Abstract
Background
In soft fruits, the differential expression of many genes during development and ripening is responsible for changing their organoleptic properties. In strawberry fruit, although some genes involved in the metabolic regulation of the ripening process have been functionally characterized, some of the most studied genes correspond to transcription factors. High throughput transcriptomics analyses performed in strawberry red receptacle (Fragaria x ananassa) allowed us to identify a ripening-related gene that codes an atypical HLH (FaPRE1) with high sequence homology with the PACLOBUTRAZOL RESISTANCE (PRE) genes. PRE genes are atypical bHLH proteins characterized by the lack of a DNA-binding domain and whose function has been linked to the regulation of cell elongation processes.
Results
FaPRE1 sequence analysis indicates that this gene belongs to the subfamily of atypical bHLHs that also includes ILI-1 from rice, SlPRE2 from tomato and AtPRE1 from Arabidopsis, which are involved in transcriptional regulatory processes as repressors, through the blockage by heterodimerization of bHLH transcription factors. FaPRE1 presented a transcriptional model characteristic of a ripening-related gene with receptacle-specific expression, being repressed by auxins and activated by abscisic acid (ABA). However, its expression was not affected by gibberellic acid (GA3). On the other hand, the transitory silencing of FaPRE1 transcription by agroinfiltration in receptacle produced the down-regulation of a group of genes related to the ripening process while inducing the transcription of genes involved in receptacle growth and development.
Conclusions
In summary, this work presents for the first time experimental data that support an important novel function for the atypical HLH FaPRE1 during the strawberry fruit ripening. We hypothesize that FaPRE1 modulates antagonistically the transcription of genes related to both receptacle growth and ripening. Thus, FaPRE1 would repress the expression of receptacle growth promoting genes in the ripened receptacle, while it would activate the expression of those genes related to the receptacle ripening process.
Keywords: Atypical HLH, Fruit ripening, PRE1, Strawberry
Background
During the processes of development and ripening of soft fruits, many metabolic pathways that are responsible for organoleptic properties are differentially expressed. In strawberry fruits, it is very well established that the increase in the ABA/auxins ratio triggers the transcription of many ripening-related genes involving the different organoleptic properties such as aroma, color, taste and softening [1–10]. However, with the exception of some transcriptional factors (TFs), the functional characterization of genes involved in the regulation of these metabolic pathway is very scarce until now. Thus, it has been described the function played by a few transcription factors. FaMYB10, a R2R3 MYB TF related to the secondary metabolism, is described as a ripening-related master regulatory gene of the structural flavonoid/phenylpropanoid metabolic pathway genes [9]; and the EMISSION OF BENZENOIDS II (FaEOBII), a positive regulator of flavonoids/phenylpropanoids volatile-related genes, regulates the CINNAMYL ALCOHOL DEHYDROGENASE (FaCAD1) and the EUGENOL SYNTHASE 2 (FaEGS2), which controls the production of eugenol, a volatile phenylpropanoid, in ripe strawberry receptacles [8]. Recently, a DOF-type TF (FaDOF2) has also been identified as a positive regulator of eugenol biosynthesis in ripened strawberry receptacle. Both FaEOBII and FaDOF2 seem to act synergistically in the activation of the FaEGS2 gene transcription [11]. In this way, an ERF-MYB TF complex regulates furaneol biosynthesis by means of a quinone reductase transcription regulation [12]. The functional role played by FcMYB1, another R2R3 MYB TF, was also characterized. This TF acts as regulator of the branching point of the anthocyanins/proanthocyanidins biosynthesis [13]. Also, FaGAMYB has been described as a regulator in the transition from vegetative growth to ripening process [14]. In addition, it was demonstrated that the transient down-regulation of the C-type MADS-box TF expression (SHATTERPROOF-like gene; FaSHP) gave rise to a slightly shorter delay in the time required to reach the pink stage of ripening [7]. Besides, transcription of several ripening-related genes as well as the content of several metabolites was altered in these transiently modified fruits [7]. It was proposed that SCARECROW-LIKE 8 (FaSCL8) could modulate the transcription regulation of genes related to the flavonoid/anthocyanin biosynthesis, probably through their influence on FaMYB10 gene expression [15]. Moreover, FaMYB44.2 has been proposed to interact with FaMYB10 in sucrose accumulation, which would have an impact on the ripening process [16]. On the other hand, four TFs (FaMYB9/FaMYB11, FabHLH3 and FaTTG1) have been described as positive activators of genes that are involved in the proanthocyanidins (PAs) biosynthesis in strawberry immature fruits [17]. A comparation between a mutant whited coloured strawberry and a red natural one has discovered some TF potentially involved in the anthocyanin biosynthesis [18].
High throughput transcriptomics analyses previously performed by our group [19] have allowed us to identify a ripening-related gene that codes an atypical HLH (FaPRE1) belonging to the basic helix-loop-helix/helix-loop-helix (bHLH/HLH) TFs family. FaPRE1 was selected for its expression characteristics: a) ripening-related; b) receptacle-specific; c) negatively regulated by auxins, and d) induced by ABA [19]. According to their DNA-binding ability, these proteins are classified into two groups; DNA-binding bHLH (bHLH) and non-DNA-binding bHLH (HLH) proteins, also called atypical HLH [20–24]. bHLH TFs contain two clearly differentiated domains, a basic domain located at the amino terminus of the proteins, which contains 13–17 basic amino acids, and an HLH region, located at the carboxy terminus that comprises two amphipathic α-helices which are rich in hydrophobic amino acids and are connected by a loop of variable length. The basic domain gives the transcription factor the ability to bind to the DNA [24–26] while the presence of the HLH motif confers the ability to establish homo- or heterodimeric interactions with other bHLH proteins, which is essential for DNA recognition and DNA-binding specificity [22, 24]. On the contrary, HLH proteins are particularly diverged at the basic region, that usually lacked critical sequences for a proper DNA binding domain and, in consequence, they did not present DNA-binding ability [25]. HLH proteins may dimerize with other bHLH proteins [24, 27–29], thus acting as negative regulators of bHLH protein action through the formation of heterodimers. This interaction will avoid bHLH protein to interact with other bHLH and, in this way, with their corresponding cis sequences on the DNA [24, 29–34].
Several studies have shown that atypical HLH proteins play important regulatory roles in hormone signaling and cell elongation [20, 35–38], light signaling [39], vascular and fruit development [30, 34] or grain size [40, 41]. In this sense, functional analysis identified AtPRE1 (Arabidopsis PACLOBUTRAZOL RESISTANCE 1), an atypical HLH protein that plays an activator role of genes that respond to gibberellin, presumably downstream of DELLA proteins [20]. AtPRE1 also regulates organ elongation in response to BRs [31, 32]. Thus, AtPRE1 interacts with IBH1 (ILI1 binding bHLH 1), another atypical HLH that negatively regulates ACE1 (Activator of Cell Elongation 1). When AtPRE1 interacts with IBH1, it prevents its binding to ACE1 and restores the transcription ability of ACE to induce cell elongation [31, 32]. Thus, this triantagonistic bHLH system, which is generally used for these transcriptional regulators to perform its function, seems to be important in determining the final size of plant cells [31, 32].
Furthermore, in tomato, the PRE-like gene SlStyle2.1 controls both the elongation and length of floral style, and has also been related to the evolution of self-pollination flowers in cultivated varieties [42]. In all these cases, the balance of triantagonistic bHLH proteins might be important to determine both the size of plant cells and the regulation of cell elongation, acting downstream of multiple external and endogenous signals [31, 32, 43].
Very little is known about the role of bHLH/HLH regulators in fruit ripening. In fruits, only an atypical HLH has been described in tomato (SlPRE2) that seems to participate in the development of the immature fruit but not in the stages of fruit ripening [33, 34]. This transcriptional factor is predominantly expressed in the fruit development and the silencing of its transcription diminished fruit size due to a thinning of the fruit pericarp [34]. Furthermore, SlPRE2 transcription was GA3-inducible in immature green fruits. The authors suggest that SlPRE2 may regulate fruit size through the regulation of the cell expansion [34].
However, the specific role played by the bHLH/HLH in the fruit ripening process is not known. In this paper, we present the functional characterization, along the ripening of the strawberry receptacle, of an atypical HLH protein (FaPRE1). The transcription pattern of this gene is receptacle specific and clearly inducible along the ripening stages. In addition, the FaPRE1 transcription is regulated positively for the internal concentration of abscisic acid (ABA) in the receptacle but not for the content of GA3. The transitory silencing of FaPRE1 transcription by agroinfiltration in receptacle produced the down-regulation of a group of genes related to the ripening process while it induced the transcription of genes involved in receptacle growth. All these results indicate that FaPRE1 plays a novel and important pivotal functional role along the receptacle ripening process differentially coordinating the antagonistic transcription of genes related to the receptacle growth and of those genes involved in receptacle ripening.
Results
FaPRE genes encode atypical HLH proteins
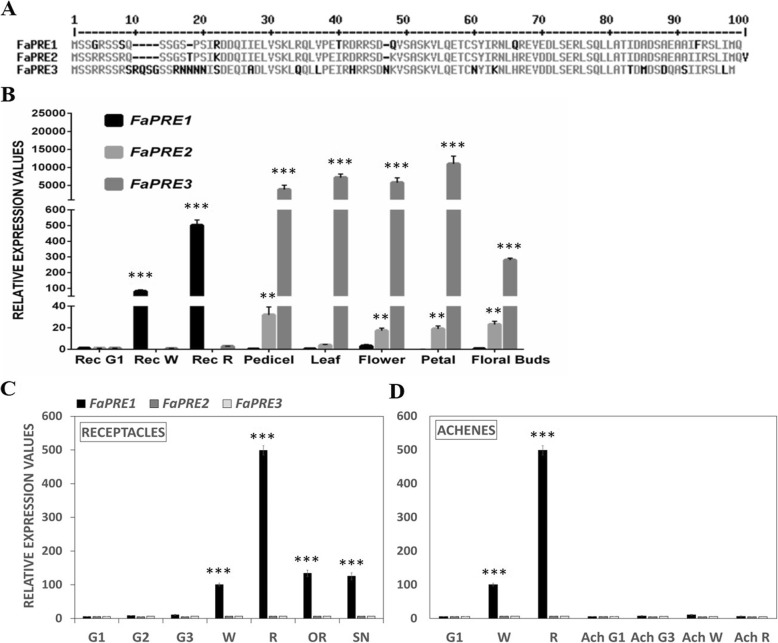
Bioinformatics analysis of the available Fragaria vesca (v2.0.a2) [44] and Fragaria x ananassa genome (v1.0-a1) [45] has allowed us to identify three PRE genes (FaPRE) in strawberry genome that we have named FaPRE1 (gene30478), FaPRE2 (gene28320) and FaPRE3 (gene03986). The comparison of the deduced proteins from FaPRE genes had a 90% amino acid sequence identity among them (Fig. 1a). Phylogenetic analysis showed that FaPRE1, FaPRE2 and FaPRE3 proteins can be classified into the atypical HLH subgroup 16 of the 32 plant bHLH/HLH subfamilies [22] (Additional files 1, 2).
Fig. 1.
