Abstract
Poor growth has long been a characteristic feature of cystic fibrosis (CF) and is significantly linked to lung function and overall health status. Improvements in pulmonary and nutrition care for patients with cystic fibrosis (CF) have resulted in better growth outcomes; however, height gains have not paralleled the improvements in weight in children with CF, and patients with more severe CF mutations remain significantly more affected. Many factors affect the growth hormone-IGF-1 axis and the growth plate of the long bones, including the chronic inflammatory state associated with CF. There are also increasing data on the direct effects of CFTR on bone and implications for CFTR modulators in attaining optimal growth. Treatments aimed at improving growth in CF are also reviewed here.
Keywords: growth, short stature, cystic fibrosis, growth delay
1. Background
Growth is a key indicator of health status in children with cystic fibrosis (CF) and is strongly linked with CF outcomes such as nutrition and pulmonary function (1-5). While monitoring growth and nutrition status at every visit is a standard component of optimal CF care, particularly critical times for meticulous attention to growth include the 12 months after initial diagnosis, whether made prenatally, through newborn screening, or later diagnosis, and the peripubertal period (6). Lower weight-for-age and height-forage during the toddler years are associated with subsequently lower pulmonary function (2), and weight-for-age <50th percentile (4) and lower height (1) are risk factors for early mortality by young adulthood. Stunting, defined as <5th percentile for height, is an independent risk factor for mortality after adjusting for factors such as CFTR genotype, FEV1, exocrine pancreatic insufficiency, or bacterial colonization (7). Early intervention to improve growth can mitigate this risk; for infants with CF who attain weight z-score at or above birth weight by age 2 years, lung function is significantly better at age 6 years (3), and higher weight-for-age at age 4 years is associated with improved pulmonary function, height, and pubertal progression (5).
The advent of newborn screening for CF has allowed for earlier implementation of pancreatic enzyme replacement therapy, and other aggressive nutritional interventions have resulted in overall better growth (8, 9). However, improvements in height have continued to lag behind improvements in weight. In a cohort of infants diagnosed on newborn screening, 23.9% of infants remained below 10th percentile of length for age at 1 year of age, compared with only 13.6% below the 10th percentile of weight- for- age (9). In a 2012 analysis of another cohort of children diagnosed with CF by newborn screening, only 32% had attained predicted mid-parental height by puberty (10), while data from the CF Foundation registry that year indicated that 54% of children were at goal of 50th percentile for body mass index (BMI) (11). Of note, because the calculation of BMI is dependent on height, in instances of stunted linear growth, BMI would provide an incomplete assessment of growth status, as weight gain without linear growth would still result in higher BMI. Indeed, in children with CF and a BMI between the 25th and 50th percentiles, 16.8% had weight-for-age below the 10th percentile and 26.6% had height- for- age below the 10th percentile (12).
2. Mechanisms and Pathophysiology
The hypothalamic – pituitary – growth axis is the main determinant of postnatal growth. In response to hypothalamic signals, growth hormone (GH) is secreted by the anterior pituitary gland, thereby increasing production of insulin-like growth factor −1 (IGF-1) from both the liver and target tissues. In combination with GH, IGF-1 acts on the cartilaginous growth plates of the long bones to effect linear growth (13). Normally, growth rates are highest in utero, during infancy, and during puberty, with an intervening period of slower growth in childhood (14). At pubertal onset, there is a slowing of growth velocity prior to a pubertal acceleration; estrogen and particularly testosterone transiently stimulate pituitary GH secretion and growth acceleration, with peak height velocity occurring during mid- to late puberty, slightly earlier in girls than boys (15). Many chronic illnesses of childhood are associated with pubertal delay and impaired growth; children with CF can demonstrate this decrease in pubertal peak height velocities (16, 17) and delayed pubertal growth spurt, with greater delays in those with more severely affected lung function (16). Accordingly, patients with CF may display more severe growth impairment during puberty than in the prepubertal years (beyond infancy) (18-20).
2.1. CFTR, linear growth, and the GH-IGF-1 axis
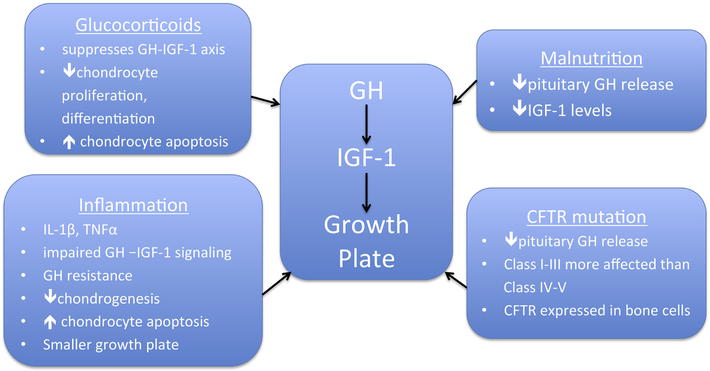
Contributors to poor growth in CF are illustrated in Figure 1. CFTR mutation class continues to demonstrate a relationship between the severity of mutation and growth impairment that becomes more prominent over time (11). The CF Foundation Patient Registry Data Report for 2017 indicates that for infants under 24 months of age, those with the most severe mutations (class I-III) continue to have lower weight and substantially lower length percentiles than those with class IV and V mutations; with median weight percentile of 41.8 for I-III versus 51.4 for IV-V, and median length percentile of 29.5 for I-III versus 35.5 for IV-V(11). For children aged 2 to 19 years, the differences in mutation severity become more pronounced with the median weight percentile of 44.6 for class I-III compared with 61.0 for class IV-V, and a dramatic difference in median height percentile of 35.5 for class I-III versus 51.6 for Class IV-V (11).
Figure 1. Contributors to Poor Growth in CF.
