Abstract
Background
Bacterial infections cause a serious public health crisis due to the emergence of resistance towards multiple conventional antibacterial drugs. In particular, multidrug-resistant (MDR) Enterococcus faecium which belongs to “ESKAPE” organisms is causing significant problems worldwide. Hence, there is an urgent need to find alternative therapies. Recently, substituted benzene guanidine compounds have been used as lead structures to discover new promising drugs in both synthetic and medicinal chemistry.
Purpose
Here we investigated the antimicrobial activity of a new substituted benzene guanidine analog, isopropoxy benzene guanidine, against Enterococci.
Material and methods
The isopropoxy benzene guanidine was synthesized by Guangzhou Insighter Biotechnology Co., Ltd and tested on both reference bacterial strain and 32 clinical MDR Enterococci strains. The in vitro antibacterial activity was evaluated by microdilution method and kill kinetic assays. The potential antibacterial mechanism was measured by fluorescence spectrometry using fluorescent membrane potential probe 3, 3-diethyloxacarbocyanine iodide (DiOC2 (3)).
Results
Isopropoxy benzene guanidine exhibited potent bactericidal activity against both reference strain and MDR Enterococci isolates. The minimum inhibitory concentration (MIC) range for isopropoxy benzene guanidine was 1–4 μg/mL. Minimum bactericidal concentration (MBC) was about 2-8-fold of its MIC values. Time-kill studies showed that isopropoxy benzene guanidine provided superior bactericidal effect against reference and MDR strains within 12 hrs at 2×MIC. Furthermore, isopropoxy benzene guanidine could cause a large reduction in the magnitude of the generated membrane potential compared to that of the untreated cells.
Conclusion
The present study highlights the potent bactericidal activity of isopropoxy benzene guanidine on Enterococci by disrupting the cell membrane potential. These findings demonstrate that isopropoxy benzene guanidine may be a good chemical lead for further medicinal chemistry and pharmaceutical development and could be used as a therapeutic agent for infectious diseases caused by MDR Enterococci.
Keywords: isopropoxy benzene guanidine, MDR Enterococci, DiOC2, 3, cell membrane potential, bactericidal activity
Introduction
The rapid emergence of multidrug resistance combined with the limited number of novel antibacterial agents has caused a public health crisis.1,2 The ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species) were known for their ability to escape the inhibitory action of and develop high levels of resistance to traditional antibiotic drugs. These pathogens have recently been identified as the leading global cause of multidrug-resistant bacterial infections. Furthermore, resistance of these important pathogens to first-line and last-resort antibiotics has been reported worldwide and can lead to untreatable infections. Without a doubt, new antibacterial agents and novel approaches to counter drug-resistant infections are urgently needed.
Enterococci are commensal bacteria found in the gastrointestinal tract of humans and many other animals. Currently, Enterococcus faecalis and Enterococcus faecium have emerged as a major cause of zoonotic and nosocomial infections worldwide.3,4 A wide armamentarium of natural resistance, along with the capacity to acquire and disseminate multiple antibiotic resistance and virulence determinants in Enterococci is of significant concern with limited therapeutic alternatives.5–7 Concurrent with the declining discovery rate of novel antibiotics, there are some strains of Enterococci have become resistant to last-resort drugs.8–10 In general, Enterococci are considered as a significant antibiotic resistance threat and pose a risk to public health as a whole.
The attractive way to address this problem is to repurpose the Food and Drug Administration (FDA) approved drugs in clinical use as potential antimicrobials or reexamination of compounds previously developed for use to support animal health as candidates for further structural modification.11 Historically, repurposing drugs have emerged as an innovation stream of pharmaceutical development that offers known safety and development pathways for drug developers and has resulted in great success in various disease areas.12,13 Recently, research efforts have focused on the development of novel antibacterial agents that is distinct from currently used antibiotics, with the objectives of avoiding cross-resistance and reducing the emergence of resistance.14
Substituted benzene guanidine compounds belonging to amino-guanidine compound class have been used in the treatment of a broad range of diseases and emerged as candidates for further structural modification for new promising drugs.15–17 For example, robenidine was synthesized as an anticoccidial agent widely used to prevent coccidian infections since the early 1970s.18 Recently, robenidine analogues have been shown as Gram-positive antibacterial agents, including Staphylococcus aureus and vancomycin-resistant Enterococci. Moreover, robenidine analog NCL195 displayed bactericidal activity against Streptococcus pneumonia and S. aureus by disrupting the cell membrane potential.19 Hence, utilization of guanidines as candidates for further structural modification against Gram-positive bacteria is highly attractive.
Recently, we have assessed the antibacterial activity of a series of substituted benzene guanidine derivatives against E.faecalis ATCC 29212 using broth microdilution method. We found that isopropoxy benzene guanidine (IBG) has the highest antibacterial activity. This compound is similar to robenidine analogues. However, the effect of robenidine analogues against Enterococci was only focused on the MIC of vancomycin-resistant Enterococci. Hence, in-depth in vitro evaluation of this compound is needed. In the present study, we evaluated the bactericidal activity of IBG against a collection of clinical MDR Enterococci and the related potential mechanism was also investigated. This presents an attractive prospect for this compound by expanding its chemical space with further medicinal chemistry for potential development as a novel drug against Enterococci.
