Abstract
Background
Cryptorchidism is a frequent endocrinopathy in boys that has been associated with an increased risk of developing testicular cancer and infertility. The condition is curable by combined surgery and hormonal treatment during early pre-pubertal stages using gonadotropin releasing hormone agonist (GnRHa). However, whether the treatment also alters the expression of testicular long non-coding RNAs (lncRNAs) is unknown. To gain insight into the effect of GnRHa on testicular lncRNA levels, we re-analyzed an expression dataset generated from testicular biopsies obtained during orchidopexy for bilateral cryptorchidism.
Results
We identified EGFR-AS1, Linc-ROR, LINC00221, LINC00261, LINC00282, LINC00293, LINC00303, LINC00898, LINC00994, LINC01121, LINC01553, and MTOR-AS1 as potentially relevant for the stimulation of cell proliferation mediated by GnRHa based on their direct or indirect association with rapidly dividing cells in normal and pathological tissues. Surgery alone failed to alter the expression of these transcripts.
Conclusion
Given that lncRNAs can cooperate with chromatin-modifying enzymes to promote epigenetic regulation of genes, GnRHa treatment may act as a surrogate for mini-puberty by triggering the differentiation of Ad spermatogonia via lncRNA-mediated epigenetic effects. Our work provides additional molecular evidence that infertility and azoospermia in cryptorchidism, resulting from defective mini-puberty cannot be cured with successful orchidopexy alone.
Keywords: Spermatogenesis, Cryptorchidism, Male infertility, GnRHa, lncRNAs, Antisense lncRNAs, lincRNAs, Mitosis
Résumé
Contexte
La cryptorchidie est. une endocrinopathie fréquente chez les garçons. Elle est. associée à un risque élevé de cancer des testicules et d’infertilité. La cryptorchidie peut être soignée par une thérapie incluant une intervention chirurgicale et un traitement hormonal par l’agoniste de l’hormone GnRH. Alors que l’effet de la thérapie sur l’expression des ARNm a été analysé, ses conséquences pour la transcription des longs ARNs non codants (ARNlnc) testiculaires restent inconnues. Afin de mieux comprendre les effets du GnRHa sur les concentrations cellulaires des ARNlnc dans le testicule, nous avons analysé des données d’expression d’ARN par séquençage (ARN-Seq) générées en utilisant des biopsies testiculaires obtenues dans le cadre d’une orchidopexie pour cryptorchidie bilatérale.
Résultats
Nous avons identifié les ARNlnc EGFR-AS1, Linc-ROR, LINC00221, LINC00261, LINC00282, LINC00293, LINC00303, LINC00898, LINC00994, LINC01121, LINC01553, et MTOR-AS1 comme potentiellement pertinents pour la stimulation de la prolifération cellulaire induite par le GnRHa. Cette conclusion fait référence à leur association directe ou indirecte avec la croissance et division cellulaire mitotique rapide dans les tissus normaux et pathologiques. Nous constatons également que la chirurgie seule n’a pas d’effet détectable par ARN-Seq sur l’expression de ces ARNlnc.
Conclusion
Étant donné que certains ARNlnc coopèrent avec des enzymes ayant un effet sur la structure chromatinienne et la régulation épigénétique des gènes, le traitement par GnRHa pourrait substituer la mini-puberté en déclenchant la différenciation des spermatogonies Ad par un mécanisme épigénétiques qui dépendrait des ARNlnc. Notre travail révèle des nouvelles pistes moléculaires soutenant l’hypothèse que l’infertilité et l’azoospermie associées avec la cryptorchidie sont la conséquence d’une anomalie de la mini-puberté. Cela explique pourquoi une thérapie efficace de cette pathologie ne nécessite pas seulement l’orchidopexie mais aussi un traitement hormonal.
Mots-clés: Spermatogénèse, cryptorchidie, GnRHa, ARNlnc, ARNlnc antisense, mitose
Introduction
Long non-coding RNAs (lncRNAs) have emerged as key regulators of gene expression in embryonic stem-cell (ESC) self-renewal and differentiation. In ESCs, lncRNAs are regulated at the genetic level by transcription factor binding to lncRNA gene promoters. A major function of lncRNAs is the regulation of specific gene expression at multiple steps, including the recruitment and expression of basal transcription machinery, post-transcriptional modifications, and epigenetics [1]. LncRNAs have also been proposed to play a targeting role by binding to certain methyltransferases and demethylases, and directing them to specific genomic locations. Depending on the biological context, certain methylation events are stably maintained (e.g., methylation involved in inheritance through mitosis of a silenced heterochromatin state), whereas others have to be amenable to change (e.g., when cells differentiate or respond to environmental cues) [2–5]. The so-called natural antisense transcripts (NATs) have been shown to regulate gene expression by affecting transcription and mRNA stability [2–5]. Almost 80% of the mammalian genome is transcribed, and many genomic loci produce RNAs from both sense and antisense DNA strands [6–8], though the functional importance of most of these transcripts is only poorly characterized.
We have previously demonstrated that the presence of type A dark (Ad) spermatogonia in the testis is a marker of low infertility risk (LIR), whereas low or absent levels (below a critical threshold) indicate high infertility risk (HIR) [9, 10]. Treatment with a gonadotropin-releasing hormone agonist (GnRHa, buserelin) enables the Ad spermatogonia population to recover, significantly improving fertility in HIR patients [11]. GnRHa induces a broad transcriptional response, including genes encoding proteins involved in pituitary development, the hypothalamic-pituitary-gonadal axis, and testosterone synthesis [12]. Earlier work focused on protein-coding mRNAs; consequently, nothing is known about the expression of lncRNAs in the treatment of cryptorchidism.
We identified several hundred GnRHa-responsive lncRNAs, which were grouped into long intergenic non-coding RNAs (lincRNAs) and antisense (AS) lncRNAs. We selected candidates on the basis of their expression profiles and then prioritized them for roles in cell growth, differentiation, and disease based on a literature search in PubMed (www.ncbi.nlm.nih.gov/pubmed/). We also included in this search protein-coding genes located upstream or downstream lincRNAs and sense genes overlapping AS-lncRNAs. In addition, we explored the RNA-RNA interaction data available in the RISE database (http://rise.life.tsinghua.edu.cn). Finally, we interpreted lncRNA expression signals in the context of protein/RNA profiling data published by the Human Protein Atlas (www.proteinatlas.org) and RNA-sequencing data from HIR/LIR patients [12]. We propose that certain hormone-responsive lncRNAs may play a role in establishing adult spermatogenesis during pre-pubertal stages of development by controlling testicular cell proliferation.
Materials and methods
Study population and biopsy sample collection
The samples used in this study have been described elsewhere [12–14]. A cryptorchid testis is defined as a testis localized outside of the scrotum and incapable of being brought into a stable scrotal position. Sixteen boys with isolated bilateral cryptorchidism who underwent orchidopexy were prospectively included in this study (Fig. 1). The patients had a median age of 18.5 months (range 8–59 months). During the first orchidopexy, biopsies of the ipsilateral testicle were obtained from all patients. Based on histological evaluation, biopsies were categorized into two groups, Ad- (or HIR) and Ad+ (or LIR),. The Ad- group included biopsies with no Ad, and the Ad+ group included testes with Ad spermatogonia (Fig. 1). Cryptorchid boys in the Ad- group had 8-times lower plasma LH levels (0.11 IU/L) than the Ad+ group (0.89 IU/L, p < 0.009), indicating hypogonadotropic hypogonadism [13]. Five boys (Ad- group) were randomly included in each arm. One HIR patients was excluded (Fig. 1.) In the GnRHa-treated group, the median total germ cell count per tubule (S/T) increased from 0.11 to 0.42 (p = 0.03, paired-samples Wilcoxon test, one-tailed). In the surgery only group, the median S/T did not change and none of the testes had Ad spermatogonia. In contrast, in the GnRHa treated group, all testes completed the transition from gonocytes to Ad spermatogonia (p = 0.008; Fisher test, 2-tailed) [14].
