Abstract
Background:
Lymph node (LN) status is an important predictor of overall survival for resected IHCC, yet current guidelines for the optimal extent of LN dissection for IHCC are not evidence-based. The aim of this study was to evaluate whether the number of LNs resected at the time of surgery is associated with overall survival for IHCC.
Methods:
Patients undergoing resection for IHCC between 2004 and 2012 were identified within the US National Cancer Database. LN thresholds were evaluated using maximal chi-square testing and Kaplan-Meier and Cox regression methods were used to model five-year overall survival.
Results:
Of 2,000 eligible patients undergoing R0 or R1 resection, 57% (n=1,132) had one or more LNs examined. In the 631 patients (56%) undergoing R0 resection with pathologic N0 disease, the maximal chi-square statistic was reached when 3 or more LNs were examined. Only 39% of this cohort met this threshold. This threshold was not associated with overall survival (p=0.186). Similarly, the current American Joint Committee on Cancer recommendation of examining ≥ 6 LNs was not associated with overall survival (p=0.318).
Conclusion:
Despite the results of maximal chi-square testing in evaluating the optimal number of LNs to examine for IHCC, no threshold was associated with overall survival. In determining the extent and utility of lymphadenectomy for IHCC, surgeons should carefully consider the prognostic benefit in the absence of survival benefit.
INTRODUCTION
Defining evidence-based standards for the surgical management of intrahepatic cholangiocarcinoma (IHCC) is essential, as surgery remains the only treatment with curative potential (1, 2). Currently, there is no consensus on the utility or optimal extent of lymph node dissection in IHCC despite evidence that lymph node status is an important prognostic indicator (3, 4). Current practice guidelines from the National Comprehensive Cancer Network (NCCN) suggest that lymphadenectomy is “reasonable for staging purposes”, yet retrospective reports have shown that resection and pathologic evaluation of LNs occurs in only 55–59% of patients undergoing surgical resection for IHCC (4–6). Further, the recent 8th edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual recommends resection of 6 or more lymph nodes but this is not evidence-based and multiple series have reported that less than 25% of cases have even four or more nodes examined (5–7).
As IHCC is difficult to study prospectively due to its low incidence, large databases have proven useful in studying the effect of lymphadenectomy on this condition (2, 8, 9). Jutric et al. (2016) reviewed lymph node dissection within the National Cancer Database (NCDB) and suggested that lymphadenectomy should be performed for staging purposes but determined that the extent of lymphadenectomy was not associated with improved outcomes (5). Retrospective reports for other biliary tract cancers have used additional techniques to evaluate optimal thresholds of lymph node evaluation and have set the standard of evidence-based surgical practice and staging systems(10, 11). Therefore, the aim of this study was to apply a similar method to determine the optimal number of lymph nodes to be resected and pathologically examined for IHCC.
METHODS
Data Source and Study Population
This study was approved as exempt from review by the Institutional Review Board at Washington University in St. Louis. Patients undergoing resection for IHCC diagnosed between 2004 and 2012 were identified from the liver and intrahepatic bile duct files of the NCDB (SEER ICD-O-3 site codes C220 and C221). IHCC was defined using SEER ICD-O-3 Histology Validation codes 8160, 8161, and 8180(12). Klatskin tumors (8162) were specifically excluded. Patients were not eligible if their surgery was coded as a liver transplant, if they had a prior or concurrent malignancy, or if they received all treatment decisions and therapies at a facility other than the NCDB reporting facility.