Multiple factors are implicated in poor growth in CF affect the GH-IGF-1-growth plate axis and can impair growth through actions on GH-IGF-1 signaling and through direct effects on the growth plate.
The relationship between CFTR mutations and impaired growth may be partially explained by exocrine pancreatic insufficiency (PI), which is highly linked to genotype (21). Within the first 6 weeks of life, up to 85% of infants with class I-III mutations are already pancreatic insufficient (22), with 100% progression to PI by age 12 months in infants with class I and II mutations (23). Deficiency of pancreatic lipases results in macronutrient malabsorption, with particularly high caloric losses from fat malabsorption, leading to poor growth through worsening malnutrition.
The GH-IGF-1 axis is suppressed in states of malnutrition and inflammation, as seen in other chronic pro-inflammatory conditions of childhood such as juvenile idiopathic arthritis, inflammatory bowel disease, and also CF (24). However, growth impairment has also been reported in individuals with CF without substantial malnutrition or inflammation. For example, several studies have demonstrated that birth weight is already lower in neonates with CF (9, 25-27) prior to the onset of chronic lung infections or malabsorption, with subsequent faltering in length by age 12 months despite maintenance of adequate correction of weight with nutritional management (9), thus implicating a more direct role for CFTR mutations in growth impairment. Birth length has also been reported to be lower in neonates with CF (18), although not consistently (9). Hypotheses regarding the etiology of in utero growth failure include increased energy requirements and energy expenditure due to meconium ileus, which could shunt energy balance away from growth (27), despite the lack of dependence on the gut for nutrition during fetal life, and possible effects of defective CFTR expressed in placental tissue. The finding that placental CFTR may mediate placental aquaporin activity (28) raises the question of the role of impaired amniotic fluid and nutrient solute exchange as a factor in impaired prenatal growth. However, data to support these hypotheses remain limited.
Whether CFTR dysfunction may directly affect the GH-IGF-1 axis is also unknown. Lower IGF-1 levels are noted in both children and adults with CF (29, 30) and has previously been attributed to malnutrition, but has also been noted to be already lower in the neonatal period in both humans and animal models. Specifically, IGF-1 levels measured from blood spot newborn screening samples indicated that newborns with CF (n=23) already have a small but statistically significant reductions in IGF-1 when compared to newborns without CF (n=41) (31). At birth, CFTR-null (CFTR−/−) pigs demonstrate decreased pituitary GH release (from pituitary cell cultures), lower serum IGF-1 levels, and decreased bone mineral content compared to non-CF controls, despite non-significantly lower weight (31). Lower IGF-1 concentrations have also been found in CF-mice, although not until 3 weeks of postnatal life (28), indicating some interspecies variability of impairments in the GH-IGF axis in the context of CF.
Despite consistent reports of lower IGF-1 concentrations in both humans and animal models of CF (28-32), some controversy exists over the adequacy of GH hormone production in CF. Whereas some studies have suggested a decreased production of GH and IGF-1 in individuals with CF, other studies imply normal GH and/or IGF-1 concentrations in CF (33-35). Further complicating our understanding of the etiology of GH axis dysfunction in CF, in a single study of patients with CF and significant growth failure who underwent provocative testing for GH deficiency (n=18), two-thirds of subjects met standard diagnostic criteria for GH deficiency (36). Thus, CF may demonstrate significant variability along the GH-IGF-1 axis at multiple levels, and CF-related growth impairment and may have features both similar to and distinct from other states of GH deficiency or impaired GH-IGF-1 action.
CFTR is expressed in human bone and has been identified in neonatal osteoblasts, osteocytes, and osteoclasts (37). CFTR-deficient mice, CFTR-deficient rats and neonatal CFTR−/− pigs have all demonstrated reduced bone length in addition to diminished bone quality (31, 32, 38). CF rats revealed both reduced bone thickness and reduced IGF-1 levels when compared to wild-type controls (32). Taken together, these data suggest multiple mechanisms for direct effects of CF mutations on poor growth, which are distinct from and additive to the effects of malabsorption-related malnutrition.
The presence of CFTR in human bone (37) raises the possibility of an intrinsic defect in linear growth related to CFTR mutations; as the linear growth from the growth plate involves a multitude of cellular interactions that could be impacted either directly or indirectly by CFTR. Furthermore, differences have been demonstrated in the growth plates—specifically of the hypertrophic zone—of CF rats compared to wildtype littermates (32). Perhaps poor linear growth is a combination of intrinsic factors, hormonal influences, and biochemical influences all interrelated to overall bone health and linear growth in the CFTR deficient state.
2.2. Inflammation and glucocorticoids
The chronic pro-inflammatory state of CF is associated with increased levels of the pro-inflammatory cytokines IL-1β and TNFα, resulting in a smaller growth plate from decreased chondrogenesis (39) and increased chondrocyte apoptosis (40). IL-1β and TNFα inhibit the growth of metatarsal bones in culture and have synergistic effects on growth reduction when applied in combination (41). The mechanism for these effects may be related to impaired intracellular signaling along the GH-IGF-1 axis, as IL-1β has been shown to inhibit GH signaling in hepatocytes, resulting in GH resistance in the liver (42) and impairment of IGF-1 downstream signal activation in myoblasts (43). However, data to support this hypothesis in chondrocytes remain limited. Thus, in addition to the low IGF-1 levels as outlined above, there may be a component of inflammation-induced GH resistance through impaired signaling along the GH axis, resulting in decreased GH-IGF-1 action and further exacerbating the poor growth seen in patients with CF.
