −23.4 ± 25.4 | 0.161 |
| Urea, mmol/l | 0.43 ± 1.46 | 0.22 ± 2.90 | 0.821 | −0.46 ± 2.71 | 1.16 ± 3.04 | 0.172 |
| Uric acid, mmol/l | 9.08 ± 20.1 | 23.2 ± 38.0 | 0.355 | −22.3 ± 61.0 | 21.3 ± 55.5 | 0.104 |
| Biomarkers | ||||||
| Tocopherol, µmol/l | 14.2 ± 34.1 | 12.5 ± 30.3 | 0.893 | 35.2 ± 20.8 | 4.48 ± 17.7 | 0.002 * |
| TNFR1, pg/ml | 78.9 ± 56.2 | 57.3 ± 57.0 | 0.427 | 73.1 ± 59.7 | 72.2 ± 34.2 | 0.969 |
| MDA, ng/ml | 103 ± 468 | −138 ± 305 | 0.251 | −229 ± 689 | −519 ± 511 | 0.316 |
| VCAM-1, ng/ml | 8.30 ± 20.3 | 3.16 ± 56.4 | 0.821 | –9.40 ± 33.7 | 29.4 ± 30.7 | 0.022 * |
| Thromboxane-B2, pg/ml | 20.3 ± 53.5 | 27.8 ± 57.4 | 0.793 | 49.6 ± 49.8 | 47.3 ± 31.7 | 0.913 |
All data presented as mean ± standard deviation.
Data represents mean difference between baseline and 12 weeks post-supplementation. A positive value denotes an increase whereas a negative value denotes decrease.
Significant at p < 0.05.
p values obtained using independent t test comparing change in intervention group against change in placebo group.
eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; MDA, malondialdehyde; TNFR-1, tumor necrosis factor receptor-1; UACR, urine albumin:creatinine ratio; VCAM-1, vascular cell adhesion molecule-1.
Six to nine months washout
A total of 18 out of the 54 patients in the original cohort did not return, as such only 36 patients were analyzed post-washout. The patients who came back have ceased Tocovid supplementation for 6–9 months: Table 5 lists the mean differences of renal parameters from end of 12 weeks of supplementation to after 6–9 months of washout between the intervention and control groups. p values were obtained by comparing the mean difference between groups. There was a significant difference in serum creatinine. The serum creatinine of the intervention group remained relatively stable whereas it had increased steadily in the control group. This change was reflected in a difference in eGFR as well, however this difference was not statistically significant.
Table 5.
Mean difference of all parameters after washout.
| Parameters | Intervention group (n = 18) |
Control group (n = 18) |
p valuea | Effect size | ||||
|---|---|---|---|---|---|---|---|---|
| At 12-weeks of supplementation | 6–9 months washout | Change | At 12-weeks of supplementation | 6–9 months washout | Change | |||
| General | ||||||||
| HbA1c, % | 8.26 ± 1.21 | 8.31 ± 1.16 | 0.04 ± 0.78 | 8.11 ± 1.16 | 8.06 ± 1.20 | −0.05 ± 0.85 | 0.743 | 0.21 |
| Renal parameters | ||||||||
| eGFR, ml/min/1.73 m2 | 60.4 ± 25.6 | 58.6 ± 22.3 | −1.75 ± 6.54 | 55.4 ± 24.1 | 49.4 ± 22.2 | −6.00 ± 8.88 | 0.144 | 0.41 |
| Serum creatinine, µmol/l | 105.9 ± 40.4 | 106.7 ± 37.3 | 0.82 ± 8.33 | 120.3 ± 51.0 | 131.6 ± 59.8 | 11.3 ± 15.5 | 0.031 * | 0.50 |
| UACR, mg/mmolb | 18.8 (40.0) | 25.1 (24.5) | 7.9 (22.6) | 25.1 (55.2) | 37.3 (71.9) | 7.20 (62.3) | 0.614b | 0.12b |
| Biomarkers | ||||||||
| Tocopherol, µmol/l | 76.8 ± 24.7 | 78.6 ± 38.2 | 23.0 ± 36.6c | 50.4 ± 21.8 | 56.0 ± 16.8 | 11.1 ± 14.5c | 0.329 | 0.77 |
| TNFR1, pg/ml | 121.8 ± 72.3 | 85.3 ± 85.5 | −36.5 ± 44.7 | 116.8 ± 63.7 | 111.3 ± 59.1 | −5.4 ± 57.0 | 0.202 | 0.35 |
| MDA, ng/ml | 1259 ± 543 | 437 ± 169 | −821 ± 604 | 915 ± 249 | 458 ± 200 | −457 ± 273 | 0.054 | 0.11 |
| VCAM-1, ng/ml | 94.1 ± 46.4 | 148.1 ± 70.3 | 54.0 ± 47.5 | 85.7 ± 61.3 | 118.4 ± 52.6 | 32.7 ± 50.1 | 0.279 | 0.48 |
| Thromboxane-B2, pg/ml | 172.7 ± 100.7 | 104.1 ± 35.8 | −68.6 ± 108.4 | 135.6 ± 38.4 | 114.6 ± 74.3 | −21.0 ± 77.4 | 0.235 | 0.18 |
All data presented as mean ± standard deviation.
