Abstract
Introduction
Stage IV large cell neuroendocrine carcinoma (LCNEC) of the lung generally presents as disseminated and aggressive disease with a Ki-67 proliferation index (PI) 40–80%. LCNEC can be subdivided in two main subtypes: the first harboring TP53/RB1 mutations (small-cell lung carcinoma (SCLC)-like), the second with mutations in TP53 and STK11/KEAP1 (non-small-cell lung carcinoma (NSCLC)-like). Here we evaluated 11 LCNEC patients with only a solitary brain metastasis and evaluate phenotype, genotype and follow-up.
Methods
Eleven LCNEC patients with solitary brain metastases were analyzed. Clinical characteristics and survival data were retrieved from medical records. Pathological analysis included histomorphological analysis, immunohistochemistry (pRB and Ki-67 PI) and next-generation sequencing (TP53, RB1, STK11, KEAP1 and MEN1).
Results
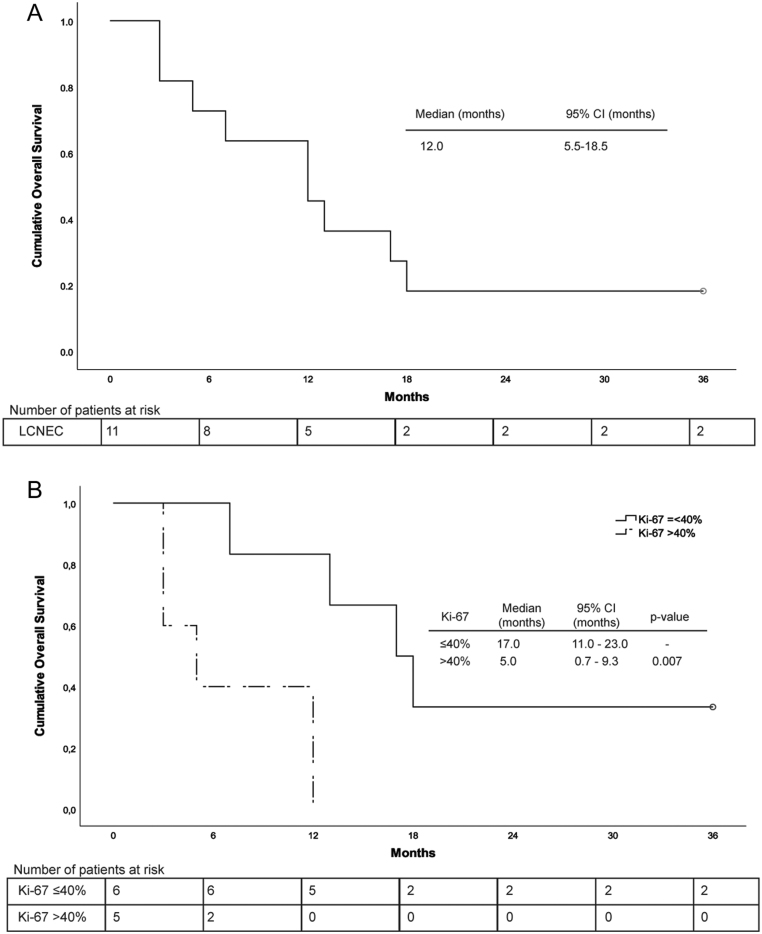
All patients had N0 or N1 disease. Median overall survival (OS) was 12 months (95% confidence interval (CI) 5.5–18.5 months). Mean Ki-67 PI was 59% (range 15–100%). In 6/11 LCNEC Ki-67 PI was ≤40%. OS was longer for Ki-67 ≤40% compared to >40% (17 months (95% CI 11–23 months) vs 5 months (95% CI 0.7–9 months), P = 0.007). Two patients were still alive at follow-up after 86 and 103 months, both had Ki-67 ≤40%. 8/11 patients could be subclassified, and both SCLC-like (n = 6) and NSCLC-like (n = 2) subtypes were present. No MEN1 mutation was found.
