Atrial fibrillation (AF) is becoming a global epidemic and is predicted to affect 6–12 million people in the USA by 2050 and 17.9 million in Europe by 2060.1–4 Concurrently, with the advent of newer cancer therapies, global cancer survival has dramatically increased, with an expected overall survival of over 18 million persons by 2030.5 Increasingly, the profile of patients presenting with AF has disproportionately shifted to encompass patients with current or prior cancer diagnoses.
The ‘REasons for Geographic and Racial Differences in Stroke’ (REGARDS) cohort of nearly 15 500 patients reported a 20% higher adjusted risk of AF in patients with cancer compared to those without cancer.6 Notably, the incidence of AF was appreciated to be 3.0–4.5 times higher within the first year of a cancer diagnosis compared to later years.1,7 Conversely, an increased risk of cancer among patients presenting with AF has also been appreciated.4 For example, in a Danish cohort of 270 000 patients with new-onset AF, the standardized rate incidence ratio of cancer diagnosis was 5.11 in the first 3 months after diagnosis of AF.8 Similarly, Kim et al.5 reported a 2.6-fold increased incidence of a cancer diagnosis at 1 year after developing AF.
Moreover, the cardiovascular and overall prognosis with AF is worse among cancer patients compared to those without cancer, with a two-fold higher adjusted risk for thromboembolic complications, and a six-fold higher adjusted risk for heart failure,9 as well as a 10-fold higher risk of adjusted 30-day mortality.9
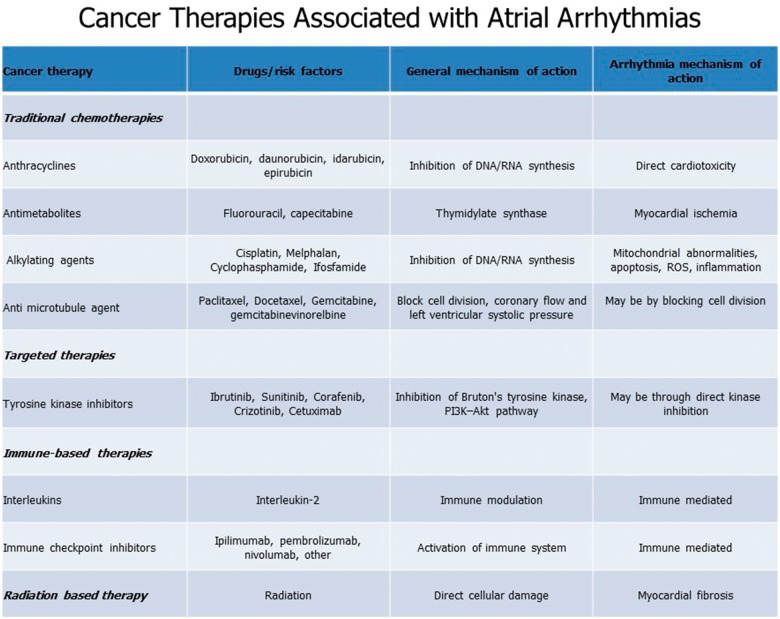
The increased incidence of AF in the setting of malignancy may be due to a variety of factors, including the cancer itself as well as the potential medical and surgical treatments associated with cancer (Figure 1). For example, in cancer states such as chronic lymphocytic leukaemia, the incidence of AF is increased irrespective of the treatment delivered.10 Similarly, the incidence of AF during the perioperative period of cancer surgery ranges from 6–28% for lung resection, 4–5% for colectomy, and 9–11% for oesophagectomy.10,11 Moreover, AF is a known cardiotoxicity of several chemo-, targeted-, and immuno-therapeutics, including anthracyclines, melphalan, ibrutinib, checkpoint inhibitors, and interleukin-2.12 Broadly, proposed mechanisms driving cancer therapy-related arrhythmogenicity include myocardial ion-channel interactions, excess oxidative stress, fibrosis, and increased levels of inflammatory mediators11,13 (Figure 2). However, more study is needed, particularly with emerging targeted and immune-based therapies.
Figure 1.
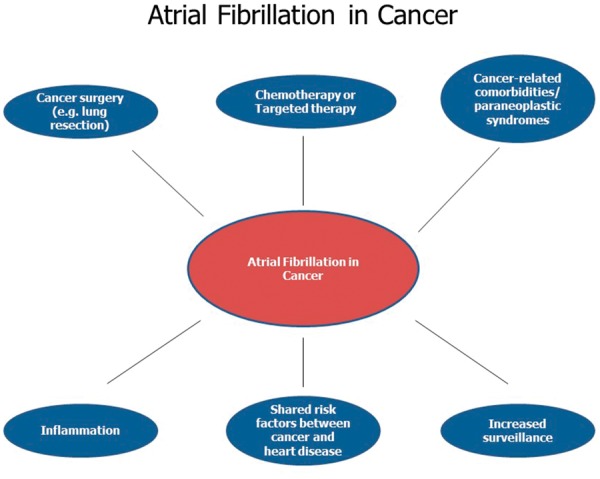
Common risk factors between atrial fibrillation and cancer.
Figure 2.
Mechanism of action between cancer therapies and associated atrial fibrillation.