Definitions
The inclusion dates of this study spanned the 6th and 7th editions of the AJCC Cancer Staging Manual(6, 13, 14), so T stage was harmonized into the AJCC 8th edition using the alignment in Supplemental Material, Table S1. These definitions do not have complete alignment across editions, so the variables used to define T stage – tumor size, vascular invasion, and multiple intrahepatic tumors – were individually incorporated in the multivariable models. Pathologic nodal disease was defined using the variable “number of regional nodes positive”. Where data for clinical nodal or metastatic disease was missing, data was inferred from the variable for clinical stage using the AJCC edition appropriate for that patient’s year of diagnosis or the variable for metastasis at diagnosis. Metastatic disease was defined as a distant metastatic deposit or distant nodal disease as defined by the AJCC Collaborative Stage Data Collection System for Intrahepatic Bile Duct tumors(15). Curative-intent resection was defined as R0 (microscopically-negative margins) or R1 (microscopically-positive margins) resection.
Statistical Analysis
Between-group comparisons were performed using chi-square and Wilcoxon-Mann-Whitney tests.
Survival analyses were performed using Kaplan-Meier analysis and univariate and multivariable Cox proportional hazards regression. A single multivariable model was employed throughout the analysis and was derived using variables with a known(3) or clinically suspected association with overall survival in IHCC: age, sex, extent of surgery, metastatic disease, vascular invasion, multiple lesions, pathologic tumor grade, tumor size ≥ 5cm, receipt of adjuvant chemotherapy, and receipt of adjuvant radiation. Backwards selection was used, with p<0.3 required to enter and p<0.15 to remain in the model. To reduce guarantee time bias, no patients were entered into regression analyses if they received surgery more than 90 days after diagnosis(16). Missing data was assumed to be missing at random and was retained as a separate category, in line with NCDB recording, except for tumor size, where patients with missing data (n=41, 2.1%) were excluded from the multivariable analyses.
Evaluation of the optimal number of LNs examined was performed using maximum chi-square testing. This test is similar to a sensitivity analysis in that it examines a predefined range of thresholds to identify the threshold with the strongest statistical significance; the optimal threshold is the one at which the model’s chi-square statistic is maximized(17). This method was carried out for patients who received R0 resection and had confirmed node-negative disease, as has previously been done for other biliary tract cancers(10, 11, 17). Thresholds needed to contain at least 10% of the population above or below to qualify for evaluation. Chi-square statistics were derived from univariate and multivariable Cox regression models. All maximal chi-square results are presented without adjustment for violation of the proportional hazards assumption, as no time-interaction variables with any LN threshold were significant at p<0.05 on multivariable models. The results of all maximal chi-square subgroups testing and all thresholds are presented in Table S2.
All tests were two-sided and statistical significance was set at p<0.05. Analyses were performed using SAS v9.4 (SAS Institute, Cary, N.C.). Reporting follows the STROBE guidelines, version 4(18).
RESULTS
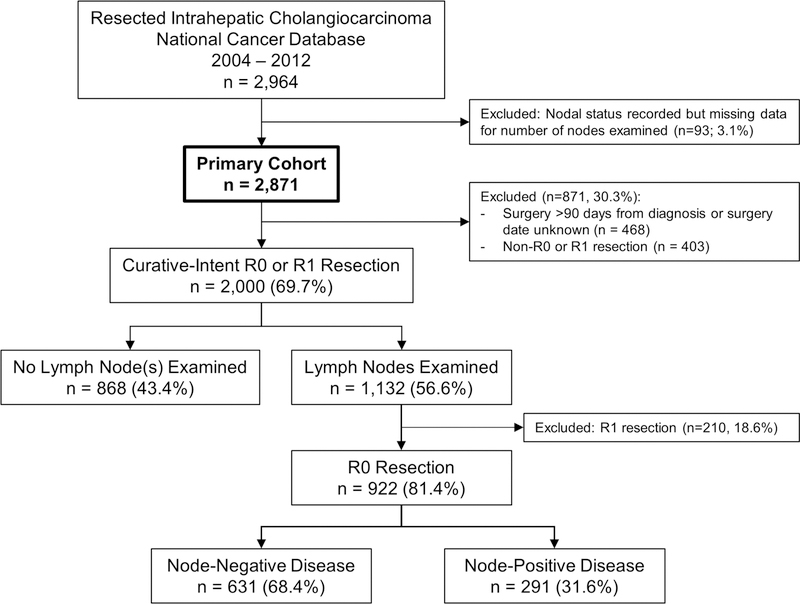
2,871 patients were diagnosed with IHCC between 2004 and 2012 within the NCDB and met all inclusion criteria (Figure 1). Demographic and clinical information is presented in Table 1. The median time from diagnosis to surgery was 32 days (IQR 10 – 62). The median duration of follow-up was 23 months (IQR 11 – 41). Median overall survival was 30 months from diagnosis (95% confidence interval, CI, 28.6 – 32.0). Five-year overall survival was 31%.
