Abstract
Colorectal cancer (CRC) can be effectively prevented or detected with guideline concordant screening, yet Medicaid enrollees experience disparities. We used microsimulation to project CRC screening patterns, CRC cases averted, and life-years gained in the population of 68,077 Oregon Medicaid enrollees 50–64 over a five year period starting in January 2019. The simulation estimated the cost-effectiveness of five intervention scenarios - academic detailing plus provider audit and feedback (Detailing+), patient reminders (Reminders), mailing a Fecal Immunochemical Test (FIT) directly to the patient’s home (Mailed FIT), patient navigation (Navigation), and mailed FIT with Navigation (Mailed FIT+Navigation) – compared to usual care. Each intervention scenario raised CRC screening rates compared to usual care, with improvements as high as 11.6 percentage points (Mailed FIT+Navigation) and as low as 2.5 percentage points (Reminders) after one year. Compared to usual care, Mailed FIT+Navigation would raise CRC screening rates 20.2 percentage points after five years - averting nearly 77 cancer cases (a reduction of 113 per 100,000) and exceeding national screening targets. Over a five year period, Reminders, Mailed FIT and Mailed FIT+Navigation were expected to be cost effective if stakeholders were willing to pay $230 or less per additional year up-to-date (at a cost of $22, $59, and $227 respectively per additional person-year up-to-date), whereas Detailing+ and Navigation were more costly for the same benefits. To approach national CRC screening targets, health system stakeholders are encouraged to implement Mailed FIT with or without Navigation and Reminders.
Keywords: Colorectal Cancer, Simulation, Screening, Cancer Prevention & Control, Disparities, Intervention Selection, Implementation, Medicaid
INTRODUCTION
Colorectal cancer (CRC) is a leading cause of cancer-related deaths in the United States.1 Screening in accordance with clinical guidelines (e.g., colonoscopy every 10 years or Fecal Immunochemical Tests (FIT) annually) can help prevent and/or detect CRC in adults ages 50–75.2 However, only 62.6% of age-eligible adults reported being up-to-date with CRC screening nationally as of 2015, well below national targets.3,4 Moreover, CRC screening rates are routinely 15–30% lower among Medicaid enrollees, ethnically diverse populations, and in rural settings.3,5–8
Numerous interventions can increase CRC screening. Recent systematic reviews demonstrate the effectiveness of directly mailing a FIT to a patient’s home, patient navigation, and patient reminders in increasing CRC screening rates.9–12 Research also demonstrates that multicomponent interventions – which use two or more strategies to increase community demand, community access or to improve provider delivery systems – are more effective in increasing CRC screening than single interventions alone.13 However, limited guidance exists to help clinics or health plan leaders determine the best interventions to implement in their specific settings and populations.
Regional and national health reform efforts underway in the United States focus on improving the delivery of clinical preventive services such as CRC screening, and on attenuating disparities.14,15 For example, the Affordable Care Act of 2010 included provisions to increase access to preventive care and to support formation of accountable care organizations (ACOs). ACOs are designed to create coordinated care systems that can help achieve triple aim objectives of improving experience and quality of care while controlling costs for a defined population.14 While ACOs originated in Medicare, more than ten states have active Medicaid ACO programs and 12 more states were pursuing this possibility as of 2018.16,17 Oregon initiated formation of Medicaid ACOs, called Coordinated Care Organizations (CCOs), in 2012. CCOs are responsible to the state for annual performance on selected clinical quality incentive metrics, which include CRC screening. CCO stakeholders are especially eager for information on which effective interventions they should implement to improve CRC screening.
We conducted this microsimulation study to estimate the impact of five evidence-based interventions on CRC screening rates, CRC cases averted, life-years gained and cost-effectiveness compared to usual care in age-eligible Oregon Medicaid enrollees. Microsimulation is an individual-based modeling technique that simulates a population of individuals with varying characteristics who are evolving over time and transitioning between different health states. This approach provides an opportunity to compare the population’s projected health outcomes associated with particular interventions, and is especially helpful when randomized controlled trials are not feasible or practical.18 Moreover, microsimulation modeling provides critical information to help policy and health system leaders make informed decisions regarding healthcare resource allocation and anticipated impact.19,20 Here, we focus on Medicaid enrollees aged 50–64, before they reached eligibility for Medicare, given lower CRC screening rates in this population both in Oregon and nationwide6,21 and the stated needs of our community and health system partners.22–24 Findings can help stakeholders choose interventions based on anticipated impact and cost while ultimately reducing CRC screening disparities in Medicaid enrollees.
METHODS
We used an individual-based microsimulation model to compare the impact and cost-effectiveness of five evidence-based interventions implemented among Oregon Medicaid enrollees age-eligible for CRC screening.25,26 Our model simulates baseline polyp/cancer development and can estimate each intervention’s impact on lifetime CRC screening, intervention costs, and outcomes (e.g., cancer incidence, cancers averted, life-years gained) compared to the usual care scenario (i.e., in the absence of any implemented intervention). We simulated outcomes over a five year period from January 1, 2019 through December 31, 2023 and report findings at one and five years to inform intervention selection and policy decisions for the short and longer term. This research was approved by the Oregon Health & Science University Institutional Review Board with a waiver of informed consent (IRB #8865).
Study Population
Our synthetic population was built on the RTI Synthetic Household Population and prior work by our study team.25,27 The synthetic population was generated to represent an accurate sociodemographic representation of Oregon residents using the American Community Survey Use Microdata Sample data from 2007–2011 (e.g., gender, age, race, ethnicity, household income, insurance coverage, marital status).27,28 We simulate the life course of Oregon Medicaid enrollees ages 50–64 given observed CRC screening disparities and health system stakeholder interests. Although CRC screening is recommended to age 75, we exclude patients over 65 given the anticipated transition of Medicaid enrollees to Medicare.
Simulation Model Description and Validation
Our individual-based simulation model was developed using AnyLogic Simulation Modeling Software;29 specifications are detailed elsewhere and summarized here.25,26,30 The model simulates the life course of each synthetic individual and tracks polyp and adenoma progression, of which some may eventually become malignant.31,32 Each individual has a chance of developing CRC over his or her lifetime, and the probability of experiencing an incident polyp is differentiated by age, race, and gender.33 Cancer can progress over each person’s lifespan through stages I to IV as defined by the American Joint Committee on Cancer;34 in the simulation model CRC can be detected by either symptoms or CRC screening at any stage. For the current simulation, transition rates are similar in structure to the model developed by Subramanian and Bobashev.31 The natural history model was recently recalibrated to current cancer incidence by stage and to account for racial differences.30 The estimated lifespan for each individual, in the absence of a CRC event, is derived based on race- and gender-specific life tables from the United States Census Bureau.35
Individuals simulated in the model are recommended to receive routine screening based on U.S. Preventive Services Task Force (USPSTF) guidelines starting at age 50.2 Each age-eligible individual is considered to be up-to-date if they have received a colonoscopy within the past 10 years or a FIT within the past year; we limited our analyses to these tests since they are most commonly used modalities.6,36 We differentiate factors affecting an individual’s default screening modality (FIT or colonoscopy) and the predicted probability of compliance (i.e., whether or not CRC screening is completed), consistent with prior work.37 The logistic regression models are based on observational claims data from Oregon Medicaid enrollees and allow for varying effects of individual attributes (i.e., race, gender, ethnicity, urban/rural residence, distance to endoscopy facility) as well as county of residence to account for regional factors. Members of the synthetic population who are not up-to-date with CRC screening are exposed to individual intervention scenarios.