Although the prognosis of AF is generally worse in cancer patients, specific guidelines directing the treatment of AF in cancer patients are lacking.5 Nevertheless, the 2019 American College of Cardiology/Heart Rhythm Society (ACC/HRS) update on the management of AF suggested several strategies that may be applicable to cancer patients.14 As with all patients with AF, management of AF in the setting of cancer should start with a calculation of CHA2DS2-VASc score and those with elevated score (≥2 in men and ≥3 in women) should be offered anticoagulation.14 The direct applicability of the CHA2DS2-VASc (or the CHADS2) score in cancer patients is uncertain.15 However, in a recent study, patients with cancer and AF had higher rates of thromboembolism only at low CHA2DS2-VAsc scores (0–1) when compared with non-cancer patients.16 Another study demonstrated that CHADS2 score was insufficient to predict thromboembolic complications in cancer patients with new-onset AF.17 As such, a scoring system specific to cancer patients may be needed to optimize outcomes.
The most effective anticoagulant(s) to prevent AF-associated thromboembolism in cancer patients remains an area of active investigation. Although low-molecular weight heparin (LMWH) was recommended as the standard of care in treating cancer-associated venous thromboembolic events,18,19 there is limited high-quality data on the use of LMWH in thromboprophylaxis of AF.20 Current ACC/HRS guidelines recommend the use of a direct oral anticoagulant (DOAC) over warfarin in DOAC-eligible patients including those with cancer.13,14 A sub-study of the ENGAGE AF TIMI 48 trial comparing edoxaban to warfarin demonstrated significant improvement in the composite endpoint of ischaemic stroke/systemic embolism/myocardial infarction in patients with active cancer compared to those without malignancy.21 A sub-analysis of the ARISTOTLE study evaluating patients with a history of active or prior cancer demonstrated superior efficacy of apixaban vs. warfarin at preventing the primary endpoint when compared with patients without cancer.22 Comparative effectiveness data of patients with AF and cancer have consistently shown DOACs to be associated with similar (or lower) risks of bleeding and stroke compared with conventional strategies, such as warfarin.23
Despite their efficacy in preventing AF-associated thromboembolic complications, increased scrutiny must be utilized when prescribing DOACs in cancer patients. Cancer patients frequently have thrombocytopenia and other bleeding diatheses. If the bleeding risk is substantial and overall life-expectancy is limited (<12 months), foregoing anticoagulation may be a reasonable choice even at higher CHA2DS2-VASc scores.24 Moreover, frequent drug–drug interactions with cancer therapeutics and/or renal impairment may limit the use of anticoagulants, particularly, the DOACs.13
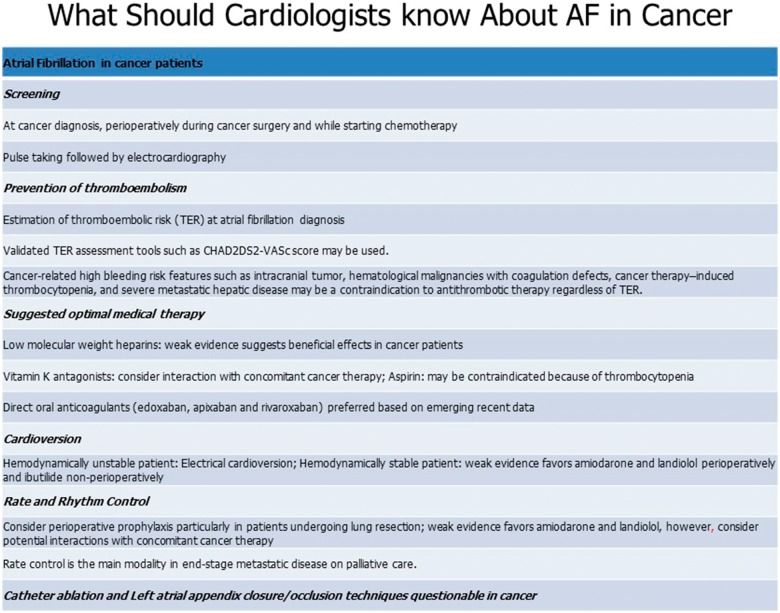
In patients that are unable to tolerate long-term anticoagulation, percutaneous left atrial appendage closure may be an option, however, this procedure has not been specifically studied in the cancer population.14 In general, the management of AF in cancer patients should follow a shared decision-making model.20 Though dedicated studies in cancer patients are lacking, AF catheter ablation may be a reasonable option in highly symptomatic patients or those with heart failure with reduced ejection fraction who can tolerate at least short-term anticoagulation.14 In addition, patients who are overweight or obese should be advised weight loss and combination of risk factor modification is recommended.14 Finally, it is essential to recognize the potential interactions of antiarrhythmic medications or anticoagulants with different cancer therapeutics in order to minimize toxicity and mitigate risk. Specific details about management of AF and caveats specific to cancer are presented in (Figure 3).
Figure 3.
Management of atrial fibrillation in cancer.
In conclusion, AF is an increasingly frequent presenting cardiovascular condition among cancer patients. Shared epidemiology and risk factors contribute to the association between cancer and AF. However, AF may also be a manifestation of cardiotoxicity of specific cancer treatments. The optimal AF management strategy in cancer patients is uncertain due to the lack of dedicated prospective studies in this population. Direct oral anticoagulants represent a convenient and patient-centred anticoagulation strategy to minimize AF-associated thromboembolism, with emerging data supporting their safety and efficacy in the care of cancer patients. Nonetheless, the application of traditional risk scores to determine the likelihood of AF-associated thromboembolism may not be sufficient in this population.
It is evident that the management of AF in cancer patients can be challenging and is best accomplished with a longitudinal multidisciplinary approach with frequent clinical assessment by cardio-oncology specialists.
Conflict of interest: none declared.
References
References are available as supplementary material at European Heart Journal online.






Supplementary Material
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.