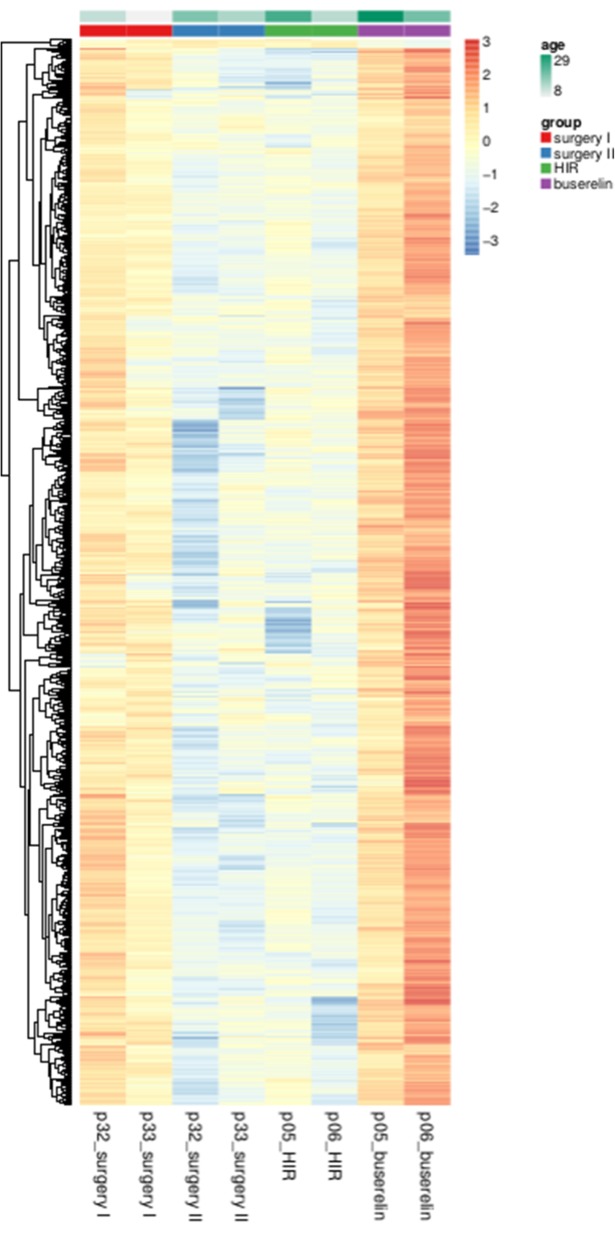
Fig. 1.
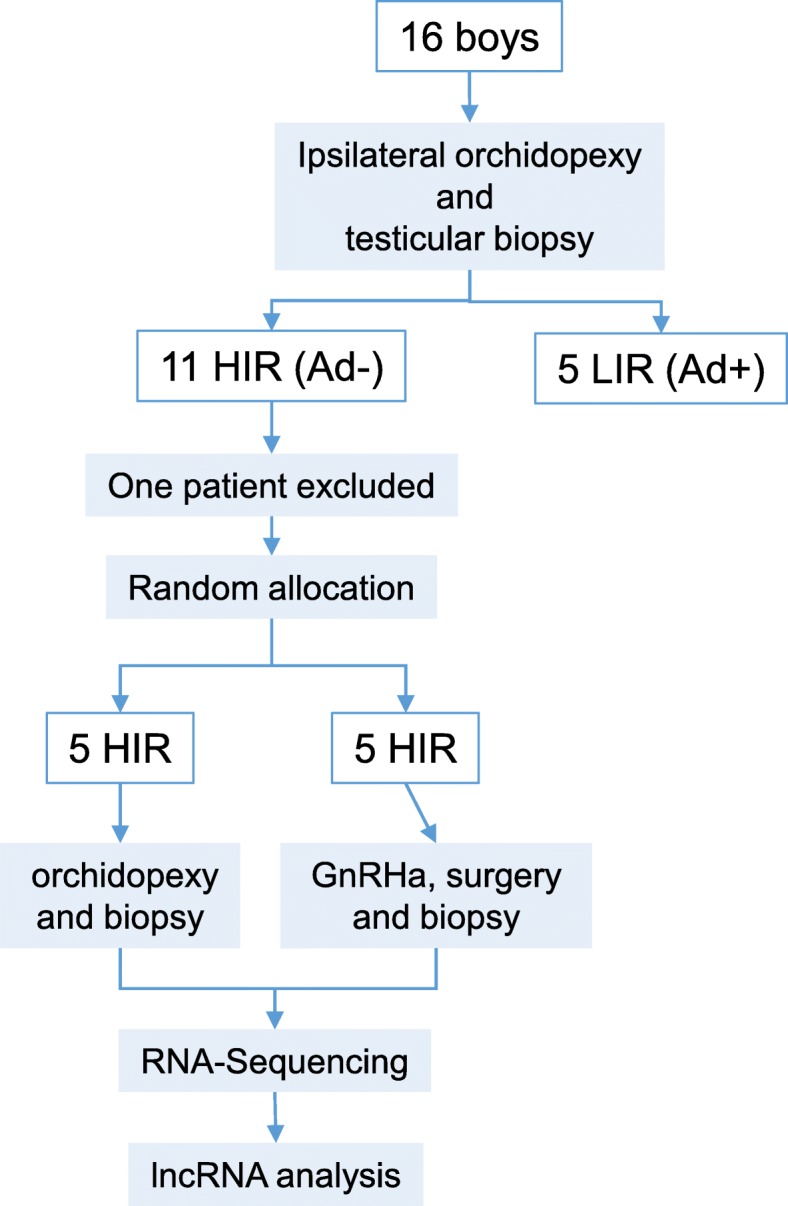
Flow chart of the study design and the selection of patients and samples for RNA-sequencing based on expression profiling. High infertility risk (HIR) and low infertility risk (LIR) patients are indicated. Classification is based on the absence (Ad-) or presence (Ad+) of Ad spermatogonia
RNA-sequencing data analysis
The workflow from RNA isolation to purification, library preparation, sequencing, data analysis, and expression level analysis has been described in detail elsewhere [12]. Determination of differentially expressed genes, statistical analyses, and model design were carried out as described previously [12]. Genomic coordinates for known lncRNAs were obtained from the Bioconductor package TxDb.Hsapiens.UCSC.hg19.lincRNAsTranscripts (version 3.2.2). Only genes with at least one read per million, in at least two samples were included. P-values and fold-changes were calculated for the treatment factor, and differentially expressed genes were defined as those with a false discovery rate (FDR) of less than 0.05. Raw data files are available at the Database of Genotypes and Phenotypes (dbGaP) under accession number phs001275.v1.p1. Expression signals are given in standard RKPM units. They are calculated as follows: the number of reads mapped to a gene sequence is divided by the length of the gene sequence over 1000 multiplied by the total number of mapped reads per sample over 1′000’000.
LncRNA data interpretation
We analyzed lincRNA and AS-lncRNA expression in cryptorchid patients with HIR before and after GnRH treatment to identify all RNAs annotated as antisense (AS) transcripts, as well as all RNAs annotated as lincRNAs with logFC > 1.0. In addition, we compared lincRNA and AS- lncRNA expression between the HIR and LIR groups of cryptorchid patients and analyzed those with lower expression in the HIR group. LincRNAs and AS- lncRNAs were prioritized based on RNA-RNA interactions, revealing the lncRNAs, AS- lncRNAs, or mRNAs encoding proteins involved in spermatogenesis or fertility, and important lncRNAs or mRNAs encoding proteins involved in cell division/growth, signaling pathways, and cancer. Furthermore, we included five lincRNA directly related to spermatogenesis that had a log FC > 1.0. After the AS- lncRNA candidates were identified, they were prioritized based on a PubMed literature search of themselves and their overlapping sense mRNA/protein, revealing roles in spermatogenesis, fertility, cell division/growth, signaling pathways, and cancer (www.ncbi.nlm.nih.gov/pubmed). The RNA annotation was verified using Ensembl (www.ensembl.org; release 97). The lincRNA/mRNA expression was interpreted using GermOnline (www.germonline.org; version 4.0), Human Protein Atlas (www.proteinatlas.org; version 18), and Genevestigator (www.genevestigator.com; version 7.3.1). Experimentally validated RNA-RNA interaction data were retrieved from RISE (http://rise.life.tsinghua.edu.cn; version 1.0).
Results
Global effects on testicular lncRNA levels in response to GnRHa treatment
First, we identified significantly differentially expressed lncRNAs in duplicate testicular biopsies from LIR and HIR patients who underwent surgical correction of undescended testis (Fig. 2, lanes 1–4).
Fig. 2.
RNA-sequencing data for lncRNAs. A false color heatmap (red is high, blue is low) shows data from pairs of testes, analyzed. Horizontal bars at the top indicate patient categories and age. Four sample from two HIR patients (p32 and p33; biopsy number) having had surgery “only” treatment; line 1 and 2 during first surgery obtained from ipsilateral testis (I), line 3 and 4 show results from contralateral testis six months after first orchidopexy (II) Buserelin treated patients p5 and p6; line 5 an 6 before treatment and line 7 and 8 contralateral testis after hormonal treatment. Color scales for expression (red/blue) and age (green) are shown
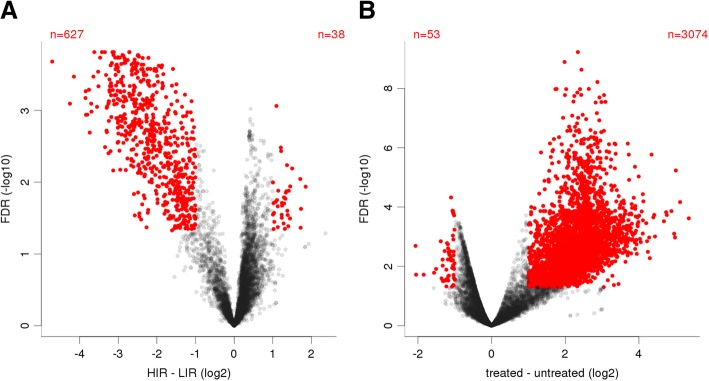
Next, we compared samples obtained from HIR patients at the time of initial surgery (Fig. 2, lanes 5 and 6) and after six months of treatment with GnRHa (Fig. 2, lanes 7 and 8). The genes were ordered using an unsupervised clustering method (hierarchical clustering with complete linkage using Euclidean distances) and are shown in a false-color heatmap relative to the mean expression of each gene over all samples in Fig. 2. The results indicate that a large number of lncRNAs accumulate at low levels in the testes of boys with HIR compared to LIR, and that a substantial fraction of these transcripts is up-regulated by GnRHa treatment. In contrast, surgery alone had no significant impact on lncRNA expression. We explored the dataset using Volcano plots that display statistical significance (false discovery rate, FDR) against fold-change of expression signals allowing the selection of genes for which large and significant differences in expression levels were observed (Fig. 3).