Conclusion
Stage IV LCNEC with a solitary brain metastasis and N0/N1 disease show in the majority of cases Ki-67 PI ≤40% and prolonged survival, distinguishing them from general LCNEC. This unique subgroup can be both of the SCLC-like and NSCLC-like subtype.
Keywords: large cell neuroendocrine carcinoma, LCNEC, solitary brain metastasis, prognosis, Ki-67
Introduction
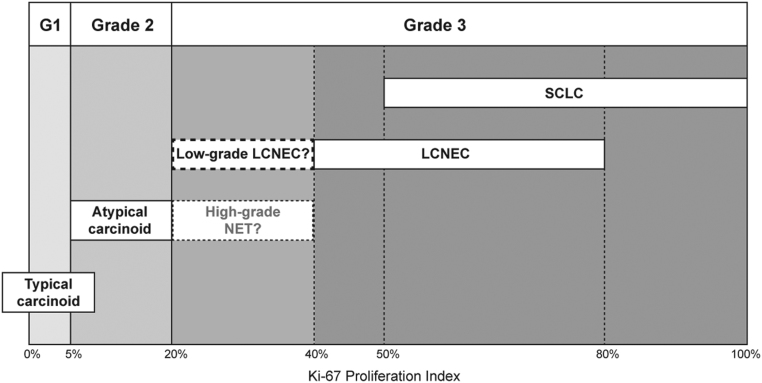
Neuroendocrine neoplasms can originate in various organ systems and are subdivided in neuroendocrine tumors (NET) and neuroendocrine carcinoma (NEC) (1). The most common NEC is small-cell lung carcinoma (SCLC), followed by pulmonary large cell neuroendocrine carcinoma (LCNEC) (2). Although LCNEC is the second most frequent NEC, it represents only 1–3% of all types of lung cancer (3, 4). Generally, stage IV LCNEC presents with extensive metastatic disease and poor survival rates (<10 months), comparable to SCLC (3, 5). Furthermore, Ki-67 proliferation index (PI) of LCNEC is approximately in the same range as SCLC (40–80%), whereas the PI is distinctly lower in well-differentiated neuroendocrine tumors such as typical and atypical carcinoid (0–20%) (Fig. 1) (6). Based on mutational analysis, LCNEC can be separated into two main molecular subtypes: the first with mutations in TP53/RB1 (a hallmark of SCLC), the other with mutations in TP53/STK11 and/or KEAP1 genes and retained pRB protein expression (non-small-cell lung carcinoma (NSCLC)-like) (7, 8). In addition, a LCNEC subtype with lower Ki-67 PI was identified having a MEN1 mutation and, more recently, a study showed overlapping molecular alterations between atypical carcinoid and LCNEC for TP53, RB1 and MEN1 (7, 9).
Figure 1.
Ki-67 proliferation indices (PIs) in the spectrum of pulmonary neuroendocrine neoplasms. Carcinoids have a Ki-67 PI ≤20%, whereas LCNEC and SCLC generally have a Ki-67 PI >40%. The group with Ki-67 PI >20% and ≤40% might be considered an intermediate NEN group, including high-grade NET and/or low-grade LCNEC, not specified in current WHO criteria. The majority of LCNEC patients with solitary brain metastases have a Ki-67 PI in this category. G1, grade 1; LCNEC, large cell neuroendocrine carcinoma; NET, neuroendocrine tumor; SCLC, small-cell lung carcinoma.
In contrast to these high grade neuroendocrine carcinomas, a subgroup of NSCLC presents with a solitary metastasis, limited to the brain. This subgroup comprises 7% of NSCLC and shows prolonged survival compared to NSCLC with extensive metastatic disease (10). According to current guidelines, local radical treatment of the lesions may be considered in patients with solitary brain metastases and a good performance score (11).
In this study, we present a unique subgroup of 11 stage IV LCNEC patients harboring a synchronous solitary brain metastasis as only metastatic site. We hypothesized that those tumors had a lower KI-67 PI than general LCNEC and that those tumors were of the NSCLC-like molecular subtype. Therefore, tumors were evaluated for Ki-67 PI, pRB expression and gene mutations.