In the simulation model, the likelihood that an individual is up-to-date with screening is increased above usual care estimates of screening, based on the best available evidence for each intervention for effectiveness and uptake (where relative risk values greater than one correspond to increased effectiveness). Our team used best practices throughout all stages of the modeling process.38,39 Transparency and validation of the CRC national history model is well described in prior publications and a public website.26,30 Development of the intervention models and selection of parameter values for each intervention scenario was based on the best available research evidence and published systematic reviews (see Intervention Scenarios, below).10,38,40–47 Like most microsimulation models, we could not predictively or externally validate intervention outcomes (e.g., CRC screening, mortality) since the scenarios are hypothetical and occur in the future (see limitations).20,39 However, we applied multiple forms of validation (e.g., internal validity, face validity, cross validity).39 We assessed internal validity (verification) through internal testing and debugging to ensure that the mathematical calculations were accurate and consistent with model specifications. Face validity was ensured through regular review of model specifications and outcomes (e.g., cancer stage of detection, mortality) with study team members and health system stakeholders to ensure the values produced were within acceptable limits. Cross validation occurred by reviewing and comparing outcomes against model simulations from published and ongoing studies.25,48
Usual Care (Control) Scenario and Model Calibration
Our usual care scenario was designed to serve as the control scenario across the intervention period. Usual care model estimates were designed to align with Oregon state policies, which included implementation of the Affordable Care Act in 2010 and Medicaid expansion in 2014.49 We calibrated the simulation model’s predicted probability of being up-to-date with CRC screening in the usual care scenario using retrospective analysis of Oregon’s Medicaid claims from 2010 to 2013 and annual Oregon Health System Transformation Reports on CRC screening by CCO.36,50 Together, these data and literature sources were used to adjust screening probabilities so that the results generated by the model matched the reported percent up-to-date in the reference years.
Evidence-based Intervention Scenarios
As detailed in Table 1, we estimated the impact of five intervention scenarios as compared to usual care: 1) academic detailing plus provider audit and feedback (Detailing+);10,40 2) patient reminders (Reminders);10,41,42 3) mailing a FIT directly to the patient’s home (Mailed FIT);10,43 4) patient navigation (Navigation);10,44,45 and 5) direct mail with patient navigation (Mailed FIT+Navigation).10,46,47 We used a multistep participatory process to select these interventions. First, we generated a list of proposed interventions based on an evaluation of interventions used by Oregon CCOs22 and recommendations from the community guide for preventive services.13 Second, we reviewed proposed interventions with our advisory board of health system stakeholders and removed interventions with limited effectiveness based on recent systematic reviews.10,40–47 This resulted in a prioritized list of interventions that were effective in similar populations, had low risk of bias, and aligned with interventions utilized or under consideration by Oregon CCOs. We provide estimates of relative risk effectiveness for each scenario based on published literature. We estimated intervention costs using published literature and consultation with experts as summarized in Table 1; and further detailed in Appendix A.40,44,45,51–58
Table 1.
Simulated Interventions and Estimates of Effectiveness and Cost.
| Interventiona | Intervention Description | Estimated Relative Risk of Effectivenessb (Range) |
Cost (per patient unless otherwise noted) |
|---|---|---|---|
| Academic detailing & provider assessment and feedback (Detailing+) | A clinic-level intervention that consists of provider education and monitoring of CRC screening practices. The onsite provider training covers the importance of CRC screening, how to talk to patients about CRC screening, and best-practices for encouraging patients to get screened for CRC. Each provider receives an individual quarterly report describing progress in boosting CRC screening rates amongst patients, including specialized recommendations for improvement. |
1.27 (1.20, 1.30)10,40 |
$1,583.04 per clinic40,51,52 |
| Patient reminders (Reminders) | Patients receive automated call(s) to notify them that they are overdue for CRC screening. The automated message includes the notification that they are overdue, brief information about why CRC screening is important, and information about various options for screening (e.g., FIT or colonoscopy). |
1.26 (1.20, 1.30)10,42,43 |
$1.4353 |
| Mailing a Fecal Immunochemical Test (FIT) directly to the patient’s home (Mailed FIT) | Patients receive a notification mailed to their house to alert them that they are due for CRC screening and will be receiving a FIT in the mail that they can complete at home and return to the clinic via mail (or that they can contact the clinic to schedule a colonoscopy). Shortly after, patients receive a package by mail that includes: a low-literacy information sheet about CRC and why screening is important, a FIT, directions for how to complete the FIT, and a pre-addressed envelope with a stamp to return the FIT for processing. Patients also receive up to two automated phone calls to remind them to complete the FIT if they have not yet mailed it back. |
2.17 (2.10, 2.25)10,43 |
$20.8453,54 |
| Patient navigation (Navigation) | A trained patient navigator calls eligible patients to help “navigate” them towards getting screened for CRC with the goal of overcoming any barriers to screening, and to support diagnostic testing and treatment initiation. Patients receive individualized assistance such that the navigator’s actions are dependent on what each patient needs. Navigation may include explaining why CRC screening is important, describing where and how to get screened, helping to arrange transportation to a screening center, ordering a FIT to the patient’s house, and answering questions about CRC screening. |
1.65 (1.60, 1.76)10,44,45 |
$1,159.6453,55,57,58 |
| Mailed FIT + patient navigation (Mailed FIT +Navigation) | The combination of Mailed FIT and Navigation, both described above. In this intervention, navigators focus more commonly on FIT completion or follow-up after an abnormal FIT. |
2.43 (2.40, 2.55)10,46,47 |
$1,180.4853,55,57,58 |
Although observed in use in Oregon, we did not model the impact of mass media, patient incentives, or provider incentives because of limited evidence of effectiveness with low or medium risk of bias.
The likelihood that an individual is up-to-date with screening is increased above usual care estimates of screening where relative risk values greater than one correspond to increased effectiveness.
Individuals are simulated to complete CRC screening using their probabilistically assigned modality (i.e., FIT or colonoscopy) unless the opportunity to switch modalities occurs, as is the case in interventions which include Mailed FIT. For Mailed FIT, if an individual with an assigned preference for colonoscopy is not up-to-date, his or her modality is temporarily changed to FIT, and the likelihood of compliance is increased according to the proportional increase in effectiveness of the Mailed FIT intervention. Once the intervention is complete, each individual’s screening modality defaults to his or her initially assigned screening modality. Detailing+ is the only intervention that affects all individuals within the intervention window, regardless of up-to-date status, since we assumed all clinics within Oregon received this training during year 1. Implementation of Detailing+ only incurred costs during the first year of implementation while the other interventions accrue implementation costs over all five years of implementation.
Intervention scenarios were simulated over a period of five years with 30 replications for each intervention. We conducted sensitivity analyses for the number of replications and relative risk estimates. We decided 30 replications was sufficient given findings that adding the 30th replication led to less than a 1% difference in the average percent up-to-date for CRC screening, cancer cases, and total life years compared to the average for 29 replications. In addition, we provide 90% uncertainty intervals across replications for each primary outcome of interest, where the lower bound is the 5th percentile and the upper bound is the 95th percentile. Anticipating distal outcomes (e.g., CRC cases, life years) will be more uncertain than proximal outcomes (e.g., up-to-datedness with CRC screening) and that uncertainty intervals across interventions might overlap, we also calculated average rankings of intervention scenarios across replications. Rankings from the intervention’s impact across all replications are robust to uncertainty for all the outcomes under consideration (data not shown). For relative risk estimates, our sensitivity analysis found that none of the outcome values varied by more than 5%, with the majority at 1% or less compared to the model estimate, when using the higher and lower relative risk estimates shown in Table 1.