Fig. 3.
Volcano plots of lncRNA expression ratios: (a) Between the high (HIR) and low infertility risk (LIR) groups or (b) in the HIR group before and after GnRHa treatment. Genes with no significant difference in expression between the two groups compared in each panel are in black. Differentially expressed genes are shown in red. The most upregulated genes on the right, the most downregulated genes on the left, and the most significant genes at the top
We found that 627 and 38 lncRNAs were expressed at lower and higher levels, respectively, in HIR versus LIR samples (Fig. 3a). We concluded that the vast majority of differentially expressed lncRNAs are detected at lower levels in HIR testes. Comparing HIR testes before and after GnRHa treatment, we found that 3074 lncRNAs were increased, whereas 53 were decreased (Fig. 3b). Thus, hormonal treatment induces a considerable number of lncRNAs. In the following section the novel lncRNAs were organized based on their known functions or roles that were attributed to their potential target genes.
Certain testicular lincRNAs upregulated by GnRHa treatment are involved in stem cell renewal, signaling, and cell differentiation
We also sought to gain insight into the potential roles that hormone-responsive RNAs might play by interpreting their genomic location, association with protein coding genes in sense/antisense pairs, and RNA-RNA interactions. We selected 11 of 77 lincRNAs and four of 46 AS-lncRNAs with > 2.0-fold change after GnRHa treatment because their expression patterns lead us to hypothesize that they are important for the development of Ad spermatogonia (Table 1). In this section we focus on novel potential regulatory lincRNAs and we provide context information about their putative protein-coding target genes. This includes previously published expression data obtained with samples from HIR and LIR patients (fold change and FDR values) [12] and functional information relevant for germ cells growth and differentiation from the literature.
Table 1.
Testicular lincRNAs and AS-lncRNAs that increase after GnRHa treatment and are involved in stem cell renewal and differentiation
| Gene ID | RPKM before GnRHa Median MAD |
RPKM after GnRHa Median MAD |
log2FC GnRH | p-value | FDR |
|---|---|---|---|---|---|
| LINC-ROR | 0.044 / 0.06 | 0.40 / 0.24 | 2,68 | 0,0004 | 0,002 |
| LINC00261 | 0.11 / 0.04 | 1.11 / 0.68 | 2.60 | 3.588E-08 | 4.35E-06 |
| LINC00293 | 0.05 / 0.04 | 0.66 / 0.49 | 2.80 | 0.0001 | 0.001 |
| LINC00303 | 0.22 / 0.07 | 1.25 / 0.45 | 2.57 | 0.0001 | 0.0008 |
| LINC00520 | 0.15 / 0.07 | 0.79 / 0.64 | 2.76 | 0.0002 | 0.001 |
| LINC00898 | 0.04 / 0.03 | 0.35 / 0.25 | 2.72 | 0.0002 | 0.001 |
| LINC00974 | 0.07 / 0.04 | 0.48 / 0.46 | 3.9 | 0.0008 | 0.003 |
| LINC00994 | 0.20 / 0.10 | 1.41 / 0.81 | 2.73 | 5.773E-06 | 0.0001 |
| LINC01016 | 0.15 / 0.03 | 1.32 / 0.70 | 3,36 | 2.078E-09 | 6.43E-07 |
| LINC01121 | 0.25 / 0.04 | 1.84 / 0.45 | 2.89 | 2.049E-09 | 6.43E-07 |
| LINC01553 | 0.09 / 0.06 | 1.26 / 0.67 | 3.60 | 0.0002 | 0.001 |
| EGFR-AS1 | 0.03 / 0.05 | 0.58 / 0.30 | 2.99 | 3.850E-07 | 2.28E-0.5 |
| HOTTIP | 0.04 / 0.03 | 0.47 / 0.37 | 2.22 | 0.0010 | 0.004 |
| MTOR-AS1 | 0.21 / 0.19 | 1.90 / 1.33 | 4.96 | 6.67E-06 | 0.0001 |
| OTX2-AS1 | 0.09 / 0.007 | 0.91 / 0.45 | 2.37 | 2.95E-0.6 | 8.83E-05 |
The log-fold changes (FC), p-value, false discovery rate (FDR), median expression values in reads per kilobase per million (RPKM) (Median), and the median absolute deviation (MAD) for LINC samples before and after GnRHa treatment are given
LINC01016 is a so-called hub RNA that binds many mRNAs (encoding epigenetic regulators and transcription factors) and lincRNAs (including XIST). This feature distinguishes a hub RNA from most other transcripts that interact with few, one or no other RNAs. LINC01016 is expressed in the same direction as MLN (Motilin) and is a transcriptional target of the estrogen receptor [15] (Table 1).
LINC01121 is expressed upstream of SIX2 and may influence its proximal promoter regions. SIX2 interacts with TCF7L2 and OSR1 in a canonical WNT signaling-independent pathway, preventing the transcription of differentiation genes in cap mesenchyme, such as WNT4 [16–18] (Table 1).
LINC00261 is expressed in the 3’regions of PAX1 and FOXA2, which encode transcription factors. It is also a hub lncRNA that binds mRNAs and lncRNAs, including HOTAIR, and is an epigenetically regulated tumor suppressor that is essential for activation of the DNA damage response [19]. FOXA2 is involved in androgen receptor regulation [19–21] and upregulated after GnRHa treatment (log2FC = 1.69; FDR = 0.0004;). Furthermore, LINC00261 is a tumor suppressor that blocks cellular proliferation by activating the DNA damage response [22].
LINC00303 is expressed upstream of SOX13, a developmental factor expressed in mouse Leydig cells and germ cells [23]. Therefore, this lncRNA could be involved in SOX13 regulation. LINC00293 is expressed upstream of SPIDR, which is involved in double-stranded break repair and genome integrity and binds two TTTY type testis-specific lncRNAs. Several lincRNAs involved in DNA damage repair were increased after GnRHa treatment, including LINC00994 (expressed upstream of PSMD6), LINC00898 (binds mRNA encoding USP1), and testis-specific LINC01553 (interacts with mRNA encoding TIMELESS). TIMELESS plays an important role in the control of DNA replication, the maintenance of genome stability throughout normal DNA replication, and regulation of the circadian clock [24]. (Table 1).
EGFR-AS1, which is involved in determining period length and in the DNA damage-dependent phase advancing the circadian clock [25], interacts with NEU3 mRNA. NEU3 activity enhances EGFR activation without affecting EGFR expression. This may indicate a regulatory mechanism involving a feedback loop. EGFR-AS1 is weakly expressed in adult testis and highly expressed in liver and liver cancer. Intense EGFR immunostaining was found in men with high plasma FSH levels and in all patients who received exogenous FSH, supporting a possible gonadotropin role in the modulation of EGFR expression [25]. GnRHa treatment increased the plasma FSH level and EGFR-AS1, but decreased EGFR expression (log2FC = − 0.58; FDR = 0.01;). Epidermal growth factor receptor signaling is associated with the pathogenesis of cutaneous squamous cell carcinoma. LINC00520-targeted EGFR inhibition might result in inactivation of the PI3K/Akt pathway, thereby inhibiting cancer development [26].
HOTTIP mediates the regulation of CXCL genes, which are implicated in Ad spermatogonia differentiation [12]. HOTTIP is antisense to HOXA13 and modulates cancer stem cell properties in human pancreatic cancer by regulating HOXA9 [27, 28]. OTX2-AS1 is a NAT RNA that plays an important role in eye development and exhibits sequence complementarity to the exon sequences in its corresponding sense gene, OTX2, in both mice and humans [12]. OTX2 is downregulated in HIR (log2FC = − 1.73; FDR = 0.02;) and upregulated after GnRH treatment (log2FC = 1.24; FDR = 0.03) [12]. Though no role has been found for OTX2-AS1, deletion of its sense gene OTX1 was found in six patients with genitourinary defects. Three of these individuals were diagnosed with cryptorchidism [29]. MTOR, the key regulator of spermatogenesis [30], is downregulated in boys with HIR (log2FC = − 0.42; FDR = 0.03;) and remains downregulated after GnRHa treatment (log2FC = − 0.53; FDR = 0.02;). Its antisense gene, MTOR-AS1, was up-regulated 4.9-log2 by GnRHa treatment (Table 1). Thus far, nothing is known about the function of MTOR-AS1.