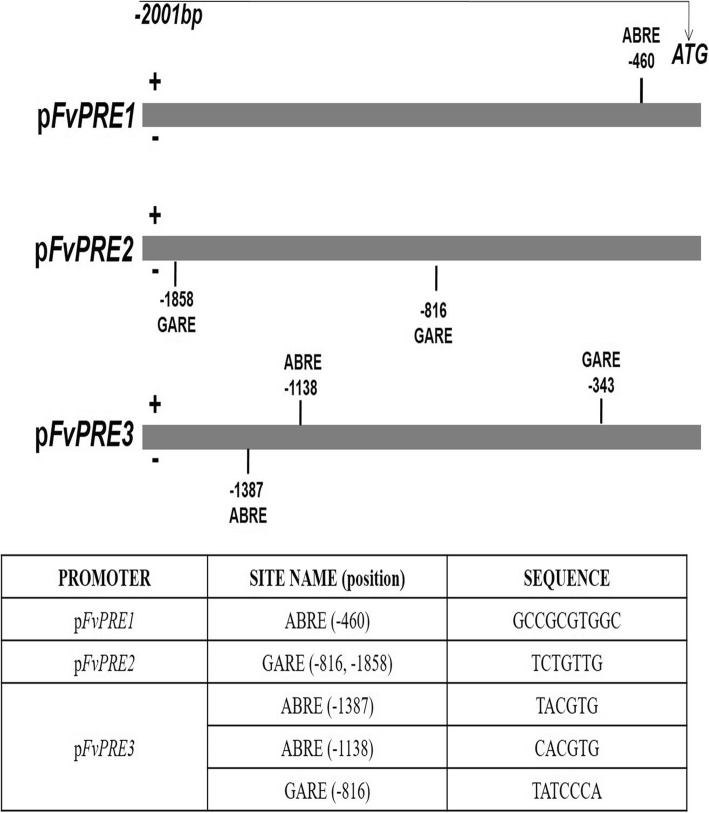
a Alignment of the predicted amino acid sequences of FaPRE1, FaPRE2 and FaPRE3 proteins. b-d Analysis by qRT-PCR of FaPRE genes expression in different tissues of Fragaria x ananassa “Camarosa” plants. b Analysis of FaPRE genes expression in some selected developing receptacles compared with vegetative tissues. Analysis of FaPRE genes expression in receptacles (c) and achenes (d) in different stages of development. Rec G1 and G1, small-sized green fruit; G2, middle-sized green fruits; G3, full-sized green fruit (G1 and G3: stages of development); Rec W and W, white stage; Rec R and R, ripe stage; OR, overripe stage; SN, senescent stage. Results were obtained using 3’UTR specific primers and quantification is based on Ct values. Relative expression values were calculated relative to receptacles G1 stage Ct value, which was assigned an arbitrary value equal to unity. Values are mean ± SD of five independent experiments. Statistical significance with respect to the reference sample was determined by the Student’s t-test: **p < 0.01, ***p < 0.001
In addition, the analysis of the FaPREs amino acid deduced sequence with InterProScan software revealed that, as in other similar PRE-like proteins, FaPREs lack the basic region at the amino terminal end of the protein which is characteristic of the bHLH transcription factors and responsible for their specific DNA binding ability. The atypical HLHs interact with bHLHs transcription factors and, in this way, interfere with their regulatory activity by blocking its binding to the cis-regulatory sequences positioned on the gene promoters that regulate. In this sense, the existence of a putative helix-loop-helix (HLH) domain, which is important for the interaction with other HLH transcription factors, was observed in the three FaPREs deduced proteins (Additional file 3B). It is noteworthy that this HLH domain is highly conserved in all PRE family members from A. thaliana [20], as well as in other plants as rice and grape (Additional file 3C) [23, 46]. Using the Plant-mPLoc program (http://www.csbio.sjtu.edu.cn/cgi-bin/PlantmPLoc.cgi) to determine the bioinformatic prediction of FaPREs, a nuclear subcellular localization for these proteins was predicted (Additional file 3D), as has previously been described in other plant species [47, 48].
FaPRE1 protein is located in nucleus
To confirm bioinformatics predictions related to the subcellular location of FaPRE1 protein, we carry out in vivo heterologous studies in N. benthamiana. For that, a N-terminal translational fusion protein construct between FaPRE1 and GFP proteins was driven under the control of a CaMV35S promoter. Confocal imaging analysis of the agroinfiltrated leaves indicated that the fusion protein co-localized with the nucleus marker DAPI (Fig. 2).
Fig. 2.
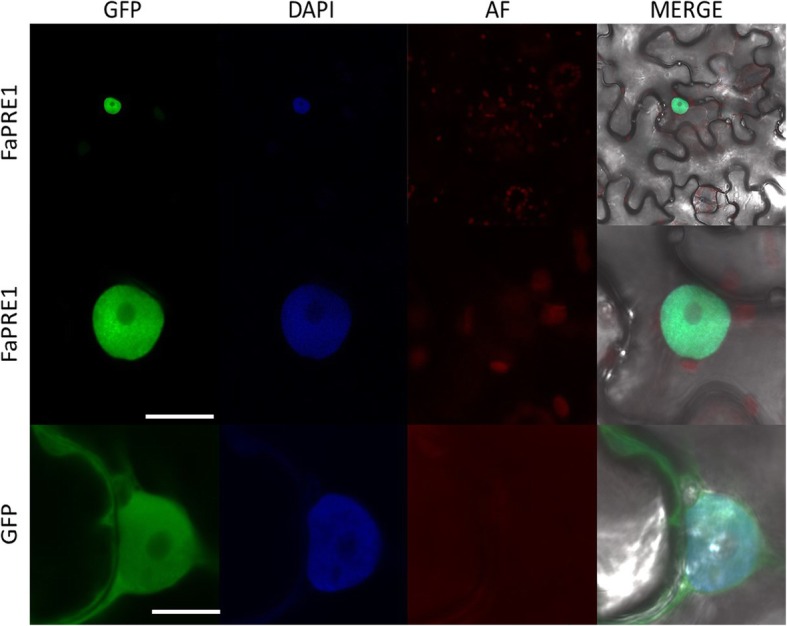
Subcellular localization of FaPRE1-GFP or free GFP upon transient expression in Nicotiana benthamiana leaves. Leaves from N. benthamiana were agroinfiltrated with translational constructs 35S-GFP-FaPRE1 and with 35S-GFP as control. Fluorescence signal detected using a confocal microscope. GFP, green fluorescent protein; DAPI, 4′,6-Diamidino-2-Phenylindole; AF, Autofluorescence; MERGE, merged view of the GFP and DAPI images. Scale bar: 5 μm
The spatio-temporal expression of FaPRE genes indicates that FaPRE1 is a ripening-related gene
qRT-PCR studies were performed to determine the spatial expression of the three FaPRE genes. Our analysis showed that FaPRE2 and FaPRE3 transcription was restricted to vegetative tissues with a scarce or negligible transcription in the receptacle. However, FaPRE1 was almost exclusively expressed in ripe receptacle (Fig. 1b). For this reason, a more detailed spatio-temporal study of FaPRE1 expression was carried out in the strawberry receptacle at different stages of growth and ripening. The data indicated that the amount of FaPRE1 transcript increased steadily along the development and receptacle ripening stages, reaching their highest levels of transcription in the fully ripe stage (R). Afterwards, a slight decrease of transcript was observed in the overripe stage (OR), that was more pronounced in the senescent stage (SN), where only a low transcription level was detected (Fig. 1c). In contrast, transcript levels in achenes, corresponding to the different development and ripening stages, were negligible with respect to the values observed in the receptacle (Fig. 1d). Besides, the FaPRE1 expression was not significant in vegetative tissues. All these data taken together suggest the participation of FaPRE1 in the strawberry receptacle ripening process while FaPRE2 and FaPRE3 would develop their function in the vegetative tissues of the plant.
Hormonal regulation of FaPRE genes transcription
Considering that FaPRE1 is a ripening-related gene, its regulation by auxins and ABA was studied. It has been previously reported that achenes removal from the surface of immature G3-stage fruits decreases the inner concentration of auxins in the receptacle, which induces the transcription of many ripening-related genes [9, 19]. Similarly, the FaPRE1 transcription increased in de-achened receptacles (G3-achenes) with respect to that observed in control receptacles (G3) (Fig. 3a). As expected, this induction was abolished by the external application of the synthetic auxin IAA (Fig. 3a). Both results suggest that FaPRE1 gene transcription was negatively regulated by the internal content of auxins in immature receptacles. On the other hand, and supporting the previous data, the transcription of FaPRE1 decreased in receptacles where ABA production was diminished either by the inhibition of FaNCED1 enzymatic activity through the fruit treatment with NDGA or by the transitory silencing of the FaNCED1 transcription (Fig. 3b) [9]. This differential hormonal expression pattern shows that, as in the case of many ripening-related genes, FaPRE1 gene transcription is regulated, directly or indirectly, by the ratio ABA/auxins [19].
Fig. 3.
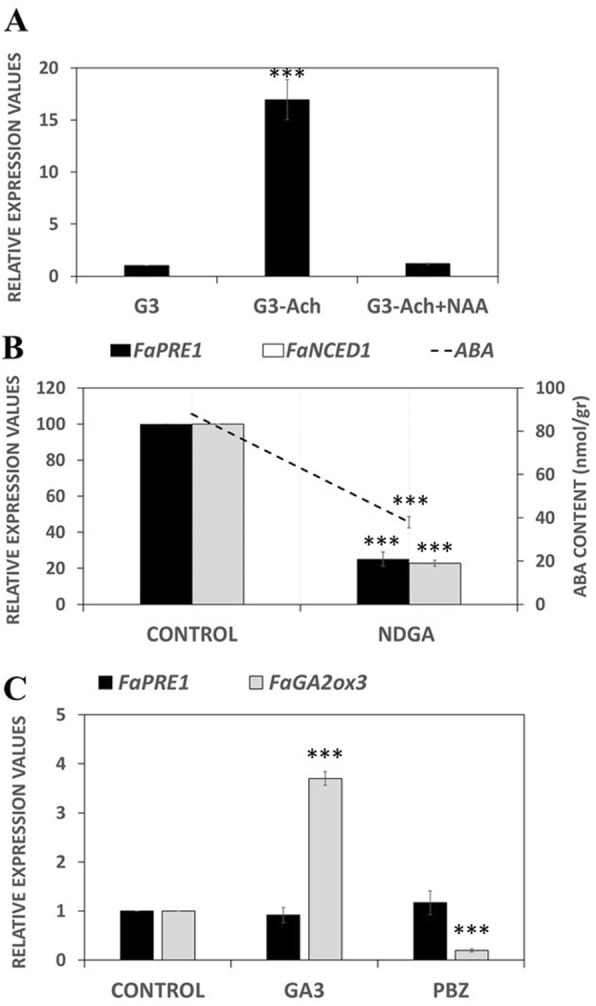
Hormonal effects in FaPRE1 gene expression. a Analysis by qRT-PCR of the effects of removing achenes from G3 developing fruits on FaPRE1 gene expression. b Analysis of FaPRE1 and FaNCED1 gene expression (bars) in G-W fruits treated with NDGA in both experimental situations; line indicates the ABA content in the analyzed fruits. c Analysis of the effects of gibberellins on FaPRE1 and FaGA2ox3 expression which was used as a control. The increase in the mRNA value was relative to the CONTROL Ct value of each experiment. Values are mean ± SD of five independent experiments. Statistical significance with respect to the reference sample was determined by the Student’s t-test: ***p < 0.001
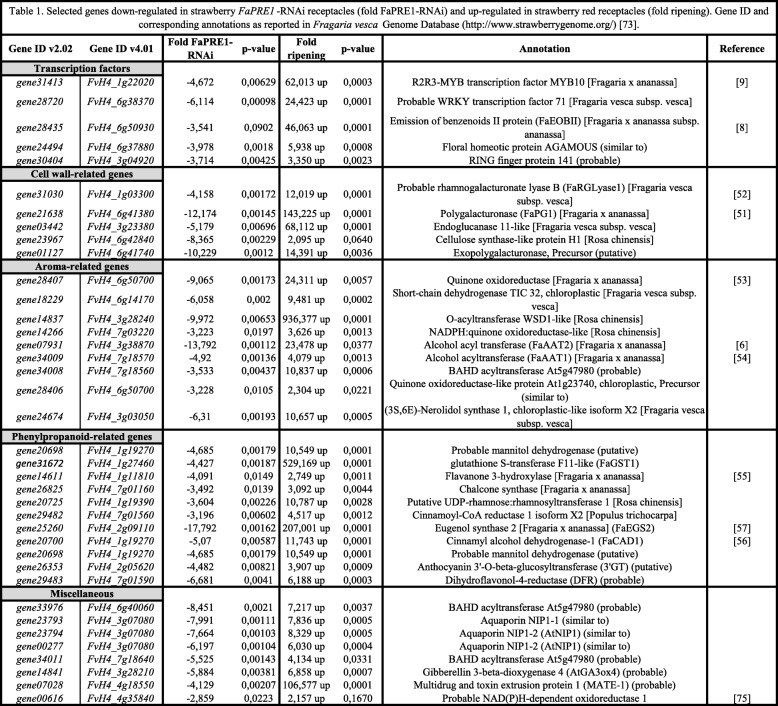
In A. thaliana, it has been previously demonstrated that gibberellic acid (GA) regulates cell elongation through the increase of AtPRE1 transcription [20]. On the other hand, SlPRE2 also shows an important role in the cell enlargement along tomato fruit development through GAs [34]. In strawberry fruit receptacles, although endogenous GA content has been measured along receptacle development and ripening [49, 50], the relationship between GAs and fruit ripening has not been established. To determine whether FaPRE1 transcription is under the control of GAs, strawberry fruit were injected with gibberellic acid (GA3) or paclobutrazol (PBZ), a compound that blocks gibberellin biosynthesis. Interestingly, in both cases, no significant changes were detected in FaPRE1 transcription between treated fruits versus untreated control fruits while GA2ox3, a control gene related with the GA degradation in strawberry fruit, was induced by GAs and repressed by PBZ treatment respectively (Fig. 3c). Besides, no phenotypic changes were observed in treated fruits compared to controls (data not shown). These results discard that gibberellins affect the FaPRE1 gene transcription in ripe fruits. This fact was reinforced by the bioinformatic analysis of the pFaPRE1 promoter that showed the absence of GARE cis-regulatory sequences (DNA recognition sites of gibberellin response) in this promoter (Fig. 4).
