Abstract
Purpose
Colistin alone may not be sufficient for treating carbapenem-resistant Acinetobacter baumannii (CRAB); thus, efforts are needed to increase treatment success rates. We compared the effects of colistin plus carbapenem therapy versus colistin monotherapy in treating pneumonia caused by CRAB and attempted to identify specific populations or factors that could benefit from combination therapy.
Methods
We retrospectively collected data on cases of CRAB pneumonia. The patients were divided into colistin plus carbapenem therapy and colistin monotherapy groups. The primary outcome was 14-day mortality. The secondary outcomes were in-hospital mortality, clinical improvement at days 2 and 14, and microbiological improvement at day 14.
Results
Of 160 cases meeting criteria for CRAB pneumonia, 83 (52%) and 77 (48.0%) were treated with carbapenem combination therapy or colistin monotherapy, respectively. Among these patients, 50 (63.3%) in the combination group and 27 (39.7%) in the monotherapy group had Acute Physiologic Assessment and Chronic Health Evaluation (APACHE) II scores >24 points (p=0.010). Overall, there was no significant difference in 14-day mortality between the combination and monotherapy groups (24.1% vs 20.8%, p=0.616). Clinical improvement and sputum-negative conversion also showed no significant difference. After adjusting for disease severity according to APACHE II score, the 14-day mortality was significantly lower in the combination group than in the monotherapy group among patients with APACHE II scores of 25–29 points (9.1% vs 53.8%, P=0.020).
Conclusion
Despite more severe conditions, compared with colistin monotherapy, colistin plus carbapenem combination therapy showed equivalent primary mortality outcome in treating CRAB pneumonia. Combination therapy was more effective in patients with APACHE II score ranging from 25 to 29 points.
Keywords: carbapenem-resistant Acinetobacter baumannii, CRAB, pneumonia, colistin, combination therapy, risk factor
Introduction
Acinetobacter spp. are glucose-non-fermentative, non-motile, non-fastidious, catalase-positive, oxidative-negative, aerobic gram-negative coccobacilli. Among Acinetobacter spp., Acinetobacter baumannii is the most important member associated with hospital-acquired infections worldwide. A. baumannii has emerged as a major cause of nosocomial infections, especially in intensive care units.1
A. baumannii has high rates of resistance to many available antibiotics in clinical practice, with the minimum inhibitory concentration (MIC) of imipenem increasing significantly in a worldwide collection of clinical samples between 2004 and 20092 and resistance to imipenem in over 70% of A. baumannii isolates in more recent data.3 There are limited therapeutic options in carbapenem-resistant Acinetobacter infection and colistin monotherapy has been the only available treatment. However, colistin monotherapy is not an optimal treatment.4,5 The mortality of A. baumannii infection is over 40% in the recent era.6 This rate is partially due to the fact that colistin is a very narrow-spectrum antibiotic, as well as its complicated pharmacokinetics/pharmacodynamics and barriers to use such as nephrotoxicity and neurotoxicity.
Due to concerns that colistin monotherapy might not be sufficient, efforts have been made to increase treatment success rates. The most common effort is antibiotic combination therapy, especially the addition of carbapenem to colistin for a synergistic effect.
The present study compared colistin plus carbapenem therapy versus colistin monotherapy in treating pneumonia caused by carbapenem-resistant A. baumannii (CRAB) and attempted to identify specific populations or factors that could benefit from colistin plus carbapenem therapy.
Materials and Methods
Study Design and Patient Population
We retrospectively collected data on cases of CRAB pneumonia from July 2007 through October 2018 at Kangdong Sacred Hospital, a university-affiliated 680-bed, secondary-care hospital in Seoul, Republic of Korea. Patients older than 18 years of age were included in the study. We searched the electronic medical record (EMR) systems by 10th revision of the International Statistical Classification of Diseases and Related Health Problems code7 for eligible patients with CRAB pneumonia. The inclusion criteria were a) at least one positive respiratory sample (sputum, endotracheal aspirate, or broncho-alveolar lavage) for CRAB; b) a clinical course compatible with pneumonia (as defined later). The exclusion criteria were patients without definitive pneumonia and those without colistin treatment or with only inhaled colistin therapy. This study was performed and described in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology guidelines for cohort studies. The Institutional Review Board of the Kangdong Sacred Heart Hospital approved the study protocol (2019-09-010). A waver for patient parental consent to review their medical records was granted by the Institutional Review Board. The handling of the patient data confidentiality strictly followed the rules set by the institution and were in compliance with the Declaration of Helsinki.
