ABSTRACT
Systemic lupus erythematosus (SLE) is an autoimmune multisystem disease that commonly affects the kidneys. It is characterized by persistent autoantibody production that targets a multitude of self-antigens. B-cells, plasmablasts and plasma cells, as the source of these autoantibodies, play a major role in the development of lupus nephritis (LN), and are therefore promising therapeutic targets. To date, however, randomized clinical trials of B-cell therapies in LN have not lived up to expectations, whereas uncontrolled cohort and observational studies of B-cell antagonists have been more promising. In this article, we will review the current experience with B-cell therapy in LN and highlight the pitfalls that may have limited their success. We will conclude by suggesting B-cell-centric approaches to the management of LN based on what has been learned from the overall B-cell experience in SLE.
Keywords: BAFF, B-cell, lupus nephritis, rituximab, SLE
INTRODUCTION
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by loss of self-tolerance resulting in persistent generation of autoantibodies. These autoantibodies are classically directed against nuclear antigens, but can also target many others including cytoplasmic, cell membrane and phospholipid-associated antigens, blood cells and endothelial cells, with the total number of autoantibody specificities identified exceeding a hundred [1]. Kidney injury is a major morbidity of SLE and most often is caused by immune-complex accumulation in the kidney [lupus nephritis (LN)]. The management of LN usually involves an ‘induction’ phase that aims to decrease inflammation and suppress the autoimmune pathways contributing to it, and a ‘maintenance’ phase that aims to keep those pathways at bay, limiting relapses. The currently available medications for the management of LN are associated with significant adverse effects and have limited success rates, thus the need for new agents is high and urgent.
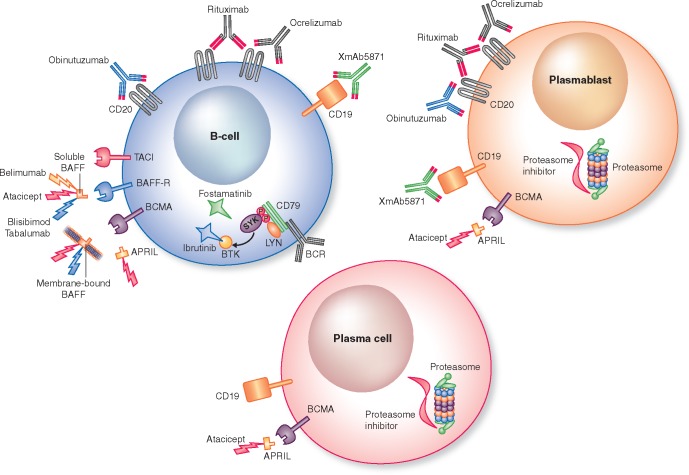
Our understanding of SLE and LN pathogenesis has evolved over the past few decades, and the multiple and diverse roles of the various effector cells of the immune system have been better elucidated. In this regard B-cells have received considerable attention. Although an in-depth review of B-cells in the pathogenesis of SLE is beyond the scope of this article, some key studies are important to note. Shlomchik et al. [2] and Vlahakos et al. [3] demonstrated that lupus-prone mice lacking B-cells did not develop nephritis, and injection of antibodies from lupus mice into normal mice resulted in glomerular immune-complex deposition and development of a lupus-like nephritis. The relevance of B-cells to LN is not limited to autoantibody production. Mice with B-cells that do not secrete antibodies were still able to develop nephritis [4]. B-cells also act as antigen-presenting cells, can promote T-cell activation and produce inflammatory cytokines [5]. These and other data (reviewed in [6–8]) have positioned B-cells as a potentially high-value therapeutic target for the treatment of SLE and LN. The evolution and application of B-cell therapies (Figure 1) to human LN will be reviewed here.
FIGURE 1.
A schematic of the current targets of B-cell therapy. LYN, Lck/Yes novel tyrosine kinase; SYK, spleen tyrosine kinase; BTK, Bruton’s tyrosine kinase.
THERAPEUTIC B-CELL STRATEGIES
B-cell depletion
CD20 promotes B-cell activation and was one of the first B-cell surface antigens exploited for B-cell depletion. Lupus-prone MRL/lpr mice treated with anti-CD20 monoclonal antibodies (mAbs) had a fall in anti-double-stranded DNA (dsDNA) antibody titers and attenuation of their autoimmune nephritis [9] (Figure 1). Lupus-prone NZB/W F1 mice treated with anti-CD20 mAbs showed a delay in disease onset and decreased progression of nephritis [10]. These findings were not solely autoantibody-mediated as there was a reduction in T-cell activation after B-cells regenerated and antibody levels returned to baseline [10]. B-cell depletion was also shown to impair protein and pathogen CD4+-mediated T-cell activation and clonal expansion [11].
Another target antigen that can decrease the number of B-cells when engaged is CD22, a transmembrane protein mainly expressed on mature B-cells (but not plasma cells or memory B-cells) [12, 13]. Lupus-prone mice were found to express functionally deficient CD22 [14], which can contribute to enhanced autoimmunity as CD22 is believed to be an inhibitor of B-cell receptor (BCR) signaling [15]. In addition, polymorphisms in the CD22 gene were identified in human SLE [16].
Importantly, neither CD20 nor CD22 is expressed on plasmablasts or plasma cells [17], and depletion with an anti-CD20 mAb did not affect plasma cell survival in mice [18]. Plasmablasts and plasma cells produce antibodies and autoantibodies in SLE. Plasma cell depletion resulted in decreased autoantibody production, improved survival and LN in NZB/W F1 and MRL/lpr mice [19–21]. Plasma cells are also thought to be the source of persistent antigen-specific antibodies [22], which decreased when B-cells and plasma cells were depleted at the same time [18], suggesting that targeting B-cells and their progeny, plasma cells, might have a synergistic effect on B-cell-driven autoimmunity.