LINC-ROR is induced 6.5-fold by GnRHa. (Table 1) This lncRNA controls stem cell renewal and acts as an miRNA sponge via gene silencing, which indicates that the transcript itself has a biological role [31–33]. In addition, we found that BOD1L2, a testis-specific gene, is located downstream of LINC-ROR and may be transcriptionally regulated by the lncRNA. BOD1L2 plays a role in chromosome biorientation through the detection or correction of syntelic attachments in mitotic spindles [34, 35]. Buserelin treatment increases BOD1L2 gene expression (log2FC = 1.72 l; FDR = 0.003;), indicating a possible role for it in Ad spermatogonia differentiation.
LincRNAs downregulated in HIR testes and stimulated after GnRHa treatment are associated with cancer and the transition of ad spermatogonia
We previously reported different lncRNA expression in patients with HIR compared to LIR; some of these RNAs participate in epigenetic processes, including AIRN, ERICH-AS1, FENDRR, HAGLR, and XIST [12]. Here, we focuse on seven lncRNAs with decreased gene expression in HIR, indicating abrogated mini-puberty, and increased expression after GnRHa treatment (Tables 2 and 3).
Table 2.
Testicular lincRNAs downregulated in HIR testes compared to LIR
| lincRNA | LIR Median/MAD |
HIR Median/ MAD |
log2FC | p-value | FDR |
|---|---|---|---|---|---|
| LINC00922 | 0.47 0.17 | 0.10 0.07 | - 1.47 | 0.002 | 0.01 |
| LINC00221 | 0.64 0.27 | 0.27 0.07 | - 1.20 | 0.0007 | 0.008 |
| LINC01249 | 0.43 0.28 | 0.15 0.08 | - 1.42 | 0.001 | 0.01 |
| LINC00701 | 0.29 0.03 | 0.11 0.06 | - 0.84 | 0.002 | 0.02 |
| HOTAIR | 0.49 0.23 | 0.13 0.09 | - 1.74 | 0.0001 | 0.002 |
| DLX6-AS1 | 0.42 0.09 | 0.20 0.06 | - 0.92 | 0.006 | 0.03 |
| LINC01446 | 0.57 0.09 | 0.31 0.08 | - 1.21 | 0.0002 | 0.003 |
The log-fold changes (FC), p-value, false discovery rate (FDR), median expression values in reads per kilobase per million (RPKM) (Median), and the median absolute deviation (MAD) for LINC samples are presented
Table 3.
LncNRAs downregulated in HIR testes and stimulated after GnRHa treatment
| lincRNA (RPKM) | before GnRHa Median MAD |
after GnRHa Median MAD |
log2FC | p-value | FDR |
|---|---|---|---|---|---|
| LINC00922 | 0.10 0.07 | 0.85 0.40 | 1.41 | 0.02 | 0.04 |
| LINC00221 | 0.27 0.07 | 1.12 0.42 | 1.23 | 0.01 | 0.03 |
| LINC01249 | 0.15 0.08 | 0.92 0.56 | 1.38 | 0.003 | 0.01 |
| LINC00701 | 0.11 0.06 | 0.72 0.48 | 1.28 | 0.0009 | 0.003 |
| HOTAIR | 0.13 0.09 | 1.04 0.87 | 1.14 | 0.01 | 0.03 |
| DLX6-AS1 | 0.20 0.06 | 1.13 0.81 | 2.02 | 1.55E-05 | 0.0002 |
| LINC01446 | 0.31 0.08 | 1.49 0.82 | 1.14 | 0.0007 | 0.003 |
The log-fold changes (FC), p-value, false discovery rate (FDR), median expression values in reads per kilobase per million (RPKM) (Median), and the median absolute deviation (MAD) for LINC samples before and after GnRHa treatment are shown
LINC00922 is expressed upstream of cadherins CDH5 and CDH11; the latter encodes a calcium-dependent cell adhesion protein that may play a role in testicular architecture [36].
LINC00221 binds mRNA encoding DCBLD2, which is involved in negative regulation of cell growth. Significant downregulation of DCBLD2 occurred after GnRHa treatment (log2FC = − 1.0; FRD = 0.0002;). LINC00221 interacts with VPS53’s mRNA. The protein acts as component of the GARP complex involved in retrograde transport from early and late endosomes to the trans-Golgi network [37].
LINC01249 is expressed upstream of SOX11, which is important for embryonic neurogenesis and tissue modeling. SOX11 is upregulated after GnRHa treatment (log2FC = 0.7; FDR = 0.008). It has been suggested that, together with SOX4, SOX11 may function as a transcriptional repressor in fetal testes, contributing to the precise regulation of SRY and SOX9 [23].
LINC01446 promotes glioblastoma progression by modulating the miR-489-3p/TPT1 pathway [38].
The testis expression of LINC00701 is developmental stage-specific and associated with SLC25A37, encoding a solute carrier localized in the inner mitochondrial membrane. The protein functions as an essential iron importer for the synthesis of mitochondrial heme and iron-sulfur clusters [39].
HOX antisense intergenic RNA (HOTAIR) is an lncRNA that coordinates with chromatin-modifying enzymes, regulates gene silencing, and is transcriptionally induced by estradiol (E2) [12, 40, 41].
Distal-less homeobox6 antisense (DLX6-AS1) was downregulated in HIR and responded positively to GnRHa treatment. This supports the observation in mice that DLX6 participates in the control of steroidogenesis [42]. DLX5 and DLX6 showed low or no expression in HIR samples.
TINCR, an lncRNA required for the induction of key differentiation genes, is downregulated in HIR testes (log2FC = − 1.07 l; FDR = 0.002). Seven epigenetic modifiers found to bind TINCR were upregulated in HIR and downregulated after GnRHa treatment (Table 4).
Table 4.
Seven epigenetic modifiers that bind TINCR
| Gene ID | Name | Log2FC HIR/LIR | FDR HIR/LIR |
Log2FC HIR/ GnRHa |
FDR HIR/ GnRHa |
|---|---|---|---|---|---|
| SETD7 | SET domain containing lysine methyltransferase 7 | + 0.22 | 0.042 | −0.85 | 0.0006 |
| ARID4B | AT-rich ineraction domain 4B | + 0.18 | 0.041 | −0.63 | 0.008 |
| ARID5B | AT-rich interaction domain 5B | + 0.35 | 0.004 | −0.49 | 0.003 |
| KDM6A | lysine demethylase 6A | + 0.20 | 0.017 | −0.81 | 0.0009 |
| CHD6 | chromodomain helicase DNA binding protein 6 | + 0.21 | 0.040 | −0.54 | 0.03 |
| MBD2 | methyl-CpG binding domain protein 2 | + 0.23 | 0.029 | −0.68 | 0.004 |
| BPTF | bromodomain PHD finger transcription factor | + 0.19 | 0.055 | −0.61 | 0.01 |
The log-fold changes (FC), p-value, false discovery rate (FDR), comparing HIR and LIR cryptorchid testes as well as results from HIR group following GnRHa treatment are presented
Discussion
In this study, we aimed to gain molecular insight into the effect on testicular lncRNA expression levels of a curative treatment for cryptorchidism and related infertility that combines surgery and nasal administration of GnRHa [11, 12, 14]. We found hundreds of lncRNAs that respond to treatment, including a subset that is present at lower levels in testicular samples from boys with HIR. A detailed interpretation of the expression data revealed candidate lncRNAs that may play important regulatory roles in establishing adult spermatogenesis during early postnatal development in humans. Our data are consistent with the hypothesis that hypogonadotropic hypogonadism in boys with altered mini-puberty is the consequence of a profoundly altered gene expression program involving protein-coding genes and lncRNAs. The results point to molecular mechanisms that underlie the ability of GnRHa to rescue fertility.
Study design for human testicular RNA profiling experiments
When working with human samples, a critical issue is the number of cases included in a given analysis. The number of replicates affects the statistical confidence level, and human tissue samples exhibit intrinsic variability that needs to be controlled. In this exploratory lncRNA profiling study, we included first seven patients chosen sequentially from a study based on randomized patient samples [12, 14]. Their inclusion in the cohorts to be treated or to remain untreated was completely unbiased by any parameter other than undescended testes, which were surgically corrected. This sample size, while small, is enough for an initial transcriptome study as presented here.