Fig. 4.
Schematic diagram of FvPRE1, FvPRE2 and FvPRE3 promoters from Fragaria vesca. The bar on top indicates the length of the promoter fragment relative to the ATG codon. ABRE and GARE motif are marked within their position in promoter sequence
On the contrary, in vitro strawberry plants treated with GA3 showed morphological changes that resulted in elongated plants while PBZ treated-plants displayed a dwarfed phenotype (Additional file 4). The analysis of FaPRE genes transcription in leaves, pedicel and root of the treated versus untreated plants showed that FaPRE2 increased its transcription in all the analyzed tissues from GA3 treated-plants while FaPRE3 transcription did not vary with respect to the control (Additional file 4B-D). In addition, FaPRE2 and FaPRE3 transcription was significantly reduced in all tissues in the presence of PBZ (Additional file 4 B-D). This suggests that both FaPRE2 and FaPRE3 are under the regulation of GA3 and probably play an active role in gibberellin signaling in vegetative tissues but not in fruits. Moreover, the promoter analysis of both genes presents GARE-motifs. In the pFaPRE2 promoter region two GARE-motifs were present, whereas in pFaPRE3 only one was present (Fig. 4). These data not only support the idea that the FaPRE2 and FaPRE3 transcription is regulated by gibberellins but relate the transcript level of each gene in response to this hormone with the number of GARE-motifs identified in their promoter sequences. Furthermore, these results support the proposal that the transcription of FaPRE1 is independent of GA3 levels.
High-throughput transcriptional analysis of transgenic receptacle where the FaPRE1 transcription was silenced
Considering that FaPRE1 is a transcriptional co-regulator, to determine the putative functional role that FaPRE1 plays along the ripening process, we proceeded to transitorily silence its transcription in ripened fruit by RNAi-FaPRE1 agroinfiltration approaches. RNAi-FaPRE1 silenced receptacles did not shown any phenotypical changes when compared to control receptacles (data not shown). Using a custom-made oligo-based microarray platform [19], a transcriptomic comparison between transgenic receptacle, where the FaPRE1 transcription was silenced, versus control receptacles was carried out (Additional files 5, 6) and the obtained data were validated by qRT-PCR (Additional file 7). The transcriptomic results and their comparison with red receptacle transcriptomes [19], showed that the transcription of 227 genes was down-regulated in FaPRE1 silenced ripen receptacles, out of which160 (70%) were also ripening-related genes (Additional files 8, 9). By way of contrast, the transcription of 276 genes was up-regulated in RNAi-FaPRE1 receptacles, out of whom 211 (76%) were overexpressed in immature strawberry receptacles (Additional files 8, 10).
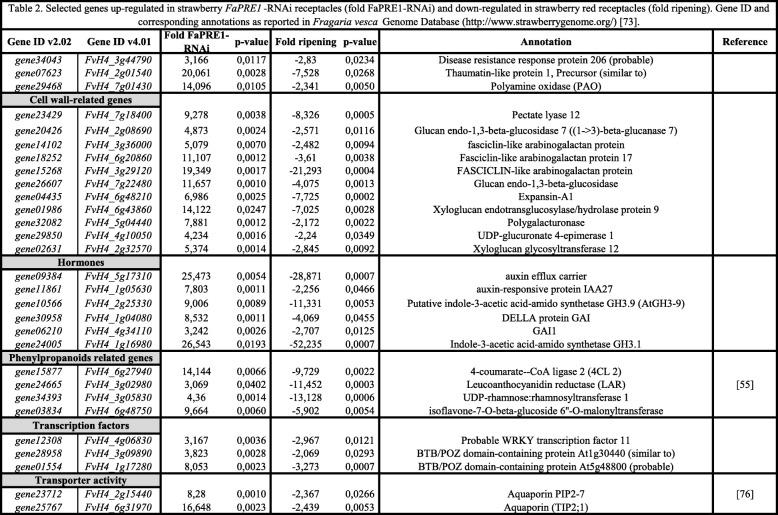
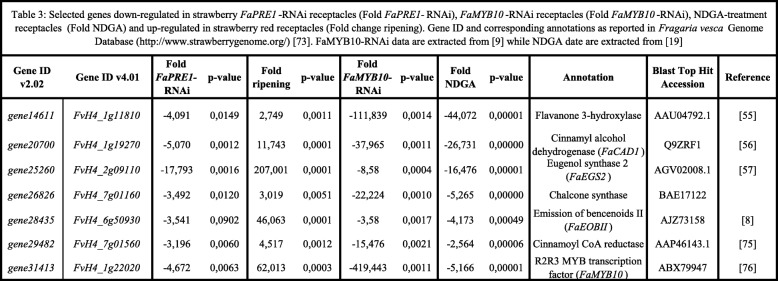
Among the ripening related genes whose transcription was downregulated in RNAi-FaPRE1 ripen receptacles, we found transcription factors as FaMyb10 (gene31413) and FaEOBII (gene28435) [8, 9] (Additional files 9, 10). Both TFs, with a regulatory role in the flavonoid/phenylpropanoid pathway during ripening, were significantly down-regulated in transgenic receptacle with FaPRE1 transcription silenced (Table 1). The same behavior was shown by other genes whose function is described in strawberry during its ripening process, such as gene21638 (FaPG1, polygalacturonase-1 [51]) and gene31030 (FaRGlyaseI, rhamnogalacturonate lyase-1 [52]), which synthesize hydrolytic enzymes related with the cell wall dismantling during the ripening; gene28407 (FaQR, Quinone oxidoreductase [53]), gene07931 (FaAAT2, Alcohol acyl transferase-2 [6]) and gene34009 (FaAAT1, Alcohol acyl transferase-1 [54]), which synthesize enzymes involved in the biosynthesis of esters that contribute to the final aroma of the fruit; and gene14611 (FaF3H, Flavanone 3-hydroxylase [55]), gene20700 (FaCAD1, cinnamyl alcohol dehydrogenase-1 [56]) and gene25260 (FaEGS2, Eugenol synthase-2 [57]), related to the phenylpropanoids biosynthesis in strawberry ripe fruit (Table 1). All these results seem to indicate that FaPRE1 gene might have a regulatory function in the strawberry ripening process.
Table 1.
Selected genes down-regulated in strawberry FaPRE1-RNAi receptacles (fold FaPRE1-RNAi) and up-regulated in strawberry red receptacles (fold ripening). Gene ID and corresponding annotations as reported in Fragaria vesca Genome Database (https://www.rosaceae.org/) [73]
On the other hand, the FaPRE1 silencing induced the expression of genes whose transcription was higher in immature green receptacles, in the development and growth stages, but not in ripening stages. Most of these genes are related with the metabolism and remodeling of the cell wall, both vital processes for the fruit growth and development. Thus, the FaPRE1 silencing induced clearly the transcription of the gene01986 and gene02631 that encode a Xyloglucan endotransglucosylase/hydrolase and a Xyloglucan glycosyltransferase respectively (Table 2). These genes could be related with the hydroxylation and reconnection of xyloglucan fragments during the wall growth [58]. Similarly, gene23429 (Pectate lyase 12) and gene04435 (Expansin-A1) are also up-regulated in FaPRE1-RNAi receptacles. PLs and Expansins have been related to cell elongation and cell wall extensibility [58] (Table 2). In addition, the transcription of gene20426 and gene26607 was also induced in the same receptacles. Both genes encode beta-glucosidases, enzymes that are potentially involved in cellulose degradation [58] (Table 2). Otherwise, the transcription of gene09384, gene11861 and gene24005, that encode an Auxin efflux carrier, an Auxin-responsive protein IAA27, and an Indole-3-acetic acid-amido synthetase GH3.1 respectively, was additionally over-expressed in FaPRE1-RNAi receptacles (Table 2). These three genes are related to the response to auxins which is the hormone that regulates the strawberry receptacle growth and development [59].
Table 2.
Selected genes up-regulated in strawberry FaPRE1-RNAi receptacles (fold FaPRE1-RNAi) and down-regulated in strawberry red receptacles (fold ripening). Gene ID and corresponding annotations as reported in Fragaria vesca Genome Database (https://www.rosaceae.org/) [73]
Discussion
In this article, we present the functional characterization of the FaPRE1, a gene belonging to the strawberry FaPRE family (FaPRE1, FaPRE2 and FaPRE3), which are the putative orthologous of the AtPRE genes from Arabidopsis thaliana.
FaPRE1 gene was classified as member of the subfamily of atypical bHLHs by lacking a DNA binding domain. In this subfamily are also included both the ILI-1 gene from rice [32], SlPRE2 from tomato [33], as well as the members of the Arabidopsis PRE family [22] (Additional files 1, 2, 11). AtPRE1 [20], ATBS1 [39], PGL1 and APG [40, 60], and IBH1 [31] which are involved in transcriptional regulatory processes as repressors, through the blockage by heterodimerization of bHLH transcription factors. As expected by bioinformatic analysis, a nuclear localization of FaPRE1 (Fig. 2, Additional file 3D) supports their relationship with transcriptional regulatory processes. According to our experimental data the FaPRE1 could play a similar role in strawberry fruit ripening process.