Another approach to achieve a more comprehensive depletion of B-cells (and plasma cells) is targeting CD19, which has a broader range of B-cell lineage expression including plasmablasts and plasma cells. Its use is currently being evaluated in autoimmune diseases in humans [23]. Alternatively, CD19+ B-cells can be suppressed (rather than depleted) when CD19 is co-engaged with FcγRIIb [24]. This was demonstrated using XmAb5871, an engineered anti-CD19 mAb that also binds FcγRIIb. XmAb5871 suppressed humoral immunity and reduced serum immunoglobulin levels without depleting B-cells in severe combined immunodeficiency (SCID) mice engrafted with human SLE peripheral blood mononuclear cells [25].
B-cell neutralization
B-cell activating factor (BAFF, also known as B-lymphocyte stimulator) and a proliferation-inducing ligand (APRIL) are two members of the tumor necrosis factor (TNF) superfamily that promote B-cell survival and differentiation (Figure 1) [26–28]. They are predominantly secreted by dendritic cells, macrophages and neutrophils. BAFF is known to bind three receptors; BAFF receptor (BAFF-R), transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI) and B-cell maturation antigen (BCMA), while APRIL only binds to TACI and BCMA. B-cells express all three receptors, but plasma cells predominantly express BCMA.
There is ample evidence suggesting a role for BAFF in SLE. Circulating BAFF levels are higher in NZB/W F1 and MRL/lpr mice during the onset and progression of SLE, and treatment of NZB/W F1 mice with a soluble TACI-Ig or BAFF-R-Ig fusion protein prolonged survival and inhibited development of proteinuria [29, 30]. BAFF-deficient NZM lupus-prone mice had less severe proteinuria and reduced mortality [31]. In addition, in patients with SLE, CD19+ B-cell expression of BAFF and APRIL correlated with disease severity (as measured by the SLE disease activity index) and anti-dsDNA titers, and plasma cells had increased BAFF and APRIL expression [32]. Interestingly, BAFF levels are elevated in African-Americans with SLE compared with patients of European ancestry [33], which may partially explain their greater degree of B-cell activation [34] and possibly a worse prognosis.
B-cell depletion and neutralization strategies have been applied to the treatment of human LN with some mixed but also some promising results. These studies will be discussed in the remainder of this review to provide insight into how B-cell therapies may be best deployed for patients with LN.
B-CELL DEPLETION FOR LN INDUCTION
The first multinational randomized controlled trial of B-cell therapy to improve renal response rates in LN was the lupus nephritis assessment with rituximab (LUNAR) study [35]. Rituximab is a chimeric IgG mAb directed against CD20, and is currently used in the management of B-cell dyscrasias and autoimmune diseases such as rheumatoid arthritis. LUNAR randomized 144 patients with proliferative LN (ISN/RPS Class III/IV ± V), and a mean serum creatinine concentration and urine protein-to-creatinine ratio (uPCR) of 1.0 ± 0.5 mg/dL and 4.0 ± 2.9 mg/mg, respectively, to receive rituximab (four 1 g doses on Days 1, 15, 168, 182) or placebo in addition to standard-of-care [pulse methylprednisolone followed by oral prednisone and mycophenolate mofetil (MMF)]. The study did not demonstrate significantly more complete or partial renal responses than placebo at 52 weeks. The overall renal response (complete plus partial) was 57% for the rituximab group and 46% for the placebo group. However, patients treated with rituximab had more improvements in complement and anti-dsDNA levels, and a larger decrease in proteinuria than the controls.
Ocrelizumab, a humanized anti-CD20 mAb was subsequently evaluated in 223 patients with Class III/IV ± V LN in the BELONG trial [36]. Patients were randomized to placebo or one of two ocrelizumab doses plus either MMF or cyclophosphamide as background immunosuppression. Although BELONG was stopped early due to an imbalance in serious infections in the ocrelizumab groups, 148 patients completed >32 weeks of therapy. Reminiscent of the LUNAR trial, the ocrelizumab-treated patients showed a nonsignificant increase (12% over control) in overall renal response, but significant improvement in complement and anti-dsDNA levels.
These negative results were unexpected given the established role of the B-cell in SLE. Several concerns over trial design were raised. These included adding B-cell therapy to intense background therapy with corticosteroids and immunosuppression, insufficient sample size, insufficient sensitivity in determining complete B-cell depletion, failure to account for tissue B-cells that may be harder to kill, lack of effect on plasma cells and insufficient length of follow-up [37]. These concerns have led to some innovations in LN trial design as outlined below, and importantly, have not discouraged the evaluation of anti-B-cell therapies in LN (Table 1).
Table 1.