Figure 1.
Inclusion schema.
Table 1. Demographic and clinical information for eligible patients diagnosed with Intrahepatic Cholangiocarcinoma within the National Cancer Database, 2004–2012.
Clinical T, N, and M stages are defined according to 8th edition of the AJCC Cancer Staging Manual. Statistical comparison is made within the cohort of patients undergoing curative-intent resection within 90 days of diagnosis, between the patients undergoing nodal examination (n=1,132) and those without nodal examination (n=868). cT3 disease is blank, as the 8th edition definition does not align with the 6th and 7th editions used for this cohort in the NCDB.
| Curative-Intent Resection Within 90 Days of Diagnosis |
|||||||
|---|---|---|---|---|---|---|---|
| No Lymph Nodes Examined | Lymph Nodes Examined | p | |||||
| n or median | % or IQR | n or median | % or IQR | n or median | % or IQR | ||
| n | 2,871 | 868 | 1,132 | ||||
| Age | 63 years | 54 – 71 | 64 years | 56 – 72 | 60 years | 53 – 70 | <0.001 |
| Female | 1,535 | 53.5% | 431 | 49.7% | 655 | 57.9% | <0.001 |
| Charlson Comorbidity Index | <0.001 | ||||||
| 0 | 1,983 | 69.1% | 557 | 64.2% | 824 | 72.8% | |
| 1 | 605 | 21.1% | 210 | 24.2% | 210 | 18.6% | |
| ≥ 2 | 283 | 9.9% | 101 | 11.6% | 98 | 8.7% | |
| Race/Ethnicity | 0.011 | ||||||
| White | 2,219 | 77.3% | 647 | 74.5% | 915 | 80.8% | |
| Black | 208 | 7.2% | 70 | 8.1% | 62 | 5.5% | |
| Hispanic/Latino | 171 | 6.0% | 54 | 6.2% | 59 | 5.2% | |
| Asian | 152 | 5.3% | 61 | 7.0% | 49 | 4.3% | |
| Other | 61 | 2.1% | 15 | 1.7% | 21 | 1.9% | |
| Missing/Unknown | 60 | 2.1% | 21 | 2.4% | 26 | 2.3% | |
| Insurance Status | 0.078 | ||||||
| Private | 1,316 | 45.8% | 371 | 42.7% | 555 | 49.0% | |
| Medicare | 1,160 | 40.4% | 386 | 44.5% | 456 | 40.3% | |
| Medicaid/Other Government | 225 | 7.8% | 66 | 7.6% | 73 | 6.4% | |
| None | 73 | 2.5% | 21 | 2.4% | 25 | 2.2% | |
| Missing/Unknown | 97 | 3.4% | 24 | 2.8% | 23 | 2.0% | |
| Treating Facility | 0.261 | ||||||
| Academic | 1,954 | 68.1% | 583 | 67.2% | 787 | 69.5% | |
| Other | 917 | 31.9% | 285 | 32.8% | 345 | 30.5% | |
| Clinical T Stage | <0.001 | ||||||
| T1a/b | 728 | 25.4% | 283 | 32.6% | 269 | 23.8% | |
| T2 | 699 | 24.3% | 194 | 22.4% | 262 | 23.1% | |
| T3 | - | - | - | - | - | - | |
| T4 | 165 | 5.7% | 29 | 3.3% | 57 | 5.0% | |
| Unknown or T0 | 1,267 | 44.1% | 362 | 41.7% | 544 | 48.1% | |
| Clinical N Stage | <0.001 | ||||||
| 0 | 1,504 | 52.4% | 530 | 61.1% | 549 | 48.5% | |
| 1 | 240 | 8.4% | 19 | 2.2% | 120 | 10.6% | |
| Missing/Unknown | 1,127 | 39.3% | 319 | 36.8% | 463 | 40.9% | |
| Clinical M Stage | |||||||
| 0 | 2,463 | 85.8% | 833 | 96.0% | 1083 | 95.7% | 0.921 |
| 1 | 181 | 6.3% | 34 | 3.9% | 48 | 4.2% | |
| Missing/Unknown | 308 | 10.7% | 1 | 0.1% | 1 | 0.1% | |
| Tumor Size | 5.5 cm | 3.5 – 8.4 | 5.1 cm | 3.5 – 8.0 | 6.0 cm | 3.5 – 8.6 | 0.009 |