Significant at p < 0.05.
Effect size between groups was calculated using sample means and pooled standard deviation at post-washout visit.
p values obtained using independent t test comparing change in intervention group against change in placebo group.
UACR presented as median (interquartile range), p value obtained using Mann–Whitney test.
Change in serum tocopherol was measured from baseline as we expect it to decrease after cessation.
eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; MDA, malondialdehyde; TNFR-1, tumor necrosis factor receptor-1; UACR, urine albumin:creatinine ratio; VCAM-1, vascular cell adhesion molecule-1.
Analysis of serum tocopherol and biomarkers were performed at 6–9 months post-treatment washout period. As expected, slow wash out of serum tocopherol in the intervention group led to a negative value, the mean difference of tocopherol was taken from baseline to post-treatment washout instead.
There was still a residual increase in serum tocopherol concentration with large effect size. However, this difference is not statistically significant. There was a greater decrease in MDA in the intervention group, but there were no significant differences between the means of both cohorts (MDA at washout: intervention 437 ng/ml versus control 458 ng/ml). Otherwise, there were no significant differences between groups for TNFR1, VCAM-1, and thromboxane B2.
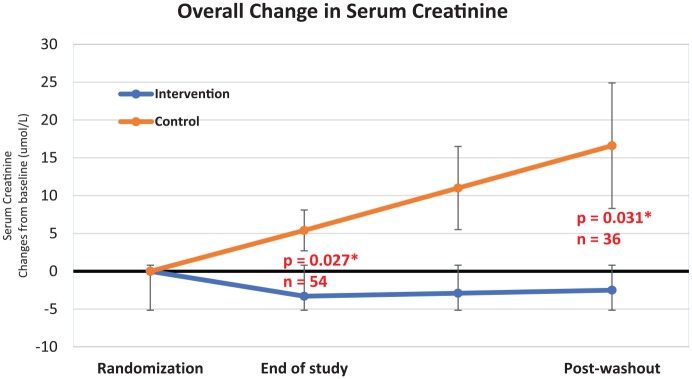
Figure 2 illustrates the trend of serum creatinine in patients taking tocotrienol-rich vitamin E compared with the placebo group. There was a downwards trend of serum creatinine in the intervention group while there was a steady rise in serum creatinine in the control group.
Figure 2.
Graph of overall trend in serum creatinine.
Error lines represent standard error of mean.
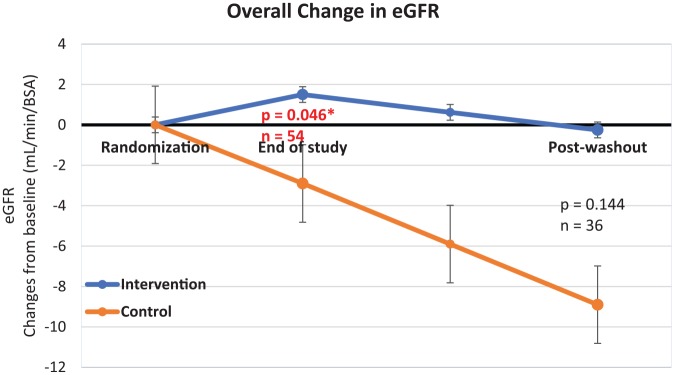
The increasing trend of serum creatinine in the control group was reflected by a steady decrease of renal function as shown in Figure 3. In contrast, the eGFR of the intervention group remained stable around the baseline value.