FaPRE1 and fruit ripening
FaPRE1 presented an expression model characteristic of a ripening-related gene, with transcription values negligible in both immature receptacles and vegetative tissues, but high in ripened stages. In strawberry, this is a common transcription pattern that is shared by the vast majority of ripening-related genes [19]. This model of expression is characterized by being a) ripening-related; b) receptacle-specific; c) negatively regulated by auxins, and d) induced by ABA. FaPRE1 follows these criteria since the amount of FaPRE1 transcript increases along receptacle ripening (Fig. 1c) and is preferentially expressed in mature red receptacle (Fig. 1b-c). Otherwise, the FaPRE1 transcription was also negatively regulated by auxins but positively by ABA (Fig. 3a-b). The spatial-temporal and the hormonal transcription profile of FaPRE1 are in agreement with the above-mentioned criteria and are also in accordance with the proposal of [59], who suggested that the ABA/auxins ratio determines the transition from the development to the ripening stage in the strawberry receptacle. Thus, auxins would be produced in immature achenes and released to the receptacle promoting its growth but preventing premature ripening. Afterwards, the auxin production would be arrested and subsequently the endogenous biosynthesis of ABA in the receptacle would be stimulated increasing the ABA/auxins ratio and thus promoting the ripening process [59]. This proposal was experimentally demonstrated recently [2, 5, 9]. A similar transcription pattern has been found in other ripening-related genes that encode transcription factors such as FaMYB10 [9], FaEOBII [8] or FaDOF2 [11]. Furthermore, it has been reported that PRE-like genes are under the positive regulation of GAs [20, 43]. That is not the case of the FaPRE1 gene since the treatment of receptacles with GA3 did not result in an increase of its expression, unlike theGA2ox3 control gene, discarding any involvement of this hormone in the regulation of FaPRE1 transcription in ripe strawberry fruit (Fig. 3c). This assumption is reinforced by the absence of regulatory sequences response to GAs (GARE-motifs) in the pFvPRE1 promoter (Fig. 4). However, these motifs are present in the pFvPRE2 and pFvPRE3 promoters, two genes that have a specific expression of vegetative tissues. All these expression data suggest that FaPRE1 plays a different physiological role than FaPRE2 and FaPRE3, mainly focused on the process of fruit ripening.
In soft fruits, with the exception of tomato, the functional role played by PRE-like genes during the fruit ripening process has not been studied. Very recently, the relationship between a PRE-like atypical HLH gene (SlPRE2) and the growth of tomato fruit has been established [33, 34]. Thus, in tomato immature fruit, SlPRE2 seems to have a repressor role of chlorophyll accumulation and chloroplast development. In addition, it represses the transcription of genes involved in the carotenoid’s biosynthesis during the fruit ripening process [33]. However, SlPRE2 presents a different expression pattern from that observed for FaPRE1. Thus, while SlPRE2 was expressed in both fruit and vegetative tissues as root, young leaf, mature leaf, senescent leaf, flower and sepal, the FaPRE1 transcription was restricted to the ripened receptacle. In tomato vegetative tissues, the highest transcription levels were found in young leaf and in flowers where the elongation processes are more active. This is not the case of FaPRE1, whose transcription is mainly limited to the final stages of receptacle ripening, in which the processes of cellular elongation are not significant. Besides, SlPRE2 was expressed strongly in small tomato immature fruit, but its transcript level decreased with the growth, although at a later point its transcription increased slightly along the ripening process [33, 34]. On the contrary, the FaPRE1 transcription raised continuously throughout the receptacle ripening. The expression data suggest that FaPRE1 plays a physiological function different from that played by SlPRE2 in tomato fruit. Otherwise, the SlPRE2 overexpression (35S-SlPRE2) in tomato fruits gave rise to a decrease of both chlorophyll and carotenoid content in unripe and ripe fruits respectively. This fact was accompanied by a down-regulation of the transcript levels of genes related to chlorophyll metabolism and light signaling as GLK2, HY5, RbcS and Cab7 in fruits. Additionally, the transcription of genes involved in the biosynthesis of carotenoids such as phytoene synthase1 (PSY1), phytoene desaturase (PDS), and ζcarotene desaturase (ZDS) was significantly down-regulated in 35S-SlPRE2 transgenic ripe fruits, with a concomitant reduction of lycopene content [33]. These findings indicated that SlPRE2 regulates the chlorophyll and carotenoid content by repressing the expression of these chlorophyll and carotenoid biosynthetic genes. Besides, SlPRE2 determine fruit size probably through a pathway GA3-dependent that regulate the pericarp cell expansion [34]. However, in the strawberry ripening process, the regulatory FaPRE1 function seems to be quite different from that of SlPRE2. Certainly, the comparative analysis carried out between the FaPRE1-RNAi and control receptacles transcriptomes have shown that FaPRE1 plays a dual function regulating the transcription of two groups of genes whose expression models are antagonistic. One of these groups includes those genes that are ripening-related and mainly expressed in the receptacle during the fruit ripening process, while the other group contains those genes that have an expression profile that is more related to the vegetative growth of the receptacle (Additional files 9, 10).
Among the genes whose transcription may be influenced by FaPRE1 in ripened receptacles, we found genes involved in several metabolic processes related to the organoleptic properties of fruit. For instance, genes involved in the regulation of the transcription of those genes belonging to the flavonoid/phenylpropanoid metabolism and that codes two R2R3 MYB transcriptional factors as FaMYB10 and FaEOBII [8, 9]. We have previously demonstrated that FaMYB10 regulates the transcription of most of the Early-regulated Biosynthesis Genes (EBGs) and Late-regulated Biosynthesis Genes (LBGs) involved in the flavonoid/phenylpropanoid pathway, including flavonol-3-hidroxylase (FaF3H), chalcone synthase (FaCHS), dihidroflavonol reductase (DFR), cinnamoyl -CoA reductase (FaCCR), cinnamyl alcohol dehydrogenase (FaCAD1), and eugenol synthase-2 (FaEGS2) [9]. Also, FaMYB10 regulates the FaEOBII expression, which in turn regulates the transcription of the gene that encodes the FaEGS2, an enzyme involved in the biosynthesis of the phenylpropanoid volatile eugenol [8, 57]. The transcription of all these genes was also down-regulated in FaPRE1-RNAi receptacles (Table 3). These results suggest that FaPRE1 would play an important regulatory role of the phenylpropanoids pathway, probably through the regulation of the FaMYB10 transcription through the sequestering of a bHLH whose function would be to suppress the gene expression of this FT in immature receptacle.
Table 3.
Selected genes down-regulated in strawberry FaPRE1-RNAi receptacles (Fold change FaPRE1-RNAi), FaMYB10-RNAi receptacles (Fold change FaMYB10-RNAi) and NDGA-treatment receptacles and up-regulated in strawberry red receptacle (Fold change ripening). Gene ID and corresponding annotations as reported in Fragaria vesca Genome Database (https://www.rosaceae.org/) [73]. FaMYB10-RNAi data are extracted from [9] while NDGA data are extracted from [19]
In addition, the expression of cell wall-related genes as FaPG1 and FaRGlyaseI [51, 52], that has been previously demonstrated that are involved in the cell wall disassembly, was also down-regulated in RNAi-FaPRE1 receptacles (Table 1).
The same behavior was shown by other genes whose function has been described in strawberry during its ripening process and related with aroma production. For instance, one of the genes whose transcription was down-regulated in RNAi-FaPRE1 strawberry receptacles was the ripening-related FaQR gene, that encodes a quinone oxidoreductase. We have demonstrated that this enzyme is crucial for the furaneol (4-hydroxy-2,5-dimethyl-3(2H)-furanone; HDMF) biosynthesis, one of the most important components of the strawberry fruit aroma [61]. Besides, the transcription of genes which encode two enzymes involved in the biosynthesis of the key esters that contribute to the final aroma of the ripened fruit, such as FaAAT1 (Alcohol acyl transferase-1) and FaAAT2 (Alcohol acyl transferase-2) [6, 54], was also down-regulated in RNAi-FaPRE1 strawberry receptacles. All these genes share a common expression profile as receptacle ripening-related genes (Table 1). In general, all these results seem to indicate that FaPRE1 gene has a regulatory function in the strawberry ripening process.
As mentioned above, PREs are HLH proteins which lack the basic domain required for DNA binding but dimerize with DNA binding factors bHLH to inhibit their DNA binding ability [20, 33, 62, 63]. In this sense, we hypothesize that FaPRE1 might exert its transcriptional regulatory properties through the formation of an inactive FaPRE1 HLH: bHLHa heterodimeric complex that would withdraw the amount of bHLHa available to form a homo or heterodimeric transcriptionally active complex. We propose that this heterodimerization should inhibit the formation of a putative heterodimeric (bHLHa:bHLHb) transcriptional activator. This complex would up-regulate the transcription of non-ripening related genes that are expressed specifically in the immature receptacle. These genes are related to the growth and development stages and must be silenced in ripened receptacles. On the contrary, in ripened receptacles, FaPRE1 would inhibit the formation of another bHLH heterodimeric negative regulatory complex that determines the down regulation of ripening-related genes, but in non-ripened immature receptacles. This repressor would be constituted by a heterodimer of two DNA binding basic helix-loop-helices (bHLHa:bHLHc). Thus, the formation of a repressor complex in ripened receptacle would be inhibited by sequestering one of the monomer partners (bHLHa). In this way, the repression of ripening-related genes would be avoided thus facilitating its expression.
A similar but not identical mechanism of interaction between FaPRE-like genes and bHLHs in response to different signals, including light, temperature, BRs and GAs, has been described [31, 32, 43]. For instance, three PRE genes (PRE1, PRE3/ATBS1, PRE6/KIDARI), positively regulate organ elongation in response to GAs, BRs and light signaling [20, 35, 39] through its interaction with other bHLH transcription factors that negatively regulate cell elongation, as AtIBH1, AIFs and HFR1 [35, 39, 64]. In Arabidopsis thaliana, a triantagonistic bHLH system cascade negatively regulates cell elongation in response to multiple hormonal and environmental signaling pathways [43]. In this system, the homodimer HBI1:HBI1 is directly bound to the promoter of two EXPANSIN genes activating its transcription. Otherwise, the interaction of IBH1 with HBI1 inhibits, by heterodimerization, the production of the activator homodimer which in turn determines the repression of both EXPANSIN genes. In addition, PRE1 activates the DNA binding capacity of HBI1 by sequestering its inhibitor IBH1 throughout the PRE1:IBH1 heterodimer formation [43]. Also, this triantagonistic system has been demonstrated in the interactions between the bHLH Activator of Cell Elongation 1(ACE1) and two atypical HLH proteins, AtIBH1 and PRE1, in Arabidopsis [31] and between ACE1 and ATBS1 interaction factors (AIF2, AIF3 and AIF4) or PRE1 in response to BRs and light [32]. Likewise, through a similar regulatory system, the ARF/BZR/PIF interaction stimulates the hypocotyl elongation in Arabidopsis [38].
Apparently, in strawberry, FaPRE1 does not play a similar function to that of SlPRE2 and cannot be considered an orthologous gene. However, its function is clearly involved in the fruit ripening process. In fact, the genes regulated by FaPRE1 are different to those regulated by SlPRE2.
Conclusions
In summary, this work presents, for the first time in strawberry ripened fruits, experimental data that support an important novel function for the atypical HLH FaPRE1 during fruit ripening. FaPRE1 antagonistically coordinated the transcription of genes related to both receptacle growth and ripening. Thus FaPRE1, in ripened receptacle, represses the transcription of receptacle growth promoting genes while activating the transcription of those genes related to the receptacle ripening process.
