Abstract
In the era of combined antiretroviral therapy (cART), HIV-1 infection has transformed from a death sentence to a manageable, chronic disease. Although the life expectancy of HIV+ individuals is comparable to that of the uninfected subjects paradoxically, there is increased prevalence of age-associated comorbidities such as atherosclerosis, diabetes, osteoporosis & neurological deficits in the context of HIV infection. Drug abuse is a common comorbidity of HIV infection and is often associated with increased neurological complications. Chronic neuroinflammation (abnormal microglial and astrocyte activation) and neuronal synaptodendritic injury are the features of CNS pathology observed in HIV (+) individuals that are taking cART & that abuse drugs. Neuroinflammation is the driving force underlying premature aging associated with HIV (+) infection, cART and drugs of abuse. Autophagy is a highly conserved process critical for maintaining cellular homeostasis. Dysregulated autophagy has been shown to be linked with abnormal immune responses & aging. Recent emerging evidence implicates the role of HIV/HIV proteins, cART, & abused drugs in disrupting the autophagy process in brain cells such as microglia, astrocytes, and neurons. It can thus be envisioned that co-exposure of CNS cells to HIV proteins, cART and/or abused drugs could have synergistic effects on the autophagy process, thereby leading to exaggerated microglial/astrocyte activation, ultimately, promoting the aging process. Restoration of autophagic function could thus provide an alternative therapeutic strategy for mitigating neuroinflammation & ameliorating the premature aging process. The current review aims to unravel the role of dysregulated autophagy in the context of single or co-exposure of microglia, astrocytes, and neurons to HIV/HIV proteins, drugs of abuse &/or cART and will also discuss the pathways involved in dysregulated autophagy-mediated neuroinflammation.
1. Introduction
In the era of combined antiretroviral therapy (cART), HIV(+) individuals continue to enjoy longer lifespans with life-expectancy comparable to that of the HIV negative population [1–3]. The quality of life of those infected with HIV-1, however, is compromised, owing to the prevalence of range of mild cognitive deficits and memory loss, commonly referred to as HIV-associated neurological disorders (HAND), that afflicts about 30–60% of infected individuals [1–3]. In addition to HAND, comorbidities such as atherosclerosis, diabetes, osteoporosis, and cardiovascular disease are also highly prevalent in those infected with HIV [4, 5]. Most of these end-organ comorbidities are often observed as a consequence of the aging process. It can thus be speculated that HIV infection in the face of cART leads to an accelerated aging phenotype or pre-mature aging compared to uninfected individuals. Adding further complexity to this is also the comorbidity of drug abuse in HIV-infected individuals [6, 7]. Abused drugs including cocaine, opiates, and methamphetamine have been shown to promote premature aging [8, 9]. It is thus likely that drugs of abuse in combination with HIV/HIV proteins and cART can further potentiate the aging process. Another possible factor contributing to the aging process is the long-term use of cART. Antiretrovirals (ARVs) have been suggested to confer toxicity in various types of cells including the neurons, microglia, and endothelial cells [10–12]. Additionally, previous studies have reported increased inflammation in the brains of HIV (+) individuals on cART [11, 13–22]. The summary of the neuroinflammation status in these previous studies is shown in Table 1. It is also recognized that drugs of abuse can result in lack of adherence of ARVs, thereby decreasing the efficiency of ARVs in HIV (+) individuals. It can thus be surmised that the interactions among HIV/HIV proteins, drugs of abuse & cART can form a vicious tripartite loop contributing to the accelerated aging process.
Table 1:
Summary of neuroinflammation status in vivo
| Authors | Species | Neuroinflamation | cART | Method |
|---|---|---|---|---|
| Alakkas et al. (2019) | Human | Yes | Yes | MRI |
| Chaganti et al. (2019) | Human | Yes | Yes | MRI |
| Coughlin et al. (2014) | Human | No | Yes | PET |
| Garvey et al. (2014) | Human | Yes | Yes | PET |
| Hammoud et al. (2005) | Human | No | Yes | PET |
| Meulendyke et al. (2014) | Monkey | Yes | Yes | ELISA |
| Rubin et al. (2018) | Human | Yes | Yes | PET |
| Tavazzi et al. (2014) | Human | Yes | No | Immunohistology |
| Vera et al. (2016) | Human | Yes | Yes | PET |
| Wiley et al. (2006) | Human | No | Yes | PET |
| Zulu et al. (2018) | Mice | Yes | Yes | RT-PCR |
The underlying mechanism(s) responsible for premature aging in chronic HIV (+) subjects that abuse drugs remains unclear. The sustained low level of inflammation both in the periphery and in the central nervous system (CNS) has been considered as the “driving force” that promotes the aging process. Previous studies have shown a correlation between neuroinflammation and the aging process, with the presence of increased neuroinflammation owing to enhanced microglial activation in the brains of aging people. [23]. Changes in microglial numbers and morphology (decreased process length and increased cell body) have been found in the brains of HIV (+) individuals [24]. Many in vitro studies have focused on dissecting the detailed molecular abuse and cART on CNS cells. Mechanism(s) involved in this process include, but are not limited to, dysregulated toll-like receptor signaling [25], upregulated endoplasmic reticulum (ER) stress/reactive oxygen species (ROS) axis [26, 27], & activation of the NLRPs inflammasome [28]. Recent findings from us [25–27] and others [29–31] have implicated the role of dysregulated autophagy as a central player in promoting neuroinflammation in the context of HIV proteins, cART, and drugs of abuse, which is the focus of this review.
Autophagy is one of highly conserved cellular processes in almost all of the eukaryotic cells [32, 33]. In mammalian cells, there are three primary types of autophagy: microautophagy, macroautophagy, and chaperone-mediated autophagy (CMA) [32, 33]. Microautophagy is a process wherein the lysosomes, via the protrusion and invagination of their membranes, directly capture the cargos for degradation[34]. CMA uses chaperones to identify cargo proteins that contain a particular pentapeptide motif. These targeting proteins are then unfolded and translocated directly across the lysosomal membrane for degradation [35]. Of note, this process does not use membranous structures to sequester cargo. In contrast to microautophagy and CMA, macroautophagy (therefore referred as autophagy and the focus of this review), is involved plays a role in the de novo synthesis of double-membrane vesicles (autophagosomes) for sequestration of cargos, and subsequent transport to the lysosomes for degradation.
Autophagy is a highly regulated process involving multiple autophagy-related proteins (ATG) and molecular mechanism(s) such as transcriptional modulation, post-translational modifications (phosphorylation & ubiquitination), as well as protein-protein interactions, to form protein complexes [36]. Briefly, “preinitiation” complex (also termed as the ULK complex) is regulated by upstream kinases resulting in phosphorylation of ULK1/2 proteins. Subsequently, the preinitiation complex activates the “initiation complex” (also called the Class III PI3K complex including VPS34, VPS15, ATG14L and beclin1) through ULK-dependent phosphorylation and via disruption of the binding of Bcl-2 to beclin1. In this step, upregulation of beclin1 is considered as a marker of autophagy initiation. The Class III phosphoinositide 3-kinase (PI3K) complex subsequently generates Phosphatidylinositol-3-phosphate (PI3P) at the site of nucleation of the isolated membranes, leading, in turn, to the binding of PI3P-binding proteins (such as WIPI/II) and other recruited proteins which are involved in the “elongation reaction” step to form the autophagosomes. The phosphatidylethanolamine-conjugated form of the formation. Other proteins including the E1 ligase ATG7, the E2 ligase ATG3, and the E3 ligase complex ATG12/ATG5/ATG16L also contribute to the formation of autophagosomes and stably associate with the mature autophagosomes. The final step of autophagy involves fusion of the autophagosomes with the lysosomes to form autolysosomes. The sequestered contents are degraded in the autolysosomes and released into the cytoplasm for recycling. This step is also called the maturation step (autophagy flux) with the change in expression of p62 considered as a marker of autophagy flux.
