Abstract
BACKGROUND:
The high value of the specific absorption rate (SAR) of radio-frequency (RF) energy arising from the series of RF refocusing pulses in T2-weighted (T2-w) turbo spin echo (TSE) MRI hampers its clinical application at 7.0 Tesla (7T). T2-w gradient and spin echo (GRASE) uses the speed from gradient refocusing in combination with the chemical-shift/static magnetic field (B0) inhomogeneity insensitivity from spin-echo refocusing to acquire T2-w images with a limited number of refocusing RF pulses, thus reducing SAR.
OBJECTIVES:
To investigate whether low SAR T2-w GRASE could replace T2-w TSE in detecting white matter (WM) disease in MS patients imaged at 7T.
METHODS:
The .7 mm3 isotropic T2-w TSE and T2-w GRASE images with variable echo times (TEs) and echo planar imaging (EPI) factors were obtained on a 7T scanner from postmortem samples of MS brains. These samples were derived from brains of 3 female MS patients. WM lesions (WM-Ls) and normal-appearing WM (NAWM) signal intensity, WM-Ls/NAWM contrast-to-noise ratio (CNR) and MRI/myelin staining sections comparisons were obtained.
RESULTS:
GRASE sequences with EPI factor/TE = 3/50 and 3/75 ms were comparable to the SE technique for measures of CNR in WM-Ls and NAWM and for detection of WM-Ls. In all sequences, however, identification of areas with remyelination, Wallerian degeneration, and gray matter demyelination, as depicted by myelin staining, was not possible.
CONCLUSIONS:
T2-w GRASE images may replace T2-w TSE for clinical use. However, even at 7T, both sequences fail in detecting and characterizing MS disease beyond visible WM-Ls.
Keywords: Multiple sclerosis, white matter lesions, 7.0 Tesla magnetic resonance imaging, T2-weighted turbo spin echo imaging, T2- weighted gradient and spin echo imaging
Introduction
The interest in the use of 7.0 Tesla (7T) MRI to study brain disease in patients with MS is growing.1–17 The increase in signal-to-noise ratio (SNR) and contrast-to-noise ratio (CNR) at 7T can be used to increase spatial resolution and reduce scanning time. The combination of these factors may ultimately translate into: (1) better delineation of white matter (WM)/gray matter (GM) tissue and lesions and (2) increased ability to detect disease not otherwise visible at lower magnetic fields MRI.5,10,13
Currently, T2-weighted (T2-w) 2-dimensional (2D) spin echo (SE) imaging and T2-w 2D Fluid-Attenuated Inversion Recovery (FLAIR) techniques are the clinical gold standard to identify MS-induced WM-lesions (WM-Ls) by MRI.18–20 WM-L load on T2-w SE images is an important imaging metric of disease presence and progression for monitoring patients not only in daily clinical practice but also during experimental clinical trials.
One of the drawbacks associated with the acquisition of T2-w SE images is the relatively high specific absorption rate (SAR) of radio-frequency (RF) energy in the human body arising from the series of RF refocusing pulses in the sequence. The high values of SAR hamper the clinical application of T2-w SE images at 7T. Recent work has shown that an approach employing power independent of number of slices RF pulses combined with multiband imaging reduces SAR sufficiently to enable turbo spin echo (TSE) imaging at 7T.21 However, acquiring volumetric T2-w SE and T2-w FLAIR images with spatially uniform image quality remains difficult at 7T. The inhomogeneity of the RF transmit field (B1) leads to significant signal loss due to imperfect RF refocusing (for T2-w FLAIR and T2-w SE) and/or incomplete inversion recovery signal nulling (for T2-w FLAIR).
T2-w GRASE, a combined SE and gradient echo (GRE) technique,22 is a viable alternative to T2-w SE and FLAIR imaging at 7T. GRASE is a rapid MRI technique that uses the speed from gradient refocusing in combination with the chemical-shift/static magnetic field (B0) inhomogeneity insensitivity from spin-echo refocusing to acquire T2-w images with a limited number of refocusing RF pulses.23,24 Besides reducing SAR, the lower number of refocusing RF pulses in GRASE sequence relative to conventional SE makes GRASE less vulnerable to B1 inhomogeneity (at the cost of a small increase in sensitivity to B0). Because GRASE achieves similar contrast to T2-w SE images with lower RF deposition and higher signal uniformity at high field, GRASE could be a compelling alternative approach for clinical MRI at 7T.
