Abstract
Introduction
Robotic surgery has been increasingly used in fashioning various surgical anastomoses. Our aim was to collect and analyze outcomes related to anastomoses performed using a robotic approach and compare them with those done using laparoscopic or open approaches through meta-analysis.
Methods
A systematic review was conducted for articles comparing robotic with laparoscopic and/or open operations (colectomy, low anterior resection, gastrectomy, Roux-en-Y gastric bypass (RYGB), pancreaticoduodenectomy, radical cystectomy, pyeloplasty, radical prostatectomy, renal transplant) published up to June 2019 searching Medline, Scopus, Google Scholar, Clinical Trials and the Cochrane Central Register of Controlled Trials. Studies containing information about outcomes related to hand-sewn anastomoses were included for meta-analysis. Studies with stapled anastomoses or without relevant information about the anastomotic technique were excluded. We also excluded studies in which the anastomoses were performed extracorporeally in laparoscopic or robotic operations.
Results
We included 83 studies referring to the aforementioned operations (4 randomized controlled and 79 non-randomized, 10 prospective and 69 retrospective) apart from colectomy and low anterior resection. Anastomoses done using robotic instruments provided similar results to those done using laparoscopic or open approach in regards to anastomotic leak or stricture. However, there were lower rates of stenosis in robotic than in laparoscopic RYGB (p=0.01) and in robotic than in open radical prostatectomy (p<0.00001). Moreover, all anastomoses needed more time to be performed using the robotic rather than the open approach in renal transplant (p≤0.001).
Conclusion
Robotic anastomoses provide equal outcomes with laparoscopic and open ones in most operations, with a few notable exceptions.
Keywords: anastomosis, hand-sewn, leak, stenosis, stricture, robotic, laparoscopic, open
Introduction
The introduction of laparoscopic techniques is considered to be one of the most prominent changes in surgical practice in the last decade of the twentieth century.1 Since the first laparoscopic cholecystectomy in 1987, minimally invasive approaches have become the mainstay of most abdominal surgical procedures. The benefits of minimally invasive surgery are well established and include reduced analgesic requirements, reduced wound-related complications, shorter length of hospital stay, and faster return to normal daily activities.2
Laparoscopic surgery, however, has its technical limitations. As described by Ruurda et al, open procedures offer the surgeon unlimited flexibility in his/her body, arm, and hand positions. A surgeon’s actions can be controlled by visual and haptic feedback. In laparoscopic surgery, however, natural dexterity is compromised by the restricted degree of motion in laparoscopic instruments.1 Hand-eye coordination is reduced by the need to move an instrument in the opposite direction from the desired target on the monitor. Furthermore, depth perception is compromised by the two-dimensional image, and the need to rely on an assistant to operate the camera takes away the surgeon’s control over the field of view.2 Most importantly, the length of rigid laparoscopic instruments results in the ready transmission and exaggeration of tiny movements from the surgeon, making delicate procedures, particularly fine anastomoses difficult.3 As a result, although the majority of abdominal general surgical operations can be performed laparoscopically, techniques for more complex surgery are less easily reproducible and left in the hands of only a limited number of experts.1 Open approaches for complex operations, where delicate and accurate anastomoses with minimal risk of complication are of critical importance, have hence remained the gold standard over the last 60 years.4
The introduction of the da Vinci® Surgical System (Intuitive Surgical Inc., Sunnyvale, CA, US) in 2000, however, has allowed some of the technical limitations of laparoscopic surgery to be overcome. One of the main advantages is that of enhanced dexterity (increased degrees of freedom of movement) in the instruments and filtering of tremor, which enables the surgeon to operate in a similar manner to open surgery, thus enabling fine microsurgery and microanastomoses to be performed.1 Other advantages include a three-dimensional view of the operative field, which allows for better depth perception, the ability of the surgeon to control of the view of the operative field, and an ergonomically designed workstation where the surgeon assumes a comfortable sitting position.2
Since its introduction almost 20 years ago, robotic surgery has been successfully applied to key colorectal, gastric, pancreatic, urological and transplantation procedures where anastomoses form a critical part of the operation. The aim of this study was to review the current literature surrounding robotic surgery in the abdomen, with particular reference to a comparison between robotically fashioned anastomoses and similar anastomoses performed via a laparoscopic and/or open approach.
Methods
Search Strategy
A systematic review and meta-analysis were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines.5 We systematically searched the following databases for articles published up to June 2019: Medline, Scopus, Google Scholar, Clinical Trials and Cochrane Central Register of Controlled Trials. We used the following search terms: “robotic”, “robotic-assisted”, “robot-assisted”, “colectomy”, “low anterior resection”, “anterior resection”, “rectal resection”, “gastrectomy”, “gastric bypass”, “pancreaticoduodenectomy”, “Whipple”, “cystectomy”, “ileal conduit”, “neobladder”, “pyeloplasty”, “prostatectomy”, “renal transplant”, “kidney transplant”, combined with the Boolean operators AND, OR.
Inclusion and Exclusion Criteria
We included only original articles written in English that compared robotic with laparoscopic and/or open procedures regarding the aforementioned types of operations, included data about anastomotic leak, anastomotic stricture and/or anastomotic time and the anastomoses were hand-sewn. We excluded reviews, case reports, congress abstracts, animal studies, original articles referring only to robotic operations without comparing them with either laparoscopic or open ones and original articles comparing robotic with laparoscopic and/or open operations, but without having data about anastomotic leak, anastomotic stricture and/or anastomotic time or without the anastomoses being hand-sewn or with the anastomoses performed extracorporeally in laparoscopic or robotic cases, and articles written in languages other than English.
Review and Analysis
We extracted data about patients’ number, gender and age, type of operation, approach (robotic, laparoscopic or open), anastomotic technique, anastomotic leak, anastomotic stricture and anastomotic time.
Statistical Analysis
Meta-analysis was performed using Review Manager Version 5.3. Dichotomous variables were assessed using risk ratio (RR), whereas continuous variables were assessed using mean difference. The random-effects model was chosen due to the heterogeneity among the included studies. Comparisons between dichotomous or continuous variables were made with the inverse variance method. Statistical heterogeneity was assessed with the Higgin’s I2 statistic. Ninety-five percent confidence intervals (CI) were noted for all results. Results were considered statistically significant if p-value was less than 0.05.
Results
Search Results
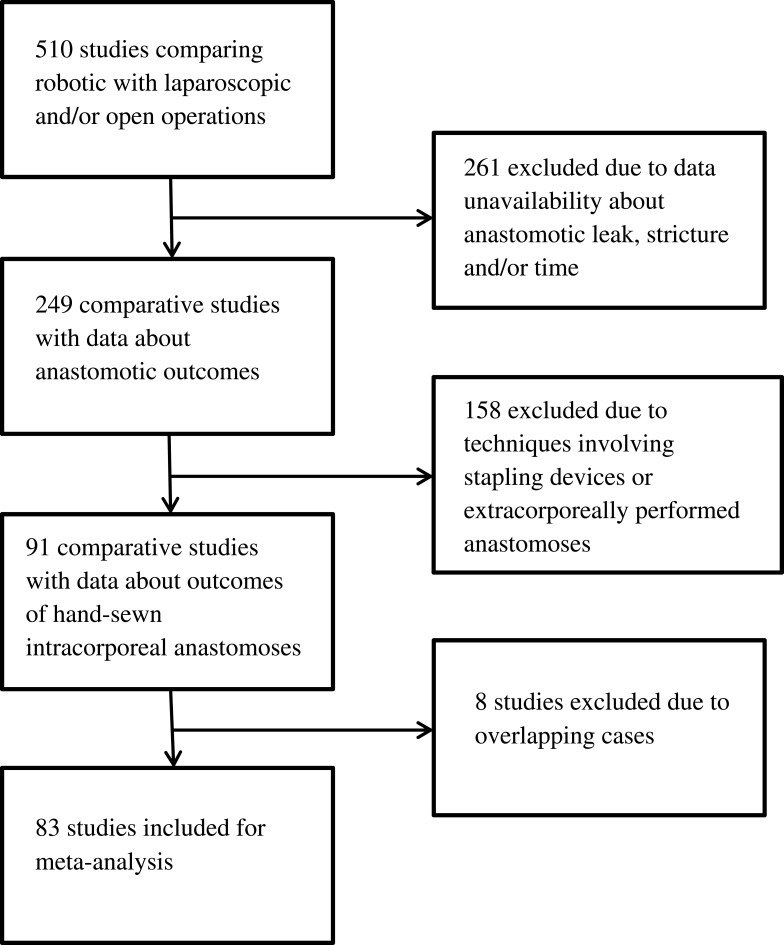
The initial database search yielded 510 studies comparing robotic with laparoscopic and/or open operations in regards to the aforementioned types of procedures. Out of the initial 510 articles, 261 were excluded due to data unavailability about anastomotic leak, anastomotic stricture and/or anastomotic time. Out of the remaining 249 articles, 158 were excluded due to anastomotic techniques involving stapling devices or extracorporeally performed anastomoses in laparoscopic or robotic cases. Finally, another 8 studies were excluded due to overlapping cases, leaving 83 studies to be included in our analysis (4 randomized controlled and 79 non-randomized, 10 prospective and 69 retrospective). In particular, the distribution of included articles according to the exact type of operation was the following: colectomy: 0, low anterior resection: 0, gastrectomy: 4, Roux-en-Y gastric bypass (RYGB): 5, pancreaticoduodenectomy: 16, radical cystectomy: 1, pyeloplasty: 20, radical prostatectomy: 36, renal transplant: 1. Figure 1 shows the study flowchart.