Figure 3.
Graph of overall trend in estimated glomerular filtration rate (eGFR).
Error lines represent standard error of mean.
Safety and adverse events
There were no significant differences detected in all safety tests done, which were blood pressure, lipid profile [total cholesterol, triglycerides, high-density lipoprotein (HDL)-cholesterol and low-density lipoprotein (LDL)-cholesterol], liver functions [aspartate transaminase (AST) and alanine transaminase (ALT)], and ECG tests. There were also no significant differences in adverse events between both groups at 12 weeks and after washout.
There was a total of 28 adverse events recorded among all the patients in both arms. The proportion of patients experiencing any adverse event was similar between intervention and control groups: 13 (48.1%) and 15 (55.6%), respectively. The exact breakdown and category of adverse events are given in Table 6.
Table 6.
Adverse events recorded.
| Adverse event | Intervention group | Control group |
|---|---|---|
| Serious adverse events | 2 | 1 |
| Intra-retinal hemorrhage | 2 | 1 |
| Sleepiness | 0 | 4 |
| Pedal edema | 1 | 0 |
| Dermatological | 2 | 0 |
| Neurological | 2 | 2 |
| Vascular | 0 | 1 |
| Respiratory | 1 | 0 |
| Postural hypotension | 1 | 1 |
| Musculoskeletal | 2 | 2 |
| Urological | 0 | 3 |
| Total | 13 (48.1%) | 15 (55.6%) |
There were three recorded incidents of serious adverse events: two in the intervention group and one in the control group. In the intervention group, one patient was admitted to the hospital for 3 days due to septic shock secondary to bronchopneumonia; another patient had a minor cerebrovascular event (left anterior cerebral infarct). In the control group, one patient was admitted to the hospital for 8 days owing to septic shock secondary to left leg cellulitis. All the patients were discharged well and there were no mortalities during the period of study.
Discussion
This study is a continuation of our previous started in 2018, where it was found that 8 weeks of Tocovid supplementation reduced serum creatinine in patients with diabetic nephropathy but not HbA1c, blood pressure, AGE, RAGE, Nε-CML, and Cystatin C. The patients were assessed at the end of a 12 weeks treatment period and again at 6–9 months following the end of the study, the washout period. Data was then compared from baseline to end of 12 weeks of treatment and between 12 weeks of treatment and 6–9 months after washout.
First, the results at 12 weeks of supplementation confirmed the previous reported findings at 2 months.16 At 12 weeks of supplementation, there was a difference in serum creatinine and eGFR between both cohorts. This effect is significant and has moderate effect size. The overall net difference of serum creatinine between groups is approximately 11.1 mmol/l, which led to a corresponding 5.0 ml/min/1.73 m2 difference in eGFR.
There was a reduction in eGFR of 2.9 ml/min/1.73 m2 in 12 weeks in the control group. This decrease matches the normal deterioration of eGFR in patients with DN. A retrospective cohort study presented by Goderis et al. shows that the natural decline in eGFR of patients with DN can range from 2 to 10 ml/min/1.73 m2 per year.23 In contrast, patients in the intervention group had an improvement in kidney function despite no differences in HbA1c, blood pressure, weight control, duration of diabetes, and chronological age between both groups. Differences between groups stem from the reduction in the control group, rather than direct improvement in the intervention group. This suggests that 12 weeks of tocotrienol-rich vitamin E supplementation prevents deterioration of DN rather than improving it.
It was postulated that tocotrienol-rich vitamin E ameliorates DN by reducing toxic AGEs in the body. This was because an animal study by Cheng et al. found that tocotrienol-rich vitamin E decreased AGE and RAGE levels in diabetic rats.15 This study was not able to replicate the finding in human subjects which may be due to the different biology in humans. Another study done in rats by Kuhad et al. had shown that tocotrienol-rich vitamin E ameliorates DN by inhibiting the NF-κB pathway, which leads to downstream inflammation and further oxidative stress.14,24 NF-κB leads to an increase in interleukin-6, VCAM-1, TNF-α, and biomarkers of oxidative stress such as MDA.25 These were the biomarkers that were tested throughout this study.
