Abstract
An alkyl–alkyl cross-coupling of alkylpyridinium salts and organoboranes, formed in situ via hydroboration of alkenes, has been developed. This method utilizes the abundance of both alkyl amine precursors and alkenes to form C(sp3)─C(sp3) bonds. This strategy is also effective with alkynes, enabling a C(sp3)─C(sp2) cross-coupling. Under these mild conditions, a broad range of functional groups, including protic groups, is tolerated. As seen with previous alkylpyridinium cross-couplings, mechanistic studies support an alkyl radical intermediate.
Graphical Abstract
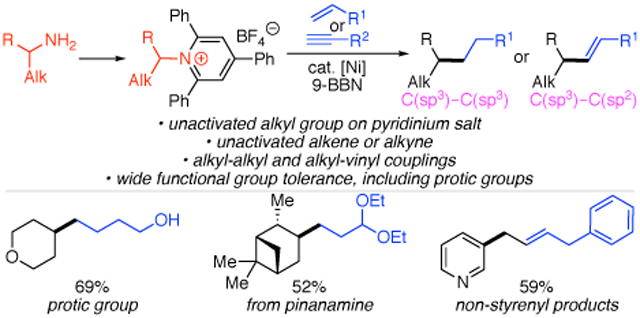
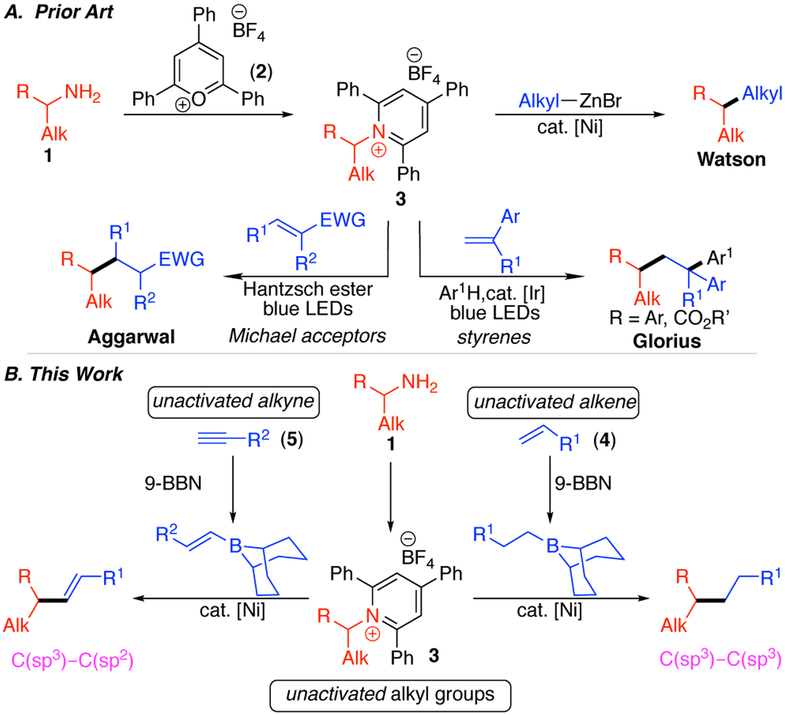
Alkyl amines are inexpensive and widely abundant feedstock chemicals, making them ideal precursors for further functionalization.1 The amino group is also present in many advanced intermediates and products, enabling opportunities for late-stage derivatization.1-2 Although reactions of both simple and complex alkyl amines have classically centered on the preparation of nitrogen-conaining products, deaminative reactions via C─N bond activation of Katritzky pyridinium salts 3 have emerged as useful transformations of the highly versatile amino functional group.3 Specifically, we and others have developed arylations,4 vinylations,5 alkynylations,6 allylations,7 and borylations8 of pyridinium salts. However, deaminative alkylation of pyridinium salts to form C(sp3)─C(sp3) bonds remains limited, despite the potential of such reactions as a powerful, albeit noncanonical, disconnection in synthesis, particularly if both starting materials can arise from ubiquitous substrate classes. Towards such a deaminative alkylation, we reported a Negishi alkylation of alkyl pyridinium salts (Scheme 1A).9 Although this cross-coupling tolerated primary and secondary alkyl pyridinium salts and a range of functional groups, the harsh conditions prevented the use of benzylic pyridinium salts and base-sensitive functional groups. Han, Wang, and Yan recently reported several examples of reductive alkylation, but these were limited to forging C─C bonds between primary alkyl groups.4g We thus pursued the use of an alternative, milder nucleophilic partner to provide broad scope in both primary and secondary alkyl pyridiniums, as well as excellent functional group tolerance. In particular, we envisioned that the use of alkyl-boranes, generated in situ via hydroboration of simple alkenes, would fulfill our requirement for mild, neutral conditions and also enable the use of abundant alkenes as starting materials.10
Scheme 1.
