Abstract
Background
Vaccination remains the mainstay of prevention of hepatitis B virus (HBV) including birth dose and hepatitis B immunoglobulins (HBIGs). National estimates of vaccination coverage exclude migrants. The objective of this study is to investigate documentation practices of HBV-related infant vaccinations in Northern Thailand including migrants.
Methods
This is a retrospective review of hospital records of women who birthed infants in 2015 at Maharaj Nakorn Hospital, Chiang Mai (CM) or on the Thailand-Myanmar border, Tak.
Results
Of 2522 women, 987 were from CM (861 Thai nationals, 126 migrants) and 1535 were from Tak (651 Thai residence and 884 Myanmar residence). In CM, documentation for the birth dose vaccine (999 of 999, 100%) and HBIG was complete. In Tak, documentation was 1441 of 1549 (93%) for birth dose and 26 of 34 (76.5%) for HBIG, with missed opportunities including home delivery, delay in obtaining hepatitis B e-antigen status, and limitations of the records. Expanded Program of Immunization (EPI) documentation of 3 follow-up vaccinations dwindled with subsequent doses and distance, and complete documentation of 3 HBV EPI vaccines at the hospital of birth was low, 41.5% (1056 of 2547), but equitable for Thai or migrant status.
Conclusions
This review provides strong support for excellent documentation of HBIG and birth dose vaccination in urban and rural settings, and in migrants, consistent with Thailand’s vaccination policy and practice. Documentation of the 3 HBV EPI at the hospital of birth decreases with sequential doses, especially in families further away. Innovative data linkage is required to prove coverage and identify gaps.
Keywords: childhood, coverage, EPI, hepatitis B, immunization
Hepatitis B Immunoglobulin and birth dose vaccination was almost 100% and equal in Thais and migrants. Documentation of EPI vaccination at the hospital of birth declined with sequential doses. Birth hospital documentation for complete EPI hepatitis B vaccination was available for 41.4% infants. Confirmation of EPI completion requires innovations to link various sources of vaccination documentation.
Hepatitis B virus (HBV) is highly endemic in Southeast Asia (SEA), and mother-to-child transmission (MTCT) is the most common route of infection [1]. Vaccination is the main preventive strategy, and the birth dose of HBV vaccination within the first 24 hours of life is the most important, with additional protection obtained from hepatitis B immunoglobulins (HBIGs) within 72 hours [2]. This immunoprophylaxis is followed by 3 HBV vaccines according to the Expanded Program of Immunization (EPI) schedule. The combination of HBIG with the 3 follow-up vaccines reduces hepatitis B occurrence more effectively compared with only vaccination in infants born to hepatitis B surface antigen (HBsAg)-positive mothers (relative risk = 0.54; 95% confidence interval [CI], 0.41–0.73) [3]. However, even with optimal preventive strategies—birth dose vaccination, HBIG after birth in hepatitis B e-antigen (HBeAg)-positive mothers, in addition to the 3 EPI vaccinations—MTCT occurs in an estimated 8%–32% of cases [1, 4].
The recommended HBV birth dose vaccination was introduced in 2015 but was estimated to be missed in 66% of infants born in that year in SEA, especially in areas with home deliveries [5, 6]. Even when hospital birth can be encouraged, uptake may be inhibited by out-of-pocket expenditure for HBIG. The cost of HBIGs is equal to a month’s salary for the average family in some settings [7]. Refugees and migrants face barriers to proper access to vaccination due to social, economic, and political circumstances, leading to low levels of vaccine uptake, high HBV prevalence, and poorer health outcomes [8–10]. The reported prevalence of HBsAg positivity among pregnant women in Northern Thailand was 6.2% in 2014 [11], and on the Thailand-Myanmar border it 6.2%–8.3% between 2012 and 2016 [11–13].
Vaccination coverage, defined as the proportion of infants that receive a vaccine in relation to the overall infant population, can be estimated from registries, routine administrative reports, or household surveys. Vaccination coverage is difficult to document in refugees and migrants who have been excluded from the World Health Organization (WHO) estimates [5]. In rural areas, the documentation is either not kept or not accessible for analyses [14, 15]. Because the population denominator can be inaccurate in rural areas, more reliable data can be acquired from large-scale, multipurpose surveys [16].
The reported HBV vaccination coverage for Thailand is approximately 98% [17]. In Myanmar, HBV vaccination was introduced in 2003 with HBV birth dose supported by the Vaccine Alliance (Gavi), but reported coverage varies between the 14 states of the country. In Eastern Myanmar, the estimated vaccination coverage of the 3 EPI HBV vaccinations was reported as <70% in the Shan State and 80%–89% in Kayin State in 2014 [18]. In a survey of vaccination cards and mother recall, the uptake of HBV vaccinations differed greatly between urban and rural Myanmar with 75.2% coverage of the 3 EPI doses in urban versus 57.8% in rural areas [19]. Coverage surveys are at risk for selection and information bias, especially when conducted in marginalized populations.
The objective of this investigation is to verify individual documentation of HBIG, birth dose, and the 3 hepatitis vaccination doses in the EPI in Thai nationals and migrants in urban and rural areas of Northern Thailand.
METHODS
Study Design
This is a retrospective review of hospital records of women and their infants born in 2015 at Maharaj Nakorn Hospital, Chiang Mai (CM), or at Shoklo Malaria Research Unit (SMRU) on the Thailand border with Myanmar, Tak.
Setting
The Faculty of Medicine of Chiang Mai University (CMU) was the first medical school to be established outside of Bangkok, the capital of Thailand, in 1965. The women who come to antenatal care (ANC) and give birth at Maharaj Nakorn Hospital are usually people living in CM. It is recognized as the best regional center for birth and the main tertiary referral center in Northern Thailand.
The SMRU was established in 1986 and has an office in Mae Sot, Thailand. The SMRU has offered ANC and birth services to marginalized Karen and Myanmar populations residing on both sides of the Thailand-Myanmar border since 1998. Women come for antenatal visits at migrant clinics, Wang Pha and Mawker Thai, which are based north and south of Mae Sot in rural settings in Tak. It is one of the few services established to provide language appropriate services for migrants and is acknowledged as a site for vaccine provision by the Tak Public Health Office, Thailand.