Current clinical trials evaluating B-cell therapies in SLE and LN
| Target | Drug | Study title | Study status | Disease target | Study phase | Identifier |
|---|---|---|---|---|---|---|
| CD20 | Rituximab (anti-CD20 mAb) | Rituximab objective outcome measures trial in SLE | Recruiting | SLE | Phase 2 | NCT03054259 |
| RING | Recruiting | LN | Phase 3 | NCT01673295 | ||
| Obinutuzumab (anti-CD20 mAb) | A study to evaluate the safety and efficacy of obinutuzumab compared with placebo in participants with LN | Recruiting | LN | Phase 2 | NCT02550652 | |
| CD19 | XmAb5871 | A study of the effect of XmAb®5871 in patients with SLE | Active | SLE | Phase 2 | NCT02725515 |
| BAFF/APRIL | RC18 (TACI-antibody fusion protein) | Study of RC18 administered subcutaneously to subjects with SLE | Recruiting | SLE | Phase 2 | NCT02885610 |
| Belimumab (anti-BAFF) | Efficacy and safety of belimumab in patients with active LN | Active | LN | Phase 3 | NCT01639339 | |
| AMG 570 (bisepcific peptibody—BAFF and ICOS ligand) | Single ascending dose study of AMG 570 in healthy subjects | Active | SLE | Phase 1 | NCT02618967 | |
| Immunoproteasome | KZR-616 | A study of KZR-616 in patients with SLE with and without nephritis | Recruiting | SLE | Phase 1/2 | NCT03393013 |
| Combination | Rituximab, belimumab | Rituximab and belimumab for LN | Active | LN | Phase 2 | NCT02260934 |
| Synergetic B-cell immodulation in SLE | Recruiting | SLE | Phase 2 | NCT02284984 | ||
| A study to evaluate the efficacy and safety of belimumab administered in combination with rituximab to adult subjects with SLE–BLISS–BELIEVE | Recruiting | SLE | Phase 3 | NCT03312907 |
Add-on trials in which a novel therapeutic is given with standard-of-care background immunosuppression are common in LN because of the potential for rapid disease progression and the uncertain efficacy of the novel drug being tested. However, the benefits of immunosuppressive therapies beyond those which may be attributed to corticosteroids alone can take >1–2 years to become apparent [38, 39], raising the possibility that any beneficial effects of B-cell therapies added to high-dose corticosteroids may have been masked. For example, in BELONG, ocrelizumab showed no additive improvement for patients receiving >1 g of intravenous methylprednisolone, who had a higher rate of renal response regardless of concomitant therapy, but did appear to have efficacy in patients who had been given less methylprednisolone initially. Rituximab has also been shown to be ‘steroid-sparing’ in the treatment of LN. In a single-center cohort 50 consecutive patients with ISN/RPS Class III, IV or V LN were treated with rituximab (two 1 g doses on Days 1, 15), methylprednisolone (two 500 mg doses on Days 1, 15) and MMF without any oral steroids, and achieved excellent renal responses with 90% complete or partial remission after a median follow-up of 37 weeks [40]. Finally, a small study examined the potential of rituximab to facilitate rapid weaning of corticosteroids and minimization of maintenance immunosuppression in severe SLE and LN. A total of 12 patients with multiorgan involvement, including LN (three patients with Class IV, four with Class III + V and five with Class V), were treated with rituximab administered as four weekly doses of 375 mg/m2, followed by two additional doses 1 and 2 months after the last weekly infusion, cyclophosphamide (10 mg/kg on Days 4 and 17) and methylprednisolone (15 mg/kg on Days 1, 4 and 8). Oral prednisone was started at 0.8 mg/kg/day for 2 weeks, but rapidly tapered to 5 mg at 3 months and was maintained afterward. At 12 months all patients were in complete renal remission (defined by 24 h proteinuria <0.5 g/day and GFR within 10% of baseline). After a mean follow-up time of 51.6 months, 9 out of 12 patients remained in remission with minimal maintenance immunosuppression [41]. Taken together, these findings suggest that it may be necessary to design future LN trials with less background corticosteroid.
The concern that LUNAR and BELONG failed because of incomplete B-cell depletion in the periphery, lymphoid tissues or the kidney is being addressed by the NOBILITY trial (NCT02550652). NOBILITY is similar to LUNAR, but the B-cell drug is obinutuzumab, a new-generation humanized anti-CD20 mAb with superior antibody-dependent cellular cytotoxicity and antibody-dependent cellular phagocytosis. In trials of B-cell malignancies, obinutuzumab was more effective at depleting peripheral and lymphoid tissue B-cells than rituximab [42–44]. The expectation of NOBILITY is that the enhanced cytotoxicity will translate into improved outcomes, as the degree of B-cell depletion has been associated with clinical response in LN [45].
The lack of plasma cell depletion with anti-CD20 mAbs might be circumvented by targeting CD19 instead, which is expressed on B-cells and plasma cells. An anti-CD19 mAb, XmAb5871 is currently under study in human SLE (NCT02725515), but proliferative LN is excluded. Alternatively, plasma cells can be targeted with proteasome inhibitors (PIs). Proteasomes are the major nonlysosomal degradation system for proteins, and proteasome inhibition leads to the accumulation of misfolded proteins within the endoplasmic reticulum. Plasma cells are particularly sensitive to proteasome inhibition due to their high rate of antibody synthesis [46]. PIs have effects other than plasma cell depletion that could also be relevant for the treatment of LN. For example, in mice bortezomib can abrogate Type-I interferon activity [20], probably by inducing apoptosis in Toll-like receptor-activated plasmacytoid dendritic cells [47]. Bortezomib also prevents degradation of IκBα, thus inhibiting the activation of the transcription factor NF-κB [48, 49]. NF-κB plays multiple roles in SLE including B- and T-cell development, maturation of dendritic cells and cytokine secretion [50]. NF-κB mediates the synthesis of TNF-α, interleukin (IL)-1β, IL-6 and IL-8, and is one of the most important regulators of proinflammatory gene expression [51]. Thus PIs may have a role in reducing inflammation in LN.
Preliminary studies have examined PI inhibition in LN. Bortezomib depleted peripheral and bone marrow plasma cells, reduced interferon-α activity, decreased anti-dsDNA antibodies, decreased proteinuria and led to improvement in C3 levels in 12 patients with SLE, 5 of whom had active LN previously treated with cyclophosphamide or MMF [52]. Unfortunately, bortezomib was discontinued in seven patients due to adverse reactions. In five patients with refractory LN and high histological activity on kidney biopsy bortezomib resulted in a partial renal remission in four patients [53]. Most patients also had an improvement in serological markers. With these encouraging findings, bortezomib is currently being evaluated in a Phase 2 trial for SLE (NCT02102594) and ixazomib, another PI, is being evaluated in LN (NCT02176486). Ixazomib differs from bortezomib as it is a second-generation PI, that is given orally and may have less neurotoxicity [54]. Newer PIs that selectively inhibit the immunoproteosome, but not the constitutive proteasome, are being developed for use in autoimmunity (NCT03393013). Immunoproteasomes are typically expressed in hematopoietic cells but can also be expressed in other cells exposed to an inflammatory milieu. The selectivity of immunoproteasome inhibitors may reduce the risk of adverse effects observed with inhibition of all cellular proteosomal activity [55], and help specifically target sites of inflammation.