Curative hormone treatment affects signaling pathways
During GnRHa treatment, increased LH and testosterone secretion induced the transition of gonocytes and undifferentiated spermatogonia into Ad spermatogonia. In this context, it is interesting that the expression of LINC-ROR, a key regulator of pluripotent stem cell reprogramming, increased after hormone treatment. LINC-ROR influences cell differentiation, in part, by acting as a sponge for miR-138 and miR-145 and by activating both the canonical and non-canonical WNT/β-catenin signaling pathways [31]. Importantly, an increase in mTOR-AS1 expression after GnRHa treatment may have resulted in the suppression of mTOR activity, allowing spermatogonial stem cells to undergo self-renewal. WNT3 induces many transcription factors associated with mesoderm and is downregulated in the testes of men with HIR testes [12]. WNT interacts with mTOR signaling to affect cancer cell growth and tumor metabolism [43], as well as the formation of spermatozoa [30]. We propose that WNT3 is the signaling component that regulates early expression of Brachyury (T) [44], a mesodermal factor known to determine the fate of Ad spermatogonia. T is a classical and conserved mesodermal factor essential for robust activation of the germline determinants PRDM1 and PRDM14 [45]. T directly upregulates these genes, thereby delineating the downstream primordial germ cell program. In mutant mice lacking Bmp4, a program induced by WNT3 prevents T from activating PRDM1 and PRDM14, demonstrating a permissive role of Bmp4 in primordial gem cell specification [45]. We found that DMRTC2, PAX7, T, and TERT are downregulated in testes with defective mini-puberty and respond to GnRHa treatment [46]. Furthermore, we found lower levels of PRDM1, PRDM6, PRDM9, PRDM13, and PRDM14 mRNA in the testes of patients with HIR compared to LIR, and PRDM7, PRDM9, PRDM12, and PRDM16 were significantly induced after GnRHa treatment. Thus, GnRHa treatment induces an alternate pathway to stimulate PRDM9 for Ad spermatogonia specification without the permissive role of BMP4, but increased the expression of BMP5 (log2FC = 2.31; FDR = 0.0001;) [12, 47]. LINC01121 is expressed upstream of SIX2 and may therefore influence its expression. SIX2 and WNT regulate the self-renewal and commitment of nephron progenitors through shared gene regulatory networks [16–18]. SIX2 also activates the expression of GDNF and plays a role in cell proliferation and migration. Testes in men with HIR expressed lower levels of SIX2 than LIR testes (log2FC = − 1.76; FDR = 0.0008). GnRHa treatment increased SIX2 (log2FC = 1.61; FDR = 0.014) and GDNF (log2FC = 1.46; FDR = 0.003) expression. Taken together, our results support the notion that LINC-ROR and mTOR are involved in Ad spermatogonia development and specification via the WNT signaling pathway.
Hormone treatment influences epigenetic factors
LINC00261 stimulates the expression of HOTAIR and HOTTIP genes to stabilize androgen receptor (AR) together with FOXA1. Specifically, the DNA-binding domain (DBD)/hinge region of AR directly interacts with the fork head domain of FOXA1, thereby acting as an AR-collaborating factor [48].
HOTAIR’s promoter contains multiple functional estrogen response elements (EREs). HOTAIR mediates the recruitment of H3K27 methyltransferase and H3K4 demethylase, which leads to efficient repression of certain loci. The levels of histone H3 lysine-4 trimethylation, histone acetylation, and RNA polymerase II recruitment are increased at the HOTAIR promoter in the presence of E2, and knock-down of ERs downregulated E2-induced HOTAIR expression. Thus, like the transcription of protein-coding genes, E2 induces the transcription of antisense RNAs [27, 49, 50]. HIR patient’s exhibit decreased HOTAIR levels, [12] and GnRHa treatment induced HOTAIR expression, indicating that LH, testosterone and/or converted E2 have a positive effect on HOTAIR and, thus, Ad spermatogonia differentiation [12]. Furthermore, HOTAIR was previously shown to mediate tumorigenesis by recruiting EZH2 [51]. This is of interest, as GnRHa induces expression of HOTAIR, downregulates EZH2 (log2FC = − 0.6; FDR = 0.01) and implicates the regulation of HOTTIP gene transcription, which then transcriptionally regulates the HOXA cluster. As a result, an increase occurs in HOXA2 (log2FC = 2.38; FDR = 0.007), HOXA3 (log2FC = 1.68; FDR = 0.007), HOXA11 (log2FC = 1.77; FDR = 0.02), and HOXA-AS3 (log2FC = 2.68; FDR = 0.0001).
Gamma-aminobutyric acid (GABA) plays a key developmental role in the regulation of GnRH neuron migration from the olfactory placodes into the forebrain during fetal development [52], and co-expression of DLX3 and PAX6 proteins, correlates with acquisition of the olfactory placode fate [53]. Moreover, GABA-A receptors and GABA transporter 1 (GAT1) have been reported to be involved in the proliferation of Leydig cells, testosterone production, and spermatogenesis [54]. GABRA 3 (log2FC = − 2.49; FDR = 0.0001;) and GABR5 (log2FC = − 2.59; FDR = 0.0006;) were downregulated in HIR testes. Following GnRHa treatment, an increase expression was observed in DLX3, PAX6 [12], and TP63 (log2FC = 0.91; FDR = 0.002;), whereby the latter was downregulated in HIR (log2FC = − 1.58; FDR = 0.001;). Berghoff et al. proposed a model in which DLX6-AS1 inhibits the ultraconserved DNA methylation mark in DLX5/6, facilitating antagonistic interactions between repressive and activating transcription factors MECP2 and DLX [53]. DLX2, DLX3, DLX5 and DLX6 showed low or no expression in HIR samples [12] Loss of DLX1/2 increases site-specific methylation of the DLX5/6 ultraconserved enhancer [53]. Tp63 regulates DLX5 and DLX6 transcription, at least in part, via cis-acting regulation at the promoter level [55]. These interactions allow differential control of adjacent genes by shared DNA regulatory elements [56]. It was also shown that deletion of Dlx5 and Dlx6 in the mouse leads to decreased testosterone levels and an abnormal masculinization phenotype [42]. Thus, impaired steroidogenesis during mini-puberty in HIR boys may be induced by altered GABRA-A receptor signaling, silenced DLX6 expression as well as destabilization of AR and ERs.
GnRHa treatment affects genes associated with normal and pathological cell division
Several recent studies have reported roles for lncRNAs in cancer, including MALAT1 and GAS, strongly indicating that lncRNAs not only control gene regulatory pathways in normal cells and tissue, but also during tumor development [57, 58]. The occurrence and progression of cancer is the result of a combination of multiple factors. Furthermore, cancer-specific lncRNA expression patterns appear to be more tissue- and stage-specific than those of protein-coding genes, supporting the potential development of lncRNAs as efficient biomarkers and therapeutic targets [58]. Interestingly, GnRHa treatment increased LH and testosterone secretion and HOTAIR expression, and probably its recruitment to chromatin, whereas the expression of MALAT1 was reduced (log2FC = − 0.64; FDR = 0.03; REF), suggesting opposite regulation and functions of these lncRNAs during normal testis development. It was reported that LINC-ROR promote liver cancer stem cell growth by upregulating TERT and C-MYC [59]. Notably, GnRHa treatment stimulates the expression of TERT (log2FC = 0.91; FDR = 0.002;) [46], which is decreased in HIR (log2FC = − 1.58; FDR = 0.001;) [46] but downregulates C-MYC signaling (log2FC = − 0.58; FDR = 0.01;).
Variation in lincRNA expression may be associated with cancer progression. For example, LINC00261, which plays an important role in gastric cancer, is stimulated by GnRHa, and exerts tumor suppressive activity by reducing cancer cell invasion via suppression of the epithelial-mesenchymal transition process [60]. Another lincRNA, TINCR, is involved in normal tissue differentiation and plays a critical role in cancer and metastasis. TINCR expression is downregulated in HIR (log2FC = − 1.07; FDR = 0.002;) [12]. We observed that TINCR depletion in HIR testes resulted in the induction of key epigenetic modifiers, seven of which were downregulated following GnRHa treatment (Table 4). One of them, SETD7, is an epigenetic modifier and regulates AR [61]. SETD7 binds TINCR and may mediate the formation of AR-associated coactivator complexes. Taken together, the results indicate that the HIR group of cryptorchid boys with abortive mini-puberty expresses several cancer genes. Gonadotropin-releasing hormone treatment may protect against testicular tumor development by downregulating oncogenes, such as MALAT1, mTOR, C-MYC, and EZH2 to enable normal germ cell development.
Conclusions
Two major goals in the field of male reproductive biology are to elucidate the molecular mechanisms that underlie cryptorchidism and to develop an effective treatment for its long-term consequences. According to the mini-puberty hypothesis, early-life exposure to gonadal hormones during a specific window of sensitivity triggers sex-specific developmental processes. To preserve molecular features of differentiated cells, it is crucial that transcriptional alterations triggered by external or intrinsic signals continue beyond the initial stimulus. One possible mechanism involves epigenetic histone variant replacement, chromatin remodeling factors, and lncRNAs associated with epigenetic factors. We found that many of the lincRNAs responding to GnRHa treatment are associated with somatic cancer. This may reflect the fact that the hormone stimulates germ stem cell growth and Leydig, as well as Sertoli, cell division. The mechanisms involved are likely rather diverse and may include promoter/enhancer activity, miRNA sponge activity, or control of gene expression via RNA-RNA interactions. We propose that the lncRNAs identified in this study may be involved in establishing normal male fertility by acting at early stages of spermatogonia stem cell development and by affecting other testicular cells capable of responding to GnRHa treatment. Our results provide information’s for further functional analysis of long-noncoding RNA in relation to the infertility development. We propose that the HOTAIR and DLX pathways, as well as both canonical and non-canonical WNT pathways, are involved in Ad spermatogonia growth and differentiation.