Hepatitis B Guidelines and Administration of Expanded Program of Immunization for Infants
The Thailand Practice Guideline in 2015 recommended antiretroviral treatment for those who plan to be pregnant and have an indication for treatment; however, in 2015, payment for the medication was not supported by the government so there was no uptake reported in this review [16, 18]. The guidelines recommended HBIG for infants within 12 hours of birth in HBsAg-positive mothers in Thailand [20] and in HBeAg-positive mothers in Myanmar [21] (Table 1). In addition, all newborns should be offered the HBV vaccination within the first 24 hours of birth and at 2, 4, and 6 months of age [16, 18, 22] in CM and Tak.
Table 1.
Differences in Hepatitis B Screening, Vaccination, and Treatment Policies Between Chiang Mai Migrant and Tak Migrant Program per Pregnancy Stage in 2015
| Variable | Chiang Mai | Tak |
|---|---|---|
| Population | Thai Nationals and registered migrants (urban and rural) | Mostly unregistered migrants (rural) |
| Study site | Tertiary referral, University Hospital | Primary health center with birth facilities |
| Guideline used | Thai | Myanmar |
| Accessibility | EPI vaccine HBV covered by all 3 major types of insurance scheme (universal coverage, civil servant medical benefit, and social security service) | EPI vaccine HBV from Tak Public Health |
| HBIG not covered by insurance scheme | HBIG procured at cost by SMRU | |
| Pregnancy | ||
| Screening | HBsAg is included in first ANC laboratory screening, if positive, HBeAg and LFT ± viral load (VL not covered by any of the insurance schemes) | Point-of-care HBsAg, if positive confirmation HBsAg and HBeAg |
| Antiretroviral therapy | Available since 2015 | Not available (currently being studied) |
| Delivery | ||
| HBIG | Infants born to HBsAg-positive mother | Infants born to HBeAg-positive mothers |
| Birth dose vaccine | Yes | Yes (but not for home births) |
| Infant serology | No testing at delivery | Not available |
| Postpartum | ||
| Vaccination schedule | Month 1–2: EPI vaccine HBV | Month 2: EPI vaccine HBV |
| Month 4: EPI vaccine HBV with DTP | Month 4: EPI vaccine HBV | |
| Month 6: EPI vaccine HBV | Month 6: EPI vaccine HBV | |
| Postdelivery TDF care mother | Available but no guideline from institution | Available but not documented |
| Postvaccine serology infant | Consider HBsAg and anti-HBs at 9–12 years old | Not routine, not available |
Abbreviations: ANC, antenatal care; DTP, diphtheria, tetanus, pertussis; HBeAg, hepatitis B e-antigen; HBIG, hepatitis B immunoglobulins; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; EPI, Expanded Program on Immunization; LTF, liver function test; SMRU, Shoklo Malaria Research Unit; TDF, tenofovir disoproxil fumarate; VL, viral load.
Definition of Documentation of Vaccination
For the purpose of this survey, documentation of a dose of HBIG, the birth dose, and the 3 EPI vaccinations were considered verified if the specified field/s representing each specific vaccination was completed with at least 1 date in digital datasets of hospital records. Discrepancies at CM and Tak could be verified by tracing back to paper-based hospital records when required.
Timeliness varied with the type of vaccine: for HBIG, this was from birth up to72 hours of life; for the birth dose, this was within 24 hours of life; and for subsequent routine, 3 EPI HBV vaccinations at month 2, 4, and 6 were defined to be timely if a vaccination was given within 30 days of the recommended interval [23]. Nontimely HBIG and HBV vaccinations were considered as delayed. Infants that weigh <2 kilograms at birth received delayed vaccination for HBV per protocol based on a reduced immune responses in these infants [24].
Participants
Women who had been screened for HBV and gave birth from January to December 2015 to a live born infant with an estimated gestational age of 28 weeks or more were eligible together with their infants. Exclusion criteria included neonatal deaths (died in the first 28 days of life).
There are approximately 3.5 million migrants in Thailand [25]. Chiang Mai is the economic epicenter of Northern Thailand and is rapidly urbanizing with growing numbers of migrant workers from Myanmar and Lao People’s Democratic Republic. In CM in 2013, approximately 10% of the population of the CM metropolitan area were migrant workers. The largest group of migrants originate from Myanmar, 98.4%, and are mainly from Shan State (87.8%) [25]. There are documented and undocumented migrant workers [26], and more than one quarter of the migrant workers work without work permits or legal status in Thailand [27].
Mothers who gave birth at CMU hospital were classified using the 13-digit Thai identification (ID) card [28] with the number “1” to “5” in the first digit. Migrants in CM were defined as those having the first digit as “0” or “6” to “8.” The latter numbers indicated that they were non-Thais with intent to stay on temporary basis or non-Thais who converted their nationality after May 1984. Mothers from Tak were classified by their residence reported at the time they registered their pregnancy. There is no 13-digit code system available in the SMRU clinics, but each patient has a unique ID. At the border in Tak, the migrant population are predominantly Karen or Myanmar people. The population is largely used in agricultural work on both sides of the border predominantly carried out by migrant workers originally from Myanmar.
Variables
Demographic and delivery characteristics were included when they were available for both cohorts: reported district and/or country of residence at first ANC visit, age, gravidity, parity, human immunodeficiency virus status, mode of delivery (vaginal delivery, caesarean section), and the place of delivery (home, SMRU clinic, hospital). Hepatitis B virus status was categorized into HBsAg negative, HBsAg positive, and HBeAg negative and both HBsAg and HBeAg positive.
Data Sources
For CM and Tak, data digital datasets of records of mothers who delivered in 2015 were extracted from the centralized electronic health record systems. All ANC demographic data of the mother and birth records and vaccinations were derived from these data sources, which were held independently at each site.
Statistical Methods
Data were analyzed using SPSS version 23. Demographic characteristics were compared using the χ 2 test for categorical variables and the Student’s t test or Mann-Whitney U test for continuous data.
Ethics
A retrospective review of anonymized data from antenatal records was approved by the local Tak Community Advisory Board and the Research Ethics Committee, Faculty of Medicine, CMU (058/2017) and Oxford University (OxTREC 49-16).