Methods
We identified 10 stage IV LCNEC patients who underwent surgical resection of synchronous solitary brain metastases by screening of pathological reports, making use of the nationwide network and registry of histo- and cytopathology in the Netherlands (PALGA, 2003-2012) (12, 13). Furthermore, we identified one additional LCNEC patient treated in our own hospital with lobectomy and stereotactic radiotherapy targeting his solitary brain metastasis (2015). Clinical characteristics and survival data were retrieved from medical records.
All histological samples were centrally reviewed to confirm LCNEC diagnosis according to the criteria described in the World Health Organization (WHO) classification of lung tumors, 2015 (14). Immunohistochemistry (IHC) was performed with antibodies against Ki-67 (MIB-1) and pRB (13A10) as described earlier (13). Ki-67 PI was assessed semi-quantitatively by an experienced pulmonary pathologist (LH) as is done in usual care in our center (15). Targeted next-generation sequencing (NGS) for TP53, RB1, STK11 and KEAP1 was performed on tumor tissue from available formalin-fixed paraffin-embedded (FFPE) blocks of the primary tumor and/or the brain metastasis (13). In addition mutational analysis for MEN1 was performed by NGS (13).
Median overall survival (OS) was evaluated by Kaplan–Meier analysis and differences in survival between low and high Ki-67 PI (arbitrary threshold ≤40 vs >40%) were tested for significance with log-rank test. P < 0.05 was considered significant.
The study protocol was approved by the Medical Ethical Committee of the Maastricht University Medical Centre (METC azM/UM 14-4-043). The study is performed according to the Dutch ‘Federa, Human Tissue and Medical Research: Code of conduct for responsible use (2011)’ regulations not requiring patients’ informed consent.
Results
Eleven LCNEC patients with a synchronous solitary brain metastasis were included in the analysis (Table 1). Mean age at diagnosis was 59 years (range 34–72), 9/11 patients were male. For five patients, smoking history was available and mean packyears exceeded 40 years. Seven out of 11 patients had N0 disease, the other four patients had N1 disease (4/11). Nine out of 11 patients were treated with definitive therapy. Seven of those patients underwent lobectomy/pneumonectomy and surgical resection of the brain metastasis with all resection margins histopathologically free of tumor cells (Table 1: patients A-E, J and K). Of the other two patients with definitive therapy, one underwent metastasectomy and stereotactic radiotherapy + chemotherapy for the primary tumor (G). The other one underwent a lobectomy and stereotactic radiotherapy for his metastasis (F).
Table 1.
Characteristics of 11 LCNEC patients with solitary brain metastases.
| Gender | Age | Smoking (PY) | TNM | Initial treatment | Recurrence | Treatment recurrence | OS (months) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| T | N | PFS (months) | Location | |||||||
| SCLC-likea | ||||||||||
| A | Male | 70 | N/a | 2 | 1 | Lobectomy + metastasectomy + WBRT | 2 | Liver | No treatment | 3 |
| B | Male | 59 | >40 | 1a | 1 | Lobectomy + metastasectomy | 5 | Brain, liver | RTx 30Gy | 7 |
| C | Male | 71 | 50 | 4 | 0 | Lobectomy + metastasectomy + neo-adjuvant chemotherapy (cis-eto 1x) | 5 | Thorax, adrenal gland, liver | Eto-carbo | 12 |
| D | Male | 49 | N/a | 1 | 0 | Lobectomy + metastasectomy + WBRT | 12 | At least brain | Resection | 18 |
| E | Male | 55 | >40 | 1a | 0 | Lobectomy + metastasectomy + chemotherapy (cis-pem, PD) | 4 | Brain | SRT + WBRT | 12 |
| F | Male | 60 | 50 | 2a | 0 | Lobectomy + SRT brain | 12 | Liver, brain | – | 17 |
| NSCLC-likea | ||||||||||
| G | Female | 34 | N/a | 1 | 0 | Metastasectomy + SRT lung + chemotherapy (gem-cis, 4x, PR) | – | – | – | >86 |
| H | Male | 63 | N/a | 1b | 1 | Metastasectomy + SRT brain | Unknown | – | – | 13 |
| Indefinitea | ||||||||||
| I | Male | 72 | N/a | 1b | 1 | Metastasectomy | Unknown | – | – | 5 |
| J | Female | 60 | >25 | 2a | 0 | Lobectomy + metastasectomy | 3 | Liver | Cis-gem | 3 |
| K | Male | 58 | N/a | 2 | 0 | Lobectomy + metastasectomy + chemotherapy | 51 | Lung | Resection | >103 |
aSCLC-like: RB1 mutation and/or no pRB expression. NSCLC-like: RB1 wildtype and retained pRB expression. Indefinite: no classification could be made on basis of immunohistochemistry and mutational results.