Data Collection and Analysis
Medical records based on EMR systems for clinical and microbiological data were reviewed and collected. Only the first episode was counted if multiple CRAB pneumonia events occurred. The patients were divided into the combination (those administered colistin plus carbapenem therapy) and monotherapy (those administered colistin monotherapy) groups. The following data were collected: demographic data, microorganisms isolated from blood and sputum cultures, antibiotic susceptibility, comorbidities (e.g., hypertension, diabetes mellitus, acquired immune deficiency syndrome, chronic kidney disease, solid or hematologic malignancies, liver cirrhosis, rheumatic disease, central nervous system [CNS] surgery, chronic lung disease, etc.), previous steroid use, need for intensive care, ventilator use, presence of hypoxia, time to defervescence, duration of antimicrobial therapy, invasive procedures, and treatment outcomes (mortality, clinical improvement, and microbiological improvement). We also collected the first laboratory data at the time of pneumonia diagnosis, with respect to white blood cell (WBC) counts, segmented neutrophil percentage, absolute neutrophil count, hemoglobin, platelet count, erythrocyte sedimentation rate, international normalized ratio, protein, albumin, total bilirubin, aspartate aminotransferase, alanine aminotransferase, C-reactive protein, procalcitonin, lactic acid, blood urea nitrogen, and creatinine. Microbiological data were obtained from the database at our clinical microbiology laboratory.
The primary outcome was 14-day mortality. The secondary outcomes were in-hospital mortality, clinical improvement at day 2, clinical improvement at day 14, and microbiological improvement at day 14.
Definitions
Pneumonia: a) new-onset respiratory symptoms (cough, sputum, and dyspnea) or rales or desaturation; b) new-onset or progressive infiltrative lesion on chest radiography suggestive of pneumonia; c) fever (≥38.0°C) or hypothermia (≤35.0°C) for more than 48 hrs or abnormal WBC count (≥10,000/mm3or ≤4500/mm3).
Quality of sputum: only fair or good sputum was counted.
CRAB culture criteria: CRAB documented by a culture study of sputum samples (>106 CFU/mL), airway aspirates (>105 CFU/mL), or bronchoalveolar fluid (>104 CFU/mL); protected specimen brushing cultures 103 CFU/mL
Initial sputum or blood culture: the first positive sputum or blood culture at the time of the pneumonia episode.
Follow-up sputum cultures: more than one separate sputum culture taken more than 24 hrs after an initial sputum culture.
True bacteremia: at least one positive blood culture, not otherwise considered a contaminant.
Contaminant: a positive blood culture in which the isolate was a common skin organism (such as diphtheroids, micrococci, or coagulase-negative staphylococci) isolated in one bottle, or when the medical records reported positive cultures as contaminants.
Febrile: patients were considered febrile if their recorded temperature was ≥100.4°F (38°C).
Antimicrobial susceptibility: identified with a Microscan WalkAway 96 instrument (Siemens Healthcare Diagnostics, Deerfield, Illinois) using Clinical and Laboratory Standards Institute criteria and guidelines.8 When an isolate was resistant to both cefotaxime and ceftazidime, it was suspected of producing extended-spectrum β-lactamase, the production of which was confirmed by an automated system with a standard identification card and the modified broth microdilution method.
Carbapenem-resistant: isolate resistant to at least one of the carbapenems tested except for ertapenem (meropenem, imipenem, doripenem).
Carbapenem-susceptible: isolate susceptible to all carbapenems tested.
Clinical improvement: more than any two of the following: 1) improvement of chest radiography; 2) defervescence; 3) resolution or improvement of symptoms such as sputum, cough, rhinorrhea, and dyspnea; and 4) improvement of oxygenation or ventilator weaning.
Statistical Analyses
Results are expressed as means ± standard deviation and as incidences in the study population. Student’s t- and Mann–Whitney tests were used to compare continuous variables, while chi-square and Fisher’s exact tests were used for categorical variables. To control for confounding variables such as severity, a logistic regression model and layering analysis were used. All P-values were two-tailed and P-values <0.05 were considered statistically significant. Variables that were statistically significant in the univariate analyses were candidates for multivariate analysis in addition to the main variables of clinical importance. IBM SPSS Statistics for Windows, version 24.0 (IBM Corp., Armonk, NY, USA) was used for the analyses.