After supplementation, there was a significant increase in serum tocopherol levels, because Tocovid contained 30% tocopherol. This confirms the compliance rates. Tocotrienol levels were measurable 2–4 h post-ingestion of Tocovid 200 mg, but not at 12 h after the last dose.
Despite the large increase in serum tocopherol levels, there were no significant differences in circulating MDA (a marker of oxidative stress), TNFR-1, and thromboxane B2. There was a significant difference in VCAM-1 when comparing the mean difference between groups, however there was no difference between sample means. Thus, this change is likely due to regression to the mean.
In addition to creatinine, urea is another waste product produced in the body from dietary proteins. In established kidney failure, urea builds up in the blood causing uremia. Urea is a less reliable marker of kidney injury as its concentration fluctuates with diet. As shown in Table 2, there was an increase in urea in both groups, but this increase was lower in the intervention group compared with the control group. This difference is consistent with the improvement in renal function by the reduction in serum creatinine.
Subgroup analyses have revealed that Tocovid supplementation significantly improved renal function in patients with low baseline vitamin E levels. In contrast patients with high baseline tocopherol levels, Tocovid supplementation did not increase serum tocopherol levels, nor did it improve renal function. This finding complements the finding by the meta-analysis done by Suksomboon et al., where vitamin E supplementation improved HbA1c in diabetic patients with low baseline serum tocopherol, but not those with high baseline levels.7
The significant improvement in VCAM-1 after 12-week supplementation in the low vitamin E group may suggest the potential mechanism of action. VCAM-1 is an adhesion molecule that is a mediator in the inflammation process. VCAM-1 is upregulated when exposed to AGEs and it facilitates the transmigration of leukocytes.24 A cross-sectional study presented by Liu et al. shows that in Asians, VCAM-1 is associated with eGFR and albuminuria.26 The anti-inflammatory effects of tocotrienol are well-documented.9 Our team hypothesizes that tocotrienol supplementation improves renal function in patients with low baseline serum tocopherol by reducing VCAM-1. To confirm this finding, multiple regression should be done but the sample size was inadequate.
To the best of the authors’ knowledge, this is the first time a study has been done to demonstrate the persistent effect of tocotrienol-rich vitamin E on DN. Tocotrienol is a fat-soluble antioxidant which is stored mainly in adipose tissue. This acts as a form of depot for vitamin E which is slowly released in the circulation. Patel et al. has shown that circulating tocotrienol rapidly washes out after 2 months of cessation, whereas serum tocopherol tends to remain for longer periods of time.27 To determine the presence of tocotrienol in the body, adipose tissue sampling is required.
Serum tocopherol concentrations in the intervention group remained persistently high as shown in Table 5. However, there were no statistical differences when compared with placebo, likely due to insufficient sample size. Mean concentration of serum tocopherol was 78.6 µmol/l in the intervention group and 56.0 µmol/l in the control group after washout. Despite this, its effects persist as shown in Table 5, serum tocopherol was 76.8 µmol/l after 12 weeks of supplementation and it was still 78.6 µmol/l even after 6–9 months of cessation. This shows that a brief 12 weeks of Tocovid supplementation can persistently elevate serum tocopherol concentrations by up to 9 months. One of the exclusion criteria is that patients must not be taking any fat-soluble antioxidants in the past 2 months. We have shown that tocopherol concentrations can be elevated for as long as 9 months. In future, studies which research the potential benefits of tocotrienol may need to take this into account. This may also explain why the effects on renal parameters were only seen in the cohort with low tocopherol levels.
In addition, patients in the intervention group still had stable renal function even after cessation of Tocovid. This is reflected in eGFR as it only decreased by 1.75 ml/min/1.73 m2 in the intervention group over 1 year (baseline 60.5 ml/min/1.73 m2 to post-washout 58.6 ml/min/1.73 m2) compared with the control group, which decreased by 6.0 ml/min/1.73 m2. However, this difference is not statistically significant, which may be due to insufficient sample size.
