Abstract
Introduction
The objective of this study was to assess the clinical significance of determining the levels of matrix metalloproteinase-2 (MMP-2), MMP-9, tissue inhibitor of matrix metalloproteinase-1 (TIMP-1), and TIMP-2 in the peripheral blood of patients with differentiated thyroid carcinoma (DTC).
Methods
Forty-nine patients with benign thyroid lesions and 57 patients with DTC were examined using the enzyme-linked immunosorbent assay method preoperatively and 1 month after operation.
Results
The levels of MMP-2, MMP-9, TIMP-1, and TIMP-2 in the peripheral blood of patients with DTC were significantly higher than those measured in patients with benign thyroid disease (P<0.05). After surgery, these levels in the peripheral blood of patients with benign thyroid lesions were not significantly changed (P>0.05). However, after operation, these levels in the peripheral blood of patients with DTC were significantly lower (P<0.05). These levels in the serum of patients with DTC which were tumor-node-metastasis stage, tumor diameter ≥l cm, infiltrating capsula outside or existing lymph metastasis were significantly higher than those reported in patients with early tumor-node-metastasis stage, tumor diameter <l cm or absence of lymph metastasis (P<0.05).
Discussion
Detecting the levels of these factors in peripheral blood is helpful in the diagnosis of benign and malignant thyroid lesions, and can be used as a basis for the prognosis of DTC.
Keywords: differentiated thyroid carcinoma, matrix metalloproteinase-2, matrix metalloproteinase-9, tissue inhibitor of matrix metalloproteinase-1
Introduction
Thyroid tumor is a type of tumor with the fastest growing incidence rate in the previous 20 years. Particularly, thyroid carcinoma has an annual growth rate of 4–6.2%,1,2 and is pathologically classified as papillary carcinoma, follicular carcinoma, medullary carcinoma, and undifferentiated carcinoma. Papillary and follicular carcinomas are also termed differentiated thyroid carcinoma (DTC), accounting for approximately 90% of all thyroid carcinomas.3 Studies show that the extracellular matrix (ECM) is the main barrier to tumor metastasis. When the tumor breaks through the basement membrane and enters ECM, it is the beginning of tumor metastasis and invasion. Similar to other carcinomas, DTC is mainly featured by biological effects, such as the destruction of the ECM and basement membranes. Matrix metalloproteinases (MMPs) play the most important role in this process,4 MMPs can degrade almost all extracellular matrix except polysaccharides. Tissue inhibitors of metalloproteinases (TIMPs) are specific inhibitors of MMPs, which hamper tumor development.5 In recent years, MMPs and TIMPs have been the focus of tumor research. In this experiment, we detected the levels of MMP-2, MMP-9, TIMP-1, and TIMP-2 in the peripheral blood of patients with DTC and benign thyroid tumors using the enzyme-linked immunosorbent assay (ELISA) method. The aim was to explore their clinical significance in peripheral blood during the treatment of DTC.
Materials and Methods
Patients
We selected patients with thyroid adenoma who visited the Department of General Surgery at Tianjin First Central Hospital (Tianjin, China) for treatment and surgery. The exclusion criteria were hypertension, coronary heart disease, cerebrovascular event, diabetes, tuberculosis, and other systemic tumors. There were 49 patients with benign thyroid lesions (27 thyroid tumor cases and 22 nodular goiter cases). Of those, 11 and 38 patients were males and females, respectively. The median age of patients in this group was 37 years (range: 21–65 years). There were 57 patients with DTC (52 papillary thyroid cancer cases and 5 follicular carcinoma cases). Of those, 18 and 39 patients were males and females, respectively. The median age of patients in this group was 43 years (range: 27–69 years). According to the Union for International Cancer Control staging standard for thyroid carcinoma, there were 17, 34, and 6 cases of stage I, II, and III disease, respectively. In addition, 20 healthy volunteers were included as a control group.
Levels of MMPs and TIMPs Detected Through ELISA
ELISA was used to detect the levels of MMP-2, MMP-9, TIMP-1, and TIMP-2 in the serum of patients. Peripheral blood (2 mL) was extracted from all fasting patients on the day of surgery (in the morning) and 1 month after surgery (in the morning). The specimens were centrifuged, collected, and stored in the refrigerator. The expression of MMP-1, MMP-9, TIMP-1, and TIMP-2 in the serum was detected using the double sandwich ELISA method according to the instructions provided by the manufacturer. The testing kit was purchased from Boster Biological Technology Co. Ltd.