RESULTS
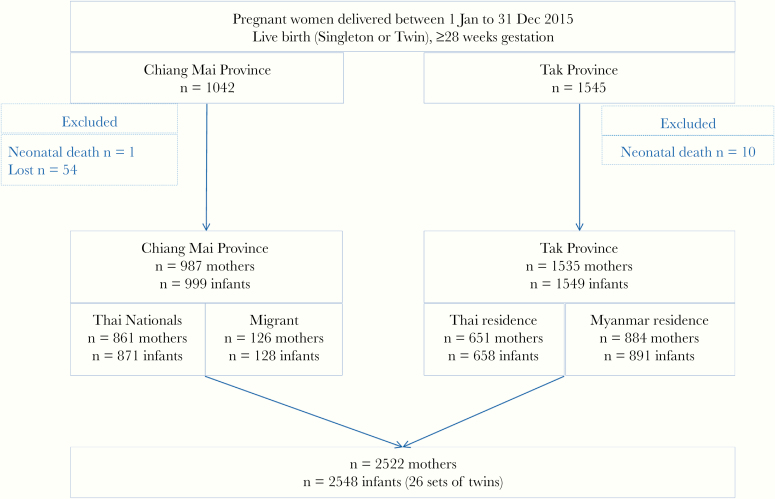
After exclusions, 2522 women were included: 987 from CM (861 Thai nationals, 126 migrants) and 1535 from Tak (651 migrants reporting Thai residence and 884 migrants reporting Myanmar residence). There were 2548 eligible infants with 999 from CM (871 Thai nationals, 128 migrants) and 1549 from Tak (658 Thai residence and 891 Myanmar residence) (Figure 1). There were 26 sets of twins in the cohort, 12 born in CM and 14 born in Tak.
Figure 1.
Study flow chart.
Characteristics of Women in the Two Areas
Migrants from CM (126 of 987, 12.8%), were mostly from Myanmar; in Tak (651 of 1535, 42.4%), the migrants with Thai residence were mostly Karen from Myanmar (Table 2). The proportion of women that were HBsAg positive was not different between the study sites with 60 of 987 (6.1%; 95%; CI, 4.9%–8.0%) positive in CM and 106 of 1535 (6.9%; 95% CI, 5.7%–8.1%) positive in Tak. Thai nationals had a lower proportion compared with migrant women in the CM (51 of 861, 5.9% vs 9 of 126, 7.1%, P = .552). Migrants from the 2 sites had similar characteristics except for home deliveries, which occurred in 1 in 10 deliveries (199 of 1535, 13.0%) in Tak and were significantly higher for migrant women reporting Myanmar residence compared with Thai residence (16.6% vs 8.0%, P < .001). The overall rate of caesarean section was 5.7% (144 of 2523) (Table 2).
Table 2.
Demographic Characteristics of Included Women Who Gave Birth in 2015 and Followed Antenatal Care in Chiang Mai and Tak
| Chiang Mai | P Valuea | Tak | P Valuea | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | All n = 987 | Thai Nationals n = 861 | Migrants n = 126 | All n = 1535 | Migrants Thai Residence n = 651 | Migrants Myanmar Residence n = 884 | ||
| Stage of Pregnancy at First ANC | ||||||||
| 1st trimester | 637 (68.3)c | 571 (70.5) | 66 (53.7) | .001 | 466 (30.4) | 182 (28.0) | 284 (32.1) | .14 |
| 2nd trimester | 259 (27.8)c | 211 (26.0) | 48 (39.0) | 725 (47.2) | 311 (47.8) | 414 (46.8) | ||
| 3rd trimester | 37 (4.0)c | 28 (3.5) | 9 (7.3) | 344 (22.4) | 158 (24.3) | 186 (21.0) | ||
| Age years, mean ± SD (min-max) | 29 ± 6 (15–45) | 29 ± 6 (16–45) | 29 ± 6 (15–44) | .34 | 26 ± 7 (14–50) | 26 ± 7 (14–48) | 26 ± 7 (14–50) | .93 |
| Age <25 years (%) | 224 (22.7) | 185 (21.5) | 39 (31.0) | .02 | 745 (48.5) | 321 (49.3) | 424 (48.0) | .61 |
| Born after HBV added to EPI Thailand in 1992 (%) | 173 (17.5) | 142 (16.5) | 31 (24.6) | .03 | 667 (43.5) | 29 (44.7) | 376 (42.5) | .40 |
| Born after HBV added to EPI in Myanmar in 2003 (%) | 0 | 0 | 0 | NA | 0 | 0 | 0 | NA |
| Gravidity median [IQR] (min-max) | 2 [1–2] (1–13) | 2 [1–2] (1–13) | 2 [1–2] (1–11) | .13 | 2 [1–3] (1–11) | 2 [1–3] (1–10) | 2 [1–3] (1–11) | .21 |
| Parity median [IQR] (min-max) | 0 [0–1] (0–4) | 0 [0–1] (0–4) | 1 [0–1] (0–4) | .01 | 1 [0–2] (0–8) | 1 [0–2] (0–8) | 1 [0–2] (0–8) | .55 |
| Primigravida (%) | 418 (42.4) | 369 (42.9) | 49 (38.9) | .44 | 552 (36.0) | 251 (38.6) | 301 (34.0) | .08 |
| HBsAg positive (%) | 60 (6.1) | 51 (5.9) | 9 (7.1) | .55 | 106 (6.9) | 47 (7.2) | 59 (6.7) | .69 |
| HBeAg positiveb (%) | 18/60 (30.0) | 14/51 (27.5) | 4/9 (44.4) | .51 | 34/106 (32.1) | 14/47 (29.8) | 20/59 (33.9) | .69 |
| HIV positive (%) | 17 (1.7) | 11 (1.3) | 6 (4.8) | .01 | 5 (0.3) | 4 (0.6) | 1 (0.1) | .17 |
| Home delivery (%) | 0 | 0 | NA | 199 (13.0) | 52 (8.0) | 147 (16.6) | <.001 | |
| Mode of Delivery (%) | ||||||||
| Vaginal delivery | 925 (93.7) | 803 (93.3) | 122 (96.8) | .32 | 1452 (94.6) | 614 (94.5) | 838 (94.8) | .47 |
| Caesarean section | 62 (6.3) | 58 (6.7) | 4 (3.2) | .17 | 82 (5.3) | 36 (5.5) | 46 (5.2) | .82 |
Abbreviations: ANC, antenatal care; EPI, Expanded Programme on Immunization; HBeAg, hepatitis B e-antigen; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HIV, human immunodeficiency virus; IQR, interquartile range; min-max, minimum-maximum; NA, not applicable; SD, standard deviation.
