Abstract
Glaucoma is a very serious disease that can lead to blindness in severe cases. In an attempt to increase the efficacy of the drugs used in treating this disease, many particulate systems (micro/nano and lipid-based/nonlipid-based) have been exploited. In this study, the meta-analysis approach was implemented in order to explore the published studies and extract the literature-based evidence (proof-of-concept studies = 16) that the particulate systems increase the efficiency of the investigated intraocular pressure drugs as demonstrated by the increase in the area under effect curve. Comparison of micron particles versus nanoparticles on the one hand and lipid-based versus nonlipid-based on the other hand, as subgroups of the meta-analysis, was also included in the study where the latter comparison led to insignificant differences, whereas the former has proven the superiority of the nanoparticles over the micronized counterparts.
1. Introduction
Glaucoma is a pathological eye condition that is usually associated with an elevated intraocular pressure (IOP), and it is the second cause of blindness worldwide that it is currently named as the silent blinder.1 Several attempts have been made in order to deliver antiglaucomatous drugs and increase their residence and contact with the cornea and to augment their penetration abilities. Among these attempts are the usage of particulate systems which include the lipid-based particles such as the liposomal vesicles,2,3 solid lipid nanoparticles,4 and nanostructured lipid carriers5 on one hand and the utilization of other nonlipid particles such as the polymeric,6 protein-based particles like gelatin-based particles7 and surfactant-based counterparts such as the niosomes8 on the other hand. These carriers were highly variable according to their size, encountering both micron- and nanosized particles throughout the investigated research articles.
Meta-analysis is an advanced statistical technique that combines data emerging from multiple studies on a particular topic. It increases the accuracy and precision of the study estimates. Actually, meta-analyses play fundamental roles in evidence-based healthcare-related topics. Compared to other study designs (such as randomized controlled trials or cohort studies), meta-analysis comes in at the top of the “levels of evidence” pyramid.9
Currently, the use of meta-analysis is gaining more grounds and is considered crucial in both clinical and industrial decision-making,10 though not fully explored in the drug delivery discipline. Additionally, with the advent of the Internet and computer softwares, the exploitation of literature and data is becoming easier and the mining of this treasure is becoming more warranted.11−13 Meta-analysis, the quantitative synthesis of information from independent sources (studies), forms a cornerstone of evidence-based medicine.14 It is indeed a powerful tool that can be used to compare any intervention with a control.10
The aim of this study was to provide a quantitative summary of the existing literature which utilized the particulate systems approach for the enhancement of the pharmacodynamics of glaucoma treatments represented by the area under effect (intraocular pressure, IOP) curve (AUEC), which is additionally considered an indirect marker of the bioavailability of the delivered drugs. The significance of this approach was assessed. Furthermore, two covariates were evaluated, namely, the type of material used (whether lipid-based or not) and the size of the particulate system (whether micronized or nanosized). Such information could be very helpful and insightful for the drug delivery and formulation scientists who wish to adopt this kind of approach to increase the efficacy of glaucoma therapy.
2. Results and Discussion
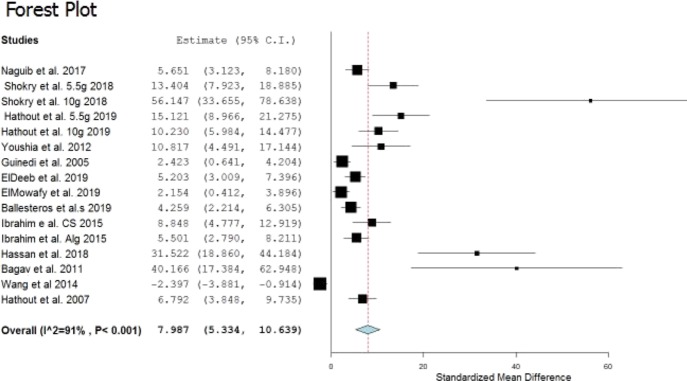
Table 1 shows the summary of the performed meta-analysis study after calculating the standardized mean difference (SMD) for each study and the lower and upper confidence intervals (CIs). All the investigated studies were proven significant with CIs always lying on one side of the zero as a cutoff (i.e., either both positive or both negative) as demonstrated by the generated forest plot from the used software (Figure 1). Usually, an individual study is considered significant if its SMD with its two CIs are all positive or all negative without crossing or passing the zero cutoff (i.e., in the current analysis, the particulate treatment significantly increases the AUEC or significantly decreases it as compared to the control, the drug solution).10
Table 1. Summary of the Meta-Analysis of the Published Studies Investigating the Pharmacodynamic Effects of Antiglaucomatous Drug Particulate Systems.