RESULTS
Demographic characteristics of the simulated population on December 31, 2018 (prior to intervention implementation) are provided in Appendix B. A total of 68,077 Medicaid enrollees aged 50–64 were included and 50.3% (n-34,236) were estimated to be up-to-date with CRC screening. Eligible enrollees were 50.4% female, 82.6% white and 7.1% Hispanic. Forty-one percent resided in a rural region of the state.
Interventions’ Impact on Being Up-To-Date with Screening Recommendations
Under usual care, 50.1% of eligible Medicaid enrollees would be up-to-date with CRC screening at the end of the first year; slightly lower than the prior year due to an increase in members age eligible for CRC screening (Table 2a). Slight differences in screening rates were seen by gender, race, ethnicity, geography, and age. Relative to usual care, after one year the implemented interventions had increased CRC screening by 11.6 percentage points for Mailed FIT+Navigation, 10.0 percentage points for Mailed FIT, 6.3 percentage points for Navigation, 2.5 percentage points for Reminders and 2.6 percentage points for Detailing+ (Table 2a).
Table 2.
Simulated Age-Eligible Oregon Medicaid Population Up-to-Date with Recommended Colorectal Cancer Screening After One and Five Years.a
| 2a. Impact after One Year (December 31, 2019) | ||||||
|---|---|---|---|---|---|---|
| Percentage-Point Change in CRC Screening Under Each Intervention Scenario Compared with Screening as Usual | ||||||
| Variable | Screening as Usual, % | Detailing+ | Reminders | Mailed FIT | Navigation | Mailed FIT + Navigation |
| Overall | 50.1% | 2.6% | 2.5% | 10.0% | 6.3% | 11.6% |
| Uncertainty Interval | (49.7%, | (2.2%, | (2.1%, | (9.8%, | (6.2%, | (11.4%, |
| (5th, 95th Percentile) | 50.4%) | 3.1%) | 2.9%) | 10.4%) | 6.6%) | 13.0%) |
| By gender | ||||||
| Male | 48.1% | 2.5% | 2.5% | 9.9% | 6.2% | 11.7% |
| Female | 52.0% | 2.7% | 2.5% | 10.0% | 6.3% | 11.5% |
| By race | ||||||
| White | 49.9% | 2.6% | 2.4% | 9.8% | 6.2% | 11.4% |
| African American | 49.3% | 2.3% | 2.5% | 10.3% | 6.1% | 12.4% |
| Other | 50.8% | 2.9% | 2.7% | 11.0% | 6.8% | 12.8% |
| By ethnicity | ||||||
| Hispanic | 50.0% | 2.9% | 2.8% | 11.5% | 7.1% | 13.4% |
| Non-Hispanic | 50.1% | 2.6% | 2.4% | 9.7% | 6.1% | 11.3% |
| By geography | ||||||
| Urban | 50.9% | 2.8% | 2.6% | 10.5% | 6.6% | 12.1% |
| Rural | 48.8% | 2.4% | 2.3% | 9.2% | 5.8% | 10.9% |
| By age | ||||||
| 50–54 | 48.3% | 2.8% | 2.8% | 11.2% | 7.0% | 13.0% |
| 55–59 | 51.1% | 2.4% | 2.2% | 8.9% | 5.6% | 10.5% |
| 60–64 | 52.2% | 2.5% | 2.2% | 8.8% | 5.6% | 10.3% |
| 2b. Impact after Five Years (December 31, 2023) | ||||||
| Percentage-Point Change in CRC Screening Under Each Intervention Scenario Compared with Screening as Usual | ||||||
| Variable | Screening as Usual, % | Detailing+ | Reminders | Mailed FIT | Navigation | Mailed FIT + Navigation |
| Overall | 50.1% | 7.2% | 5.8% | 10.0% | 14.1% | 20.2% |
| Uncertainty Interval | (49.7%, | (6.8%, | (5.2%, | (9.6%, | (13.7%, | (19.9%, |
| (5th, 95th Percentile) | 50.4%) | 7.6%) | 6.5%) | 10.4%) | 14.7%) | 20.8%) |
| By gender | ||||||
| Male | 48.2% | 6.8% | 5.9% | 10.0% | 14.3% | 21.8% |
| Female | 52.0% | 7.5% | 5.8% | 9.9% | 13.9% | 18.8% |
| By race | ||||||
| White | 50.0% | 7.2% | 5.9% | 9.7% | 14.2% | 20.5% |
| African American | 50.5% | 6.9% | 5.8% | 11.1% | 13.8% | 19.5% |
| Other | 50.8% | 7.2% | 5.8% | 10.9% | 13.8% | 19.3% |
| By ethnicity | ||||||
| Hispanic | 49.8% | 7.1% | 5.9% | 11.3% | 14.1% | 20.1% |
| Non-Hispanic | 50.2% | 7.2% | 5.8% | 9.6% | 14.1% | 20.3% |
| By geography | ||||||
| Urban | 51.1% | 7.3% | 5.8% | 10.3% | 14.0% | 19.4% |
| Rural | 48.6% | 7.0% | 5.9% | 9.4% | 14.4% | 21.6% |
| By age | ||||||
| 50–54 | 48.0% | 7.3% | 6.0% | 10.3% | 14.5% | 21.1% |
| 55–59 | 50.9% | 7.2% | 5.8% | 10.1% | 14.1% | 20.0% |
| 60–64 | 52.9% | 6.9% | 5.5% | 9.1% | 13.5% | 19.1% |
Aged 50–64 years of age during the 5 year study period (January 1, 2019 – December 31, 2023).
CRC screening rates remained stable under the usual care scenario after five years at 50.1% (see Table 2b). However, the percentage of Medicaid enrollees estimated to be up-to-date with CRC screening would increase by 20.2 percentage points with Mailed FIT+Navigation, 14.1 percentage points with Navigation, 10.0 percentage points with Mailed FIT, 7.2 percentage points with Detailing+ and 5.8 percentage points with Reminders. Interventions had different effects for different sub-populations of interest over time. For example, implementation of Mailed FIT+Navigation over five years demonstrated higher improvements in screening for rural (21.6 percentage points) compared to urban patients (19.4 percentage points). Mailed FIT programs at both one and five years were more effective in Hispanic patients (11.3 percentage point increase versus 9.6 percentage point increase for non-Hispanic patients over 5 years). The 90% uncertainty intervals for CRC screening at one and five years are tightly controlled (see Tables 2a and 2b).