Figure 1.
Study flowchart.
Gastrectomy
Four studies referring to gastrectomy were taken into account for our analysis, including 689 adult patients in total (451 men, 238 women). All studies included both total and subtotal gastrectomies.6–9 There was one prospective randomized study conducted in China comparing robotic with laparoscopic gastrectomy. There were only two cases of anastomotic leak in the laparoscopic group (61 patients), while there were no cases of anastomotic leak in the robotic group (102 patients) (p=0.139).7
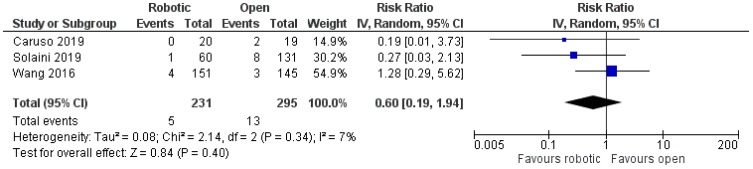
The other three articles compared robotic with open gastrectomy and these included two retrospective non-randomized studies, one from Spain6 and one from Italy,8 and one prospective randomized study from China.9 There was no significant difference in the rates of anastomotic leak between the two treatment groups [robotic: 5/231 (2.2%), open: 13/295 (4.4%), RR: 0.6, 95% CI: 0.19 to 1.94, p=0.4; I2: 7%, p=0.34]6,8,9 (Figure 2). Data about anastomotic stricture were available only in one study, in which there was only one case of anastomotic stenosis in the robotic group (20 patients), whereas there was no similar case in the open group (19 patients) (p=1).6
Figure 2.
Comparison between robotic and open gastrectomy (anastomotic leak).
Roux-En-Y Gastric Bypass
Five studies referring to RYGB were considered for our analysis, which included 2155 adult patients in total (490 men, 1665 women).10–14 Out of these five studies, one was a prospective randomized trial conducted in the USA comparing robotic with laparoscopic RYGB,14 three were retrospective non-randomized done in the USA comparing again robotic with laparoscopic RYGB,10,11,13 and one was a retrospective non-randomized Swiss study with three treatment arms (robotic, laparoscopic, open).12
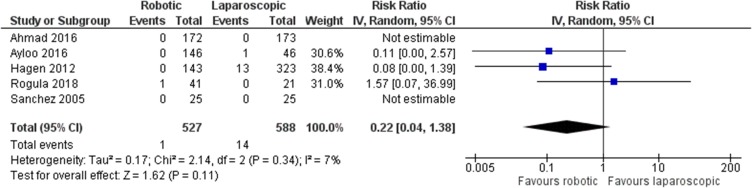
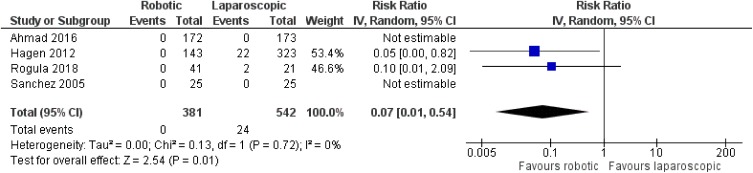
There was no statistically significant difference between robotic and laparoscopic RYGB in regards to anastomotic leak [robotic: 1/527 (0.2%), laparoscopic: 14/588 (2.4%), RR: 0.22, 95% CI: 0.04 to 1.38, p=0.11; I2: 7%, p=0.34]10–14 (Figure 3). However, there was an advantage of the robotic approach over the laparoscopic one in terms of anastomotic stricture [robotic: 0/381 (0%), laparoscopic: 24/542 (4.4%), RR: 0.07, 95% CI: 0.01 to 0.54, p=0.01; I2: 0%, p=0.72]10,12–14 (Figure 4). The only study that compared robotic with open procedures concluded that there is no statistically significant difference between robotic and open operations as far as anastomotic leak [robotic: 0/143 (0%), open: 10/524 (1.9%), p=0.21] or stricture [robotic: 0/143 (0%), open: 6/524 (1.1%), p=0.23] are concerned.12
Figure 3.
Comparison between robotic and laparoscopic RYGB: anastomotic leak.
Figure 4.
Comparison between robotic and laparoscopic RYGB: anastomotic stricture.
Pancreaticoduodenectomy
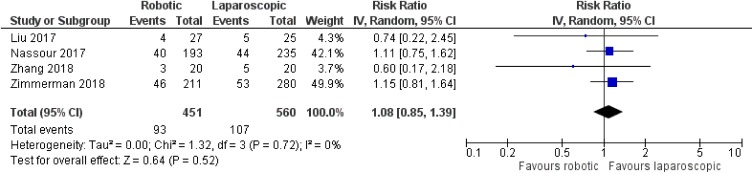
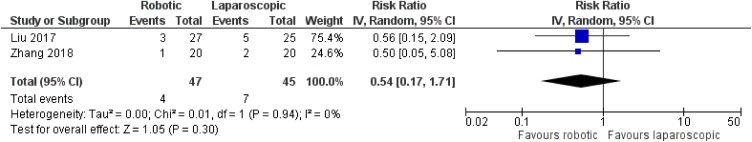
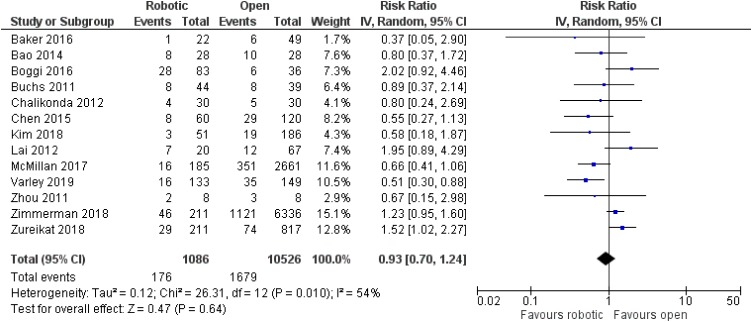
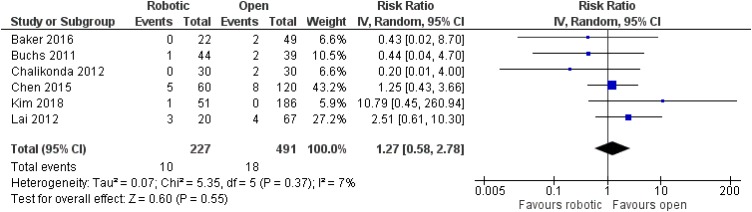
Sixteen studies referring to pancreaticoduodenectomy were taken into account for our analysis, including 12,529 adult patients in total (6627 men, 5902 women).15–30 All included studies were retrospective non-randomized,15–19,21–30 apart from one prospective non-randomized study from China.20 Nine articles were from the USA,15,16,18,19,24–26,29,30 five from China,20,22,23,27,28 one from Italy17 and one from South Korea.21 Three articles compared robotic with laparoscopic operations,23,25,27 12 articles compared robotic with open procedures,15–22,24,26,28,30 and one article compared all three options.29
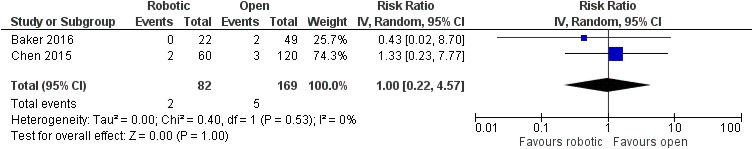
No significant difference was noted between robotic and laparoscopic pancreaticoduodenectomy in regards to pancreatic leak [robotic: 93/451 (20.6%), laparoscopic: 107/560 (19.1%), RR: 1.08, 95% CI: 0.85 to 1.39, p=0.52; I2: 0%, p=0.72]23,25,27,29 (Figure 5) or bile leak [robotic: 4/47 (8.5%), laparoscopic: 7/45 (15.6%), RR: 0.54, 95% CI: 0.17 to 1.71, p=0.3; I2: 0%, p=0.94]23,27 (Figure 6). Similarly, no significant difference was detected between robotic and open pancreaticoduodenectomy in regards to pancreatic leak [robotic: 176/1086 (16.2%), open: 1679/10,526 (16%), RR: 0.93, 95% CI: 0.7 to 1.24, p=0.64] [although there was significant heterogeneity among studies (I2: 54%, p=0.01)]15–22,24,26,28–30 (Figure 7), bile leak [robotic: 10/227 (4.4%), open: 18/491 (3.7%), RR: 1.27, 95% CI: 0.58 to 2.78, p=0.55; I2: 7%, p=0.37]15,18–22 (Figure 8) or leak from gastrointestinal anastomoses [robotic: 2/82 (2.4%), open: 5/169 (3%), RR: 1, 95% CI: 0.22 to 4.57, p=1; I2: 0%, 0.53]15,20 (Figure 9). There were no available data regarding anastomotic strictures.