| study | year | number of animals receiving the particulate system (Gp A) | mean AUEC of Gp A | SD of Gp A | number of animals receiving the drug solution (Gp B) | mean AUEC of Gp B | SD of Gp B | SMD | lower CI | upper CI | type of materiala | size of particulate systemb |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Naguib et al.20 | 2017 | 6 | 99.08 | 20.1 | 6 | 11.78 | 1.54 | 5.651 | 3.123 | 8.18 | 1 | 2 |
| Shokry et al.21c | 2018 | 6 | 459 | 10.76 | 6 | 331.9 | 6.11 | 13.404 | 7.923 | 18.885 | 2 | 1 |
| Shokry et al.22d | 2018 | 6 | 455.1 | 5.43 | 6 | 200.7 | 2.34 | 56.147 | 33.655 | 78.638 | 2 | 1 |
| Hathout et al.23c | 2019 | 6 | 567.6 | 15.12 | 6 | 369.5 | 7.98 | 15.121 | 8.966 | 21.275 | 1 | 2 |
| Hathout et al.24d | 2019 | 6 | 528.8 | 13.27 | 6 | 385.4 | 12.59 | 10.23 | 5.984 | 14.477 | 1 | 2 |
| Youshia et al.5 | 2012 | 3 | 64.2 | 5.25 | 3 | 10.9 | 1.83 | 10.817 | 4.491 | 17.144 | 1 | 1 |
| Guinedi et al.25 | 2005 | 6 | 50.3 | 20.3 | 3 | 3.5 | 0.5 | 2.423 | 0.641 | 4.204 | 1 | 2 |
| Eldeeb et al.26 | 2019 | 7 | 409.2 | 82.72 | 7 | 81.46 | 10.44 | 5.203 | 3.009 | 7.396 | 2 | 2 |
| Elmowafy et al.27 | 2019 | 4 | 106.7 | 29.09 | 4 | 44.3 | 20.5 | 2.154 | 0.412 | 3.896 | 2 | 2 |
| GómezGomez-Ballesteros et al.28 | 2019 | 6 | 137.4 | 15 | 6 | 58.9 | 18.8 | 4.259 | 2.214 | 6.305 | 1 | 1 |
| Ibrahim e al.29e | 2015 | 5 | 80.3 | 7.79 | 5 | 25.94 | 0.91 | 8.848 | 4.777 | 12.919 | 2 | 1 |
| Ibrahim et al.30f | 2015 | 5 | 71.79 | 10.6 | 5 | 25.95 | 0.91 | 5.501 | 2.79 | 8.211 | 2 | 1 |
| Hassan et al.31 | 2018 | 6 | 759.7 | 16.7 | 6 | 322.3 | 7 | 31.522 | 18.86 | 44.184 | 1 | 1 |
| Bagav et al.32 | 2011 | 3 | 268.09 | 4.89 | 3 | 38.4 | 4.21 | 40.166 | 17.384 | 62.948 | 2 | 1 |
| Wang et al.33 | 2014 | 6 | 126.74 | 17.73 | 6 | 171.17 | 16.45 | –2.397 | –3.881 | –0.914 | 1 | 1 |
| Hathout et al.34 | 2007 | 6 | 26.03 | 2.6 | 6 | 7.825 | 2.34 | 6.792 | 3.848 | 9.735 | 1 | 2 |
Type of material (lipid or nonlipid).
Size of particulate system (micro or nano).
5.5 g plunger of the tonometer.
10 g plunger of the tonometer.
Chitosan nanoparticles.