RNA Extraction and cDNA Synthesis
The peripheral blood total RNA extraction kit and Quant cDNA first-strand synthesis kit were purchased from Beijing Tiangen Biochemical Technology Co. Ltd. Cells were lysed by using the guanidine/thiocyanate method; the RNA was isolated and purified via silicon matrix adsorption. Subsequently, the RNA integrity was determined through agar-agar gel electrophoresis. The yield and purity were detected using an ultraviolet spectrophotometer. The first strand of cDNA was synthesized using Oligo-dT15 as primer.
Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
The primers for the MMPs, TIMPs, and the reference protein β-actin were synthesized by Nanjing Kingsley Company (Table 1). After 1% agarose gel electrophoresis, the PCR products were photographed under ultraviolet light. The results were analyzed using the Kodak DC 290 gel imaging system. The results were expressed as the ratio of optical density (OD) (OD ratio of objective gene=OD MMPs(TIMPs)/ODβ-actin).
Table 1.
The Primers for MMPs, TIMPs, and β-Actin
| Sense Strand | Antisense Strand | Product (bp) | |
|---|---|---|---|
| MMP-2 | 5ʹ-TGGCACCCATTTACACCTAC-3’ | 5ʹ-CCTCGTATACCGCATCAATC-3’ | 315 |
| MMP-9 | 5ʹ-CCTTCTACGGCCACTACTGT-3’ | 5ʹ-TCCACCTGGTTCAACTCACT-3’ | 575 |
| TIMP-1 | 5ʹ-ACTTCCACAGGTCCCACAAC-3’ | 5ʹ-AGAAAGATGGGAGTGGGAAC-3’ | 405 |
| TIMP-2 | 5ʹ-CAGAGAAGAACATCAACGGG-3’ | 5ʹ-CGAAGCCCCAGACACATAGA-3’ | 369 |
| β-actin | 5ʹ-GGGACGACATGGAGAAAATC-3’ | 5ʹ-GATCTGGGTCATCTTCTCGC-3’ | 131 |
Statistical Analyses
All data are expressed as the mean ± standard deviation. Comparison among the treatment groups was performed using one-way analysis of variance followed by post hoc Student–Newman–Keuls tests. The SPSS 17.0 software was used for the analyses (SPSS Inc., Chicago, IL, USA). A P<0.05 denoted statistical significance.
Results
Levels of MMPs and TIMPs in Peripheral Blood
The levels of MMP-2, MMP-9, TIMP-1, and TIMP-2 in patients with benign thyroid disease were not significantly different compared with those measured in the control group (P>0.05). Prior to surgery, these levels in the peripheral blood of 57 patients with DTC were significantly higher than those recorded in patients with benign thyroid disease (P<0.05). After surgery, there were no obvious changes in these levels in the peripheral blood of patients with benign thyroid lesions (P>0.05). However, as shown in Table 2, these levels in the peripheral blood of patients with DTC were significantly lower than those noted preoperatively (P<0.05).
Table 2.
MMPs and TIMPs in the Peripheral Blood of Patients with DTC and Benign Thyroid Tumors )
)
| MMP-2 (ng/mL) | MMP-9 (ng/mL) | TIMP-1 (ng/mL) | TIMP-2 (ng/mL) | ||
|---|---|---|---|---|---|
| Benign thyroid tumors (n=49) | Preoperative | 38.25±15.20c | 47.60±20.10c | 53.71±32.49c | 49.81±17.26c |
| Postoperative | 35.20±17.70d | 43.27±10.27d | 55.15±13.74d | 50.78±14.23d | |
| DTC (n=57) | Preoperative | 100.24±27.32a | 134.70±32.52a | 117.78±26.53a | 89.43±18.05a |
| Postoperative | 42.71±16.20b | 51.46±18.34b | 48.43±13.02b | 52.36±12.27b | |
| Healthy individuals (n=20) | 30.25±11.70 | 40.52±10.20 | 47.10±12.30 | 46.74±12.53 | |
Notes: aCompared with healthy individuals and benign thyroid tumors, P<0.05. bCompared with DTC patients prior to surgery, P<0.05. cCompared with healthy individuals, P>0.05. dCompared with benign thyroid tumor patients prior to surgery, P>0.05.