Glucocorticoids adversely affect growth through interactions with the GH-IGF-1 axis and through direct actions on chondrocytes at the growth plate. Glucocorticoid receptors are present in the human growth plate (44), and glucocorticoids are well known to have several adverse effects on bone with chronic systemic use, ranging from suppression of growth to osteoporosis. Systemic glucocorticoid use is associated with decreased GH secretion, decreased IGF-1, and direct inhibition of growth plates through increased chondrocyte apoptosis and decreased chondrocyte proliferation and differentiation (24, 45). The use of inhaled corticosteroids, although at a significantly lower dose than systemic steroids, may still present potential concerns. In children with asthma, daily doses of inhaled corticosteroids (ICS) have been shown to temporarily reduce prepubertal growth velocity and reduce mean adult height by a small but statistically significant 1.2 cm when compared with unexposed control subjects (46). A recent Cochrane analysis of 25 trials studying the effects of ICS on growth in children with asthma concluded that use of ICS results in a mean reduction of 0.48 cm/yr in growth velocity, with the maximal impact noted during the first year of treatment with ICS and attenuated thereafter (47). In patients with CF and features of asthma requiring ICS, this relatively small effect may still represent a concern when considered in the context of overall growth impairment and its relationship to other CF outcomes.
3. Diagnostic Work Up
Careful assessment of height and weight gain remains essential to initial evaluation of growth failure. This is particularly true in the first year after diagnosis of CF, and during the peripubertal years. Length (for supine measurements) and height (standing) which is more than 2 standard deviation scores (SDS) below the mean for age, or below the 5th percentile for age, are concerning predictors of mortality (1, 7). Height velocity should be assessed and is concerning if less than 4-5 cm per year for prepubertal children; decline in height velocity can identify growth failure earlier than may be detected by height percentiles alone. When assessing growth as the patient nears the age of pubertal onset, assessment of pubertal status with Tanner staging is indicated, and is concerning for pubertal delay if there are no signs of puberty by age 12-13 years in girls or age 14 years in boys. The reader is referred to the Delayed Puberty, Article 11 in this issue, for further details. Bone age x-ray of the left hand may assist in quantifying the degree of bone maturation and / or pubertal delay, if present. Other causes of growth failure should be ruled out such as thyroid disorders, liver / kidney disease, or celiac disease (as celiac disease and cystic fibrosis tend to affect the same population of individuals of Northern European descent). Declining growth may be an early sign of development of cystic fibrosis related diabetes seen 1-2 years before the diagnosis of CFRD (48, 49).
Due to the pulsatile nature of GH secretion, which can include undetectable GH levels in between pulses in non-GH-deficient children, random GH levels are uninterpretable for diagnosing GH deficiency; provocative testing with agents (insulin, arginine, levodopa, clonidine, or glucagon) to stimulate a peak level of GH response are often used in diagnosing GH deficiency (50). However, these tests are imperfect even in patients without CF, due to variability in GH assays, poor reproducibility, and the non-physiological nature of the testing conditions (50). In patients with CF, given that GH deficiency is not the only underlying cause of growth failure for the mechanistic reasons outlined above, a “normal” response to GH with peak levels above 10ng/mL may be of little clinical utility. Since a normal response on provocative testing may indicate that there is no evidence of classic GH deficiency, but still does not account for other causes of poor linear growth intrinsic to CF, provocative GH testing remains controversial in patients with CF and should not be undertaken routinely in patients with CF and growth failure.
4. Routine Management
For optimal growth, nutritional and pulmonary status should be optimized, and if necessary, CFRD should be diagnosed and treated. Growth-impairing medications such as ICS should be reduced to the minimum dose and duration necessary. At this point, other pharmacologic agents may, in some individuals, be considered.
4.1. Recombinant human growth hormone
Recombinant human growth hormone (rhGH) has been studied for effects not only on growth but also on pulmonary function, bone health, and lean body mass. In the most recent update of a Cochrane review of randomized controlled trials of rhGH treatment in patients with CF, eight trials (age range, 5-23 years, n=291) were included, with rhGH dose for most studies of 0.3mg/kg/week and total treatment time of 1 year (51). Height velocity was significantly increased with rhGH treatment compared with placebo (52-55), along with improved lean body mass (46, 52-54, 56). FEV1 was improved in some trials (54, 55) but not in others (56-58). Data on bone mineral content, exercise tolerance and quality of life suggest improvement with rhGH treatment (52, 56, 57, 59), but data remain limited. A retrospective analysis of patients with CF treated with rhGH for 1 year suggests reduction in delayed puberty as compared with controls (60). Due to the known increase in insulin resistance associated with GH, theoretical concerns of inducing hyperglycemia with rhGH treatment have been raised, but there was no difference in development of impaired glucose tolerance or CFRD in rhGH-treated patients compared with control (55) and no differences in fasting plasma glucose levels (53, 55, 58). Other potential side effects regarding rhGH include increased intracranial pressure, which has been reported in 1 patient with CF (55), and scoliosis, which has not been reported in CF patients. Also, CF is not a currently approved indication for rhGH by the Food and Drug Administration (US) or the European Medicines Agency (Europe), and there are no data regarding a cost-benefit analysis of rhGH in CF.
4.2. Oxandrolone
Oxandrolone is an orally administered weak androgen with anabolic effects that has been used to enhance growth in multiple conditions. A meta-analysis of oxandrolone-rhGH combination treatment in children with Turner Syndrome indicated greater height velocity than with rhGH alone(61). Potential side effects of oxandrolone of particular concern in females include deepening of the voice, hirsutism, acne, and clitoromegaly, which appears to be dose-dependent (61). Published data on oxandrolone use in CF are limited; in a retrospective study of patients with CF aged 8.5-14.5 years (3 male, 2 female), treatment with oxandrolone 2.5mg daily for at least 8 months resulted in improved height velocity and BMI z-score, with no adverse effects reported (specifically, neither female subject developed hirsutism, clitoromegaly, or other hyperandrogenism) (62).