carbo, carboplatin; cis, cisplatin; eto, etoposide; gem, gemcitabine; N/a, not available; OS, overall survival; PD, progressive disease; pem, pemetrexed; PFS, progression-free survival; PR, partial response; PY, packyears; RTx, radiotherapy; SRT, stereotactic radiotherapy; WBRT, whole brain radiotherapy.
Mean Ki-67 PI was 59% (range 15–100%, Table 2). In 6/11 LCNEC Ki-67 PI was ≤40%. Both tumors with a low Ki-67 PI of 15% were diagnosed as LCNEC because of the presence of necrosis and a mitotic index of 14 and >30 per 10 high power fields, respectively (patients F and H). The patients had a median OS of 12 months (95% confidence Interval (CI) 5.5–18.5 months). A significant prolonged OS was seen in patients with a Ki-67 PI ≤40% compared to >40% (17 months (95% CI 11.0–23.0 months) vs 5 months (95% CI 0.7–9.3 months), P = 0.007; Fig. 2). Two patients were still alive after 5 years, a remarkable longer time than average in stage IV LCNEC patients (Tables 1 and 2: patients G and K). A male patient of 58 years with T2N0 disease who underwent lobectomy and metastasectomy (largest tumor part 25 × 20 × 20 mm, Ki-67 PI 30%), had pulmonary recurrence after 51 months but was still alive at follow-up after 103 months. A woman of 34 years with T1N0 disease underwent a metastasectomy (two parts of tumor tissue, cross sections 8 mm and 22 mm, Ki-67 PI 40%) and was treated with chemotherapy and radiotherapy for the primary tumor. She was still alive after 86 months of follow-up, without recurrence of disease.
Table 2.
Mutational and immunohistochemical characteristics of 11 LCNEC patients with solitary brain metastases.
| OS (months) | Immunohistochemistry | Mutational status | |||||
|---|---|---|---|---|---|---|---|
| Ki-67 PI prim | Ki-67 PI meta | pRB prim | pRB meta | Primary | Metastasis | ||
| SCLC-likea | |||||||
| A | 3 | N/a | 90% | neg | neg | TP53/RB1 | TP53/RB1 |
| B | 7 | 40% | 40% | neg | neg | RB1 | RB1 |
| C | 12 | 90% | N/a | neg | neg | TP53/RB1 | TP53/RB1 |
| D | 18 | N/a | 30% | neg | neg | TP53/RB1 | TP53/RB1 |
| E | 12 | 90% | 80% | neg | N/a | TP53 | TP53 |
| F | 17 | 15% | N/a | neg | N/a | TP53 | N/a |
| NSCLC-likea | |||||||
| G | >86 | N/a | 40% | N/a | pos | N/a | TP53/STK11/KEAP1 |
| H | 13 | N/a | 15% | N/a | pos | N/a | TP53 |
| Indefinitea | |||||||
| I | 5 | N/a | 100% | N/a | pos | N/a | TP53/RB1 |
| J | 3 | 90% | 70% | neg | neg | KEAP1 | KEAP1 |
| K | >103 | N/a | 30% | N/a | neg | TP53/KEAP1 | TP53 (different)/RB1/KEAP1/STK11 |
aSCLC-like: RB1 mutation and/or no pRB expression. NSCLC-like: RB1 wildtype and retained pRB expression. Indefinite: no classification could be made on basis of immunohistochemistry and mutational results.
