Abstract
Patient: Female, 19-year-old
Final Diagnosis: Status epilepticus and stress induced cardiomyopathy
Symptoms: Seizure
Medication: —
Clinical Procedure: —
Specialty: Toxicology
Objective:
Unusual clinical course
Background:
Synthetic cannabinoids have a higher affinity for the cannabinoid receptors CB1 and CB2 than natural cannabinoids. Their use can be associated with cardiovascular disease and neurological complications. A case is reported of status epilepticus and stress cardiomyopathy following the recreational use of the synthetic cannabinoid, UR-144.
Case Report:
A 19-year-old woman presented to the emergency department in status epilepticus after smoking the synthetic cannabinoid known as ‘space’. Recurring seizure activity was controlled after three hours. On hospital day 3, the patient developed severe biventricular failure. Cardiac magnetic resonance imaging (MRI) confirmed the diagnosis of stress cardiomyopathy. A comprehensive urine drug screen was performed using gas chromatography-mass spectrometry (GC-MS), which was positive for UR-144, or (1-pentyl-1H-indol-3-yl) (2,2,3,3-tetramethylcyclopropyl)-methanone, and negative for all other illicit recreational drugs. The patient improved at one week following admission, with a left ventricular ejection fraction (LVEF) of 40%. She was discharged home on hospital day 10.
Conclusions:
The use of the synthetic cannabinoid, UR-144, may be associated with prolonged status epilepticus and stress cardiomyopathy. Physicians should be aware of these potentially lethal complications associated with the recreational use of this and other illicit synthetic cannabinoids.
MeSH Keywords: Cannabinoids, Drug Overdose, Heart Failure, Status Epilepticus
Background
Synthetic cannabinoids were originally developed to study endogenous cannabinoid receptors and for therapeutic drug development [1,2]. They have much greater affinity for the cannabinoid receptors CB1 and CB2 than naturally occurring cannabinoids. Production and manufacturing of these compounds for illicit use have become attractive, as these compounds are perceived to give a more intense ‘high’ than natural cannabinoids, and they escape detection by traditional urine drug screens [1,2].
Several synthetic cannabinoids are commercially available, including but not limited to JWH 018, JWH 157, JWH 176, HU 210, and UR-144 [1,2]. Synthetic cannabinoids are marketed under brand names, such as ‘spice,’ ‘K2,’ or ‘space.’ They are labeled as being not for human consumption or aroma-therapy use only. Synthetic cannabinoids produce physiological and psychoactive effects similar to delta-9-tetrahydrocannabinol but with greater intensity. Their use has been associated with several complications, including seizures, myocardial infarction, ischemic strokes, and acute renal failure [1].
A case is reported of status epilepticus and stress cardiomyopathy following the recreational use of the synthetic cannabinoid, UR-144.
Case Report
A 19-year-old woman, weighing 67.8 kg, presented with generalized tonic-clonic seizures after smoking the synthetic cannabinoid known as ‘space’ (Figure 1) [1]. She had no history of other drug use, and she was previously healthy with no medical history and no allergies. She was transferred to a small, regional emergency department, Foothills Medical Centre in Calgary, Alberta, Canada, where she experienced a further generalized tonic-clonic seizure.
Figure 1.

Photograph of the synthetic cannabinoid known as ‘space,’ which was used illicitly by the patient
On examination following hospital transfer, her vital signs included a heart rate (HR) of 138 beats per minute (bpm), blood pressure (BP) of 90/60, a respiratory rate (RR) of 28 per min, and a temperature of 37.1°C. Her blood glucose was 10.1 mmol/L. The seizures were refractory to treatment with 8 mg of intravenous (IV) lorazepam, 10 mg of IV midazolam, and 1400 mg of IV phenytoin. The patient was subsequently intubated and transferred to a tertiary care hospital. After approximately three hours of intermittent seizure activity, her seizures were finally controlled with intravenous infusions of midazolam, propofol, and 1500 mg of IV levetiracetam. The patient required intermittent blood pressure support with intravenous norepinephrine and intubation. Computed tomography (CT) imaging of the head was normal. Her metabolic workup was unremarkable with a normal glucose level, and a blood ethanol level of <2 mmol/l. A lumbar puncture was performed and showed no cerebral spinal fluid (CSF) abnormalities.