Alginate nanoparticles.
Figure 1.
Overall forest plot of the investigated studies.
The overall meta-analysis study was extremely significant with a P value < 0.001 with a pooled estimate of 7.987 and CI (5.334, 10.639), confirming the significance of the results and the presence of the real effect of the use of the particulate systems on the pharmacodynamic responses of the investigated drugs, as revealed by AUEC.
The particulate systems are usually successfully uptaken by the cells present in the different biological membranes such as the cornea that normally consists of stratified squamous epithelium (the hardest-to-penetrate) using different mechanisms such as passive diffusion, clathrate–claveolate-mediated endocytosis, and micropinocytosis.15−18
In another encounter, the heterogeneity of meta-analysis was high, with the degree of heterogeneity (I2) scoring 91%. The source of heterogeneity comes from the different years of study, different animals, different kinds of measurements, different climates, and different number of animals.19
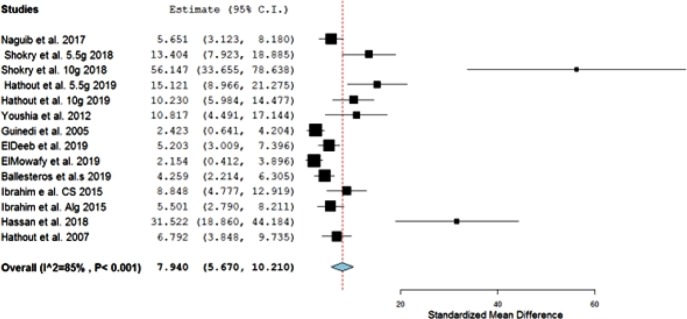
Additionally, a leave-one-out meta-analysis was carried out to exclude the study of the highest weight (Table 2) Wang et al. 2014. The pooled estimate changed to 7.940 (5.670, 10.210), and the heterogeneity dropped to 85% (Figure 2).
Table 2. Investigated Study Weights.
| study names | weights (%) |
|---|---|
| Naguib et al. 2017 | 7.78 |
| Shokry et al. 5.5 g 2018 | 6.167 |
| Shokry et al. 10 g 2018 | 1.193 |
| Hathout et al. 5.5 g 2019 | 5.771 |
| Hathout et al. 10 g 2019 | 6.893 |
| Youshia et al. 2012 | 5.671 |
| Guinedi et al. 2005 | 8.067 |
| ElDeeb et al. 2019 | 7.918 |
| ElMowafy et al. 2019 | 8.08 |
| Ballesteros et al. 2019 | 7.975 |
| Ibrahim e al. CS 2015 | 6.993 |
| Ibrahim et al. Alg 2015 | 7.699 |
| Hassan et al. 2018 | 2.879 |
| Bagav et al. 2011 | 1.167 |
| Wang et al. 2014 | 8.158 |
| Hathout et al. 2007 | 7.589 |
Figure 2.
Leave-one-out meta-analysis forest plot of the investigated studies.
Further exploring, the investigated studies were subdivided into two groups according to the main material used to fabricate the particulate system: subgroup 1––lipid-based encoded (1) and subgroup 2––nonlipid-based encoded (2). Subgroup meta-analysis was implemented, where subgroup 1 scored a pooled estimate of 8.266 with CIs (5.209, 11.322), whereas subgroup 2 scored a pooled estimate of 9.166 with CIs (4.927, 13.405). The overlapping CIs indicate nonsignificant differences between the two subgroups. Surprisingly, the lipid-based particulate systems were not superior to the nonlipid counterparts. Though lipids such as the phospholipids or triglycerides are compatible with the endothelial corneal layers, the surfactant-based particles are still compatible as usually nonionic surfactants are the ones used for ocular delivery. Additionally, even the polymeric and protein-based particles are still highly compatible with other corneal layers such as the stroma.35,36
Moreover, the investigated studies were subdivided into two other subgroups according to the size range of the used particulate system: subgroup 1––nanosized particles encoded (1) and subgroup 2––micron-sized particles encoded (2). The subgroup meta-analysis was reimplemented changing the covariate. Subgroup 1 was proven significantly superior in increasing the AUEC as compared to the control, scoring a pooled estimate of 13.649 with CIs (8.462, 18.837), whereas subgroup 2 scored 5.906 with CIs (6.007, 10.712). The results are significant as the CIs do not overlap.