Materials and Methods
Bacterial Strains
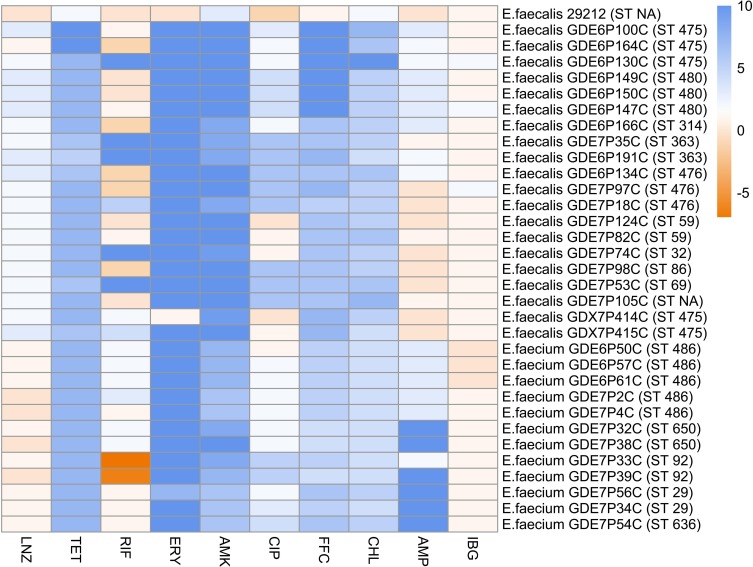
E. faecalis ATCC 29212 was stored in our laboratory. Twenty MDR E. faecalis strains and 12 MDR E. faecium strains were isolated from various livestock farms (Figure 1). Strain identification was performed by MALDI-TOF MS (Bruker Daltonik GmbH, Germany) and was further confirmed by16S rDNA sequencing using universal prokaryotic primers. Multilocus sequence typing (MLST) was conducted according to the reference MLST database20 (http://efaecalis.mlst.net/; http://efaecium.mlst.net/). All strains were grown in Mueller-Hinton (MH) broth.
Figure 1.
The MIC and MLSTs of all studied Enterococci. The colors present the value of log2 MIC of corresponding antibiotics.
Abbreviations: VAN, vancomycin; LZD, linezolid; TET, tetracycline; RIF, rifamycin; ERY, erythromycin; AMK, amikacin; CIP, ciprofloxacin; FFC, florfenicol; CHL, chloramphenicol; AMP, ampicillin; IBG, isopropoxy benzene guanidine.
Antimicrobial Agents and Medicinal Chemistry
Isopropoxy benzene guanidine (IBG) (batch number: 20150506, content: 99.9%) was synthesized by Guangzhou Insighter Biotechnology Co., Ltd (Guangzhou, China). Dimethyl Sulphoxide (DMSO) (Dmreagent, Tianjin, China) was utilized as solvent to dissolve IBG. Fetal bovine serum (FBS) was from Zhejiang Tianhang Biotechnology Co., Ltd (Zhejiang, China). MH broth was from HuanKai Microbial (Guangzhou, China). Trixon X-100, phosphate buffer solution (PBS) was from Sangon Biotech (Shanghai, China). Methylthiazoletetrazolium (MTT) was from Sigma-Aldrich (USA) and 3, 3ʹ-diethyloxacarbocyanine iodide (DiOC2 (3)) was from Thermo Fisher Scientific (Germany).
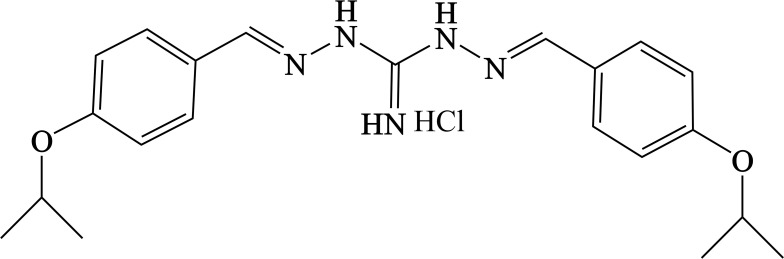
Synthesis of IBG (1, 3-Bis (P-Isopropoxydibenzylamine) Guanidine Hydrochloride; Isopropoxy Benzene Guanidine)
A suspension of p-isopropoxy benzaldehyde (25 g, 0.153 mol, 2 eq) and diaminoguanidine monohydrochloride (0.7–1.5 eq) in ethyl alcohol (EtOH) was heated at reflux until it becomes clear. The reaction mixture was cooled down to 10°C. The resulting precipitate was collected and washed twice with EtOH to afford the isopropoxy benzene guanidine as a white powder (Figure 2).
Figure 2.
Chemical structure of isopropoxy benzene guanidine (IBG).