The Results of RT-PCR
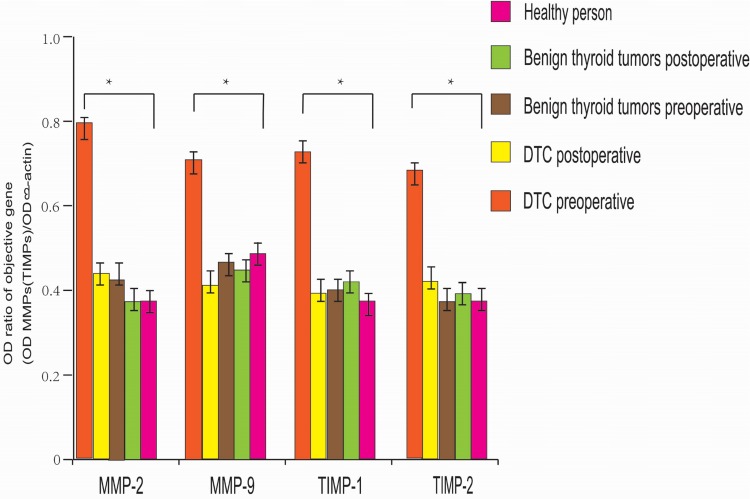
The expression levels of MMP-2, MMP-9, TIMP-1, and TIMP-2 in patients with benign thyroid disease were not significantly different compared with those detected in the control group (P>0.05). Notably, there was no significant difference between the preoperative and postoperative levels (P>0.05). However, the expression levels of MMP-2, MMP-9, TIMP-1, and TIMP-2 in the peripheral blood of patients with DTC were significantly higher than those observed in patients with benign thyroid lesions (P<0.05); and the preoperative expression levels of MMP-2, MMP-9, TIMP-1, and TIMP-2 were significantly higher than the postoperative levels (P<0.05) (Figures 1 and 2).
Figure 1.
The expression levels of MMPs and TIMPs in patients with benign thyroid tumors were not significantly different from those in healthy persons, and there was no significant change after surgery (P>0.05). The expression levels of TIMPs and MMPs in DTC patients before surgery were significantly higher than those in benign thyroid tumor patients and healthy people. The expression levels of TIMPs and MMPs after surgery were significantly lower (*P<0.05).
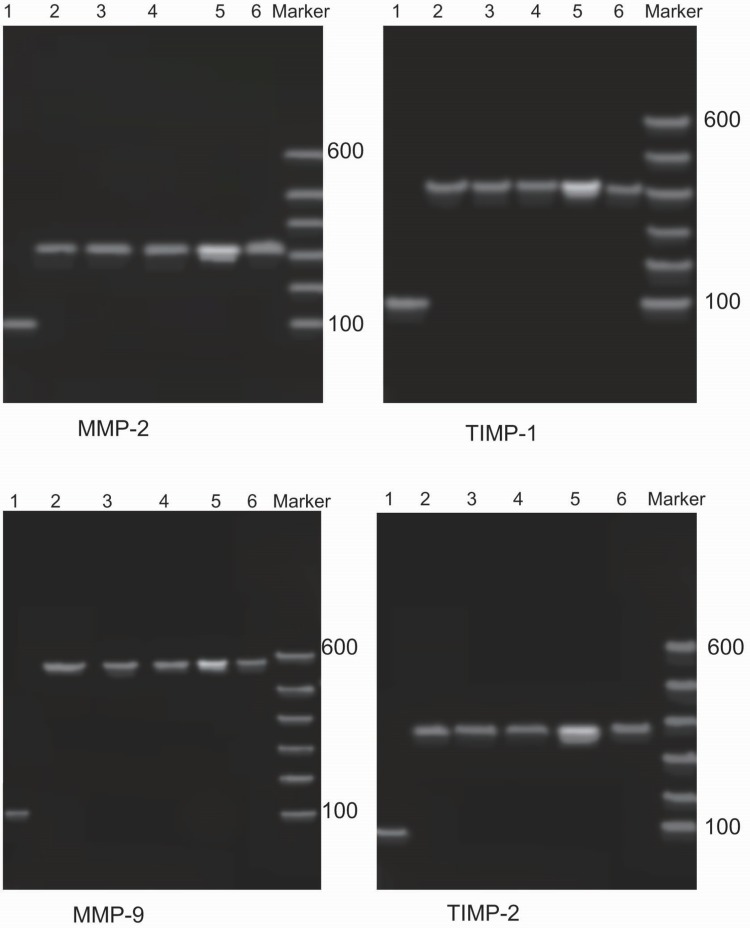
Figure 2.