Deaminative Alkyl–Alkyl Couplings
Other groups have also utilized alkenes in alkylations of alkylpyridinium salts. Glorius and Aggarwal reported photocatalytic generation of alkyl radicals, which were subsequently trapped with either styrenes or electron-poor alkenes (Scheme 1A).11,12 However, these reactions are limited to activated alkenes, as well as activated alkyl groups on the pyridinium salt for Glorius’s three-component coupling. While this manuscript was in preparation, Martin published a cross-coupling of alkylpyridinium salts with alkenes, reduced in situ with a silane.13 Herein, we report our development of an alkyl–alkyl cross-coupling of alkylpyridinium salts with alkenes, including unactivated examples, via in situ formation of organoboranes. These conditions also enable vinylation when alkyne starting materials are used.
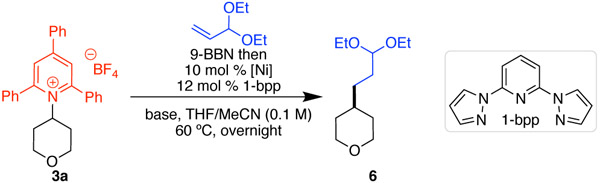
We selected the reaction of pyridinium salt 3a and commercially available acrolein acetal for our initial studies. We used (9-BBN) as our hydroboration agent based on its high hydroboration regioselectivities and precedent in the use of this type of alkylborane in other cross-couplings.10, 14 Our initial studies focused on examining both ligand and base in the cross-coupling of pyridinium 3a and preformed alkylborane using high-throughput experimentation (HTE) techniques (scale: 8.3 μmol pyridinium 3a). We used a combination of Ni(acac)2 and Ni(cod)2 to ensure successful in situ formation of Ni(I).15 Among the 36 ligands examined, 2,6-bis(pyrazol-1-yl)pyridine (1-bpp) provided the best yield of desired product 6.16 In addition, we found that only KF provided product of the eight activating agents tested. Using 1-bpp and KF, quantitative yield of 6 was observed on HTE scale. When we applied these conditions to a 0.1 mmol-scale experiment, however, only 16% yield was observed (Table 1, entry 1). Under these conditions, we found that the use of air-stable NiCl2·DME provided a comparable, albeit low, yield (entry 2), and thus switched to this simpler catalyst system to determine why yields were inconsistent between the HTE and 0.1-mmol scale reactions. In our HTE campaign, we used spray-dried and carefully sieved KF, but had used only oven-dried KF in the 0.1-mmol experiments. By switching to spray-dried and sieved KF on 0.1-mmol scale, yield increased substantially (entry 3). With Ni(acac)2, an even higher yield of 67% was observed (entry 4). Notably, other fluoride sources, such as CsF, resulted in a significant drop in yield (entry 5). Under these conditions, the equivalents of (9-BBN)2, alkene, and base could be decreased without lowering the yield (entry 6). Considering the sensitivity of the yield to the fluoride activator, we hypothesized that efficient formation of the boronate via fluoride co-ordination to the organoborane was critical. To promote formation of this intermediate, KF was added in the hydroboration step; by combining (9-BBN)2, alkene, and KF at 60 °C before the addition of the other reagents, we increased the yield to 95% (entry 7). Control experiments showed the importance of this pre-ligation and the need for both nickel and base in this reaction (entries 8–10).