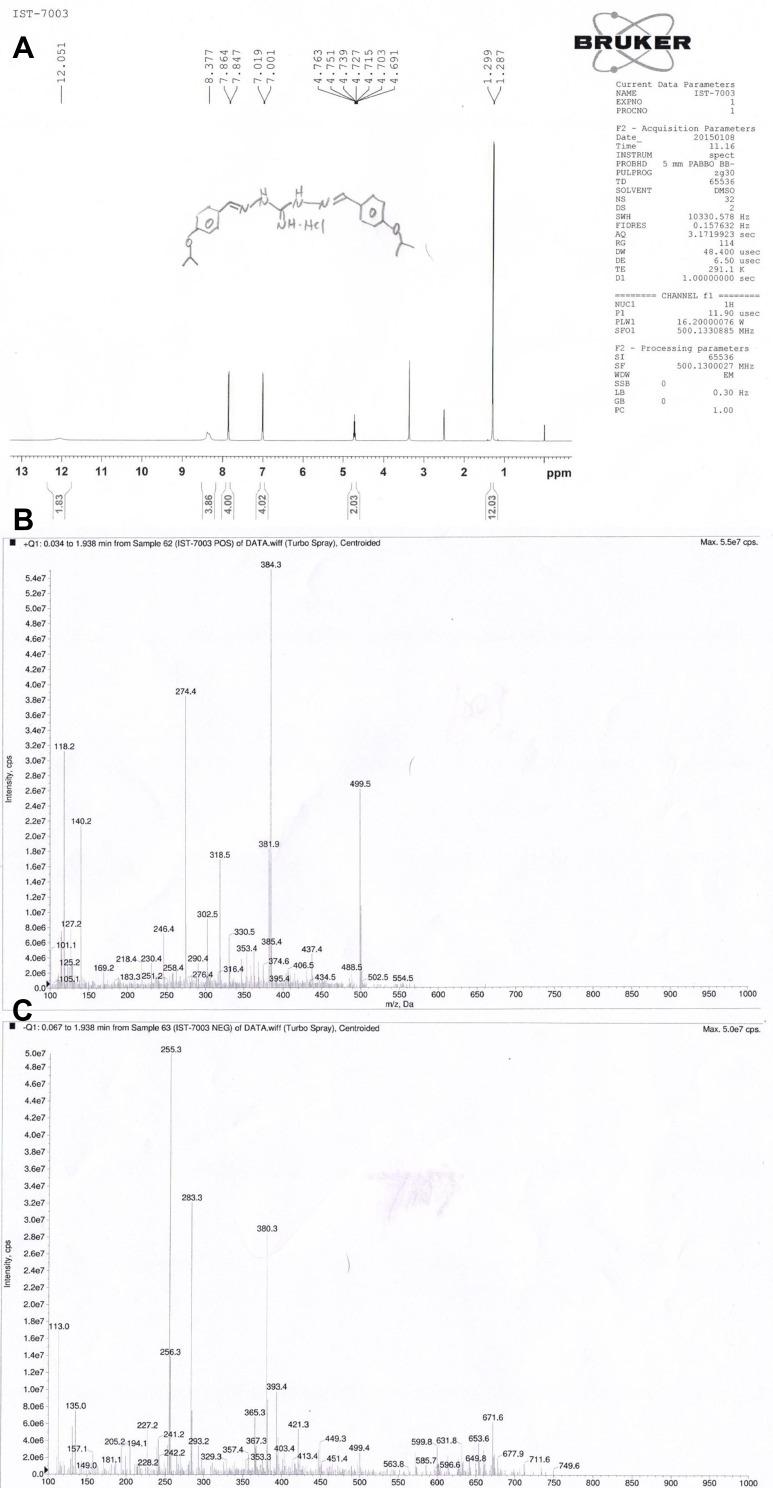
Nuclear magnetic resonance (NMR) and MS spectrum of IBG are shown in Figure 3. 1H NMR (DMSO, 500 MHz) δ 11.77 (2H, s), 8.29 (4H, s), 7.85 (4H, d), 7.01 (4H, d), 4.72 (2H, m), 1.29 (12H, d).
Figure 3.
NMR and MS spectrum of isopropoxy benzene guanidine (IBG). (A) NMR spectrum of IBG (B) MS spectrum of IBG in positive ion mode (C) MS spectrum of IBG in negative ion mode.
Minimum Inhibitory Concentration (MIC) Determination
The MIC was determined by broth microdilution method recommended by CLSI.21 Mueller-Hinton (MH) broth was used in this experiment and cell concentration was adjusted to approximately 5 × 105 CFU/mL. Briefly, strains were cultured in MH broth and incubated at 37°C for about 5–6 hrs until the cell suspension was about 108 CFU/mL. The culture was then diluted 1:200 in MH broth, serial two-fold dilutions of drugs were added to the wells. After 16–20 hrs of incubation at 37°C, the MIC was defined as the lowest concentration of antibiotic with no visible growth. Experiments were performed with three biological replicates.
Minimum Bactericidal Concentration (MBC)
The MBC was further determined according to the CLSI guidelines. Briefly, after determination of the MIC, 20 μL aliquots were taken from all the wells from MIC and spotted onto Brain-Heart Infusion (BHI) agar. The colonies were enumerated after incubating for 24 h at 37°C. The MBC is defined as the lowest concentration where a 99.9% colony count reduction was observed. Experiments were performed in triplicates.
Time-Kill Kinetics
The time-dependent killing for E. faecalis ATCC 29212, MDR E. faecalis GDE6P130C and MDR E. faecium GDE6P50C with various concentrations of IBG, vancomycin (VAN) and linezolid (LNZ) were investigated.22 The initial inoculum of 2×106 CFU/mL cells in 5 mL MH broth was challenged with IBG, VAN, LNZ at 2× MIC, 4× MIC or 10× MIC. At 0, 4, 8, 12, 24 hrs, 100 μL aliquots were serially diluted 10-fold in phosphate-buffered saline (PBS) and plated with BHI agar medium. The plates were incubated at 37°C for 16–24 hrs. Plates with around 30 to 300 colonies were counted and CFU/mL for each time point was calculated. All experiments were replicated.
Membrane Potential Assay
To examine the perturbation of the cell membrane of Enterococci by IBG, the membrane potential of the cells was measured by fluorescence spectrometry using fluorescent membrane potential probe 3, 3-diethyloxacarbocyanine iodide DiOC2 (3) as described previously.23 E. faecalis ATCC 29212 cells were grown in LB broth for 12 hrs, then centrifuged at 4000×g for 10 mins at room temperature, washed twice in PBS and resuspended in PBS to OD600nm=0.5. For the fluorescence assay, 2 mL of this suspension was added in a quartz cuvette, the mixture was stirred gently for 5 mins (with or without addition of 1× IBG, 2× IBG, 4× IBG, 10× IBG, using 16 μg/mL ampicillin as control), the cuvette was then placed in a Hitachi F-7000 Fluorescence Spectrometer set at Ex.486 nm/Em.620 nm, with excitation and emission slit widths at 5 nm and 10 nm, respectively. The background fluorescence of each suspension was followed for 1 min after which DiOC2 (3) was added to a final concentration of 10μM and the fluorescence monitored until it plateaued. Cells were then re-energized with 0.5% glucose and fluorescence further monitored until it plateaued, after which 10 μM of the proton ionophore carbonyl cyanide-chlorophenylhydrazone (CCCP) was added and fluorescence followed again until plateaued. All assays were performed in triplicates.