NOTE: Data are n (%), mean ± SD (min-max); median IQR (min-max).
a P value: comparisons between the 2 populations within each with proportions compared by 2 × 2 χ 2 test, means by Student’s t test; median by the Mann-Whitney U test.
bOnly tested if HBsAg positive.
cMissing data for 54 patients in Chiang Mai (51 Thai Nationals and 3 migrants).
Hepatitis B Antigen Status of Pregnant Women
The HBV groups differed significantly for maternal age, gravidity, and parity (Table 3). Only 1 of 3 of all women in the combined cohort (173 of 987, 17.5% CM; 668 of 1535, 43.5% Tak; 841 of 2522, 33.3% both sites combined) of pregnant women were born after the HBV vaccination was included to the EPI schedule in Thailand (in 1992). Women who were HBsAg and HBeAg positive were significantly younger compared with HBsAg-positive and HBeAg-negative women (24 ± 5 vs 29 ± 6, P = .002). There were no significant differences observed in the mode of delivery by HBV status.
Table 3.
Demographic Characteristics of 2523 Women Who Gave Birth in 2015 From Both Areas According to Hepatitis B Antigen Status
| Variable | HBs Antigen Negative | HBs Antigen Positive, HBe Antigen Negative | HBs Antigen Positive, HBe Antigen Positive | P Valuea |
|---|---|---|---|---|
| CM | 927/987 (93.9) | 42/987 (4.3) | 18/987 (1.8) | .48 |
| Thai Nationals | 810 (87.4) | 37 (88.1) | 14 (77.8) | .48 |
| Migrant | 117 (12.6) | 5 (11.9) | 4 (22.2) | .48 |
| Age years, mean ± SD (min-max) | 29 ± 6 (15–45) | 31 ± 4 (23–39) | 29 ± 4 (21–36) | .09 |
| Born after HBV added to EPI Thailand in 1992 (%) | 171 (18.4) | 1 (2.4) | 1 (5.6) | .01 |
| Tak | 1429/1535 (93.1) | 72/1535 (4.7) | 34/1535 (2.2) | .83 |
| Thai residence | 604 (42.3) | 33 (45.8) | 14 (41.2) | .83 |
| Myanmar residence | 825 (57.7) | 39 (54.2) | 20 (58.8) | .83 |
| Age years, mean ± SD (min-max) | 26 ± 7 (14–50) | 28 ± 7 (17–47) | 22 ± 5 (15–32) | .01 |
| Born after HB added to EPI Thailand in 1992 (%) | 623 (43.6) | 23 (31.9) | 22 (64.7) | .01 |
| CM and Tak Combined Data | ||||
| Total (CM+Tak) | 2356/2522 (93.4) | 114/2522 (4.5) | 52/2522 (2.1) | |
| Age years, mean ± SD (min-max) | 27 ± 7 (14–50) | 29 ± 6 (17–47) | 24 ± 5 (15–36)b | <.01 |
| Gravidity median [IQR] (min-max) | 2 [1–3] (1–13) | 2 [2–3] (1–10) | 2 [1–2] (1–7) | .03 |
| Parity median [IQR] (min-max) | 1 [0–2] (0–8) | 1 [0–2] (0–8) | 1 [0–1] (0–6) | .02 |
| Primigravida | 923/2356 (39.2) | 28/114 (24.6) | 19/52 (36.5) | .01 |
| HIV positive | 22/2356 (0.9) | 0 | 0 | NA |
| Home delivery | 184/2356 (7.8) | 12/114 (10.5) | 3/52 (5.8) | .49 |
| Mode of Delivery | ||||
| Vaginal delivery | 2223/2356 (94.4) | 105/114 (92.1) | 50/52 (96.2) | .28 |
| Caesarean section | 133/2356 (5.6) | 9/114 (7.9) | 2/52 (3.8) | .51 |
Abbreviations: CM, Chiang Mai; EPI, Expanded Programme of Immunization; HBe, hepatitis e; HBs, hepatitis B surface; HIV, human immunodeficiency virus; HBV, hepatitis B virus; IQR, interquartile range; min-max, minimum-maximum; NA, not applicable; SD, standard deviation.
NOTE: Data are n (%), mean ± SD (min-max); median IQR (min-max).
a P value: proportions compared by overall χ 2 test, means by Student’s t test; median by Mann-Whitney U test.
bSignificantly different from HBs antigen-positive and HBe antigen-negative group.
Verification of Documentation of Hepatitis B Immunoglobulins, Birth Dose, and Three Hepatitis B Virus Expanded Program of Immunization Vaccinations
Of the 2548 included infants, there was a higher proportion of preterm birth (95 of 999 [9.5%] vs 109 of 1549 [7.0%]) in CM compared with Tak (Table 4). In CM, 60 of 60 (100.0%) babies born to HBsAg-positive mothers received HBIG as per protocol [20]. Of the 52 infants that were born to mothers that were HBeAg positive, 44 received HBIG (84.6%), all within 72 hours of life, with 100% coverage in CM compared with 76.5% from Tak. The 8 that did not receive HBIG included the following: 2 home births where the window for HBIG had passed when the infant first presented to the clinic; 2 women gave birth within 24 hours after first presenting to the clinic, which allowed no time to confirm the HBeAg status at the time of birth; in 2 cases, HBIG left the central pharmacy in time for the HBeAg-positive birth, but there was no documentation in the records available for verification; and for the remaining 2, the reason could not be determined.
Table 4.