B-CELL NEUTRALIZATION FOR LN INDUCTION
There have been three randomized controlled trials of BAFF antagonists for the treatment of SLE. Although active or severe LN was excluded from these trials some information about the effects of BAFF inhibition on LN can be gleaned from their results. Belimumab is a humanized monoclonal antibody that inhibits the soluble form of BAFF, and was shown to be effective for nonrenal SLE in two large trials (BLISS-52 and BLISS-76) [56, 57]. Although patients with active nephritis (defined as a serum creatinine >2.5 mg/dL or >6 g/day of proteinuria) were excluded, a post hoc analysis of patients with a history of LN demonstrated a dose-dependent reduction in renal flares compared with placebo during follow-up [58]. Belimumab is currently being tested as an add-on agent for LN induction therapy in the BLISS–LN trial (NCT01639339). Given the questions surrounding LUNAR regarding insufficient duration of follow-up, patients in BLISS–LN will be observed for 2 years rather than 1 year.
Two large studies (>2000 patients) of tabalumab, an mAb that binds soluble and membrane-bound BAFF, were done in nonrenal SLE [59, 60]. Patients with severe active LN, defined as an uPCR >200 mg/mmol, creatinine clearance <30 mL/min or by physician assessment were excluded. In contrast to the BLISS trials, the outcome of these studies was not clear cut, and post hoc analysis did not demonstrate any difference in renal outcomes for patients who had LN and were treated with tabalumab compared with those treated with placebo [61].
Blisibimod is a fusion protein of antibody Fc region and BAFF binding domains that also binds soluble and membrane-bound BAFF. Blisibimod was evaluated in a Phase 2 trial of predominantly Hispanic patients with moderate-to-severe SLE, but not severe LN (PEARL-SC) [62]. Although treatment with blisibimod did not significantly improve the SLE response index-5, a subgroup analysis of patients with baseline proteinuria (uPCR 1–6 mg/mg) demonstrated a significantly greater reduction in proteinuria in those treated with blisibimod, along with improved levels of C3, C4 and anti-dsDNA. It remains to be seen whether these results hold up in CHABLIS-SC1 (NCT01395745), a recently completed Phase 3 study of blisibimod in patients with SLE.
Taken together, the results of BAFF inhibition in LN are mixed, but seem to support investigation of BAFF inhibitors to prevent LN flares. The blisibimod data may also support induction treatment of LN, a question that will be answered by BLISS–LN.
Another approach to B-cell growth factor inhibition was through atacicept, a human recombinant fusion protein of TACI and the Fc portion of human IgG1 (TACI-IgG). Atacicept blocks BAFF and APRIL. A Phase 2/3 study adding atacicept to corticosteroids plus MMF in LN was terminated early because of a high incidence of hypogammaglobulinemia [63]. A trial of atacicept in moderate-to-severe SLE was also terminated early due to a higher incidence of death in the atacicept group [64]. A third study of atacicept in nonrenal SLE (ADDRESS II NCT01972568) did not show a safety signal, but showed only a nonsignificant trend toward improvement [65].
B-CELL THERAPY FOR LN MAINTENANCE
The post hoc analysis of BLISS-52 and BLISS-76 suggested belimumab could prevent LN flares and may therefore be useful in the maintenance of remission [58]. The CALIBRATE trial (NCT02260934) is the first prospective investigation to test B-cell directed therapies for induction and maintenance in LN. In CALIBRATE, patients initially receive rituximab plus cyclophosphamide, given as two 1 g and 750 mg doses, respectively, 2 weeks apart. Patients are then randomized to placebo or belimumab (10 mg/kg Weeks 4, 6, 8, then every 4 weeks). This unique design tackles the observation that B-cell depletion results in a marked increase in serum BAFF levels [66], and that in SLE patients treated with rituximab, relapse was associated with significantly elevated serum BAFF levels after B-cell repopulation [67]. Animal studies in nonautoimmune mice not only showed similar results after B-cell depletion with cyclophosphamide, but also demonstrated that repopulation of B-cells in a high BAFF environment led to cells with an autoimmune phenotype and glomerular immunoglobulin deposition [68]. The underlying hypothesis of CALIBRATE is that B-cell repopulation in a BAFF-poor environment may attenuate reactivation of autoimmunity and prevent disease flares.
B-CELL THERAPY IN REFRACTORY LN
The efficacy of rituximab in refractory LN is currently being evaluated prospectively in a randomized clinical trial: rituximab for lupus nephritis with remission as a goal (RING, NCT01673295). The RING trial is supported by several studies describing the use and utility of rituximab for LN refractory to conventional approaches. For example, an Italian multicenter observational cohort described 145 SLE patients in whom rituximab was added on to 76% of the cohort after they had not responded to at least one other immunosuppressant [69]. LN was present in 68% of patients (mean serum creatinine 1.09 ± 0.63 mg/dL; mean proteinuria 4.04 ± 2.91 g/day). A complete or partial renal response was seen in 94% of patients. Proteinuria decreased from 4.1 ± 2.9 at baseline to 1.1 ± 1.9 g/day (P = 0.021) after 12 months.