In vitro Cell Cytotoxicity
A thiazolyl blue tetrazolium bromide (MTT) assay was used to assess the in vitro cytotoxicity of IBG against A549 cells with a previously reported protocol.24 Briefly, human lung epithelial (A549) cells were seeded at a density of 5×103 cells per well in 96-well plates and then incubated for 24 hrs. Then, the growth medium was rinsed with PBS and replaced with fresh medium containing different concentrations of the IBG. Control wells were treated with an equivalent volume of IBG-free and DMSO-medium. The cells were incubated at 37°C for 48 hrs. After incubation, the medium was removed, and MTT solution (5 mg/mL) was added to each well, and the plate was incubated for 4 hrs, thus allowing the viable cells to convert the yellow MTT into purple formazan crystals. Finally, the medium was completely removed, and 150 μL of DMSO was added to each well to dissolve the purple formazan crystals. The absorbance was measured at 490 nm using a multifunctional microplate reader (Thermo Fisher Scientific, Germany). The IC50 values were calculated using nonlinear regression analysis, and cell cytotoxicity was assessed by quantifying the IC50 values of the IBG.
Hemolytic Activity
The hemolysis assay was performed as previously described.25 Blood was collected from the posterior orbital venous plexus of KM mice and centrifuged at 1000×g for 5 mins. After centrifugation, the supernatant was discarded and obtained the fresh mice red blood cells (RBCs). Then, cells were washed with PBS (pH 7.4) three times, centrifuged at 1000g for 5 mins, and resuspended in PBS to attain a dilution of ~4% (v/v) of the erythrocyte. A total of 150 μL mice RBCs were added to the wells of a 96-well U-bottom plate and serial dilution of IBG was added to the wells resulting in a final concentration ranging from 1.25 to 2560 μg/mL. After 1 hr at 37°C, cells were centrifuged at 1000×g. The supernatant was transferred to 96-well plates and A450nm (OD450) measured using a multifunctional microplate reader. The mRBCs in PBS and 1% Triton X-100 were used as negative and positive controls, respectively. Experiments were performed with biological replicates. The percentage of hemolysis was calculated using the following equation:
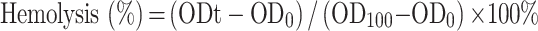 ;
;
Ethics Statement
All specific-pathogen-free female KM mice (Southern Medical University, Guangdong, China) were 6–8-week old, weighing 20±2 g. Mouse studies were approved by the Animal Research Committee of South China Agricultural University [ID: 2018030]. All experiments were conducted in full compliance with the guidelines of Guangdong Laboratory Animal Welfare and Ethics and the Institutional Animal Care and Use Committee of the South China Agricultural University.
Results
Inhibitory Activities of IBG
To examine the antimicrobial activities of IBG, we measured MIC against 32 Enterococci isolates containing diverse multilocus sequence types and antibiotic-resistant phenotypes. The MIC range for IBG was 1–4 μg/mL. Then, we performed MBC against 9 E. faecalis and 5 E. faecium with different ST types. The MBC range for IBG was 2–16 μg/mL, which was 2-8-fold of its MICs. In addition, in the presence of 10% FBS, the MIC range increased 4-8-fold to 16 μg/mL.