Lymph Node Assessment
Of this primary cohort of 2,871 patients, 1,580 (55.0%) had documented lymph node examination of any extent. The median number of lymph nodes examined was two (IQR 1 – 5), with 37.2% (n=587) of patients having four or more lymph nodes examined and only 12.4% (n=357) of patients having six or more lymph nodes examined. Those patients who were eventually found to have node-positive disease on pathology (n=572, 36.2%) tended to have a greater number of lymph nodes examined (median 4, IQR 2 – 8). Node-positive (N1) disease was associated with significantly worse median and five-year overall survival (17.3 months versus 40.0 months; 13.4% versus 37.5%; p<0.001 for both).
In a subset of patients undergoing curative-intent (R0 or R1) resection within 90 days of diagnosis (n=2,000), 1,132 patients had lymph nodes examined (56.6%; Figure 1). Patient-specific variables associated with the receipt of lymph node resection are evaluated in Table 1. Five-year survival was lower for the group of patients with documented lymph node resection than those without lymph node resection (29.9% versus 36.5%; p=0.008). However, this difference was nonsignificant (p=0.208) after adjusting for the variables with significant (p<0.05) between-group imbalances (Table 1). The median number of lymph nodes examined increased over the duration of the inclusion period (2 to 3, p=0.013), as did the mean number of lymph nodes examined (4.1 in 2004 to 4.8 in 2012, p=0.038).
Optimal Number of Lymph Nodes
On univariate analysis, an increasing number of lymph nodes examined was associated with worse five-year overall survival (HR 1.02, 95% CI 1.01 – 1.03; p=0.009). However, within the multivariable model including the impact of node-positive disease on overall survival, the number of lymph nodes examined was not associated with overall survival (p=0.998). Therefore, as with prior studies, exploration of the optimal extent of lymphadenectomy was restricted to a subset of patients with node-negative disease who underwent R0 resection with at least one lymph node examined (n=631, 68.4%; Figure 1).
In this cohort, the median number of lymph nodes examined was 2 (IQR 1 – 4, range 1 – 26). On univariate regression, the number of lymph nodes examined was not significantly associated with five-year overall survival (p=0.652).
Maximal chi-square testing was then performed using cutoffs for the number of lymph nodes ranging from ≥ 2 to ≥ 7, the highest cutoff to include at least 10% of the cohort on either side of the cutoff. On univariate survival regression, the maximal chi-square statistic was reached at ≥ 3 lymph nodes examined (chi-square statistic = 2.193). Only 38.8% of the cohort (n=245) had ≥ 3 lymph nodes examined. Variables associated with greater extent of lymphadenectomy (≥ 3 lymph nodes) are reviewed in Table 2.
Table 2.