Additional evidence for rituximab in refractory LN comes from a systematic review of 26 studies that reported the outcomes of 300 refractory LN patients given rituximab [70]. Most (60%) of the patients had Class III/IV ± V LN, and all had been treated previously with cyclophosphamide, MMF or both. Complete or partial remission was achieved in 74% after receiving rituximab. However, a cautious interpretation of these results is warranted. Kidney pathology was not available in 30% of patients, the definition of refractory LN was inconsistent and not explained for 30% of patients, the duration of prior immunosuppression was not analyzed, rituximab was added to ongoing immunosuppression in 62% of the patients, and rituximab dosing was not uniform. Rituximab was generally given as four weekly doses of 375 mg/m2 or two doses of 1 g given 2 weeks apart, but some trials gave two doses of 750 mg/m2 given 2 weeks apart, and others gave 500 mg every 2 weeks for two to four doses. Assuming depletion of peripheral blood CD19 cells as measured by routine flow cytometry represents the correct biomarker to follow for rituximab efficacy, it is conceivable that rituximab regimens can be personalized for individual patients by using peripheral B-cell counts to determine when to dose and how much to use.
Future directions
Although there is considerable circumstantial evidence that B-cell-targeted therapies may be effective in the initial treatment of LN, this still needs to be verified in a randomized controlled trial. Based on the presumptive mechanisms of actions of B-cell therapies, and the results of pre-clinical and clinical trials we suggest three regimens that may be worth consideration for future randomized clinical trials (Table 2). As a disclaimer, we are not suggesting these regimens be used off-label, but rigorously evaluated in trials.
Table 2.
Proposed future B-cell-targeted strategies for management of ISN/RPS Class III/IV ± V LN
| Proposed regimen | Target | Timeline | Expected response |
|---|---|---|---|
| 1 | Proteasome inhibitor | At flare |
|
| Anti-CD20 | At flare |
|
|
| Anti-BAFF/APRIL | Post-peripheral B-cell depletion |
|
|
| 2 | Anti-CD19 | At flare |
|
| Pulse methylprednisolone | At flare |
|
|
| Anti-BAFF/APRIL | Post-peripheral B-cell depletion |
|
|
| 3a | Anti-CD20 | At flare |
|
| Pulse methylprednisolone | At flare |
|
|
| MMF/cyclophosphamide | At flare |
|
|
| MMF | Maintenance |
|
In Regimen 3, if the anti-CD20 agent is given as a loading regimen, that is expected to deplete peripheral B-cells and there is no further anti-CD20 dosing, we suggest MMF be given after anti-CD20 and continued for maintenance; if the anti-CD20 agent is continued for two doses beyond loading we suggest cyclophosphamide be given in between anti-CD20 doses and possibly no maintenance immunosuppression will be needed.
Regimen 1 is corticosteroid-free. This protocol takes advantage of the anti-inflammatory activities of PIs to eliminate the need for high-dose corticosteroids to immediately attenuate intra-renal inflammation. This may not be sufficient, and one could consider adding a complement inhibitor with the proteasome inhibitor to help control inflammation without corticosteroids, as has been demonstrated recently in vasculitis-associated glomerulonephritis [71]. The single published study of proteasome inhibition in human LN showed a very rapid (within the first 11-day cycle of bortezomib) depletion of peripheral blood plasma cells [52], so one induction course will likely be sufficient. Anti-CD20 therapy would be started mid-proteasome course to ensure that B-cells would be depleted by the time the proteasome inhibitor was stopped, because plasma cells returned within 10 days of withdrawing bortezomib [52]. Notably, a similar regimen was tested in murine LN and was shown to effectively deplete long-lived plasma cells, delay onset of nephritis and improve overall survival [72]. After B-cells have been depleted anti-BAFF therapy could be given to prevent B-cell repletion in an environment conducive to autoreactive cell development. The duration of anti-BAFF therapy is unknown, nor is it clear whether or what type of maintenance immunosuppression would be needed; however, the CALIBRATE trial should answer these questions, as there no maintenance immunosuppression will be given in that trial.
Regimens 2 and 3 (Table 2) incorporate high-dose pulse methylprednisolone to rapidly control inflammation while at the same time depleting B-cells and plasma cells (Regimen 2). Both regimens are otherwise corticosteroid-free, and both offer the possibility of no maintenance immunosuppression. Of note, evaluating corticosteroid-free/reduced regimens in a lupus trial is challenging, possibly because of prevailing attitudes on how lupus must be treated, and the almost ubiquitous use of corticosteroids for anyone with LN. For example, the RITUXILup trial (NCT01773616) [40] inspired Regimen 3, MMF version (Table 2), but failed to enroll adequately due to difficulty in identifying and recruiting patients that had not been exposed to corticosteroids, and was closed down.
Finally, there are other ways to target B-cells in LN. For example, Bruton’s tyrosine kinase and spleen tyrosine kinase are important in BCR signaling, and inhibitors of these tyrosine kinases have shown efficacy in murine LN models. Similarly, an oral JAK2 kinase inhibitor has been shown to ameliorate experimental LN and, among other anti-inflammatory activities, also depleted autoreactive plasma cells [73]. Tyrosine kinase inhibition in glomerular disease has recently been reviewed in this journal [74].
FUNDING
This work was supported in part by DK096927 (BHR).
CONFLICT OF INTEREST STATEMENT
All of B.H.R.’s potential COIs are managed through the Ohio State University office of research risks and protections; none of B.H.R.’s associations or positions have impacted the current manuscript.