Although one may think that the micronized particles may reside in the cul-du-sac and in more contact with the cornea because of their larger particle size preventing their easy drainage, it seems that the higher penetration ability of the nanoparticles supersedes this property and enables the drugs to surpass the corneal barrier more efficiently.35
3. Conclusions
There is a strong literature-driven evidence based on meta-analysis that the particulate systems (micro/nano) potentiate the pharmacodynamic effects of antiglaucomatous drugs on animals. Furthermore, the study gives evidence that the nanoparticles are more efficient in lowering the IOP than the micronized counterparts and hence pose more efficient glaucoma therapies. The material with which the nanosystem is produced does not appear to influence its eye performance.
4. Methodology
4.1. Data Mining
A computer-based search was conducted on databases such as Medlineand Embase and on a search engine, Google Scholar. SciFinder was also used to reconduct a further computer-based research for any relevant patents or commentaries.
The following keywords were utilized in the search: micro, nano, vesicles, lipid, polymer, polymeric, nanoparticles, glaucoma, ocular, and liposomes. All the searches were performed in English language.
4.2. Inclusion Data and their Criteria
The mined articles were considered eligible for evaluation if they contained the methodology and discussion related to particulate systems that are used as carriers for drugs to lower the IOP on animal studies through the topical ocular route. All initially eligible articles were further screened in detail by analyzing the abstract and full text. All the articles should contain original data (research articles). AUC or AUEC (starting from the baseline) should have been reported or can be calculated from IOP tables. The optimization or the formulation scoring the highest AUEC was selected in case of the studies containing several formulations.
The following data were collected from articles fulfilling the inclusion criteria: the name of the author and year of publication, the studied drug, type of the carrier (lipid-based or nonlipid-based: surfactants or polymers), particle size (micron or nano), and AUEC as a pharmacodynamic parameter compared to a control (drug solution).
4.3. Meta-Analysis
The meta-analysis was performed to confirm the augmentation of the pharmacodynamic effects of the antiglaucomatous drugs compared to the drug solution as a control, as revealed by AUEC, and this was considered the basis of the effect size of the experiment. As meta-analysis combines the results of different studies and gives an overall conclusion, “heterogeneity” should be considered.
The effect size (AUEC) and the study sample size were fed into OpenMetaAnalyst (http://www.cebm.brown.edu/openMeta/) to perform meta-analysis of the investigated studies and provide forest plots.
As the studies in this meta-analysis are variable in their animal numbers (sample size), they do not meet the underlying assumption of a fixed effect model that only the sampling error is the source of variability; hence, the overall effect size was estimated using the random effects model and utilizing the Der Simonian–Laird method. The random effects model takes into account the variability between studies such as measurements and sample size and was therefore claimed adequate for the purpose of this meta-analysis. Heterogeneity was assessed using two parameters: the Q statistic and the I2 index. The Q statistic gives an indication of the presence or absence of heterogeneity among a set of studies related to differences in the measurements, year of study, and conditions, whereas the I2 index gives an indication of the degree of heterogeneity. The mean percent increase and a 95% confidence interval (CI) was calculated and represented in the forest plot. Significance was employed by the P value. The sensitivity and consistency of the study were evaluated using the leave-one-out meta-analysis.
The effect size was calculated as follows
| 1 |
where N is the sample size (number of animals).
SMD was calculated as
| 2 |
where
| 3 |
where na is the number of animals that received the particulate formulation, nb is the number of animals that received the drug solution as a control, Sa is the standard deviation of the particulate formulation mean effect, whereas Sb is the standard deviation of the drug solution mean effect.
Every study weight was calculated as follows
| 4 |
where SE is the standard error of each study.