Intraoperative and postoperative variables for patients undergoing R0 resection, lymph node examination, and with node-negative IHCC, stratified by the number of lymph nodes examined.
| Number of Lymph Nodes Examined | ||||||
|---|---|---|---|---|---|---|
| 1 or 2 | ≥ 3 | p Value | ||||
| n or median | % or IQR | n or median | % or IQR | Univariate | Multivariable* | |
| n | 386 | 61.2% | 245 | 38.8% | ||
| Tumor Size ≥ 5cm | 247 | 64.0% | 131 | 53.5% | 0.007 | |
| Extent of Surgery | <0.001 | |||||
| Wedge Resection | 15 | 3.9% | 15 | 6.1% | ||
| Segmentectomy (up to 3) | 121 | 31.3% | 44 | 18.0% | ||
| Hemihepatectomy | 143 | 37.0% | 80 | 32.7% | ||
| Extended Hepatectomy | 49 | 12.7% | 50 | 20.4% | ||
| Other or NOS | 58 | 15.0% | 56 | 22.9% | ||
| Post-Operative Length of Stay | 7 days | 5 – 10 | 7 days | 5 – 11.5 | 0.160 | |
| Unplanned 30-Day Readmissions | 31 | 8.0% | 21 | 8.6% | 0.802 | |
| 30-Day Postoperative Mortality | 13 | 3.4% | 17 | 6.9% | 0.040 | 0.109 |
| 90-Day Postoperative Mortality | 23 | 6.0% | 25 | 10.2% | 0.050 | 0.095 |
IQR = interquartile range.
Multivariable logistic regression adjusting for the effect of tumor size and extent of surgery
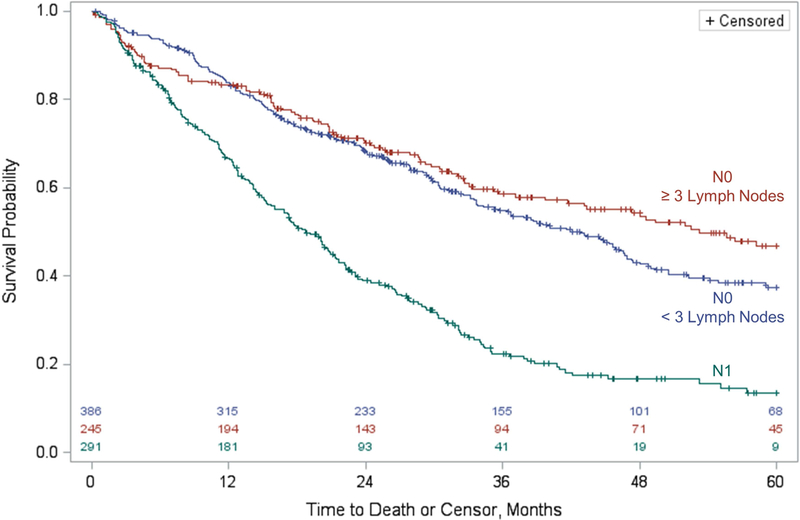
On Kaplan-Meier analysis, patients with ≥ 3 lymph nodes examined had median survival 10 months longer than those with less than 3 lymph nodes examined (53.3 months vs 42.8 months; Figure 2, with statistical comparison of these values invalid due to crossing survival curves). On univariate survival regression, the difference in five-year overall survival was not statistically significant (p=0.139; 46.8% for ≥ 3 lymph nodes examined and 37.5% for 1 or 2 lymph nodes examined).
Figure 2.
Five-year overall survival for patients with resected Intrahepatic Cholangiocarcinoma with lymph node evaluation, stratified by lymph node status and, for N0 patients, further stratified based on the optimal threshold for the number of lymph nodes to examine based on maximal chi-square testing. Number at risk is included along the X axis.
Maximal chi-square testing was then performed within a multivariable model. The maximal chi-square statistic was again reached at a threshold of ≥ 3 lymph nodes examined (chi-square statistic = 1.749). Covariate-adjusted overall survival was 5% greater in the group with ≥ 3 lymph nodes examined (Figure S1). However, examination of ≥ 3 lymph nodes was not associated with five-year overall survival (p=0.186; Hazard Ratio 0.85, 95% CI 0.66 – 1.08; Table 3). Similarly, examining six or more lymph nodes, in line with current AJCC guidelines, was not associated with five-year overall survival (p=0.318; HR 1.20, 95% CI 0.84 – 1.69).