Results
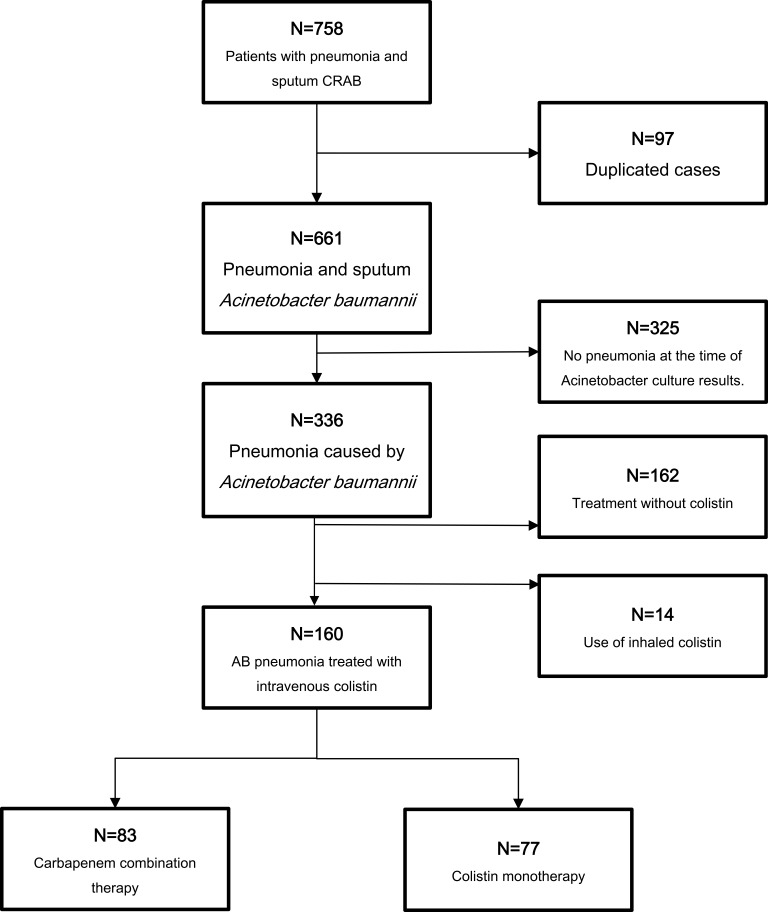
During the study period, 758 patients with pneumonia and sputum culture positive for CRAB were identified. After removing duplicated cases, 661 patients were included. A total of 325 patients were excluded because they did not have pneumonia following medical record review. A further 162 cases were excluded because they were administered therapy without colistin. An additional 14 cases were excluded due to the administration of an inhalation regimen. Finally, 160 cases were enrolled in the analysis. Of the remaining 160 patients with CRAB pneumonia, 83 (52%) were treated with carbapenem combination therapy (combination group) and 77 (48.0%) were treated with colistin monotherapy (monotherapy group) (Figure 1). Minimum amount of treatment duration required for enrollment was single dose. Meropenem, doripenem, imipenem was used in the carbapenem combination therapy group. The median time of therapy received were 12 days for the colistin monotherapy group and 14 days for the carbapenem combination group. The median time to therapy was 3 days after culture identification in both groups. The monotherapy group had received colistin 150 mg every 12 h and dose was adjusted according to renal function and additional standard dose of carbapenem by their renal function were given to the carbapenem combination group.
Figure 1.
Study population.
Abbreviations: CRAB, carbapenem-resistant Acinetobacter baumannii; AB, Acinetobacter baumannii.
The baseline characteristics of the patients are shown in Table 1. The median age of the study population was 71.6 (interquartile range=14.5) years and 71.2% were male. The most common underlying diseases were hypertension, diabetes mellitus, and chronic lung disease (Table 1). By Acute Physiologic Assessment and Chronic Health Evaluation (APACHE) II score, 50 (63.3%) of the combination group and 27 (39.7%) of the monotherapy group had scores >24 (P = 0.010) (Table 1).
Table 1.
Baseline Characteristics of Patients with Carbapenem-Resistant Acinetobacter Baumannii (CRAB) Pneumonia (N = 160)
| Characteristic (unit) | Carbapenem Combination (n = 83) | Colistin Monotherapy (n = 77) | P value |
|---|---|---|---|
| Male sex | 61 (73.5%) | 53 (68.8%) | 0.515 |
| Age, median ± IQR (years) | 71.9 ± 14.37 | 71.53 ± 14.61 | 0.878 |
| Underlying disease | |||
| Diabetes mellitus | 37 (45.1%) | 27 (35.1%) | 0.302 |
| Hypertension | 51 (61.4%) | 43 (55.8%) | 0.522 |
| Hematologic malignancy | 2 (2.4%) | 2 (2.6%) | 1.000 |
| Solid malignancy | 14 (16.9%) | 13 (16.9%) | 1.000 |
| Chronic kidney disease | 7 (8.4%) | 7 (9.0%) | 0.760 |
| Chronic lung disease | 34 (41.0%) | 28 (36.4%) | 0.505 |
| CNS surgery | 8 (9.6%) | 6 (7.8%) | 0.783 |
| Any surgery | 17 (20.5%) | 12 (15.6%) | 0.538 |
| Neurologic disease | 51 (62.7%) | 39 (50.6%) | 0.151 |
| Previous steroid use | 7 (8.4%) | 3 (3.9%) | 0.332 |
| Previous CARB | 35 (42.2%) | 40 (51.9%) | 0.267 |
| Previous carbapenem use | 57 (71.7%) | 48 (62.3%) | 0.093 |
| Previous hospitalization | 30 (36.1%) | 27 (35.1%) | 1.000 |
| Severity index | |||
| APACHE II Score >24 | 50 (63.3%) | 27 (39.7%) | 0.010 |
| Sepsis | 5 (6.0%) | 1 (1.3%) | 0.212 |
| Initial laboratory data (unit) | Mean ± SD | Mean ± SD | |
| WBC (×103/µL) | 16.13 ± 27.00 | 14.14 ± 7.28 | 0. 533 |
| Hemoglobin (g/dL) | 9.73 ± 1.55 | 9.97 ± 1.44 | 0. 320 |
| Platelet count (/mm3) | 229.12 ± 154.76 | 281.20 ± 133.26 | 0. 024 |
| Segmented neutrophil (%) | 92.67 ± 80.69 | 82.71 ± 13.32 | 0. 287 |
| PT INR (INR) | 1.38 ± 0.51 | 1.47 ± 0.66 | 0. 647 |
| ESR (mm/hr) | 36.25 ± 19.53 | 72.25 ± 31.68 | 0. 101 |
| CRP (g/dL) | 116.20 ± 75.75 | 105.59 ± 85.79 | 0. 408 |
| Procalcitonin (mg/dL) | 3.37 ± 6.83 | 3.65 ± 7.22 | 0. 895 |
| Protein (g/dL) | 5.43 ± 0.89 | 5.80 ± 0.86 | 0. 010 |
| Albumin (g/dL) | 2.6 ± 0.4 | 2.7 ± 0.4 | 0. 457 |
| Total bilirubin (mg/dL) | 1.41 ± 1.69 | 1.09 ± 2.53 | 0. 346 |
| AST (U/L) | 42.95 ± 32.94 | 56.78 ± 162.63 | 0. 352 |
| ALT (U/L) | 43.96 ± 83.24 | 56.78 ± 166.09 | 0. 534 |
| BUN (mg/dL) | 29.37 ± 21.38 | 33.04 ± 49.93 | 0. 577 |
| Creatinine (mg/dL) | 0.99 ± 0.73 | 0.94 ± 0.81 | 0. 667 |
Abbreviations: N, number; IQR, interquartile range; Steroid use, more than 20 mg/day over 14 days; CNS, central nervous system; APACHE, Acute Physiologic Assessment and Chronic Health Evaluation; SD, standard deviation; WBC, white blood cell; PT, prothrombin time; INR, international normalized ratio; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; AST, Aspartate aminotransferase; ALT, Alanine aminotransferase; BUN, blood urea nitrogen.
