Abstract
Collection of antimicrobial peptides (CAMP), CAMPSign, and ClassAMP are open‐access resources that have been developed to enhance research on antimicrobial peptides (AMPs). Comprehensive information on AMPs and machine learning‐based predictive models are made available for users through these resources. As of date, CAMPR3 has 10,247 sequences, 757 structures, and 114 family‐specific signatures of AMPs along with associated tools for AMP sequence and structure analysis. CAMPSign uses family‐specific sequence conservation, in the form of patterns and hidden Markov models for identification of AMPs. ClassAMP can be used to classify AMPs as antibacterial, antifungal, or antiviral based on sequence information. Here we describe CAMP and its derivatives and illustrate, with a few examples, the contribution of these online resources to the advancement of our current understanding of AMPs.
Keywords: antimicrobial peptides, CAMP database, CAMPSign, ClassAMP, family‐specific signatures, machine learning
1. INTRODUCTION
Antimicrobial peptides (AMPs) are multifaceted host defense molecules that are produced by organisms ranging from microbes to mammals.1 AMPs kill microbes via pleiotropic mechanisms of action, such as destruction of the microbial membrane and inhibition of macromolecule synthesis.2, 3, 4 These molecules exhibit broad range antimicrobial activity, rapid killing kinetics, reduced toxicity, and reduced microbial resistance. Apart from their antimicrobial activity, a few AMPs also regulate physiological functions such as inflammation, angiogenesis, and wound healing.5
Identification of AMPs from natural sources in the 1980's stimulated research on their isolation and characterization. Further, the threat posed by antibiotic resistance accelerated research on AMPs. This resulted in a rapid increase in the number of identified AMPs, which in turn demanded efficient AMP data registration, organization, and retrieval methods. Responding to this need, a few databases on AMPs were developed.6, 7, 8, 9, 10, 11, 12, 13, 14 However, these databases were limited to AMPs from a specific source, that is, either natural or synthetic, or from a particular source organism. To address this issue, we developed the collection of antimicrobial peptides (CAMP) database15 in the year 2010. The manually curated sequence information of AMPs was further used to develop tools for AMP prediction and annotation. The databases and tools, that were developed by our group and updated over time, are discussed below.
2. CAMP DATABASE
2.1. CAMPR1
CAMP was an open‐access resource that provided information on sequences of natural as well as synthetic AMPs on a single platform. AMP sequence information obtained from the publicly available National Center for Biotechnology Information (NCBI) database was systematically categorized as (a) experimentally validated, (b) predicted, and (c) patents based on the reference literature. Furthermore, information on the target organism, MIC values and hemolytic activity was manually annotated from literature. It was the first AMP database to have information on patented AMPs. The database also hosted machine learning (ML) based algorithms like random forests (RF), support vector machines (SVM), and discriminant analysis (DA) for AMP prediction.
2.2. CAMPR2
CAMP was expanded in 2014 to include information on structures of AMPs.16 An additional feature of CAMPR2 was the introduction of family information for sequences present in the database. AMPs belong to diverse families such as cathelicidins, defensins, temporins, and so forth, having characteristic sequence composition. The database had manually annotated information on 53 AMP families. This information was meticulously sourced from (a) UniProtKB,17 (b) protein family databases such as Pfam,18 InterPro,19 and (c) literature databases such as PubMed. The database was updated with newly identified AMPs and the prediction algorithms were retrained using the updated sequence information.
2.3. CAMPR3
The inclusion of AMP family‐specific signatures represented by patterns and hidden Markov models (HMMs) and the AMPs retrieved from online databases using these signatures, mainly constituted the third update of CAMP. Users can access CAMPR3 20 for information such as family signatures, sequences, structures, activity profile, source, target organisms, hemolytic activity and links to external databases such as UniProtKB, PDB, PubMed, and NCBI Taxonomy for AMPs from eukaryotic and prokaryotic sources. Presently, CAMPR3 holds information on 10,247 sequences, 757 structures, and 114 family‐specific signatures of AMPs along with associated tools for AMP analysis. Thus, CAMP evolved from a simple repository of AMP sequences to a comprehensive database containing sequences, structures, and family signatures along with associated tools for AMP analysis. The evolution of the CAMP database from its inception to the present state is described in Table 1.
Table 1.