Table 1.
Optimizationa
 | |||
|---|---|---|---|
| entry | [Ni] | Base | yield (%)b |
| 1 | Ni(cod)2/Ni(acac)2 | KF | 16 |
| 2c,d | NiCl2·DME | KF | 12 |
| 3d,e | NiCl2·DME | KF | 55 |
| 4d,e | Ni(acac)2 | KF | 67 |
| 5d | Ni(acac)2 | CsF | 30 |
| 6d,e,f | Ni(acac)2 | KF | 68 |
| 7d,e,f,g | Ni(acac)2 | KF | 95 |
| 8e,f,g | Ni(acac)2 | KF | 81 |
| 9e,f,g | None | KF | 7 |
| 10e,f,g | Ni(acac)2 | none | n.d.h |
Conditions: alkene (3.0 equiv) and 9-BBN (0.5 M in THF, 3.0 equiv), then pyridinium salt 3a (0.10 mmol, 1.0 equiv), [Ni] (10 mol %), ligand (12 mol %), KF (3.3 equiv), 3:2 THF:MeCN (0.1 M), 60 °C, 24 h, unless noted otherwise.
Determined by 1H NMR using 1,3,5-trimethoxybenzene as internal standard.
KF oven-dried.
Nickel and 1-bpp stirred for 15 min in MeCN before addition to other reagents.
KF spray-dried, oven-dried, and sieved.
9-BBN (0.5 M in THF, 2.5 equiv), alkene (2.5 equiv), KF (2.5 equiv), 1:1 THF:MeCN (0.1 M).
9-BBN, KF, and alkene heated at 60 °C for 30 min before addition of other reagents.
n.d. = not detected.
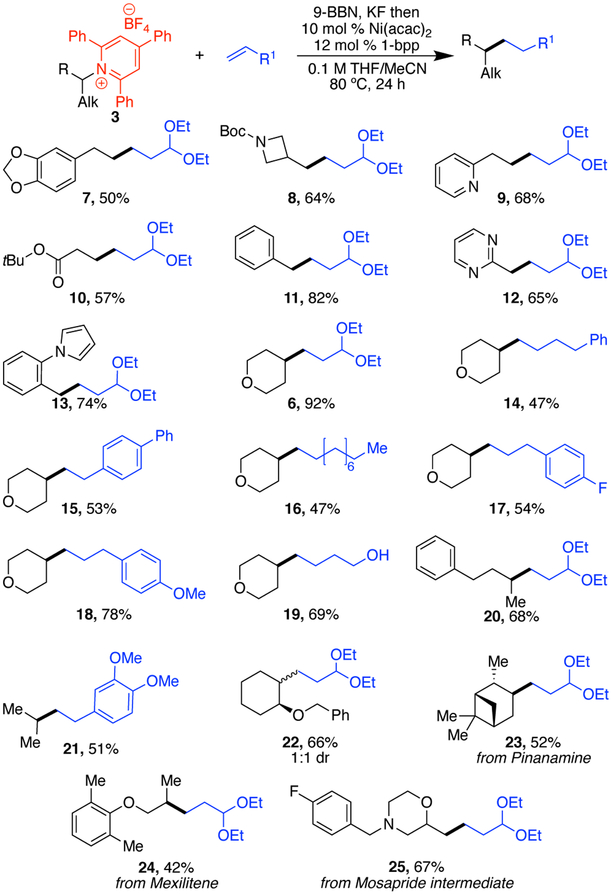
Under these optimized conditions, a variety of both primary and secondary alkylpyridinium salts were successfully alkylated. Notably, although benzylic pyridinium salts failed under our previous, more basic Negishi alkylation conditions,9 benzylic pyridinium salts (11–13) worked under these conditions.17 This method also shows high tolerance for a variety of functional groups on the pyridinium salt, including acetals (7), protected amines (8), esters (10), ethers (6, 14–19, 22, 24, 25), tertiary amines (25), and aryl fluorides (25). These examples include base-sensitive substrates, such as the pyridinium salt of a β-amino ester (10). Our previous Negishi coupling failed for these types of substrates.9 Pyridinium salts containing a range of heterocycles also underwent alkylation in good yields: azetidines (8), pyridines (9), pyrimidines (12), pyrroles (13), pyrans (6, 14–19), and morpholines (25). With a diastereomerically pure pyridinium salt prepared from cyclohexane amino ether (22), a 1:1 ratio of diasteromeric products was isolated, consistent with a radical intermediate. With a more constrained system (23), a single diastereomer of product was isolated. To highlight the utility of this method for late-stage functionalization of amines, pharmaceutical intermediates and natural products were investigated. Both pinanamine and mexilitine were successfully alkylated (23, 24).18 Alkylation of the pyridinium salt derived from an amine intermediate in the Mosapride synthesis also worked well (25).19