Ki-67 PI, Ki-67 proliferation index; meta, metastatic lesion; N/a, not available; neg, negative; OS, overall survival; pos, positive; prim, primary tumor.
Figure 2.
(A) Overall survival of LCNEC patients with solitary brain metastases (censored at 36 months). (B) Overall survival of LCNEC patients with solitary brain metastases, exhibiting a Ki-67 proliferation index ≤40% or >40% in the primary tumor and/or metastasis (censored at 36 months).
Tissue material of all patients was examined with IHC and NGS (Table 2 and Supplementary Table 1, see section on supplementary data given at the end of this article). In seven patients samples from both primary tumors and brain lesions were available. Four LCNEC patients (A-D) had a RB1 (and TP53) mutation with loss of pRB protein expression in IHC analysis, classifying as SCLC-like subtype. Two LCNEC patients (E and F) had a TP53 mutation in combination with loss of pRB expression and were therefore also regarded as SCLC subtype. Absence of RB1 mutation and retained pRB expression was observed in two LCNEC patients (G and H), classifying them as NSCLC-like subtype. Both NSCLC-like tumors had low Ki-67 PI (40 and 15%, respectively). One LCNEC (I) had a RB1 mutation, but retained pRB expression. Another tumor (J) was RB1 wildtype and had a KEAP1 mutation, but showed also loss of pRB expression. The last LCNEC (K) had KEAP1 and TP53 mutations in the primary tumor (no pRB available) and additional STK11 and RB1 mutations as well as a different TP53 mutation in the metastatic lesion. Therefore, those last three tumors could not definitely be classified as SCLC-like or NSCLC-like LCNEC. No MEN1 mutations were identified in the LCNEC cases.
Discussion
We here present the clinical and molecular features of a unique Dutch multicenter cohort of 11 LCNEC patients with synchronous solitary brain metastases. Whereas the majority of stage IV LCNEC patients endure an aggressive disease, this subgroup presents with limited disease and a relatively low Ki-67 PI. Stage IV LCNEC thus is a heterogeneous disease.
In this series, OS was 12 months and two long-term survivors (>5 years) were observed. On the contrary, stage IV LCNEC generally presents as disseminated disease with limited survival time (3, 5). So far, only few series including oligometastatic LCNEC patients have been reported, and this is the first series describing solely LCNEC patients with solitary brain metastases (16, 17). Furthermore, only a minority of patients with stage IV LCNEC present with N0/N1 disease. In our recent study, 27% of patients had N0/N1 disease (extracted from (13)). Remarkably, in this series of patients with solitary metastases, 64% of patients have N0 disease and 36% N1 disease.
The prolonged survival of patients in this study with a Ki-67 PI ≤40% suggests that Ki-67 PI might be used as a prognostic factor in LCNEC patients with solitary brain metastases. A prognostic role for Ki-67 PI has already been shown in pulmonary neuroendocrine neoplasms, specifically separating favorable subgroups with Ki-67 PI <25 vs ≥25% (18). The current WHO guideline for lung cancer does not include Ki-67 PI for classification of neuroendocrine neoplasms (14). However, Ki-67 PI has been shown to be ≤20% for pulmonary NET and >40% for NEC (6). Although the mean value of Ki-67 PI in this study was 59% and therefore falls within the NEC category, the majority of the patients had a Ki-67 PI ≤40%. This implicates that a subgroup of neuroendocrine neoplasms with a Ki-67 PI >20% but ≤40% does exist (Fig. 1). This subgroup might comprise high-grade NET, which has been recently described in several studies although not recognized in current WHO classification (19, 20, 21). However, in those series, the tumors had a carcinoid morphology and absence of TP53 and RB1 mutations. In contrast, in our study all patients had LCNEC morphology and all exhibited TP53 and/or RB1 mutations or loss of pRB expression but no MEN1 mutations. Therefore, the patients in this study more likely comprise low-grade LCNEC with a Ki-67 PI >20 but ≤40% (Fig. 1).