Time-Dependent Assay
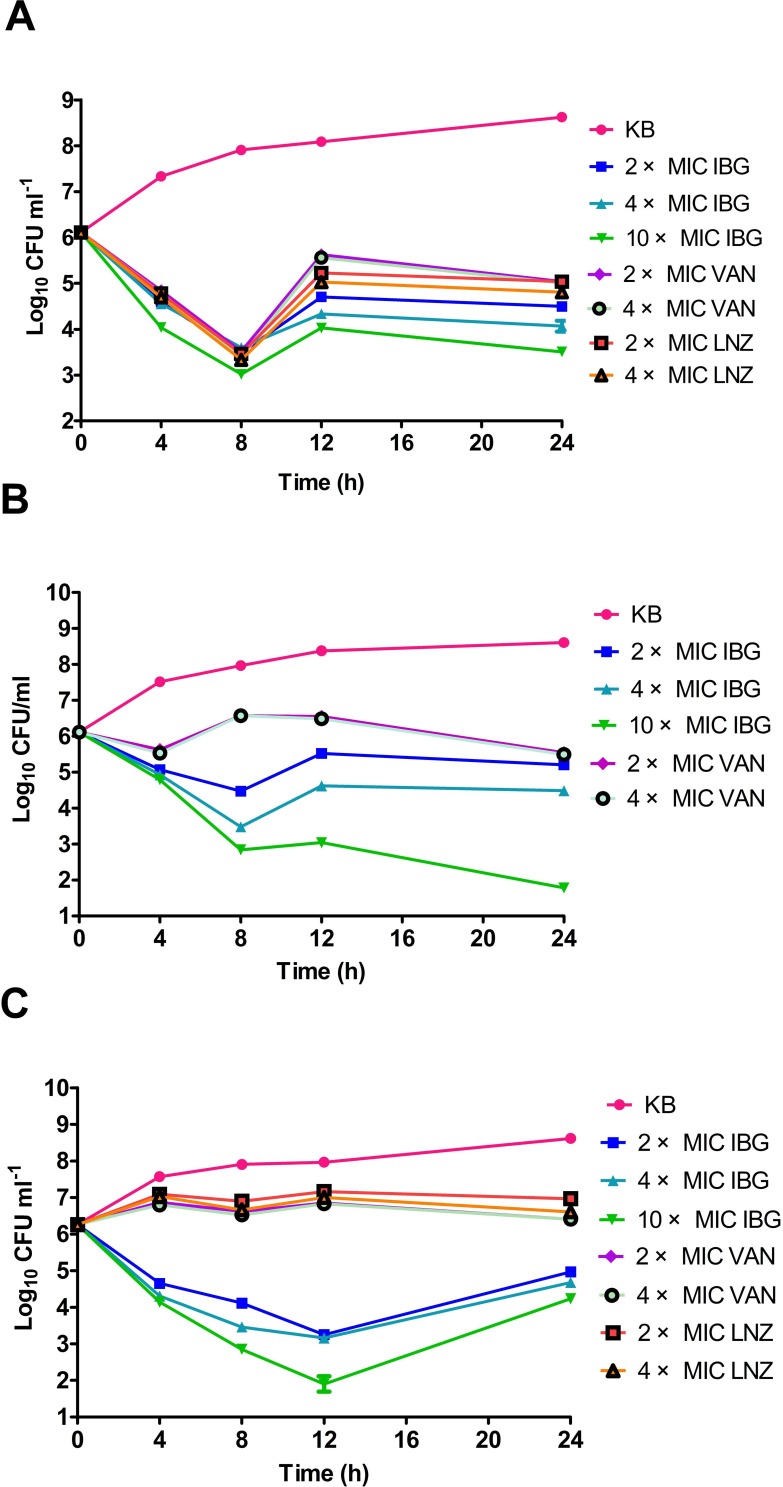
We performed a kill kinetic assay to analyze the killing rate of IBG and to compare it with that of conventional antibiotics frequently used against E. faecalis ATCC 29212, E. faecalis GDE6P130C and E. faecium GDE6P50C. For E. faecalis ATCC 29212, after 8 h of exposure, at least a 3-fold reduction in viable cells at 2 × MIC of VAN, LNZ, and IBG was observed. However, at 12 h of exposure, some E. faecalis regrowth was observed for all antibiotics (Figure 4A). For MDR E. faecalis GDE6P130C, VAN had a low bactericidal activity at 2 × and 4 × MIC and substantial regrowth was observed after 8 h. In comparison, there was rapid killing activity of IBG at 4× MIC, with a two-log reduction in viable cells following an 8-h exposure. The E. faecalis regrowth was observed for all antibiotics at 24 h of exposure (except at 10 × MIC of IBG) (Figure 4B). For E. faecium GDE6P50C, VAN and LNZ (at 2 × and 4× MIC) had no effect on bacterial growth. While the use of IBG even at 2 × MIC could decrease bacterial cell counts by3 log10 CFU/mLat 12 h (Figure 4C).
Figure 4.
Time-kill studies of antibiotics against Enterococci. (A) E. faecalis ATCC 29212 was grown in 5 mL MH broth in the presence of 2 × MIC, 4 × MIC, 10 × MIC of isopropoxy benzene guanidine (IBG), 2 × MIC, 4 × MIC of vancomycin (VAN), 2 × MIC, 4 × MIC of linezolid (LNZ). (B) E. faecalis GDE6P130C was grown in 5 mL MH broth in the presence of 2 × MIC, 4 × MIC, 10 × MIC of IBG, 2 × MIC, 4 × MIC of VAN. (C) E. faecium GDE6P50C was grown in 5 mL MH broth in the presence of 2 × MIC, 4 × MIC, 10 × MIC of IBG, 2 × MIC, 4 × MIC of VAN, 2 × MIC, 4 × MIC of LNZ.
IBG Exerts Its Antibacterial Action on the Cell Membrane of E. faecalis
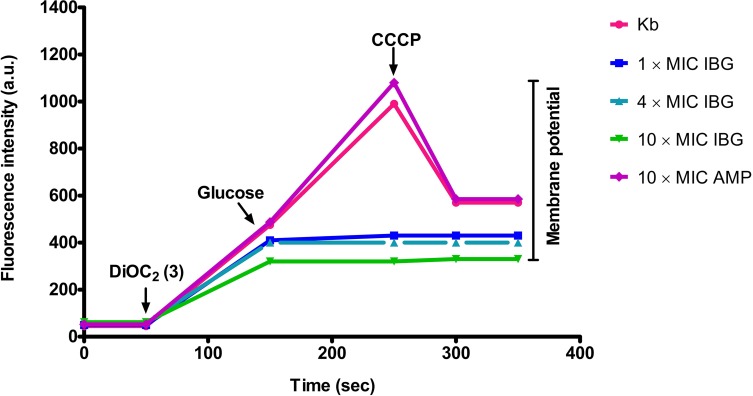
To gain insight into how IBG exerts its antibacterial activity on E. faecalis, we investigate cell membrane perturbation by IBG using DiOC2 (3). Bacterial cells were energized by the addition of glucose to establish a proton motive force (negative and basic inside the cell) and de-energized by incubation with the proton ionophore CCCP. This led to variation in fluorescence associated with DiOC2 (3). When E. faecalis ATCC 29212 was pre-incubated with IBG, a large reduction in fluorescence compared to that of the untreated cells and cells in the presence of ampicillin was observed (shown in Figure 5).
Figure 5.
IBG dissipates the membrane potential of E. faecalis ATCC 29212. Bacterial suspensions were exposed to isopropoxy benzene guanidine (IBG) or ampicillin (AMP, control) for 5 mins after which DiOC2 (3) was added and the fluorescence monitored until it plateaued. Cells were then re-energized with 0.5% glucose and the establishment of a membrane potential was measured as an increase in fluorescence until it plateaued. The membrane potential was then disrupted by the addition of the proton ionophore (CCCP). Data presented are representative of two experiments.