Q is the amount of observed heterogeneity that
will be compared to the amount of expected heterogeneity by chance,
whereas I2 index is the quantitative degree
of heterogeneity and is calculated as: 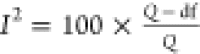 , where df is the degree of freedom taken
as the number of studies – 1.
, where df is the degree of freedom taken
as the number of studies – 1.
Furthermore, the mined studies were divided into subgroups according to the following:
-
(a)
Particle type designed as covariate factors encoded (1) and (2) for lipid-based and nonlipid-based, respectively.
-
(b)
Particle size also designed as other covariate factors encoded (1) and (2) for the nano- and micron-sized particles, respectively.
The author declares no competing financial interest.
References
- Sun J.; Lei Y.; Dai Z.; Liu X.; Huang T.; Wu J.; Xu Z. P.; Sun X. Sustained Release of Brimonidine from a New Composite Drug Delivery System for Treatment of Glaucoma. ACS Appl. Mater. Interfaces 2017, 9, 7990–7999. 10.1021/acsami.6b16509. [DOI] [PubMed] [Google Scholar]
- Hathout R. M.; Gad H. A.; Metwally A. A. Gelatinized-core liposomes: Toward a more robust carrier for hydrophilic molecules. J. Biomed. Mater. Res., Part A 2017, 105, 3086–3092. 10.1002/jbm.a.36175. [DOI] [PubMed] [Google Scholar]
- Hassan D. H.; Abdelmonem R.; Abdellatif M. M. Formulation and Characterization of Carvedilol Leciplex for Glaucoma Treatment: In-Vitro, Ex-Vivo and In-Vivo Study. Pharmaceutics 2018, 10, 197. 10.3390/pharmaceutics10040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F.; Chen L.; Zhang D.; Jiang S.; Shi K.; Huang Y.; Li R.; Xu Q. Methazolamide-loaded solid lipid nanoparticles modified with low-molecular weight chitosan for the treatment of glaucoma: vitro and vivo study. J. Drug Target. 2014, 22, 849–858. 10.3109/1061186x.2014.939983. [DOI] [PubMed] [Google Scholar]
- Youshia J.; Kamel A.; El Shamy; Mansour Design of cationic nanostructured heterolipid matrices for ocular delivery of methazolamide. Int. J. Nanomed. 2012, 7, 2483–2496. 10.2147/ijn.s28307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim M. M.; Abd-Elgawad A.-E. H.; Soliman O. A.-E.; Jablonski M. M. Natural Bioadhesive Biodegradable Nanoparticle-Based Topical Ophthalmic Formulations for Management of Glaucoma. Transl. Vis. Sci. Technol. 2015, 4, 12. 10.1167/tvst.4.3.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathout R. M.; Metwally A. A.. Gelatin Nanoparticles. Pharmaceutical Nanotechnology; Methods in Molecular Biology; Springer, 2019; Vol. 2000, pp 71–78. [DOI] [PubMed] [Google Scholar]
- Guinedi A. S.; Mortada N. D.; Mansour S.; Hathout R. M. Preparation and evaluation of reverse-phase evaporation and multilamellar niosomes as ophthalmic carriers of acetazolamide. Int. J. Pharm. 2005, 306, 71–82. 10.1016/j.ijpharm.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Burns P. B.; Rohrich R. J.; Chung K. C. The levels of evidence and their role in evidence-based medicine. Plast. Reconstr. Surg. 2011, 128, 305–310. 10.1097/prs.0b013e318219c171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills E.; Bansback N.; Ghement I.; Tholund K.; Kelly S.; Puhan M. A.; Wright G. Multiple treatment comparison meta-analyses: a step forward into complexity. Clin. Epidemiol. 2011, 3, 193–202. 10.2147/clep.s16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathout R. M.; Metwally A. A. Towards better modeling of drug-loading in solid lipid nanoparticles: Molecular dynamics, docking experiments and Gaussian Processes machine learning. Eur. J. Pharm. Biopharm. 2016, 108, 262. 10.1016/j.ejpb.2016.07.019. [DOI] [PubMed] [Google Scholar]