Demographic Characteristics of Included Infants That Were Born in 2015 at Both Sites
| Chiang Mai | P Valuea | Tak | P Valuea | |||||
|---|---|---|---|---|---|---|---|---|
| Variable | All n = 999 | Thai Nationals n = 871 | Migrants n = 128 | All n = 1549 | Thai Residence n = 658 | Myanmar Residence n = 891 | ||
| Gestation, weeks mean ± SD (min-max) | 39 ± 2 (29–42) | 39 ± 2 (30–42) | 39 ± 2 (29–41) | .76 | 39 ± 2 (28–43) | 39 ± 2 (30–43) | 39 ± 2 (28–43) | .28 |
| Preterm birth (<37 weeks) | 95 (9.5) | 85 (9.8) | 10 (7.8) | .63 | 109 (7.0) | 40 (6.1) | 69 (7.7) | .23 |
| Birthweight <72 hours after birth | 999 (100.0) | 871 (100.0) | 128 (100.0) | NA | 1373 (88.6) | 603 (92.1) | 770 (86.4) | .001 |
| Birthweight, grams mean ± SD (min-max) | 3049 ± 453 (1050–4865) | 3053 ± 445 (1050–4865) | 3017 ± 499 (1215–4140) | .41 | 2968 ± 462 (1080–4200) | 2960 ± 449 (1360–4200) | 2975 ± 473 (1080–4100) | .55 |
| Gender male | 513 (51.4) | 449 (51.5) | 64 (50.0) | 1.00 | 812 (52.4) | 341 (51.8) | 471 (52.9) | .72 |
| HBIG in HBs antigen-positive mothers (n = 61b) | 61 (100.0) | 51 (100.0) | 9 (100.0) | NA | NA | NA | NA | NA |
| HBIG in HBe antigen-positive mothers (n = 34) | NA | NA | NA | NA | 26 (76.5) | 10 (71.4) | 16 (80.0) | .70 |
| Birth dose | 999 (100.0) | 871 (100.0) | 128 (100.0) | .50 | 1441 (93.0) | 617 (93.9) | 824 (92.6) | .53 |
| HBV EPI vaccine 2 months | 291 (29.2) | 256 (29.3) | 36 (28.1) | .84 | 1140 (73.6) | 469 (71.3) | 671 (75.3) | .01 |
| HBV EPI vaccine 4 months | 277 (27.7) | 244 (28.0) | 33 (25.8) | .68 | 962 (62.1) | 381 (57.9) | 581 (65.2) | <.01 |
| HBV EPI vaccine 6 months | 264 (26.4) | 234 (26.9) | 30 (23.4) | .45 | 800 (51.6) | 309 (47.0) | 491 (55.1) | <.01 |
| Three HBV EPI vaccines | 255 (25.5) | 225 (25.8) | 30 (23.4) | .59 | 800 (51.6) | 309 (47.0) | 491 (55.1) | <.01 |
Abbreviations: EPI, Expanded Programme on Immunization; HBe, hepatitis e; HBIG, hepatitis B immunoglobulins; HBs, hepatitis B surface; HBV, hepatitis B virus; min-max, minimum-maximum; NA, not applicable; SD, standard deviation.
NOTE: Data are n (%) unless otherwise stated. Values are reported as mean ± SD.
a P value: proportions comparing the 2 populations within each by overall χ 2 test, means by Student’s t test; median by Mann-Whitney U test.
bIncluding 1 twin.
Documentation of the birth dose was high with a total of 2440 of 2548 (95.8%) infants receiving the birth dose and only a small proportion (44 of 2548, 1.7%) reported as delayed. As per guidelines, delay was expected in infants with a birthweight of less than 2000 grams, and this accounted for 10 of 44 (22.7%) of delayed birth dose in both sites.
In Tak, there were 108 of 1549 (6.8%) newborns where documentation of the birth dose was not available. The reasons included the following: home delivery (40 of 108, 37.0%) where it is certain no vaccine was provided; hospital delivery (52 of 108, 48.1%) where birth dose was most likely provided as per the Thai protocol, but documentation was not available; and an additional 16 newborns that were born in the border clinics (4 with a birthweight of less than 2000 grams, 7 who were unwell and admitted to the special care baby unit, and 5 where the reason for lack of documentation was not apparent).
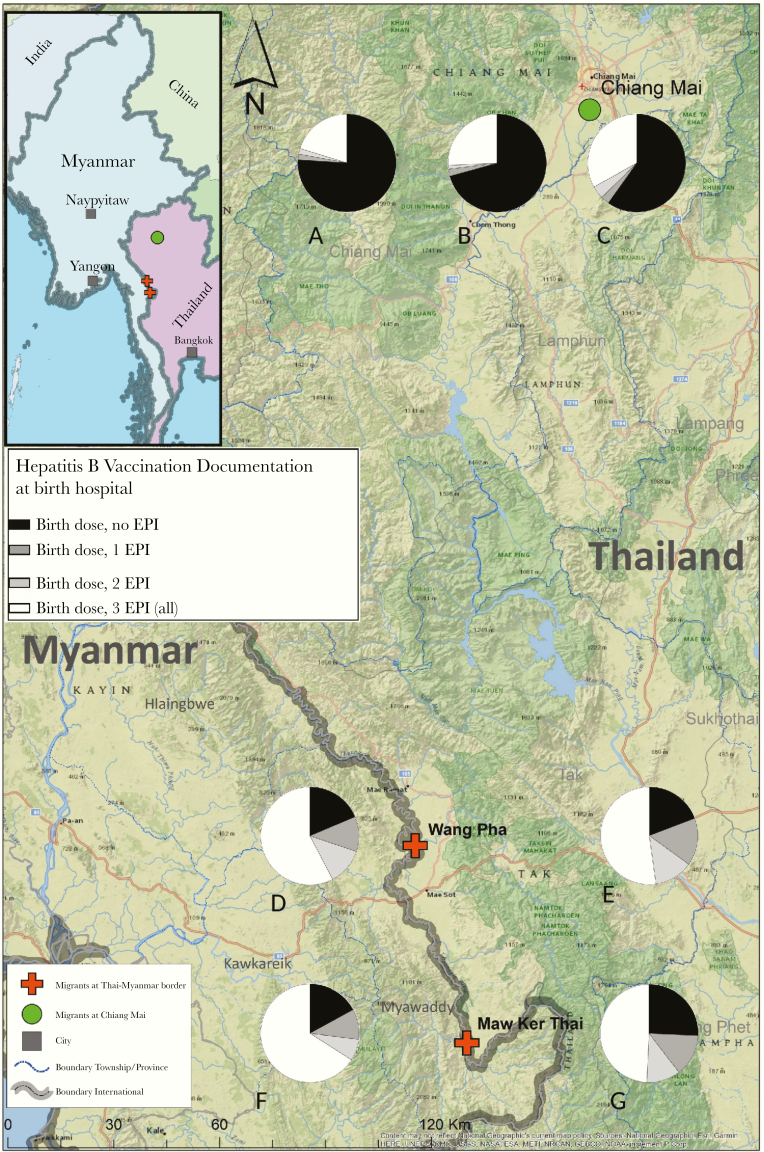
The proportion of infants documented to have received the 3 EPI HBV vaccinations at their hospital of birth, decreased at each subsequent EPI dose, in both sites (Table 4). Documentation of an infant having completed the 1st, 2nd, and 3rd dose was low at 1055 of 2549 (41.4%). In CM, there was no significant difference in the proportion of documentation of completed vaccination in Thai nationals (225 of 871 [25.8%]) or migrants (30 of 128 [23.4%]) (P = .59). In Tak, the highest proportion with completed documentation of the 3-dose EPI schedule was in migrants reporting Myanmar residence (491 of 891, 55.1%), significantly higher than migrants reporting Thai residence (309 of 658, 47.0%) (P = .002) (Figure 2). Documentation of delay at the 1st, 2nd, and 3rd EPI dose was uncommon in CM (4 of 1000, 0.4%), but it occurred in 88 of 1549 (5.7%), 93 of 1549 (6.0%), and 98 of 1549 (6.3%) per vaccination, respectively, in Tak.