Methods
Plant material
Fragaria × ananassa Duch. (cv. Camarosa) plants were grown under field conditions in Huelva (S.W. Spain). Strawberry fruits and achenes were harvested at different stages of development and ripening: small-sized green fruits (G1, 2–3 g), middle-sized green fruits (G2, 3–5 g), full-sized green fruits (G3, 4–7 g), white fruits (W, 5–8 g), full-ripe red fruits (R, 10–20 g), over-ripe fruits (OR, 10–20 g) and senescent fruits (SN, 10–20 g). Flowers, floral buds, petals and vegetative tissues such as pedicels and expanding leaves were also collected. Nicotiana benthamiana and strawberry plants (F. × ananassa Duch. cv. Elsanta) used for infiltration were grown in plant chamber at 25 °C, 10.000 lx and 80% humidity. All tissues analyzed were immediately frozen in liquid nitrogen and then stored at − 80 °C. The strawberry plants were acquired in “Viveros California S.L.” (Huelva, Spain) while Nicotiana benthamiana seeds were a gift from Dr. Muñoz-Alamillo.
Hormonal treatments
With the objective to eliminate the auxins of the fruit, the achenes were carefully removed from two sets of 50 full-sized green fruits (G3) each, in accordance with [9]. Briefly, one set of de-achened G3 fruits was covered with lanolin paste containing indole-3-acetic acid (IAA) 1 mM in 1% (w/v) dimethyl sulphoxide (DMSO). The other group of de-achened fruits (control group) was covered with the same paste but without IAA. Sample collection and analysis were performed following the instructions by [9].
For the gibberellic acid (GA3) treatment, strawberry fruits were injected at G2 stage with paclobutrazol (PBZ) 100 μM and GA3100 μM. PBZ is a triazole that slows plant growth blocking the synthesis of gibberellins [65]. Control fruits were injected with water. For each treatment, 30 fruits were infiltrated. Fruits were harvested after 24 h of experimentation. In parallel, in vitro strawberry plants (F. × ananassa Duch. cv. Chandler), micropropagated in a N30K medium supplemented with 2.2 mM kinetin [66], were treated with gibberellic acid. Two groups of six independent clones were grown in MS medium supplemented with GA3 (100 μM) and PBZ (100 μM), respectively, and kept in a growth chamber for 11 days prior harvest. Untreated plants were used as control. All samples and tissues after collection were immediately frozen in liquid nitrogen and then stored at − 80 °C.
In order to block ABA biosynthesis, 20 strawberry fruits (F. x ananassa cv. Elsanta) in G3 stage of development were injected with nordihydroguaiaretic acid (NDGA) 100 μM. NDGA is an ideal inhibitor of the 9-cis-epoxycarotenoid dioxygenase (NCED) enzyme activity [67] and it has previously been demonstrated to decrease endogenous ABA concentration in ripe fruit receptacle [9]. The fruits were injected with 1–2 ml of NDGA solution or water (control fruits) and harvested after 8d of treatment, frozen in liquid nitrogen and stored at − 80 °C until use. These samples were used for measurement of the ABA content and relative expression of FaPREs and FaNCED1 genes.
Quantification of abscisic acid content
Deuterated abscisic acid (dABA) was used as an internal standard. Both the dABA preparation and ABA extraction from strawberry samples were performed following the instructions by [9]. In order to determine the ABA amount, a HPLC-MS system (VARIAN 1200 L Triple Quadrupole) was used with a column (150 × 2.1 mm i.d. Phenomenex C18 with 3 μm particle) (California, USA). The conditions and procedure used for the analysis were the same described by [9].
Bioinformatic resources
Resources of National Center for Biotechnology Information (NCBI) (Bethesda, MD) (http://www.ncbi.nlm.nih.gov) and the European Bioinformatics Institute server (EBI) (http://www.ebi.ac.uk/) were used for in silico study of FaPRE genes sequences against databases. Multiple sequence alignment and phylogenetic tree construction were performed with the EBI ClustalW2 program or the MegAlign program (from the Lasergene DNASTAR software package) as well as the FigTree program (http://tree.bio.ed.ac.uk/software/figtree/). The prediction of domains and functional sites was performed with an InterProScan database (version 4.8) (www.ebi.ac.uk/Tools/pfa/iprscan/) and the prediction of protein localization sites in cells was performed with a Plant-mPLoc computer program (http://www.csbio.sjtu.edu.cn/cgi-bin/PlantmPLoc.cgi). BlastN was also used to localize the genes position in F. vesca and F. x ananassa genome at a GDR databank (https://rosaceae.org). Available F. vesca (v 2.0.a2) genome [44] were used to determine FaPRE promoter sequences. The promoter analysis of FaPRE genes were performed with the PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/).
Generation of RNAi constructs and transfection of strawberry fruits by agroinfiltration
A fragment of 626-pb (RNAi-fragment) from FaPRE1 cDNA was PCR amplified and cloned into pCR®8/GW/TOPO® vector (Invitrogen). Later, the RNAi-fragment was transferred to the pFRN binary vector (courtesy of Marten Denekamp) by LR recombination. The RNAi construct (pFRN-FaPRE1) generated was tested by sequencing and restriction analyses prior to transformation of strawberry fruit. The RNAi-FaPRE1 construct was transformed into Agrobacterium tumefaciens strain AGL1. RNAi-construct was used to obtain transient transgenic strawberry fruit with the FaPRE1 expression silenced by agroinfiltration [68]. The injection of RNAi-construct was performed with a syringe into the base on the entire fruits attached to the strawberry plant following the indications of [68]. 30–40 fruits were inoculated and analysed of a total of 15–25 strawberry plants.
Subcellular localization analysis
The construct used for localization studies was derived from the binary vector pK7WGF2, which allows for the N-terminal fusion of the selected protein with GFP [69]. The 282-bp CDS of the strawberry FaPRE1 gene was amplified from F. x ananassa cDNA using specific primers (Additional file 12) and cloned into the pDONR™221. The PCR-product was then transferred to the pK7WGF2 destination vector, resulting in 35S-GFP-FaPRE1 fusion construct. The generated construct was tested through sequencing prior to N. benthamiana leaves agroinfiltration. The procedures used for N. benthamiana agroinfiltration have been previously described [8, 10]. N. benthamiana plants were agroinfiltrated with clones to express FaPRE1-GFP and GFP. The samples were imaged 2 days after agroinfiltration on a Leica TCS SP8 point scanning confocal microscope using the pre-set settings for GFP with Ex:488 nm, Em:500-550 nm. For nuclear staining, samples were stained with a solution of 40 μg/ml DAPI 10 min before imaging with Ex:405 nm, Em: 448–525 nm.
RNA isolation
Total RNA was isolated from three independent pools (10 fruits per pool) of strawberry fruits at different development stages and plant vegetative tissues following the indications of [70]. When strawberry fruits were used, the achenes were always removed before extracting the RNA from the samples. In any case, the RNA extracted was always incubated with DNase I (RNase free) (Invitrogen) to eliminate the genomic DNA contamination following manufacturer’s instructions. The RNA quality and integrity were checked using an Agilent 2100 Bioanalyzer (Agilent Technologies, Deutschland). Only samples with a RIN value ≥8 were used for subsequent transcriptomic analyses.
Microarray generation and analysis
The transcriptomic changes produced by the FaPRE1 silencing were determined using a custom-made oligo microarray platform (60-mer length; FraGenomics 35 k) containing a total of 34.616 singletons corresponding to those sequences published in the strawberry genome project (http://www.strawberry.org). We compared the transcriptomes from control red receptacles injected with the empty pFRN vector versus red receptacles injected with the RNAi-FaPRE1 construct. The same microarray platform was also used for the transcriptomic analysis of the strawberry ripening fruit process comparing the transcriptomes from green (G1) receptacle versus red (R) receptacle [19]. The corresponding data were deposited in the GEO database (www.ncbi.nlm.nih.gov/geo/) with the GSE125995 for silencing data and GSE126220 for ripening data [19]. The criteria for the selection of the differentially expressed genes were log2 fold change > ±2 and p ≤ 0.05 in both analyses. The microarray characteristics, hybridization and processing conditions were as described in [8].
Validation of microarray data and expression analysis by quantitative real-time PCR
Expression analyses of the genes herein studied in different physiological conditions and for microarray validation were performed by quantitative real-time PCR (qRT-PCR) using iCycler system (BioRad), as previously described by [9, 71]. Specific primers of the 3’UTR regions were designed to analyze the expression of the PRE-like genes (FaPRE1, FaPRE2 and FaPRE3) identified in the strawberry genome. Besides, to validate the expression data obtained in the microarray analysis, specific primers were designed on several genes that showed differential expression in the experimental situations analyzed. Additional file 12 depicts the primer sequences used for all quantitative amplifications. The relative increase or decrease of gene expression in the samples in comparison to that in the control gene was calculated in accordance with Pedersen and [72]. Interspacer 26S–18S gene was selected as control gene owing to its constitutive expression.
Statistical analysis of data
Statistical significance was tested with a Student’s t-test using SPSS software.
Supplementary information
Additional file 1. Phylogenetic tree of 184 bHLH/HLH transcription factors. FaPREs taxa is written in black and grey clade contains sequences belonging to subgroup 16. The tree was constructed using the IQTREE web software (http://iqtree.cibiv.univie.ac.at/) by the neighbor-joining method with 1000 bootstrap replicates.
Additional file 2. Subfamily classification of 182 plant bHLH/HLH sequences examined in this study and additional information.
Additional file 3. A. Table containing additional information of the atypical HLH sequences belonging to subgroup 16. B. Screenshot corresponding to the prediction of domains performed with InterProScan database (version 5) (http://www.ebi.ac.uk/Tools/pfa/iprscan5). C. Sequence alignment of bHLH proteins. Identical amino acids are shaded in black. The two helices are indicated with sets of black arrows and the loop is indicated with a grey line. Numbers indicate amino acid positions. D. Screenshot corresponding to the result of protein localization sites prediction in cells performed with the Plant-mPLoc computer program (http://www.csbio.sjtu.edu.cn/cgi-bin/PlantmPLoc.cgi).
Additional file 4. Phenotypic analysis of F. × ananassa “Chandler” in vitro plants grown in N30K medium supplemented with hormones. (A) General view of control plants (CONTROL) and treated plants with gibberellic acid (GA3) and paclobutrazol (PBZ) after 11 dpt. Analysis by qRT-PCR of FaPRE1, FaPRE2 and FaPRE3 expression in leaves (B), pedicels (C) and roots (D) from in vitro strawberry plants (F. × ananassa “Chandler”) treated with GA3 and PBZ. Mean values ± SD of three independent experiments are shown. CONTROL, plants in N30K medium; GA3, plants in N30K medium supplemented with GA3 100 μM; PBZ, plants in N30K medium supplemented with paclobutrazol 100 μM. Statistical significance with respect to the reference sample (Control) was determined by the Student’s t-test: *p < 0.05.
Additional file 5. Analysis by qRT-PCR of FaPRE1, FaPRE2 and FaPRE3 gene expression in strawberry transgenic receptacle agroinfiltrated with the RNAi-FaPRE1 construct. Control: receptacle agroinfiltrated with the empty pFRN vector; Pool 1, 2 and 3: receptacles agroinfiltrated with FaPRE1-pFRN construct.
Additional file 6 Total microarray data from transcriptomic comparison between transgenic receptacles agroinfiltrated with FaPRE1-RNAi construct and no-transgenic control receptacles. Gene ID and corresponding annotations as reported in Fragaria vesca Genome Database (https://www.rosaceae.org/) [73].