Updates of the CAMP database
| Database | Primary structure | 3D structure | Family description | Patterns and HMMs |
|---|---|---|---|---|
| CAMP (2010) | 4,020 | – | – | – |
| CAMPR2 (2014) | 6,756 | 682 | 3,111 (53 families) | – |
| CAMPR3 (2016) | 10,247 | 757 | 5,241 (53 families) | 114 |
Abbreviations: CAMP, collection of antimicrobial peptides; HMMs, hidden Markov models.
3. ONLINE WEBSERVERS FOR ANALYSIS OF AMPs
3.1. CAMPSign
AMPs belong to diverse families with conserved sequence composition and this can be leveraged to efficiently identify/predict AMPs from a large pool of sequences. CAMPSign is an open‐access webserver that aids in identification of AMPs and their families using family‐specific signatures represented by patterns and HMMs.21 CAMPSign, presently can predict members of 45 AMP families.
3.2. ClassAMP
While few AMPs exhibit broad‐spectrum activity, many of them are target‐specific. ClassAMP is an online prediction tool for classification of peptides as antibacterial, antifungal and/or antiviral using sequence‐based features.22 It employs ML algorithms such as SVM and RF for classification.
4. RESOURCES AVAILABLE AT CAMP AND ITS DERIVATIVES
4.1. Database search
The CAMP database can be searched to retrieve sequences, structures and signatures of AMPs. The database provides basic and advanced search options. Basic search feature enables keyword‐based search for all fields or restricted to a specific field. The advanced search has a query builder by which users can combine multiple queries using logical AND or OR operators. Users can search the database using AMP name, sequence, source organism, target organism, activity, and so forth. Users can also query for AMP family members and retrieve family signatures in the form of patterns and HMMs.
4.2. Data analysis tools available through CAMP
4.2.1. AMP prediction
Users can input sequences and obtain a variety of information including the following: (a) Predict if the sequence/s is/are antimicrobial; (b) Predict whether antimicrobial regions are present within a protein; (c) Rationally design single‐residue mutants and predict the effect of substitutions on antimicrobial activity. The outcome of the prediction analysis is in the form of probability scores (Figure 1). The higher the score (max = 1), the greater is the likelihood of the sequence having antimicrobial activity. The prediction tools aid in the identification and rational design of novel AMPs.
Figure 1.
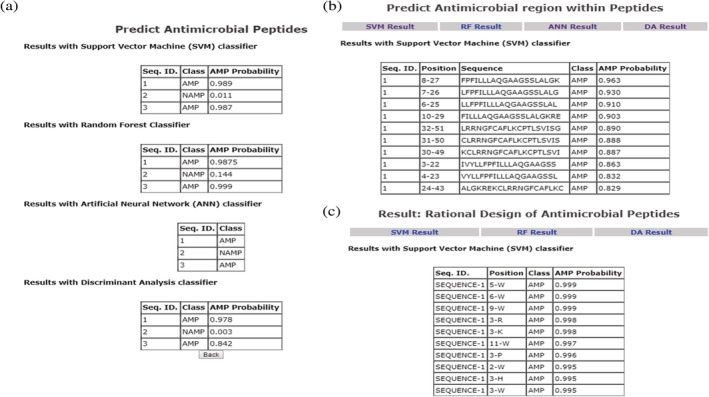
Snapshots of analysis reports from prediction modules available in CAMP. (a) Prediction of antimicrobial peptides based on various ML classifiers. (b) Prediction of antimicrobial regions within the input sequence. (c) Prediction of single‐residue mutants with enhanced antimicrobial activity. A probability threshold of 0.5 was heuristically set to classify peptide as being antimicrobial. CAMP, collection of antimicrobial peptides; ML, machine learning
4.2.2. Basic local alignment search tool
Users can query peptides of interest and their homologs in the entire CAMP database or restricted to various subdatasets of CAMP, for example, structure, patent, experimentally validated, predicted, and predicted based on signature data sets using the basic local alignment search tool (BLAST) tool23 available in CAMP.
4.2.3. Links to third‐party analysis tools
The CAMP database provides access to third‐party tools for sequence and structure analysis such as Clustal Omega,24 Vector Alignment Search Tool (VAST),25 PRATT,26 ScanProsite,27 PHI‐BLAST,28 and jackhmmer29 for increasing the data analysis options available to users.