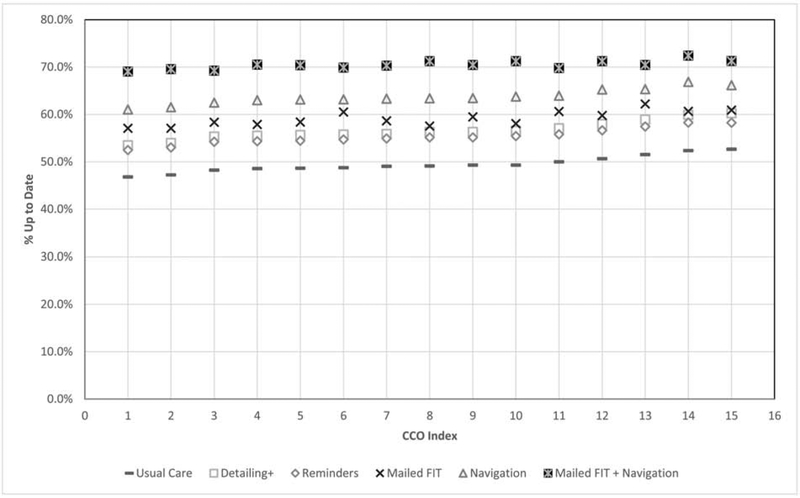
At the level of Oregon’s 15 individual CCOs, when implemented over a five year period, all interventions had a positive impact on CRC screening up-to-date rates compared to usual care (Figure 1). Across all CCOs Mailed FIT+Navigation performed the best, followed by Navigation, Mailed FIT, Detailing+ and Reminders. In addition to increasing screening rates overall, all interventions helped close the performance gap between CCOs. For example, under the usual care scenario, the range in percent-up-to-date was 46.8% to 52.6% across CCOs, a 5.8 percentage point difference. Following implementation of Mailed FIT+Navigation the range in the percent up-to-date was 69.0% to 72.4%, a 3.4 percentage point difference.
Figure 1. Simulated Age-Eligiblea Oregon Medicaid Population Up-to-Date with Recommended Colorectal Cancer Screening on December 31, 2023 By CCO.
Cancer Cases Averted and Life-Years Gained
As detailed in Table 3, our model estimates a rate of 1,435 CRC cancer cases per 100,000 patients in the usual care scenario. Over the five-year period, the number of cancer cases averted would increase with all implemented interventions, from a high of 113 cases averted per 100,000 for Mailed FIT+Navigation to a low of 27 cases averted per 100,000 with Detailing+. In addition, because screening catches early cancers and precancerous polyps before they can progress to more advanced disease, the majority of cancers prevented are the more costly and deadly advanced stages III and IV (see Table 3). The 90% uncertainty intervals for CRC cases averted are wider than those for CRC screening given that cases averted are less frequent as well as a more distal outcome.
Table 3.
CRC Cases Averted among Medicaid Patients Exposed to Five Years of Intervention, simulated impact over their full life course and reported as an unadjusted rate per 100,000 people.
| Variable | Cancer Cases under care as usual | Number of Cancer Cases Averted Under Each Intervention Scenario Compared with Screening as Usual | n | ||||
|---|---|---|---|---|---|---|---|
| Detailing+ | Reminders | Mailed FIT | Navigation | Mailed FIT + Navigation | |||
| Overall | 1,435 | 27 | 32 | 77 | 77 | 113 | 106,673 |
| Uncertainty Interval (5th, 95th Percentile) | (1,298, 1,635) | (0, 51) | (0, 57) | (46, 129) | (42, 109) | (74, 146) | |
| By gender | |||||||
| Male | 811 | 12 | 16 | 42 | 41 | 66 | 52,618 |
| Female | 623 | 15 | 16 | 35 | 36 | 47 | 54,055 |
| By race | |||||||
| White | 1,136 | 22 | 26 | 58 | 58 | 88 | 86,676 |
| African American | 45 | 0 | 1 | 1 | 3 | 3 | 2,644 |
| Other | 254 | 5 | 5 | 18 | 16 | 22 | 17,354 |
| By ethnicity | |||||||
| Hispanic | 266 | 4 | 5 | 16 | 16 | 22 | 19,268 |
| Non-Hispanic | 1,169 | 23 | 27 | 61 | 61 | 91 | 87,406 |
| By geography | |||||||
| Urban | 882 | 19 | 21 | 49 | 48 | 69 | 64,287 |
| Rural | 553 | 8 | 11 | 28 | 29 | 44 | 42,386 |
| Stage | |||||||
| I | 682 | 5 | 4 | 9 | 12 | 18 | |
| II | 232 | 4 | 4 | 7 | 11 | 15 | |
| III | 278 | 9 | 13 | 27 | 25 | 37 | |
| IV | 242 | 9 | 10 | 35 | 29 | 43 | |
An increasing number of CRC screenings and cancers prevented translates to greater life-years gained at a population level, as detailed in Table 4. For example, compared to usual care, five years’ implementation of Mailed FIT+Navigation would result in 1,315 life-years gained (90% Uncertainty Interval: 778, 2112).
Table 4.
Total Life Years Gained by all Patients Receiving Interventions Over Five Years
| Number of Life Years Gained Under Each Intervention Scenario Compared with Screening as Usual | ||||||
|---|---|---|---|---|---|---|
| Variable | Life Years as Usual | Detailing+ | Reminders | Mailed FIT | Navigation | Mailed FIT+ Navigation |
| Overall | 3,153,151 | 326 | 393 | 976 | 969 | 1,315 |
| Uncertainty Interval (5th, 95th Percentile) | (3,141,775, 3,172,292) | (39, 705) | (62, 726) | (366, 1637) | (311, 1697) | (778, 2112) |
| By gender | ||||||
| Male | 1,455,572 | 143 | 169 | 525 | 519 | 726 |
| Female | 1,697,579 | 183 | 225 | 451 | 450 | 589 |
| By race | ||||||
| White | 2,611,835 | 264 | 321 | 765 | 749 | 1,056 |
| African American | 71,061 | 18 | 18 | 30 | 43 | 48 |
| Other | 470,255 | 44 | 55 | 182 | 177 | 212 |
| By ethnicity | ||||||
| Hispanic | 559, 385 | 33 | 55 | 150 | 143 | 192 |
| Non-Hispanic | 2,593,766 | 293 | 339 | 826 | 825 | 1,123 |
| By geography | ||||||
| Rural | 1,259,635 | 131 | 151 | 399 | 403 | 571 |
| Urban | 1,893,516 | 195 | 243 | 577 | 565 | 744 |
Costs and Cost Effectiveness
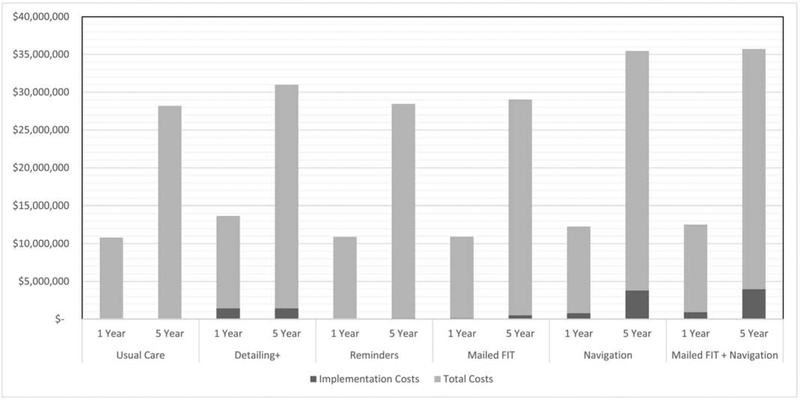
As summarized in Figure 2 and detailed in Appendix C, the implementation costs for the five interventions are a small percentage of the total costs, which include implementation (cost of implementing the intervention), procedure (cost of follow-up screenings and polyp removal, polyp pathology, addressing any adverse events resulting from colonoscopies), and cancer treatment costs. After one year, total costs were just under $11K for usual care and ranged from $11K to $12K for the interventions. For the five intervention scenarios, implementation costs made up .2% (Reminders) to 11.7% (Detailing+) of total costs and ranged from $21K (Reminders) to $915K (Mailed FIT+Navigation). After five years, total costs were $28M for usual care and ranged from $28M (i.e., Reminders, Mailed FIT) to nearly $32M for Mailed FIT+Navigation.