Figure 5.
Comparison between robotic and laparoscopic pancreaticoduodenectomy: pancreatic leak.
Figure 6.
Comparison between robotic and laparoscopic pancreaticoduodenectomy: bile leak.
Figure 7.
Comparison between robotic and open pancreaticoduodenectomy: pancreatic leak.
Figure 8.
Comparison between robotic and open pancreaticoduodenectomy: bile leak.
Figure 9.
Comparison between robotic and open pancreaticoduodenectomy: leak from gastrointestinal anastomoses.
Radical Cystectomy
Only one retrospective non-randomized study concerning radical cystectomy was included in our analysis, which was conducted in South Korea and compared robotic with open radical cystectomy.31 It included 139 adult patients (116 men, 23 women). Out of the 139 cases, a neobladder with hand-sewn urethroneovesical anastomosis was formed in 41. There were 19 cases of open and 22 cases of robotic radical cystectomy. No postoperative anastomotic leak in the open group [0/19 (0%)], but there were three cases of postoperative anastomotic leak in the robotic group [3/22 (13.6%)]. However, this difference was not significant when we compared the two groups with Fisher’s exact test (p=0.235). There was no available information about anastomotic strictures.31
Pyeloplasty
Twenty studies referring to pyeloplasty were considered for our analysis, which included 1158 patients in total.32–51 Ten articles referred to paediatric patients,32,33,38,43,45–49,51 five articles referred to adult patients34,35,37,42,44 and five articles referred to both paediatric and adult patients.36,39–41,50 Gender distribution was mentioned in 17 articles,32–34,36,37,40–51 which included 625 male and 432 female patients in total. All included studies were retrospective non-randomized,32–41,43–51 with the exception of one, which was prospective non-randomized.42 Eleven studies were from the USA,32,33,35,36,38,42,44,45,47,50,51 one from Italy,37 one from Switzerland,48 one from Israel,43 one from Turkey,34 two from India,39,41 two from China40,49 and one from South Korea.46 Fourteen articles compared robotic with laparoscopic operations,35–45,48–50 four articles compared robotic with open procedures,32,33,47,51 and two articles compared all three options.34,46
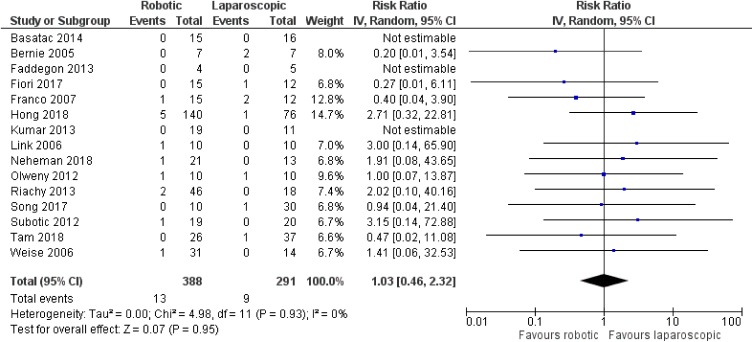
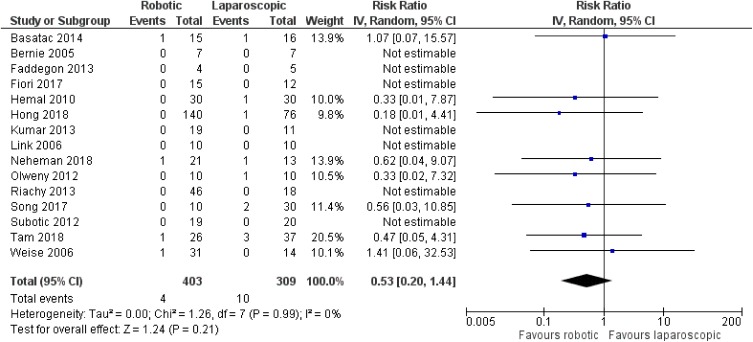
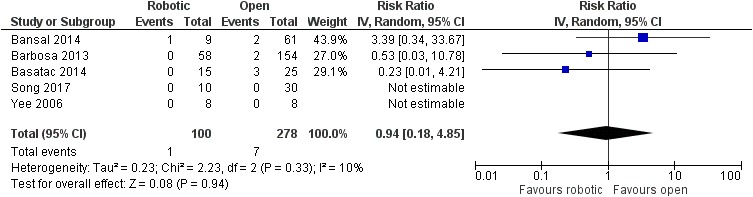
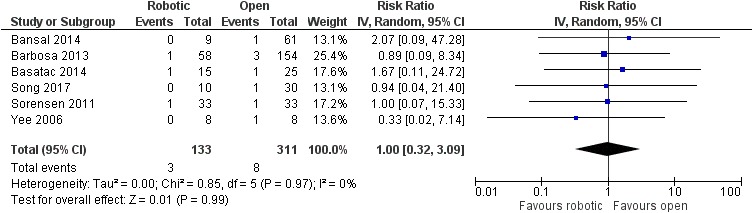
There was no significant difference between robotic and laparoscopic pyeloplasty concerning anastomotic leak [robotic: 13/388 (3.4%), laparoscopic: 9/291 (3.1%), RR: 1.03 95% CI: 0.46 to 2.32, p=0.95; I2: 0%, p=0.93]34–38,40–46,48–50 (Figure 10) or anastomotic stricture/failure [robotic: 4/403 (1%), laparoscopic: 10/309 (3.2%), RR: 0.53, 95% CI: 0.2 to 1.44, p=0.21; I2: 0%, p=0.99]34–37,39–46,48–50 (Figure 11). Similarly, no significant difference was detected between robotic and open pyeloplasty regarding anastomotic leak [robotic: 1/100 (1%), open: 7/278 (2.5%), RR: 0.94, 95% CI: 0.18 to 4.85, p=0.94; I2: 10%, p=0.33]32–34,46,51 (Figure 12) or anastomotic stricture/failure [robotic: 3/133 (2.2%), open: 8/311 (2.6%), RR: 1, 95% CI: 0.32 to 3.09, p=0.99; I2: 0%, p=0.97]32–34,46,47,51 (Figure 13).
Figure 10.
Comparison between robotic and laparoscopic pyeloplasty: anastomotic leak.
Figure 11.