4.3. Predict AMPs based on family signatures
The CAMPSign webserver can be used to predict AMPs based on family‐specific sequence conservation. Users can scan the sequence of interest against all or specific AMP family signatures comprising of patterns and HMMs. Results are generated in a tabular format and have information on the AMP family and number of patterns and HMMs that match user‐defined sequence/s. Users can obtain a detailed view of the patterns and HMMs that match/align to the sequence through the hyperlinks in the results page (Figure 2).
Figure 2.

Screenshot and description of the analysis report of CAMPSign
4.4. Predict AMPs based on target organisms
The ClassAMP webserver can be used to predict the propensity of a peptide to have antibacterial, antifungal, or antiviral properties based on sequence features trained using SVM or RF based algorithms. The sequences are predicted as antibacterial, antifungal, or antiviral and a probability score (0–1) is provided. The higher the probability score, the higher the likelihood of correct classification.
5. CONTRIBUTION OF CAMP, CAMPSIGN, AND CLASSAMP TO RESEARCH ON AMPs
5.1. Creation of other AMP databases
The data present in CAMP was used to create other AMP databases like ADAM—a database of AMPs,30 InverPep—a database of invertebrate AMPs,31 YADAMP—yet another database of AMPs,32 LAMP—a database linking AMPs,33 C‐PAmP—a database containing computationally predicted AMPs from plants,34 Hemolytik—a database of experimentally determined hemolytic and nonhemolytic peptides,35 dbAMP—a resource for exploring AMPs with functional activities and physicochemical properties on transcriptome and proteome data,36 and ANTISTAPHYBASE—a database of AMPs and essential oils against methicillin‐resistant Staphylococcus aureus (MRSA) and Staphylococcus aureus.37
5.2. Creation of prediction algorithms on antimicrobial activity
The data present in CAMP have been used either for training or testing algorithms developed for AMP prediction and/or classification.38, 39, 40, 41, 42, 43, 44, 45
5.3. Identification of peptides from natural sources
The AMP prediction algorithm, available through CAMP, has been successfully used to identify/predict peptides with antimicrobial activity from natural sources such as Protaetia brevitarsis larvae,46 Sichuan pepper,47 Litopenaeus vannamei,48 milk proteins,49 human sweat,50 Thermophilic geobacillus sp. Strain ZGt‐1,51 Varanus komodoensis (Komodo Dragon),52 American alligator plasma,53 human basal tear sample,54 Oxya chinensis sinuosa (grasshopper),55 lily leaves,56 Chrysochromulina tobin,57 and marine mussels.58 Few of these peptides have been experimentally validated using wet‐lab methods.
5.4. Rational design of AMPs
Using CAMP data, Joker,59 an algorithm was developed that aids in rational design of AMPs. CAMP with BLAST tool has been widely used to identify AMP sequences homologous to the user‐defined sequences.60, 61, 62, 63, 64, 65, 66 SVM model of CAMP was used for prediction of 10,000 double mutants of Bactenecin 2A and subsequently 17 peptides were shortlisted for experimental validation.67
5.5. Relatedness of novel peptides to AMP families
The novelty of an antiviral peptide identified from the Asian medicinal plant Acacia catechu was evaluated by comparing it with members of the AMP families present in CAMPSign.68 Differentially expressed peptides from serous ovarian cancer tissues were found to exhibit similarity to the members of the aurein AMP family based on sequence analysis using CAMPSign.69
6. CONCLUSIONS
CAMPR3, ClassAMP, and CAMPSign are available online at http://www.camp.bicnirrh.res.in, http://www.bicnirrh.res.in/classamp, and http://www.campsign.bicnirrh.res.in, respectively. Open access to CAMP and its derivatives has accelerated research on AMPs. These resources have improved AMP identification, prediction, and rational design. The citation reports are indicative of the world‐wide usage of these resources (Figure 3). We hope to improve the functionality of these resources, as more data on AMPs are made available in the public domain.
Figure 3.