With our study, we aim to investigate the value of 7T T2-w GRASE sequences compared to T2-w turbo TSE images in detecting MS-induced WM brain disease at 7T. The ultimate goal is assessing whether the use of T2-w GRASE could replace that of T2-w TSE when imaging MS patients at 7T.
Materials and Methods
This is a collaborative project between Vanderbilt University (Nashville, TN, USA) and the Center for Brain Research of the Medical University in Vienna (Austria, EU).
The samples used for this study are derived from 3 MS patients. The Rocky Mountain MS Center Brain Bank (Englewood, CO, USA) donated the tissue samples to Vanderbilt University. Patients were known to be not affected by any other neurological condition apart from MS. Causes of death were not due to neurological diseases in all cases although 1 patient died because of systemic MS complications. Ages of death were 39, 69, and 72 years. The donors were all females. Each donor consented to the study prior to death and the study was conducted in accordance with HIPAA compliance.
Whole brain tissue was fixed in paraformaldehyde from 2 to 18 hours after death in each case. Details on tissue collection and preparation for the MRI have been previously described.25
Image Acquisition
Scans were acquired using a whole body 7T Achieva MRI scanner (Philips Healthcare, Cleveland, OH) equipped with a volume transmit and 32-channel receive head coil (NOVA Medical, Wilmington, MA).
For each 2D acquisition, 40 contiguous slices of .7 mm3 isotropic resolution over a constant field of view (18 cm × 24 cm), repetition time (TR = 23,000 ms), turbo SE factor (TSE = 4), sensitivity encoding parallel imaging acceleration factor (SENSE = 2, Right-Left), and flip angle (90) were obtained. For T2-w SEs, we examined multiple TE values to assess the sensitivity to WM-Ls. For T2-w GRASE, we examined various combinations of EPI train length and TE.
TEs from 50 to 75 with 5 ms increments were sampled with various combinations of EPI factors (EPI = 3 for TEs = 50/75; EPI = 5 for TEs = 60/75; EPI = 7 for TEs = 70/75; n = 12 sequences). For T2-w TSE acquisitions, we studied TEs ranging from 35 to 75 with increments of 5 ms (n = 9 sequences). To allow for SNR and CNR calculations, 2 repetitions of each scan were acquired. Scanning time for each volume ranged from 4:13 minutes (T2-w GRASE TE=75, EPI=7) to 25:18 minutes (T2-w SE TE = 75). Estimated SAR levels ranged from <1.5 W/kg (GRASE sequence) to <1.6 W/kg (TSE sequence). Details of SAR and acquisition time of each sequence are reported in Table 1.
Table 1.
SAR and Acquisition Time of Each Sequence
| SAR | Acquisition | |
|---|---|---|
| T2-w TSE Images | Level (W/kg) | Time (Min:Secs) |
| (TE = ms) | ||
| TE 35 | <1.6 | 25:18 |
| TE 40 | <1.6 | 25:18 |
| TE 45 | <1.6 | 25:18 |
| TE 50 | <1.6 | 25:18 |
| TE 55 | <1.6 | 25:18 |
| TE 60 | <1.6 | 25:18 |
| TE 65 | <1.6 | 25:18 |
| TE 70 | <1.6 | 25:18 |
| TE 75 | <1.6 | 25:18 |
| T2-w GRASE Images (EPI/TE = ms) | ||
| TE/EPI 3/50 | <1.5 | 8:49 |
| TE/EPI 3/55 | <1.5 | 8:49 |
| TE/EPI 3/60 | <1.5 | 8:49 |
| TE/EPI 3/65 | <1.5 | 8:49 |
| TE/EPI 3/70 | <1.5 | 8:49 |
| TE/EPI 3/75 | <1.5 | 8:49 |
| TE/EPI 5/60 | <1.5 | 5:45 |
| TE/EPI 5/65 | <1.5 | 5:45 |
| TE/EPI 5/70 | <1.5 | 5:45 |
| TE/EPI 5/75 | <1.5 | 5:45 |
| TE/EPI 7/70 | <1.5 | 4:13 |
| TE/EPI 7/75 | <1.5 | 4:13 |
Image Postprocessing
Images were visualized and processed using the Medical Image Processing, Analysis, and Visualization application (MIPAV version 3.1.6; http://mipav.cit.nih.gov).