Comparison between robotic and laparoscopic pyeloplasty: anastomotic stricture/failure.
Figure 12.
Comparison between robotic and open pyeloplasty: anastomotic leak.
Figure 13.
Comparison between robotic and open pyeloplasty: anastomotic stricture/failure.
Radical Prostatectomy
Thirty-six studies referring to radical prostatectomy were taken into account for our analysis, including 40,313 adult male patients in total.52–87 Twenty-seven studies were retrospective non-randomized,52–57,60,62,63,65–70,72,73,75,76,78,79,81–84,86,87 eight studies were prospective non-randomized58,59,61,64,71,74,77,85 and one study was prospective randomized.80 Twelve studies were from the USA,52,56,58,64,66,67,69,70,74,75,79,87 one from the UK,78 one from Germany,85 three from France,61,80,82 one from Belgium,53 three from Italy,62,71,81 one from Norway,68 two from Sweden,57,86 one from Switzerland,59 three from Australia,60,77,88 one from Canada,73 three from South Korea,65,83,84 one from Taiwan,76 one from Thailand54 and one from Venezuela,72 while one study was multinational.63 Thirteen articles compared robotic with laparoscopic operations,53,54,63,65,67,68,71,76,79–81,84,87 19 articles compared robotic with open procedures,52,55–59,61,62,66,69,72–75,77,82,85,86 and four articles compared all three options.60,70,78,83
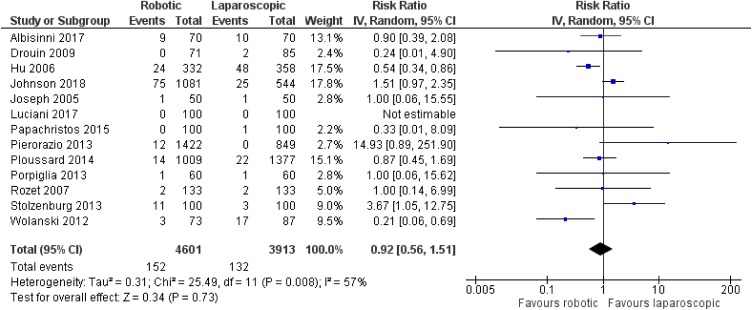
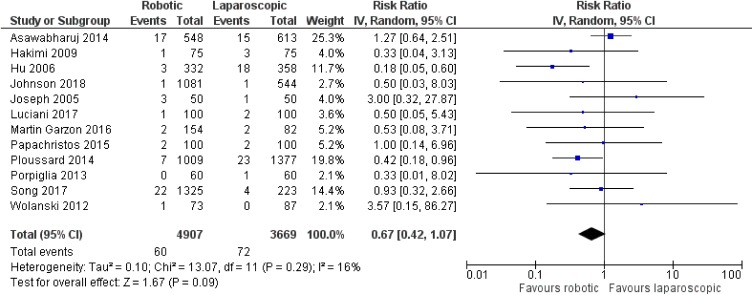
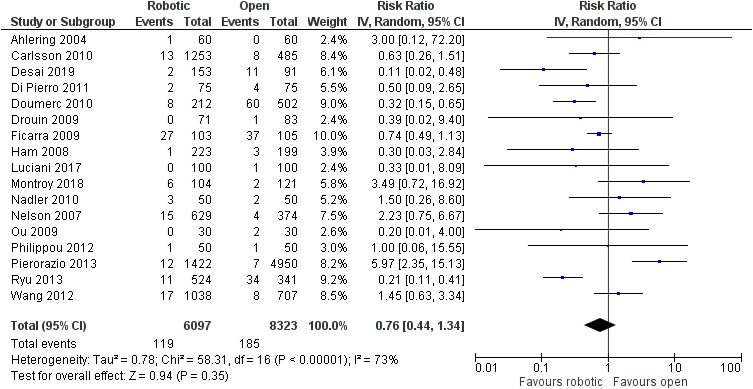
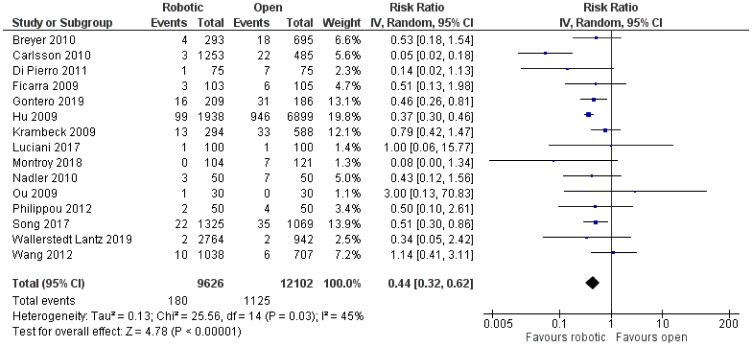
There was no significant difference between robotic and laparoscopic radical prostatectomy in terms of leak from the vesicourethral anastomosis [robotic: 152/4601 (3.3%), laparoscopic: 132/3913 (3.4%), RR: 0.92, 95% CI: 0.56 to 1.51, p=0.73] [although there was significant heterogeneity among studies (I2: 57%, p=0.008)]53,60,65,67,68,70,76,78–81,84,87 (Figure 14) or anastomotic stricture/contracture [robotic: 60 4907 (1.2%), laparoscopic: 72/3669 (2%), RR: 0.67, 95% CI: 0.42 to 1.07, p=0.09; I2: 16%, p=0.29]54,63,65,67,68,70,71,76,79,80,83,87 (Figure 15). When we compared robotic with open radical prostatectomy, no significant difference was found in regards to leak from the vesicourethral anastomosis [robotic: 119/6097 (2%), open: 185/8323 (2.2%), RR: 0.76, 95% CI: 0.44 to 1.34, p=0.35]52,56–61,64,70,72–75,77,78,82,86 (Figure 16), but there were lower rates of anastomotic stricture/contracture in robotic approach [robotic: 180/9626 (1.9%), open: 1125/12,102 (9.3%), RR: 0.44, 95% CI: 0.32 to 0.62, p<0.00001]55,56,58,61,62,66,69,70,72,73,75,77,83,85,86 (Figure 17). However, we have to mention that there was significant heterogeneity among the included studies for both comparisons between robotic and open procedures (anastomotic leak: I2: 73%, p<0.00001, anastomotic stricture/contracture: I2: 45%, p=0.03).
Figure 14.
Comparison between robotic and laparoscopic prostatectomy: anastomotic leak.
Figure 15.
Comparison between robotic and laparoscopic prostatectomy: anastomotic stricture/contracture.
Figure 16.
Comparison between robotic and open radical prostatectomy: anastomotic leak.
Figure 17.
Comparison between robotic and open radical prostatectomy: anastomotic stricture/contracture.
Renal Transplant
Only one retrospective non-randomized study concerning renal transplant was included in our analysis, which was conducted in Turkey and compared robotic with open renal transplant.88 It included 80 adult patients (53 men, 27 women). All three anastomoses (arterial, venous, ureterovesical) were performed faster with the open than the robotic approach. In particular, the mean (SD) anastomotic times for arterial, venous and ureterovesical anastomoses were 18.45 min (5.73), 20.92 min (6.57) and 21.30 min (4.73), respectively, in robotic cases, whereas they were 14.97 min (2.59), 16.02 min (2.3) and 14.95 min (1.56), respectively, in open cases. All differences were statistically significant (p≤0.001). There was no available information about anastomotic leaks/bleeding or strictures.88
Discussion
Since its introduction almost 20 years ago, robotic surgery has been successfully applied to key colorectal, gastric, pancreatic, urological and transplantation procedures where anastomoses form a critical part of the operation. Anastomoses are often time critical, in particular in transplantation where minimising warm ischaemia of the organ is critical to the graft function and outcome.89,90 Furthermore, anastomotic complications such as leak of urine or intestinal contents result in significant morbidity including the need for salvage surgery. The incidence of strictures or stenoses is also of importance in the functional outcome of an anastomosis and may necessitate intervention or even revision surgery.91 The integrity of the anastomosis, therefore, may represent a crucial factor when comparing the overall benefits of a robotic surgical approach to laparoscopic or open alternatives.