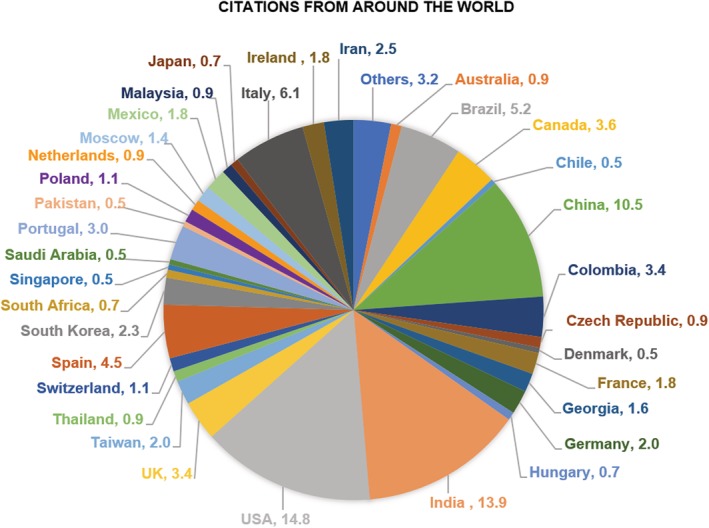
Country‐wise share of citations (in %) of CAMP and its derivatives. Countries with fewer share of citations (<0.5%) were grouped as Others and these included Algeria, Argentina, Belgium, Cuba, Croatia, Ecuador, Finland, Egypt, Uruguay, Tunisia, Sweden, Russia, Norway, and Indonesia. CAMP, collection of antimicrobial peptides
CONFLICT OF INTEREST
None declared.
ACKNOWLEDGMENTS
The authors are grateful to Dr. Smita D. Mahale for all the assistance and support. They acknowledge contributions from all members of the CAMP team for data collection, curation, analysis, and representation. The authors also acknowledge Ms. Ulka Gawde for helping with the figures.
Waghu FH, Idicula‐Thomas S. Collection of antimicrobial peptides database and its derivatives: Applications and beyond. Protein Science. 2020;29:36–42. 10.1002/pro.3714
Funding information Department of Science and Technology, Government of India, Grant/Award Numbers: SB/S3/CE/028/2013, SR/S3/CE/52/2007; Indian Council of Medical Research
REFERENCES
- 1. Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. [DOI] [PubMed] [Google Scholar]
- 2. Haney EF, Petersen AP, Lau CK, Jing W, Storey DG, Vogel HJ. Mechanism of action of puroindoline derived tryptophan‐rich antimicrobial peptides. Biochim Biophys Acta. 2013;1828:1802–1813. [DOI] [PubMed] [Google Scholar]
- 3. Roy RN, Lomakin IB, Gagnon MG, Steitz TA. The mechanism of inhibition of protein synthesis by the proline‐rich peptide oncocin. Nat Struct Mol Biol. 2015;22:466–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang S, Thacker PA, Watford M, Qiao S. Functions of antimicrobial peptides in gut homeostasis. Curr Protein Pept Sci. 2015;16:582–591. [DOI] [PubMed] [Google Scholar]
- 5. Hancock RE, Sahl HG. Antimicrobial and host‐defense peptides as new anti‐infective therapeutic strategies. Nat Biotechnol. 2006;24:1551–1557. [DOI] [PubMed] [Google Scholar]
- 6. Wang Z, Wang G. APD: The antimicrobial peptide database. Nucleic Acids Res. 2004;32:D590–D592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li Y, Chen Z. RAPD: A database of recombinantly‐produced antimicrobial peptides. FEMS Microbiol Lett. 2008;289:126–129. [DOI] [PubMed] [Google Scholar]
- 8. Hammami R, Ben Hamida J, Vergoten G, Fliss I. PhytAMP: A database dedicated to antimicrobial plant peptides. Nucleic Acids Res. 2009;37:D963–D968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hammami R, Zouhir A, Ben Hamida J, Fliss I. BACTIBASE: A web‐accessible database for bacteriocin characterization. BMC Microbiol. 2007;7:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Seebah S, Anita S, Zhuo SW, et al. Defensins knowledgebase: A manually curated database and information source focused on the defensins family of antimicrobial peptides. Nucleic Acids Res. 2006;35:D265–D268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gueguen Y, Garnier J, Robert L, et al. PenBase, the shrimp antimicrobial peptide penaeidin database: Sequence‐based classification and recommended nomenclature. Dev Comp Immunol. 2006;30:283–288. [DOI] [PubMed] [Google Scholar]
- 12. Whitmore L, Wallace BA. The Peptaibol database: A database for sequences and structures of naturally occurring peptaibols. Nucleic Acids Res. 2004;32:D593–D594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wade D, Englund J. Synthetic antibiotic peptides database. Protein Pept Lett. 2002;9:53–57. [DOI] [PubMed] [Google Scholar]