4.3. Recombinant IGF-1
A single double-blind placebo-controlled study of 6 months of recombinant IGF-1 treatment in seven prepubertal children with CF did not show differences in growth velocity, weight gain, lean body mass, or FEV1 when compared with placebo (63); no additional studies of recombinant IGF-1 have been undertaken in CF.
Other agents that have been studied for growth effect in CF include insulin and CFTR modulators. Other agents used in instances of growth failure may have differential results on weight versus height improvement, depending on whether they are intended to stimulate appetite or have direct effects on linear growth, and are summarized in Table 1. Overall, published data on safety and efficacy of appetite stimulants in CF remain limited (64).
Table 1.
Pharmacologic Agents Studied for Effects on Growth and Nutrition in CF.
| Drug | Mechanism of Action |
Dosage | Adverse Effects |
|---|---|---|---|
| Megestrol acetate | Appetite stimulant, ↑neuropeptide Y | 400-800mg/day or 7.5-15mg/kg/day | Glucose dysregulation, insomnia, hyperactivity, irritability, adrenal suppression, concern for increased fat vs lean body mass (76, 77). There are no data regarding a safe duration for long-term use. |
| Cyproheptadine | Appetite stimulant, antihistamine and serotonin agonist | 4mg BID-QID or 0.5mg/kg/day | Transient mild sedation, pill fatigue (78) |
| Dronabinol | Appetite enhancement via central cannabinoid receptors | 2.5mg daily - 5mg BID | Anxiety, confusion, euphoria, somnolence (79) |
| Olanzapine | Appetite stimulant, atypical antipsychotic | 5-20mg daily | Liver dysfunction, sleepiness, hyperglycemia (80) |
| Mirtazapine | Appetite stimulant, antidepressant - central presynaptic alpha2-adrenergic antagonist, potent antagonist of 5-HT2 and 5-HT3 serotonin receptors and H1histamine receptor | 15-45mg daily | Mild sedation, dry mouth, somnolence (81) |
| Oxandrolone | Anabolic steroid, ↑protein synthesis and skeletal muscle growth | 0.03-0.075mg/kg/d, once daily, max 2.5mg per day for children >9-10yrs | Hepatic dysfunction, androgenic effects in females when used at high doses, rapid progression of puberty in males in high doses (61, 62) |
| rhGH | Anabolic agent, stimulates linear growth of linear bone, skeletal muscle | 0.3-0.5 mg/kg/week dose, given as a daily SQ injection | Potential for hyperglycemia (not found to be clinically significant to date), increased intracranial pressure, additional treatment burden due to requirement for daily injections(51-55) |
| Ivacaftor | Potentiates epithelial cell chloride ion transport of defective (G551D mutant) cell-surface CFTR protein | 150mg every 12 hours for age 6 years and up | Skin rash / hypersensitivity reaction, transaminitis, cough(82) |
5. Potential Impact of CFTR Modulation
The advent of CFTR modulators, which target specific defects in the CFTR protein, have drastically altered the outlook for the future of CF care. The CFTR potentiator ivacaftor improves CFTR function in those with G551D-CFTR mutations (65) and has been shown to improve lung function, weight, and BMI, along with decreasing resting energy expenditure and gut inflammation (66-68). In an analysis of data pooled from 83 prepubertal children treated with ivacaftor in 2 clinical trials, height and weight z-scores along with height velocity increased significantly from baseline (69). In comparison to those treated with placebo, there was an improvement of +1.08cm/year in the treatment group when compared over 48 weeks (69). Thus, there are encouraging signs that improvement of CFTR function may provide additional benefits in linear growth and bone health through correction of the underlying defect.
6. Future Directions
Studies of the CFTR modulators ivacaftor and the lumacaftor/ivacaftor combination for the very young child, under 5 years of age, are underway (70-73). Ongoing follow up of patients already treated with CFTR modulators will provide additional longitudinal information on the effect of CFTR modulators on the growth patterns of peripubertal children with regard to pubertal growth acceleration and mid-pubertal peak growth velocity. Ivacaftor provided dramatic improvements in CFTR activity for individuals with G551D genotypes (74). New combination therapies are on the horizon which have the potential to provide similar improvements for a wider range of CF individuals. The impact of these therapies on linear growth will provide a better understanding of the pathogenesis of growth restriction in CF. Due to the increased burden of care associated with daily subcutaneous injections of rhGH, there has been ongoing interest in the development of longer acting growth hormone formulations, through the development of rhGH fusion proteins with longer half-lives or through microencapsulation of the molecule for slower release (75), but currently these agents remain investigational.
7. Clinical Practice Points
Careful attention to both height percentile and height velocity are indicated along with meticulous assessment of weight status at each visit for patients with CF, as height has additional prognostic significance in CF outcomes.
Optimization of nutrition status remains a cornerstone of a treatment plan for poor growth, but may still be inadequate to attain adequate improvement in height.
Inhaled corticosteroids should be used at the minimum effective dose and duration of treatment to reduce the impact on linear growth.
Critical times to assess growth are during the first year after initial diagnosis, whether in the neonatal period or with later diagnosis, and at the time of expected pubertal onset.
Delayed puberty or poor pubertal progression can have significant impacts on both peak growth velocity at mid-puberty and final adult height and should be addressed as part of a complete evaluation for growth failure.
Screening for CF-related diabetes with an oral glucose tolerance test should be performed, as early glucose abnormalities may contribute to growth failure.
Consider early referral to endocrinology if growth velocity is faltering during infancy, slower than expected during the childhood years (less than 4-5cm/year), or with delayed pubertal onset (no signs of breast bud in girls by age 12 years, no increase in testicular size by age 14 in boys, although family history and timing of pubertal development must also be considered), or height less than −2 SDS.