The autophagy machinery functions by delivering cytosolic materials to the lysosomes for degradation. Removal of damaged organelles such as the mitochondria (Mito) is termed as “mitophagy”, with the role of proteins, Pink1 and Parkin critical for mitophagy induction. Pink1 is constitutively imported into the functional Mito and is degraded by presenilin-associated rhomboid-like protein (PARL). Transmembrane potential of the inner mitochondrial membrane is critical for this process. Mito damage (loss of this potential) causes Pink1 accumulation on the cytosolic face of the outer mitochondrial membrane, leading, in turn, to recruitment and activation of Parkin (an ubiquitin E3 ligase), that is known to ubiquitinate mitochondrial proteins including p62, NBR1, and optineurin. The ubiquitin-binding proteins bind to LC3 to initiate the formation of autophagosomes which contain the damaged Mito. The expression levels of Pink1, Parkin1, and optineurin can regulate induction of mitophagy. The schematic of autophagy and mitophagy processes is shown in Fig. 1.
Figure 1:
The schematic of autophagy and mitophagy
Autophagy is a homeostatic process critical for the elimination of misfolded cytosolic proteins and damaged organelles in the cells. Basal/constitutive levels of autophagy aid in regulating quality control of proteins while protecting cells from damaged protein aggregation. Under cellular stress such as nutrient depletion, abnormal temperature or oxidative stress, autophagy is induced to maintain cell survival [32, 33]. Autophagy and innate immune responses are mutually regulated functionally [33]. Downregulation or upregulation of the autophagy flux can either increase or decrease the immune responses, respectively. Pro-inflammatory factors such as LPS has the ability to stimulate autophagy [37]. Interestingly, autophagy process is downregulated during the aging process, further supporting the critical role of dysregulated autophagy/inflammation during aging [38–40].
Recently, emerging evidence indicates that HIV/HIV proteins, abused drugs, and cART each can dysregulate autophagy which, in turn, plays a key role in neuroinflammation (microglial/astrocyte activation). Based on the linkage between inflammation and aging process, it is possible that upregulation of neuroinflammation due to autophagy/mitophagy dysregulation plays a critical role in pre-mature aging during chronic HIV infection in the context of abused drugs.
2. Impaired autophagy during the normal aging process.
Normal aging process is defined as a chronic deterioration of various physiological systems (cognitive, endocrine, and motor) with advancing age. Decline of these essential body functions leads to increased vulnerability to external stimuli/insults, ultimately resulting in higher risk for succumbing to various diseases and eventually leading to death. Aging is characterized by an entangled dysregulation of several biological processes including decreased mitochondrial functioning, increased oxidative stress, altered energy metabolism & accumulation of misfolded proteins. Many diseases such as diabetes, cancer, osteoporosis, neurodegenerative disorders, and cardiovascular diseases are associated with aging [41, 42]. Dysregulation of autophagy process in the brain and peripheral compartments has been demonstrated during the aging process [38–40]. In the aging brain, there is downregulation of three autophagy proteins - beclin1, ATG5, & ATG7, compared to the brains of younger individuals [43, 44]. It has also been reported that the expression of both LC3 and HDAC6 (histone deacetylase 6), which modulate the chromatin structure to affect transcriptional activity, was downregulated in the hippocampus during aging [45, 46]. Levels of PINK1 were found to be downregulated in older women [47]. Interestingly, expression of Bcl-2, an inhibitor of beclin1 was upregulated in older man suggesting a decrease in autophagic activity [48]. In cardiovascular system, cardiac aging was found to be associated with hypertrophy, fibrosis, inflammation, and decreased contractility [49, 50]. Interestingly, cardiac autophagy was found to decrease with age with a concomitant accumulation of misfolded proteins and dysfunctional Mito in the aging heart [49, 50]. Lung aging was associated with progressive functional impairment, reduced capacity to respond to environmental stresses, and an injury that was characterized by enlarged alveolar spaces and loss of lung elasticity in the elderly [51]. Mechanistically, increased levels of oxidative stress are responsible for accelerated ageing through multiple mechanisms including stem cells depletion, impaired autophagy and reduced antioxidant responses [51]. Another important organ, the kidney demonstrated higher basal levels of autophagy in older for upregulation of autophagic flux in response to metabolic stress was significantly reduced in older mice and, this was associated with age-related kidney diseases [52]. In summary, it is well-accepted that autophagy/mitophagy dysfunction is associated with aging in many organ systems. There is overwhelming evidence demonstrating that upregulation of autophagy either through pharmacological or genetic approaches, can extend the life-expectancy in various model systems including yeast, nematodes, fruit flies, and even mice [32, 53]. Mice injected with rapamycin (autophagy inducer) or spermidine (an inhibitor of histone acetyltransrase p300 that regulates transcription of genes via chromatin remodeling) demonstrated induction of autophagy and increased life expectancy [54–57]. Along these lines, mice overexpressing ATG5 demonstrated extended lifespans involving activation of autophagy [58]. Similarly, disruption of the beclin1-Bcl2 complex inhibited age-induced apoptotic cell death, cardiac hypertrophy and fibrosis, thereby leading to delayed cardiac aging [59]. Mitophagy has also been shown to delay the aging process. For example, Pink1 knockout mice demonstrated age-dependent impairment of mitochondrial respiration and increased numbers of larger mitochondria in the cortex [60]. Similarly Parkin deficient mice were found to accumulate abnormal Mito in the heart as they aged [61]. Reciprocally, Parkin transgenic mice showed increased levels of mitophagy and were resistant to cardiac aging with amelioration of decline in cardiac functional and decreased cellular senescence and inflammation [39]. In summary, during the normal aging process there is downregulation of autophagy/mitophagy that is accompanied with increased inflammation in multiple systems/organs throughout the body. Restoration of autophagy levels could thus delay the aging process. In chronic HIV+ individuals that abuse illicit drugs, there is accelerated aging that is accompanied with sustained neuroinflammation, compared with HIV negative individuals. Herein we discuss the roles of HIV/HIV proteins, abused drugs, and cART on autophagy dysregulation and propose that autophagy dysregulation underlies the potential linkage of neuroinflammation with accelerating aging.
3. The effects of HIV/HIV proteins on autophagy process.
It has been well recognized that HIV/HIV proteins disrupt the autophagy process in various cell types including macrophages, T cells, microglia, & astrocytes in vitro [62, 63]. In the brains of individuals with HIV-1 associated encephalitis, there is significant upregulation of the expression of beclin1, Atg5, Atg7, and LC3II compared with autophagy process [64]. It has been suggested that HIV hijacks the ATGs including ATG7, ATG8, and ATG12 to maximize its replication and at the same time inhibits degradative ability of the lysosomes as a mechanism to avoid the degradation of newly synthesized virions [65]. Among the complex interactions between HIV-1 replication and autophagy process, several HIV proteins including the envelope glycoprotein (Env), transactivator of transcription (TAT), & negative regulatory factor (Nef), have been shown to exert their modulatory effects through distinct mechanism(s) as described below.