REFERENCES
- 1. Sherer Y, Gorstein A, Fritzler MJ. et al. Autoantibody explosion in systemic lupus erythematosus: more than 100 different antibodies found in SLE patients. Semin Arthritis Rheum 2004; 34: 501–537 [DOI] [PubMed] [Google Scholar]
- 2. Shlomchik MJ, Madaio MP, Ni D. et al. The role of B cells in lpr/lpr-induced autoimmunity. J Exp Med 1994; 180: 1295–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vlahakos D, Foster MH, Ucci AA. et al. Murine monoclonal anti-DNA antibodies penetrate cells, bind to nuclei, and induce glomerular proliferation and proteinuria in vivo. J Am Soc Nephrol 1992; 2: 1345–1354 [DOI] [PubMed] [Google Scholar]
- 4. Chan OT, Hannum LG, Haberman AM. et al. A novel mouse with B cells but lacking serum antibody reveals an antibody-independent role for B cells in murine lupus. J Exp Med 1999; 189: 1639–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chan O, Shlomchik MJ.. A new role for B cells in systemic autoimmunity: B cells promote spontaneous T cell activation in MRL-lpr/lpr mice. J Immunol 1998; 160: 51–59 [PubMed] [Google Scholar]
- 6. Alarcón GS. Multiethnic lupus cohorts: what have they taught us? Reumatol Clin 2011; 7: 3–6 [DOI] [PubMed] [Google Scholar]
- 7. Dörner T, Giesecke C, Lipsky PE.. Mechanisms of B cell autoimmunity in SLE. Arthritis Res Ther 2011; 13: 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dörner T, Jacobi AM, Lipsky PE.. B cells in autoimmunity. Arthritis Res Ther 2009; 11: 247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ahuja A, Shupe J, Dunn R. et al. Depletion of B cells in murine lupus: efficacy and resistance. J Immunol 2007; 179: 3351–3361 [DOI] [PubMed] [Google Scholar]
- 10. Bekar KW, Owen T, Dunn R. et al. Prolonged effects of short-term anti-CD20 B cell depletion therapy in murine systemic lupus erythematosus. Arthritis Rheum 2010; 62: 2443–2457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bouaziz JD, Yanaba K, Venturi GM. et al. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc Natl Acad Sci USA 2007; 104: 20878–20883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ding C, Foote S, Jones G.. B-cell-targeted therapy for systemic lupus erythematosus: an update. BioDrugs 2008; 22: 239–249 [DOI] [PubMed] [Google Scholar]
- 13. Dorner T, Goldenberg DM.. Targeting CD22 as a strategy for treating systemic autoimmune diseases. Ther Clin Risk Manag 2007; 3: 953–959 [PMC free article] [PubMed] [Google Scholar]
- 14. Mary C, Laporte C, Parzy D. et al. Dysregulated expression of the Cd22 gene as a result of a short interspersed nucleotide element insertion in Cd22a lupus-prone mice. J Immunol 2000; 165: 2987–2996 [DOI] [PubMed] [Google Scholar]
- 15. Dorner T, Shock A, Goldenberg DM. et al. The mechanistic impact of CD22 engagement with epratuzumab on B cell function: implications for the treatment of systemic lupus erythematosus. Autoimmun Rev 2015; 14: 1079–1086 [DOI] [PubMed] [Google Scholar]
- 16. Hatta Y, Tsuchiya N, Matsushita M. et al. Identification of the gene variations in human CD22. Immunogenetics 1999; 49: 280–286 [DOI] [PubMed] [Google Scholar]
- 17. Gopal AK, Press OW.. Clinical applications of anti-CD20 antibodies. J Lab Clin Med 1999; 134: 445–450 [DOI] [PubMed] [Google Scholar]
- 18. DiLillo DJ, Hamaguchi Y, Ueda Y. et al. Maintenance of long-lived plasma cells and serological memory despite mature and memory B cell depletion during CD20 immunotherapy in mice. J Immunol 2008; 180: 361–371 [DOI] [PubMed] [Google Scholar]
- 19. Neubert K, Meister S, Moser K. et al. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat Med 2008; 14: 748. [DOI] [PubMed] [Google Scholar]
- 20. Ichikawa HT, Conley T, Muchamuel T. et al. Beneficial effect of novel proteasome inhibitors in murine lupus via dual inhibition of Type I interferon and autoantibody-secreting cells. Arthritis Rheum 2012; 64: 493–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seavey MM, Lu LD, Stump KL. et al. Novel, orally active, proteasome inhibitor, delanzomib (CEP-18770), ameliorates disease symptoms and glomerulonephritis in two preclinical mouse models of SLE. Int Immunopharmacol 2012; 12: 257–270 [DOI] [PubMed] [Google Scholar]
- 22. Radbruch A, Muehlinghaus G, Luger EO. et al. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat Rev Immunol 2006; 6: 741–750 [DOI] [PubMed] [Google Scholar]
- 23. Mei HE, Schmidt S, Dörner T.. Rationale of anti-CD19 immunotherapy: an option to target autoreactive plasma cells in autoimmunity. Arthritis Res Ther 2012; 14: S1–SS [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Szili D, Cserhalmi M, Banko Z. et al. Suppression of innate and adaptive B cell activation pathways by antibody coengagement of FcgammaRIIb and CD19. mAbs 2014; 6: 991–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Horton HM, Chu SY, Ortiz EC. et al. Antibody-mediated coengagement of FcgammaRIIb and B cell receptor complex suppresses humoral immunity in systemic lupus erythematosus. J Immunol 2011; 186: 4223–4233 [DOI] [PubMed] [Google Scholar]
- 26. Mackay F, Schneider P.. Cracking the BAFF code. Nat Rev Immunol 2009; 9: 491–502 [DOI] [PubMed] [Google Scholar]
- 27. Mackay F, Schneider P, Rennert P. et al. BAFF AND APRIL: a tutorial on B cell survival. Annu Rev Immunol 2003; 21: 231–264 [DOI] [PubMed] [Google Scholar]