On the alkene side, broad functional group tolerance was also observed, including acetals (6–13, 20, 22–25), ethers (18, 21), and aryl fluorides (17). Notably, unprotected alcohols (19) are even tolerated, highlighting the mild conditions and representing a significant advance over our previous Negishi conditions.9 In addition to aliphatic alkenes, styrenes can also be used in this chemistry (15, 21). We were also pleased to find that allylic arenes can serve as the alkene partner (17, 18); alkene isomerization did not pose a major problem. Unfortunately, however, 1,1- and 1,2-disubstituted alkenes were not effective in this reaction.
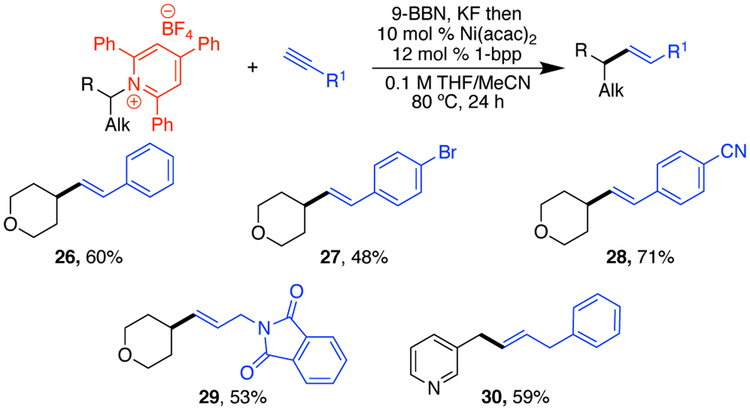
While investigating the scope of this alkylation method, we were also intrigued by the possibility of starting with a simple alkyne. We have previously reported the vinylation of benzylic pyridinium salts with vinyl boronic acids,5a and installation of styrenyl groups can also be accomplished with boronic acids or via a Heck-type reaction.5b-d Excitingly, our hydroboration/cross-coupling conditions can be applied to alkyne substrates, providing a vinylated product. The functional group tolerance includes ethers (26–29), aryl bromides (27), nitriles (28), phthalimides (29). Notably, this vinylation is successful with nonbenzylic pyridinium salts and can install non-styrenyl vinyl groups (30), complimenting the methods previously developed.
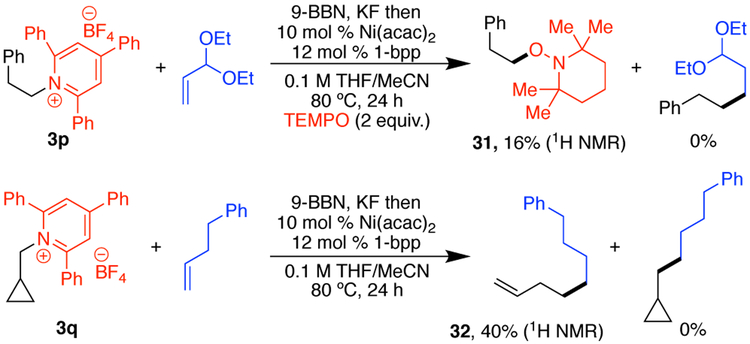
Similar to previously developed pyridinium cross-couplings,3d, 4a we propose that this reaction proceeds through a single-electron transfer (SET) from a Ni(0) or Ni(I) intermediate to the alkyl pyridinium salt. Fragmentation of the neutral pyridyl radical gives an alkyl radical, which can recombine with a Ni(I) or Ni(II) intermediate to provide the product after reductive elimination. Consistent with the formation of an alkyl radical, TEMPO adduct 31 is observed upon addition of TEMPO, and cyclopropane 3q underwent ring-opening (Scheme 4).