Acknowledgments
We thank Manuel Kohler, Department of Biosystems Science and Engineering (D-BSSE), ETH Zurich, for helping us perform the RNA sequencing.
Abbreviations
- 1PRDM6
PR/SET domain 6
- AIRN
Antisense of IGF2R (insulin like growth factor 2 receptor) non-protein coding RNA
- AR
Androgen receptor
- ARID4B
AT-rich interaction domain 4B
- ARID5B
AT-rich interaction domain 5B
- AS-lncRNA
Antisense long non-coding RNA
- Bmp4
Bone morphogenetic protein 4
- BMP5
Bone morphogenetic protein 5
- BOD1L2
Biorientation of chromosomes in cell division 1 like 2
- BPTF
Bromodomain PHD finger transcription factor
- CDH11
Cadherin 11
- CDH5
Cadherin 5
- CDH6
Cadherin 6
- C-MYC
v-myc avian myelocytomatosis viral oncogene homolog
- CXCL
Chemokine
- DBD
DNA-binding domain
- dbGaP
Database of Genotypes and Phenotypes
- DCBLD2
Discoidin, CUB and LCCL domain containing 2
- DLX
Distal-less homeobox
- DLX2
Distal-less homeobox 2 [Homo sapiens (human)]
- DLX3
Distal-less homeobox 3 [Homo sapiens (human)]
- Dlx5
Distal-less homeobox 5 [Mus musculus (house mouse)]
- Dlx6
Distal-less homeobox 6 [Mus musculus (house mouse)]
- DLX6-AS1
Distal-less homeobox 6 antisense RNA 1
- DMRTC2
DMRT (doublesex and mab-3 related transcription factor) like family C2
- E2
Estradiol
- EGFR
Epidermal growth factor receptor
- EGFR-AS1
Epidermal growth factor receptor antisense RNA 1
- EREs
Estrogen response elements
- ERICH-AS1
Glutamate rich protein antisense RNA 1
- ERs
Estrogen receptors
- ESC
Embryonic stem-cell
- EZH2
Enhancer of zeste 2 polycomb repressive complex 2 subunit
- FDR
False discovery rate
- FENDRR
FOXF1 (forkhead box F1) adjacent non-coding developmental regulatory RNA
- FOXA1
Forkhead box A1
- FOXA2
Forkhead box A2
- FSH
Follicle stimulating hormone
- GABA
Gamma-aminobutyric acid
- GABA-A
Gamma-aminobutyric acid type A
- GABRA-A
gamma-aminobutyric acid (GABA) A receptor, alpha 1
- GABRA-A5
Gamma-aminobutyric acid (GABRA) A receptor 5
- GARP
Golgi-associated retrograde protein
- GAS
Growth arrest specific
- GAT1
GABA (gamma-aminobutyric acid) transporter 1
- GDNF
Glial cell derived neurotrophic factor
- GnRHa
Gonadotropin releasing hormone antagonist
- H3K27
Histone H3 Lysine 27
- H3K4
Histone H3 Lysine 4
- HAGLR
HOXD (homeobox D cluster) antisense growth-associated long non-coding RNA
- HIR
High infertility risk
- HOTAIR
HOX (homeobox) transcript antisense RNA
- HOTTIP
HOXA (homeobox A cluster) distal transcript antisense RNA
- HOXA11
Homeobox A11
- HOXA13
Homeobox A13
- HOXA2
Homeobox A2
- HOXA3
Homeobox A3
- HOXA9
Homeobox A9
- HOXA-AS3
HOXA (Homeobox A) cluster antisense RNA 3
- KDM6A
Lysine demethylase 6A
- LH
Luteinizing hormone
- LINC00221
Long intergenic non-protein coding RNA 221
- LINC00261
Long intergenic non-protein coding RNA 261
- LINC00282
long intergenic non-protein coding RNA 282
- LINC00293
Long intergenic non-protein coding RNA 293
- LINC00303
Long intergenic non-protein coding RNA 303
- LINC00520
Long intergenic non-protein coding RNA 520
- LINC00701
Long intergenic non-protein coding RNA 701
- LINC00898
Long intergenic non-protein coding RNA 898
- LINC00922
Long intergenic non-protein coding RNA 922
- LINC00974
Long intergenic non-protein coding RNA 974
- LINC00994
Long intergenic non-protein coding RNA 994
- LINC01016
Long intergenic non-protein coding RNA 1016
- LINC01121
Long intergenic non-protein coding RNA 1121
- LINC01249
Long intergenic non-protein coding RNA 1249
- LINC01446
Long intergenic non-protein coding RNA 1446
- LINC01553
Long intergenic non-protein coding RNA 1553
- lincRNA
Long intergenic non-protein coding RNA
- Linc-ROR
Long intergenic non-protein coding RNA, regulator of reprogramming
- LIR
Low infertility risk
- lncRNA
Long non-coding RNA
- log2FC
log2 fold change
- MALAT1
Metastasis associated lung adenocarcinoma transcript 1
- MBD2
Methyl-CpG binding domain protein 2
- MECP2
methyl-CpG binding protein 2
- miR-138
microRNA 138
- miR-145
microRNA 1
- miR-489-3P
microRNA 489
- MLN
Motilin
- MTOR
mechanistic target of rapamycin kinase
- MTOR-AS1
MTOR (mechanistic target of rapamycin kinase) antisense RNA 1
- NAT RNA
N-acetyltransferase RNA
- NATs
Natural antisense transcripts
- NEU3
Neuraminidase 3
- OSR1
Odd-skipped related transcription factor 1
- OTX1
Orthodenticle homeobox 1
- OTX2
Orthodenticle homeobox 2
- OTX2-AS1
Orthodenticle homeobox 2 antisense RNA 1 (head to head)
- PAX1
Paired box 1
- PAX6
Paired box 6
- PAX7
Paired box 7
- PI3K/Akt pathway
Phosphatidylinositol 3-kinase, putative, and protein kinase B pathway
- PRDM1
PR domain containing 1, with ZNF domain
- PRDM12
PR/SET domain 12
- PRDM14
PR/SET domain 14
- PRDM16
PR/SET domain 16
- PRDM7
PR/SET domain 7
- PRDM9
PR/SET domain 9
- PRMD13
PR/SET domain 13
- PSMD6
Proteasome 26S subunit, non-ATPase (adenosine triphosphatase) 6
- RPKM
Reads per kilo base per million mapped reads
- S/T
Total germ cell count per tubule
- SETD7
SET domain containing 7, histone lysine methyltransferase
- SIX2
SIX homeobox 2
- SLC25A37
Solute carrier family 25 member 37
- SOX11
SRY (sex determining region Y)-box transcription factor 11
- SOX13
SRY (sex determining region Y)-box transcription factor 13
- SOX4
SRY (sex determining region Y)-box transcription factor 4
- SPIDR
Scaffold protein involved in DNA repair
- SRY
Sex determining region of Y
- T
Brachyury
- TCF7L2
Transcription factor 7 like 2
- TERT
Telomerase reverse transcriptase
- TIMELESS
Timeless circadian regulator
- TINCR
TINCR (Tissue differentiation-inducing non-protein coding RNA) ubiquitin domain containing
- TP63
Tumor protein p63
- TPT1
Tumor protein, translationally-controlled 1
- TTTY
Testis-specific transcript, Y-linked
- USP1
Ubiquitin specific peptidase 1
- VPS53
VPS53 (Vacuolar protein sorting 53 homolog) subunit of GARP (Golgi-associated retrograde protein) complex
- Wnt
Wingless-related integration site
- WNT3
Wnt family member 3
- WNT4
Wnt family member 4
- XIST
Inactive X specific transcripts
Authors’ contributions
FH conceived and designed the study, interpreted the data, and organized and wrote the manuscript. GV performed experiments, analyzed the data, and read the paper. BV performed experiments and read the paper. MBS analyzed and interpreted the data, contributed analysis tools, and read the paper.
Funding
This study was supported in part by the European Social Fund under the Global Grant measure. Work in the Stadler group is supported by funding from the MetastasiX project of SystemsX.ch.
Availability of data and materials
Raw data files were deposited at the Database of Genotypes and Phenotypes (dbGaP) under accession number phs001275.v1.p1.
Ethics approval and consent to participate
Investigations were carried out in accordance with the Declaration of Helsinki of 1975 (revised in 2008). The study was approved by the Institutional Review Board and the Independent Ethics Committee of Vilnius University (Vilnius Regional Biomedical Research Ethics Committee, No. 158200–580-PPI-17, 11 June 2013).
Consent for publication
Written informed consent was obtained from the patients’ guardians after approval by the ethical committee.
Competing interests
The authors declare no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Faruk Hadziselimovic, Email: liestal@kindermedizin-zentrum.ch.
Gilvydas Verkauskas, Email: gilvydas.verkauskas@santa.lt.
Beata Vincel, Email: beata.vincel@santa.lt.