Table 3. Multivariable analysis modeling five-year overall survival for patients with margin-negative and node-negative intrahepatic cholangiocarcinoma.
The optimal threshold for the number of lymph nodes examined, ≥ 3, as determined by maximal chi-square testing, was not significantly associated with five-year overall survival. Patients with missing data for tumor size were excluded (n=23).
| n | % | Hazard Ratio, Five-Year Overall Survival | 95% CI | |
|---|---|---|---|---|
| Number of Lymph Nodes Examined | ||||
| 1 – 2 | 372 | 61.2% | 1.00 | - |
| ≥ 3 | 236 | 38.8% | 0.85 | 0.66 – 1.08 |
| Tumor Size | ||||
| < 5cm | 230 | 37.8% | 1.00 | - |
| ≥ 5cm | 378 | 62.2% | 1.37 | 1.05 – 1.78 |
| Sex | ||||
| Female | 376 | 61.8% | 1.00 | - |
| Male | 232 | 38.2% | 1.58 | 1.25 – 2.01 |
| Metastatic Disease | ||||
| No | 502 | 82.6% | 1.00 | - |
| Yes | 16 | 2.6% | 3.24 | 1.79 – 5.84 |
| Unknown | 90 | 14.8% | 1.15 | 0.83 – 1.61 |
| Vascular Invasion | ||||
| No | 291 | 47.9% | 1.00 | - |
| Yes | 170 | 28.0% | 1.51 | 1.12 – 2.02 |
| Unknown | 147 | 24.2% | 1.34 | 0.87 – 2.07 |
| Multiple Lesions | ||||
| No | 375 | 61.7% | 1.00 | - |
| Yes | 67 | 11.0% | 2.49 | 1.78 – 3.47 |
| Unknown | 166 | 27.3% | 1.31 | 0.89 – 1.92 |
The same analyses were performed for patients with node-positive disease after R0 resection (n=291; 31.6%). On univariate regression, the number of nodes examined was not associated with five-year overall survival (p=0.725). Maximal chi-square testing for node-positive patients failed to reveal a threshold resulting in an appreciable difference in median or five-year overall survival.
DISCUSSION
In a retrospective analysis of the US National Cancer Database, maximal chi-square testing was used to suggest that ≥ 3 lymph nodes is the optimal threshold for the extent of lymphadenectomy for resected IHCC. Examination of three or more lymph nodes was not associated with adverse postoperative outcomes. However, this threshold was not associated with overall survival. Therefore, it is the authors’ conclusion that, from currently available data, there is no optimal threshold of lymph nodes to resect to improve overall survival for resected IHCC.
The optimal extent of lymphadenectomy has been studied for other hepatobiliary malignancies(10, 11). These studies, although limited due to small cohorts from a single institution, demonstrated statistically-significant differences in disease-specific survival at the identified thresholds, shaping guidelines for the surgical management of these diagnoses. However, the extent of lymphadenectomy had had not been examined for IHCC despite evidence that lymph node status is perhaps the strongest predictor of overall survival for patients undergoing curative-intent resection for IHCC(3). A prior report of lymph node status for IHCC using the NCDB determined that the number of lymph nodes examined was not associated with survival, although this was in a subset of patients with node-positive disease(5).
The current study, incorporating data from 2,871 patients in the NCDB, is the largest series describing the current practice of LN evaluation for IHCC. Only 57% of the cohort undergoing curative-intent resection had documented lymph node evaluation, although the inclusion dates of this analysis span the 6th and 7th editions of the AJCC Cancer Staging Manuals, a time in which the surgical management of this disease has evolved due to analyses similar to the current study evaluating the utility of lymphadenectomy as well as the separation of AJCC staging guidelines for intrahepatic bile duct cancers from those of hepatocellular carcinoma. In this cohort, the number of nodes examined did increase over time.