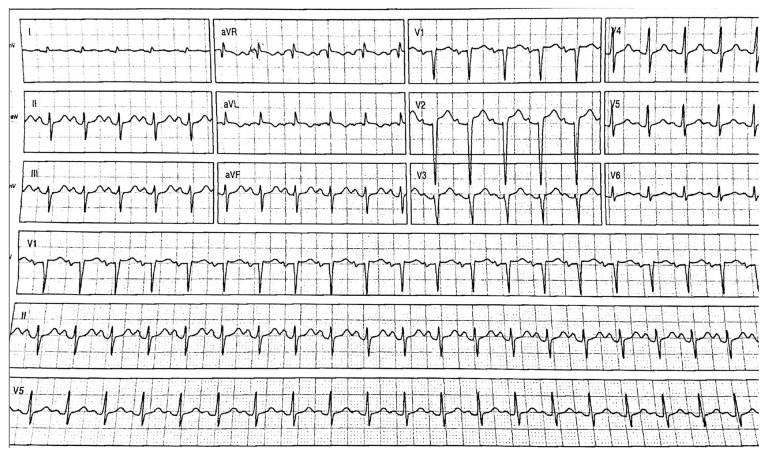
She was admitted to the intensive care unit (ICU), where she was extubated the following day. At this time, the patient had a normal mental status and was stable. However, she was tachycardic with a heart rate of approximately 150 bpm. On day 3 following admission, the patient started to complain of shortness of breath and was found to be tachypneic and hypoxic. An electrocardiogram (ECG) showed dynamic ST-T wave changes in the anteroseptal precordial leads and the development of pathologic Q waves (Figure 2). A chest X-ray showed pulmonary edema. Her troponin levels peaked at 268 ng/l. An echo-cardiogram showed severe global hypokinesia with an ejection fraction (EF) of 16% and apical ballooning. The basal portions of the ventricular walls were hyperdynamic, while the mid to apical regions were akinetic. She was treated with noninvasive positive pressure ventilation (PPV), low-dose ramipril, and titrated doses of intravenous nitroglycerin.
Figure 2.
The findings of the 12-lead electrocardiogram (ECG) on hospital admission. The 12-lead ECG shows non-specific ST and T wave abnormalities and the development of pathologic Q waves in leads V2 through V6.
Further history from the patient revealed no prior use of cocaine, ethanol, or any sympathomimetic substances, but she admitted to smoking the synthetic cannabinoid known as ‘space’ multiple times for at least an hour to two hours before her initial presentation. She had no significant past medical history and no prior seizures. Because conventional urine drug assays are not able to detect synthetic cannabinoids, a comprehensive urine drug screen using gas chromatography-mass spectrometry (GC-MS) was performed. Urine screening was positive for the synthetic cannabinoid UR-144 and negative for cocaine, amphetamines, tetrahydrocannabinol (THC) and other synthetic cannabinoids. The GC-MS comprehensive urine drug screen detected the following 11 synthetic cannabinoid metabolites: RSC-4 [N-(5-carboxypentyl), N-(5-hydroxypentyl)]; JWH-018 [Parent and N-Pentanoic acid, N- 4/5 hydroxypentyl) metabolites]; JWH-073 [Parent and N-Butanoic acid, N-(3-hydroxybutyl), and N-(4-hydroxybutyl) metabolites]; JWH-250 [N-(5-carboxypentyl), N-(4/5-hydroxypentyl) metabolites]; JWH-200 [4-hydroxyindole metabolite]; JWH-122 [N-(4/5 hydroxypentyl) metabolites]; URR-144 [N-pentanoic acid, N-(4/5 hydroxypentyl) metabolites]; AM-2201 [N-(hydroxypentyl) metabolite]; JWH-398 [N-(5-hydroxypentyl) metabolites]; MAM-2201 [N-pentanoic acid]; and XLR-11 [N-pentanoic acid, N-(4/5 hydroxypentyl).