- 14. de Jong A, van Hijum SA, Bijlsma JJ, Kok J, Kuipers OP. BAGEL: A web‐based bacteriocin genome mining tool. Nucleic Acids Res. 2006;34:W273–W279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thomas S, Karnik S, Barai RS, Jayaraman VK, Idicula‐Thomas S. CAMP: A useful resource for research on antimicrobial peptides. Nucleic Acids Res. 2010;38:D774–D780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Waghu FH, Gopi L, Barai RS, Ramteke P, Nizami B, Idicula‐Thomas S. CAMP: Collection of sequences and structures of antimicrobial peptides. Nucleic Acids Res. 2014;42:D1154–D1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. UniProt Consortium . Update on activities at the Universal Protein Resource (UniProt) in 2013. Nucleic Acids Res. 2013;41:D43–D47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Finn RD, Bateman A, Clements J, et al. Pfam: The protein families database. Nucleic Acids Res. 2014;42:D222–D230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mitchell A, Chang HY, Daugherty L, et al. The InterPro protein families database: The classification resource after 15 years. Nucleic Acids Res. 2015;43:D213–D221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Waghu FH, Barai RS, Gurung P, Idicula‐Thomas S. CAMPR3: A database on sequences, structures and signatures of antimicrobial peptides. Nucleic Acids Res. 2016;44:D1094–D1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Waghu FH, Barai RS, Idicula‐Thomas S. Leveraging family‐specific signatures for AMP discovery and high‐throughput annotation. Sci Rep. 2016;6:24684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Joseph S, Karnik S, Nilawe P, Jayaraman VK, Idicula‐Thomas S. ClassAMP: A prediction tool for classification of antimicrobial peptides. IEEE/ACM Trans Comput Biol Bioinform. 2012;9:1535–1538. [DOI] [PubMed] [Google Scholar]
- 23. Altschul SF, Madden TL, Schäffer AA, et al. Gapped BLAST and PSI‐BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sievers F, Wilm A, Dineen D, et al. Fast, scalable generation of high‐quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 2011;7:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gibrat JF, Madej T, Bryant SH. Surprising similarities in structure comparison. Curr Opin Struct Biol. 1996;6:377–385. [DOI] [PubMed] [Google Scholar]
- 26. Jonassen I, Collins JF, Higgins DG. Finding flexible patterns in unaligned protein sequences. Protein Sci. 1995;4:1587–1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Castro E, Sigrist CJ, Gattiker A, et al. ScanProsite: Detection of PROSITE signature matches and ProRule‐associated functional and structural residues in proteins. Nucleic Acids Res. 2006;34:W362–W365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang Z, Schäffer AA, Miller W, et al. Protein sequence similarity searches using patterns as seeds. Nucleic Acids Res. 1998;26:3986–3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Finn RD, Clements J, Eddy SR. HMMER web server: Interactive sequence similarity searching. Nucleic Acids Res. 2011;39:W29–W37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee HT, Lee CC, Yang JR, Lai JZ, Chang KY. A large‐scale structural classification of antimicrobial peptides. Biomed Res Int. 2015;2015:475062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gómez EA, Giraldo P, Orduz S. InverPep: A database of invertebrate antimicrobial peptides. J Glob Antimicrob Resist. 2017;8:13–17. [DOI] [PubMed] [Google Scholar]
- 32. Piotto SP, Sessa L, Concilio S, Iannelli P. YADAMP: Yet another database of antimicrobial peptides. Int J Antimicrob Agents. 2012;39:346–351. [DOI] [PubMed] [Google Scholar]
- 33. Zhao X, Wu H, Lu H, Li G, Huang Q. LAMP: A database linking antimicrobial peptides. PLoS One. 2013;8:e66557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Niarchou A, Alexandridou A, Athanasiadis E, Spyrou G. C‐PAmP: Large scale analysis and database construction containing high scoring computationally predicted antimicrobial peptides for all the available plant species. PLoS One. 2013;8:e79728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gautam A, Chaudhary K, Singh S, et al. Hemolytik: A database of experimentally determined hemolytic and non‐hemolytic peptides. Nucleic Acids Res. 2014;42:D444–D449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jhong JH, Chi YH, Li WC, Lin TH, Huang KY, Lee TY. dbAMP: An integrated resource for exploring antimicrobial peptides with functional activities and physicochemical properties on transcriptome and proteome data. Nucleic Acids Res. 2019;47:D285–D297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zouhir A, Taieb M, Lamine MA, et al. ANTISTAPHYBASE: Database of antimicrobial peptides (AMPs) and essential oils (EOs) against methicillin‐resistant Staphylococcus aureus (MRSA) and Staphylococcus aureus . Arch Microbiol. 2017;199:215–222. [DOI] [PubMed] [Google Scholar]