Scheme 4.
Mechanistic studies
In summary, we have developed a nickel-catalyzed alkyl–alkyl cross-coupling of alkyl pyridinium salts with alkenes, via organoborane intermediates. This method harnesses ubiquitous functional groups (amines and alkenes) in both partners, and is successful even with unactivated alkenes. In addition, this method can also be applied to alkynes to effectively provide the vinylation of unactivated pyridinium salts. Broad functional group tolerance, including benzylic pyridinium salts and protic functional groups, is seen with both of these methods.
Supplementary Material
Scheme 2. Scope of alkyl–alkyl couplinga.
a Conditions: alkene (2.5 equiv), 9-BBN (0.5 M in THF, 2.5 equiv), KF (2.75 equiv), then pyridinium salt 3 (1.0 mmol, 1.0 equiv), [Ni] (10 mol %), 1-bpp (12 mol %), MeCN (0.5 mL), 80 °C, 24 h. Average isolated yield of duplicate experiments (±4%).
Scheme 3. Vinylation Scopea.
a Conditions: alkene (2.5 equiv), 9-BBN (0.5 M in THF, 2.5 equiv), KF (2.75 equiv), then pyridinium salt 3 (1.0 mmol, 1.0 equiv), [Ni] (10 mol %), 1-bpp (12 mol %), MeCN (0.5 mL), 80 °C, 24 h.
ACKNOWLEDGMENT
We thank NIH (R01 GM111820, R35 GM131816) and University of Delaware for a Bigelow Summer Scholars Fellowship (M.E.D.). We thank Olivia Bercher (UD) for providing several pyridinium salts. Data were acquired at UD on instruments obtained with assistance of NSF and NIH funding (NSF CHE0421224, CHE1229234, CHE0840401, and CHE1048367; NIH P20 GM104316, P20 GM103541, and S10 OD016267).
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website.
Experimental details and data (PDF)
Any additional relevant notes should be placed here.
REFERENCES
- 1. (a).Lawrence SA, Amines: Synthesis, Properties and Applications. Cambridge University Press: New York, NY, 2004; [Google Scholar]; (b) Nugent TC, Chiral Amine Synthesis. Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, 2010. [Google Scholar]
- 2. (a).Ruiz-Castillo P; Buchwald SL, Applications of Palladium-Catalyzed C─N Cross-Coupling Reactions. Chem. Rev 2016, 116 (19), 12564–12649; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) McGrath NA; Brichacek M; Njardarson JT, A Graphical Journey of Innovative Organic Architectures That Have Improved Our Lives. J. Chem. Educ 2010, 87 (12), 1348–1349; [Google Scholar]; (c) Liu Y; Ge H, Site-selective C─H arylation of primary aliphatic amines enabled by a catalytic transient directing group. Nat. Chem 2017, 9 (1), 26–32. [Google Scholar]
- 3. (a).Bapat JB; Blade RJ; Boulton AJ; Epsztajn J; Katrizky AR; Lewis J; Molina-Buendia P; Nie P-L; Ramsden CA, Pyridines as Leaving Groups in Synthetic Transformations: Nucleophilic Displacements of Amino Groups, and Novel Preparations of Nitriles and Isocyanates. Tetrahedron Lett. 1976, 31, 2691–2694; [Google Scholar]; (b) Katrizky AR; Marson CM, Pyrylium Mediated Transformations of Primary Amino Groups into Other Functional Groups. Angew. Chem., Int. Ed 1984, 23, 420–429; [Google Scholar]; (c) Sowmiah S; Esperança JMSS; Rebelo LPN; Afonso CAM, Pyridinium salts: from synthesis to reactivity and applications. Org. Chem. Front 2018, 5, 453–493; [Google Scholar]; (d) Kong D; Moon PJ; Lundgren RJ, Radical Coupling from Alkyl Amines. Nat. Catal 2019, 2, 473–476. [Google Scholar]