Image Analysis
CNR Measurements
CNR between different regions of interest (ROIs) were computed on T2-w TSE image with different TEs (n = 9) and T2-w GRASE images with variable combinations of TEs and EPIs (n = 12). CNR measurement between 2 ROIs, namely ROI-A and ROI-B was given by the following formula: (SI-ROI-A/σ) – (SI-ROI-B/σ), where SI is the signal intensity (SI) for specific ROI computed on the average of 2 repetitions, and σ is the pooled standard deviation of the images noise estimated using a single large rectangular ROI placed entirely within the boundaries of the specimen on the subtraction image of the 2 repetitions.26 The subtraction method for estimating noise is described elsewhere in the literature.27
ROI Selection for CNR Measurements
Two investigators, blinded to the other’s ROI placement, traced ROIs using graphic tools available in MIPAV. Each observer placed 10 ROIs of 12 voxels (8.4 mm3) in WM-Ls28 and 10 ROIs of 12 voxels (8.4 mm3) in the NAWM for the measurements of SI. To avoid partial volume effects, we selected slices where each ROI could be positioned away from boundary regions (ie, at the interface GM/WM or normal/disease tissue). The distance to any boundary was greater than 10 pixels.29 Care was also taken to place ROIs far from each other. In case of large lesions encompassing several slices, whenever possible, care was taken to avoid selecting the same lesions twice, even if on separate slices.
One large ROI of 12,870 (observer-1, 9.009 mm3) and 12,980 (observer-2, 9.086 mm3) pixels was placed entirely within the boundaries of the specimen on the subtraction image for the estimation of σ.
Histology
After scanning, formalin-fixed brain tissue was dehydrated and embedded in paraffin. Whole hemispheric slices were cut with a microtome to have a thickness 7–10 μm and mounted on slides with appropriate size. For basic evaluation of general pathology and demyelination, hematoxylin and eosin, and Luxol fast blue (LFB) myelin stains were performed. Immunohistochemical staining for proteolipid protein (PLP) was done for detailed evaluation of demyelination, remyelination, and primary Wallerian degeneration with secondary demyelination.30 The primary antibody against PLP was applied twice, that is, a first time overnight at 4 °C and a second time for 1 hour at room temperature after 5 rinses with wash buffer.
WM-Ls Maps
For the generation of the lesion maps, sections stained for LFB and PLP were scanned with an Agfa Duoscan™ scanner at resolution of 4,000 pixels per inch. Images were stored as JPEG files and transferred to a workstation, where they were displayed on a screen while simultaneously inspected in detail with a Reichert™ Microstar IV microscope at high magnification. Areas of demyelination were manually outlined and marked on the LFB-stained sections using Adobe Photoshop CS4®. MS lesions were indicated using green (complete demyelination), yellow (remyelination), red (Wallerian degeneration with secondary demyelination), and orange (deep GM demyelination). On a separate workstation, these images were displayed along with each corresponding 7T image for visual comparison.