The platelet count was lower in the combination group (229.12±154.76 and 281.20±133.26/mm3 in the combination and monotherapy groups, respectively, P = 0.024), and the protein level was lower in the combination group (5.43±0.89 and 5.80±0.86 g/dL in the combination and monotherapy groups, respectively, P =0.010). The other laboratory data were similar between groups (Table 1). In the combination group, the most common carbapenem antibiotic used in combination was meropenem (77 cases, 92.7%), followed by imipenem (six cases, 7.3%). No patient in our study was administered doripenem or ertapenem (data not shown).
There was no significant difference in 14-day mortality between the combination and monotherapy groups (24.1% vs 20.8%, P = 0.616). The clinical improvement at day 2, clinical improvement at day 14, and sputum culture negative conversion on day 14 were also comparable between the groups (Table 2). In-hospital mortality was significantly lower in the colistin monotherapy group (55.4% and 37.7% in the combination and monotherapy group, respectively) (Table 2).
Table 2.
Outcome Differences Between Carbapenem Combination Therapy and Colistin Monotherapy
| Characteristics | Combination Therapy (n = 83) | Colistin Monotherapy (n = 77) | P-value |
|---|---|---|---|
| Primary outcome | |||
| 14-day mortality | 20 (24.1%) | 16 (20.8%) | 0.616 |
| Secondary outcome | |||
| In-hospital mortality | 46 (55.4%) | 29 (37.7%) | 0.024 |
| Clinical improvement at day 14 | 47 (58.8%) | 46 (73.1%) | 0.076 |
| Negative conversion at day 14 | 34 (41.0%) | 37 (48.7%) | 0.328 |
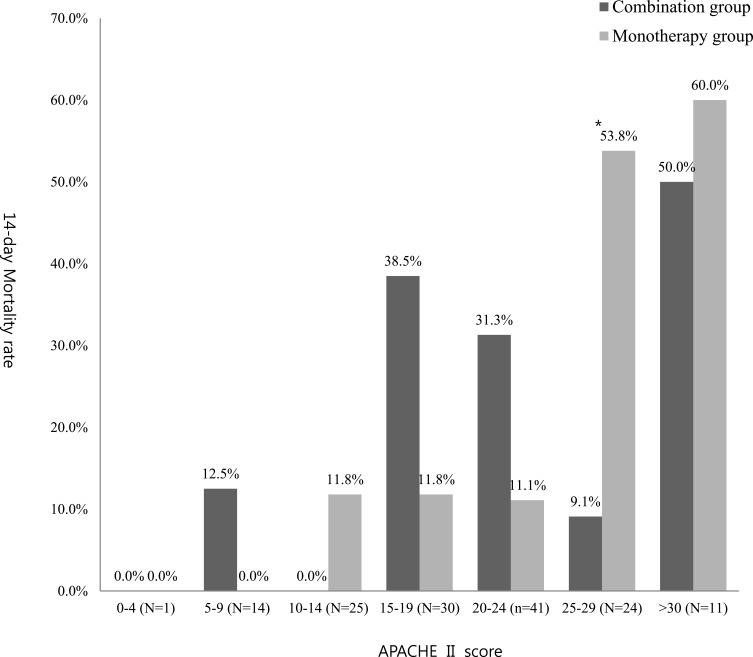
To remove the effect of disease severity (disturbance variables), the outcomes were adjusted by APACHE II score (Table 3 and Figure 2). Although the overall results were similar after adjustment, the 14-day mortality was significantly lower in the combination group than that in the monotherapy group (9.1% vs 53.8%, P = 0.020) among those with APACHE II mortality scores of 25–29 points.
Table 3.