Figure 2. Implementation and Total Costs of Interventions at One and Five Years.
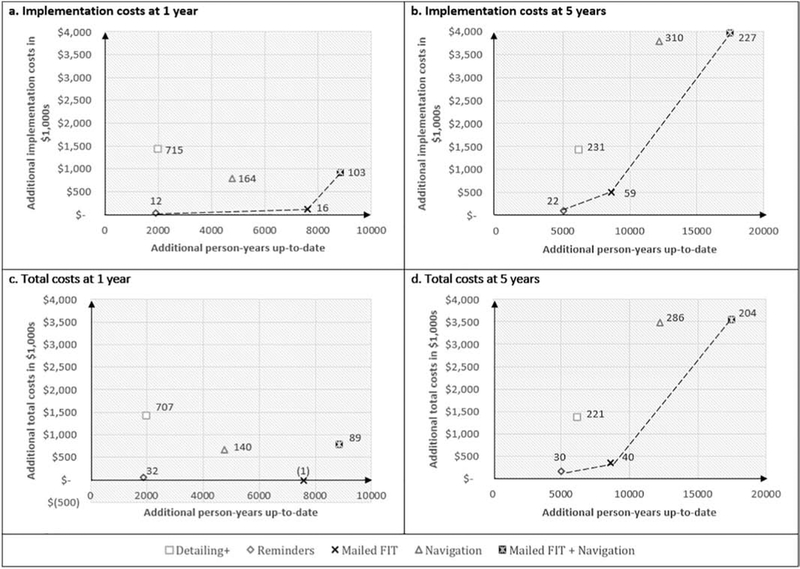
Figure 3 summarizes the implementation and total costs per additional year up-to-date for each intervention (compared to usual care), at both one and five years. Detailing+ and Navigation are dominated – more costly and less effective than the other interventions – when considering implementation costs only or total costs. In terms of implementation costs at one year (Figure 3a). Reminders, Mailed FIT and Mailed FIT+Navigation fall on the efficiency frontier – the line that indicates if an intervention is more cost effective than others -- with cost per additional person-year up-to-date of $12, $16 and $103 respectively as compared to usual care. When we consider total costs after one year (Figure 3c), Mailed FIT turns out to be both beneficial and cost saving; this is because the intervention costs are low enough that the savings from the few cancer treatments averted offset the cost of implementing the intervention. After five years, Reminders, Mailed FIT, and Mailed FIT+Navigation remained cost effective as long as decision makers are willing to pay up to $230 per person-year up-to-date (at a cost of $22, $59, and $227 respectively per additional person-year up-to-date), whereas Detailing+ and Navigation were more costly for the same benefits (see Figure 3b).
Figure 3. Cost Effectiveness Efficiency Frontiers at One and Five Years Based on Implementation and Total Costs.
Figure 3 shows the costs involved with implementing each intervention (x-axis) compared to the number of person-years up-to-date gained through the intervention (y-axis; each compared to usual care) after one year and five years of intervention. Figures 3a and 3b present findings in relation to implementation costs in 1,000s. Figures 3c and 3d present findings in relation to total costs in 1,000s. The data labels present the incremental cost effectiveness ratio (ICER) for each intervention compared to usual care, and tells us the additional amount of money that must be spent in order to gain one additional person-year up-to-date. The interventions on the efficiency frontier (dashed line) are those that are cost-effective to implement (dominant interventions). These interventions may be more or less desirable based on a stakeholder’s willingness to pay for each additional person-year up-to-date. Moving along the efficiency frontier from left to right, interventions can be selected with greater impact, but at greater cost. The interventions above the efficiency frontier are dominated by, or less cost effective, relative to those on the frontier. Panel 3c does not have an efficiency frontier because Mailed FIT is unequivocally cost-efficient when one considers the incremental total costs involved over the first year of implementation.
DISCUSSION
Our findings suggest that if implemented over five years, Mailed FIT with or without Navigation and Reminders, may enable Oregon Medicaid beneficiaries to approach and potentially surpass current CRC screening targets set by Healthy People 2020.59 The multicomponent intervention bundle consisting of Mailed FIT+Navigation was estimated to be the most effective approach to increase CRC screening among Oregon Medicaid beneficiaries, resulting in a nearly 12 percentage point increase in the overall proportion of the age-eligible population up-to-date with screening recommendations after one year of implementation, compared to usual care, at an implementation cost of $103 per additional person screened. After five years, Mailed FIT+Navigation resulted in a more than 20 percentage point increase in the proportion up-to-date at an implementation cost of $227 per additional person screened, compared to usual care. When costs associated with CRC screening, testing, and cancer treatment are considered in addition to intervention implementation costs over five years, Mailed FIT+Navigation had a total cost of $204 per additional person screened relative to the usual care. Improvements in in screening following Mailed FIT+Navigation implementation over five years would avert nearly 77 cancer cases (a reduction of 113 per 100,000) and increase life-years by 1,315 compared to usual care.
A 10 to 20 percentage point gain in CRC screening at the population level, as projected to be achieved by implementation of these interventions, is substantial. Prior simulation modeling work by our team focused on North Carolina and found only modest improvements in overall screening rates (0.2–0.5 percentage points) over a 10-year period for four intervention scenarios: mailed reminders for Medicaid enrollees, mass media campaigns targeting African Americans, colonoscopy vouchers for the uninsured, and expanding endoscopy facilitators.25 Health system stakeholders may be more or less likely to implement the current interventions based on their willingness and ability to pay for improvements in screening. Reminders, Mailed FIT and Mailed FIT+Navigation fall along the efficiency frontier at both one and five years of implementation and may be cost effective, depending on stakeholder willingness to pay up to $103 for each additional person year up-to-date at one year or $227 at five years compared to usual care. Importantly, when total costs were considered, including follow-up and cancer treatment costs, Mailed FIT alone was cost-saving (i.e., costs less and results in better outcomes) at one year, compared to usual care. Navigation and Detailing+ were dominated by other interventions at both one and five years such that these interventions cost more than the other interventions for the same level of benefit.
It is notable that the interventions in this scenarios served to increase CRC screening rates and to attenuate observed disparities across Oregon’s CCOs. Thus, although CCOs may start with higher or lower CRC screening rates based on historical artifacts such as the type of screening used (colonoscopy versus FIT)36 or characteristics of the population served,6 implementation of these scenarios reduced variation in the proportion of patients up-to-date across the CCOs (e.g., from a 5.8 to 3.8 percentage point difference). Implementing Mailed FIT, with or without Navigation, may also help improve CRC screening within Medicaid sub-populations that experience disparities (e.g., Rural, Latino). If select interventions were only implemented in higher performing CCOs, existing disparities in screening and outcomes may be exacerbated.22 Given the impact of these interventions across Oregon’s 15 CCOs, we anticipate that findings would translate to other states implementing Medicaid ACOs and to dually eligible Medicaid-Medicare patients. Given that the simulation model accounts for patient demographic characteristics (e.g., race/ethnicity, gender, geography) and baseline screening rates, the potential impact of these interventions in other settings may vary accordingly.