Since the solitary metastatic state is clinically more comparable to NSCLC than to SCLC, we hypothesized that most LCNEC patients with solitary metastases would be of the NSCLC-like subtype. However, six patients were classified as SCLC-like and only two as NSCLC-like. The remaining three patients could not definitively be subclassified. Interestingly, mutations were identical in six out of seven patients with available samples of both primary tumor and metastatic lesion. This suggests that mutation of TP53, RB1 and/or STK11/KEAP1 occurs prior to tumor cell dissemination in LCNEC (8). In one patient, a TP53 and KEAP1 mutation was found in the primary and metastatic lesion, whereas another TP53 and additional RB1 and STK11 mutations were also found in the metastasis. This suggests that primary and metastatic lesions of this patient were clonally related and additional mutations in the metastasis probably developed later in tumorigenesis. Mutational characteristics have not been reported before for LCNEC patients with solitary brain metastases or oligometastatic disease.
Nine of 11 patients in this series were treated with definitive therapy (resection or stereotactic radiotherapy) for both primary and metastatic lesions, instead of standard treatment for stage IV LCNEC with palliative chemotherapy. Retrospective studies in NSCLC with solitary brain metastases have shown extended OS in patients treated with definitive therapy for primary and metastatic tumors (22). No data regarding definitive therapy is available for solitary metastases in SCLC and LCNEC. However, limited data on this subject is available for oligometastatic SCLC and LCNEC, revealing prolonged OS after definitive therapy (17, 23). Since retrospective datasets are prone to confounding by indication, prospective randomized trials are necessary to confirm the effect of definitive local treatment.
Conclusion
We present 11 LCNEC patients with a solitary brain metastasis and relatively low Ki-67 PI in the majority of the patients. Although presence of solitary brain metastases resembles NSCLC more than SCLC, presence of a solitary metastasis was not restricted to NSCLC-like LCNEC. Our data indicate that stage IV LCNEC is a heterogeneous disease, not justifying standard treatment with palliative chemotherapy in all patients. Instead, in those patients a curative treatment strategy for primary and metastatic lesions might be considered to improve OS, especially in LCNEC with relatively low Ki-67 PI.
Supplementary Material
Declaration of interest
B C M H reports grants from Bristol-Myers Squibb, non-financial support from Abbvie, outside the submitted work. J L D reports personal fees from BMS, personal fees from Pfizer, personal fees from Boehringer-Ingelheim, personal fees from Novartis, personal fees from Ipsen, outside the submitted work. E J M S reports grants from Bristol-Myers Squibb, Astra Zeneca, Pfizer, Novartis and MSD, personal fees from AbbVie and Roche, non-financial support from Abbvie, outside the submitted work. A-M C D reports grants from Bristol-Myers Squibb, personal fees from Roche, BMS, Eli Lily, Takeda and Boehringer Ingelheim, non-financial support from Abbvie, outside the submitted work. The other authors have nothing to disclose.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Statement of ethics
This study is performed in accordance with the World Medical Association Declaration of Helsinki and the Dutch ‘Federa, Human Tissue and Medical Research: Code of conduct for responsible use (2011)’ regulations not requiring patients’ informed consent.
Author contribution statement
E J M Speel and A-M C Dingemans contributed equally to this work.