Subgroup Analysis of Primary Outcome (14-Day Mortality) by APACHE II Score
| APACHE II Score (No. of Patients) | Group (No. of Patients) | 14-Day Mortality (%) | P-value |
|---|---|---|---|
| 0–4 (1) | Combination group (0) | NA | NA |
| Monotherapy group (1) | 0 (0%) | ||
| 5–9 (14) | Combination group (8) | 1(12.5%) | 0.369 |
| Monotherapy group (6) | 0 (0%) | ||
| 10–14 (25) | Combination group (8) | 0 (0%) | 0.312 |
| Monotherapy group (17) | 2 (11.8%) | ||
| 15–19 (30) | Combination group (13) | 5 (38.5%) | 0.087 |
| Monotherapy group (17) | 2 (11.8%) | ||
| 20–24 (41) | Combination group (32) | 10 (31.3%) | 0.228 |
| Monotherapy group (9) | 1 (11.1%) | ||
| 25–29 (24) | Combination group (11) | 1 (9.1%) | 0.020 |
| Monotherapy group (13) | 7 (53.8%) | ||
| 30–34 (11) | Combination group (6) | 3 (50.0%) | 0.740 |
| Monotherapy group (5) | 3 (60.0%) | ||
| >34 (1) | Combination group (1) | 0 (0%) | NA |
| Monotherapy group (0) | NA |
Abbreviations: APACHE, Acute Physiologic Assessment and Chronic Health Evaluation; N, number.
Figure 2.
Subgroup analysis of primary outcome (14-day mortality) by APACHE II score.
Note: *P=0.02.
Abbreviations: APACHE, Acute Physiologic Assessment and Chronic Health Evaluation.
The risk factors for 14-day mortality were analyzed by univariate and multivariate analyses (Tables 4 and 5). In univariate analysis, age, previous steroid use, and clinical improvement at day 2 differed significantly between the survival and mortality groups. Multivariate analysis that included factors with P-value <0.1 revealed that previous steroid use and clinical improvement at day 2 were significantly associated with 14-day mortality (odds ratios [ORs] 4.771 [P=0.031] and 0.326 [P=0.011], respectively) (Table 5).
Table 4.
Risk Factors for 14-Day Mortality in Carbapenem-Resistant Acinetobacter Baumannii (CRAB) Pneumonia: Univariate Analysis
| Characteristic (unit) | Survived (n = 124) | Death (n =36) | P-value |
|---|---|---|---|
| Male sex | 87 (70.2%) | 9 (25%) | 0.572 |
| Age, median ± IQR (years) | 70.29 ± 15.25 | 76.55 ± 10.02 | 0.022 |
| Underlying disease | |||
| Diabetes mellitus | 50 (40.7%) | 13 (36.1%) | 0.167 |
| Hypertension | 74 (59.7%) | 20 (55.6%) | 0.658 |
| Hematologic malignancy | 4 (3.2%) | 0 (0%) | 0.275 |
| Solid malignancy | 20 (16.1%) | 7 (19.4%) | 0.640 |
| Chronic kidney disease | 8 (6.5%) | 3 (8.3%) | 0.694 |
| Chronic lung disease | 46 (37.1%) | 16 (44.4%) | 0.116 |
| CNS surgery | 12 (9.7%) | 2 (5.6%) | 0.441 |
| Any surgery | 22 (17.7%) | 7 (19.4%) | 0.815 |
| Neurologic disease | 72 (58.1%) | 19 (52.8%) | 0.573 |
| Previous steroid use | 5 (4.0%) | 5 (13.9%) | 0.031 |
| Previous CARB | 60 (48.4%) | 15 (41.7%) | 0.477 |
| Previous carbapenem use | 88 (70.7%) | 28 (72.3%) | 0.757 |
| Previous hospitalization | 44 (35.5%) | 13 (36.1%) | 0.945 |
| Severity index | |||
| APACHE II Score >24 | 52 (46.5%) | 25 (71.4%) | 0.064 |
| Sepsis | 4 (3.2%) | 2 (5.6%) | 0.517 |
| Initial laboratory data (unit) | Mean ± SD | Mean ± SD | |
| WBC (×103/µL) | 15.03 ± 22.39 | 15.67 ± 8.01 | 0.867 |
| Hemoglobin (g/dL) | 9.794 ± 1.41 | 10.05 ± 1.78 | 0.375 |
| Platelet count (/mm3) | 265.94 ± 152.79 | 213.69 ± 116.43 | 0.060 |
| Segmented neutrophil (%) | 87.92 ± 66.81 | 87.73 ± 8.11 | 0.987 |
| PT INR (INR) | 1.41 ± 0.57 | 1.52 ± 0.72 | 0.611 |
| ESR (mm/hr) | 56.00 ± 33.11 | 42.00 ± 31.68 | 0.706 |
| CRP (g/dL) | 116.02 ± 84.09 | 94.11 ± 65.70 | 0.152 |
| Procalcitonin (mg/dL) | 3.07 ± 6.42 | 4.58 ± 8.27 | 0.501 |
| Protein (g/dL) | 5.61 ± 0.91 | 5.58 ± 0.86 | 0.883 |
| Albumin (g/dL) | 2.7 ± 0.4 | 2.6 ± 0.40 | 0.163 |
| Total bilirubin (mg/dL) | 1.12 ± 2.03 | 1.74 ± 2.38 | 0.124 |
| AST (U/L) | 52.33 ± 128.52 | 46.86 ± 44.05 | 0.802 |
| ALT (U/L) | 47.22 ± 131.65 | 59.88 ± 122.43 | 0.607 |
| BUN (mg/dL) | 30.28 ± 40.83 | 34.85 ± 24.94 | 0.525 |
| Creatinine (mg/dL) | 0.91 ± 0.67 | 1.18 ± 1.01 | 0.059 |
Abbreviations: N, number; IQR, interquartile range; CNS, central nervous system; Steroid use, more than 20 mg/day over 14 days; APACHE, Acute Physiologic Assessment and Chronic Health Evaluation; SD, standard deviation; WBC, white blood cell; PT, prothrombin time; INR, international normalized ratio; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; BUN, blood urea nitrogen.