3. 1. Env dysregulates autophagy in various types of brain cells:
HIV-1 Env gene encodes an envelope glycoprotein precursor (gp) which is further cleaved into two subunit proteins: gp120 and gp41 transmembrane glycoprotein [66]. Gp120 is responsible for facilitating virus entry into the host cells through its binding to the CD4 receptor and a co-receptor, C-C chemokine receptor type 5(CCR5) or C-X-C chemokine receptor type 4 (CXCR4). It has been shown that gp120 induced beclin1 expression in uninfected CD4+ T lymphocytes via CXCR4 binding [67] and gp41, induced autophagy in uninfected CD4+ T lymphocytes [68]. Envs derived from both X4 and R5 HIV induced autophagy induction in uninfected CD4+ T cells [69]. In the neuroblastoma SK-NSH cell line, overexpression of either CXCR4- or CCR5- type gp120 increased the levels of autophagy markers including beclin1, Atg5, Atg7, and LC3II suggesting thereby that HIV-1 gp120 could induce autophagy in neuronal cells, thus implicating its role in HIV-associated complications of the CNS [64]. In microglial BV2 cells, gp120 was capable of inducing autophagy and this was associated with microglial activation [70]. Similarly it was shown that gp120 could also regulate autophagy in SVG astrocytes leading to increased expression of LC3II [71]. Interestingly, till date no reports have revealed the effects of gp120 on dysregulation of autophagy in oligodendrocytes, another important glial cell type of the brain.
3. 2. TAT dysregulates autophagy in various types of cells.
HIV TAT plays a key role in HIV-1 replication by upregulating the transcription of the 5’ long terminal repeat-containing promoter [72]. The effects of TAT on autophagy have been well-studied in vitro in various cell types. In monocytes/macrophages, TAT has been shown to inhibit autophagy process either through Src-AKT & STAT3 or IFN-γ/STAT1 signaling [73, 74]. TAT has also been shown to induce neurotoxicity &/or neuronal death in neuroblastoma SH-SY5Y cells, with the involvement of increased autophagosome formation in this process [75]. Other studies have reported that TAT resulting in block in fusion of lysosomes with the autophagosomes, leading resulting in neuronal injury in primary hippocampal neurons [76, 77]. More recently, Spector lab has demonstrated that TAT can induce mitochondrial (Mito) fragmentation and incomplete mitophagy in human primary neurons [78]. Findings from our group have also demonstrated that TAT exerts similar deleterious effects on Mito also in BV2 cells as well as in mouse primary microglia [79]. We have shown that exposure of HIV TAT to mouse primary microglia resulted in altered mitochondrial membrane potential with increased expression of mitophagy markers including Pink1, Parkin, and dynamin 1-like as well as autophagy markers beclin1and LC3II. TAT significantly upregulated the expression levels of p62 implying thereby the blockade of mitophagy flux (the fusion of mitophagosome with the lysosome). Functional studies revealed that TAT downregulated the rate of extracellular acidification and mitochondrial oxygen consumption. Our findings demonstrated the role of defective mitophagy in TAT-mediated microglial activation [79]. TAT has similar effects on autophagy dysregulation in astrocytes. In human primary glioblastoma cells (U87), HIV TAT upregulated the expression of BAG3 in an NF-κB-dependent manner to induce autophagy and, suppression of BAG3 or inhibition of NF-κB activity blocked TAT- mediated induction of autophagy Studies assessing the effects of TAT on autophagy dysregulation in oligodendrocytes are scant and warrant further investigation.
3. 3. Nef dysregulates autophagy process in various types of brain cells.
The accessory protein Nef plays fundamental roles in modulating host membrane trafficking machinery and major histocompatibility complex I (MHC-I) receptor downregulation during virus replication [81]. Nef has been shown to decrease the expression levels of transmembrane factor SERINC5 (serine incorporator 5) - an inhibitor of HIV replication, by directing SERING5 to the Rab7-positive endosomal compartments, ultimately leading to increased HIV replication [82]. In macrophages, Nef acts as an anti-autophagic maturation factor through its interactions with the autophagy regulatory factor beclin1, to protect HIV-1 from degradation [83]. Alternatively, Nef has the ability to sequester transcription factor EB (TFEB), a master regulator of lysosomal functioning, to inhibit autophagy maturation, in macrophage [84]. Similarly, Nef-mediated blocking of the autophagosome-lysosome fusion were also observed in human primary astrocytes [85].
4. The effects of abused drugs on autophagy process
HIV infection is often accompanied by the comorbidity of drug abuse including drugs such as cocaine, methamphetamine (Meth), & morphine. In fact, HIV (+) individuals with a history of abused drugs show worse neurological complications compared with infected individuals not abusing drugs. For example, cocaine abusers exhibit accelerated aging process as evidenced by the annual rate of global gray matter volume loss in cocaine-dependent individuals at twice the rate of healthy volunteers [86]. Herein we summarize the recent progress on the effects of these abused drugs on the autophagy process.
4. 1. The effect of cocaine on autophagy process in various types of brain cells.
Cocaine has been well-recognized for its stimulating effects on neurons involving increase of multiple endogenous transmitters such as dopamine and glutamate in the brain [87]. Emerging evidence demonstrates that cocaine can also induce neuroinflammation by activating microglia and astrocytes, with the involvement of dysregulated autophagy. Findings from our group for the first time demonstrated cocaine-mediated dysregulated autophagy in both microglia and astrocytes [26, 27]. We demonstrated that cocaine exposure induced autophagy in both BV2 microglial cells and primary rat microglia with time- and dose- dependent upregulation of beclin1, ATG5, & LC3II. LC3II puncta was increased in cocaine exposed microglial cells. Furthermore, cocaine-induced autophagy was inhibited in cells pre-treated with the autophagy inhibitors (3-MA and wortmannin). We also showed that cocaine-mediated induction of autophagy involved upstream activation of two ER stress pathways. Increased autophagy as evidenced by enhanced expression of beclin1, ATG5, & LC3II was also observed in the brains of mice administered cocaine compared with the untreated animals. Functionally, dysregulated autophagy contributed to cocaine-mediated activation of microglia. In summary, our findings suggested that cocaine-mediated dysregulation of autophagy played a role in neuroinflammation. In a separate study, we showed that cocaine also mediated induction of autophagy in human astrocyte cell line A157 as well as human primary astrocytes, leading ultimately to astrocyte activation [27]. Our findings were also validated by others wherein it was shown that cocaine dysregulated autophagy also in SVGA astrocytes involving multiple molecules including the sigma 1 receptor, PI3K, mTOR, Atg5/7, and beclin1 [29]. In addition to glia, cocaine also dysregulated autophagy in mouse primary hippocampal neurons leading to neurotoxicity via the nitric oxide-GAPDH signaling cascade [88]. In addition, cocaine was capable of disrupting autophagy also in both primary human brain vascular pericytes as well as mouse primary pericytes and, this dysregulation was associated with cocaine-mediated pericyte immune responses which contributed to neuroinflammation [89]. In our recent study, we also demonstrated that cocaine was able to upregulate the expression of Pink1, Parkin, and optineurin (OPTN) with a concomitant decrease in the rate of mitochondrial oxygen consumption and impaired mitochondrial function in BV2 cells and primary mouse microglial cells, leading to defective mitophagy [90]. Taken together, cocaine can dysregulate both the autophagy/mitophagy processes in various types of brain cells.
4. 2. The effects of Meth on autophagy process in various types of brain cells.