Figure 2.
Map of clinic sites and hepatitis B vaccination documentation at birth hospitals, according to residence. (A) Outside of Chaing Mai; (B) Regional Chiang Mai; (C) Chiang Mai District; (D) Wang Pha Myanmar residence; (E) Wang Pha Thailand residence; (F) Maw Ker Thai Myanmar residence; (G) Maw Ker Thai Thailand residence. Clarification: Pie charts represent hepatitis B virus (HBV) vaccination at the birth hospital per clinic site and per area of residence for Chiang Mai and country of residence for Tak. Birth dose, birth dose HBV vaccination; EPI, Expanded Program on Immunization consisting of 3 vaccinations.
In CM, the greater the distance from the birth hospital, the lower the proportion of documentation of HBV EPI vaccinations (Figure 2). The highest proportion of completion of the 3 EPI vaccinations at Wang Pha may relate to the proximity of the population (255 of 635, 40%) Myanmar migrants at Shwe Ko Ko, directly opposite Wang Pha Clinic.
Discussion
Among 2 independent cohorts in CM and Tak, Northern Thailand, documentation of HBIG and the birth dose was high, in contrast to low documentation of the 3 HBV EPI doses in records from the hospital of birth. In CM, documentation for Thai nationals and migrants for HBIG, birth dose, or 3 HBV EPI vaccines was equitable. The overall coverage for HBIG and birth dose for both CM and Tak in migrants is commendable and a positive example for Asia, the most affected region globally in terms of hepatitis B, and with significant populations of migrants [27].
The Myanmar EPI started later than the Thai EPI, in 2003 and 1992, respectively. This means that the current reproductive age of women fall largely outside the benefits of that program: in this study cohort, 2 of 3 of the pregnant women were older than 23 and did not get the vaccine in the EPI. Until the generation that was born before the period Hepatitis B became part of the EPI are no longer child bearing aged, significant effort will be required to prevent perinatal transmission in Southeast Asia.
Hepatitis B Immunoglobulin Documentation
The first step in prevention of MTCT of HBV is the administration of HBIG to the newborn within 72 hours of birth. This is currently recommended for infants of HBsAg-positive women in Thailand [20] and HBe-positive women in Myanmar [21], who are at highest risk of infection from vertical transmission. In HBeAg-positive mothers provided with optimal intervention (HBIG and birth dose), prevention of MTCT fails in an estimated 8%–32% of cases [1]. In Tak at the border, the documentation of HBIG provision was lower than in CM (76.5% vs 100%), indicating a need to improve. Difficulties observed in the Tak cohort included the following: a delay in receiving HBeAg results from referral laboratories, which could be solved with a point-of-care rapid diagnostic test for HBeAg, as is already the case for HBsAg; case-by-case ordering of HBIG due to the cost, cold chain, and short shelf life of HBIG resulted in issues with the stock, and although this could be improved by issuing the HBIG to all infants born to mothers who are HBsAg positive, a source of funding or donation would be required; homebirths and presentation of the infant to a clinic after 5 days of life requires resources to overcome access to clinics where HBIG is available; and HBIG as a nonroutine vaccination requires staff to be alert to provision and documentation, which could be improved by training.
Birth Dose Documentation
In the studied population where births occurred in clinics and/or hospitals, 95.8% of all infants received the birth dose, which is better than the global estimate of 43% [29, 30]. In other low- and middle-income countries, these numbers range between 24% and 50% [31], and, although these numbers are low, they are likely to exceed population estimates for rural Myanmar due to high rates of home births, which is typically 64% [6, 29]. Effective delivery of the birth dose requires a 24-hour service so advising women to give birth in centers that can provide this is optimal. Progress on total government health expenditure in Myanmar has risen from 1% to 3% from 2005 to 2012, but rural areas remain neglected [32].
The birth dose vaccine for HBV is of high importance and has proven efficacious in the prevention of MTCT in many other countries [33, 34]. Adding the birth dose increases prevention of new HBV infections in infants by 25% in comparison to follow-up vaccinations alone [34, 35].
Three Hepatitis B Virus Expanded Program of Immunization Vaccines Documentation
In this cohort, documentation of the 3-dose HBV EPI vaccinations was low at the hospital of birth, declining in time, in both Thai nationals and in migrants. In CM, a higher proportion of the 3 HBV EPI doses were documented when the participants resided closer to the birth hospital, and this is similar for Thai nationals and migrants. The low proportion of documentation of the HBV EPI vaccines in CM is expected. This reflects the National Health system where childbirth is centralized at the Nakhorn Maharaj hospital, but vaccinations can be obtained in a range of peripheral health structures that do not have data linkage to hospital records. The fact that immunization occurred outside the CMU electronic records is supported by surveys of the Thai population for immune markers describing “declining rates of HBs Antigen carriers and natural HBV infection in people who were born after the universal HBV vaccine was included in the EPI program” [36]. The WHO estimates completion rates of 70%–90% and 98.3% of the 3 HBV vaccines as part of the EPI for Myanmar and Thailand [17, 18]. However, it would be important to know whether migrants who are excluded from national estimates reach similar results for protective markers for HBV or completion of EPI vaccinations by survey. A previous study described that vaccination coverage among migrant children from Myanmar between the age 1 and 2 years was lower compared to Thai children [37].