Hemolytic Activity and Cytotoxicity of IBG
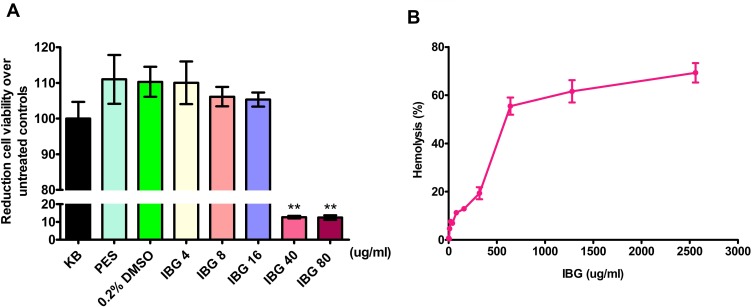
We evaluated the toxicity profile of IBG against A549 cell using MTT assay and haemolytic activity against mice RBCs. The results showed that IC50 values of 28μg/mL for IBG (Figure 6A). Hemolytic activity of IBG showed that the concentration that induces 50% hemolysis (HC50) for IBG was 443.1μg/mL, indicating that IBG is well tolerated by RBCs (Figure 6B).
Figure 6.
Isopropoxy benzene guanidine (IBG) demonstrates limited cytotoxicity to mammalian cell lines. (A) Cell viability was measured on a multifunctional microplate reader for A549 cell using MTT assay (B) Cell viability was measured on a multifunctional microplate reader for mice red blood cells. Data are means (±SD) for each treatment (in duplicate).
Discussion
The major challenge to treatment and control of leading bacterial pathogens is the rapid emergence and global spread of multidrug-resistant clones that are refractory to last-resort antimicrobial therapy.26 To address this problem, we have examined the substituted benzene guanidine compounds as a parent scaffold for developing a new antimicrobial class. Here we identified the promising antibacterial compound IBG, which possesses anti-bactericidal activity against MDR Enterococci. Furthermore, we demonstrated that IBG could disrupt the cell membrane potential of Enterococci.
This study examined strains that originate from agriculture; however, we deliberately collected 20 E. faecalis isolates belong to the ST 475, 480, 314, 363, 69, 476, 32, 86, 59, and 12 E. faecium isolates belong to the ST 486, 650, 92, 29, 636. Furthermore, the cfr and optrA genes were found in these strains. Notably, the gene cfr and/or optrA in ST59, ST314, ST476, ST480 E. faecalis, and ST29 E. faecium have been reported in human clinical infections.27–29 These results indicate that Enterococci can be a reservoir for resistance as well as transfer resistance genes between humans and animals. Hence, they often showed resistant to a wide range of antibiotics, necessitating the development of novel compounds that are effective against this species.
The MIC of IBG against 32 clinically Enterococci isolates was 1–4 μg/mL, which was equal or superior to that of robenidine with MIC values of 4.7 μg/mL against vancomycin-resistant Enterococci. According to the structure–activity relationship in robenidine analogues, the methylation saw a modest activity improvement, but only with the methoxy group (3-OCH3) (16). However, our compound with the isopropoxy group (3-OCH(CH3)2) displayed a significant reduction in the MIC values against Enterococci. This indicated the introduction of isopropoxy group has an important role on improving activity of robenidine analogue against Enterococci . In addition, IBG was more active than the robenidine analogue 26 which has a replacement with 4-CH(CH3)2. The finding that the MICs of IBG against strains of Enterococci were increased 4–8-fold in the presence of serum suggests a high protein binding. This may be one of the factors allowing enhanced stability and plasma lifetime without necessarily reducing its effectiveness in vivo.30
In the present study, the rate of bactericidal activity was determined for IBG, vancomycin, and linezolid against Enterococci. Interestingly, IBG displayed superior bactericidal activity compared to vancomycin and linezolid, which used as last-resort antibiotic for Enterococci infections. IBG caused a similar rate of bactericidal activity at 2×MIC, 4×MIC, indicating time dependent rather than concentration-dependent killing.
It should be noted that regrowth was observed for all antibacterial agents, although this occurred at a much slower rate for IBG. The regrowth was observed after treatment with vancomycin and linezolid could be explained by selective amplification of less-susceptible sub-populations and is consistent with previous studies performing the time-kill kinetics of vancomycin and linezolid.31,32 As for IBG, regrowth at 24 hrs is not uncommon and has previously been reported for robenidine analog NCL195 against S. aureus. In addition, we confirmed that the rebound growth was neither due to chemical instability of IBG or emergence of a resistant population by adding 105 CFU of Enterococci in LB broth containing 2 × MIC, 4 × MIC and 10 × MIC of IBG over 24–72 h. After 72 h incubation in the presence of antibiotic, a few colonies were obtained from broth containing 105 CFU of Enterococci to which 2 × MIC, 4 × MIC and 10 × MIC of IBG was added. We subjected the colonies that grew after 72 h to MIC testing and these returned 1× MIC for IBG.
As the guanidine compounds possess a mechanism of action that targets the cell membrane, they could be more effective than other bactericidal concentration-dependent antimicrobials that have intracellular targets, such as fluoroquinolones and aminoglycosides. DiOC2 (3) has been used to measure the magnitude and stability of Δψ in bacterial cells and proteoliposomes.33 In our fluorescence membrane potential measurements, when treated with IBG, a large reduction in the magnitude of the generated membrane potential was observed, suggesting that IBG could permeabilize the cytoplasmic membrane of E. faecalis, which is in corroboration with the results obtained in previous studies.