Meth is another drug that is commonly abused by chronic HIV (+) individuals. Similar to cocaine, Meth has the ability to interact with the autophagy process resulting in organ/tissue dysfunction [91]. Several investigations have demonstrated an association between Meth-mediated autophagy and neurotoxicity in vitro in PC12 and SH-SY5Y neuroblastoma cells. Mechanistically, C/EBPβ-mediated signaling [92], mTOR [93], CHOP-Trib3-mediated ER stress [94], DNA damage-inducible transcript 4 [95] and kappa opioid receptor [96] have been implicated in autophagy dysregulation in neuronal cells exposed to Meth in vitro. In addition to neurons, Meth also has the ability to exert similar effects on autophagy in other types of cells. In astrocytes, Meth induced autophagy via the opioid receptor and metabotropic glutamate receptor type 5 receptor-mediated signaling. Furthermore, autophagy inhibition was shown to exacerbate Meth-induced cytotoxicity [71]. In endothelial cells, Meth induced autophagy as a pro-survival response against apoptotic cell death involving the kappa opioid receptor [96]. Till date, there is no direct evidence demonstrating Meth-mediated dysregulation of autophagy process in either the microglia or in oligodendrocytes. In summary, Meth dysregulates autophagy in multiple types of cells through different mechanisms, with the effects on cell viability being cellular-context dependent.
4. 3. The effects of opiate drugs on autophagy process in various types of brain cells.
Opiate-like drugs have been widely used in the clinical setting owing to their analgesic effects. In HIV (+) individuals, abuse of heroin (morphine is its main in vivo metabolite) can accelerate the pathogenesis of HAND and also reduce the efficacy of cART [97, 98]. Numerous studies have demonstrated that similar to psychostimulants such as cocaine and Meth, morphine can also dysregulate autophagy process both in vitro and in vivo. Morphine exposure has been shown to increase intracellular pH coinciding with a reduction in the formation of acidic vesicular organelles and autophagolysosome formation in microglia; detailed mechanisms underlying this phenomenon however, remain elusive [30, 99]. In astrocytes, morphine dysregulates the ER stress/autophagy axis resulting in astrocytosis by increasing the levels of beclin1, LC3II, & p62 [98, 100]. Morphine-mediated dysregulated autophagy process has also been described in neurons. We have shown that exposure of mouse primary hippocampal neurons to morphine resulted in an imbalance between excitatory and inhibitory synapses and these effects were mediated by up-regulation of intracellular ROS/ER stress and autophagy signaling [101]. In a separate study, morphine was shown to result in memory loss by disrupting the autophagy process and, restoration of autophagy ameliorated memory impairment in vivo [102]. Studies by Yao et al., demonstrated that in cultured primary midbrain neurons, morphine exposure reduced the total dendritic length and complexity of neurons, which could be reversed by knockdown of Atg5 or Atg7 [103]. Furthermore, mice deficient in Atg5 or Atg7 specifically in the dopaminergic neurons were less sensitive to developing a morphine reward response, behavioral sensitization, analgesic tolerance and physical dependence compared to wild-type mice [103]. Interestingly, morphine has also been suggested to disrupt Pink/Parkin1-mediated mitophagy process in spinal cord neurons implying the role of mitophagy in antinociceptive tolerance [104]. Till date, the effects of morphine on autophagy in oligodendrocytes remain unexplored although there are reports suggesting that morphine can alter the morphology of oligodendrocytes while also increasing apoptosis [105].
5. The effects of cART on autophagy process in various types of brain cells
Recent, emerging evidence has shown that antiretrovirals (ARV) can interrupt multiple cellular processes including autophagy in vitro leading to cellular dysfunction which, in turn, has been linked to accelerate aging in HIV (+) individuals on cART. In microglia, efavirenz and other drugs including darunavir, atazanavir and nevirapine inhibited the activity of arginase (ARG), resulting in activation of microglia in vitro; dysregulation of autophagy however, was not the focus of this study [106]. In neurons, tenofovir, efavirenz, & emtricitabine induced dendritic simplification and neurotoxicity through mitochondrial dysfunction [107, 108]. Nucleoside analogs including azidothymidine, stavudine, and lamivudine induced autophagy in primary cortical neurons in a p53-independent manner [109]. The studies on ARV-mediated autophagy dysregulation are new in the field and further investigations on mechanisms underlying ARV-mediated autophagy in other cell types such as microglia and oligodendrocytes are warranted.
6. Combined effects of HIV/HIV proteins, abused drugs, & cART on the autophagy process
As discussed above, single exposure of brain cells to either HIV/HIV proteins, abused drugs or cART can interrupt the autophagy process leading to cellular dysfunction. In real life scenario however, all these factors coexist in chronically infected HIV (+) individuals that are addicted to abused-drugs and hence can interact with leading to synergistic effects on cellular dysfunction. In fact, co-exposure of HIV proteins and abused drugs have additive/synergistic effects on multiple cellular processes including autophagy. For example, cocaine and HIV-TAT synergistically decreased both Mito membrane potential and ATP production thereby implying Mito damage in human and rat primary hippocampal neurons [110]. Co-exposure of morphine and TAT induced exacerbated dysfunction of autophagy in pulmonary endothelial cells has been shown to be associated with HIV-related pulmonary arterial hypertension [111]. The effects of TAT and morphine co-exposure on autophagy process in primary neurons is rather more complex exhibiting a biphasic modulation such that while morphine and TAT individually can significantly inhibit the autophagic flux and reduce dendrite length, co-exposure of TAT and morphine results in lower autophagic activity at an earlier time point after co-exposure and in contrast, higher levels of autophagy flux at later times [112]. The mechanisms responsible for this however, remains unknown. The combined effects of Meth and TAT on autophagy process have also been investigated in neurons. While both TAT and Meth individually can induce the autophagosome formation, there was even more increase in autophagosome formation in SH-SY5Y cells exposed to both TAT and Meth [75]. These findings were further validated in primary midbrain neurons exposed to both TAT and Meth [113]. Meth increased the expression levels of LC3II and beclin1 and, these effects were significantly enhanced by TAT. Meth was also shown to potentiate gp120-mediated autophagy in astrocytes [71]. Till date most investigations have focused on the combined effects of abused drugs and TAT on autophagy process with fewer studies on the combined effects of abused drugs and gp120. There is also currently no study on the combined effects of abused drugs and Nef on autophagy. Very few studies are extant on assessing the combined effects of HIV proteins, abused drugs, & ARVs on autophagy in vitro. In one study neuron-glia mixed cultures were exposed to ARVs, Meth, & gp120 singly or in combination to assess the neuronal injury, ATP levels, & autophagy process in vitro [114]. These findings suggested that the overall protective effect of cART on HIV infection was accompanied by detectable neurotoxicity, which in turn, could be aggravated by Meth.