8. Summary
Careful ongoing measurement of both height and weight are critical in ensuring optimal growth for patients with CF. Improvements in linear growth have lagged behind improvements in weight despite earlier diagnosis of CF and earlier pulmonary and nutrition interventions. Because CFTR appears to have direct effects on linear growth and the GH-IGF-1-growth plate axis, treatments aimed directly at the GH axis and CFTR may better address suboptimal linear growth and improve height-related CF outcomes.
Highlights.
Careful attention to both height and weight at each visit for patients with CF, is important, as height has additional prognostic significance in CF outcomes.
Optimization of nutrition status may still be inadequate to attain improvement in height.
Consider early endocrinology referral at any time that growth velocity is faltering.
Acknowledgments:
The authors would like to thank the Cystic Fibrosis Foundation and the faculty mentor members of the EnVision: Emerging Leaders in CF Endocrinology Program, for their ongoing support and mentorship of the program awardees.
Conflicts of Interest: TNL, AA, MSP, and VT have no conflicts of interest. MSS has served on medical advisory boards for Vertex Pharmaceuticals. TNL, AA, and MSP received grant support through the Cystic Fibrosis Foundation, Emerging Leaders in CF Endocrinology (EnVision) Program. MSP received grant support from the National Institutes of Health (K23DK102600 and R01DK119699). MSS currently receives grant support through the CFF (PROMISE-ENDO18KO and BEGIN-STALVE19KO) and NIH-NIDDK (DK072482).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Beker LT, Russek-Cohen E, Fink RJ. Stature as a prognostic factor in cystic fibrosis survival. J Am Diet Assoc. 2001;101(4):438–42. [DOI] [PubMed] [Google Scholar]
- 2.Konstan MW, Butler SM, Wohl ME, Stoddard M, Matousek R, Wagener JS, et al. Growth and nutritional indexes in early life predict pulmonary function in cystic fibrosis. J Pediatr. 2003;142(6):624–30. [DOI] [PubMed] [Google Scholar]
- 3.Lai HJ, Shoff SM, Farrell PM, Wisconsin Cystic Fibrosis Neonatal Screening G. Recovery of birth weight z score within 2 years of diagnosis is positively associated with pulmonary status at 6 years of age in children with cystic fibrosis. Pediatrics. 2009;123(2):714–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McColley SA, Schechter MS, Morgan WJ, Pasta DJ, Craib ML, Konstan MW. Risk factors for mortality before age 18 years in cystic fibrosis. Pediatr Pulmonol. 2017;52(7):909–15. [DOI] [PubMed] [Google Scholar]
- 5.Yen EH, Quinton H, Borowitz D. Better nutritional status in early childhood is associated with improved clinical outcomes and survival in patients with cystic fibrosis. J Pediatr. 2013;162(3):530–5 e1. [DOI] [PubMed] [Google Scholar]
- 6.Borowitz D, Baker RD, Stallings V. Consensus report on nutrition for pediatric patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2002;35(3):246–59. [DOI] [PubMed] [Google Scholar]
- 7.Vieni G, Faraci S, Collura M, Lombardo M, Traverso G, Cristadoro S, et al. Stunting is an independent predictor of mortality in patients with cystic fibrosis. Clin Nutr. 2013;32(3):382–5. [DOI] [PubMed] [Google Scholar]
- 8.Gaskin KJ. Nutritional care in children with cystic fibrosis: are our patients becoming better? Eur J Clin Nutr. 2013;67(5):558–64. [DOI] [PubMed] [Google Scholar]
- 9.Leung DH, Heltshe SL, Borowitz D, Gelfond D, Kloster M, Heubi JE, et al. Effects of Diagnosis by Newborn Screening for Cystic Fibrosis on Weight and Length in the First Year of Life. JAMA Pediatr. 2017;171(6):546–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Z, Lindstrom MJ, Farrell PM, Lai HJ, Wisconsin Cystic Fibrosis Neonatal Screening G. Pubertal Height Growth and Adult Height in Cystic Fibrosis After Newborn Screening. Pediatrics. 2016;137(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cystic Fibrosis Foundation Patient Registry 2017 Annual Data Report to the Center Directors. Bethesda, MD; 2018. [Google Scholar]
- 12.Konstan MW, Pasta DJ, Wagener JS, VanDevanter DR, Morgan WJ. BMI fails to identify poor nutritional status in stunted children with CF. J Cyst Fibros. 2017;16(1):158–60. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan SA, Cohen P. The somatomedin hypothesis 2007: 50 years later. J Clin Endocrinol Metab. 2007;92(12):4529–35. [DOI] [PubMed] [Google Scholar]
- 14.Tanner JM, Davies PS. Clinical longitudinal standards for height and height velocity for North American children. J Pediatr. 1985;107(3):317–29. [DOI] [PubMed] [Google Scholar]
- 15.Rogol AD, Roemmich JN, Clark PA. Growth at puberty. J Adolesc Health. 2002;31(6 Suppl):192–200. [DOI] [PubMed] [Google Scholar]
- 16.Assael BM, Casazza G, Iansa P, Volpi S, Milani S. Growth and long-term lung function in cystic fibrosis: a longitudinal study of patients diagnosed by neonatal screening. Pediatr Pulmonol. 2009;44(3):209–15. [DOI] [PubMed] [Google Scholar]
- 17.Byard PJ. The adolescent growth spurt in children with cystic fibrosis. Ann Hum Biol. 1994;21(3):229–40. [DOI] [PubMed] [Google Scholar]