In the ICU, she developed respiratory fatigue and was intubated. Subsequently, a diagnostic coronary angiography was performed, which showed no coronary artery dissection or stenosis and did not suggest any anomalous coronary artery origin. She then underwent myocardial biopsy, which showed mild myocardial cell hypertrophy and fibrosis but was not diagnostic for myocarditis, amyloidosis, sarcoidosis, or hemochromatosis. Hemodynamic and respiratory parameters improved, and the patient was subsequently extubated. A cardiac MRI was performed and confirmed the findings of stress cardiomyopathy (Figures 3, 4). Repeat echocardiography showed a significant improvement in left ventricular function a week following her initial presentation. Her ejection fraction improved to 40%, although the apical portion of the ventricles remained hypokinetic. The ECG changes resolved, and she was discharged on hospital day 10 with bisoprolol 5 mg per day and lisinopril10 mg per day with a diagnosis of stress cardiomyopathy.
Figure 3.
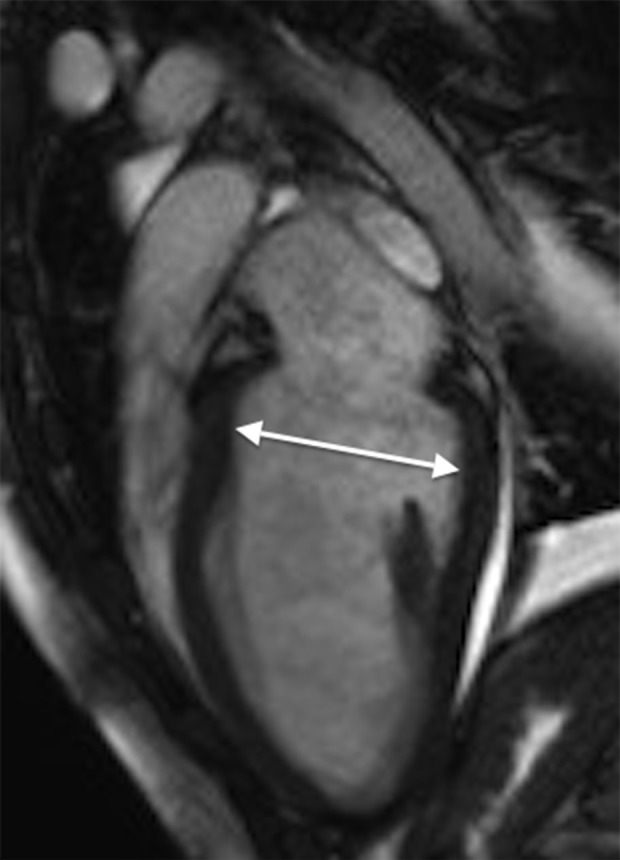
Cardiac magnetic resonance imaging (MRI) on hospital admission. Steady-state free precision (SSFP) cine 2-dimensional (2D) cardiac MRI in diastole showing apical ballooning. The arrows indicate ventricular relaxation.
Figure 4.
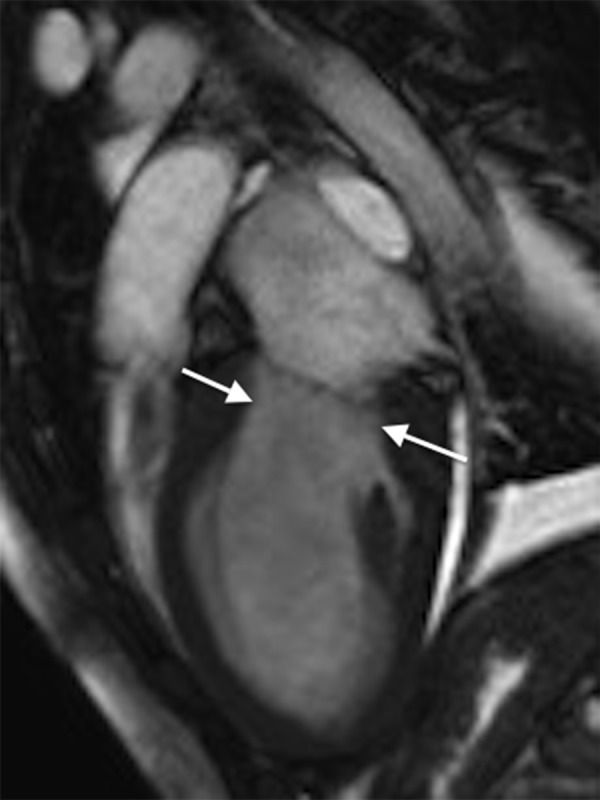
Cardiac magnetic resonance imaging (MRI) on hospital admission. Steady-state free precision (SSFP) cine 2-dimensional (2D) cardiac MRI in diastole showing apical ballooning. The arrows indicate ventricular contraction.