7. Autophagy-mediated regulation of neuroinflammation in the context of HIV/HIV proteins, abused drugs, & cART.
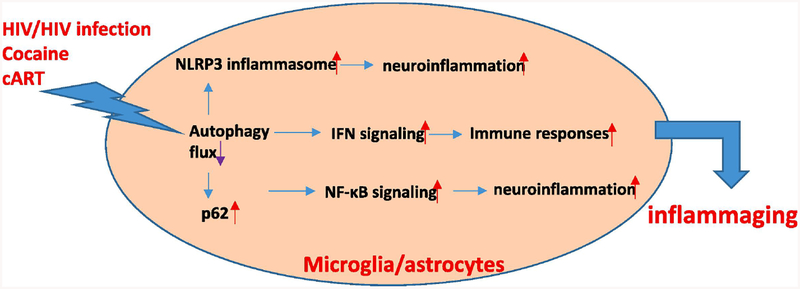
Although the detailed molecular mechanism(s) underlying dysregulated autophagy-mediated neuroinflammation remain unclear in the context of HIV/HIV proteins, abused drugs, & cART, several pathways have been implicated in this regulation. For example, interferon (IFN)-mediated signaling is induced by HIV infection and this response acts as a host defense mechanism to eliminate the virus [115]. On the other hand, sustained IFN signaling leads to abnormal immune responses and elevated neuroinflammation promoting the pathogenesis of neuroHIV [115]. Autophagy negatively regulates IFN production through two mechanisms: (1) inhibiting retinoic-acid inducible gene I (RIG-I)-like receptor-mediated induction of type I IFN production through mitophagy [116]; (2) reducing the activation of STING, a transmembrane protein that promotes type I IFN activation and pro-inflammatory cytokine production in vitro [117]. Interestingly, cocaine is also capable of activating IFN signaling in astrocytes in vitro [118]. Sustained elevation of IFN-γ has been found in HIV (+) individuals after initiation of cART therapy [119]. Elevation of IFN levels has been thought to be a phenotype of late senescence and has contributed to the maintenance of the senescence-associated secretory phenotype (aging) [120]. it is thus likely that in the context of HIV/HIV proteins, abused, drugs, & cART, elevated IFN signaling due to autophagy impairment, could lead to the exaggerated pre-mature aging process in vivo. In addition to abnormal IFN signaling, autophagy protein p62 has also been demonstrated to increase the activity of the pro-inflammatory transcription factor NF-κB, resulting in enhanced levels of IL6, TNFα, and IL1β [121]. Consistently, the levels of p62 were elevated in microglial cells or macrophage exposed to either HIV, morphine/cocaine exposure [90, 100, 122]. Another underlying mechanism of autophagy-mediated regulation of neuroinflammation could involve a functional interaction between the autophagy process and the inflammasome protein complex, which in turn, could lead to the increased activation of inflammasome. The inflammasome protein complex contains NOD-like receptor cryopyrin proteins, the adaptor protein ASC and caspase 1 functioning to promote the maturation of IL1β and IL18, the major determinants for microglial activation [123]. Previous studies demonstrated that beclin1-driven autophagy inhibited microglial inflammatory response by increasing the degradation of the NLRP3 inflammasome. NLRP3 has been shown to co-localizewith LC3-positive vesicles indicating thereby that NLRP3 in the autophagosomes could be slated for degradation [124]. HIV TAT, in addition dysregulating the autophagy signal 1 and 2 as reported in our previous study [28]. Taken together, autophagy dysregulation in the context of HIV/HIV proteins, abused drugs, and cART, coupled with abnormal IFN signaling, p62-mediated NF-κB activation, and increased NLRP3 inflammasome activity co-operate to contribute to chronic neuroinflammation, which in turn, promotes the premature aging process (Fig. 2).
Figure 2:
7. Autophagy-mediated regulation of neuroinflammation in the context of HIV/HIV proteins, abused drugs, & cART.
8. Conclusions
Autophagy plays essential role in modulating inflammation/neuroinflammation in vivo. Emerging evidence shows that autophagy dysregulation is critical for sustained inflammation in the brain, resulting in accelerated aging process observed in chronic HIV (+) individuals on cART that abuse drugs. Most investigations have focused on the effects of single exposure of HIV proteins, abused drugs, or ARVs on the autophagy process in various types of brain cells with less emphasis on the combined effects of these three factors. In summary, autophagy dysregulation plays critical role in neuroinflammation and pre-mature aging in the context of HIV, abused drugs, & cART. Restoration of autophagy could thus provide an alternative therapeutic approach to alleviate the symptoms of neuroHIV and improve the quality of life of chronic HIV (+) individuals abusing drugs.
Chronic HIV (+) individuals with abused drugs have accelerated aging process compared to HIV negative population.
The accelerating aging process is linked with chronic inflammation due to the dysregulated autophagy process.
HIV/HIV proteins, abused drugs, and cART could dysregulate autophagy in multiple types of brains cells, individually.
Co-exposure of HIV/HIV proteins, abused drugs, and cART could lead to combined/synergistic effects on neuroinflammation in vivo.
Acknowledgement:
This work was supported by the NIH NIDA grants: DA044586, DA043138, R01DA050545, and DA047156.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing financial interests:
The authors declare no competing financial interests.
Reference
- 1.Paul R, Neurocognitive Phenotyping of HIV in the Era of Antiretroviral Therapy. Curr HIV/AIDS Rep, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLaurin KA, Booze RM, and Mactutus CF, Diagnostic and prognostic biomarkers for HAND. J Neurovirol, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alford K and Vera JH, Cognitive Impairment in people living with HIV in the ART era: A Review. Br Med Bull, 2018. 127(1): p. 55–68. [DOI] [PubMed] [Google Scholar]
- 4.Tedaldi EM, Minniti NL, and Fischer T, HIV-associated neurocognitive disorders: the relationship of HIV infection with physical and social comorbidities. Biomed Res Int, 2015. 2015: p. 641913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerkhof PLM and Khamaganova I, Sex-Specific Cardiovascular Comorbidities with Associations in Dermatologic and Rheumatic Disorders. Adv Exp Med Biol, 2018. 1065: p. 489–509. [DOI] [PubMed] [Google Scholar]
- 6.Cai Y, et al. , Multiple Faceted Roles of Cocaine in Potentiation of HAND. Curr HIV Res, 2016. 14(5): p. 412–416. [DOI] [PubMed] [Google Scholar]
- 7.Corsi KF, et al. , Interventions to Reduce Drug Use Among Methamphetamine Users at Risk for HIV. Curr HIV/AIDS Rep, 2019. 16(1): p. 29–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sen N, Epigenetic regulation of memory by acetylation and methylation of chromatin: implications in neurological disorders, aging, and addiction. Neuromolecular Med, 2015. 17(2): p. 97–110. [DOI] [PubMed] [Google Scholar]
- 9.Searby A, Maude P, and McGrath I, Growing Old With Ice: A Review of the Potential Consequences of Methamphetamine Abuse in Australian Older Adults. J Addict Nurs, 2015. 26(2): p. 93–8. [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Perez E, et al. , Behavioral and electrophysiological abnormalities in two rat models of antiretroviral drug-induced neuropathy. Pain, 2015. 156(9): p. 1729–36. [DOI] [PubMed] [Google Scholar]
- 11.Garvey LJ, et al. , Increased microglia activation in neurologically asymptomatic HIV-infected patients receiving effective ART. AIDS, 2014. 28(1): p. 67–72. [DOI] [PubMed] [Google Scholar]
- 12.Chen YF, et al. , Coenzyme Q10 Alleviates Chronic Nucleoside Reverse Transcriptase Inhibitor-Induced Premature Endothelial Senescence. Cardiovasc Toxicol, 2019. [DOI] [PubMed] [Google Scholar]
- 13.Alakkas A, et al. , White matter damage, neuroinflammation, and neuronal integrity in HAND. J Neurovirol, 2019. 25(1): p. 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaganti J, et al. , Imaging correlates of the Blood Brain Barrier disruption in HIV associated neurocognitive disorder and therapeutic implications. AIDS, 2019. [DOI] [PubMed] [Google Scholar]