- 18.Haeusler G, Frisch H, Waldhor T, Gotz M. Perspectives of longitudinal growth in cystic fibrosis from birth to adult age. Eur J Pediatr. 1994;153(3):158–63. [DOI] [PubMed] [Google Scholar]
- 19.Lai HC, Kosorok MR, Sondel SA, Chen ST, FitzSimmons SC, Green CG, et al. Growth status in children with cystic fibrosis based on the National Cystic Fibrosis Patient Registry data: evaluation of various criteria used to identify malnutrition. J Pediatr. 1998;132(3 Pt 1):478–85. [DOI] [PubMed] [Google Scholar]
- 20.Morison S, Dodge JA, Cole TJ, Lewis PA, Coles EC, Geddes D, et al. Height and weight in cystic fibrosis: a cross sectional study. UK Cystic Fibrosis Survey Management Committee. Arch Dis Child. 1997;77(6):497–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerem E, Corey M, Kerem BS, Rommens J, Markiewicz D, Levison H, et al. The relation between genotype and phenotype in cystic fibrosis--analysis of the most common mutation (delta F508). N Engl J Med. 1990;323(22):1517–22. [DOI] [PubMed] [Google Scholar]
- 22.Cipolli M, Castellani C, Wilcken B, Massie J, McKay K, Gruca M, et al. Pancreatic phenotype in infants with cystic fibrosis identified by mutation screening. Arch Dis Child. 2007;92(10):842–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walkowiak J, Sands D, Nowakowska A, Piotrowski R, Zybert K, Herzig KH, et al. Early decline of pancreatic function in cystic fibrosis patients with class 1 or 2 CFTR mutations. J Pediatr Gastroenterol Nutr. 2005;40(2):199–201. [DOI] [PubMed] [Google Scholar]
- 24.Wong SC, Dobie R, Altowati MA, Werther GA, Farquharson C, Ahmed SF. Growth and the Growth Hormone-Insulin Like Growth Factor 1 Axis in Children With Chronic Inflammation: Current Evidence, Gaps in Knowledge, and Future Directions. Endocr Rev. 2016;37(1):62–110. [DOI] [PubMed] [Google Scholar]
- 25.Darrah R, Nelson R, Damato EG, Decker M, Matthews A, Hodges CA. Growth Deficiency in Cystic Fibrosis Is Observable at Birth and Predictive of Early Pulmonary Function. Biol Res Nurs. 2016;18(5):498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Festini F, Taccetti G, Repetto T, Reali MF, Campana S, Mergni G, et al. Gestational and neonatal characteristics of children with cystic fibrosis: a cohort study. J Pediatr. 2005;147(3):316–20. [DOI] [PubMed] [Google Scholar]
- 27.Muller AE, Thamm B, Lietz T, Handrick W, Walter S. Cystic fibrosis: a cause of reduced birth weight? Eur J Pediatr. 1999;158(3):264. [DOI] [PubMed] [Google Scholar]
- 28.Darrah R, Bederman I, Vitko M, Valerio DM, Drumm ML, Hodges CA. Growth deficits in cystic fibrosis mice begin in utero prior to IGF-1 reduction. PLoS One. 2017;12(4):e0175467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boguszewski MC, Kamoi TO, Bento Radominski R, Boguszewski CL, Rosberg S, Filho NA, et al. Insulin-like growth factor-1, leptin, body composition, and clinical status interactions in children with cystic fibrosis. Horm Res. 2007;67(5):250–6. [DOI] [PubMed] [Google Scholar]
- 30.Laursen EM, Juul A, Lanng S, Hoiby N, Koch C, Muller J, et al. Diminished concentrations of insulin-like growth factor I in cystic fibrosis. Arch Dis Child. 1995;72(6):494–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rogan MP, Reznikov LR, Pezzulo AA, Gansemer ND, Samuel M, Prather RS, et al. Pigs and humans with cystic fibrosis have reduced insulin-like growth factor 1 (IGF1) levels at birth. Proc Natl Acad Sci U S A. 2010;107(47):20571–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stalvey MS, Havasi V, Tuggle KL, Wang D, Birket S, Rowe SM, et al. Reduced bone length, growth plate thickness, bone content, and IGF-I as a model for poor growth in the CFTR-deficient rat. PLoS One. 2017;12(11):e0188497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Schepper J, Van Blerk M, Hachimi-Idrissi S, Dab I, Smitz J. Plasma insulin-like growth factor-I determinations in patients with cystic fibrosis: Influence of the nutritional and liver status. Clin Nutr. 1992;11(5):298–302. [DOI] [PubMed] [Google Scholar]
- 34.Lee JA, Dickinson LS, Kilgore BS, Warren RH, Elders MJ. Somatomedin activity in cystic fibrosis and reserpinized rats: possible explanation for growth retardation. Ann Clin Lab Sci. 1980;10(3):227–33. [PubMed] [Google Scholar]
- 35.Rosenfeld RG, Landon C, Lewiston N, Nagashima R, Hintz RL. Demonstration of normal plasma somatomedin concentrations in cystic fibrosis. J Pediatr. 1981;99(2):252–4. [DOI] [PubMed] [Google Scholar]
- 36.Ciro D, Padoan R, Blau H, Marostica A, Fuoti M, Volpi S, et al. Growth retardation and reduced growth hormone secretion in cystic fibrosis. Clinical observations from three CF centers. J Cyst Fibros. 2013;12(2):165–9. [DOI] [PubMed] [Google Scholar]