- 28. Chan VS, Tsang HH, Tam RC. et al. B-cell-targeted therapies in systemic lupus erythematosus. Cell Mol Immunol 2013; 10: 133–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gross JA, Johnston J, Mudri S. et al. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature 2000; 404: 995–999 [DOI] [PubMed] [Google Scholar]
- 30. Kayagaki N, Yan M, Seshasayee D. et al. BAFF/BLyS receptor 3 binds the B cell survival factor BAFF ligand through a discrete surface loop and promotes processing of NF-kappaB2. Immunity 2002; 17: 515–524 [DOI] [PubMed] [Google Scholar]
- 31. Jacob CO, Pricop L, Putterman C. et al. Paucity of clinical disease despite serological autoimmunity and kidney pathology in lupus-prone New Zealand mixed 2328 mice deficient in BAFF. J Immunol 2006; 177: 2671–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chu VT, Enghard P, Schurer S. et al. Systemic activation of the immune system induces aberrant BAFF and APRIL expression in B cells in patients with systemic lupus erythematosus. Arthritis Rheum 2009; 60: 2083–2093 [DOI] [PubMed] [Google Scholar]
- 33. Ritterhouse LL, Crowe SR, Niewold TB. et al. B lymphocyte stimulator levels in systemic lupus erythematosus: higher circulating levels in African American patients and increased production after influenza vaccination in patients with low baseline levels. Arthritis Rheum 2011; 63: 3931–3941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Menard LC, Habte S, Gonsiorek W. et al. B cells from African American lupus patients exhibit an activated phenotype. JCI Insight 2016; 1: e87310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rovin BH, Furie R, Latinis K. et al. Efficacy and safety of rituximab in patients with active proliferative lupus nephritis: the lupus nephritis assessment with rituximab study. Arthritis Rheum 2012; 64: 1215–1226 [DOI] [PubMed] [Google Scholar]
- 36. Mysler EF, Spindler AJ, Guzman R. et al. Efficacy and safety of ocrelizumab in active proliferative lupus nephritis: results from a randomized, double-blind, phase III study. Arthritis Rheum 2013; 65: 2368–2379 [DOI] [PubMed] [Google Scholar]
- 37. Parikh SV, Rovin BH.. Current and emerging therapies for lupus nephritis. J Am Soc Nephrol 2016; 27: 2929–2939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Houssiau FA, Vasconcelos C, D'Cruz D. et al. Immunosuppressive therapy in lupus nephritis: the Euro-Lupus Nephritis Trial, a randomized trial of low-dose versus high-dose intravenous cyclophosphamide. Arthritis Rheum 2002; 46: 2121–2131 [DOI] [PubMed] [Google Scholar]
- 39. Gourley MF, Austin HA 3rd, Scott D. et al. Methylprednisolone and cyclophosphamide, alone or in combination, in patients with lupus nephritis. A randomized, controlled trial. Ann Intern Med 1996; 125: 549–557. [DOI] [PubMed] [Google Scholar]
- 40. Condon MB, Ashby D, Pepper RJ. et al. Prospective observational single-centre cohort study to evaluate the effectiveness of treating lupus nephritis with rituximab and mycophenolate mofetil but no oral steroids. Ann Rheum Dis 2013; 72: 1280–1286 [DOI] [PubMed] [Google Scholar]
- 41. Roccatello D, Sciascia S, Baldovino S. et al. A 4-year observation in lupus nephritis patients treated with an intensified B-lymphocyte depletion without immunosuppressive maintenance treatment—clinical response compared to literature and immunological re-assessment. Autoimmun Rev 2015; 14: 1123–1130 [DOI] [PubMed] [Google Scholar]
- 42. Reddy V, Klein C, Isenberg DA. et al. Obinutuzumab induces superior B-cell cytotoxicity to rituximab in rheumatoid arthritis and systemic lupus erythematosus patient samples. Rheumatology (Oxford, England) 2017; 56: 1227–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Goede V, Fischer K, Busch R. et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 2014; 370: 1101–1110 [DOI] [PubMed] [Google Scholar]
- 44. Mossner E, Brunker P, Moser S. et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new Type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood 2010; 115: 4393–4402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vital EM, Dass S, Buch MH. et al. B cell biomarkers of rituximab responses in systemic lupus erythematosus. Arthritis Rheum 2011; 63: 3038–3047 [DOI] [PubMed] [Google Scholar]
- 46. Basler M, Mundt S, Bitzer A. et al. The immunoproteasome: a novel drug target for autoimmune diseases. Clin Exp Rheumatol 2015; 33: S74–S79 [PubMed] [Google Scholar]
- 47. Hirai M, Kadowaki N, Kitawaki T. et al. Bortezomib suppresses function and survival of plasmacytoid dendritic cells by targeting intracellular trafficking of Toll-like receptors and endoplasmic reticulum homeostasis. Blood 2011; 117: 500–509 [DOI] [PubMed] [Google Scholar]
- 48. Coppo R. Proteasome inhibitors in progressive renal diseases. Nephrol Dial Transplant 2014; 29: i25–i30 [DOI] [PubMed] [Google Scholar]
- 49. Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene 2006; 25: 6680–6684 [DOI] [PubMed] [Google Scholar]
- 50. Zubair A, Frieri M.. NF-kappaB and systemic lupus erythematosus: examining the link. J Nephrol 2013; 26: 953–959 [DOI] [PubMed] [Google Scholar]
- 51. Tak PP, Firestein GS.. NF-κB: a key role in inflammatory diseases. J Clin Invest 2001; 107: 7–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Alexander T, Sarfert R, Klotsche J. et al. The proteasome inhibitior bortezomib depletes plasma cells and ameliorates clinical manifestations of refractory systemic lupus erythematosus. Ann Rheum Dis 2015; 74: 1474–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhang H, Liu Z, Huang L. et al. The short-term efficacy of bortezomib combined with glucocorticoids for the treatment of refractory lupus nephritis. Lupus 2017; 26: 952–958 [DOI] [PubMed] [Google Scholar]