Discussion
To our knowledge, this is the first reported case that describes the development of transient severe biventricular failure in association with recent exposure to synthetic cannabinoids. One previously reported case was of a 16-year-old male patient who presented with chest pain 60–90 min after smoking the synthetic cannabinoid, K2 [3]. This previously reported case developed persistent ST-segment elevation and had a peak troponin level of 8.29 ng/ml [3]. Another case series described three cases of myocardial injury also associated with the recent use of K2 [4]. In all cases, ST-segment elevation was reported with increased troponin levels, but there was no global ventricular dysfunction. In the patient presented in the present report, two days after smoking the synthetic cannabinoid, ‘space,’ there was evidence of myocardial dysfunction and severe biventricular failure requiring advanced airway protection and inotropic support.
Synthetic cannabinoids are molecules that have much higher affinity for cannabinoid receptors than delta-9 tetrahydrocannabinol, which is the most physiologically active ingredient in marijuana) [2]. Marijuana has previously been associated with cardiovascular complications after following its use [5–9]. Therefore, it is not surprising that synthetic cannabinoids lead to similar complications. Marijuana has been shown to increase the risk of myocardial infarction by 4.8-fold in the first hour after smoking [8]. Synthetic cannabinoids have been implicated in ischemic stroke in three previously reported cases [10,11].
In the patient presented in this report, the synthetic cannabinoid, UR-144, was detected in urine. UR-144 was developed as a research chemical by Abbott Laboratories in 2006 [12]. Structurally, it is similar to JWH-018, one of the first synthetic cannabinoids detected in herbal preparations for illicit use [13,14]. UR-144 has a much higher affinity for CB2 receptors and a lower affinity for CB1 receptors [12]. CB1 receptors are thought to be responsible for the psychoactive effects experienced with synthetic cannabinoids [15]. Despite the weak CB1 affinity, UR-144 has been reported by drug users in internet forums to have positive psychoactive properties [16]. To our knowledge, one case report in Poland described the development of prolonged seizure activity in a 22-year-old man after smoking a ‘joint’ that was later discovered to include UR-144 [16]. However, the patient did not develop cardiovascular complications and was discharged from the emergency department on the same day of admission [16].
The mechanism of transient cardiomyopathy resulting from the use of synthetic cannabinoids is unclear. Whether it is a direct effect from synthetic cannabinoid itself or an indirect effect from the other complications of synthetic cannabinoid use, for example, from prolonged seizure activity, remains to be elucidated. Synthetic cannabinoids cause hypertension and tachycardia, probably as a result of stimulation of the sympathetic nervous system combined with parasympathetic blockade [17]. There have been previous reports of cannabinoid-induced coronary vasospasm [18,19]. Also, CB1 and CB2 receptors are found in myocardial tissue [20,21]. Although endogenous cannabinoids (endocannabinoids) have been proposed to be cardioprotective under situations of stress, the activation of CB1 receptors cause depressed myocardial contractility and hypotension [20,21]. The effects of the catecholamine surge associated with coronary vasospasm might have resulted in an increased demand to supply ratio that could have caused or exacerbated the myocardial damage and the stress cardiomyopathy induced in this patient. Also, cannabinoid smoking is associated with an increase in carboxyhemoglobin levels, which can reduce the oxygen-carrying capacity of the blood, further exacerbating myocardial ischemia [22,23].