In future, long-term phase III multicenter trials need to be conducted to determine the benefits of long-term supplementation. Clinical trials in the future will need to be event-driven similarly to the CREDENCE study to determine whether tocotrienol can prevent ESRD. Future study would require more than 500 patients and multiple years of follow up to test this hypothesis. This would inevitably require funding and sponsors from pharmaceutical companies.
Hence, Tocovid may be used as an adjunct to standard diabetic therapy to delay the progression of DN. Patients in this study have relatively stable control and have access to standard diabetic care. As such, Tocovid should not be treated as a panacea but rather an adjunct to good, stable glycemic control.
Limitations
One limitation of the study was that tocotrienol measurements were not accurate. The concentration of plasma tocotrienol is highly variable depending on when the patient took their last dose of Tocovid. Plasma tocotrienol concentrations were inconsistent when measured as some patients took Tocovid before coming for their visits whereas others took their last dose the night before. In patients who took Tocovid the night prior to scheduled visits, plasma tocotrienol concentrations were nearly undetectable. This phenomenon needs to be considered for future studies regarding tocotrienol supplementation.
Conclusion
DN is the leading cause of dialysis worldwide and it is a huge burden to the individual and the society at large. Current standard of care is strict glycemic, blood pressure, and cholesterol control. Despite that, patients still have declining renal function and are at risk of developing ESRD. Tocovid has been shown to attenuate the progression of DN. Twelve weeks of Tocovid supplementation resulted in statistically significant improvement in renal function despite having no effect on glycemia. The beneficial effect on serum creatinine persists even after 6–9 months of washout while serum tocopherol concentrations remain elevated even after cessation of supplementation. There was also a statistically significant improvement in VCAM-1 in patients who have low baseline vitamin E, alluding to a possible mechanism of action.
There were no changes in TNFR-1, MDA, and thromboxane B2. This finding, in addition to the finding where Tocovid did not reduce AGE, RAGE, Cystatin C, and Nε-CML, shows that Tocovid improves renal function irrespective of these markers. The pathway utilized by tocotrienol-rich vitamin E is still elusive and further studies are warranted to investigate this.
Supplemental Material
Supplemental material, CONSORT_page_1 for Tocotrienol-rich vitamin E improves diabetic nephropathy and persists 6–9 months after washout: a phase IIa randomized controlled trial by Gerald Chen Jie Tan, Suzanne May Quinn Tan, Sonia Chew Wen Phang, Yeek Tat Ng, En Yng Ng, Badariah Ahmad, Uma Devi M. Palamisamy and Khalid Abdul Kadir in Therapeutic Advances in Endocrinology and Metabolism
Supplemental Material
Supplemental material, CONSORT_page_2 for Tocotrienol-rich vitamin E improves diabetic nephropathy and persists 6–9 months after washout: a phase IIa randomized controlled trial by Gerald Chen Jie Tan, Suzanne May Quinn Tan, Sonia Chew Wen Phang, Yeek Tat Ng, En Yng Ng, Badariah Ahmad, Uma Devi M. Palamisamy and Khalid Abdul Kadir in Therapeutic Advances in Endocrinology and Metabolism
Acknowledgments
We would like to extend our gratitude to ExcelVite, Malaysia for subsidizing the cost of Tocovid SupraBio, and the placebo. In addition, we would like to thank Monash University for providing the necessary equipment and research center to conduct the study. This research would have been impossible without the staff in Sunway and Johor Bahru Clinical Research Centers, namely Noras’kin Mohamad, Ungku Zulaikha, Savithri Gopal, Chui Chor Sin, and Pang Pei Ling.
Footnotes
Trial registration: ANZCTR identifier: ACTRN12619001568101
Online view: https://anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12619001568101
Author contributions: Conceptualization, KAK; methodology, KAK; data collection, GCJT, SMQT, SCWP, YTN, EYN, BA, KAK; analysis of specimen, SCWP, UP; data curation, GCJT, YTN; writing—original draft preparation, GCJT; writing—review and editing, GCJT, BA, KAK; supervision, KAK; project administration, BA; funding acquisition, SMQT, BA.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the FRGS grant from the Malaysian Ministry of Higher Education (grant number FRGS/1/2018/SKK02/MUSM/02/1) and by Monash University Malaysia under the Tropical Medicine and Biology (TMB) grant (grant number TMB-2018-CR3185140918-SuzTMQ/KAK).