Additional file 7 Expression data of selected genes in FaPRE1-silenced receptacles obtained by QRT-PCR and microarray analysis.
Additional file 8 Venn diagrams showing the number of genes down-regulated (A) and up-regulated (B) in strawberry FaPRE1-RNAi receptacles respectively and up-regulated in strawberry red receptacle.
Additional file 9 All the genes down-regulated in strawberry FaPRE1-RNAi receptacles (fold change RNAi) and up-regulated in strawberry red receptacle (fold ripening). Gene ID and corresponding annotations as reported in Fragaria vesca Genome Database (https://www.rosaceae.org/) [73].
Additional file 10 All the genes up-regulated in strawberry FaPRE1-RNAi receptacles (fold change RNAi) and down-regulated in strawberry red receptacle (fold ripening). Gene ID and corresponding annotations as reported in Fragaria vesca Genome Database (https://www.rosaceae.org/) [73].
Additional file 11 Phylogenetic tree of some functionally characterized atypical HLH transcription factors. The length of each pair of branches represents the distance between sequence pairs, while the units at the bottom of the tree indicate the number of substitution events. FaPRE1(KM655802; Fragaria x ananassa); FaPRE2 (XM_004296502; F. x ananassa); FaPRE3 (XM_004297270; F. x ananassa); SlStyle2.1 (NM_001247361; Solanum lycopersicum) [42]; SlPRE2 (XP_004233358.1; S. lycopersicum) [34]; AtPRE1 (At5g39860; Arabidopsis thaliana) [20]; BNQ3 (NP_190355.2; A. thaliana) [62]; KIDARI (NP_849712; A. thaliana) [35]; ATBS1 (NP_177590; A. thaliana) [32]; AtPRE3 (At1g74500; A. thaliana) [30]; PGL2 (Os02g0747900; Oryza sativa) [40]; AIF2 (At3g06590; A. thaliana) [36]; AIF3 (At3g17100; A. thaliana) [36]; AIF4 (At1g09250; A. thaliana) [36]; PAR1 (At2g42870; A. thaliana) [74]; PAR2 (At3g58850; A. thaliana) [74]. Sequences were aligned using MegAlign (MegAlign 5.00; DNASTAR).
Additional file 12. Primer sequences used in this work. Fw: forward; Rv: reverse. Up: upper; Low: lower.
Acknowledgements
Authors thanks to Dr. Josefa Muñoz-Alamillo (Department of Botany, Ecology and Plant Physiology, University of Cordoba, Spain) for Nicotiana benthamiana seeds and Dr. Marten Denekamp for pFRN binary vector (Department of Molecular Cell Biology, University of Utrecht, The Netherlands).
Abbreviations
- 2,4-D
2,4-dichlorophenoxyacetic acid
- ABA
Abscisic acid
- bHLH
Basic helix-loop-helix
- CDS
Coding DNA sequence
- dABA
Deuterated abscisic acid
- DAPI
4′6-diamino-2-phenylindole dihydrochloride
- G1
Green1 stage
- G2
Green2 stage
- G3
Green3 stage
- GA3
Gibberellic acid
- GFP
Green fluorescent protein
- HLH
Helix-loop-helix
- NCED
9-cis-epoxycarotenoid dioxygenase
- NDGA
Nordihydroguaiaretic acid
- OR
Overripe stage
- PBZ
Paclobutrazol
- PRE1
Paclobutrazol resistance 1
- qRT-PCR
Quantitative real time PCR
- R
Ripe stage
- RNAi
RNA interference
- SN
Senescent stage (the seven subjective stages of strawberry fruit development)
- W
White stage
Authors’ contributions
LM-P and FJM-H carried out some of the experiments and made some figures. FJM-R also carried out some experiments, made some figures, the tables and supplementary material; JAM participated in the maintenance of plants in greenhouses; JLC, EM and AR-F contributed to the analysis and interpretation of microarray data; RB-P carried out several experiments, made figures, co-conducted the work, collaborated in writing and revising the manuscript; JM-B conducted the work, conceived the study and contributed to the discussion regarding the results obtained. All authors have read and approved the manuscript.
Funding
This work was supported by the Spanish Ministerio de Ciencia e Innovación (AGL2014-55784-C2-2-R) by the Spanish Ministerio de Educación y Ciencia (AGL2017-86531-C2-2-R) within the framework of the FPU program (PhD fellowship to MP), and by MINECO within the Ramon y Cajal Program (BP) from the Spanish Government (RYC-2014-15111). The funders have no role in the study design, data analysis and interpretation, and manuscript writing, but just provide the financial support.
Availability of data and materials
The datasets generated and analyzed during the current study are available in the GEO repository (www.ncbi.nlm.nih.gov/geo/) (GSE125995 for silencing data and GSE126220 for ripening data). The data are public from October 18, 2019.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Laura Medina-Puche, Félix J. Martínez-Rivas, Francisco J. Molina-Hidalgo contributed equally to this work.
Contributor Information
Laura Medina-Puche, Email: laura@sibs.ac.cn.
Félix J. Martínez-Rivas, Email: b02marif@uco.es
Francisco J. Molina-Hidalgo, Email: b52mohif@uco.es
José A. Mercado, Email: mercado@uma.es
Enriqueta Moyano, Email: bb2mocae@uco.es.
Antonio Rodríguez-Franco, Email: arfranco@uco.es.
José L. Caballero, Email: bb1carej@uco.es
Juan Muñoz-Blanco, Email: bb1mublj@uco.es.
Rosario Blanco-Portales, Email: bb2blpor@uco.es.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12870-019-2092-4.
References
- 1.Koyama K, Sadamatsu K, Goto-Yamamoto N. Abscisic acid stimulated ripening and gene expression in berry skins of the cabernet sauvignon grape. Funct Integr Genomics. 2010;10:367–381. doi: 10.1007/s10142-009-0145-8. [DOI] [PubMed] [Google Scholar]
- 2.Chai YM, Jia HF, Li CL, Dong QH, Shen YY. FaPYR1 is involved in strawberry fruit ripening. J Exp Bot. 2011;62:5079–5089. doi: 10.1093/jxb/err207. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Liu Y, Liu H, Kang L, Geng J, Gai Y, et al. Identification and expression analysis of MATE genes involved in flavonoid transport in blueberry plants. PLoS One. 2015;10:e0118578. doi: 10.1371/journal.pone.0118578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J, Liu D, Jiang Y, Zhao M, Shan W, Kuang J, et al. Molecular characterization of a strawberry FaASR gene in relation to fruit ripening. PLoS One. 2011;6:e24649. doi: 10.1371/journal.pone.0024649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jia H-F, Chai Y-M, Li C-L, Lu D, Luo J-J, Qin L, et al. Abscisic acid plays an important role in the regulation of strawberry fruit ripening. Plant Physiol. 2011;157:188–199. doi: 10.1104/pp.111.177311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cumplido-Laso G, Medina-Puche L, Moyano E, Hoffmann T, Sinz Q, Ring L, et al. The fruit ripening-related gene FaAAT2 encodes an acyl transferase involved in strawberry aroma biogenesis. J Exp Bot. 2012;63:4275–4290. doi: 10.1093/jxb/ers120. [DOI] [PubMed] [Google Scholar]
- 7.Daminato M, Guzzo F, Casadoro G. A SHATTERPROOF-like gene controls ripening in non-climacteric strawberries, and auxin and abscisic acid antagonistically affect its expression. J Exp Bot. 2013;64:3775–3786. doi: 10.1093/jxb/ert214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Medina-Puche L, Molina-Hidalgo FJ, Boersma M, Schuurink RC, López-Vidriero I, Solano R, et al. An R2R3-MYB transcription factor regulates eugenol production in ripe strawberry fruit receptacles. Plant Physiol. 2015;168:598–614. doi: 10.1104/pp.114.252908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medina-Puche L, Cumplido-Laso G, Amil-Ruiz F, Hoffmann T, Ring L, Rodríguez-Franco A, et al. MYB10 plays a major role in the regulation of flavonoid/phenylpropanoid metabolism during ripening of Fragaria × ananassa fruits. J Exp Bot. 2014;65:401–417. doi: 10.1093/jxb/ert377. [DOI] [PubMed] [Google Scholar]
- 10.Molina-Hidalgo FJ, Medina-Puche L, Gelis S, Ramos J, Sabir F, Soveral G, et al. Functional characterization of FaNIP1;1 gene, a ripening-related and receptacle-specific aquaporin in strawberry fruit. Plant Sci. 2015;238:198–211. doi: 10.1016/j.plantsci.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Molina-Hidalgo FJ, Medina-Puche L, Cañete-Gómez C, Franco-Zorrilla JM, López-Vidriero I, Solano R, et al. The fruit-specific transcription factor FaDOF2 regulates the production of eugenol in ripe fruit receptacles. J Exp Bot. 2017;68:4529–4543. doi: 10.1093/jxb/erx257. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Yuanyuan, Yin Xueren, Xiao Yuwei, Zhang Zuying, Li Shaojia, Liu Xiaofen, Zhang Bo, Yang Xiaofang, Grierson Donald, Jiang Guihua, Klee Harry J., Chen Kunsong. An ETHYLENE RESPONSE FACTOR-MYB Transcription Complex Regulates Furaneol Biosynthesis by Activating QUINONE OXIDOREDUCTASE Expression in Strawberry. Plant Physiology. 2018;178(1):189–201. doi: 10.1104/pp.18.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salvatierra A, Pimentel P, Moya-León MA, Herrera R. Increased accumulation of anthocyanins in Fragaria chiloensis fruits by transient suppression of FcMYB1 gene. Phytochemistry. 2013;90:25–36. doi: 10.1016/j.phytochem.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 14.Vallarino JG, Osorio S, Bombarely A, Casañal A, Cruz-Rus E, Sánchez-Sevilla JF, et al. Central role of FaGAMYB in the transition of the strawberry receptacle from development to ripening. New Phytol. 2015;208:482–496. doi: 10.1111/nph.13463. [DOI] [PubMed] [Google Scholar]
- 15.Pillet J, Yu H-W, Chambers AH, Whitaker VM, Folta KM. Identification of candidate flavonoid pathway genes using transcriptome correlation network analysis in ripe strawberry ( Fragaria × ananassa ) fruits. J Exp Bot. 2015;66:4455–4467. doi: 10.1093/jxb/erv205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei L, Mao W, Jia M, Xing S, Ali U, Zhao Y, et al. FaMYB44.2, a transcriptional repressor, negatively regulates sucrose accumulation in strawberry receptacles through interplay with FaMYB10. J Exp Bot. 2018;69:4805–4820. doi: 10.1093/jxb/ery249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaart JG, Dubos C, Romero De La Fuente I, van Houwelingen AMML, de Vos RCH, Jonker HH, et al. Identification and characterization of MYB-bHLH-WD40 regulatory complexes controlling proanthocyanidin biosynthesis in strawberry (Fragaria × ananassa) fruits. New Phytol. 2013;197:454–467. doi: 10.1111/nph.12017. [DOI] [PubMed] [Google Scholar]
- 18.Zhao F, Li G, Hu P, Zhao X, Li L, Wei W, et al. Identification of basic/helix-loop-helix transcription factors reveals candidate genes involved in anthocyanin biosynthesis from the strawberry white-flesh mutant. Sci Rep. 2018;8:2721. doi: 10.1038/s41598-018-21136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medina-Puche L, Blanco-Portales R, Molina-Hidalgo FJ, Cumplido-Laso G, García-Caparrós N, Moyano-Cañete E, et al. Extensive transcriptomic studies on the roles played by abscisic acid and auxins in the development and ripening of strawberry fruits. Funct Integr Genomics. 2016;16:671–692. doi: 10.1007/s10142-016-0510-3. [DOI] [PubMed] [Google Scholar]