The PCR results for MMPs and TIMPs. Agarose gel electrophoresis showing that the lengths of the mRNA fragments of MMP-2, MMP-9, TIMP-1, and TIMP-2 were 320 bp, 582 bp, 405 bp, and 372 bp, respectively. The PCR bands of MMPs and TIMPs in patients with DTC were the brightest; after surgery, the brightness of these bands was significantly reduced. Moreover, there were no significant differences compared with the control group and the benign thyroid tumor group. 1: β-actin; 2: healthy individual; 3: benign thyroid tumor (prior to surgery); 4: benign thyroid tumor (after surgery); 5: DTC (prior to surgery); 6: DTC (after surgery).
Relationship of Clinicopathological Features of DTC Patients with the Levels of MMPs and TIMPs in Peripheral Blood
The levels of MMP-2, MMP-9, TIMP-1, and TIMP-2 in the peripheral blood of DTC patients with advanced tumor-node-metastasis stage, tumor diameter ≥l cm, tumor invasion in the surrounding tissue, or lymph node metastasis were significantly higher those measured in patients with early tumor-node-metastasis stage patients, tumor diameter <l cm, lymph node metastasis, and presence of the tumor limited in the thyroid (Table 3).
Table 3.
Clinicopathological Features of DTC and the Levels of MMPs and TIMPs in Peripheral Blood )
)
| Pathological Characteristics | n | MMP-2 | MMP-9 | TIMP-1 | TIMP-2 | |
|---|---|---|---|---|---|---|
| (ng/mL) | (ng/mL) | (ng/mL) | (ng/mL) | |||
| TNM | Phase I | 17 | 80.14±17.30 | 108.43±21.30 | 78.44±20.37 | 62.23±21.52 |
| Phase II, III | 40 | 140.57±32.30a | 157.20±24.58a | 130.27±28.43a | 102.17±23.38a | |
| Tumor size | <1.0 cm | 10 | 76.54±20.32 | 90.28±26.30 | 86.27±20.30 | 60.49±21.30 |
| ≥1.0 cm | 47 | 143.27±27.54b | 148.24±31.46b | 140.42±28.19b | 112.20±29.58b | |
| Extent of infiltration | Limited to the thyroid | 41 | 75.24±25.37 | 101.27±25.32 | 102.63±20.49 | 58.47±23.57 |
| Outside the thyroid | 16 | 147.19±26.33c | 156.36±17.54c | 154.24±26.50c | 119.43±19.35c | |
| Lymph metastasis | No | 37 | 81.46±20.39 | 91.20±21.52 | 100.20±20.73 | 59.71±16.33 |
| Yes | 20 | 152.28±24.62d | 150.98±26.74d | 152.23±21.27d | 120.20±22.36d | |
Notes: aCompared with Phase I, P<0.05. bCompared with patients with tumor size <1.0 cm, P<0.05. cCompared with patients with tumor limit in thyroid, P<0.05. dCompared with patients without lymph metastasis, P<0.05.