In most comparisons, the robotic approach appears to provide similar results with the laparoscopic and open approaches in terms of anastomotic leak or stenosis. In particular, when we compared robotically performed anastomoses with laparoscopically performed anastomoses, no significant differences were found concerning leak or stricture after gastrectomy, leak after RYGB, pancreatic leak or bile leak after pancreaticoduodenectomy, leak or stricture/failure after pyeloplasty, and leak or stricture/contracture after radical prostatectomy. On the other hand, there were lower rates of anastomotic stricture after RYGB with the robotic technique. As far as the comparison of anastomoses performed with the robotic approach with those performed with the open approach, no significant differences were detected regarding leak or stricture after RYGB, pancreatic leak, bile leak or gastrointestinal leak after pancreaticoduodenectomy, leak or stricture/failure after pyeloplasty, leak after radical cystectomy, and leak after radical prostatectomy. On the contrary, there were lower rates of anastomotic stricture/contracture after radical prostatectomy with the robotic technique. Furthermore, it appears that all anastomoses (arterial, venous, ureterovesical) in renal transplant are performed more quickly via the open than the robotic approach. To conclude, robotically performed anastomoses provide similar results to those performed via laparoscopic or open approach, with the exception of RYGB, where the incidence of stenosis is less frequent in robotic than in laparoscopic operations, radical prostatectomy, where stenosis is less common in robotic than in open operations, and renal transplant, where the duration of anastomoses is longer in the robotic than the open approach.
It is important to note that the main focus of this analysis was a comparison of the anastomotic technique employed in these procedures, and the relevant anastomosis-related complications, which are deemed to confer significant morbidity, particularly in the early and mid-term post-operative period. This represents one of the limitations of this review, as it does not take into account oncological outcomes, incidence of nerve damage, incision size or incidence of incision-related complications, or ease of access, in particular for deep pelvic surgery. These are all factors that would undoubtedly contribute heavily when determining the overall benefit and/or superiority of robotic approaches as compared to open or laparoscopic approaches. For example, in robotic radical prostatectomy, superior vision and more intricate operating, facilitated by the robotic platform, has enabled improved nerve-sparing techniques resulting in superior functional outcomes, with no compromise in oncological outcomes.92 Our review also excluded procedures where a significant part of the operation is performed via a robotic approach, but not the relevant anastomosis – for example, in low rectal surgery for cancer. Current evidence suggests that the robotic approach for rectal cancer is of particular benefit in difficult cases such as patients with previous abdominal surgery and chemoradiation therapy.93 Lower conversion rates to open surgery have also been reported,94 as well as comparable,95 if not superior oncological outcomes than laparoscopic approaches, and significantly better autonomic functional outcomes.96 Furthermore, when comparing the overall advantages of robotic surgery to open or laparoscopic alternatives, cost-effectiveness is undoubtedly a key consideration. However, in many cases, the increased costs associated with a robotic system and its maintenance may well be offset by a shorter length of hospital stay and lower complication rates.97–100
In conclusion, this review shows equivalent outcomes of robotically performed anastomoses with those performed via laparoscopic or open approach, apart from the few aforementioned exceptions. This means that the other benefits of robotic operations are not compromised by any deficit in anastomotic outcomes. This is with particular reference to abdominal operations where the anastomoses and its related complications are deemed to be a significant factor contributing to the overall morbidity and outcome. The choice of robotic over current gold-standard approaches for these operations, therefore, may lie with other demonstrable benefits such as superior vision, better access to the pelvis, and superior functional or oncological outcomes, as briefly discussed above.
Disclosure
Ioannis Loukopoulos reports grants from Intuitive Surgical Operations Inc., during the conduct of the study. Nicos Kessaris reports grants from Intuitive Surgical (Clinical Research Grant), outside the submitted work. The authors report no other conflicts of interest in this work.
References
- 1.Ruurda JP, Can Vroonhoven TJ, Broeders IA. Robot-assisted surgical systems: a new era in laparoscopic surgery. Ann R Coll Surg Engl. 2002;84:223–226. doi: 10.1308/003588402320439621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanfranco AR, Castellanos AE, Desai JP, et al. Robotic surgery: a current perspective. Ann Surg. 2004;239:14–21. doi: 10.1097/01.sla.0000103020.19595.7d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prasad SM, Ducko CT, Stephenson ER, et al. Prospective clinical trial of robotically assisted endoscopic coronary grafting with 1 year follow-up. Ann Surg. 2001;233:725–732. doi: 10.1097/00000658-200106000-00001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pein U, Girndt M, Markau S, et al. Minimally invasive robotic versus conventional open living donor kidney transplantation. World J Urol. 2019. doi: 10.1007/s00345-019-02814-7 [DOI] [PubMed] [Google Scholar]
- 5.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caruso R, Vicente E, Quijano Y, et al. Robotic assisted gastrectomy compared with open resection: a case-matched study. Updates Surg. 2019;71:367–373. doi: 10.1007/s13304-018-0533-5 [DOI] [PubMed] [Google Scholar]
- 7.Pan HF, Wang G, Liu J, et al. Robotic versus laparoscopic gastrectomy for locally advanced gastric cancer. Surg Laparosc Endosc Percutan Tech. 2017;27:428–433. doi: 10.1097/SLE.0000000000000469 [DOI] [PubMed] [Google Scholar]
- 8.Solaini L, Bazzocchi F, Pellegrini S, et al. Robotic vs open gastrectomy for gastric cancer: a propensity score-matched analysis on short- and long-term outcomes. Int J Med Robot. 2019;15(5):e2019 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 9.Wang G, Jiang Z, Zhao J, et al. Assessing the safety and efficacy of full robotic gastrectomy with intracorporeal robot-sewn anastomosis for gastric cancer: a randomized clinical trial. J Surg Oncol. 2016;113:397–404. doi: 10.1002/jso.v113.4 [DOI] [PubMed] [Google Scholar]
- 10.Ahmad A, Carleton JD, Ahmad ZF, et al. Laparoscopic versus robotic-assisted Roux-en-Y gastric bypass: a retrospective, single-center study of early perioperative outcomes at a community hospital. Surg Endosc. 2016;30:3792–3796. doi: 10.1007/s00464-015-4675-y [DOI] [PubMed] [Google Scholar]
- 11.Ayloo S, Roh Y, Choudhury N. Laparoscopic, hybrid, and totally robotic Roux-en-Y gastric bypass. J Robot Surg. 2016;10:41–47. doi: 10.1007/s11701-016-0559-y [DOI] [PubMed] [Google Scholar]
- 12.Hagen ME, Pugin F, Chassot G, et al. Reducing cost of surgery by avoiding complications: the model of robotic Roux-en-Y gastric bypass. Obes Surg. 2012;22:52–61. doi: 10.1007/s11695-011-0422-1 [DOI] [PubMed] [Google Scholar]