Table 5.
Risk Factors for 14-Day Mortality in CRAB Pneumonia: Multivariate Analysis
| Risk Factors (Unit) | OR | 95% CI | P value |
|---|---|---|---|
| Age, median ± IQR (years) | 0.747 | 0.283–1.975 | 0557 |
| Previous steroid use | 4.771 | 1.150–19.801 | 0.031 |
| Platelet count | 0.999 | 0.996–1.002 | 0.418 |
| APACHE II score >24 | 0.571 | 0.250–1.305 | 0.184 |
| Creatinine initial (mg/dL) | 1.521 | 0.964–2.401 | 0.072 |
Abbreviations: IQR, interquartile range; OR, odds ratio; CI, confidence interval; APACHE, Acute Physiologic Assessment and Chronic Health Evaluation.
Discussion
A.baumannii is one of the most successful pathogens responsible for hospital-acquired nosocomial infections in the modern healthcare system. CRAB, Pseudomonas aeruginosa, and Enterobacteriaceae (specifically K. pneumoniae) were recognized in February 2017 by the World Health Organization as critical-priority pathogens posing utmost threats to human health.9
Colistin monotherapy has been the only available treatment for CRAB; however, it is not an optimal treatment.4,5 Thus, efforts have been made to increase treatment success rates. The most common effort was the use of carbapenem with colistin, which was expected to result in a synergistic effect. The suggested mechanisms of this synergistic effect may be based on the combined effect of the two molecules on bacterial cells.4 Colistin interferes with the outer membrane, changing its permeability, which in turn allows meropenem to enter the bacteria in higher amounts. Higher concentrations of meropenem in the periplasmic space could reduce the effect of resistance mechanisms, thereby rendering meropenem active against resistant bacteria. Similarly, addition of beta-lactam to beta-lactam resistant MRSA therapy combined with daptomycin proved some additional benefits in other study.10
Studies have indicated that meropenem plus colistin was superior to colistin monotherapy in carbapenem-resistant Klebsiella spp.11 and extensive drug-resistant P. aeruginosa.4 In the case of A. baumannii, one study on in vitro pharmacokinetic/pharmacodynamics reported the superiority of carbapenem combination therapy;12 however, but recently, in vivo studies have failed to support that colistin plus carbapenem combination is superior to colistin monotherapy.13–15
To identify the specific populations that would most benefit from the combination therapy, we narrowed the indications to pneumonia, adult, CRAB. However, like previous studies, our study also failed to prove the superiority of colistin plus carbapenem combination therapy over colistin monotherapy.
However, the severity differed between the two groups. The combination group had significantly more patients with APACHE II scores >24. These findings suggest that the combination group included a higher proportion of serious cases. If more severe patients were included in the combination group and similar mortality was obtained, combination therapy may have the potential to be more effective. Furthermore, the 14-day mortality was significantly lower in the combination group than in the monotherapy group (9.1% vs 53.8%, P = 0.020) for some patients with APACHE II scores of 25–29. The reason for this result is not clear but the following explanation is possible; With APACHE < 24, there does not appear to benefit because mortality is low regardless. On the other hand, patients may be “beyond saving” with APACHE >30 as mortality exceeds 50%. Patients with APACHE II scores of 25–29 may be moderately serious condition enough to gain maximal benefit from combination therapy. Additional studies with more cases are needed to evaluate the effectiveness of combination therapy according to disease severity.
Finally, physicians might choose to use combination therapy in patients with severe disease that may not be reflected by APACHE II only. For this reason, it is difficult to conduct a well-designed randomized study. A recent randomized trial compared meropenem plus colistin versus colistin monotherapy in carbapenem-resistant gram-negative bacterial infections.16 The study demonstrated that the addition of meropenem to colistin did not improve clinical failure in severe A. baumannii infections. However, the study did not measure drug concentrations and did not use MIC for treatment. The overall failure rate was 81% in A. baumannii; therefore, it is too soon to make a final decision that carbapenem combination therapy has no benefits.