- 15.Coughlin JM, et al. , Regional brain distribution of translocator protein using [(11)C]DPA-713 PET in individuals infected with HIV. J Neurovirol, 2014. 20(3): p. 219–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hammoud DA, et al. , Imaging glial cell activation with [11C]-R-PK11195 in patients with AIDS. J Neurovirol, 2005. 11(4): p. 346–55. [DOI] [PubMed] [Google Scholar]
- 17.Meulendyke KA, et al. , Combination fluconazole/paroxetine treatment is neuroprotective despite ongoing neuroinflammation and viral replication in an SIV model of HIV neurological disease. J Neurovirol, 2014. 20(6): p. 591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rubin LH, et al. , Microglial activation is inversely associated with cognition in individuals living with HIV on effective antiretroviral therapy. AIDS, 2018. 32(12): p. 1661–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tavazzi E, et al. , Brain inflammation is a common feature of HIV-infected patients without HIV encephalitis or productive brain infection. Curr HIV Res, 2014. 12(2): p. 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vera JH, et al. , Neuroinflammation in treated HIV-positive individuals: A TSPO PET study. Neurology, 2016. 86(15): p. 1425–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wiley CA, et al. , Positron emission tomography imaging of peripheral benzodiazepine receptor binding in human immunodeficiency virus-infected subjects with and without cognitive impairment. J Neurovirol, 2006. 12(4): p. 262–71. [DOI] [PubMed] [Google Scholar]
- 22.Zulu SS, et al. , Effect of long-term administration of antiretroviral drugs (Tenofovir and Nevirapine) on neuroinflammation and neuroplasticity in mouse hippocampi. J Chem Neuroanat, 2018. 94: p. 86–92. [DOI] [PubMed] [Google Scholar]
- 23.Mecca C, et al. , Microglia and Aging: The Role of the TREM2-DAP12 and CX3CL1-CX3CR1 Axes. Int J Mol Sci, 2018. 19(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen NC, et al. , Fate of microglia during HIV-1 infection: From activation to senescence? Glia, 2017. 65(3): p. 431–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Periyasamy P, et al. , Cocaine-Mediated Downregulation of miR-124 Activates Microglia by Targeting KLF4 and TLR4 Signaling. Mol Neurobiol, 2018. 55(4): p. 3196–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo ML, et al. , Cocaine-mediated microglial activation involves the ER stress-autophagy axis. Autophagy, 2015. 11(7): p. 995–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Periyasamy P, Guo ML, and Buch S, Cocaine induces astrocytosis through ER stress-mediated activation of autophagy. Autophagy, 2016. 12(8): p. 1310–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chivero ET, et al. , HIV-1 Tat Primes and Activates Microglial NLRP3 Inflammasome-Mediated Neuroinflammation. J Neurosci, 2017. 37(13): p. 3599–3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cao L, et al. , Cocaine-Mediated Autophagy in Astrocytes Involves Sigma 1 Receptor, PI3K, mTOR, Atg5/7, Beclin1 and Induces Type II Programed Cell Death. Mol Neurobiol, 2016. 53(7): p. 4417–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lapierre J, et al. , Critical Role of Beclin1 in HIV Tat and Morphine-Induced Inflammation and Calcium Release in Glial Cells from Autophagy Deficient Mouse. J Neuroimmune Pharmacol, 2018. 13(3): p. 355–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fields J, et al. , Age-dependent molecular alterations in the autophagy pathway in HIVE patients and in a gp120 tg mouse model: reversal with beclin-1 gene transfer. J Neurovirol, 2013. 19(1): p. 89–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubinsztein DC, Marino G, and Kroemer G, Autophagy and aging. Cell, 2011. 146(5): p. 682–95. [DOI] [PubMed] [Google Scholar]
- 33.Zhong Z, Sanchez-Lopez E, and Karin M, Autophagy, Inflammation, and Immunity: A Troika Governing Cancer and Its Treatment. Cell, 2016. 166(2): p. 288–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mijaljica D, Prescott M, and Devenish RJ, Microautophagy in mammalian cells: revisiting a 40-year-old conundrum. Autophagy, 2011. 7(7): p. 673–82. [DOI] [PubMed] [Google Scholar]
- 35.Massey A, Kiffin R, and Cuervo AM, Pathophysiology of chaperone-mediated autophagy. Int J Biochem Cell Biol, 2004. 36(12): p. 2420–34. [DOI] [PubMed] [Google Scholar]
- 36.Green DR and Levine B, To be or not to be? How selective autophagy and cell death govern cell fate. Cell, 2014. 157(1): p. 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chae U, et al. , Drp1-dependent mitochondrial fission regulates p62-mediated autophagy in LPS-induced activated microglial cells. Biosci Biotechnol Biochem, 2019. 83(3): p. 409–416. [DOI] [PubMed] [Google Scholar]
- 38.Taneike M, et al. , Inhibition of autophagy in the heart induces age-related cardiomyopathy. Autophagy, 2010. 6(5): p. 600–6. [DOI] [PubMed] [Google Scholar]
- 39.Hoshino A, et al. , Cytosolic p53 inhibits Parkin-mediated mitophagy and promotes mitochondrial dysfunction in the mouse heart. Nat Commun, 2013. 4: p. 2308. [DOI] [PubMed] [Google Scholar]
- 40.Shinmura K, et al. , Impact of long-term caloric restriction on cardiac senescence: caloric restriction ameliorates cardiac diastolic dysfunction associated with aging. J Mol Cell Cardiol, 2011. 50(1): p. 117–27. [DOI] [PubMed] [Google Scholar]
- 41.Brooks RW and Robbins PD, Treating Age-Related Diseases with Somatic Stem Cells. Adv Exp Med Biol, 2018. 1056: p. 29–45. [DOI] [PubMed] [Google Scholar]
- 42.Kumar S, et al. , MicroRNAs as Peripheral Biomarkers in Aging and Age-Related Diseases. Prog Mol Biol Transl Sci, 2017. 146: p. 47–94. [DOI] [PubMed] [Google Scholar]
- 43.Shibata M, et al. , Regulation of intracellular accumulation of mutant Huntingtin by Beclin 1. J Biol Chem, 2006. 281(20): p. 14474–85. [DOI] [PubMed] [Google Scholar]
- 44.Lipinski MM, et al. , Genome-wide analysis reveals mechanisms modulating autophagy in normal brain aging and in Alzheimer’s disease. Proc Natl Acad Sci U S A, 2010. 107(32): p. 14164–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guebel DV and Torres NV, Sexual Dimorphism and Aging in the Human Hyppocampus: Identification, Validation, and Impact of Differentially Expressed Genes by Factorial Microarray and Network Analysis. Front Aging Neurosci, 2016. 8: p. 229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee JH, et al. , Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell, 2010. 141(7): p. 1146–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qu D, et al. , BAG2 Gene-mediated Regulation of PINK1 Protein Is Critical for Mitochondrial Translocation of PARKIN and Neuronal Survival. J Biol Chem, 2015. 290(51): p. 30441–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang XH, et al. , Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature, 1999. 402(6762): p. 672–6. [DOI] [PubMed] [Google Scholar]
- 49.Dutta D, et al. , Contribution of impaired mitochondrial autophagy to cardiac aging: mechanisms and therapeutic opportunities. Circ Res, 2012. 110(8): p. 1125–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.El’darov Ch M, et al. , Morphometric Examination of Mitochondrial Ultrastructure in Aging Cardiomyocytes. Biochemistry (Mosc), 2015. 80(5): p. 604–9. [DOI] [PubMed] [Google Scholar]