Statistical Analysis
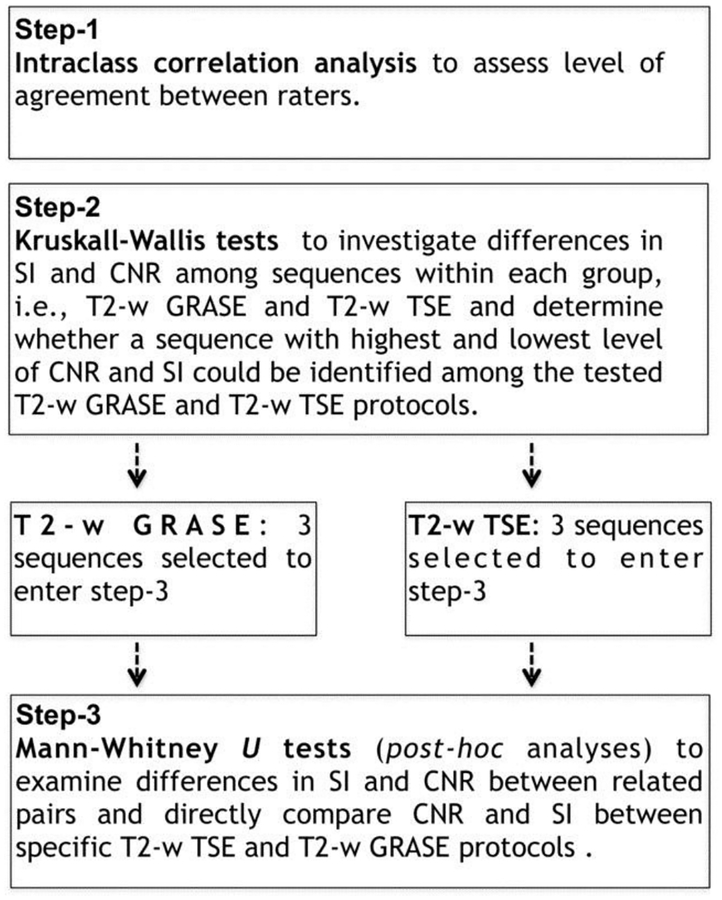
Several analyses were performed and Figure 1 is a schematic of the different processes. Intraclass correlation analysis evaluated the level of agreement between raters generating intraclass correlation coefficients (ICC) and F-Test with True Value 0 in the measurements of SI of WM-Ls (WM-Ls SI), NAWM ROIs (NAWM ROIs SI), and CNR.
Fig 1.
Flow-chart of proposed analyses.
First, all T2-w TSE sequences (n = 9) and T2-w GRASE sequences (n = 12) were treated as 2 separated groups. Within each group, differences in SI and CNR among images were evaluated. Kruskall-Wallis tests were used to investigate differences in SI and CNR among sequences. The aim of this first set of comparisons was to evaluate whether a sequence with highest and lowest level of CNR and SI could be identified among the tested T2-w GRASE and T2-w TSE protocols.
A second set of analyses was performed to test differences in SI and CNR among sequences found to show a significantly higher or lower level of SI and/or CNR on the basis of the first set of comparisons. Mann-Whitney U-tests were used for post hoc analyses to examine differences in SI and CNR between pairs. In these analyses, we aimed to directly compare CNR and SI between specific T2-w TSE and T2-w GRASE protocols.
In all comparisons, nonparametric approaches were preferred to parametric tests because the assumption that data were normally distributed was not satisfied. Kruskall-Wallis and Mann-Whitney U-tests were preferred since measurements of SI and CNR obtained from each sequence were treated as independent variables.
Bonferroni correction for multiple comparisons was applied. The statistical analyses were performed using the Statistical Package for Social Science (SPSS, Inc, Chicago, IL, USA) version 19.0.
Results
Measures of Interobserver Variability
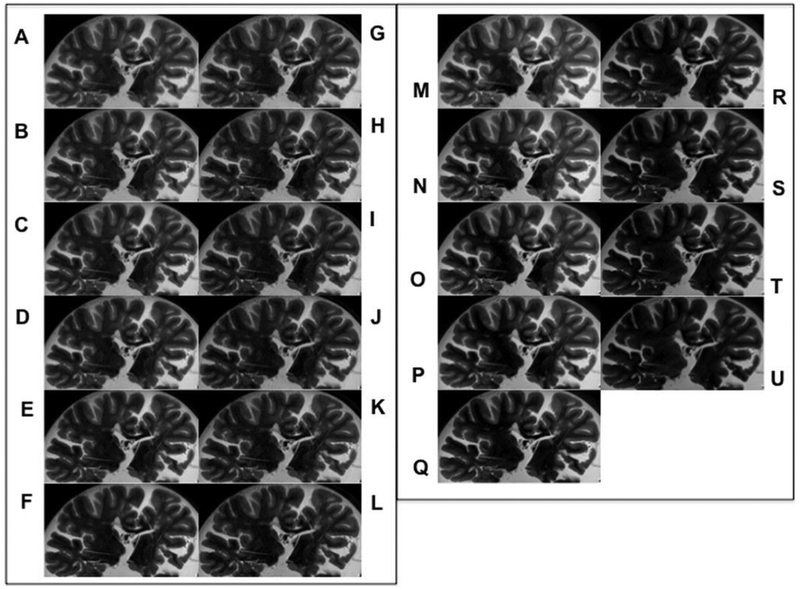
There were no identifiable differences in the number and size of WM-Ls across sequences by visual inspection (Fig 2).