- 13.Rogula T, Koprivanac M, Janik MR, et al. Does robotic Roux-en-Y gastric bypass provide outcome advantages over standard laparoscopic approaches? Obes Surg. 2018;28:2589–2596. doi: 10.1007/s11695-018-3228-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sanchez BR, Mohr CJ, Morton JM, et al. Comparison of totally robotic laparoscopic Roux-en-Y gastric bypass and traditional laparoscopic Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2005;1:549–554. doi: 10.1016/j.soard.2005.08.008 [DOI] [PubMed] [Google Scholar]
- 15.Baker EH, Ross SW, Seshadri R, et al. Robotic pancreaticoduodenectomy: comparison of complications and cost to the open approach. Int J Med Robot. 2016;12:554–560. doi: 10.1002/rcs.v12.3 [DOI] [PubMed] [Google Scholar]
- 16.Bao PQ, Mazirka PO, Watkins KT. Retrospective comparison of robot-assisted minimally invasive versus open pancreaticoduodenectomy for periampullary neoplasms. J Gastrointest Surg. 2014;18:682–689. doi: 10.1007/s11605-013-2410-3 [DOI] [PubMed] [Google Scholar]
- 17.Boggi U, Napoli N, Costa F, et al. Robotic-assisted pancreatic resections. World J Surg. 2016;40:2497–2506. doi: 10.1007/s00268-016-3565-3 [DOI] [PubMed] [Google Scholar]
- 18.Buchs NC, Addeo P, Bianco FM, et al. Robotic versus open pancreaticoduodenectomy: a comparative study at a single institution. World J Surg. 2011;35:2739–2746. doi: 10.1007/s00268-011-1276-3 [DOI] [PubMed] [Google Scholar]
- 19.Chalikonda S, Aguilar-Saavedra JR, Walsh RM. Laparoscopic robotic-assisted pancreaticoduodenectomy: a case-matched comparison with open resection. Surg Endosc. 2012;26:2397–2402. doi: 10.1007/s00464-012-2207-6 [DOI] [PubMed] [Google Scholar]
- 20.Chen S, Chen JZ, Zhan Q, et al. Robot-assisted laparoscopic versus open pancreaticoduodenectomy: a prospective, matched, mid-term follow-up study. Surg Endosc. 2015;29:3698–3711. doi: 10.1007/s00464-015-4140-y [DOI] [PubMed] [Google Scholar]
- 21.Kim HS, Han Y, Kang JS, et al. Comparison of surgical outcomes between open and robot-assisted minimally invasive pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. 2018;25:142–149. doi: 10.1002/jhbp.522 [DOI] [PubMed] [Google Scholar]
- 22.Lai EC, Yang GP, Tang CN. Robot-assisted laparoscopic pancreaticoduodenectomy versus open pancreaticoduodenectomy - a comparative study. Int J Surg. 2012;10:475–479. doi: 10.1016/j.ijsu.2012.06.003 [DOI] [PubMed] [Google Scholar]
- 23.Liu R, Zhang T, Zhao ZM, et al. The surgical outcomes of robot-assisted laparoscopic pancreaticoduodenectomy versus laparoscopic pancreaticoduodenectomy for periampullary neoplasms: a comparative study of a single center. Surg Endosc. 2017;31:2380–2386. doi: 10.1007/s00464-016-5238-6 [DOI] [PubMed] [Google Scholar]
- 24.McMillan MT, Zureikat AH, Hogg ME, et al. A propensity score-matched analysis of robotic vs open pancreatoduodenectomy on incidence of pancreatic fistula. JAMA Surg. 2017;152:327–335. doi: 10.1001/jamasurg.2016.4755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nassour I, Wang SC, Porembka MR, et al. Robotic versus laparoscopic pancreaticoduodenectomy: a NSQIP analysis. J Gastrointest Surg. 2017;21:1784–1792. doi: 10.1007/s11605-017-3543-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varley PR, Zenati MS, Klobuka A, et al. Does robotic pancreaticoduodenectomy improve outcomes in patients with high risk morphometric features compared to the open approach? HPB (Oxford). 2019;21:695–701. doi: 10.1016/j.hpb.2018.10.016 [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Hong D, Zhang C, et al. Total laparoscopic versus robot-assisted laparoscopic pancreaticoduodenectomy. Biosci Trends. 2018;12:484–490. doi: 10.5582/bst.2018.01236 [DOI] [PubMed] [Google Scholar]
- 28.Zhou NX, Chen JZ, Liu Q, et al. Outcomes of pancreatoduodenectomy with robotic surgery versus open surgery. Int J Med Robot. 2011;7:131–137. doi: 10.1002/rcs.380 [DOI] [PubMed] [Google Scholar]
- 29.Zimmerman AM, Roye DG, Charpentier KP. A comparison of outcomes between open, laparoscopic and robotic pancreaticoduodenectomy. HPB (Oxford). 2018;20:364–369. doi: 10.1016/j.hpb.2017.10.008 [DOI] [PubMed] [Google Scholar]
- 30.Zureikat AH, Postlewait LM, Liu Y, et al. A multi-institutional comparison of perioperative outcomes of robotic and open pancreaticoduodenectomy. Ann Surg. 2016;264:640–649. doi: 10.1097/SLA.0000000000001869 [DOI] [PubMed] [Google Scholar]
- 31.Sung HH, Ahn JS, Seo SI, et al. A comparison of early complications between open and robot-assisted radical cystectomy. J Endourol. 2012;26:670–675. doi: 10.1089/end.2011.0372 [DOI] [PubMed] [Google Scholar]
- 32.Bansal D, Cost NG, DeFoor WR Jr, et al. Infant robotic pyeloplasty: comparison with an open cohort. J Pediatr Urol. 2014;10:380–385. doi: 10.1016/j.jpurol.2013.10.016 [DOI] [PubMed] [Google Scholar]
- 33.Barbosa JA, Kowal A, Onal B, et al. Comparative evaluation of the resolution of hydronephrosis in children who underwent open and robotic-assisted laparoscopic pyeloplasty. J Pediatr Urol. 2013;9:199–205. doi: 10.1016/j.jpurol.2012.02.002 [DOI] [PubMed] [Google Scholar]
- 34.Başataç C, Boylu U, Önol FF, et al. Comparison of surgical and functional outcomes of open, laparoscopic and robotic pyeloplasty for the treatment of ureteropelvic junction obstruction. Turk J Urol. 2014;40:24–30. doi: 10.5152/tud.2014.06956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernie JE, Venkatesh R, Brown J, et al. Comparison of laparoscopic pyeloplasty with and without robotic assistance. JSLS. 2005;9:258–261. [PMC free article] [PubMed] [Google Scholar]
- 36.Faddegon S, Granberg C, Tan YK, et al. Minimally invasive pyeloplasty in horseshoe kidneys with ureteropelvic junction obstruction: a case series. Int Braz J Urol. 2013;39:195–202. doi: 10.1590/S1677-5538.IBJU.2013.02.07 [DOI] [PubMed] [Google Scholar]
- 37.Fiori C, Bertolo R, Manfredi M, et al. Robot-assisted laparoendoscopic single-site versus mini-laparoscopic pyeloplasty: a comparison of perioperative, functional and cosmetic results. Minerva Urol Nefrol. 2017;69:604–612. doi: 10.23736/S0393-2249.17.02833-8 [DOI] [PubMed] [Google Scholar]
- 38.Franco I, Dyer LL, Zelkovic P. Laparoscopic pyeloplasty in the pediatric patient: hand sewn anastomosis versus robotic assisted anastomosis - is there a difference? J Urol. 2007;178:1483–1486. doi: 10.1016/j.juro.2007.06.012 [DOI] [PubMed] [Google Scholar]
- 39.Hemal AK, Mukherjee S, Singh K. Laparoscopic pyeloplasty versus robotic pyeloplasty for ureteropelvic junction obstruction: a series of 60 cases performed by a single surgeon. Can J Urol. 2010;17:5012–5016. [PubMed] [Google Scholar]
- 40.Hong P, Ding G, Zhu D, et al. Head-to-head comparison of modified laparoscopic pyeloplasty and robot-assisted pyeloplasty for ureteropelvic junction obstruction in China. Urol Int. 2018;101:337–344. doi: 10.1159/000492337 [DOI] [PubMed] [Google Scholar]
- 41.Kumar R, Nayak B. Robotic versus conventional laparoscopic pyeloplasty: a single surgeon concurrent cohort review. Indian J Urol. 2013;29:19–21. doi: 10.4103/0970-1591.109978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Link RE, Bhayani SB, Kavoussi LR. A prospective comparison of robotic and laparoscopic pyeloplasty. Ann Surg. 2006;243:486–491. doi: 10.1097/01.sla.0000205626.71982.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neheman A, Kord E, Zisman A, et al. Comparison of robotic pyeloplasty and standard laparoscopic pyeloplasty in infants: a bi-institutional study. J Laparoendosc Adv Surg Tech A. 2018;28:467–470. doi: 10.1089/lap.2017.0262 [DOI] [PubMed] [Google Scholar]