Conflict of interest statement: The authors declare no conflict of interest. The sponsors had no role in the design of the study that included data collection, analyses or interpretation, the writing of the manuscript and in the decision to publish the results.
ORCID iD: Gerald Chen Jie Tan  https://orcid.org/0000-0001-6856-9365
https://orcid.org/0000-0001-6856-9365
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Gerald Chen Jie Tan, Jeffrey Cheah School of Medicine and Health Sciences, Monash University Malaysia, Bandar Sunway, Selangor, 46150, Malaysia.
Suzanne May Quinn Tan, Jeffrey Cheah School of Medicine and Health Sciences, Monash University Malaysia, Selangor, Malaysia.
Sonia Chew Wen Phang, Jeffrey Cheah School of Medicine and Health Sciences, Monash University Malaysia, Selangor, Malaysia.
Yeek Tat Ng, Jeffrey Cheah School of Medicine and Health Sciences, Monash University Malaysia, Selangor, Malaysia.
En Yng Ng, Jeffrey Cheah School of Medicine and Health Sciences, Monash University Malaysia, Selangor, Malaysia.
Badariah Ahmad, Jeffrey Cheah School of Medicine and Health Sciences, Monash University Malaysia, Selangor, Malaysia.
Uma Devi M. Palamisamy, Jeffrey Cheah School of Medicine and Health Sciences, Monash University Malaysia, Selangor, Malaysia
Khalid Abdul Kadir, Jeffrey Cheah School of Medicine and Health Sciences, Monash University Malaysia, Selangor, Malaysia.
References
- 1. World Health Organization. Global Report on Diabetes. 2016. Geneva: WHO. [Google Scholar]
- 2. National Kidney Foundation. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis 2012; 60: 850–886. [DOI] [PubMed] [Google Scholar]
- 3. Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. Epub ahead of print 4 April 2019. DOI: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 4. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res 2010; 107: 1058–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vlassara H. The AGE-receptor in the pathogenesis of diabetic complications. Diabetes Metab Res Rev 2001; 17: 436–443. [DOI] [PubMed] [Google Scholar]
- 6. Heart Outcomes Prevention Evaluation Study Investigators. Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Lancet 2000; 355: 253–259. [PubMed] [Google Scholar]
- 7. Suksomboon N, Poolsup N, Sinprasert S. Effects of vitamin E supplementation on glycaemic control in type 2 diabetes: systematic review of randomized controlled trials. J Clin Pharm Ther 2011; 36: 53–63. [DOI] [PubMed] [Google Scholar]
- 8. Xu R, Zhang S, Tao A, et al. Influence of vitamin E supplementation on glycaemic control: a meta-analysis of randomised controlled trials. PLoS One 2014; 9: e95008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bardhan J, Chakraborty R, Raychaudhuri U. The 21st century form of vitamin E - tocotrienol. Curr Pharm Des 2011; 17: 2196–2205. [DOI] [PubMed] [Google Scholar]
- 10. Ghafoorunissa A, Hemalatha S, Rao MVV. Sesame lignans enhance antioxidant activity of vitamin E in lipid peroxidation systems. Mol Cell Biochem 2004; 262: 195–202. [DOI] [PubMed] [Google Scholar]
- 11. Serbinova E, Kagan V, Han D, et al. Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radic Biol Med 1991; 10: 263–275. [DOI] [PubMed] [Google Scholar]
- 12. Paola Palozza SV, Luca Avanzi, Silvia Vertuani, et al. Comparative antioxidant activity of tocotrienols and the novel chromanyl-polyisoprenyl molecule FeAox-6 in isolated membranes and intact cells. Mol Cell Biochem 2006; 287: 21–32. [DOI] [PubMed] [Google Scholar]
- 13. Siddiqui S, Ahsan H, Khan MR, et al. Protective effects of tocotrienols against lipid-induced nephropathy in experimental type-2 diabetic rats by modulation in TGF-beta expression. Toxicol Appl Pharmacol 2013; 273: 314–324. [DOI] [PubMed] [Google Scholar]