- 4. (a).Basch CH; Liao J; Xu J; Piane JJ; Watson MP, Harnessing Alkyl Amines as Electrophiles for Nickel-Catalyzed Cross Couplings via C─N Bond Activation. J. Am. Chem. Soc 2017, 139 (15), 5313–5316; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Liao J; Guan W; Boscoe BP; Tucker JW; Tomlin JW; Garnsey MR; Watson MP, Transforming Benzylic Amines into Diarylmethanes: Cross-Couplings of Benzylic Pyridinium Salts via C─N Bond Activation. Org. Lett 2018, 20 (10), 3030–3033; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Hoerrner ME; Baker KM; Basch CH; Bampo EM; Watson MP, Deaminative Arylation of Amino Acid-derived Pyridinium Salts. Org. Lett 2019, 21 (18), 7356–7360; [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Klauck FJR; James MJ; Glorius F, Deaminative Strategy for the Visible-Light-Mediated Generation of Alkyl Radicals. Angew. Chem., Int. Ed 2017, 56 (40), 12336–12339; [DOI] [PubMed] [Google Scholar]; (e) James MJ; Strieth-Kalthoff F; Sandfort F; Klauck FJR; Wagener F; Glorius F, Visible-Light-Mediated Charge Transfer Enables C─C Bond Formation with Traceless Acceptor Groups. Chem. Eur. J 2019, 25 (35), 8240–8244; [DOI] [PubMed] [Google Scholar]; (f) See also: Martin-Montero R; Yatham VR; Yin H; Davies J; Martin R, Ni-catalyzed Reductive Deaminative Arylation at sp(3) Carbon Centers. Org. Lett 2019, 21 (8), 2947–2951; [DOI] [PubMed] [Google Scholar]; (g) Ni S; Li C-X; Mao Y; Han J; Wang Y; Yan H; Pan Y, Ni-catalyzed Deaminative Cross-electrophile Coupling of Katritzky Salts with Halides via C─N Bond Activation. Sci. Adv 2019, 5, eaaw9516; [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Yi J; Badir SO; Kammer LM; Ribagorda M; Molander GA, Deaminative Reductive Arylation Enabled by Nickel/Photoredox Dual Catalysis. Org. Lett 2019, 21 (9), 3346–3351; [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Yue H; Zhu C; Shen L; Geng Q; Hock KJ; Yuan T; Cavallo L; Rueping M, Nickel-catalyzed C─N bond activation: activated primary amines as alkylating reagents in reductive cross-coupling. Chem. Sci 2019, 10, 4430–4435; [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Zhu Z-F; Zhang M-M; Liu F, Radical alkylation of isocyanides with amino acid-/peptide-derived Katritzky salts via photoredox catalysis. Org. Biomol. Chem 2019, 17 (6), 1531–1534. [DOI] [PubMed] [Google Scholar]
- 5. (a).Guan W; Liao J; Watson MP, Vinylation of Benzylic Amines via C─N Bond Functionalization of Benzylic Pyridinium Salts. Synthesis 2018, 50 (16), 3231–3237; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jiang X; Zhang MM; Xiong W; Lu LQ; Xiao WJ, Deaminative (Carbonylative) Alkyl-Heck-type Reactions Enabled by Photocatalytic C-N Bond Activation. Angew. Chem., Int. Ed 2019, 58 (8), 2402–2406; [DOI] [PubMed] [Google Scholar]; (c) Yang Z-K; Xu N-X; Wang C; Uchiyama M, Photoinduced C(sp3)─N Bond Cleavage Leading to the Stereoselective Syntheses of Alkenes. Chem. Eur. J 2019, 25 (21), 5433–5439; [DOI] [PubMed] [Google Scholar]; (d) Hu J; Cheng B; Yang X; Loh TP, Transition-Metal-Free Deaminative Vinylation of Alkylamines. Adv. Synth. Catal 2019. [Google Scholar]