Michael B. Stadler, Email: stadler.michael@gmail.com
References
- 1.Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43:904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pelechano V, Steinmetz LM. Gene regulation by antisense transcription. Nat Rev Genet. 2013;14:880–893. doi: 10.1038/nrg3594. [DOI] [PubMed] [Google Scholar]
- 3.Tufarelli C, Stanley JA, Garrick D, Sharpe JA, Ayyub H, Wood WG, et al. Transcription of antisense RNA leading to gene silencing and methylation as a novel cause of human genetic disease. Nat Genet. 2003;34:157–165. doi: 10.1038/ng1157. [DOI] [PubMed] [Google Scholar]
- 4.Fernandes JCR, Acuña SM, Aoki JI, Floeter-Winter LM, Muxel SM. Long non-coding RNAs in the regulation of gene expression: physiology and disease. Noncoding RNA. 2019;5(1):17. doi: 10.3390/ncrna50100175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadjicharalambous MR, Lindsay MA. Long non-coding RNAs and the innate immune response. Noncoding RNA. 2019;5(2):34. doi: 10.3390/ncrna5020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carninci P, Kasukawa T, Katayama S, Gough J, Frith MC, Maeda N, et al. The transcriptional landscape of the mammalian genome. Science. 2005;309:1559–1563. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 7.Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, et al. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forrest AR, Kawaji H, Rehli M, Baillie JK, de Hoon MJ, Haberle V, et al. FANTOM consortium and the RIKEN PMI and CLST (DGT): a promoter-level mammalian expression atlas. Nature. 2014;507:462–470. doi: 10.1038/nature13182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadziselimovic F, Herzog B. The importance of both an early orchidopexy and germ cell maturation for fertility. Lancet. 2001;358:1156–1157. doi: 10.1016/S0140-6736(01)06274-2. [DOI] [PubMed] [Google Scholar]
- 10.Hadziselimovic F, Hocht B, Herzog B, Buser MW. Infertility in cryptorchidism is linked to the stage of germ cell development at orchidopexy. Horm Res. 2007;68:46–52. doi: 10.1159/000100874. [DOI] [PubMed] [Google Scholar]
- 11.Hadziselimovic F. Successful treatment of unilateral cryptorchid boys risking infertility with LH-RH analogue. Int Braz J Urol. 2008;34:319–326. doi: 10.1590/S1677-55382008000300009. [DOI] [PubMed] [Google Scholar]
- 12.Hadziselimovic F, Gegenschatz-Schmid K, Verkauskas G, Demougin P, Bilius V, Dasevicius D, et al. GnRHa treatment of Cryptorchid boys affects genes involved in hormonal control of the HPG Axis and fertility. Sex Dev. 2017;11(3):126–136. doi: 10.1159/000471937. [DOI] [PubMed] [Google Scholar]
- 13.Verkauskas G, Malcius D, Eidukaite A, Vilimas J, Dasevicius D, Bilius V, et al. Prospective study of histological and endocrine parameters of gonadal function in boys with cryptorchidism. J Pediatr Urol. 2016;12(4):238.e1–238.e6. doi: 10.1016/j.jpurol.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Vincel Beata, Verkauskas Gilvydas, Bilius Vytautas, Dasevicius Darius, Malcius Dalius, Jones Birute, Hadziselimovic Faruk. Gonadotropin-Releasing Hormone Agonist Corrects Defective Mini-Puberty in Boys with Cryptorchidism: A Prospective Randomized Study. BioMed Research International. 2018;2018:1–5. doi: 10.1155/2018/4651218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonsson P, Coarfa C, Mesmar F, Raz T, Rajapakshe K, Thompson JF, et al. Single-molecule sequencing reveals estrogen-regulated clinically relevant lncRNAs in breast Cancer. Mol Endocrinol. 2015;29(11):1634–1645. doi: 10.1210/me.2015-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park JS, Ma W, O'Brien LL, Chung E, Guo JJ, Cheng JG, et al. Six2 and Wnt regulate self-renewal and commitment of nephron progenitors through shared gene regulatory networks. Dev Cell. 2012;23(3):637–651. doi: 10.1016/j.devcel.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Hongbing, Chen Shaowei, Yao Xiao, Li Yuwen, Chen Chao-Hui, Liu Jiao, Saifudeen Zubaida, El-Dahr Samir S. Histone deacetylases 1 and 2 regulate the transcriptional programs of nephron progenitors and renal vesicles. Development. 2018;145(10):dev153619. doi: 10.1242/dev.153619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu Xu J, Liu H, Park JS, Lan Y, Jiang R. Osr1 acts downstream of and interacts synergistically with Six2 to maintain nephron progenitor cells during kidney organogenesis. Development. 2014;141(7):1442–1452. doi: 10.1242/dev.103283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang Q, Sang L, Du S. Long noncoding RNA LINC00261 regulates endometrial carcinoma progression by modulating miRNA/FOXO1 expression. Cell Biochem Funct. 2018;36(6):323–330. doi: 10.1002/cbf.3352. [DOI] [PubMed] [Google Scholar]
- 20.Liu Z, Dai J, Shen H. Systematic analysis reveals long noncoding RNAs regulating neighboring transcription factors in human cancers. Biochim Biophys Acta Mol basis Dis. 2018;1864(9 Pt B):2785–2792. doi: 10.1016/j.bbadis.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Connelly ZM, Yang S, Chen F, Yeh Y, Khater N, Jin R, et al. Foxa2 activates the transcription of androgen receptor target genes in castrate resistant prostatic tumors. Am J Clin Exp Urol. 2018;6(5):172–181. [PMC free article] [PubMed] [Google Scholar]
- 22.Shahabi S, Kumaran V, Castillo J, Cong Z, Nandagopal G, Mullen DJ, et al. LINC00261 is an epigenetically regulated tumor suppressor essential for activation of the DNA damage response. Cancer Res. 2019;79(12):3050–3062. doi: 10.1158/0008-5472.CAN-18-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daigle M, Roumaud P, Martin LJ. Expressions of Sox9, Sox5, and Sox13 transcription factors in mice testis during postnatal development. Mol Cell Biochem. 2015;407:209–221. doi: 10.1007/s11010-015-2470-7. [DOI] [PubMed] [Google Scholar]
- 24.Shen X, Li M, Mao Z, Yu W. Loss of circadian protein TIMELESS accelerates the progression of cellular senescence. Biochem Biophys Res Commun. 2018;503(4):2784–2791. doi: 10.1016/j.bbrc.2018.08.040. [DOI] [PubMed] [Google Scholar]
- 25.Foresta C, Varotto A. Immunocytochemical localization of epidermal growth factor receptors in human testis from infertile subjects. Fertil Steril. 1994;61(5):941–948. doi: 10.1016/S0015-0282(16)56710-7. [DOI] [PubMed] [Google Scholar]
- 26.Mei XL, Zhong S. Long noncoding RNA LINC00520 prevents the progression of cutaneous squamous cell carcinoma through the inactivation of the PI3K/Akt signaling pathway by downregulating EGFR. Chin Med J. 2019;132(4):454–465. doi: 10.1097/CM9.0000000000000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin C, Wang Y, Wang Y, Zhang S, Yu L, Guo C, et al. Transcriptional and posttranscriptional regulation of HOXA13 by lncRNA HOTTIP facilitates tumorigenesis and metastasis in esophageal squamous carcinoma cells. Oncogene. 2017;36(38):5392–5406. doi: 10.1038/onc.2017.133. [DOI] [PubMed] [Google Scholar]
- 28.Cheng Y, Jutooru I, Chadalapaka G, Corton JC, Safe S. The long noncoding RNA HOTTIP enhances pancreatic cancer cell proliferation, survival and migration. Oncotarget. 2015;6(13):10840–10852. doi: 10.18632/oncotarget.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jorgez Carolina J., Rosenfeld Jill A., Wilken Nathan R., Vangapandu Hima V., Sahin Aysegul, Pham Dung, Carvalho Claudia M. B., Bandholz Anne, Miller Amanda, Weaver David D., Burton Barbara, Babu Deepti, Bamforth John S., Wilks Timothy, Flynn Daniel P., Roeder Elizabeth, Patel Ankita, Cheung Sau W., Lupski James R., Lamb Dolores J. Genitourinary Defects Associated with Genomic Deletions in 2p15 Encompassing OTX1. PLoS ONE. 2014;9(9):e107028. doi: 10.1371/journal.pone.0107028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu H, Shen L, Chen X, Ding Y, He J, Zhu J, et al. mTOR/P70S6K promotes spermatogonia proliferation and spermatogenesis in Sprague Dawley rats. Reprod BioMed Online. 2016;32(2):207–217. doi: 10.1016/j.rbmo.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Loewer S, Cabili MN, Guttman M, Loh YH, Thomas K, Park IH, et al. Large intergenic non-coding RNA-RoR modulates reprogramming of human induced pluripotent stem cells. Nat Genet. 2010;42(12):1113–1117. doi: 10.1038/ng.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Y, Xu Z, Jiang J, Xu C, Kang J, Xiao L, et al. Endogenous miRNA sponge lincRNA-RoR regulates Oct4, Nanog, and Sox2 in human embryonic stem. Dev Cell. 2013;25(1):69–80. doi: 10.1016/j.devcel.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Zou G, Liu T, Guo L, Huang Y, Feng Y, Huang Q, et al. miR-145 modulates lncRNA-ROR and Sox2 expression to maintain human amniotic epithelial stem cell pluripotency and β islet-like cell differentiation efficiency. Gene. 2016;591(1):48–57. doi: 10.1016/j.gene.2016.06.047. [DOI] [PubMed] [Google Scholar]