Although maximal chi-square testing did identify an optimal threshold for the extent of lymphadenectomy – three or more lymph nodes – this threshold was not associated with overall survival on univariate or multivariable analyses. The authors wish to point out that median overall survival was approximately 25% greater in the group with three or more lymph nodes resected (+10 months) and a 5% increase in covariate-adjusted five-year overall survival; in an aggressive disease with limited survival, these differences are not trivial, although further discussion of this point introduces the subjectivity of addressing clinically-relevant survival differences where no statistical significance exists. The authors hypothesize that this noticeable although insignificant survival advantage might be due to accuracy in staging: patients with only 1 or 2 lymph nodes resected may be falsely diagnosed as node-negative due to inadequate sampling. The current study demonstrated that node-positive patients tended to have more lymph nodes evaluated, so resecting more lymph nodes likely improves the likelihood of identifying nodal disease. Particularly in light of the low percentage of patients with at least three nodes examined, surgeons may wish to adopt a minimum threshold in their practices, either based on the results of maximal chi-square testing or the conclusion that examining more lymph patients may yield a greater likelihood of finding nodal disease to be used for prognostic information. Regardless, based on these data, these justifications are not supported by significant overall survival advantage for IHCC based on the extent of lymphadenectomy, including for the recommendation of at least 6 lymph nodes from the 8th edition of the AJCC staging manual for intrahepatic bile duct cancers(6).
There are a few notable limitations of the current study, many of which are inherent to the data source. First, the NCDB does not record locoregional recurrence or disease-specific survival. Therefore, the utility of lymph node dissection in clearing locoregional disease to reduce recurrence rates cannot be assessed. Second, the site of nodal sampling is not available, nor is tumor laterality, preventing us from understanding the impact of site-specific dissection and examination of lymph nodes within the hepatoduodenal ligament compared to more distant nodes. Finally, the NCDB variable for pathologic IHCC subtypes was largely incomplete or missing, so this important prognostic variable could not be incorporated into our models.
CONCLUSION
In a retrospective analysis of the US National Cancer Database, resection and pathologic evaluation of three or more lymph nodes was identified as the optimal threshold for the extent of lymphadenectomy for IHCC. However, this was not associated with overall survival, nor was the current AJCC 8th edition guidelines recommendation of at least six lymph nodes. Until additional studies can evaluate the site of nodal sampling and disease-specific survival with sufficient sample size, lymph node evaluation for IHCC should be performed only for staging and prognostic information, if desired.
Supplementary Material
Acknowledgments
Funding:
We thank the Alvin J. Siteman Cancer Center at Washington University School of Medicine and Barnes-Jewish Hospital in St. Louis, MO for use of the Biostatistics Shared Resource, which provided analytic support services. The Siteman Cancer Center is supported in part by a National Cancer Institute Cancer Center Support Grant (P30 CA091842; PI: Eberlein). Dr. Brauer is supported by a National Cancer Institute National Research Service Award to the Department of Surgery at Washington University School of Medicine (T32 CA009621; PIs: Eberlein, Colditz, Gillanders). Dr. Colditz is supported in part by the Foundation for Barnes-Jewish Hospital.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
The authors report no disclosures relevant to the material presented herein.
The National Cancer Data Base (NCDB) is a joint project of the Commission on Cancer of the American College of Surgeons and the American Cancer Society. The NCDB and the hospitals participating in the NCDB are the source of the de-identified data used herein; they have not verified and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Presentations:
This work was presented at the annual meeting of the Americas Hepato-Pancreato-Biliary Association, March 29 – April 2, 2017 in Miami, FL.