- 37.Shead EF, Haworth CS, Condliffe AM, McKeon DJ, Scott MA, Compston JE. Cystic fibrosis transmembrane conductance regulator (CFTR) is expressed in human bone. Thorax. 2007;62(7):650–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pashuck TD, Franz SE, Altman MK, Wasserfall CH, Atkinson MA, Wronski TJ, et al. Murine model for cystic fibrosis bone disease demonstrates osteopenia and sex-related differences in bone formation. Pediatr Res. 2009;65(3):311–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacRae VE, Farquharson C, Ahmed SF. The restricted potential for recovery of growth plate chondrogenesis and longitudinal bone growth following exposure to pro-inflammatory cytokines. J Endocrinol. 2006;189(2):319–28. [DOI] [PubMed] [Google Scholar]
- 40.Aizawa T, Kon T, Einhorn TA, Gerstenfeld LC. Induction of apoptosis in chondrocytes by tumor necrosis factor-alpha. J Orthop Res. 2001;19(5):785–96. [DOI] [PubMed] [Google Scholar]
- 41.Martensson K, Chrysis D, Savendahl L. Interleukin-1beta and TNF-alpha act in synergy to inhibit longitudinal growth in fetal rat metatarsal bones. J Bone Miner Res. 2004;19(11):1805–12. [DOI] [PubMed] [Google Scholar]
- 42.Boisclair YR, Wang J, Shi J, Hurst KR, Ooi GT. Role of the suppressor of cytokine signaling-3 in mediating the inhibitory effects of interleukin-1beta on the growth hormone-dependent transcription of the acid-labile subunit gene in liver cells. J Biol Chem. 2000;275(6):3841–7. [DOI] [PubMed] [Google Scholar]
- 43.Broussard SR, McCusker RH, Novakofski JE, Strle K, Shen WH, Johnson RW, et al. IL-1beta impairs insulin-like growth factor i-induced differentiation and downstream activation signals of the insulin-like growth factor i receptor in myoblasts. J Immunol. 2004;172(12):7713–20. [DOI] [PubMed] [Google Scholar]
- 44.Abu EO, Horner A, Kusec V, Triffitt JT, Compston JE. The localization of the functional glucocorticoid receptor alpha in human bone. J Clin Endocrinol Metab. 2000;85(2):883–9. [DOI] [PubMed] [Google Scholar]
- 45.Mazziotti G, Giustina A. Glucocorticoids and the regulation of growth hormone secretion. Nat Rev Endocrinol. 2013;9(5):265–76. [DOI] [PubMed] [Google Scholar]
- 46.Kelly HW, Sternberg AL, Lescher R, Fuhlbrigge AL, Williams P, Zeiger RS, et al. Effect of inhaled glucocorticoids in childhood on adult height. N Engl J Med. 2012;367(10):904–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang L, Prietsch SO, Ducharme FM. Inhaled corticosteroids in children with persistent asthma: effects on growth. Cochrane Database Syst Rev. 2014(7):CD009471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Terliesner N, Vogel M, Steighardt A, Gausche R, Henn C, Hentschel J, et al. Cystic-fibrosis related-diabetes (CFRD) is preceded by and associated with growth failure and deteriorating lung function. J Pediatr Endocrinol Metab. 2017;30(8):815–21. [DOI] [PubMed] [Google Scholar]
- 49.Cheung MS, Bridges NA, Prasad SA, Francis J, Carr SB, Suri R, et al. Growth in children with cystic fibrosis-related diabetes. Pediatr Pulmonol. 2009;44(12):1223–5. [DOI] [PubMed] [Google Scholar]
- 50.Richmond EJ, Rogol AD. Growth hormone deficiency in children. Pituitary. 2008;11(2):115–20. [DOI] [PubMed] [Google Scholar]
- 51.Thaker V, Carter B, Putman M. Recombinant growth hormone therapy for cystic fibrosis in children and young adults. Cochrane Database Syst Rev. 2018;12:CD008901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hardin DS, Adams-Huet B, Brown D, Chatfield B, Dyson M, Ferkol T, et al. Growth hormone treatment improves growth and clinical status in prepubertal children with cystic fibrosis: results of a multicenter randomized controlled trial. J Clin Endocrinol Metab. 2006;91(12):4925–9. [DOI] [PubMed] [Google Scholar]
- 53.Hardin DS, Ellis KJ, Dyson M, Rice J, McConnell R, Seilheimer DK. Growth hormone improves clinical status in prepubertal children with cystic fibrosis: results of a randomized controlled trial. J Pediatr. 2001;139(5):636–42. [DOI] [PubMed] [Google Scholar]
- 54.Hardin DS, Rice J, Ahn C, Ferkol T, Howenstine M, Spears S, et al. Growth hormone treatment enhances nutrition and growth in children with cystic fibrosis receiving enteral nutrition. J Pediatr. 2005;146(3):324–8. [DOI] [PubMed] [Google Scholar]
- 55.Stalvey MS, Anbar RD, Konstan MW, Jacobs JR, Bakker B, Lippe B, et al. A multi-center controlled trial of growth hormone treatment in children with cystic fibrosis. Pediatr Pulmonol. 2012;47(3):252–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hutler M, Schnabel D, Staab D, Tacke A, Wahn U, Boning D, et al. Effect of growth hormone on exercise tolerance in children with cystic fibrosis. Med Sci Sports Exerc. 2002;34(4):567–72. [DOI] [PubMed] [Google Scholar]
- 57.Schnabel D, Grasemann C, Staab D, Wollmann H, Ratjen F, German Cystic Fibrosis Growth Hormone Study G. A multicenter, randomized, double-blind, placebo-controlled trial to evaluate the metabolic and respiratory effects of growth hormone in children with cystic fibrosis. Pediatrics. 2007;119(6):e1230–8. [DOI] [PubMed] [Google Scholar]
- 58.Schibler A, von der Heiden R, Birrer P, Mullis PE. Prospective randomised treatment with recombinant human growth hormone in cystic fibrosis. Arch Dis Child. 2003;88(12):1078–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hardin DS, Ahn C, Prestidge C, Seilheimer DK, Ellis KJ. Growth hormone improves bone mineral content in children with cystic fibrosis. J Pediatr Endocrinol Metab. 2005;18(6):589–95. [DOI] [PubMed] [Google Scholar]