- 20.Lee S, Lee S, Yang K-Y, Kim Y-M, Park S-Y, Kim SY, et al. Overexpression of PRE1 and its homologous genes activates gibberellin-dependent responses in Arabidopsis thaliana. Plant Cell Physiol. 2006;47:591–600. doi: 10.1093/pcp/pcj026. [DOI] [PubMed] [Google Scholar]
- 21.Roig-Villanova I, Bou-Torrent J, Galstyan A, Carretero-Paulet L, Portolés S, Rodríguez-Concepción M, et al. Interaction of shade avoidance and auxin responses: a role for two novel atypical bHLH proteins. EMBO J. 2007;26:4756–4767. doi: 10.1038/sj.emboj.7601890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carretero-Paulet L, Galstyan A, Roig-Villanova I, Martinez-Garcia JF, Bilbao-Castro JR, Robertson DL. Genome-wide classification and evolutionary analysis of the bHLH family of transcription factors in Arabidopsis, poplar, rice, moss, and algae. Plant Physiol. 2010;153:1398–1412. doi: 10.1104/pp.110.153593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang C, Feng R, Ma R, Shen Z, Cai Z, Song Z, et al. Genome-wide analysis of basic helix-loop-helix superfamily members in peach. PLoS One. 2018;13:e0195974. doi: 10.1371/journal.pone.0195974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei K, Chen H. Comparative functional genomics analysis of bHLH gene family in rice, maize and wheat. BMC Plant Biol. 2018;18:309. doi: 10.1186/s12870-018-1529-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Massari ME, Murre C. Helix-loop-helix proteins: regulators of transcription in Eucaryotic organisms. Mol Cell Biol. 2000;20:429–440. doi: 10.1128/MCB.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toledo-Ortiz G, Huq E, Quail PH. The Arabidopsis basic/helix-loop-helix transcription factor family. Plant Cell. 2003;15:1749–1770. doi: 10.1105/tpc.013839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herold S, Wanzel M, Beuger V, Frohme C, Beul D, Hillukkala T, et al. Negative regulation of the mammalian UV response by Myc through association with Miz-1. Mol Cell. 2002;10:509–521. doi: 10.1016/S1097-2765(02)00633-0. [DOI] [PubMed] [Google Scholar]
- 28.Hernandez JM, Feller A, Morohashi K, Frame K, Grotewold E. The basic helix loop helix domain of maize R links transcriptional regulation and histone modifications by recruitment of an EMSY-related factor. Proc Natl Acad Sci. 2007;104:17222–17227. doi: 10.1073/pnas.0705629104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shin K, Lee I, Kim E, Park S, Soh M-S, Lee S. PACLOBUTRAZOL-RESISTANCE gene family regulates floral organ growth with unequal genetic redundancy in Arabidopsis thaliana. Int J Mol Sci. 2019;20:869. doi: 10.3390/ijms20040869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castelain M, Le Hir R, Bellini C. The non-DNA-binding bHLH transcription factor PRE3/bHLH135/ATBS1/TMO7 is involved in the regulation of light signaling pathway in Arabidopsis. Physiol Plant. 2012;145:450–460. doi: 10.1111/j.1399-3054.2012.01600.x. [DOI] [PubMed] [Google Scholar]
- 31.Ikeda M, Fujiwara S, Mitsuda N, Ohme-Takagi M. A triantagonistic basic helix-loop-helix system regulates cell elongation in Arabidopsis. Plant Cell. 2012;24:4483–4497. doi: 10.1105/tpc.112.105023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ikeda M, Mitsuda N, Ohme-Takagi M. ATBS1 interacting factors negatively regulate Arabidopsis cell elongation in the triantagonistic bHLH system. Plant Signal Behav. 2013;8:e23448. doi: 10.4161/psb.23448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu Z, Chen G, Guo X, Yin W, Yu X, Hu J, et al. Overexpression of SlPRE2, an atypical bHLH transcription factor, affects plant morphology and fruit pigment accumulation in tomato. Sci Rep. 2017;7:5786. doi: 10.1038/s41598-017-04092-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu Zhiguo, Liang Honglian, Chen Guoping, Li Fenfen, Wang Yunshu, Liao Changguang, Hu Zongli. The bHLH transcription factor SlPRE2 regulates tomato fruit development and modulates plant response to gibberellin. Plant Cell Reports. 2019;38(9):1053–1064. doi: 10.1007/s00299-019-02425-x. [DOI] [PubMed] [Google Scholar]
- 35.Hyun Y, Lee I. KIDARI, encoding a non-DNA binding bHLH protein, represses light signal transduction in Arabidopsis thaliana. Plant Mol Biol. 2006;61:283–296. doi: 10.1007/s11103-006-0010-2. [DOI] [PubMed] [Google Scholar]
- 36.Kim Y, Song J-H, Park S-U, Jeong Y-S, Kim S-H. Brassinosteroid-induced transcriptional repression and dephosphorylation-dependent protein degradation negatively regulate BIN2-interacting AIF2 (a BR signaling-negative regulator) bHLH transcription factor. Plant Cell Physiol cell Physiol. 2017;58:227–239. doi: 10.1093/pcp/pcw223. [DOI] [PubMed] [Google Scholar]
- 37.Lu R, Zhang J, Liu D, Wei Y-L, Wang Y, Li X-B. Characterization of bHLH/HLH genes that are involved in brassinosteroid (BR) signaling in fiber development of cotton (Gossypium hirsutum) BMC Plant Biol. 2018;18:304. doi: 10.1186/s12870-018-1523-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oh E, Zhu J-Y, Bai M-Y, Arenhart RA, Sun Y, Wang Z-Y. Cell elongation is regulated through a central circuit of interacting transcription factors in the Arabidopsis hypocotyl. Elife. 2014;3. 10.7554/eLife.03031. [DOI] [PMC free article] [PubMed]
- 39.Wang H, Zhu Y, Fujioka S, Asami T, Li J, Li J. Regulation of Arabidopsis brassinosteroid signaling by atypical basic helix-loop-helix proteins. Plant Cell. 2009;21:3781–3791. doi: 10.1105/tpc.109.072504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heang D, Sassa H. An atypical bHLH protein encoded by positive regulator of grain length 2 is involved in controlling grain length and weight of rice through interaction with a typical bHLH protein APG. Breed Sci. 2012;62:133–141. doi: 10.1270/jsbbs.62.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jang S, An G, Li H-Y. Rice leaf angle and grain size are affected by the OsBUL1 transcriptional activator complex. Plant Physiol. 2017;173:688–702. doi: 10.1104/pp.16.01653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen K-Y, Cong B, Wing R, Vrebalov J, Tanksley SD. Changes in regulation of a transcription factor lead to autogamy in cultivated tomatoes. Science (80- ) 2007;318:643–645. doi: 10.1126/science.1148428. [DOI] [PubMed] [Google Scholar]
- 43.Bai M-Y, Fan M, Oh E, Wang Z-Y. A triple helix-loop-helix/basic helix-loop-helix cascade controls cell elongation downstream of multiple hormonal and environmental signaling pathways in Arabidopsis. Plant Cell. 2012;24:4917–4929. doi: 10.1105/tpc.112.105163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li Yongping, Wei Wei, Feng Jia, Luo Huifeng, Pi Mengting, Liu Zhongchi, Kang Chunying. Genome re-annotation of the wild strawberry Fragaria vesca using extensive Illumina- and SMRT-based RNA-seq datasets. DNA Research. 2017;25(1):61–70. doi: 10.1093/dnares/dsx038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edger PP, Poorten TJ, VanBuren R, Hardigan MA, Colle M, McKain MR, et al. Origin and evolution of the octoploid strawberry genome. Nat Genet. 2019;51:541–547. doi: 10.1038/s41588-019-0356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang M, Yang D, Ma F, Zhu M, Shi Z, Miao X. OsHLH61-OsbHLH96 influences rice defense to brown planthopper through regulating the pathogen-related genes. Rice (N Y). 2019;12(9). 10.1186/s12284-019-0267-0. [DOI] [PMC free article] [PubMed]
- 47.Cui J, You C, Zhu E, Huang Q, Ma H, Chang F. Feedback regulation of DYT1 by interactions with downstream bHLH factors promotes DYT1 nuclear localization and anther development. Plant Cell. 2016;28:1078–1093. doi: 10.1105/tpc.15.00986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu Z, Liu X, He X, Xu L, Huang Y, Shao H, et al. The soybean basic helix-loop-helix transcription factor ORG3-like enhances cadmium tolerance via increased Iron and reduced cadmium uptake and transport from roots to shoots. Front Plant Sci. 2017;8:1098. doi: 10.3389/fpls.2017.01098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Csukasi F, Osorio S, Gutierrez JR, Kitamura J, Giavalisco P, Nakajima M, et al. Gibberellin biosynthesis and signalling during development of the strawberry receptacle. New Phytol. 2011;191:376–390. doi: 10.1111/j.1469-8137.2011.03700.x. [DOI] [PubMed] [Google Scholar]
- 50.Symons GM, Chua Y-J, Ross JJ, Quittenden LJ, Davies NW, Reid JB. Hormonal changes during non-climacteric ripening in strawberry. J Exp Bot. 2012;63:4741–4750. doi: 10.1093/jxb/ers147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quesada MA, Blanco-Portales R, Pose S, Garcia-Gago JA, Jimenez-Bermudez S, Munoz-Serrano A, et al. Antisense down-regulation of the FaPG1 gene reveals an unexpected central role for Polygalacturonase in strawberry fruit softening. Plant Physiol. 2009;150:1022–1032. doi: 10.1104/pp.109.138297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Molina-Hidalgo FJ, Franco AR, Villatoro C, Medina-Puche L, Mercado JA, Hidalgo MA, et al. The strawberry (Fragaria×ananassa) fruit-specific rhamnogalacturonate lyase 1 (FaRGLyase1) gene encodes an enzyme involved in the degradation of cell-wall middle lamellae. J Exp Bot. 2013;64:1471–1483. doi: 10.1093/jxb/ers386. [DOI] [PubMed] [Google Scholar]
- 53.Fu X, Cheng S, Zhang Y, Du B, Feng C, Zhou Y, et al. Differential responses of four biosynthetic pathways of aroma compounds in postharvest strawberry ( Fragaria × ananassa Duch.) under interaction of light and temperature. Food Chem. 2017;221:356–364. doi: 10.1016/j.foodchem.2016.10.082. [DOI] [PubMed] [Google Scholar]
- 54.Aharoni A, Keizer LCP, Bouwmeester HJ, Sun Z, Alvarez-Huerta M, Verhoeven HA, et al. Identification of the SAAT gene involved in strawberry flavor biogenesis by use of DNA microarrays. Plant Cell. 2000;12:647. doi: 10.2307/3870992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Almeida JRM, D’Amico E, Preuss A, Carbone F, de Vos CHR, Deiml B, et al. Characterization of major enzymes and genes involved in flavonoid and proanthocyanidin biosynthesis during fruit development in strawberry (Fragaria ×ananassa) Arch Biochem Biophys. 2007;465:61–71. doi: 10.1016/j.abb.2007.04.040. [DOI] [PubMed] [Google Scholar]
- 56.Blanco-Portales R, Medina-Escobar N, López-Ráez JA, González-Reyes JA, Villalba JM, Moyano E, et al. Cloning, expression and immunolocalization pattern of a cinnamyl alcohol dehydrogenase gene from strawberry (Fragaria x ananassa cv. Chandler) J Exp Bot. 2002;53:1723–1734. doi: 10.1093/jxb/erf029. [DOI] [PubMed] [Google Scholar]