- Metwally A. A.; Hathout R. M. Computer-Assisted Drug Formulation Design: Novel Approach in Drug Delivery. Mol. Pharm. 2015, 12, 2800–2810. 10.1021/mp500740d. [DOI] [PubMed] [Google Scholar]
- Metwally A. A.; El-Ahmady S. H.; Hathout R. M. Selecting optimum protein nano-carriers for natural polyphenols using chemoinformatics tools. Phytomedicine 2016, 23, 1764–1770. 10.1016/j.phymed.2016.10.020. [DOI] [PubMed] [Google Scholar]
- Fong S. Y. K.; Brandl M.; Bauer-Brandl A. Phospholipid-based solid drug formulations for oral bioavailability enhancement: A meta-analysis. Eur. J. Pharm. Sci. 2015, 80, 89–110. 10.1016/j.ejps.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Doherty G. J.; McMahon H. T. Mechanisms of endocytosis. Annu. Rev. Biochem. 2009, 78, 857–902. 10.1146/annurev.biochem.78.081307.110540. [DOI] [PubMed] [Google Scholar]
- Ivanov A. I.Pharmacological inhibition of endocytic pathways: is it specific enough to be useful? Methods in Molecular Biology; Springer, 2008; Vol. 440, pp 15–33. [DOI] [PubMed] [Google Scholar]
- Mehanny M.; Hathout R. M.; Geneidi A. S.; Mansour S. Studying the effect of physically-adsorbed coating polymers on the cytotoxic activity of optimized bisdemethoxycurcumin loaded-PLGA nanoparticles. J. Biomed. Mater. Res., Part A 2017, 105, 1433–1445. 10.1002/jbm.a.36028. [DOI] [PubMed] [Google Scholar]
- Mehanny M.; Hathout R. M.; Geneidi A. S.; Mansour S. Bisdemethoxycurcumin loaded polymeric mixed micelles as potential anti-cancer remedy: Preparation, optimization and cytotoxic evaluation in a HepG-2 cell model. J. Mol. Liq. 2016, 214, 162–170. 10.1016/j.molliq.2015.12.007. [DOI] [Google Scholar]
- Fong S. Y. K.; Brandl M.; Bauer-Brandl A. Phospholipid-based solid drug formulations for oral bioavailability enhancement: A meta-analysis. Eur. J. Pharm. Sci. 2015, 80, 89–110. 10.1016/j.ejps.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Naguib S. S.; Hathout R. M.; Mansour S. Optimizing novel penetration enhancing hybridized vesicles for augmenting the in-vivo effect of an anti-glaucoma drug. Drug Deliv. 2017, 24, 99–108. 10.1080/10717544.2016.1233588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokry M.; Hathout R. M.; Mansour S. Exploring gelatin nanoparticles as novel nanocarriers for Timolol Maleate: Augmented in-vivo efficacy and safe histological profile. Int. J. Pharm. 2018, 545, 229–239. 10.1016/j.ijpharm.2018.04.059. [DOI] [PubMed] [Google Scholar]
- Shokry M.; Hathout R. M.; Mansour S. Exploring gelatin nanoparticles as novel nanocarriers for Timolol Maleate: Augmented in-vivo efficacy and safe histological profile. Int. J. Pharm. 2018, 545, 229–239. 10.1016/j.ijpharm.2018.04.059. [DOI] [PubMed] [Google Scholar]
- Hathout R. M.; Gad H. A.; Abdel-Hafez S. M.; Nasser N.; Khalil N.; Ateyya T.; Amr A.; Yasser N.; Nasr S.; Metwally A. A. Gelatinized core liposomes: A new Trojan horse for the development of a novel timolol maleate glaucoma medication. Int. J. Pharm. 2019, 556, 192–199. 10.1016/j.ijpharm.2018.12.015. [DOI] [PubMed] [Google Scholar]
- Hathout R. M.; Gad H. A.; Abdel-Hafez S. M.; Nasser N.; Khalil N.; Ateyya T.; Amr A.; Yasser N.; Nasr S.; Metwally A. A. Gelatinized core liposomes: A new Trojan horse for the development of a novel timolol maleate glaucoma medication. Int. J. Pharm. 2019, 556, 192–199. 10.1016/j.ijpharm.2018.12.015. [DOI] [PubMed] [Google Scholar]