In our study, 14-day mortality was influenced by factors including age, previous steroid use, and clinical improvement in two days. These factors may be indicators for which further analysis is warranted. Several observational studies showed uniformly lower mortality for combination therapy than for carbapenem-resistant or carbapenemase-producing K. pneumoniae compared with colistin monotherapy.11 Although initial studies based on cohorts as small as 41 patients with carbapenemase (KPC)-producing K. pneumoniae bacteraemia claimed the unreserved superiority of combination therapy,17 more recent and larger studies have highlighted specific patient subgroups that might benefit from combination therapy or from specific antibiotic combinations. The potential advantage of such studies is the analysis of a large cohort of uniform, rare infections (e.g., KPC-producing K. pneumoniae bacteremia) and the inclusion of all patients in need of treatment in clinical practice. However, more elaborated studies are needed.18
Moreover, may have to develop combination regimens containing drugs other than carbapenems. We widened the range of drugs from meropenem to all carbapenems; however, most of the patients were administered meropenem as a combination drug. It is also possible that carbapenems were not the right answer. Other studies have reported other drugs to be active against CRAB19,20 and several new drugs have been introduced in the real world with effects against MDR or XDR gram-negative bacteria.21,22 Whether combination therapy with newer antibiotic agents (such as ceftolozane-tazobactam or ceftazidime-avibactam) or other agents are useful options in this clinical context remains unknown and conclusions on the efficacy of combination therapy for these infections should not be drawn from the results of this trial before evaluation of newer drugs.
This study has several strengths. First. we narrowed down the study population to a specific pathogen and specific disease, CRAB pneumonia. Because previous studies assessed CRAB or A. baumannii infection regardless of site,11,16,23 many factors required consideration when determining the effect of combination therapy. While we cannot prove an overall mortality benefit, we narrowed down the disease and pathogen to eliminate possible biases and show 14-day mortality benefits in specific populations.
Second, we tried to exclude the effect of severity on mortality by subgroup analysis according to APACHE II score. Even though we failed to prove overall mortality benefits, we observed trends in physician use of carbapenem combination regimens in severe patients; moreover, after dividing the patients into subgroups by APACHE II score, combination therapy showed benefits on 14-day mortality in those scoring 25–29 points. This finding may inform further studies on specific populations and regimens that could benefit from combination antibiotics.
This study has several limitations. First, due to its retrospective design, there may have been bias during data collection. Given that this study constituted exploratory research without an exact statistical calculation of sample size, the study could be insufficiently powered to detect weaker but potentially clinically significant effects. However, previously mentioned, the fact that clinicians tended to choose combination therapy might be evidence that monotherapy was not sufficient.
Second, as the study was conducted at a single medical center, patient characteristics and the distribution of pathogens could differ according to local epidemiology, which might have negatively influenced the effect of combination therapy.
Third, we did not analyze the effect of antimicrobial dosing, and side effects such as changes in creatinine levels or collateral damages. Although we only counted cases administered colistin for more than 7 days, this factor may have also affected the mortality.
Because the overall mortality of Acinetobacter pneumonia is high,6 it is difficult to demonstrate the superiority of colistin plus carbapenem combination therapy over colistin monotherapy. Our study also failed to show overall mortality benefits; however, combination therapy showed benefits in a certain patient group. Additional studies are needed to improve clinical, microbiological cure and eventually mortality outcomes.
Conclusions
Colistin plus carbapenem combination therapy showed no significant difference in the treatment of CRAB pneumonia compared to colistin monotherapy. However, in specific populations (APACHE II scores of 25–29 points), combination therapy showed a significant 14-day mortality benefit over colistin monotherapy. Further prospective studies are needed to evaluate the effectiveness of combination therapy in CRAB pneumonia.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Fournier PE, Richet H. The epidemiology and control of Acinetobacter baumannii in health care facilities. Clin Inf Dis. 2006;42(5):692–699. doi: 10.1086/500202 [DOI] [PubMed] [Google Scholar]
- 2.Morfin-Otero R, Dowzicky MJ. Changes in MIC within a global collection of Acinetobacter baumannii collected as part of the tigecycline evaluation and surveillance trial, 2004 to 2009. Clin Ther. 2012;34(1):101–112. doi: 10.1016/j.clinthera.2011.11.028 [DOI] [PubMed] [Google Scholar]