- 54. Scalzulli E, Grammatico S, Vozella F. et al. Proteasome inhibitors for the treatment of multiple myeloma. Expert Opin Pharmacother 2018; 19: 375–386 [DOI] [PubMed] [Google Scholar]
- 55. Ettari R, Zappala M, Grasso S. et al. Immunoproteasome-selective and non-selective inhibitors: a promising approach for the treatment of multiple myeloma. Pharmacol Ther 2018; 182: 176–192 [DOI] [PubMed] [Google Scholar]
- 56. Navarra SV, Guzman RM, Gallacher AE. et al. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet 2011; 377: 721–731 [DOI] [PubMed] [Google Scholar]
- 57. Furie R, Petri M, Zamani O. et al. A phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B lymphocyte stimulator, in patients with systemic lupus erythematosus. Arthritis Rheum 2011; 63: 3918–3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dooley MA, Houssiau F, Aranow C. et al. Effect of belimumab treatment on renal outcomes: results from the phase 3 belimumab clinical trials in patients with SLE. Lupus 2013; 22: 63–72 [DOI] [PubMed] [Google Scholar]
- 59. Isenberg DA, Petri M, Kalunian K. et al. Efficacy and safety of subcutaneous tabalumab in patients with systemic lupus erythematosus: results from ILLUMINATE-1, a 52-week, phase III, multicentre, randomised, double-blind, placebo-controlled study. Ann Rheum Dis 2016; 75: 323–331 [DOI] [PubMed] [Google Scholar]
- 60. Merrill JT, van Vollenhoven RF, Buyon JP. et al. Efficacy and safety of subcutaneous tabalumab, a monoclonal antibody to B-cell activating factor, in patients with systemic lupus erythematosus: results from ILLUMINATE-2, a 52-week, phase III, multicentre, randomised, double-blind, placebo-controlled study. Ann Rheum Dis 2016; 75: 332–340 [DOI] [PubMed] [Google Scholar]
- 61. Rovin BH, Dooley MA, Radhakrishnan J. et al. The impact of tabalumab on the kidney in systemic lupus erythematosus: results from two phase 3 randomized, clinical trials. Lupus 2016; 25: 1597–1601 [DOI] [PubMed] [Google Scholar]
- 62. Furie RA, Leon G, Thomas M. et al. A phase 2, randomised, placebo-controlled clinical trial of blisibimod, an inhibitor of B cell activating factor, in patients with moderate-to-severe systemic lupus erythematosus, the PEARL-SC study. Ann Rheum Dis 2015; 74: 1667–1675 [DOI] [PubMed] [Google Scholar]
- 63. Ginzler EM, Wax S, Rajeswaran A. et al. Atacicept in combination with MMF and corticosteroids in lupus nephritis: results of a prematurely terminated trial. Arthritis Res Ther 2012; 14: R33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Isenberg D, Gordon C, Licu D. et al. Efficacy and safety of atacicept for prevention of flares in patients with moderate-to-severe systemic lupus erythematosus (SLE): 52-week data (APRIL-SLE randomised trial). Ann Rheum Dis 2015; 74: 2006–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Merrill JT, Wallace DJ, Wax S. et al. Efficacy and safety of atacicept in patients with systemic lupus erythematosus: results of a 24-week randomized, placebo-controlled, phase IIb study. Arthritis Rheum 2018; 70: 266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vallerskog T, Heimburger M, Gunnarsson I. et al. Differential effects on BAFF and APRIL levels in rituximab-treated patients with systemic lupus erythematosus and rheumatoid arthritis. Arthritis Res Ther 2006; 8: R167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Carter LM, Isenberg DA, Ehrenstein MR.. Elevated serum BAFF levels are associated with rising anti-double-stranded DNA antibody levels and disease flare following B cell depletion therapy in systemic lupus erythematosus. Arthritis Rheum 2013; 65: 2672–2679 [DOI] [PubMed] [Google Scholar]
- 68. Kawabata D, Venkatesh J, Ramanujam M. et al. Enhanced selection of high affinity DNA-reactive B cells following cyclophosphamide treatment in mice. PLoS One 2010; 5: e8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Iaccarino L, Bartoloni E, Carli L. et al. Efficacy and safety of off-label use of rituximab in refractory lupus: data from the Italian Multicentre Registry. Clin Exp Rheumatol 2015; 33: 449–456 [PubMed] [Google Scholar]
- 70. Weidenbusch M, Rommele C, Schrottle A. et al. Beyond the LUNAR trial. Efficacy of rituximab in refractory lupus nephritis. Nephrol Dial Transplant 2013; 28: 106–111 [DOI] [PubMed] [Google Scholar]
- 71. Jayne DRW, Bruchfeld AN, Harper L. et al. Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J Am Soc Nephrol 2017; 28: 2756–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Khodadadi L, Cheng Q, Alexander T. et al. Bortezomib plus continuous B cell depletion results in sustained plasma cell depletion and amelioration of lupus nephritis in NZB/W F1 mice. PloS One 2015; 10: e0135081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lu LD, Stump KL, Wallace NH. et al. Depletion of autoreactive plasma cells and treatment of lupus nephritis in mice using CEP-33779, a novel, orally active, selective inhibitor of JAK2. J Immunol 2011; 187: 3840–3853 [DOI] [PubMed] [Google Scholar]
- 74. Ma TK, McAdoo SP, Tam FW.. Targeting the tyrosine kinase signalling pathways for treatment of immune-mediated glomerulonephritis: from bench to bedside and beyond. Nephrol Dial Transplant 2017; 32: i129–ii38 [DOI] [PMC free article] [PubMed] [Google Scholar]