- Guinedi A. S.; Mortada N. D.; Mansour S.; Hathout R. M. Preparation and evaluation of reverse-phase evaporation and multilamellar niosomes as ophthalmic carriers of acetazolamide. Int. J. Pharm. 2005, 306, 71–82. 10.1016/j.ijpharm.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Eldeeb A. E.; Salah S.; Ghorab M. Proniosomal gel-derived niosomes: an approach to sustain and improve the ocular delivery of brimonidine tartrate; formulation, in-vitro characterization, and in-vivo pharmacodynamic study. Drug Deliv. 2019, 26, 509–521. 10.1080/10717544.2019.1609622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmowafy E.; Gad H.; Biondo F.; Casettari L.; Soliman M. E. Exploring optimized methoxy poly(ethylene glycol)-block-poly(epsilon-caprolactone) crystalline cored micelles in anti-glaucoma pharmacotherapy. Int. J. Pharm. 2019, 566, 573–584. 10.1016/j.ijpharm.2019.06.011. [DOI] [PubMed] [Google Scholar]
- Gómez-Ballesteros M.; Lopez-Cano J. J.; Bravo-Osuna I.; Herrero-Vanrell R.; Molina-Martinez I. T. Osmoprotectants in Hybrid Liposome/HPMC Systems as Potential Glaucoma Treatment. Polymers 2019, 11, 929. 10.3390/polym11060929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim M. M.; Abd-Elgawad A.-E. H.; Soliman O. A.-E.; Jablonski M. M. Natural Bioadhesive Biodegradable Nanoparticle-Based Topical Ophthalmic Formulations for Management of Glaucoma. Transl. Vis. Sci. Technol. 2015, 4, 12. 10.1167/tvst.4.3.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim M. M.; Abd-Elgawad A.-E. H.; Soliman O. A.-E.; Jablonski M. M. Natural Bioadhesive Biodegradable Nanoparticle-Based Topical Ophthalmic Formulations for Management of Glaucoma. Transl. Vis. Sci. Technol. 2015, 4, 12. 10.1167/tvst.4.3.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan D. H.; Abdelmonem R.; Abdellatif M. M. Formulation and Characterization of Carvedilol Leciplex for Glaucoma Treatment: In-Vitro, Ex-Vivo and In-Vivo Study. Pharmaceutics 2018, 10, 197. 10.3390/pharmaceutics10040197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagav P.; Upadhyay H.; Chandran S. Brimonidine tartrate-eudragit long-acting nanoparticles: formulation, optimization, in vitro and in vivo evaluation. AAPS PharmSciTech 2011, 12, 1087–1101. 10.1208/s12249-011-9675-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F.; Chen L.; Zhang D.; Jiang S.; Shi K.; Huang Y.; Li R.; Xu Q. Methazolamide-loaded solid lipid nanoparticles modified with low-molecular weight chitosan for the treatment of glaucoma: vitro and vivo study. J. Drug Target. 2014, 22, 849–858. 10.3109/1061186x.2014.939983. [DOI] [PubMed] [Google Scholar]
- Hathout R. M.; Mansour S.; Mortada N. D.; Guinedi A. S. Liposomes as an ocular delivery system for acetazolamide: in vitro and in vivo studies. AAPS PharmSciTech 2007, 8, E1 10.1208/pt0801001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathout R. M.; Omran M. K. Gelatin-based particulate systems in ocular drug delivery. Pharm. Dev. Technol. 2016, 21, 379–386. 10.3109/10837450.2014.999786. [DOI] [PubMed] [Google Scholar]
- Abozeid S. M.; Hathout R. M.; Abou-Aisha K. Silencing of the metastasis-linked gene, AEG-1, using siRNA-loaded cholamine surface-modified gelatin nanoparticles in the breast carcinoma cell line MCF-7. Colloids Surf., B 2016, 145, 607–616. 10.1016/j.colsurfb.2016.05.066. [DOI] [PubMed] [Google Scholar]