References
- 1.Rindi G, Klimstra DS, Abedi-Ardekani B, Asa SL, Bosman FT, Brambilla E, Busam KJ, de Krijger RR, Dietel M, El-Naggar AK, et al. A common classification framework for neuroendocrine neoplasms: an International Agency for Research on Cancer (IARC) and World Health Organization (WHO) expert consensus proposal. Modern Pathology 2018. 1770–1786. ( 10.1038/s41379-018-0110-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korse CM, Taal BG, van Velthuysen ML, Visser O. Incidence and survival of neuroendocrine tumours in the Netherlands according to histological grade: experience of two decades of cancer registry. European Journal of Cancer 2013. 1975–1983. ( 10.1016/j.ejca.2012.12.022) [DOI] [PubMed] [Google Scholar]
- 3.Derks JL, Hendriks LE, Buikhuisen WA, Groen HJ, Thunnissen E, van Suylen RJ, Houben R, Damhuis RA, Speel EJ, Dingemans AM. Clinical features of large cell neuroendocrine carcinoma: a population-based overview. European Respiratory Journal 2016. 615–624. ( 10.1183/13993003.00618-2015) [DOI] [PubMed] [Google Scholar]
- 4.Takei H, Asamura H, Maeshima A, Suzuki K, Kondo H, Niki T, Yamada T, Tsuchiya R, Matsuno Y. Large cell neuroendocrine carcinoma of the lung: a clinicopathologic study of eighty-seven cases. Journal of Thoracic and Cardiovascular Surgery 2002. 285–292. ( 10.1067/mtc.2002.122523) [DOI] [PubMed] [Google Scholar]
- 5.Asamura H, Kameya T, Matsuno Y, Noguchi M, Tada H, Ishikawa Y, Yokose T, Jiang SX, Inoue T, Nakagawa K, et al. Neuroendocrine neoplasms of the lung: a prognostic spectrum. Journal of Clinical Oncology 2006. 70–76. ( 10.1200/JCO.2005.04.1202) [DOI] [PubMed] [Google Scholar]
- 6.Pelosi G, Rindi G, Travis WD, Papotti M. Ki-67 antigen in lung neuroendocrine tumors: unraveling a role in clinical practice. Journal of Thoracic Oncology 2014. 273–284. ( 10.1097/JTO.0000000000000092) [DOI] [PubMed] [Google Scholar]
- 7.Rekhtman N, Pietanza MC, Hellmann MD, Naidoo J, Arora A, Won H, Halpenny DF, Wang H, Tian SK, Litvak AM, et al. Next-generation sequencing of pulmonary large cell neuroendocrine carcinoma reveals small cell carcinoma-like and non-small cell carcinoma-like subsets. Clinical Cancer Research 2016. 3618–3629. ( 10.1158/1078-0432.CCR-15-2946) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George J, Walter V, Peifer M, Alexandrov LB, Seidel D, Leenders F, Maas L, Muller C, Dahmen I, Delhomme TM, et al. Integrative genomic profiling of large-cell neuroendocrine carcinomas reveals distinct subtypes of high-grade neuroendocrine lung tumors. Nature Communications 2018. 1048 ( 10.1038/s41467-018-03099-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simbolo M, Barbi S, Fassan M, Mafficini A, Ali G, Vicentini C, Sperandio N, Corbo V, Rusev B, Mastracci L, et al. Gene expression profiling of lung atypical carcinoids and large cell neuroendocrine carcinomas identifies three transcriptomic subtypes with specific genomic alterations. Journal of Thoracic Oncology 2019. 1651–1661. ( 10.1016/j.jtho.2019.05.003) [DOI] [PubMed] [Google Scholar]
- 10.Torok JA, Gu L, Tandberg DJ, Wang X, Harpole DH, Jr, Kelsey CR, Salama JK. Patterns of distant metastases after surgical management of non-small-cell lung cancer. Clinical Lung Cancer 2017. e57–e70. ( 10.1016/j.cllc.2016.06.011) [DOI] [PubMed] [Google Scholar]
- 11.Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, Mok TS, Reck M, Van Schil PE, Hellmann MD, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology 2018. iv192–iv237. ( 10.1093/annonc/mdy275) [DOI] [PubMed] [Google Scholar]