- 34.Porter IM, McClelland SE, Khoudoli GA, Hunter CJ, Andersen JS, McAinsh AD, et al. Bod1, a novel kinetochore protein required for chromosome biorientation. J Cell Biol. 2007;179(2):187–197. doi: 10.1083/jcb.200704098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cleveland DW, Mao Y, Sullivan KF. Centromere and kinetochores: from epigenetics to mitotic checkpoint signaling. Cell. 2003;112(4):407–421. doi: 10.1016/S0092-8674(03)00115-6. [DOI] [PubMed] [Google Scholar]
- 36.Hawi Z, Tong J, Dark C, Yates H, Johnson B, Bellgrove MA. The role of cadherin genes in five major psychiatric disorders: a literature update. Am J Med Genet. 2018;177(2):168–180. doi: 10.1002/ajmg.b.32592. [DOI] [PubMed] [Google Scholar]
- 37.Liewen H, Meinhold-Heerlein I, Oliveira V, Schwarzenbacher R, Luo G, Wadle A, et al. Characterization of the human GARP (Golgi associated retrograde protein) complex. Exp Cell Res. 2005;306(1):24–34. doi: 10.1016/j.yexcr.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 38.Zhang L, Wang Q, Wang F, Zhang X, Zhang L, Tang Y, et al. LncRNA LINC01446 promotes glioblastoma progression by modulating miR-489-3p/TPT1 axis. Biochem Biophys Res Commun. 2018;503(3):1484–1490. doi: 10.1016/j.bbrc.2018.07.067. [DOI] [PubMed] [Google Scholar]
- 39.Chen W, Paradkar PN, Li L, Pierce EL, Langer NB, Takahashi-Makise N, et al. Abcb10 physically interacts with mitoferrin-1 (Slc25a37) to enhance its stability and function in the erythroid mitochondria. Proc Natl Acad Sci U S A. 2009;106(38):16263–16268. doi: 10.1073/pnas.0904519106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhan A, Mandal SS. LncRNA HOTAIR: a master regulator of chromatin dynamics and cancer. Biochim Biophys Acta. 2015;1856(1):151–164. doi: 10.1016/j.bbcan.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhan A, Mandal SS. Estradiol-induced transcriptional regulation of long non-coding RNA, HOTAIR. Methods Mol Biol. 2016;1366:395–412. doi: 10.1007/978-1-4939-3127-9_31. [DOI] [PubMed] [Google Scholar]
- 42.Nishida H, Miyagawa S, Vieux-Rochas M, Morini M, Ogino Y, Suzuki K, et al. Positive regulation of steroidogenic acute regulatory protein gene expression through the interaction between Dlx and GATA-4 for testicular steroidogenesis. Endocrinology. 2008;149(5):2090–2097. doi: 10.1210/en.2007-1265. [DOI] [PubMed] [Google Scholar]
- 43.Zeng H, Lu B, Zamponi R, Yang Z, Wetzel K, Loureiro J, et al. mTORC1 signaling suppresses Wnt/β-catenin signaling through DVL-dependent regulation of Wnt receptor FZD level. Proc Natl Acad Sci U S A. 2018;115(44):E10362–E10369. doi: 10.1073/pnas.1808575115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Di Ruscio A, Ebralidze AK, Benoukraf T, Amabile G, Goff LA, Terragni J, et al. DNMT1-interacting RNAs block gene-specific DNA methylation. Nature. 2013;503:371–376. doi: 10.1038/nature12598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aramaki S, Hayashi K, Kurimoto K, Ohta H, Yabuta Y, Iwanari H, et al. A mesodermal factor, T, specifies mouse germ cell fate by directly activating germline determinants. Dev Cell. 2013;27(5):516–529. doi: 10.1016/j.devcel.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 46.Gegenschatz-Schmid Katharina, Verkauskas Gilvydas, Demougin Philippe, Bilius Vytautas, Dasevicius Darius, Stadler Michael B., Hadziselimovic Faruk. DMRTC2, PAX7, BRACHYURY/T and TERT Are Implicated in Male Germ Cell Development Following Curative Hormone Treatment for Cryptorchidism-Induced Infertility. Genes. 2017;8(10):267. doi: 10.3390/genes8100267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hadziselimovic Faruk, Cathomas Gieri, Verkauskas Gilvydas, Dasevicius Darius, Stadler Michael. PRDM Histone Methyltransferase mRNA Levels Increase in Response to Curative Hormone Treatment for Cryptorchidism-Dependent Male Infertility. Genes. 2018;9(8):391. doi: 10.3390/genes9080391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao N, Zhang J, Rao MA, Case TC, Mirosevich J, Wang Y, et al. The role of hepatocyte nuclear factor-3 alpha (Forkhead Box A1) and androgen receptor in transcriptional regulation of prostatic genes. Mol Endocrinol. 2003;17(8):1484–1507. doi: 10.1210/me.2003-0020. [DOI] [PubMed] [Google Scholar]
- 49.Huang KB, Zhang SP, Zhu YJ, Guo CH, Yang M, Liu J, et al. Hotair mediates tumorigenesis through recruiting EZH2 in colorectal cancer. J Cell Biochem. 2019;120(4):6071–6077. doi: 10.1002/jcb.27893. [DOI] [PubMed] [Google Scholar]
- 50.Aiello A, Bacci L, Re A, Ripoli C, Pierconti F, Pinto F, et al. MALAT1 and HOTAIR long non-coding RNAs play opposite role in estrogen-mediated transcriptional regulation in prostate cancer cells. Sci Rep. 2016;6:38414. doi: 10.1038/srep38414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheng Y, Jutooru I, Chadalapaka G, Corton JC, Safe S. The long non-coding RNA HOTTIP enhances pancreatic cancer cell proliferation, survival and migration. Oncotarget. 2015;6(13):10840–10852. doi: 10.18632/oncotarget.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watanabe M, Fukuda A, Nabekura J. The role of GABA in the regulation of GnRH neurons. Front Neurosci. 2014;8:38. doi: 10.3389/fnins.2014.00387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berghoff EG, Clark MF, Chen S, Cajigas I, Leib DE, Kohtz JD. Evf2 (Dlx6as) lncRNA regulates ultraconserved enhancer methylation and the differential transcriptional control of adjacent genes. Development. 2013;140(21):4407–4416. doi: 10.1242/dev.099390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaewman P, Nudmamud-Thanoi S, Thanoi S. GABAergic alterations in the rat testis after methamphetamine exposure. Int J Med Sci. 2018;15:1349–1354. doi: 10.7150/ijms.27609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lo Iacono N, Mantero S, Chiarelli A, Garcia E, Mills AA, Morasso MI, et al. Regulation of Dlx5 and Dlx6 gene expression by p63 is involved in EEC and SHFM congenital limb defects. Development. 2008;135(7):1377–1388. doi: 10.1242/dev.011759. [DOI] [PubMed] [Google Scholar]
- 56.Bhattacharyya S, Bronner-Fraser M. Competence, specification and commitment to an olfactory placode fate. Development. 2008;135(24):4165–4177. doi: 10.1242/dev.026633. [DOI] [PubMed] [Google Scholar]
- 57.Ji Qing, Liu Xuan, Fu Xiaoling, Zhang Long, Sui Hua, Zhou Lihong, Sun Jian, Cai Jianfeng, Qin Jianmin, Ren Jianlin, Li Qi. Resveratrol Inhibits Invasion and Metastasis of Colorectal Cancer Cells via MALAT1 Mediated Wnt/β-Catenin Signal Pathway. PLoS ONE. 2013;8(11):e78700. doi: 10.1371/journal.pone.0078700. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Wang Jun, Zhang Xuan, Chen Wen, Hu Xiang, Li Jing, Liu Changning. Regulatory roles of long noncoding RNAs implicated in cancer hallmarks. International Journal of Cancer. 2019;146(4):906–916. doi: 10.1002/ijc.32277. [DOI] [PubMed] [Google Scholar]
- 59.Pu H, Zheng Q, Li H, Wu M, An J, Gui X, et al. CUDR promotes liver cancer stem cell growth through upregulating TERT and C- Myc. Oncotarget. 2015;6(38):40775–40798. doi: 10.18632/oncotarget.5805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ye Y, Li L, Zheng Z, Chen S, Chen E, Hu Y. Long non-coding RNA linc00261 suppress gastric cancer progression via promoting slug degradation. J Cell Mol Med. 2017;21(5):955–967. doi: 10.1111/jcmm.13035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ko S, Ahn J, Song CS, Kim S, Knapczyk-Stwora K, Chatterjee B. Lysine methylation and functional modulation of androgen receptor by Set9 methyltransferase. Mol Endocrinol. 2011;25(3):433–444. doi: 10.1210/me.2010-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data files were deposited at the Database of Genotypes and Phenotypes (dbGaP) under accession number phs001275.v1.p1.