A growing body of evidence suggests that Mailed FIT programs can improve CRC screening and have the potential for attenuating screening disparities in rural, low-income, and racially diverse populations.8–12 The relative difference in costs and effectiveness of the various interventions modeled over time results from differences in cumulative benefits of strategies that emphasize fecal testing versus colonoscopy and the duration of up-to-datedness conferred by these two modalities, as well as the timeframe over which costs are accrued. Interventions that emphasize fecal testing, in particular, may be underestimated in terms of overall benefits in our analysis, since we do not assume that recipients of a prior FIT are more likely to receive future FITs (as evidence has indicated is true).60 Additional research could also explore if these interventions build on each other when implemented simultaneously or in sequence (e.g., Detailing+ prior to Mailed FIT).
Several limitations of our analysis should be noted. First, as with any simulation model, uncertainty governs our projections. Assumptions about the reach and duration of health benefits of interventions and of CRC screening itself are based upon best available evidence, but may be subject to change within specific contexts and over time. For example, our estimates may not capture efficiencies that health systems can develop by implementing these interventions over time or efficiencies in economies of scale. Moreover, we are unable to predictively validate our results against reality given the prospective nature of our study. Second, we focused our analysis on Medicaid enrollees and modeled interventions that were currently being implemented in Oregon and were supported by published research evidence. Future research could compare the impact of these interventions in other insured populations (e.g., commercial, Medicare, Dually insured Medicaid-Medicare patients) as well as by targeting implementation to high risk populations (e.g., newly age eligible, ethnically diverse, rural). Moreover, the intervention scenarios modeled here do not capture all possible approaches to improving CRC screening. Additional policy interventions have been projected to improve CRC screening and outcomes, and could be explored in subsequent modeling research, including waiving coinsurance for screening.61,62 Third, we assumed that any intervention that requires training of health care professionals happens at a time when they would not otherwise be making revenue, so opportunity costs of such training and professional engagement are not included. Nevertheless, our analysis provides a robust assessment of anticipated costs and benefits of different intervention alternatives specific to Medicaid beneficiaries that can improve CCO leaders’ decision making as they weigh tradeoffs to improve CRC screening coverage. Finally, our presentation of 90% uncertainty intervals demonstrates that the estimates for more distal outcomes (e.g., CRC cases averted, live years gained) are more uncertain than proximal outcomes (e.g., CRC screening) such that uncertainty intervals might overlap. However, our sensitivity analysis for the number of replications and the analysis of rankings of interventions across replications demonstrates that our findings were consistently robust for all the outcomes under consideration.
CONCLUSION
Our state-level simulation results indicate that Mailed FIT, with or without Navigation, and Reminders, implemented over a one or five year horizon, may be cost-effective or cost-saving strategies to increase CRC screening in Medicaid populations, compared to usual care. Given that there is no standard threshold for cost-effectiveness for the outcome of person-years up-to-date, decision makers should evaluate their ability and willingness to pay for Reminders and Mailed FIT interventions, with or without Navigation, to determine which approach is most likely to achieve desired gains in CRC screening rates at an affordable cost. Microsimulation studies such as ours can aid health system stakeholders, including clinic and health plan leaders, in selecting appropriate interventions, anticipating health impact and costs, and ultimately reducing disparities in cancer screening and prevention for low-income and other medically underserved populations.
HIGHLIGHTS.
Multiple intervention improve colorectal cancer (CRC) screening in Medicaid patients.
Microsimulation can inform intervention selection based on costs and impact.
Mailed fecal tests (Direct Mail), Navigation, and Reminders were cost effective.
CRC screening exceeded national targets with Direct Mail+Navigation implementation.
ACKNOWLEDGEMENTS
Many colleagues provided information that contributed to our estimates of the effectiveness and costs associated with each intervention. Michael Dougherty, MD and his team were especially helpful in providing resources to help determine the estimated effect of each selected intervention. Gloria Coronado, PhD; MD; David H. Smith, PhD, RPh; Richard T. Meenan, PhD, MPH, MBA; Roxane Waldron; and Gerry Melgar, MD provided information and unpublished work that helped to develop the cost estimates. We appreciate the help of Jadon Bachtold and Sarah Bumatay with manuscript editing.
FUNDING
This study was supported, in part, by Cooperative Agreement Number U48-DP005017-01S8 and U48 DP005017-01S8 to University of North Carolina at Chapel Hill and U48 DP005006-01S3 to Oregon Health & Science University from the Centers for Disease Control and Prevention (CDC) Prevention Research Centers (PRC) Program and the National Cancer Institute (NCI), as part of the Cancer Prevention and Control Research Network (CPCRN) (PI: Wheeler). Melinda Davis was partially supported by an NCI K07 award (1K07CA211971-01A1, PI: Davis). The funding organizations did not have a role in the design of the study, collection, analysis, and interpretation of data, or writing of the manuscript. The content provided is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Appendix A. Detailed Costs for Simulated Interventions.
| Intervention | Intervention Steps | Cost (per patient unless otherwise notes) |
|---|---|---|
| Detailing+ | Physician training sessions | $970.25, per clinic (lower: $700, upper: $1250)40,51 |
| Technical and programming staff | $207.91, per clinic (lower: $150, upper: $270)51,52 | |
| Quarterly meetings with updates | $408.5, per clinic (lower: $250, upper: $550)51,52 | |
| Reminders | Technical staff (to manage automatic calls, maintain the EHR program, track patients, etc.) | $0.7953 |
| Automatic calls/texts (to alert patients that they are not up-to-date on screening) | $0.6453 | |
| Mailed FIT | FIT kit | $3.2754 |
| Mailing costs: postage, stamps, envelopes, paper, and materials (letter from provider, fact sheet, directions for FIT use) | $1.3554 | |
| Project management staff (to fill envelopes, manage the project, etc.) | $0.5053 | |
| Technical staff (to manage automatic calls, maintain the EHR program, track patients, etc.) | $0.7953 | |
| Automated phone reminder to complete FIT | $0.6453 | |
| Navigation | Navigator staff (making the navigation calls) | $35.41 (lower: $13.50, upper: $108.03)44,45,55,56 |
| Technical staff (system development and maintenance -- keep track of who is up-to-date on screening and who needs to be called) | $0.7953 | |
| Navigator training | $1123.45 (lower: $500, upper: $1500)52,57,58 | |
| Mailed FIT+ Navigation | See above for cost estimates. Note that the effectiveness estimates for these combined interventions are not equal to the sum of each intervention alone. | |
Appendix B. Demographic Characteristics of the Simulated Population, December 31, 2018.
| Characteristic | N | % |
|---|---|---|
| Population* | 68,077 | 100 |
| Gender | ||
| Male | 33,794 | 49 .6 |
| Female | 34,283 | 50.4 |
| Race | ||
| White | 56,237 | 82 .6 |
| African American | 2,004 | 2.9 |
| Other | 9,836 | 14.4 |
| Hispanic Ethnicity | 4,864 | 7.1 |
| Rural Residence | 27,601 | 40.5 |
| Age | ||
| 50–54 | 31,502 | 46 .3 |
| 55–59 | 20,946 | 30.8 |
| 60–64 | 15,629 | 23.0 |
Oregon Medicaid Enrollees Aged 50–64 Years and Eligible for Colorectal Cancer Screening
Appendix C. Implementation and Total Costs of Interventions at One and Five Years
C.1.