Discussion
The ECM is the main barrier to tumor metastasis. Breaking through the surrounding BM and entering the ECM is the first step of tumor metastasis and invasion. As a group of major proteases secreted by connective tissues and involved in ECM degradation, MMPs play an important role in physiological and pathological processes, such as embryonic development, cell migration, angiogenesis, wound healing, atherosclerosis, invasion, and metastasis of malignant tumors.6–8 Among the five documented categories of MMPs, gelatinases (including MMP-2 and MMP-9) can specifically degrade type IV collagen (the main component of the ECM and BM). Moreover, they are considered the most direct and important MMPs in the process of tumor invasion and metastasis.9 Among the MMPs, MMP-9 is the enzyme that is the largest molecular, secreted in the form of zymogen. It is subsequently activated to form type IV collagenase for the degradation and destruction of the ECM and BM on the tumor surface. This process allows cancer cells to infiltrate10 surrounding tissues through the destroyed BM. MMP-2 can degrade type I collagen, activate MMP-9 and MMP-13, and degrade other components of the ECM.11 Numerous studies12–18 have shown that the expression of MMP-2 and MMP-9 is significantly increased in several types of cancer (breast, liver, gastric, colorectal, lung, endometrial, bladder, etc.). This expression is related to tumor cell metastasis and pathological grading. A study conducted by Liu et al found that the expression levels of MMP-9 in thyroid cancer tissue was significantly higher than those measured in normal and tumor thyroid tissues. Moreover, they stated that the expression levels of MMP-9 were significantly correlated with American Joint Committee on Cancer stage, tissue differentiation, neck lymph node metastasis, and capsular invasion of thyroid carcinoma.19 Zhang and Liu found that the expression of MMP-9 in tumor tissues of high-risk thyroid carcinoma patients was positively related with the levels of MMP-9 in peripheral blood. In addition, they concluded that determining the concentration of MMP-9 in peripheral blood can assist in detecting cancer cell invasion, metastasis, and the presence of small invasion and metastasis.20 For patients with high MMP-9 expression, close follow-up should be conducted to detect recurrence or metastasis and administer comprehensive treatments, aiming to improve the prognosis. Pasieka et al observed that the levels of MMP-2 and TIMP-2 in peripheral blood are correlated with DTC.21 In the present study, the levels of MMP-2 and MMP-9 in the peripheral blood of patients with DTC were significantly higher than those measured in patients with benign thyroid tumors. Moreover, these levels in the peripheral blood of patients with DTC were significantly decreased 1 month after operation. Hence, we speculated that MMP-2 and MMP-9 can help judge the therapeutic effect and determine the prognosis.
TIMP is a secretory protein and an inhibitor of MMP. It is expressed in both tumor and interstitial tissues.22 Structurally, TIMPs are divided into two functional regions. The cysteine residue in the N-terminal functional region is combined with the Zn2+ active center of MMPs, while its C-terminal functional area forms bonds with other parts of MMPs at a proportion of 1:1 to form the MMP-TIMP complex. Thus, the combination of MMP and substrate can be blocked to inhibit tumor invasion and metastasis. TIMPs can be divided into four types (i.e., TIMP-1, TIMP-2, TIMP-3, and TIMP-4) according to their different targets. The regulation mechanism of TIMPs on MMPs is mainly embodied in the activation phase of zymogen and activated MMPs phase.23 However, TIMPs themselves also play diverse roles in various processes of tumor development, such as cell growth, proliferation, apoptosis, angiogenesis, etc.24 Studies have found that TIMP-1 and TIMP-2 could stimulate cell proliferation and inhibit apoptosis,23,25 while TIMP-3 promotes apoptosis.26 TIMP-2 prevents self-activation of MMP-2 mainly by forming stable complexes with the MMP-2 precursor, or directly inhibits the catalytic activity of MMP-2 by combining with it.27 TIMP-1 is a specific inhibitor of MMP-9. Previous studies28 found that TIMP-1 expression could be detected in fibroblasts of DTC and thyroid adenoma, and it is closely related with tumor size, lymph node metastasis, clinical stage, and vascular invasion, while promoting recurrence. A number of scholars think that the dynamic balance between TIMP-2 and MMP-2 affects tumor invasion and metastasis, and the ratio imbalance of the two is more clinically important than a single-factor abnormality.29 Our experiments showed that the preoperative levels of TIMP-1 and TIMP-2 in patients with DTC were significantly elevated. It was considered that this elevation was induced by significant increases in the levels of MMP-2 and MMP-9. However, these increased levels of TIMP-1 and TIMP-2 were lower than those of MMP-2 and MMP-9. Our results also showed that both MMPs and TIMPs were overexpressed in peripheral blood of DTC patients, and the expression of MMPs were higher than that of TIMPs, which may promote the development of tumor because the balance between the two was broken. The interaction between TIMPs and MMPs promotes the occurrence and metastasis of DTC. The levels of MMPs in high-risk DTC patients were significantly increased compared with those of TIMPs, the balance between MMPs and TIMPs in the body was impaired, and the amount of TIMPs relative to that of MMPs decreased. These effects may be led to a weakened inhibitory capability of MMPs. Meanwhile, the enhanced activity of MMPs accelerates degradation of the ECM, promotes the spread of tumor cells beyond the BM, and may cause blood or lymphatic metastasis. The detection of MMPs and TIMPs in peripheral blood is helpful in the diagnosis of benign and malignant thyroid tumors. It assists in assessing the metastatic status of DTC and provides a reference for the development of an individualized treatment plan. The detection of MMP-2, MMP-9, TIMP-1, and TIMP-2 in peripheral blood after operation can timely detect disease recurrence in patients.