- 14. Kuhad A, Chopra K. Attenuation of diabetic nephropathy by tocotrienol: involvement of NFkB signaling pathway. Life Sci 2009; 84: 296–301. [DOI] [PubMed] [Google Scholar]
- 15. Cheng HS, Ton SH, Tan JBL, et al. The ameliorative effects of a tocotrienol-rich fraction on the AGE-RAGE axis and hypertension in high-fat-diet-fed rats with metabolic syndrome. Nutrients 2017; 9 pii: E984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tan SMQ, Chiew Y, Ahmad B, et al. Tocotrienol-rich vitamin E from palm oil (tocovid) and its effects in diabetes and diabetic nephropathy: a pilot phase II clinical trial. Nutrients 2018; 10 pii: E1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359: 1577–1589. [DOI] [PubMed] [Google Scholar]
- 18. Fournet M, Bonté F, Desmoulière A. Glycation damage: a possible hub for major pathophysiological disorders and aging. Aging Dis 2018; 9: 880–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moreno JA, Gomez-Guerrero C, Mas S, et al. Targeting inflammation in diabetic nephropathy: a tale of hope. Expert Opin Investig Drugs 2018; 27: 917–930. [DOI] [PubMed] [Google Scholar]
- 20. Peh HY, Tan WS, Liao W, et al. Vitamin E therapy beyond cancer: tocopherol versus tocotrienol. Pharmacol Ther 2016; 162: 152–169. [DOI] [PubMed] [Google Scholar]
- 21. Wan Nazaimoon WM, Sakinah O, Gapor A, et al. Effects of palm olein tocopherol and tocotrienol on lipid peroxidation, lipid profiles and glycemic control in non-insulin diabetes mellitus patients. Nutr Res 1996; 16: 1901–1911. [Google Scholar]
- 22. Liu Z, Lee HJ, Garofalo F, et al. Simultaneous measurement of three tocopherols, all-trans-retinol, and eight carotenoids in human plasma by isocratic liquid chromatography. J Chromatogr Sci 2011; 49: 221–227. [Google Scholar]
- 23. Goderis G, Van Pottelbergh G, Truyers C, et al. Long-term evolution of renal function in patients with type 2 diabetes mellitus: a registry-based retrospective cohort study. BMJ Open 2013; 3: e004029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Navarro-González JF, Mora-Fernández C, de Fuentes MM, et al. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol 2011; 7: 327. [DOI] [PubMed] [Google Scholar]
- 25. Rhee SY, Kim YS. The role of advanced glycation end products in diabetic vascular complications. Diabetes Metab J 2018; 42: 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Liu JJ, Yeoh LY, Sum CF, et al. Vascular cell adhesion molecule-1, but not intercellular adhesion molecule-1, is associated with diabetic kidney disease in Asians with type 2 diabetes. J Diabetes Complications 2015; 29: 707–712. [DOI] [PubMed] [Google Scholar]
- 27. Patel V, Khanna S, Roy S, et al. Natural vitamin E alpha-tocotrienol: retention in vital organs in response to long-term oral supplementation and withdrawal. Free Radic Res 2006; 40: 763–771. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, CONSORT_page_1 for Tocotrienol-rich vitamin E improves diabetic nephropathy and persists 6–9 months after washout: a phase IIa randomized controlled trial by Gerald Chen Jie Tan, Suzanne May Quinn Tan, Sonia Chew Wen Phang, Yeek Tat Ng, En Yng Ng, Badariah Ahmad, Uma Devi M. Palamisamy and Khalid Abdul Kadir in Therapeutic Advances in Endocrinology and Metabolism
Supplemental material, CONSORT_page_2 for Tocotrienol-rich vitamin E improves diabetic nephropathy and persists 6–9 months after washout: a phase IIa randomized controlled trial by Gerald Chen Jie Tan, Suzanne May Quinn Tan, Sonia Chew Wen Phang, Yeek Tat Ng, En Yng Ng, Badariah Ahmad, Uma Devi M. Palamisamy and Khalid Abdul Kadir in Therapeutic Advances in Endocrinology and Metabolism