Toxicity is often a major obstacle in therapeutic application of membrane-damaging antibacterial agents. Human lung epithelial cells (A549) cells are widely used to evaluate cytotoxicity in antibiotics and metals.34 In this study, IBG showed a promising safety profile with A549 cytotoxicity of IC50 28 μg/mL. Low mERG inhibition with IBG has also been demonstrated, with HC50 of >400 μg/mL. These values are at least seven times the observed MIC90 values for IBG. It is possible that in vitro cytotoxicity of IBG is not predictive of in vivo toxicity; in agreement with reports which note the importance of in vitro responses but conclude that the true profile of compound toxicity can only be determined in vivo.35,36 However, the cytotoxicity of IBG is considered to be acceptable at this stage of compound development, especially as in vivo studies have been performed with IBG which determined the oral LD50 to be ~1800 mg/kg in rat. Furthermore, no adverse effects were noted in broiler chicken and weaning piglet treated with IBG which effectively improved the average daily weight gain and material weight ratio and the production performance and reduce the rate of diarrhea as feed.37,38
Conclusion
In this study, IBG displayed potent bactericidal activity against MDR Enterococci, which is likely responsible for the disruption of the cell membrane potential. The results presented demonstrate that IBG warrants further exploration for potential use as future antimicrobial agents to treat the infections caused by MDR Enterococci.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 31672608) and Doctoral Innovative Talents (Domestic Training) Cultivation Project of South China Agricultural University (Grant No. CX2019N029).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Xiong W, Wang Y, Sun Y, et al. Antibiotic-mediated changes in the fecal microbiome of broiler chickens define the incidence of antibiotic resistance genes. Microbiome. 2018;6(1):34. doi: 10.1186/s40168-018-0419-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zeng J, Pan Y, Yang J, Hou M, Zeng Z, Xiong W. Metagenomic insights into the distribution of antibiotic resistome between the gut-associated environments and the pristine environments. Environ Int. 2019;126:346–354. doi: 10.1016/j.envint.2019.02.052 [DOI] [PubMed] [Google Scholar]
- 3.Pourmand A, Mazer-Amirshahi M, Jasani G, May L. Emerging trends in antibiotic resistance: implications for emergency medicine. Am J Emerg Med. 2017;35(8):1172–1176. doi: 10.1016/j.ajem.2017.03.010 [DOI] [PubMed] [Google Scholar]
- 4.Hasan KA, Ali SA, Rehman M, Bin-Asif H, Zahid S. The unravelled enterococcus faecalis zoonotic superbugs: emerging multiple resistant and virulent lineages isolated from poultry environment. Zoonoses Public Health. 2018;65(8):921–935. [DOI] [PubMed] [Google Scholar]
- 5.Sadowy E. Linezolid resistance genes and genetic elements enhancing their dissemination in enterococci and streptococci. Plasmid. 2018;99:89–98. doi: 10.1016/j.plasmid.2018.09.011 [DOI] [PubMed] [Google Scholar]
- 6.Lebreton F, Manson AL, Saavedra JT, Straub TJ, Earl AM, Gilmore MS. Tracing the enterococci from paleozoic origins to the hospital. Cell. 2017;169(5):849–861.e813. doi: 10.1016/j.cell.2017.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vidana R, Rashid MU, Ozenci V, Weintraub A, Lund B. The origin of endodontic enterococcus faecalis explored by comparison of virulence factor patterns and antibiotic resistance to that of isolates from stool samples, blood cultures and food. Int Endod J. 2016;49(4):343–351. doi: 10.1111/iej.12464 [DOI] [PubMed] [Google Scholar]
- 8.Krull M, Klare I, Ross B, et al. Emergence of linezolid- and vancomycin-resistant enterococcus faecium in a department for hematologic stem cell transplantation. Antimicrob Resist Infect Control. 2016;5:31. doi: 10.1186/s13756-016-0131-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bi R, Qin T, Fan W, Ma P, Gu B. The emerging problem of linezolid-resistant enterococci. J Glob Antimicrob Resist. 2018;13:11–19. doi: 10.1016/j.jgar.2017.10.018 [DOI] [PubMed] [Google Scholar]
- 10.Mercuro NJ, Davis SL, Zervos MJ, Herc ES. Combatting resistant enterococcal infections: a pharmacotherapy review. Expert Opin Pharmacother. 2018;19(9):979–992. doi: 10.1080/14656566.2018.1479397 [DOI] [PubMed] [Google Scholar]
- 11.Abraham RJ, Stevens AJ, Young KA, et al. Robenidine analogues as gram-positive antibacterial agents. J Med Chem. 2016;59(5):2126–2138. doi: 10.1021/acs.jmedchem.5b01797 [DOI] [PubMed] [Google Scholar]
- 12.Farha MA, Brown ED. Drug repurposing for antimicrobial discovery. Nat Microbiol. 2019;4(4):565–577. doi: 10.1038/s41564-019-0357-1 [DOI] [PubMed] [Google Scholar]
- 13.Savoia D. New antimicrobial approaches: reuse of old drugs. Curr Drug Targets. 2016;17(6):731–738. doi: 10.2174/1389450116666150806124110 [DOI] [PubMed] [Google Scholar]
- 14.Thangamani S, Younis W, Seleem MN. Repurposing ebselen for treatment of multidrug-resistant staphylococcal infections. Sci Rep. 2015;5:11596. doi: 10.1038/srep11596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saczewski F, Balewski L. Biological activities of guanidine compounds, 2008–2012 update. Expert Opin Ther Pat. 2013;23(8):965–995. doi: 10.1517/13543776.2013.788645 [DOI] [PubMed] [Google Scholar]