- 44.Olweny EO, Park SK, Tan YK, et al. Perioperative comparison of robotic assisted laparoendoscopic single-site (LESS) pyeloplasty versus conventional LESS pyeloplasty. Eur Urol. 2012;61:410–414. doi: 10.1016/j.eururo.2011.10.024 [DOI] [PubMed] [Google Scholar]
- 45.Riachy E, Cost NG, Defoor WR, et al. Pediatric standard and robot-assisted laparoscopic pyeloplasty: a comparative single institution study. J Urol. 2013;189:283–287. doi: 10.1016/j.juro.2012.09.008 [DOI] [PubMed] [Google Scholar]
- 46.Song SH, Lee C, Jung J, et al. A comparative study of pediatric open pyeloplasty, laparoscopy-assisted extracorporeal pyeloplasty, and robot-assisted laparoscopic pyeloplasty. PLoS One. 2017;12:e0175026. doi: 10.1371/journal.pone.0175026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sorensen MD, Delostrinos C, Johnson MH, et al. Comparison of the learning curve and outcomes of robotic assisted pediatric pyeloplasty. J Urol. 2011;185:2517–2522. doi: 10.1016/j.juro.2011.01.021 [DOI] [PubMed] [Google Scholar]
- 48.Subotic U, Rohard I, Weber DM, et al. A minimal invasive surgical approach for children of all ages with ureteropelvic junction obstruction. J Pediatr Urol. 2012;8:354–358. doi: 10.1016/j.jpurol.2011.07.004 [DOI] [PubMed] [Google Scholar]
- 49.Tam YH, Pang KKY, Wong YS, et al. From laparoscopic pyeloplasty to robot-assisted laparoscopic pyeloplasty in primary and reoperative repairs for ureteropelvic junction obstruction in children. J Laparoendosc Adv Surg Tech A. 2018;28:1012–1018. doi: 10.1089/lap.2017.0561 [DOI] [PubMed] [Google Scholar]
- 50.Weise ES, Winfield HN. Robotic computer-assisted pyeloplasty versus conventional laparoscopic pyeloplasty. J Endourol. 2006;20:813–819. doi: 10.1089/end.2006.20.813 [DOI] [PubMed] [Google Scholar]
- 51.Yee DS, Shanberg AM, Duel BP, et al. Initial comparison of robotic-assisted laparoscopic versus open pyeloplasty in children. Urology. 2006;67:599–602. doi: 10.1016/j.urology.2005.09.021 [DOI] [PubMed] [Google Scholar]
- 52.Ahlering TE, Woo D, Eichel L, et al. Robot-assisted versus open radical prostatectomy: a comparison of one surgeon’s outcomes. Urology. 2004;63:819–822. doi: 10.1016/j.urology.2004.01.038 [DOI] [PubMed] [Google Scholar]
- 53.Albisinni S, Aoun F, LE DD, et al. Comparing conventional laparoscopic to robotic-assisted extended pelvic lymph node dissection in men with intermediate and high-risk prostate cancer: a matched-pair analysis. Minerva Urol Nefrol. 2017;69:101–107. doi: 10.23736/S0393-2249.16.02799-5 [DOI] [PubMed] [Google Scholar]
- 54.Asawabharuj K, Ramart P, Nualyong C, et al. Comparison of urinary continence outcome between robotic assisted laparoscopic prostatectomy versus laparoscopic radical prostatectomy. J Med Assoc Thai. 2014;97:393–398. [PubMed] [Google Scholar]
- 55.Breyer BN, Davis CB, Cowan JE, et al. Incidence of bladder neck contracture after robot-assisted laparoscopic and open radical prostatectomy. BJU Int. 2010;106:1734–1738. doi: 10.1111/bju.2010.106.issue-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carlsson S, Nilsson AE, Schumacher MC, et al. Surgery-related complications in 1253 robot-assisted and 485 open retropubic radical prostatectomies at the Karolinska University Hospital, Sweden. Urology. 2010;75:1092–1097. doi: 10.1016/j.urology.2009.09.075 [DOI] [PubMed] [Google Scholar]
- 57.Desai A, Hudnall M, Weiner AB, et al. Contemporary comparison of open to robotic prostatectomy at a veteran’s affairs hospital. Mil Med. 2019;184:e330–e337. doi: 10.1093/milmed/usy352 [DOI] [PubMed] [Google Scholar]
- 58.Di Pierro GB, Baumeister P, Stucki P, et al. A prospective trial comparing consecutive series of open retropubic and robot-assisted laparoscopic radical prostatectomy in a centre with a limited caseload. Eur Urol. 2011;59:1–6. doi: 10.1016/j.eururo.2010.10.026 [DOI] [PubMed] [Google Scholar]
- 59.Doumerc N, Yuen C, Savdie R, et al. Should experienced open prostatic surgeons convert to robotic surgery? The real learning curve for one surgeon over 3 years. BJU Int. 2010;106:378–384. doi: 10.1111/j.1464-410X.2009.09158.x [DOI] [PubMed] [Google Scholar]
- 60.Drouin SJ, Vaessen C, Hupertan V, et al. Comparison of mid-term carcinologic control obtained after open, laparoscopic, and robot-assisted radical prostatectomy for localized prostate cancer. World J Urol. 2009;27:599–605. doi: 10.1007/s00345-009-0379-z [DOI] [PubMed] [Google Scholar]
- 61.Ficarra V, Novara G, Fracalanza S, et al. A prospective, non-randomized trial comparing robot-assisted laparoscopic and retropubic radical prostatectomy in one European institution. BJU Int. 2009;104:534–539. doi: 10.1111/j.1464-410X.2009.08419.x [DOI] [PubMed] [Google Scholar]
- 62.Gontero P, Marra G, Alessio P, et al. Salvage radical prostatectomy for recurrent prostate cancer: morbidity and functional outcomes from a large multicenter series of open versus robotic approaches. J Urol. 2019;202:725–731. doi: 10.1097/JU.0000000000000327 [DOI] [PubMed] [Google Scholar]
- 63.Hakimi AA, Blitstein J, Feder M, et al. Direct comparison of surgical and functional outcomes of robotic-assisted versus pure laparoscopic radical prostatectomy: single-surgeon experience. Urology. 2009;73:119–123. doi: 10.1016/j.urology.2008.08.491 [DOI] [PubMed] [Google Scholar]
- 64.Ham WS, Park SY, Kim WT, et al. Open versus robotic radical prostatectomy: a prospective analysis based on a single surgeon’s experience. J Robot Surg. 2008;2:235–241. doi: 10.1007/s11701-008-0111-9 [DOI] [PubMed] [Google Scholar]
- 65.Hu JC, Nelson RA, Wilson TG, et al. Perioperative complications of laparoscopic and robotic assisted laparoscopic radical prostatectomy. J Urol. 2006;175:541–546. doi: 10.1016/S0022-5347(05)00156-4 [DOI] [PubMed] [Google Scholar]
- 66.Hu JC, Gu X, Lipsitz SR, et al. Comparative effectiveness of minimally invasive vs open radical prostatectomy. JAMA. 2009;302:1557–1564. doi: 10.1001/jama.2009.1451 [DOI] [PubMed] [Google Scholar]
- 67.Johnson I, Ottosson F, Diep LM, et al. Switching from laparoscopic radical prostatectomy to robot assisted laparoscopic prostatectomy: comparing oncological outcomes and complications. Scand J Urol. 2018;52:116–121. doi: 10.1080/21681805.2017.1420099 [DOI] [PubMed] [Google Scholar]
- 68.Joseph JV, Vicente I, Madeb R, et al. Robot-assisted vs pure laparoscopic radical prostatectomy: are there any differences? BJU Int. 2005;96:39–42. doi: 10.1111/bju.2005.96.issue-1 [DOI] [PubMed] [Google Scholar]
- 69.Krambeck AE, DiMarco DS, Rangel LJ, et al. Radical prostatectomy for prostatic adenocarcinoma: a matched comparison of open retropubic and robot-assisted techniques. BJU Int. 2009;103:448–453. doi: 10.1111/j.1464-410X.2008.08012.x [DOI] [PubMed] [Google Scholar]
- 70.Luciani LG, Mattevi D, Mantovani W, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a comparative analysis of the surgical outcomes in a single regional center. Curr Urol. 2017;11:36–41. doi: 10.1159/000447192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Martín Garzón OD, Azhar RA, Brunacci L, et al. One-year outcome comparison of laparoscopic, robotic, and robotic intrafascial simple prostatectomy for benign prostatic hyperplasia. J Endourol. 2016;30:312–318. doi: 10.1089/end.2015.0218 [DOI] [PubMed] [Google Scholar]