Acknowledgment
The abstract of this paper was presented at the 2018 International Conference on Biotechnology and Bioengineering as a poster presentation/conference talk with interim findings. The poster’s abstract was published in “Poster Abstracts” in Volume 124, Issue S1 in Basic & Clinical Pharmacology & Toxicology.
Informed Consent
We have obtained written informed consent from all study participants for their data to be used in the study. In the attachment, we submitted a template of patient informed consent.
Ethics Statement
The study protocol was approved by the ethics review board of the Tianjin First Central Hospital ethics committee. We confirmed that the study was carried out in accordance with the principles of the Declaration of Helsinki.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Nix P, Nicolaides A, Coatesworth AP. Thyroid cancer review: presentation and investigation of thyroid cancer. Int J Clin Pract. 2005;59:1459–1463. doi: 10.1111/j.1368-5031.2005.00672.x [DOI] [PubMed] [Google Scholar]
- 2.Shanghai Municipal Center for Disease Control & Prevention. The incidence of malignant tumor in Shanghai in 2006. Tumor. 2010;29:918. [Google Scholar]
- 3.Davies L, Welch HG. Increasing of thyroid cancer in The United States, 1973–2002. JAMA. 2006;295:2164–2167. doi: 10.1001/jama.295.18.2164 [DOI] [PubMed] [Google Scholar]
- 4.Perigny M, Bairati I, Harvey I. Role of immunohistochemical overexpression of matrix metalloproteinases MMP-2 and MMP-1 1 in the prognosis of death by ovarian cancer. Am J Clin Pathol. 2008;129:226–231. doi: 10.1309/49LA9XCBGWJ8F2KM [DOI] [PubMed] [Google Scholar]
- 5.Velinov N, Poptodorov G, Gabrovski N. The role of matrixmetalloproteinases in the tumor growth and metastasis. Khirurgiia (Sofiia). 2010;1:44–49. [PubMed] [Google Scholar]
- 6.Murphy G, Nagase H. Progress in matrix metalloproteinase research. Mol Aspects Med. 2008;29(5):290–308. doi: 10.1016/j.mam.2008.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vizoso FJ, Gonzalez LO, Corte MD, et al. Study of matrix metalloproteinases and their inhibitors in breast cancer. Br J Cancer. 2007;96:903. doi: 10.1038/sj.bjc.6603666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hojila CV, Wood GA, Khoka R. Metalloproteinases as common effectors of inflammation and extracellular matrix breakdown in breast cancer. Breast Cancer Res. 2008;10:205. doi: 10.1186/bcr1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zeng ZS, Cohen AM, Guillem JG. Loss of basement membrane type IV collagen is associated with increased expression of metalloproteinases 2 and 9 (MMP-2 and MMP-9) during human colorectal tumorigenesis. Carcinogenesis. 1999;20(5):749. doi: 10.1093/carcin/20.5.749 [DOI] [PubMed] [Google Scholar]
- 10.Patel A, Straight AM, Mann H, et al. Matrix metalloproteinase (MMPs) expression by differentiated thyroid carcinoma of children and adolescents. J Endocrinol Invest. 2002;25:403–408. doi: 10.1007/BF03344028 [DOI] [PubMed] [Google Scholar]
- 11.Ahmed M, Uddin S, Hussain AR, et al. FoxM1 and its association with matrix metalloproteinases (MMP) signaling pathway in papillary thyroid carcinoma. J Clin Endocrinol Metab. 2012;97(1):1503–1516. doi: 10.1210/jc.2011-1506 [DOI] [PubMed] [Google Scholar]
- 12.Zhang B, Li H, Yang YS, et al. Advances in the relationship between matrix metalloproteinases and their inhibitors and breast cancer. Chin J Lab Diagn. 2013;17(18):1534–1536. [Google Scholar]
- 13.Zhou XF, Miao L, Mao WZ. Correlations between peripheral blood levels of matrix metalloproteinases and its inhibitors and thyroid carcinoma. Chin J Cancer Prev Treat. 2013;20(12):942–944. [Google Scholar]