Acute stress cardiomyopathy, or Takotsubo cardiomyopathy, is more common in women who have a history of psychiatric or neurologic disease [24]. Stress cardiomyopathy can be triggered by a variety of stimuli, including those affecting the central nervous system (CNS) [24]. The majority of cases (81%) are reversible but can clinically mimic acute coronary syndrome (ACS) [24]. However, in the patients presented in this report, there was no history of emotional stressors, and stress cardiomyopathy was probably related to the effects of the synthetic cannabinoid and the recurrent seizures.
The mechanism of seizures from synthetic cannabinoids remains unknown. Cannabinoid receptors mediate neuronal excitability via γ-aminobutyric acid (GABA) and glutamate neurotransmission [25]. Specifically, cannabinoid receptors reduce both glutamate and GABA neurotransmission in the brain. Glutamate is an excitatory amino acid, and reducing its neurotransmission will result in reduced susceptibility to seizures [25]. However, GABA is an inhibitory amino acid, and reducing its neurotransmission will increase the susceptibility to seizures [25]. Some synthetic cannabinoids may have a more potent effect in reducing GABA neurotransmission than in reducing glutamate neurotransmission [25,26], which might partly explain their overall epileptogenic mechanism. While seizures have been described previously following synthetic cannabinoid use [16,27], the status epilepticus experienced by this patient appears to be unique in the literature and may have contributed to her cardiomyopathy.
Conclusions
A case is reported of status epilepticus and stress cardiomyopathy following the recreational use of the synthetic cannabinoid, UR-144. Physicians should be aware of these potentially lethal complications associated with the recreational use of this and other illicit synthetic cannabinoids. In adults and adolescents who present with symptoms of chest pain or shortness of breath, it is important to ask about the use of synthetic cannabinoids as well as other illicit sympathomimetic drugs as part of the general clinical assessment.
Footnotes
Conflict of interest
None.
References:
- 1.Al Deeb ML, Huffman J, Kholaif N, et al. Status epilepticus and transient cardiomyopathy associated with synthetic cannabinoid UR-144. Clin Toxicol. 2015;53:675–76. [Google Scholar]
- 2.Castaneto MS, Gorelick DA, Desrosiers NA, et al. Synthetic cannabinoids: Epidemiology, pharmacodynamics, and clinical implications. Drug Alcohol Depend. 2014;144:12–41. doi: 10.1016/j.drugalcdep.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKeever RG, Vearrier D, Jacobs D, et al. K2-not the spice of life; Synthetic cannabinoids and ST elevation myocardial infarction: A case report. J Med Toxicol. 2015;11(1):129–31. doi: 10.1007/s13181-014-0424-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mir A, Obafemi A, Young A, Kane C. Myocardial infarction associated with use of the synthetic cannabinoid K2. Pediatrics. 2011;128(6):e1622–27. doi: 10.1542/peds.2010-3823. [DOI] [PubMed] [Google Scholar]
- 5.Bachs L, Mørland H. Acute cardiovascular fatalities following cannabis use. Forensic Sci Int. 2001;124(2–3):200–3. doi: 10.1016/s0379-0738(01)00609-0. [DOI] [PubMed] [Google Scholar]
- 6.Aryana A, Williams MA. Marijuana as a trigger of cardiovascular events: Speculation or scientific certainty? Int J Cardiol. 2007;118(2):141–44. doi: 10.1016/j.ijcard.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Fisher BA, Ghuran A, Vadamalai V, Antonios TF. Cardiovascular complications induced by cannabis smoking: A case report and review of the literature. Emergency Med J. 2005;22(9):679–80. doi: 10.1136/emj.2004.014969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mittleman MA, Lewis RA, Maclure M, et al. Triggering myocardial infarction by marijuana. Circulation. 2001;103(23):2805–9. doi: 10.1161/01.cir.103.23.2805. [DOI] [PubMed] [Google Scholar]