- 72.Montroy J, Elzayat E, Morash C, et al. Long-term patient outcomes from the first year of a robotic surgery program using multi-surgeon implementation. Can Urol Assoc J. 2018;12:38–43. doi: 10.5489/cuaj.4528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nadler RB, Casey JT, Zhao LC, et al. Is the transition from open to robotic prostatectomy fair to your patients? A single-surgeon comparison with 2-year follow-up. J Robot Surg. 2010;3:201–207. doi: 10.1007/s11701-009-0162-6 [DOI] [PubMed] [Google Scholar]
- 74.Nelson B, Kaufman M, Broughton G, et al. Comparison of length of hospital stay between radical retropubic prostatectomy and robotic assisted laparoscopic prostatectomy. J Urol. 2007;177:929–931. doi: 10.1016/j.juro.2006.10.070 [DOI] [PubMed] [Google Scholar]
- 75.Ou YC, Yang CR, Wang J, et al. Comparison of robotic-assisted versus retropubic radical prostatectomy performed by a single surgeon. Anticancer Res. 2009;29:1637–1642. [PubMed] [Google Scholar]
- 76.Papachristos A, Basto M, Te Marvelde L, et al. Laparoscopic versus robotic-assisted radical prostatectomy: an Australian single-surgeon series. ANZ J Surg. 2015;85:154–158. doi: 10.1111/ans.2015.85.issue-3 [DOI] [PubMed] [Google Scholar]
- 77.Philippou P, Waine E, Rowe E. Robot-assisted laparoscopic prostatectomy versus open: comparison of the learning curve of a single surgeon. J Endourol. 2012;26:1002–1008. doi: 10.1089/end.2011.0569 [DOI] [PubMed] [Google Scholar]
- 78.Pierorazio PM, Mullins JK, Ross AE, et al. Trends in immediate perioperative morbidity and delay in discharge after open and minimally invasive radical prostatectomy (RP): a 20-year institutional experience. BJU Int. 2013;112:45–53. doi: 10.1111/j.1464-410X.2012.11767.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ploussard G, de la Taille A, Moulin M, et al. Comparisons of the perioperative, functional, and oncologic outcomes after robot-assisted versus pure extraperitoneal laparoscopic radical prostatectomy. Eur Urol. 2014;65:610–619. doi: 10.1016/j.eururo.2012.11.049 [DOI] [PubMed] [Google Scholar]
- 80.Porpiglia F, Morra I, Lucci Chiarissi M, et al. Randomised controlled trial comparing laparoscopic and robot-assisted radical prostatectomy. Eur Urol. 2013;63:606–614. doi: 10.1016/j.eururo.2012.07.007 [DOI] [PubMed] [Google Scholar]
- 81.Rozet F, Jaffe J, Braud G, et al. A direct comparison of robotic assisted versus pure laparoscopic radical prostatectomy: a single institution experience. J Urol. 2007;178:478–482. doi: 10.1016/j.juro.2007.03.111 [DOI] [PubMed] [Google Scholar]
- 82.Ryu J, Kwon T, Kyung YS, et al. Retropubic versus robot-assisted laparoscopic prostatectomy for prostate cancer: a comparative study of postoperative complications. Korean J Urol. 2013;54:756–761. doi: 10.4111/kju.2013.54.11.756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Song W, Park JH, Jeon HG, et al. Comparison of oncologic outcomes and complications according to surgical approach to radical prostatectomy: special focus on the perineal approach. Clin Genitourin Cancer. 2017;15:e645–e652. doi: 10.1016/j.clgc.2017.01.015 [DOI] [PubMed] [Google Scholar]
- 84.Stolzenburg JU, Qazi HA, Holze S, et al. Evaluating the learning curve of experienced laparoscopic surgeons in robot-assisted radical prostatectomy. J Endourol. 2013;27:80–85. doi: 10.1089/end.2012.0262 [DOI] [PubMed] [Google Scholar]
- 85.Wallerstedt Lantz A, Stranne J, Tyritzis SI, et al. 90-day readmission after radical prostatectomy-a prospective comparison between robot-assisted and open surgery. Scand J Urol. 2019;53:26–33. doi: 10.1080/21681805.2018.1556729 [DOI] [PubMed] [Google Scholar]
- 86.Wang R, Wood DP Jr, Hollenbeck BK, et al. Risk factors and quality of life for post-prostatectomy vesicourethral anastomotic stenoses. Urology. 2012;79:449–457. doi: 10.1016/j.urology.2011.07.1383 [DOI] [PubMed] [Google Scholar]
- 87.Wolanski P, Chabert C, Jones L, et al. Preliminary results of robot-assisted laparoscopic radical prostatectomy (RALP) after fellowship training and experience in laparoscopic radical prostatectomy (LRP). BJU Int. 2012;110:64–70. doi: 10.1111/bju.2012.110.issue-s4 [DOI] [PubMed] [Google Scholar]
- 88.Tuğcu V, Şener NC, Şahin S, et al. Robot-assisted kidney transplantation: comparison of the first 40 cases of open vs robot-assisted transplantations by a single surgeon. BJU Int. 2018;121:275–280. doi: 10.1111/bju.2018.121.issue-2 [DOI] [PubMed] [Google Scholar]
- 89.Ferede AA, Walsh AL, Davis NF, et al. Warm ischemia time at vascular anastomosis is an independent predictor for delayed graft function in kidney transplant recipients. Exp Clin Transplant. 2019. doi: 10.6002/ect [DOI] [PubMed] [Google Scholar]
- 90.Heylen L, Pirenne J, Samuel U, et al. The impact of anastomosis time during kidney transplantation on graft loss: a eurotransplant cohort study. Am J Transplant. 2017;17:724–732. doi: 10.1111/ajt.2017.17.issue-3 [DOI] [PubMed] [Google Scholar]
- 91.Guyton KL, Hyman NH, Alverdy JC. Prevention of perioperative anastomotic healing complications: anastomotic stricture and anastomotic leak. Adv Surg. 2016;50:129–141. doi: 10.1016/j.yasu.2016.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McGuiness LA, Rai BP. Robotics in urology. Ann R Coll Surg Engl. 2018;100:45–54. doi: 10.1308/rcsann.supp1.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Scarpinata R, Aly EH. Does robotic rectal cancer surgery offer improved early postoperative outcomes? Dis Colon Rectum. 2013;56:253–262. doi: 10.1097/DCR.0b013e3182694595 [DOI] [PubMed] [Google Scholar]
- 94.Ackerman SJ, Daniel S, Baik R, et al. Comparison of complication and conversion rates between robotic-assisted and laparoscopic rectal resection for rectal cancer: which patients and providers could benefit most from robotic-assisted surgery? J Med Econ. 2018;21:254–261. doi: 10.1080/13696998.2017.1396994 [DOI] [PubMed] [Google Scholar]
- 95.Baek JH, McKenzie S, Garcia-Aguilar J, et al. Oncologic outcomes of robotic-assisted total mesorectal excision for the treatment of rectal cancer. Ann Surg. 2010;251:882–886. doi: 10.1097/SLA.0b013e3181c79114 [DOI] [PubMed] [Google Scholar]
- 96.D’Annibale A, Pernazza G, Monsellato I, et al. Total mesorectal excision: a comparison of oncological and functional outcomes between robotic and laparoscopic surgery for rectal cancer. Surg Endosc. 2013;27:1887–1895. doi: 10.1007/s00464-012-2731-4 [DOI] [PubMed] [Google Scholar]
- 97.Tyler JA, Fox JP, Desai MM, et al. Outcomes and costs associated with robotic colectomy in the minimally invasive era. Dis Colon Rectum. 2013;56:458–466. doi: 10.1097/DCR.0b013e31827085ec [DOI] [PubMed] [Google Scholar]
- 98.Halabi WJ, KAng CY, Jafari MD, et al. Robotic assisted colorectal surgery in the United States: a nationwide analysis of trends and outcomes. World J Surg. 2012;37:2782–2790. doi: 10.1007/s00268-013-2024-7 [DOI] [PubMed] [Google Scholar]
- 99.Sivathondan PC, Jayne DG. The role of robotics in colorectal surgery. Ann R Coll Surg Engl. 2018;100:42–53. doi: 10.1308/rcsann.supp2.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Khosla A, Wagner AA. Robotic surgery of the kidney, bladder and prostate. Surg Clin N Am. 2016;96:615–636. doi: 10.1016/j.suc.2016.02.015 [DOI] [PubMed] [Google Scholar]