- 12.Casparie M, Tiebosch AT, Burger G, Blauwgeers H, van de Pol A, van Krieken JH, Meijer GA. Pathology databanking and biobanking in the Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cellular Oncology 2007. 19–24. ( 10.1155/2007/971816) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Derks JL, Leblay N, Thunnissen E, van Suylen RJ, den Bakker M, Groen HJM, Smit EF, Damhuis R, van den Broek EC, Charbrier A, et al. Molecular subtypes of pulmonary large-cell neuroendocrine carcinoma predict chemotherapy treatment outcome. Clinical Cancer Research 2018. 33–42. ( 10.1158/1078-0432.CCR-17-1921) [DOI] [PubMed] [Google Scholar]
- 14.Travis WD, Brambilla E, Burke AP, Markx A, Nicholson AG. WHO Classification of Tumours of the Lung, Pleura, Thymus and Heart, 4th ed. Lyon, France: International Agency for Research on Cancer, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Warth A, Fink L, Fisseler-Eckhoff A, Jonigk D, Keller M, Ott G, Rieker RJ, Sinn P, Soder S, Soltermann A, et al. Interobserver agreement of proliferation index (Ki-67) outperforms mitotic count in pulmonary carcinoids. Virchows Archiv 2013. 507–513. ( 10.1007/s00428-013-1408-2) [DOI] [PubMed] [Google Scholar]
- 16.Kotecha R, Zimmerman A, Murphy ES, Ahmed Z, Ahluwalia MS, Suh JH, Reddy CA, Angelov L, Vogelbaum MA, Barnett GH, et al. Management of brain metastasis in patients with pulmonary neuroendocrine carcinomas. Technology in Cancer Research and Treatment 2016. 566–572. ( 10.1177/1533034615589033) [DOI] [PubMed] [Google Scholar]
- 17.Naidoo J, Santos-Zabala ML, Iyriboz T, Woo KM, Sima CS, Fiore JJ, Kris MG, Riely GJ, Lito P, Iqbal A, et al. Large cell neuroendocrine carcinoma of the lung: clinico-pathologic features, treatment, and outcomes. Clinical Lung Cancer 2016. e121–e129. ( 10.1016/j.cllc.2016.01.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rindi G, Klersy C, Inzani F, Fellegara G, Ampollini L, Ardizzoni A, Campanini N, Carbognani P, De Pas TM, Galetta D, et al. Grading the neuroendocrine tumors of the lung: an evidence-based proposal. Endocrine-Related Cancer 2014. 1–16. ( 10.1530/ERC-13-0246) [DOI] [PubMed] [Google Scholar]
- 19.Rekhtman N, Desmeules P, Litvak AM, Pietanza MC, Santos-Zabala ML, Ni A, Montecalvo J, Chang JC, Beras A, Preeshagul IR, et al. Stage IV lung carcinoids: spectrum and evolution of proliferation rate, focusing on variants with elevated proliferation indices. Modern Pathology 2019. 1106–1122. ( 10.1038/s41379-019-0248-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quinn AM, Chaturvedi A, Nonaka D. High-grade neuroendocrine carcinoma of the lung with carcinoid morphology: a study of 12 cases. American Journal of Surgical Pathology 2017. 263–270. ( 10.1097/PAS.0000000000000767) [DOI] [PubMed] [Google Scholar]
- 21.Vivero M, Scholl LM. ‘Borderline’ neuroendocrine carcinomas of the lung are clinically and genomically distinct from large cell neuroendocrine carcinoma. Modern Pathology 2016. . [Google Scholar]
- 22.Li D, Zhu X, Wang H, Qiu M, Li N. Should aggressive thoracic therapy be performed in patients with synchronous oligometastatic non-small cell lung cancer? A meta-analysis. Journal of Thoracic Disease 2017. 310–317. ( 10.21037/jtd.2017.02.21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu LM, Cheng C, Kang M, Luo J, Gong LL, Pang QS, Wang J, Yuan ZY, Zhao LJ, Wang P. Thoracic radiotherapy (TRT) improved survival in both oligo- and polymetastatic extensive stage small cell lung cancer. Scientific Reports 2017. 9255 ( 10.1038/s41598-017-09775-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a