- 14.Anand M, Van Meter TE, Fillmore HL. Epidermal growth factor induces matrix metalloproteinase-1 (MMP-1) expression and invasion in glioma cell lines via the MAPK pathway. J Neurooncol. 2011;104(3):679–687. doi: 10.1007/s11060-011-0549-x [DOI] [PubMed] [Google Scholar]
- 15.Li J, Ma JM. Research progress in matrix metalloproteinase-2,9 in gastric cancer. Chin Cancer. 2015;24(5):403–407. [Google Scholar]
- 16.Miao JT, Wang SY, Wang Y, et al. Positive expression rates of HMGB1 and MMP-9 in endometrial carcinoma and correlation analysis. Maternal Child Health Care China. 2017;32(13):3036–3039. [Google Scholar]
- 17.Jumper C, Cobos E, Lox C. Determination of the serum matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) in patients with either advanced small-cell lung cancer or non-small-cell lung cancer prior to treatment. Respir Med. 2004;98(2):173–177. doi: 10.1016/j.rmed.2003.08.014 [DOI] [PubMed] [Google Scholar]
- 18.Zhao JX, Yang LP, Wang YF, et al. Gelatinolytic activity of matrix metalloproteinase-2 and matrix metalloproteinase-9 in rat brain after implantation of 9L rat glioma cells. Eur J Neurol. 2007;14(5):510–517. doi: 10.1111/ene.2007.14.issue-5 [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Wu XK, Huang X, et al. Expression and role of matrix metalloproteinase-9 and osteopontin in thyroid carcinoma and thyroid benign tumor. Chin J Exp Surg. 2015;32(1):151–153. [Google Scholar]
- 20.Zhang JJ, Liu ZM. Expression of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in thyroid carcinoma tissue and their concentrations in serum. Chin J Bases Clin General Surg. 2007;14(4):437–441. [Google Scholar]
- 21.Pasieka Z, Stepien H, Czyz W, et al. Concentration of metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 in the serum of patients with benign and malignant thyroid tumors treated surgically. Endocr Regul. 2004;38(2):112–116. [PubMed] [Google Scholar]
- 22.Visse R, Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: structure, function, and biochemistry. Circ Res. 2003;92(8):827–839. doi: 10.1161/01.RES.0000070112.80711.3D [DOI] [PubMed] [Google Scholar]
- 23.Lanmbert E, Dasse E, Haye B, et al. TIMPs as multifacial proteins. Crit Rev Oncol Hematal. 2004;49(3):187–198. doi: 10.1016/j.critrevonc.2003.09.008 [DOI] [PubMed] [Google Scholar]
- 24.Delektorskaia W, Smirnova EA, Ponomareva MV, et al. Expression of matrix metalloproteinases 2 and 9 and their tissue inhibitors 1 and 2 in papillary thyroid cancer: an association with the clinical, morphological and ultrastructural characteristics of a tumor. Arkh Patol. 2010;72(4):3–6. [PubMed] [Google Scholar]
- 25.Islekel H, Oktay G, Terzi C, et al. Matrix metalloproteinase-9,-3 and tissue inhibitor of matrix metalloproteinase-1 in colorectal cancer: relationship to clinicopathological variables. Cell Biochem Funct. 2007;27(4):433–441. doi: 10.1002/cbf.1325 [DOI] [PubMed] [Google Scholar]
- 26.Cho Mar K, Eimoto T, Tateyama H, et al. Expression of matrix metalloproteinases in benign and malignant follicular thyroid lesions. Histopathology. 2006;48(3):286–294. doi: 10.1111/j.1365-2559.2005.02325.x [DOI] [PubMed] [Google Scholar]
- 27.Rapti M, Knauper V, Murphy G, et al. Characterization of the AB loop region of TIMP-2 Involvement in pro-MMP-2 activation[J]. J Biol Chem. 2006;281(33):23386–23394. doi: 10.1074/jbc.M604423200. [DOI] [PubMed] [Google Scholar]
- 28.Cooper DS, Doherty GM, Haugen BR, et al. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2006;16:1–33. doi: 10.1089/thy.2006.16.109 [DOI] [PubMed] [Google Scholar]
- 29.Waveren CV, Sun YB, Cheung HS, et al. Oxidative phosphorylation dysfunction modulates expression of extracellular matrix—remodeling genes and invasion. Carcinogenesis. 2006;27(3):409–418. doi: 10.1093/carcin/bgi242 [DOI] [PMC free article] [PubMed] [Google Scholar]