- 51.Mercado N, Ito K, and Barnes PJ, Accelerated ageing of the lung in COPD: new concepts. Thorax, 2015. 70(5): p. 482–9. [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto T, et al. , Time-dependent dysregulation of autophagy: Implications in aging and mitochondrial homeostasis in the kidney proximal tubule. Autophagy, 2016. 12(5): p. 801–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leidal AM, Levine B, and Debnath J, Autophagy and the cell biology of age-related disease. Nat Cell Biol, 2018. 20(12): p. 1338–1348. [DOI] [PubMed] [Google Scholar]
- 54.Martinez-Cisuelo V, et al. , Rapamycin reverses age-related increases in mitochondrial ROS production at complex I, oxidative stress, accumulation of mtDNA fragments inside nuclear DNA, and lipofuscin level, and increases autophagy, in the liver of middle-aged mice. Exp Gerontol, 2016. 83: p. 130–8. [DOI] [PubMed] [Google Scholar]
- 55.Zhang Y, et al. , Complex inhibition of autophagy by mitochondrial aldehyde dehydrogenase shortens lifespan and exacerbates cardiac aging. Biochim Biophys Acta Mol Basis Dis, 2017. 1863(8): p. 1919–1932. [DOI] [PubMed] [Google Scholar]
- 56.Madeo F, et al. , Spermidine: a physiological autophagy inducer acting as an anti-aging vitamin in humans? Autophagy, 2019. 15(1): p. 165–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nilsson BO and Persson L, Beneficial effects of spermidine on cardiovascular health and longevity suggest a cell type-specific import of polyamines by cardiomyocytes. Biochem Soc Trans, 2019. 47(1): p. 265–272. [DOI] [PubMed] [Google Scholar]
- 58.Pyo JO, et al. , Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nat Commun, 2013. 4: p. 2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fernandez AF, et al. , Disruption of the beclin 1-BCL2 autophagy regulatory complex promotes longevity in mice. Nature, 2018. 558(7708): p. 136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gautier CA, Kitada T, and Shen J, Loss of PINK1 causes mitochondrial functional defects and increased sensitivity to oxidative stress. Proc Natl Acad Sci U S A, 2008. 105(32): p. 11364–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kubli DA, Quinsay MN, and Gustafsson AB, Parkin deficiency results in accumulation of abnormal mitochondria in aging myocytes. Commun Integr Biol, 2013. 6(4): p. e24511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Leymarie O, Lepont L, and Berlioz-Torrent C, Canonical and Non-Canonical Autophagy in HIV-1 Replication Cycle. Viruses, 2017. 9(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dinkins C, Pilli M, and Kehrl JH, Roles of autophagy in HIV infection. Immunol Cell Biol, 2015. 93(1): p. 11–7. [DOI] [PubMed] [Google Scholar]
- 64.Zhou D, Masliah E, and Spector SA, Autophagy is increased in postmortem brains of persons with HIV-1-associated encephalitis. J Infect Dis, 2011. 203(11): p. 1647–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brass AL, et al. , Identification of host proteins required for HIV infection through a functional genomic screen. Science, 2008. 319(5865): p. 921–6. [DOI] [PubMed] [Google Scholar]
- 66.Checkley MA, Luttge BG, and Freed EO, HIV-1 envelope glycoprotein biosynthesis, trafficking, and incorporation. J Mol Biol, 2011. 410(4): p. 582–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Espert L, et al. , Autophagy is involved in T cell death after binding of HIV-1 envelope proteins to CXCR4. J Clin Invest, 2006. 116(8): p. 2161–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Denizot M, et al. , HIV-1 gp41 fusogenic function triggers autophagy in uninfected cells. Autophagy, 2008. 4(8): p. 998–1008. [DOI] [PubMed] [Google Scholar]
- 69.Espert L, et al. , Differential role of autophagy in CD4 T cells and macrophages during X4 and R5 HIV-1 infection. PLoS One, 2009. 4(6): p. e5787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen G, et al. , Curcumin Attenuates gp120-Induced Microglial Inflammation by Inhibiting Autophagy via the PI3K Pathway. Cell Mol Neurobiol, 2018. 38(8): p. 1465–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cao L, et al. , Methamphetamine potentiates HIV-1 gp120-mediated autophagy via Beclin-1 and Atg5/7 as a pro-survival response in astrocytes. Cell Death Dis, 2016. 7(10): p. e2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Das AT, Harwig A, and Berkhout B, The HIV-1 Tat protein has a versatile role in activating viral transcription. J Virol, 2011. 85(18): p. 9506–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Li JC, et al. , HIV-1 trans-activator protein dysregulates IFN-gamma signaling and contributes to the suppression of autophagy induction. AIDS, 2011. 25(1): p. 15–25. [DOI] [PubMed] [Google Scholar]
- 74.Van Grol J, et al. , HIV-1 inhibits autophagy in bystander macrophage/monocytic cells through Src-Akt and STAT3. PLoS One, 2010. 5(7): p. e11733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qi L, et al. , Programmed neuronal cell death induced by HIV-1 tat and methamphetamine. Microsc Res Tech, 2011. 74(12): p. 1139–44. [DOI] [PubMed] [Google Scholar]
- 76.Hui L, et al. , Role of endolysosomes in HIV-1 Tat-induced neurotoxicity. ASN Neuro, 2012. 4(4): p. 243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fields J, et al. , HIV-1 Tat alters neuronal autophagy by modulating autophagosome fusion to the lysosome: implications for HIV-associated neurocognitive disorders. J Neurosci, 2015. 35(5): p. 1921–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Teodorof-Diedrich C and Spector SA, Human Immunodeficiency Virus Type 1 gp120 and Tat Induce Mitochondrial Fragmentation and Incomplete Mitophagy in Human Neurons. J Virol, 2018. 92(22). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thangaraj A, et al. , HIV-1 TAT-mediated microglial activation: role of mitochondrial dysfunction and defective mitophagy. Autophagy, 2018. 14(9): p. 1596–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wu X, et al. , HIV-1 Tat increases BAG3 via NF-kappaB signaling to induce autophagy during HIV-associated neurocognitive disorder. Cell Cycle, 2018. 17(13): p. 1614–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pawlak EN and Dikeakos JD, HIV-1 Nef: a master manipulator of the membrane trafficking machinery mediating immune evasion. Biochim Biophys Acta, 2015. 1850(4): p. 733–41. [DOI] [PubMed] [Google Scholar]
- 82.Rosa A, et al. , HIV-1 Nef promotes infection by excluding SERINC5 from virion incorporation. Nature, 2015. 526(7572): p. 212–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kyei GB, et al. , Autophagy pathway intersects with HIV-1 biosynthesis and regulates viral yields in macrophages. J Cell Biol, 2009. 186(2): p. 255–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Campbell GR, et al. , Human Immunodeficiency Virus Type 1 Nef Inhibits Autophagy through Transcription Factor EB Sequestration. PLoS Pathog, 2015. 11(6): p. e1005018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Saribas AS, Khalili K, and Sariyer IK, Dysregulation of autophagy by HIV-1 Nef in human astrocytes. Cell Cycle, 2015. 14(18): p. 2899–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ersche KD, et al. , Cocaine dependence: a fast-track for brain ageing? Mol Psychiatry, 2013. 18(2): p. 134–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nestler EJ, Historical review: Molecular and cellular mechanisms of opiate and cocaine addiction. Trends Pharmacol Sci, 2004. 25(4): p. 210–8. [DOI] [PubMed] [Google Scholar]