REFERENCES
- 1.Olnes MJ, Erlich R. A review and update on cholangiocarcinoma. Oncology. 2004;66(3):167–79. [DOI] [PubMed] [Google Scholar]
- 2.Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248(1):84–96. [DOI] [PubMed] [Google Scholar]
- 3.Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and Prognosis for Patients With Intrahepatic Cholangiocarcinoma: Systematic Review and Meta-analysis. JAMA Surg. 2014;149(6):565–74. [DOI] [PubMed] [Google Scholar]
- 4.de Jong MC, Nathan H, Sotiropoulos GC, Paul A, Alexandrescu S, Marques H, et al. Intrahepatic cholangiocarcinoma: an international multi-institutional analysis of prognostic factors and lymph node assessment. J Clin Oncol. 2011;29(23):3140–5. [DOI] [PubMed] [Google Scholar]
- 5.Jutric Z, Johnston WC, Hoen HM, Newell PH, Cassera MA, Hammill CW, et al. Impact of lymph node status in patients with intrahepatic cholangiocarcinoma treated by major hepatectomy: a review of the National Cancer Database. HPB (Oxford). 2016;18(1):79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aloia T, Pawlik TM, Taouli B, Rubbia-Brandt L, Vauthey J-N. Intrahepatic Bile Ducts In: Amin M, Edge S, Greene F, Byrd D, Brookland R, Washington M, et al. , editors. AJCC Cancer Staging Manual. 8th ed: Springer International; 2017. [Google Scholar]
- 7.Vitale A, Moustafa M, Spolverato G, Gani F, Cillo U, Pawlik TM. Defining the possible therapeutic benefit of lymphadenectomy among patients undergoing hepatic resection for intrahepatic cholangiocarcinoma. J Surg Oncol. 2016;113(6):685–91. [DOI] [PubMed] [Google Scholar]
- 8.Khan SA, Emadossadaty S, Ladep NG, Thomas HC, Elliott P, Taylor-Robinson SD, et al. Rising trends in cholangiocarcinoma: is the ICD classification system misleading us? J Hepatol. 2012;56(4):848–54. [DOI] [PubMed] [Google Scholar]
- 9.Shaib YH, Davila JA, McGlynn K, El-Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40(3):472–7. [DOI] [PubMed] [Google Scholar]
- 10.Ito H, Ito K, D’Angelica M, Gonen M, Klimstra D, Allen P, et al. Accurate staging for gallbladder cancer: implications for surgical therapy and pathological assessment. Ann Surg. 2011;254(2):320–5. [DOI] [PubMed] [Google Scholar]
- 11.Ito K, Ito H, Allen PJ, Gonen M, Klimstra D, D’Angelica MI, et al. Adequate lymph node assessment for extrahepatic bile duct adenocarcinoma. Ann Surg. 2010;251(4):675–81. [DOI] [PubMed] [Google Scholar]
- 12.ICD-O-3 Coding Materials: National Cancer Institute Surveillance, Epidemiology, and End Results Program; [updated 9/18/2015. Available from: https://seer.cancer.gov/icd-o-3/.
- 13.AJCC Cancer Staging Handbook. 7th ed. Trotti Andy IMD, Fritz April G. CTRRHIT, Compton Carolyn C. M.D PD, Byrd David R. M.D FACS, Greene Frederick L. MD, Edge Stephen B. M.D FACS, editors. Chicago, IL: American Joint Committee on Cancer; 2010. [Google Scholar]
- 14.AJCC Cancer Staging Manual. 6th ed. New York: Springer; 2002. [Google Scholar]
- 15.Collaborative Stage Schema Chicago: American Joint Committee on Cancer; [updated 2017. Available from: https://cancerstaging.org/cstage/schema/Pages/version0205.aspx.
- 16.Giobbie-Hurder A, Gelber RD, Regan MM. Challenges of guarantee-time bias. J Clin Oncol. 2013;31(23):2963–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller R, Siegmund D. Maximally Selected Chi Square Statistics. Biometrics. 1982;38(4):1011–6. [Google Scholar]
- 18.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Annals of internal medicine. 2007;147(8):573–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.