- 38. Gautam A, Sharma A, Jaiswal S, et al. Development of antimicrobial peptide prediction tool for aquaculture industries. Probiotics Antimicrob Prot. 2016;8:141–149. [DOI] [PubMed] [Google Scholar]
- 39. Sarika IMA, Arora V, Rai A, Kumar D. Species specific approach to the development of web‐based antimicrobial peptides prediction tool for cattle. Comput Electron Agricult. 2015;111:55–61. [Google Scholar]
- 40. Meher PK, Sahu TK, Saini V, Rao AR. Predicting antimicrobial peptides with improved accuracy by incorporating the compositional, physico‐chemical and structural features into Chou's general PseAAC. Sci Rep. 2017;7:42362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vishnepolsky B, Pirtskhalava M. Prediction of linear cationic antimicrobial peptides based on characteristics responsible for their interaction with the membranes. J Chem Inf Model. 2014;54:1512–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Romani AA, Baroni MC, Taddei S, et al. In vitro activity of novel in silico‐developed antimicrobial peptides against a panel of bacterial pathogens. J Pept Sci. 2013;19:554–565. [DOI] [PubMed] [Google Scholar]
- 43. Ng XY, Rosdi BA, Shahrudin S. Prediction of antimicrobial peptides based on sequence alignment and support vector machine‐pairwise algorithm utilizing LZ‐complexity. Biomed Res Int. 2015;2015:212715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Holton TA, Pollastri G, Shields DC, Mooney C. CPPpred: Prediction of cell penetrating peptides. Bioinformatics. 2013;29:3094–3096. [DOI] [PubMed] [Google Scholar]
- 45. Tyagi A, Kapoor P, Kumar R, Chaudhary K, Gautam A, Raghava GP. In silico models for designing and discovering novel anticancer peptides. Sci Rep. 2013;3:2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li Z, Meng M, Li S, Deng B. The transcriptome analysis of Protaetia brevitarsis Lewis larvae. PLoS One. 2019;14:e0214001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hou X, Li S, Luo Q, et al. Discovery and identification of antimicrobial peptides in Sichuan pepper (Zanthoxylum bungeanum Maxim) seeds by peptidomics and bioinformatics. Appl Microbiol Biotechnol. 2019;103:2217–2228. [DOI] [PubMed] [Google Scholar]
- 48. Yang S, Huang H, Wang F, Aweya JJ, Zheng Z, Zhang Y. Prediction and characterization of a novel hemocyanin‐derived antimicrobial peptide from shrimp Litopenaeus vannamei . Amino Acids. 2018;50:995–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dziuba B, Dziuba M. New milk protein‐derived peptides with potential antimicrobial activity: An approach based on bioinformatic studies. Int J Mol Sci. 2014;15:14531–14545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yu Y, Prassas I, Muytjens CM, Diamandis EP. Proteomic and peptidomic analysis of human sweat with emphasis on proteolysis. J Proteomics. 2017;155:40–48. [DOI] [PubMed] [Google Scholar]
- 51. Alkhalili RN, Bernfur K, Dishisha T, et al. Antimicrobial protein candidates from the thermophilic Geobacillus sp. strain ZGt‐1: Production, proteomics, and bioinformatics analysis. Int J Mol Sci. 2016;17:E1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bishop BM, Juba ML, Russo PS, et al. Discovery of novel antimicrobial peptides from Varanus komodoensis (Komodo Dragon) by large‐scale analyses and de‐novo‐assisted sequencing using electron‐transfer dissociation mass spectrometry. J Proteome Res. 2017;16:1470–1482. [DOI] [PubMed] [Google Scholar]