- 88.Guha P, Harraz MM, and Snyder SH, Cocaine elicits autophagic cytotoxicity via a nitric oxide-GAPDH signaling cascade. Proc Natl Acad Sci U S A, 2016. 113(5): p. 1417–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sil S, et al. , Cocaine Mediated Neuroinflammation: Role of Dysregulated Autophagy in Pericytes. Mol Neurobiol, 2019. 56(5): p. 3576–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thangaraj A, et al. , Mitigation of cocaine-mediated mitochondrial damage, defective mitophagy and microglial activation by superoxide dismutase mimetics. Autophagy, 2019: p. 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Roohbakhsh A, Shirani K, and Karimi G, Methamphetamine-induced toxicity: The role of autophagy? Chem Biol Interact, 2016. 260: p. 163–167. [DOI] [PubMed] [Google Scholar]
- 92.Huang E, et al. , Involvement of C/EBPbeta-related signaling pathway in methamphetamine-induced neuronal autophagy and apoptosis. Toxicol Lett, 2019. 312: p. 11–21. [DOI] [PubMed] [Google Scholar]
- 93.Lazzeri G, et al. , mTOR Modulates Methamphetamine-Induced Toxicity through Cell Clearing Systems. Oxid Med Cell Longev, 2018. 2018: p. 6124745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu X, et al. , Nupr1 Modulates Methamphetamine-Induced Dopaminergic Neuronal Apoptosis and Autophagy through CHOP-Trib3-Mediated Endoplasmic Reticulum Stress Signaling Pathway. Front Mol Neurosci, 2017. 10: p. 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen R, et al. , DNA damage-inducible transcript 4 (DDIT4) mediates methamphetamine-induced autophagy and apoptosis through mTOR signaling pathway in cardiomyocytes. Toxicol Appl Pharmacol, 2016. 295: p. 1–11. [DOI] [PubMed] [Google Scholar]
- 96.Ma J, et al. , Methamphetamine induces autophagy as a pro-survival response against apoptotic endothelial cell death through the Kappa opioid receptor. Cell Death Dis, 2014. 5: p. e1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dutta R, et al. , Morphine modulation of toll-like receptors in microglial cells potentiates neuropathogenesis in a HIV-1 model of coinfection with pneumococcal pneumoniae. J Neurosci, 2012. 32(29): p. 9917–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rodriguez M, et al. , Morphine counteracts the antiviral effect of antiretroviral drugs and causes upregulation of p62/SQSTM1 and histone-modifying enzymes in HIV-infected astrocytes. J Neurovirol, 2019. 25(2): p. 263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.El-Hage N, et al. , HIV-1 and morphine regulation of autophagy in microglia: limited interactions in the context of HIV-1 infection and opioid abuse. J Virol, 2015. 89(2): p. 1024–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sil S, et al. , Morphine-Mediated Brain Region-Specific Astrocytosis Involves the ER Stress-Autophagy Axis. Mol Neurobiol, 2018. 55(8): p. 6713–6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cai Y, et al. , Regulation of morphine-induced synaptic alterations: Role of oxidative stress, ER stress, and autophagy. J Cell Biol, 2016. 215(2): p. 245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pan J, et al. , Activating Autophagy in Hippocampal Cells Alleviates the Morphine-Induced Memory Impairment. Mol Neurobiol, 2017. 54(3): p. 1710–1724. [DOI] [PubMed] [Google Scholar]
- 103.Su LY, et al. , Atg5- and Atg7-dependent autophagy in dopaminergic neurons regulates cellular and behavioral responses to morphine. Autophagy, 2017. 13(9): p. 1496–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kong H, et al. , Morphine induces dysfunction of PINK1/Parkin-mediated mitophagy in spinal cord neurons implying involvement in antinociceptive tolerance. J Mol Cell Biol, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hauser KF, et al. , HIV-1 Tat and morphine have interactive effects on oligodendrocyte survival and morphology. Glia, 2009. 57(2): p. 194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lisi L, et al. , Antiretrovirals inhibit arginase in human microglia. J Neurochem, 2016. 136(2): p. 363–72. [DOI] [PubMed] [Google Scholar]
- 107.Ciavatta VT, et al. , In vitro and Ex vivo Neurotoxic Effects of Efavirenz are Greater than Those of Other Common Antiretrovirals. Neurochem Res, 2017. 42(11): p. 3220–3232. [DOI] [PubMed] [Google Scholar]
- 108.Stauch KL, et al. , Central nervous system-penetrating antiretrovirals impair energetic reserve in striatal nerve terminals. J Neurovirol, 2017. 23(6): p. 795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gao Z, et al. , DRAM Is Involved in Regulating Nucleoside Analog-Induced Neuronal Autophagy in a p53-Independent Manner. Mol Neurobiol, 2018. 55(3): p. 1988–1997. [DOI] [PubMed] [Google Scholar]
- 110.De Simone FI, et al. , HIV-1 Tat and Cocaine Impair Survival of Cultured Primary Neuronal Cells via a Mitochondrial Pathway. J Neuroimmune Pharmacol, 2016. 11(2): p. 358–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Dalvi P, et al. , Enhanced autophagy in pulmonary endothelial cells on exposure to HIV-Tat and morphine: Role in HIV-related pulmonary arterial hypertension. Autophagy, 2016. 12(12): p. 2420–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dever SM, et al. , Differing roles of autophagy in HIV-associated neurocognitive impairment and encephalitis with implications for morphine co-exposure. Front Microbiol, 2015. 6: p. 653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li J, et al. , Autophagy Induction by HIV-Tat and Methamphetamine in Primary Midbrain Neuronal Cells of Tree Shrews via the mTOR Signaling and ATG5/ATG7 Pathway. Front Neurosci, 2018. 12: p. 921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sanchez AB, et al. , Antiretrovirals, Methamphetamine, and HIV-1 Envelope Protein gp120 Compromise Neuronal Energy Homeostasis in Association with Various Degrees of Synaptic and Neuritic Damage. Antimicrob Agents Chemother, 2016. 60(1): p. 168–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Thaney VE and Kaul M, Type I Interferons in NeuroHIV. Viral Immunol, 2019. 32(1): p. 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Tal MC, et al. , Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling. Proc Natl Acad Sci U S A, 2009. 106(8): p. 2770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Saitoh T, et al. , Atg9a controls dsDNA-driven dynamic translocation of STING and the innate immune response. Proc Natl Acad Sci U S A, 2009. 106(49): p. 20842–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cisneros IE, et al. , Cocaine evokes a profile of oxidative stress and impacts innate antiviral response pathways in astrocytes. Neuropharmacology, 2018. 135: p. 431–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Balagopal A, et al. , Continued Elevation of Interleukin-18 and Interferon-gamma After Initiation of Antiretroviral Therapy and Clinical Failure in a Diverse Multicountry Human Immunodeficiency Virus Cohort. Open Forum Infect Dis, 2016. 3(3): p. ofw118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.De Cecco M, et al. , L1 drives IFN in senescent cells and promotes age-associated inflammation. Nature, 2019. 566(7742): p. 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moscat J and Diaz-Meco MT, p62 at the crossroads of autophagy, apoptosis, and cancer. Cell, 2009. 137(6): p. 1001–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang G, et al. , Induction of a Na(+)/K(+)-ATPase-dependent form of autophagy triggers preferential cell death of human immunodeficiency virus type-1-infected macrophages. Autophagy, 2018. 14(8): p. 1359–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Saitoh T and Akira S, Regulation of innate immune responses by autophagy-related proteins. J Cell Biol, 2010. 189(6): p. 925–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Houtman J, et al. , Beclin1-driven autophagy modulates the inflammatory response of microglia via NLRP3. EMBO J, 2019. 38(4). [DOI] [PMC free article] [PubMed] [Google Scholar]