- 6.Ociepa M; Turkowska J; Gryko D, Redox-Activated Amines in C(sp3)─C(sp) and C(sp3)─C(sp2) Bond Formation Enabled by Metal-Free Photoredox Catalysis. ACS Catal. 2018, 8 (12), 11362–11367. [Google Scholar]
- 7.Zhang M-M; Liu F, Visible-light-mediated allylation of alkyl radicals with allylic sulfones via a deaminative strategy. Organic Chemistry Frontiers 2018, 5 (23), 3443–3446. [Google Scholar]
- 8. (a).Hu J; Wang G; Li S; Shi Z, Selective C─N Borylation of Alkyl Amines Promoted by Lewis Base. Angew. Chem., Int. Ed 2018, 57 (46), 15227–15231; [DOI] [PubMed] [Google Scholar]; (b) Wu J; He L; Noble A; Aggarwal VK, Photoinduced Deaminative Borylation of Alkylamines. J. Am. Chem. Soc 2018, 140 (34), 10700–10704. [DOI] [PubMed] [Google Scholar]
- 9.Plunkett S; Basch CH; Santana SO; Watson MP, Harnessing Alkyl Pyridinium Salts as Electrophiles in De-aminative Alkyl-Alkyl Cross-Couplings. J. Am. Chem. Soc 2019, 141 (6), 2257–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown HC; Chen J, Hydroboration. 57. Hydroboration with 9-borabicyclo[3.3.1]nonane of alkenes containing representative functional groups. J. Org. Chem 1981, 46 (20), 3978–3988. [Google Scholar]
- 11.Klauck FJR; Yoon H; James MJ; Lautens M; Glorius F, Visible-Light-Mediated Deaminative Three-Component Dicarbofunctionalization of Styrenes with Benzylic Radicals. ACS Catal. 2019, 9 (1), 236–241. [Google Scholar]
- 12.Wu J; Grant PS; Li X; Noble A; Aggarwal VK, Catalyst-Free Deaminative Functionalizations of Primary Amines by Photoinduced Single-Electron Transfer. Angew. Chem., Int. Ed 2019, 58 (17), 5697–5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun SZ; Romano C; Martin R, Site-Selective Catalytic Deaminative Alkylation of Unactivated Olefins. J. Am. Chem. Soc 2019, 141 (41), 16197–16201. [DOI] [PubMed] [Google Scholar]
- 14. (a).Ishiyama T; Abe S; Miyaura N; Suzuki A, Palladium-Catalyzed Alkyl-Alkyl Cross-Coupling Reaction of 9-Alkyl-9-BBN Derivatives with Iodoalkanes Possessing β-Hydrogens. Chem. Lett 1992, 21 (4), 691–694; [Google Scholar]; (b) Choi J; Fu GC, Transition metal-catalyzed alkyl-alkyl bond formation: Another dimension in cross-coupling chemistry. Science 2017, 356 (6334), 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arendt KM; Doyle AG, Dialkyl Ether Formation by Nickel-Catalyzed Cross-Coupling of Acetals and Aryl Iodides. Angew. Chem., Int. Ed 2015, 54 (34), 9876–9880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.See Supporting Information.
- 17.Only primary benzylic pyridinium salts can be prepared effectively; secondary benzylic pyridinium salts decompose upon synthesis. See reference 4b.
- 18.Koppe H; Zeile K; Kummer W; Stahle H; Danneberg P 1-(2',6'-Dimethyl-phenoxy)-2-amino-alkanes and salts thereof. US3954872A, 1976. [Google Scholar]
- 19.Kato S; Morie T; Yoshida N, Synthesis and Biological Activities of Metabolites of Mosapride, a New Gastroprokinetic Agent. Chem. Pharm. Bull 1995, 43 (4), 699–702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.