- 53. Juba ML, Russo PS, Devine M, et al. Large scale discovery and de novo‐assisted sequencing of cationic antimicrobial peptides (CAMPs) by microparticle capture and electron‐transfer dissociation (ETD) mass spectrometry. J Proteome Res. 2015;14:4282–4295. [DOI] [PubMed] [Google Scholar]
- 54. Azkargorta M, Soria J, Ojeda C, et al. Human basal tear peptidome characterization by CID, HCD, and ETD followed by in silico and in vitro analyses for antimicrobial peptide identification. J Proteome Res. 2015;14:2649–2658. [DOI] [PubMed] [Google Scholar]
- 55. Kim IW, Markkandan K, Lee JH, et al. Transcriptome profiling and in silico analysis of the antimicrobial peptides of the grasshopper Oxya chinensis sinuosa . J Microbiol Biotechnol. 2016;26:1863–1870. [DOI] [PubMed] [Google Scholar]
- 56. Lin CH, Chang MW, Chen CY. A potent antimicrobial peptide derived from the protein LsGRP1 of Lilium. Phytopathology. 2014;104:340–346. [DOI] [PubMed] [Google Scholar]
- 57. Hovde BT, Deodato CR, Hunsperger HM, et al. Genome sequence and transcriptome analyses of Chrysochromulina tobin: Metabolic tools for enhanced algal fitness in the prominent order prymnesiales (Haptophyceae). PLoS Genet. 2015;11:e1005469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Leoni G, De Poli A, Mardirossian M, et al. Myticalins: A novel multigenic family of linear, cationic antimicrobial peptides from marine mussels (Mytilus spp.). Mar Drugs. 2017;15:E261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Porto WF, Fensterseifer ICM, Ribeiro SM, Franco OL. Joker: An algorithm to insert patterns into sequences for designing antimicrobial peptides. Biochim Biophys Acta Gen Subj. 2018;1862:2043–2052. [DOI] [PubMed] [Google Scholar]
- 60. Fan L, Liu Y, Li Z, et al. Draft genome sequence of the marine Streptomyces sp. strain PP‐C42, isolated from the Baltic Sea. J Bacteriol. 2011;193:3691–3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Fan L, Bo S, Chen H, et al. Genome sequence of Bacillus subtilis subsp. spizizenii gtP20b, isolated from the Indian ocean. J Bacteriol. 2011;193:1276–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kim IW, Lee JH, Subramaniyam S, et al. De novo transcriptome analysis and detection of antimicrobial peptides of the American Cockroach Periplaneta americana (Linnaeus). PLoS One. 2016;11:e0155304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Noga EJ, Stone KL, Wood A, Gordon WL, Robinette D. Primary structure and cellular localization of callinectin, an antimicrobial peptide from the blue crab. Dev Comp Immunol. 2011;35:409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Defer D, Desriac F, Henry J, et al. Antimicrobial peptides in oyster hemolymph: The bacterial connection. Fish Shellfish Immunol. 2013;34:1439–1447. [DOI] [PubMed] [Google Scholar]
- 65. Yoo WG, Lee JH, Shin Y, et al. Antimicrobial peptides in the centipede Scolopendra subspinipes mutilans. Funct Integr Genomics. 2014;14:275–283. [DOI] [PubMed] [Google Scholar]
- 66. Mooney C, Haslam NJ, Holton TA, Pollastri G, Shields DC. PeptideLocator: Prediction of bioactive peptides in protein sequences. Bioinformatics. 2013;29:1120–1126. [DOI] [PubMed] [Google Scholar]
- 67. Zhao J, Zhao C, Liang G, Zhang M, Zheng J. Engineering antimicrobial peptides with improved antimicrobial and hemolytic activities. J Chem Inf Model. 2013;53:3280–3296. [DOI] [PubMed] [Google Scholar]
- 68. Panya A, Yongpitakwattana P, Budchart P, et al. Novel bioactive peptides demonstrating anti‐dengue virus activity isolated from the Asian medicinal plant Acacia catechu . Chem Biol Drug Des. 2019;93:100–109. [DOI] [PubMed] [Google Scholar]
- 69. Xu J, Wang X, Xu P, et al. Mass spectrometry‐based peptidome profiling of human serous ovarian cancer tissues. Int J Biochem Cell Biol. 2019;107:53–61. [DOI] [PubMed] [Google Scholar]