- 9.Mukamal KJ, Maclure M, Muller JE, Mittleman MA. An exploratory prospective study of marijuana use and mortality following acute myocardial infarction. Am Heart J. 2008;155(3):465–70. doi: 10.1016/j.ahj.2007.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Freeman MJ, Rose DZ, Myers MA, et al. Ischemic stroke after use of the synthetic marijuana “spice”. Neurology. 2013;81(24):2090–93. doi: 10.1212/01.wnl.0000437297.05570.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takematsu M, Hoffman RS, Nelson LS, et al. A case of acute cerebral ischemia following inhalation of a synthetic cannabinoid. Clin Toxicol. 2014;52(9):973–75. doi: 10.3109/15563650.2014.958614. [DOI] [PubMed] [Google Scholar]
- 12.Frost JM, Dart MJ, Tietje KR, et al. Indol-3-ylcycloalkyl ketones: Effects of N1 substituted indole side chain variations on CB(2) cannabinoid receptor activity. J Medicinal Chem. 2010;53(1):295–315. doi: 10.1021/jm901214q. [DOI] [PubMed] [Google Scholar]
- 13.Zuba D, Byrska B, Maciow M. Comparison of “herbal highs” composition. Anal Bioanal Chem. 2011;400(1):119–26. doi: 10.1007/s00216-011-4743-7. [DOI] [PubMed] [Google Scholar]
- 14.Zuba D, Byrska B. Analysis of the prevalence and coexistence of synthetic cannabinoids in “herbal high” products in Poland. Forensic Toxicol. 2013;31(1):21–30. [Google Scholar]
- 15.Huffman JW, Dai D. Design, synthesis and pharmacology of cannabimimetic indoles. Bioorg Med Chem Lett. 1993;4(4):563–66. [Google Scholar]
- 16.Adamowicz P, Zuba D, Sekula K. Analysis of UR-144 and its pyrolysis product in blood and their metabolites in urine. Forensic Sci Int. 2013;233(1–3):320–27. doi: 10.1016/j.forsciint.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Gash A, Karliner JS, Janowsky D, Lake CR. Effects of smoking marihuana on left ventricular performance and plasma norepinephrine: Studies in normal men. Ann Intern Med. 1978;89(4):448–52. doi: 10.7326/0003-4819-89-4-448. [DOI] [PubMed] [Google Scholar]
- 18.Basnet S, Mander G, Nicolas R. Coronary vasospasm in an adolescent resulting from marijuana use. Pediatric Cardiol. 2009;30(4):543–45. doi: 10.1007/s00246-009-9384-7. [DOI] [PubMed] [Google Scholar]
- 19.Rezkalla SH, Sharma P, Kloner RA. Coronary no-flow and ventricular tachycardia associated with habitual marijuana use. Ann Emergency Med. 2003;42(3):365–69. doi: 10.1016/s0196-0644(03)00426-8. [DOI] [PubMed] [Google Scholar]
- 20.Hiley CR. Endocannabinoids and the heart. J Cardiovasc Pharmacol. 2009;53(4):267–76. doi: 10.1097/FJC.0b013e318192671d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaschina E. Cannabinoids in Health and Disease. InTech Open; 2016. Cannabinoid CB1/CB2 receptors in the heart: Expression, regulation, and function; pp. 169–85. [Google Scholar]
- 22.Weiss JL, Watanabe AM, Lemberger L, et al. Cardiovascular effects of delta-9-tetrahydrocannabinol in man. Clin Pharmacol Ther. 1972;13(5):671–84. doi: 10.1002/cpt1972135part1671. [DOI] [PubMed] [Google Scholar]
- 23.Renault PF, Schuster CR, Heinrich R, Freeman DX. Marihuana: Standardized smoke administration and dose effect curves on heart rate in humans. Science (New York, NY) 1971;174(4009):589–91. doi: 10.1126/science.174.4009.589. [DOI] [PubMed] [Google Scholar]
- 24.Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373:929–38. doi: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 25.De Havenon A, Chin B, Thomas KC, Afra P. The secret “spice”: An undetectable toxic cause of seizure. Neurohospitalist. 2011;1(4):182–86. doi: 10.1177/1941874411417977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hájos N, Ledent C, Freund TF. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106(1):1–4. doi: 10.1016/s0306-4522(01)00287-1. [DOI] [PubMed] [Google Scholar]
- 27.Lapoint J, James LP, Moran CL, et al. Severe toxicity following synthetic cannabinoid ingestion. Clin Toxicol. 2011;49(8):760–64. doi: 10.3109/15563650.2011.609822. [DOI] [PMC free article] [PubMed] [Google Scholar]