One Year
| Intervention | Total Costs | Implementation Costs | Procedure Costs | Cancer Treatment Costs |
|---|---|---|---|---|
| Usual Care | $10,818,320 | $ 0 | $9,822,507 | $ 995,814 |
| Detailing+ | $12,229,765 | $ 1,427,814 | $9,851,486 | $ 950,465 |
| Reminders | $10,878,104 | $ 21,781 | $9,906,320 | $ 950,002 |
| Mailed FIT | $10,807,801 | $ 118,191 | $9,813,537 | $ 876,073 |
| Navigation | $11,486,130 | $ 784,132 | $9,852,404 | $ 849,595 |
| Mailed FIT+Navigation | $11,608,956 | $ 914,820 | $9,907,727 | $ 786,409 |
NOTE: Procedure costs includes the cost of FIT and colonoscopy, including expenses associated with polyp removal, pathology, and procedural complications.
C.2.
Five Years
| Intervention | Total Costs | Implementation Costs | Procedure Costs | Cancer Treatment Costs |
|---|---|---|---|---|
| Usual Care | $28,185,814 | $ 0 | $25,177,732 | $ 3,008,082 |
| Detailing+ | $29,551,284 | $ 1,427,814 | $25,227,708 | $ 2,895,762 |
| Reminders | $28,337,788 | $ 112,008 | $25,336,374 | $ 2,889,406 |
| Mailed FIT | $28,529,517 | $ 506,788 | $25,296,288 | $ 2,726,440 |
| Navigation | $31,667,518 | $ 3,784,027 | $25,231,658 | $ 2,651,833 |
| Mailed FIT+Navigation | $31,744,918 | $ 3,966,957 | $25,263,119 | $ 2,514,842 |
NOTE: Procedure costs includes cost of FIT and colonoscopy, including expenses associated with polyp removal, pathology, and procedural complications.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
Stephanie Wheeler receives unrelated grant funding to her institution from Pfizer. All other authors declare no conflicts of interest.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA: A Cancer Journal for Clinicians. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.US Preventive Services Task Force. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2016;315(23):2564–2575. [DOI] [PubMed] [Google Scholar]
- 3.White A, Thompson TD, White MC, et al. Cancer Screening Test Use — United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(8):201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Cancer Society. Colorectal Cancer Facts & Figures 2017–2019. Atlanta: American Cancer Society. 2017. [Google Scholar]
- 5.Cole AM, Jackson JE, Doescher M. Urban–rural disparities in colorectal cancer screening: cross-sectional analysis of 1998–2005 data from the Centers for Disease Control’s Behavioral Risk Factor Surveillance Study. Cancer Medicine. 2012;1(3):350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davis MM, Renfro S, Pham R, et al. Geographic and population-level disparities in colorectal cancer testing: A multilevel analysis of Medicaid and commercial claims data. Preventive Medicine. 2017;101:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wheeler SB, Kuo T-M, Goyal RK, et al. Regional variation in colorectal cancer testing and geographic availability of care in a publicly insured population. Health & Place. 2014;29:114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holden DJ, Jonas DE, Porterfield DS, Reuland D, Harris R. Systematic review: enhancing the use and quality of colorectal cancer screening. Annals of Internal Medicine. 2010;152(10):668–676. [DOI] [PubMed] [Google Scholar]
- 9.Davis MM, Freeman M, Shannon J, et al. A systematic review of clinic and community intervention to increase fecal testing for colorectal cancer in rural and low-income populations in the United States - How, what and when? BMC Cancer. 2018;18(1):40–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dougherty MK, Brenner AT, Crockett SD, et al. Evaluation of interventions intended to increase colorectal cancer screening rates in the United States: A systematic review and meta-analysis. JAMA Internal Medicine. 2018;178(12):1645–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rat C, Latour C, Rousseau R, et al. Interventions to increase uptake of faecal tests for colorectal cancer screening: a systematic review. European Journal of Cancer Prevention. 2018;27(3):227–236. [DOI] [PubMed] [Google Scholar]
- 12.Issaka RB, Avila P, Whitaker E, Bent S, Somsouk M. Population health interventions to improve colorectal cancer screening by fecal immunochemical tests: A systematic review. Preventive Medicine. 2019;118:113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Community Guide. Cancer Screening: Multicomponent Interventions—Colorectal Cancer. https://www.thecommunityguide.org/findings/cancer-screening-multicomponent-interventions-colorectal-cancer. Accessed April 27, 2019. [Google Scholar]
- 14.Albright BB, Lewis VA, Ross JS, Colla CH. Preventive care quality of Medicare Accountable Care Organizations: Associations of organizational characteristics with performance. Medical Care. 2016;54(3):326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joseph DA, Redwood D, DeGroff A, EL B. Use of evidence-based interventions to address disparities in colorectal cancer screening. MMWR Suppl. 2016;65(01):21–28. [DOI] [PubMed] [Google Scholar]
- 16.Center for Health Care Strategies Inc. Medicaid Accountable Care Organizations: State Update. 2018; https://www.chcs.org/resource/medicaid-accountable-care-organizations-state-update/. Accessed May 10, 2019. [Google Scholar]
- 17.Henry J Kaiser Family Foundation. Mapping Medicaid Delivery System and Payment Reform. 2017. [Google Scholar]
- 18.Çağlayan Ç, Terawaki H, Chen Q, Rai A, Ayer T, Flowers CR. Microsimulation Modeling in Oncology. JCO Clinical Cancer Informatics. 2018(2):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krijkamp EM, Alarid-Escudero F, Enns EA, Jalal HJ, Hunink MGM, Pechlivanoglou P. Microsimulation Modeling for Health Decision Sciences Using R: A Tutorial. Medical decision making : an international journal of the Society for Medical Decision Making. 2018;38(3):400–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rutter CM, Zaslavsky AM, Feuer EJ. Dynamic microsimulation models for health outcomes: a review. Medical decision making : an international journal of the Society for Medical Decision Making. 2011;31(1):10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Moor JS, Cohen RA, Shapiro JA, et al. Colorectal cancer screening in the United States: Trends from 2008 to 2015 and variation by health insurance coverage. Prev Med. 2018;112:199–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis MMGR, Pham R, Wiser A, Hassmiller Lich K, Wheeler SB, Coronado GD. Key collaborative factors when Medicaid Accountable Care Organizations work with primary care clinics to improve colorectal cancer screening: Relationships, data, and quality improvement infrastructure. Preventing Chronic Disease. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davis MM, Lindberg P, Cross S, Lowe S, Gunn R, Dillon K. Aligning systems science and community-based participatory research: A case example of the Community Health Advocacy and Research Alliance (CHARA). J Clin Transl Sci. 2018;2(5):280–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kristen D, Paul L, Marie DM. Aligning Research with Action in the Columbia Gorge – The Community Health Advocacy and Research Alliance (CHARA) In: Page-Reeves J, ed. Well-being as Multi-Dimensional Concept: Understanding Connections between Culture, Community, and Health. Lanham, Maryland: Lexington Books of Rowman & Littlefield; In press. [Google Scholar]
- 25.Hassmiller Lich K, Cornejo DA, Mayorga ME, et al. Cost-effectiveness analysis of four simulated colorectal cancer screening interventions, North Carolina. Preventing Chronic Disease. 2017;14:E18–E18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nambiar S, Mayorga ME, O’Leary MC, Lich KH, Wheeler SB. A simulation model to assess the impact of insurance expansion on colorectal cancer screening at the population level Proceedings of the 2018 Winter Simulation Conference; 2018; Gothenburg, Sweden. [Google Scholar]
- 27.Wheaton WD, Cajka JC, Chasteen BM, et al. Synthesized population databases: A US geospatial database for agent-based models. Methods Report (RTI Press) 2009:905–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.US Census Bureau. American Community Survey (ACS). N.D; https://www.census.gov/programs-surveys/acs/. Accessed June 19, 2019. [Google Scholar]
- 29.AnyLogic . AnyLogic Simulation Software. n.d; https://www.anylogic.com/. Accessed June 17, 2019. [Google Scholar]
- 30.Population Simulation Model. 2019; www.crcsim.web.unc.edu. Accessed May 23, 2019.