The highest proportion of documentation of the 3 HBV EPI doses was observed in Tak in Myanmar migrants, and this may reflect proximity of the clinic compared with other outlets, but this investigation cannot provide evidence of this. Border migrants with Thai residence may access the EPI at peripheral Thai health structures, or, at the other extreme, the risk of arrest or payments at checkpoints may inhibit EPI uptake [38–41]. In mobile migrant populations, it is important to understand vaccination rates, and this will be examined in future work.
Globally, these results reflect a strong public health initiative and provide a positive example for the current estimate of more than 1 billion migrants who support the economy of countries who depend on the migrant workforce [27]. Improved documentation contributes to a decrease in overvaccination without adversely affecting immunity and is an ideal target for e-health initiatives [42].
Limitations
There are limitations to this study that need to be considered. Vaccination services are offered across different hospitals and domains of the healthcare system in Thailand. In CM, migrant parents or caregivers can bring their infant/s back to the hospital of birth, the district hospital of their residence, the local Health Promoting Hospitals, or go to private providers for vaccination, and these data were not available. In Tak, migrant parents may also go to a Health Promoting Hospital closer to their home. Some infants of Myanmar descent may be taken back to grandparents and drop out of vaccinations services in Thailand completely. This investigation was limited to verification of documentation at the hospital of birth, and although the data provide a strong estimate of HBIG and birth dose coverage, it cannot provide reliable estimates of the EPI uptake.
Conclusions
This study provides support for excellent documentation of HBIG and birth dose vaccination in urban and rural settings and in migrants, consistent with Thailand’s policy and practice. Documentation of the EPI at the hospital of birth decreases with sequential doses, especially in families that live further away. Shifting from reporting numbers of infants vaccinated to individual patient data requires a significant investment, and the presence of in-country migrants complicates this process. Innovative methods to data linkage is required to prove coverage and identify gaps.
Acknowledgments
We thank the Thailand Department of Public Health for support and the pregnant women and their infants who attended the hospital and clinics. We are appreciate the health staff from various departments for documentation in the health system at Nakhorn Maharaj Hospital in Chiang Mai and Wang Pha and Mawker Thai clinics of Shoklo Malaria Research Unit (SMRU) in Mae Sot, Tak. Moreover, Wimol Moo and Hser Moo have been instrumental in the vaccination team at SMRU. Finally, we thank Myo Chit Min for preparing the map.
Disclaimer. The funders had no role in the collection, analysis and interpretation of the data, the writing of the article, or in submission of the paper for publication. The views expressed in the paper are those of the authors and do not represent the positions of their respective institutions or that of the funding agencies.
Financial support. This work was funded by the Wellcome-Trust Major Overseas Program in Southeast Asia (Grant Number 106698/Z/14/Z).
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Pan CQ, Duan ZP, Bhamidimarri KR, et al. An algorithm for risk assessment and intervention of mother to child transmission of hepatitis B virus. Clin Gastroenterol Hepatol 2012; 10:452–9. [DOI] [PubMed] [Google Scholar]
- 2. Chen SC, Toy M, Yeh JM, et al. Cost-effectiveness of augmenting universal hepatitis B vaccination with immunoglobulin treatment. Pediatrics 2013; 131:e1135–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee C, Gong Y, Brok J, et al. Hepatitis B immunisation for newborn infants of hepatitis B surface antigen-positive mothers. Cochrane Database Syst Rev 2006; Cd004790. [DOI] [PubMed] [Google Scholar]
- 4. Pan CQ, Duan Z, Dai E, et al. ; China Study Group for the Mother-to-Child Transmission of Hepatitis B Tenofovir to prevent hepatitis B transmission in mothers with high viral load. N Engl J Med 2016; 374:2324–34. [DOI] [PubMed] [Google Scholar]
- 5. World Health Organization. Global Hepatitis Report 2017. Geneva: World Health Organization; 2017. [Google Scholar]
- 6. Childs L, Roesel S, Tohme RA. Status and progress of hepatitis B control through vaccination in the South-East Asia Region, 1992–2015. Vaccine 2018; 36:6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O’Hara GA, McNaughton AL, Maponga T, et al. Hepatitis B virus infection as a neglected tropical disease. PLoS Negl Trop Dis 2017; 11:e0005842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu R, Li Y, Wangen KR, et al. Analysis of hepatitis B vaccination behavior and vaccination willingness among migrant workers from rural China based on protection motivation theory. Hum Vaccin Immunother 2016; 12:1155–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Antai D. Migration and child immunization in Nigeria: individual- and community-level contexts. BMC Public Health 2010; 10:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hu Y, Li Q, Chen E, et al. Determinants of childhood immunization uptake among socio-economically disadvantaged migrants in East China. Int J Environ Res Public Health 2013; 10:2845–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sirilert S, Traisrisilp K, Sirivatanapa P, Tongsong T. Pregnancy outcomes among chronic carriers of hepatitis B virus. Int J Gynaecol Obstet 2014; 126:106–10. [DOI] [PubMed] [Google Scholar]
- 12. Banks T, Kang J, Watts I, et al. High hepatitis B seroprevalence and risk factors for infection in pregnant women on the Thailand-Myanmar Border. J Infect Dev Ctries 2016; 10:384–8. [DOI] [PubMed] [Google Scholar]
- 13. Bierhoff M, Angkurawaranon C, Myat Min A, et al. Maternal hepatitis B infection burden, comorbidity and pregnancy outcome in a low-income population on the Myanmar-Thailand border: a retrospective cohort study. J Pregnancy 2019; 2019:8435019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Datar A, Mukherji A, Sood N. Health infrastructure & immunization coverage in rural India. Indian J Med Res 2007; 125:31–42. [PubMed] [Google Scholar]