- 16.Rauf MK, Imtiaz ud D, Badshah A. Novel approaches to screening guanidine derivatives. Expert Opin Drug Discov. 2014;9(1):39–53. doi: 10.1517/17460441.2013.857308 [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Li XW, Guo YW. Recent advances in the isolation, synthesis and biological activity of marine guanidine alkaloids. Mar Drugs. 2017;15(10):324. doi: 10.3390/md15100324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kantor S, Kennett RL Jr, Waletzky E, Tomcufcik AS. 1,3-Bis(p-chlorobenzylideneamino)guanidine hydrochloride (robenzidene): new poultry anticoccidial agent. Science. 1970;168(3929):373–374. doi: 10.1126/science.168.3929.373 [DOI] [PubMed] [Google Scholar]
- 19.Ogunniyi AD, Khazandi M, Stevens AJ, et al. Evaluation of robenidine analog NCL195 as a novel broad-spectrum antibacterial agent. PLoS One. 2017;12(9):e0183457. doi: 10.1371/journal.pone.0183457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larsen MV, Cosentino S, Rasmussen S, et al. Multilocus sequence typing of total genome sequenced bacteria. J Clin Micobiol. 2012;50(4):1355–1361. doi: 10.1128/JCM.06094-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, M100. 28th Informational Supplement. Clinical and Laboratory Standards Institute. Wayne, PA; 2018. [Google Scholar]
- 22.Lin S, Koh JJ, Aung TT, et al. Symmetrically substituted xanthone amphiphiles combat gram-positive bacterial resistance with enhanced membrane selectivity. J Med Chem. 2017;60(4):1362–1378. doi: 10.1021/acs.jmedchem.6b01403 [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Mowla R, Guo L, et al. Evaluation of a series of 2-napthamide derivatives as inhibitors of the drug efflux pump AcrB for the reversal of antimicrobial resistance. Bioorg Med Chem Lett. 2017;27(4):733–739. doi: 10.1016/j.bmcl.2017.01.042 [DOI] [PubMed] [Google Scholar]
- 24.Roberts J, Bingham J, McLaren AC, McLemore R. Liposomal formulation decreases toxicity of amphotericin B in vitro and in vivo. Clin Orthop Relat Res. 2015;473(7):2262–2269. doi: 10.1007/s11999-015-4232-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diaz ME, Rocha GF, Kise F, Rosso AM, Guevara MG, Parisi MG. Antimicrobial activity of an aspartic protease from salpichroa origanifolia fruits. Lett Appl Microbiol. 2018;67(2):168–174. doi: 10.1111/lam.2018.67.issue-2 [DOI] [PubMed] [Google Scholar]
- 26.Ahmed MO, Baptiste KE. Vancomycin-resistant enterococci: a review of antimicrobial resistance mechanisms and perspectives of human and animal health. Microb Drug Resist. 2018;24(5):590–606. doi: 10.1089/mdr.2017.0147 [DOI] [PubMed] [Google Scholar]
- 27.Zhang Y, Dong G, Li J, et al. A high incidence and coexistence of multiresistance genes cfr and optrA among linezolid-resistant enterococci isolated from a teaching hospital in Wenzhou, China. Eur J Clin Microbiol Infect Dis. 2018;37(8):1441–1448. doi: 10.1007/s10096-018-3269-8 [DOI] [PubMed] [Google Scholar]
- 28.Cai J, Wang Y, Schwarz S, et al. High detection rate of the oxazolidinone resistance gene optrA in enterococcus faecalis isolated from a Chinese anorectal surgery ward. Int J Antimicrob Agents. 2016;48(6):757–759. doi: 10.1016/j.ijantimicag.2016.08.008 [DOI] [PubMed] [Google Scholar]
- 29.Wang Y, Lv Y, Cai J, et al. A novel gene, optrA, that confers transferable resistance to oxazolidinones and phenicols and its presence in enterococcus faecalis and enterococcus faecium of human and animal origin. J Antimicrob Chemother. 2015;70(8):2182–2190. doi: 10.1093/jac/dkv116 [DOI] [PubMed] [Google Scholar]
- 30.Lee BL, Sachdeva M, Chambers HF. Effect of protein binding of daptomycin on MIC and antibacterial activity. Antimicrob Agents Chemother. 1991;35(12):2505–2508. doi: 10.1128/AAC.35.12.2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen GP, Bierman BC. In vitro analysis of resistance selection by linezolid in vancomycin-susceptible and -resistant enterococcus faecalis and enterococcus faecium. Int J Antimicrob Agents. 2009;34(1):21–24. doi: 10.1016/j.ijantimicag.2008.12.011 [DOI] [PubMed] [Google Scholar]
- 32.Allen GP, Cha R, Rybak MJ. In vitro activities of quinupristin-dalfopristin and cefepime, alone and in combination with various antimicrobials, against multidrug-resistant staphylococci and enterococci in an in vitro pharmacodynamic model. Antimicrob Agents Chemother. 2002;46(8):2606–2612. doi: 10.1128/AAC.46.8.2606-2612.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venter H, Shilling RA, Velamakanni S, Balakrishnan L, Van Veen HW. An ABC transporter with a secondary-active multidrug translocator domain. Nature. 2003;426(6968):866–870. doi: 10.1038/nature02173 [DOI] [PubMed] [Google Scholar]
- 34.Ahamed M, Akhtar MJ, Alhadlaq HA. Toxicology in vitro: an international journal published in association with BIBRA. Toxicol in Vitro. 2019;57:18–27. doi: 10.1016/j.tiv.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 35.Garle MJ, Fentem JH, Fry JR. In vitro cytotoxicity tests for the prediction of acute toxicity in vivo. Toxicol in Vitro. 1994;8(6):1303–1312. doi: 10.1016/0887-2333(94)90123-6 [DOI] [PubMed] [Google Scholar]
- 36.Jaroch K, Jaroch A, Bojko B. Cell cultures in drug discovery and development: the need of reliable in vitro-in vivo extrapolation for pharmacodynamics and pharmacokinetics assessment. J Pharm Biomed Anal. 2018;147:297–312. doi: 10.1016/j.jpba.2017.07.023 [DOI] [PubMed] [Google Scholar]
- 37.Xiao QX, Qiang MH, Zhang L, Ou YR, Zhang SM, Qin ZH. Effects of isopropoxy benzene guanidine as a substitute for antibiotics on performance and serum biochemical indexes of weaned piglets. Anim Sci Abroad. 2018;38(08):14–17. [Google Scholar]
- 38.Xiao QX, Qiang MH, Zhang SM, Ou YR, Peng XF. Effects of isopropoxy benzene guanidine as a substitute for antibiotics on the performance and immune organ index of broilers. Anim Sci Abroad. 2018;38(07):14–18. [Google Scholar]