- 31.Subramanian S, Bobashev G, Morris RJ. Modeling the cost-effectiveness of colorectal cancer wcreening: Policy guidance based on patient preferences and compliance. Cancer Epidemiology Biomarkers & Prevention. 2009;18(7):1971. [DOI] [PubMed] [Google Scholar]
- 32.Loeve F, Boer R, van Oortmarssen GJ, van Ballegooijen M, Habbema JD. The MISCAN-COLON simulation model for the evaluation of colorectal cancer screening. Computers and biomedical research, an international journal. 1999;32(1):13–33. [DOI] [PubMed] [Google Scholar]
- 33.Lansdorp-Vogelaar I, van Ballegooijen M, Boer R, Zauber A, Habbema JDF. A novel hypothesis on the sensitivity of the fecal occult blood test: Results of a joint analysis of 3 randomized controlled trials. Cancer. 2009;115(11):2410–2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th Edition of the AJCC Cancer Staging Manual and the Future of TNM. Annals of Surgical Oncology. 2010;17(6):1471–1474. [DOI] [PubMed] [Google Scholar]
- 35.Arias E United States life tables, 2002. National Vital Statistics Reports. 2004;53(6). [PubMed] [Google Scholar]
- 36.Davis MM, Shafer P, Renfro S, et al. Does a transition to accountable care in Medicaid shift the modality of colorectal cancer testing? BMC Health Serv Res. 2019;19(1):54–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wheeler SB, Kuo T-M, Meyer AM, et al. Multilevel predictors of colorectal cancer testing modality among publicly and privately insured people turning 50. Preventive Medicine. 2016;6:9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karnon J, Stahl J, Brennan A, Caro JJ, Mar J, Moller J. Modeling using discrete event simulation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force−−4. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2012;15(6):821–827. [DOI] [PubMed] [Google Scholar]
- 39.Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB. Model transparency and validation: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-7. Medical decision making : an international journal of the Society for Medical Decision Making. 2012;32(5):733–743. [DOI] [PubMed] [Google Scholar]
- 40.Ferreira MR, Dolan NC, Fitzgibbon ML, et al. Health Care Provider-Directed Intervention to Increase Colorectal Cancer Screening Among Veterans: Results of a Randomized Controlled Trial. Journal of Clinical Oncology. 2005;23(7):1548–1554. [DOI] [PubMed] [Google Scholar]
- 41.Mosen DM, Feldstein AC, Perrin N, et al. Automated telephone calls improved completion of fecal occult blood testing. Medical Care. 2010;48(7):604–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muller CJ, Robinson RF, Smith JJ, et al. Text message reminders increased colorectal cancer screening in a randomized trial with Alaska Native and American Indian people. Cancer. 2017;123(8):1382–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jean-Jacques M, Kaleba EO, Gatta JL, Gracia G, Ryan ER, Choucair BN. Program to improve colorectal cancer screening in a low-income, racially diverse population: a randomized controlled trial. Annals of Family Medicine. 2012;10(5):412–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dietrich AJ, Tobin JN, Cassells A, et al. Telephone care management to improve cancer screening among low-income women: a randomized, controlled trial. Annals of Internal Medicine. 2006;144(8):563–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lasser KE, Murillo J, Lisboa S, et al. Colorectal cancer screening among ethnically diverse, low-income patients: A randomized controlled trial. Archives of Internal Medicine. 2011;171(10):906–912. [DOI] [PubMed] [Google Scholar]
- 46.Gupta S, Halm EA, Rockey DC, et al. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: a randomized clinical trial. JAMA Internal Medicine. 2013;173(18):1725–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goldman SN, Liss DT, Brown T, et al. Comparative effectiveness of multifaceted outreach to initiate colorectal cancer screening in community health centers: A randomized controlled trial. Journal of General Internal Medicine. 2015;30(8):1178–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hassmiller Lich K, O’Leary M, Nambiar S, et al. The anticipated impact of insurance expansion on colorectal cancer and related costs in North Carolina: a population-level simulation analysis. In Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Leary MC, Lich KH, Gu Y, et al. Colorectal cancer screening in newly insured Medicaid members: a review of concurrent federal and state policies. 2019;19(1):298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oregon Health Authority. Oregon Health System Transformation: CCO Metrics 2017 Final Report. 2018. [Google Scholar]
- 51.Melgar G Email Correspondence. 2018. [Google Scholar]
- 52.United States Department of Labor Bureau of Labor Statistics. May 2018 State Occupational Employment and Wage Estimates: Oregon. 2019. [Google Scholar]
- 53.Smith DH, Feldstein AC, Perrin N, et al. Automated telephone calls to enhance colorectal cancer screening: economic analysis. The American Journal of Managed Care. 2012;18(11):691–699. [PMC free article] [PubMed] [Google Scholar]
- 54.Smith DH, O’Keeffe RM, Mosen DM, et al. Balancing adherence and expense: The cost-effectiveness of two-sample vs one-sample Fecal Immunochemical Test. Population Health Management. In Review;22(1):83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lairson DR, Dicarlo M, Deshmuk AA, et al. Cost-effectiveness of a standard intervention versus a navigated intervention on colorectal cancer screening use in primary care. Cancer. 2014;120(7):1042–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fiscella K, Whitley E, Hendren S, et al. Patient navigation for breast and colorectal cancer treatment: a randomized trial. Cancer Epidemiology Biomarkers & Prevention. 2012;21(10):1673–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Patient Navigator Training Collaborative. Course Pricing & Scholarships. 2019. [Google Scholar]
- 58.Harold P Freeman Patient Navigation Institute. 2019; http://www.hpfreemanpni.org. Accessed June 17, 2019. [Google Scholar]
- 59.Office of Disease Prevention and Health Promotion. Healthy People 2020. 2014; https://www.healthypeople.gov/2020/leading-health-indicators/2020-lhi-topics/Clinical-Preventive-Services/data#C-16. Accessed May 10, 2019. [Google Scholar]
- 60.Townlsey RM. An examination of individual sequential behavior modeling in the context of healthcare simulation. Raleigh, North Carolina: North Carolina State University; 2018. [Google Scholar]
- 61.Peterse EFP, Meester RGS, Gini A, et al. Value of waiving coinsurance for colorectal cancer screening in Medicare beneficiaries. Health Affairs. 2017;36(12):2151–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zerhouni YA, Trinh QD, Lipsitz S, et al. Effect of Medicaid expansion on colorectal cancer screening rates. Diseases of the colon and rectum. 2019;62(1):97–103. [DOI] [PubMed] [Google Scholar]