- 15. Ijarotimi IT, Fatiregun AA, Adebiyi OA, et al. Urban-rural differences in immunisation status and associated demographic factors among children 12-59 months in a southwestern state, Nigeria. PLoS One 2018; 13:e0206086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cutts FT, Claquin P, Danovaro-Holliday MC, Rhoda DA. Monitoring vaccination coverage: defining the role of surveys. Vaccine 2016; 34:4103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. World Health Organization. Expanded Program on Immunization Thailand. Available at: http://origin.searo.who.int/immunization/data/thailand_2018.pdf Accessed 4 December 2018. [Google Scholar]
- 18. World Health Organization. Expanded Program on Immunization Factsheet Myanmar. Available at: http://origin.searo.who.int/immunization/data/myanmar.pdf Accessed 4 December 2018. [Google Scholar]
- 19. Ministry of Health and Sports Nay Pyi Taw, Myanmar. Myanmar Demographic and Health Survey 2015–16: Key Indicators Report. Nay Pyi Taw, Myanmar, and Rockville, Maryland: Ministry of Health and Sports and ICF; International; Available at: https://dhsprogram.com/pubs/pdf/FR324/FR324.pdf. Accessed 21 May 2019. [Google Scholar]
- 20. Thai Association for the Study of the Liver. Thailand Practice Guideline for Management of Chronic Hepatitis B and C. Available at: http://www.thasl.org/files/25.Thailand%20guideline%20for%20management%20of%20CHB%20%20and%20CHC%202015.pdf. Accessed 18 December 2018. [Google Scholar]
- 21. Expanded Programme on Immunization (EPI). Myanmar: WHO Regional Office for South-East Asia; 2017. [Google Scholar]
- 22. Schalm SW, Mazel JA, de Gast GC, et al. Prevention of hepatitis B infection in newborns through mass screening and delayed vaccination of all infants of mothers with hepatitis B surface antigen. Pediatrics 1989; 83:1041–8. [PubMed] [Google Scholar]
- 23. Kaji A, Parker DM, Chu CS, et al. Immunization coverage in migrant school children along the Thailand-Myanmar border. J Immigr Minor Health 2016; 18:1038–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gagneur A, Pinquier D, Quach C. Immunization of preterm infants. Hum Vaccin Immunother 2015; 11:2556–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manoyos V, Tangmunkongvorakul A, Srithanaviboonchai K, Yangyuenkul S, Grimes RM. Sexual risk-behaviors for HIV infections among young cross-border migrant workers living in urban Chiang Mai, Thailand. J Health Res 2016; 30:347–53. [Google Scholar]
- 26. Tangmunkongvorakul A, Musumari PM, Srithanaviboonchai K, et al. “When I first saw a condom, I was frightened”: a qualitative study of sexual behavior, love and life of young cross-border migrants in urban Chiang Mai, Thailand. PLoS One 2017; 12:e0183255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. The UN migration agency Day. International Organization for Immigration. IOM Thailand National Strategy 2017–2020. Available at: https://thailand.iom.int/sites/default/files/document/publications/IOM%20Thailand%20-%20National%20Strategy%20%282017%20-%202020%29.pdf Accessed 15 June 2019. [Google Scholar]
- 28. Maynard JE. Passive immunization against hepatitis B: a review of recent studies and comment on current aspects of control. Am J Epidemiol 1978; 107:77–86. [DOI] [PubMed] [Google Scholar]
- 29. World Health Organization. Global Immunization Coverage 2017. Geneva: World Health Organization; 2017. [Google Scholar]
- 30. Kristin VanderEnde K, Gacic-Dobo M, Diallo MS, Conklin LM, Wallace AS. Global routine vaccination coverage—2017. MMWR Morb Mortal Wkly Rep 2018; 67:1261–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Purwono PB, Juniastuti, Amin M, et al. Hepatitis B virus infection in Indonesia 15 years after adoption of a universal infant vaccination program: possible impacts of low birth dose coverage and a vaccine-escape mutant. Am J Trop Med Hyg 2016; 95:674–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. World Health Organization. Global Health Expenditure Database. 2016. Available at: http://apps.who.int/nha/database/ViewData/indicators/en. Accessed 21 December 2018. [Google Scholar]
- 33. Lee C, Gong Y, Brok J, et al. Effect of hepatitis B immunisation in newborn infants of mothers positive for hepatitis B surface antigen: systematic review and meta-analysis. BMJ 2006; 332:328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Anderson S, Harper LM, Dionne-Odom J, et al. A decision analytic model for prevention of hepatitis B virus infection in sub-Saharan Africa using birth-dose vaccination. Int J Gynaecol Obstet 2018; 141:126–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hipgrave DB, Maynard JE, Biggs BA. Improving birth dose coverage of hepatitis B vaccine. Bull World Health Organ 2006; 84:65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Posuwan N, Wanlapakorn N, Sa-Nguanmoo P, et al. The success of a universal hepatitis B immunization program as part of Thailand’s EPI after 22 years’ implementation. PLoS One 2016; 11:e0150499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Prakunwisit D, Areesantichai C. Factors associated with immunization status among Myanmar migrant children aged 1–2 years in Tak Province, Thailand. J Health Res 2015; 29:121–6. [Google Scholar]
- 38. Kentikelenis A, Karanikolos M, Williams G, et al. How do economic crises affect migrants’ risk of infectious disease? A systematic-narrative review. Eur J Public Health 2015; 25:937–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stoesslé P, González-Salazar F, Santos-Guzmán J, Sánchez-González N. Risk factors and current health-seeking patterns of migrants in Northeastern Mexico: healthcare needs for a socially vulnerable population. Front Public Health 2015; 3:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Han K, Zheng H, Huang Z, et al. Vaccination coverage and its determinants among migrant children in Guangdong, China. BMC Public Health 2014; 14:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pearson R, Kusakabe K.. Thailand’s Hidden Workforce: Burmese Migrant Women Factory Workers. United Kingdom: Zed Books; 2012. [Google Scholar]
- 42. Tozzi AE, Gesualdo F, D’Ambrosio A, et al. Can digital tools be used for improving immunization programs? Front Public Health 2016; 4:36. [DOI] [PMC free article] [PubMed] [Google Scholar]