- 57.Araguez I, Osorio S, Hoffmann T, Rambla JL, Medina-Escobar N, Granell A, et al. Eugenol production in Achenes and receptacles of strawberry fruits is catalyzed by synthases exhibiting distinct kinetics. Plant Physiol. 2013;163:946–958. doi: 10.1104/pp.113.224352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barnes WJ, Anderson CT. Release, recycle, rebuild: cell-wall remodeling, autodegradation, and sugar salvage for new wall biosynthesis during plant development. Mol Plant. 2018;11:31–46. doi: 10.1016/j.molp.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 59.Perkins-Veazie P. Horticultural reviews. Oxford: John Wiley & Sons, Inc.; 1995. Growth and ripening of strawberry fruit; pp. 267–297. [Google Scholar]
- 60.Heang D, Sassa H. Antagonistic actions of HLH/bHLH proteins are involved in grain length and weight in rice. PLoS One. 2012;7:e31325. doi: 10.1371/journal.pone.0031325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raab T, López-Ráez JA, Klein D, Caballero JL, Moyano E, Schwab W, et al. FaQR, required for the biosynthesis of the strawberry flavor compound 4-hydroxy-2,5-dimethyl-3(2H)-furanone, encodes an enone oxidoreductase. Plant Cell. 2006;18:1023–1037. doi: 10.1105/tpc.105.039784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mara CD, Huang T, Irish VF. The Arabidopsis floral homeotic proteins APETALA3 and PISTILLATA negatively regulate the BANQUO genes implicated in light signaling. Plant Cell. 2010;22:690–702. doi: 10.1105/tpc.109.065946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zheng K, Wang Y, Wang S. The non-DNA binding bHLH transcription factor Paclobutrazol resistances are involved in the regulation of ABA and salt responses in Arabidopsis. Plant Physiol Biochem PPB. 2019;139:239–245. doi: 10.1016/j.plaphy.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 64.Zhang L-Y, Bai M-Y, Wu J, Zhu J-Y, Wang H, Zhang Z, et al. Antagonistic HLH/bHLH transcription factors mediate brassinosteroid regulation of cell elongation and plant development in rice and Arabidopsis. Plant Cell. 2009;21:3767–3780. doi: 10.1105/tpc.109.070441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Butcher DN, Clark JA, Lenton JR. Gibberellins and the growth of excised tomato roots: comparison of gib-1 mutant and wild type and responses to applied GA 3 and 2 S , 3 S paclobutrazol. J Exp Bot. 1990;41:715–722. doi: 10.1093/jxb/41.6.715. [DOI] [Google Scholar]
- 66.Barceló M, El-Mansouri I, Mercado JA, Quesada MA, Pliego-Alfaro F. Regeneration and transformation via agrobacterium tumefaciens of the strawberry cultivar chandler. Plant Tissue Cult Biotechnol. 1998;54:29–36. doi: 10.1023/A:1006031527413. [DOI] [Google Scholar]
- 67.Creelman RA, Bell E, Mullet JE. Involvement of a Lipoxygenase-like enzyme in abscisic acid biosynthesis. Plant Physiol. 1992;99:1258–1260. doi: 10.1104/pp.99.3.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoffmann T, Kalinowski G, Schwab W. RNAi-induced silencing of gene expression in strawberry fruit (Fragaria x ananassa) by agroinfiltration: a rapid assay for gene function analysis. Plant J. 2006;48:818–826. doi: 10.1111/j.1365-313X.2006.02913.x. [DOI] [PubMed] [Google Scholar]
- 69.Karimi M, Inzé D, Depicker A. GATEWAY vectors for agrobacterium-mediated plant transformation. Trends Plant Sci. 2002;7:193–195. doi: 10.1016/S1360-1385(02)02251-3. [DOI] [PubMed] [Google Scholar]
- 70.Asif MH, Dhawan P, Nath P. A simple procedure for the isolation of high quality rna from ripening banana fruit. Plant Mol Biol Report. 2000;18:109–115. doi: 10.1007/BF02824018. [DOI] [Google Scholar]
- 71.Benítez-Burraco A, Blanco-Portales R, Redondo-Nevado J, Bellido ML, Moyano E, Caballero JL, et al. Cloning and characterization of two ripening-related strawberry (Fragaria x ananassa cv. Chandler) pectate lyase genes. J Exp Bot. 2003;54:633–645. doi: 10.1093/jxb/erg065. [DOI] [PubMed] [Google Scholar]
- 72.Pedersen S, Amtssygehus A. Multiplex relative gene expression analysis by real-time RT-PCR using the iCycler iQ detection system. BioRadiations. 2001;107:10–11. [Google Scholar]
- 73.Shulaev V, Sargent DJ, Crowhurst RN, Mockler TC, Folkerts O, Delcher AL, et al. The genome of woodland strawberry (Fragaria vesca) Nat Genet. 2011;43:109–116. doi: 10.1038/ng.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hao Y, Oh E, Choi G, Liang Z, Wang Z-Y. Interactions between HLH and bHLH factors modulate light-regulated plant development. Mol Plant. 2012;5:688–697. doi: 10.1093/mp/sss011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Agius F, González-Lamothe R, Caballero JL, Muñoz-Blanco J, Botella MA, Valpuesta V. Engineering increased vitamin C levels in plants by overexpression of a D-galacturonic acid reductase. Nat Biotechnol. 2003;21:177–181. doi: 10.1038/nbt777. [DOI] [PubMed] [Google Scholar]
- 76.Ma N, Xue J, Li Y, Liu X, Dai F, Jia W, et al. Rh-PIP2;1, a rose aquaporin gene, is involved in ethylene-regulated petal expansion. Plant Physiol. 2008;148:894–907. doi: 10.1104/pp.108.120154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Salentijn EMJ, Aharoni A, Schaart JG, Boone MJ, Krens FA. Differential gene expression analysis of strawberry cultivars that differ in fruit-firmness. Physiol Plant. 2003;118:571–578. doi: 10.1034/j.1399-3054.2003.00138.x. [DOI] [Google Scholar]
- 78.Lin-Wang K, Bolitho K, Grafton K, Kortstee A, Karunairetnam S, McGhie TK, et al. An R2R3 MYB transcription factor associated with regulation of the anthocyanin biosynthetic pathway in Rosaceae. BMC Plant Biol. 2010;10:50. doi: 10.1186/1471-2229-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Phylogenetic tree of 184 bHLH/HLH transcription factors. FaPREs taxa is written in black and grey clade contains sequences belonging to subgroup 16. The tree was constructed using the IQTREE web software (http://iqtree.cibiv.univie.ac.at/) by the neighbor-joining method with 1000 bootstrap replicates.
Additional file 2. Subfamily classification of 182 plant bHLH/HLH sequences examined in this study and additional information.
Additional file 3. A. Table containing additional information of the atypical HLH sequences belonging to subgroup 16. B. Screenshot corresponding to the prediction of domains performed with InterProScan database (version 5) (http://www.ebi.ac.uk/Tools/pfa/iprscan5). C. Sequence alignment of bHLH proteins. Identical amino acids are shaded in black. The two helices are indicated with sets of black arrows and the loop is indicated with a grey line. Numbers indicate amino acid positions. D. Screenshot corresponding to the result of protein localization sites prediction in cells performed with the Plant-mPLoc computer program (http://www.csbio.sjtu.edu.cn/cgi-bin/PlantmPLoc.cgi).
Additional file 4. Phenotypic analysis of F. × ananassa “Chandler” in vitro plants grown in N30K medium supplemented with hormones. (A) General view of control plants (CONTROL) and treated plants with gibberellic acid (GA3) and paclobutrazol (PBZ) after 11 dpt. Analysis by qRT-PCR of FaPRE1, FaPRE2 and FaPRE3 expression in leaves (B), pedicels (C) and roots (D) from in vitro strawberry plants (F. × ananassa “Chandler”) treated with GA3 and PBZ. Mean values ± SD of three independent experiments are shown. CONTROL, plants in N30K medium; GA3, plants in N30K medium supplemented with GA3 100 μM; PBZ, plants in N30K medium supplemented with paclobutrazol 100 μM. Statistical significance with respect to the reference sample (Control) was determined by the Student’s t-test: *p < 0.05.
Additional file 5. Analysis by qRT-PCR of FaPRE1, FaPRE2 and FaPRE3 gene expression in strawberry transgenic receptacle agroinfiltrated with the RNAi-FaPRE1 construct. Control: receptacle agroinfiltrated with the empty pFRN vector; Pool 1, 2 and 3: receptacles agroinfiltrated with FaPRE1-pFRN construct.
Additional file 6 Total microarray data from transcriptomic comparison between transgenic receptacles agroinfiltrated with FaPRE1-RNAi construct and no-transgenic control receptacles. Gene ID and corresponding annotations as reported in Fragaria vesca Genome Database (https://www.rosaceae.org/) [73].
Additional file 7 Expression data of selected genes in FaPRE1-silenced receptacles obtained by QRT-PCR and microarray analysis.
Additional file 8 Venn diagrams showing the number of genes down-regulated (A) and up-regulated (B) in strawberry FaPRE1-RNAi receptacles respectively and up-regulated in strawberry red receptacle.
Additional file 9 All the genes down-regulated in strawberry FaPRE1-RNAi receptacles (fold change RNAi) and up-regulated in strawberry red receptacle (fold ripening). Gene ID and corresponding annotations as reported in Fragaria vesca Genome Database (https://www.rosaceae.org/) [73].
Additional file 10 All the genes up-regulated in strawberry FaPRE1-RNAi receptacles (fold change RNAi) and down-regulated in strawberry red receptacle (fold ripening). Gene ID and corresponding annotations as reported in Fragaria vesca Genome Database (https://www.rosaceae.org/) [73].
Additional file 11 Phylogenetic tree of some functionally characterized atypical HLH transcription factors. The length of each pair of branches represents the distance between sequence pairs, while the units at the bottom of the tree indicate the number of substitution events. FaPRE1(KM655802; Fragaria x ananassa); FaPRE2 (XM_004296502; F. x ananassa); FaPRE3 (XM_004297270; F. x ananassa); SlStyle2.1 (NM_001247361; Solanum lycopersicum) [42]; SlPRE2 (XP_004233358.1; S. lycopersicum) [34]; AtPRE1 (At5g39860; Arabidopsis thaliana) [20]; BNQ3 (NP_190355.2; A. thaliana) [62]; KIDARI (NP_849712; A. thaliana) [35]; ATBS1 (NP_177590; A. thaliana) [32]; AtPRE3 (At1g74500; A. thaliana) [30]; PGL2 (Os02g0747900; Oryza sativa) [40]; AIF2 (At3g06590; A. thaliana) [36]; AIF3 (At3g17100; A. thaliana) [36]; AIF4 (At1g09250; A. thaliana) [36]; PAR1 (At2g42870; A. thaliana) [74]; PAR2 (At3g58850; A. thaliana) [74]. Sequences were aligned using MegAlign (MegAlign 5.00; DNASTAR).
Additional file 12. Primer sequences used in this work. Fw: forward; Rv: reverse. Up: upper; Low: lower.
Data Availability Statement
The datasets generated and analyzed during the current study are available in the GEO repository (www.ncbi.nlm.nih.gov/geo/) (GSE125995 for silencing data and GSE126220 for ripening data). The data are public from October 18, 2019.