- 3.Kim YA, Park YS, Youk T, Lee H, Lee K. Abrupt increase in rate of imipenem resistance in Acinetobacter baumannii complex strains isolated from General Hospitals in Korea and correlation with carbapenem administration during 2002–2013. Ann Lab Med. 2018;38(2):179–181. doi: 10.3343/alm.2018.38.2.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montero MM, Domene Ochoa S, Lopez Causape C, et al. Colistin plus meropenem combination is synergistic in vitro against extensively drug-resistant (XDR) Pseudomonas aeruginosa, including high-risk clones. J Global Antimicrob Resist. 2019. doi: 10.1016/j.jgar.2019.04.012 [DOI] [PubMed] [Google Scholar]
- 5.Paul M, Bishara J, Levcovich A, et al. Effectiveness and safety of colistin: prospective comparative cohort study. J Antimicrob Chemother. 2010;65(5):1019–1027. doi: 10.1093/jac/dkq069 [DOI] [PubMed] [Google Scholar]
- 6.Nasir N, Mahmood SF. Mortality in patients with respiratory and nonrespiratory carbapenem resistant-multidrug resistant acinetobacter infections. J Ayub Med Coll Abbottabad. 2017;29(3):511–513. [PubMed] [Google Scholar]
- 7.Archer A, Campbell A, D’Amato C, McLeod M, Rugg D. Putting the ICD-10-CM/PCS GEMs into practice (updated). J AHIMA. 2016;87(1):48–53. [PubMed] [Google Scholar]
- 8.Clinical and Laboratory Standards Institute. CLSI supplement M100.Performance standards for antimicrobial susceptibility testing.28th ed. Wayne, PA:CLSI;2018 [Google Scholar]
- 9.Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis. 2018;18(3):318–327. doi: 10.1016/S1473-3099(17)30753-3 [DOI] [PubMed] [Google Scholar]
- 10.Dhand A, Bayer AS, Pogliano J, et al. Use of antistaphylococcal beta-lactams to increase daptomycin activity in eradicating persistent bacteremia due to methicillin-resistant Staphylococcus aureus: role of enhanced daptomycin binding. Clin Inf Dis. 2011;53(2):158–163. doi: 10.1093/cid/cir340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zusman O, Altunin S, Koppel F, Dishon Benattar Y, Gedik H, Paul M. Polymyxin monotherapy or in combination against carbapenem-resistant bacteria: systematic review and meta-analysis. J Antimicrob Chemother. 2017;72(1):29–39. doi: 10.1093/jac/dkw377 [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Zhao M, Chen Y, et al. Synergistic killing by meropenem and colistin combination of carbapenem-resistant Acinetobacter baumannii isolates from Chinese patients in an in vitro pharmacokinetic/pharmacodynamic model. Int J Antimicrob Agents. 2016;48(5):559–563. doi: 10.1016/j.ijantimicag.2016.07.018 [DOI] [PubMed] [Google Scholar]
- 13.Gutierrez-Gutierrez B, Salamanca E, de Cueto M, et al. Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect Dis. 2017;17(7):726–734. doi: 10.1016/S1473-3099(17)30228-1 [DOI] [PubMed] [Google Scholar]
- 14.Kengkla K, Kongpakwattana K, Saokaew S, Apisarnthanarak A, Chaiyakunapruk N. Comparative efficacy and safety of treatment options for MDR and XDR Acinetobacter baumannii infections: a systematic review and network meta-analysis. J Antimicrob Chemother. 2018;73(1):22–32. doi: 10.1093/jac/dkx368 [DOI] [PubMed] [Google Scholar]
- 15.Durante-Mangoni E, Utili R, Zarrilli R. Combination therapy in severe Acinetobacter baumannii infections: an update on the evidence to date. Future Microbiol. 2014;9(6):773–789. doi: 10.2217/fmb.14.34 [DOI] [PubMed] [Google Scholar]
- 16.Paul M, Daikos GL, Durante-Mangoni E, et al. Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant Gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis. 2018;18(4):391–400. doi: 10.1016/S1473-3099(18)30099-9 [DOI] [PubMed] [Google Scholar]
- 17.Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother. 2012;56(4):2108–2113. doi: 10.1128/AAC.06268-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giacobbe DR, Maraolo AE, Viscoli C. Pitfalls of defining combination therapy for carbapenem-resistant Enterobacteriaceae in observational studies. Eur j Clin Microb Infect Dis. 2017;36(10):1707–1709. doi: 10.1007/s10096-017-3010-z [DOI] [PubMed] [Google Scholar]
- 19.Fragkou PC, Poulakou G, Blizou A, et al. The role of minocycline in the treatment of nosocomial infections caused by multidrug, extensively drug and pandrug resistant acinetobacter baumannii: a systematic review of clinical evidence. Microorganisms. 2019;7:6. doi: 10.3390/microorganisms7060159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park HJ, Cho JH, Kim HJ, Han SH, Jeong SH, Byun MK. Colistin monotherapy versus colistin/rifampicin combination therapy in pneumonia caused by colistin-resistant Acinetobacter baumannii: a randomised controlled trial. J Global Antimicrob Resist. 2018;17:66–71. doi: 10.1016/j.jgar.2018.11.016 [DOI] [PubMed] [Google Scholar]
- 21.van Duin D, Lok JJ, Earley M, et al. Colistin versus Ceftazidime-Avibactam in the treatment of infections due to carbapenem-resistant enterobacteriaceae. Clin Inf Dis. 2018;66(2):163–171. doi: 10.1093/cid/cix783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodlet KJ, Nicolau DP, Nailor MD. In vitro comparison of ceftolozane-tazobactam to traditional beta-lactams and ceftolozane-tazobactam as an alternative to combination antimicrobial therapy for pseudomonas aeruginosa. Antimicrob Agents Chemother. 2017;61:12. doi: 10.1128/AAC.01350-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vardakas KZ, Mavroudis AD, Georgiou M, Falagas ME. Intravenous colistin combination antimicrobial treatment vs. monotherapy: a systematic review and meta-analysis. Int j Antimicrob Agents. 2018;51(4):535–547. doi: 10.1016/j.ijantimicag.2017.12.020 [DOI] [PubMed] [Google Scholar]