- 60.Vanderwel M, Hardin DS. Growth hormone normalizes pubertal onset in children with cystic fibrosis. J Pediatr Endocrinol Metab. 2006;19(3):237–44. [DOI] [PubMed] [Google Scholar]
- 61.Sheanon NM, Backeljauw PF. Effect of oxandrolone therapy on adult height in Turner syndrome patients treated with growth hormone: a meta-analysis. Int J Pediatr Endocrinol. 2015;2015(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Varness T, Seffrood EE, Connor EL, Rock MJ, Allen DB. Oxandrolone Improves Height Velocity and BMI in Patients with Cystic Fibrosis. Int J Pediatr Endocrinol. 2009;2009:826895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bucuvalas JC, Chernausek SD, Alfaro MP, Krug SK, Ritschel W, Wilmott RW. Effect of insulinlike growth factor-1 treatment in children with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2001;33(5):576–81. [DOI] [PubMed] [Google Scholar]
- 64.Chinuck R, Dewar J, Baldwin DR, Hendron E. Appetite stimulants for people with cystic fibrosis. Cochrane Database Syst Rev. 2014(7):CD008190. [DOI] [PubMed] [Google Scholar]
- 65.Pettit RS. Cystic fibrosis transmembrane conductance regulator-modifying medications: the future of cystic fibrosis treatment. Ann Pharmacother. 2012;46(7-8):1065–75. [DOI] [PubMed] [Google Scholar]
- 66.McKone EF, Borowitz D, Drevinek P, Griese M, Konstan MW, Wainwright C, et al. Long-term safety and efficacy of ivacaftor in patients with cystic fibrosis who have the Gly551Asp-CFTR mutation: a phase 3, open-label extension study (PERSIST). Lancet Respir Med. 2014;2(11):902–10. [DOI] [PubMed] [Google Scholar]
- 67.Davies JC, Wainwright CE, Canny GJ, Chilvers MA, Howenstine MS, Munck A, et al. Efficacy and safety of ivacaftor in patients aged 6 to 11 years with cystic fibrosis with a G551D mutation. Am J Respir Crit Care Med. 2013;187(11):1219–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stallings VA, Sainath N, Oberle M, Bertolaso C, Schall JI. Energy Balance and Mechanisms of Weight Gain with Ivacaftor Treatment of Cystic Fibrosis Gating Mutations. J Pediatr. 2018;201:229–37 e4. [DOI] [PubMed] [Google Scholar]
- 69.Stalvey MS, Pace J, Niknian M, Higgins MN, Tarn V, Davis J, et al. Growth in Prepubertal Children With Cystic Fibrosis Treated With Ivacaftor. Pediatrics. 2017;139(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.A Study to Evaluate the Safety of Long-term Ivacaftor Treatment in Subjects With Cystic Fibrosis Who Are Less Than 24 Months of Age at Treatment Initiation and Have an Approved Ivacaftor-Responsive Mutation. Retrieved from http://clinicaltrials.gov/ct2 (Identification No. NCT03277196).
- 71.A Study to Explore the Impact of Lumacaftor/Ivacaftor on Disease Progression in Subjects Aged 2 Through 5 Years With Cystic Fibrosis, Homozygous for F508del. Retrieved from http://clinicaltrials.gov/ct2 (Identification NCT03625466).
- 72.Orkambi Treatment in 2 to 5 Year Old Children With CF. Retrieved from http://clinicaltrials.gov/ct2 (Identification NCT03795363).
- 73.Ivacaftor Treatment in 1 to 2 Year Old CF Subjects. Retrieved from http://clinicaltrials.gov/ct2 (Identification NCT03783286).
- 74.Sermet-Gaudelus I Ivacaftor treatment in patients with cystic fibrosis and the G551D-CFTR mutation. Eur Respir Rev. 2013;22(127):66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saenger PH, Mejia-Corletto J. Long-Acting Growth Hormone: An Update. Endocr Dev. 2016;30:79–97. [DOI] [PubMed] [Google Scholar]
- 76.Marchand V, Baker SS, Stark TJ, Baker RD. Randomized, double-blind, placebo-controlled pilot trial of megestrol acetate in malnourished children with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2000;31(3):264–9. [DOI] [PubMed] [Google Scholar]
- 77.Eubanks V, Koppersmith N, Wooldridge N, Clancy JP, Lyrene R, Arani RB, et al. Effects of megestrol acetate on weight gain, body composition, and pulmonary function in patients with cystic fibrosis. J Pediatr. 2002;140(4):439–44. [DOI] [PubMed] [Google Scholar]
- 78.Homnick DN, Marks JH, Hare KL, Bonnema SK. Long-term trial of cyproheptadine as an appetite stimulant in cystic fibrosis. Pediatr Pulmonol. 2005;40(3):251–6. [DOI] [PubMed] [Google Scholar]
- 79.Anstead MIKR, Martyn D, Craigmyle L, Kanga JF. Dronabinol, an effective and safe appetite stimulant in cystic fibrosis. Pediatr Pulmonol. 2003;36(Suppl 25):343. [Google Scholar]
- 80.Nasr SZ, Drury D. Appetite stimulants use in cystic fibrosis. Pediatr Pulmonol. 2008;43(3):209–19. [DOI] [PubMed] [Google Scholar]
- 81.Young J DM, McColley SA, Boar SR. The role of mirtazapine as an appetite sitmulant in malnourished individiuals with CF Pediatr Pulmonol. 2000;20(Suppl 20):326. [Google Scholar]
- 82.Talamo Guevara M, McColley SA. The safety of lumacaftor and ivacaftor for the treatment of cystic fibrosis. Expert Opin Drug Saf. 2017;16(11):1305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]