Fig 2.
Image comparison. T2-w GRASE acquisitions: TEs from 50 to 75 with 5 ms increments sampled with various combinations of EPI factors. EPI = 3 for TEs = 50/75 (A-F); EPI = 5 for TEs = 60/75 (G-J); EPI = 7 for TEs = 70/75 (K-L). T2-w TSE acquisitions with TEs ranging from 35 to 75 with increments of 5 ms (M-U).
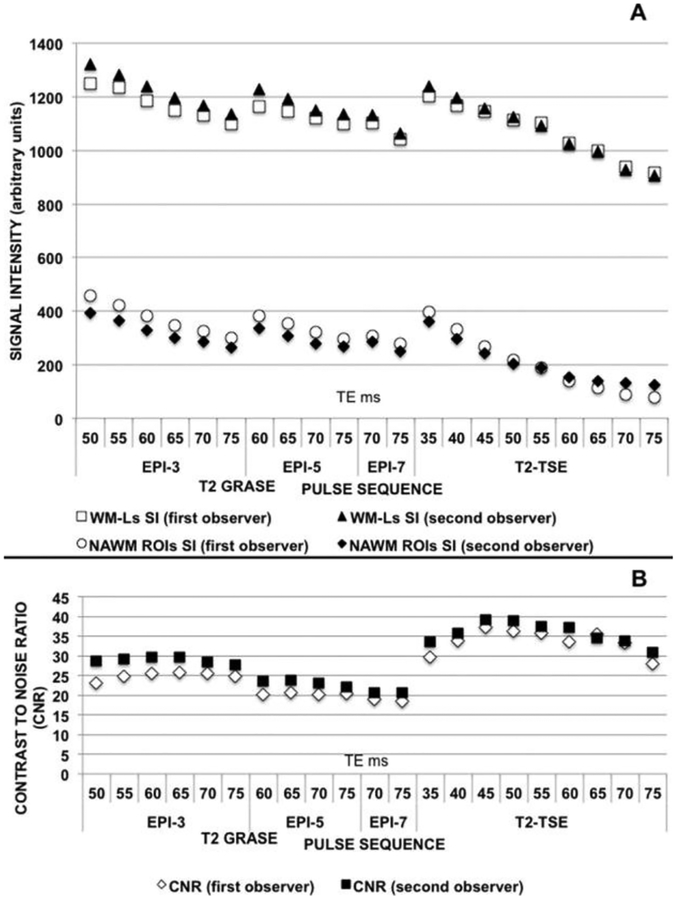
Intraclass correlation analysis displayed a high level of concordance between the 2 observers in measuring WM-Ls SI (mean ICC = .9651, F = 25,74),NAWMROIs SI (mean ICC = .9208, F=11,13) and CNR (mean ICC=.8901, F=78,03). Figure 3 shows WM-Ls SI (Fig 3A), NAWM ROIs SI (Fig 3A), and CNR (Fig 3B) as function of TE and combinations of EPI/TE for each examined T2-w TSE image and T2-w GRASE image, respectively. Measurements of each of the 2 investigators are reported using white and black markers.
Fig 3.
Interobserver variability. (A) WM-Ls SI (black triangles and white squares) and NAWM ROIs SI (white circles and black rhombi) as function of EPI/TE and TE in T2-w GRASE and T2-w TSE images. The white squares and circles represent the measurements of the first observer; the black triangles and rhombi represent the measurements of the second observer. (B) WM-Ls/NAWM CNR changes as function of EPI/TE and TE in T2-w GRASE and T2-w TSE images. White rhombi represent the measurement of the first observer and black squares represent the measurements of the second observer.
Differences in SI and CNR across Sequences
T2w-w GRASE Images
Among T2-w GRASE sequences, Kruskal-Wallis tests showed significant differences in CNR (P < .0001) but not in WM-Ls SI (P = .028) and NAWM ROIs SI (P = .021) depending on which combination of EPI/TE was used (Figs 3A, B). For this set of comparisons, the significance level was Bonferroni corrected and set at P ≤ .017 (ie, .05/3). For CNR measures, post hoc analysis with Mann-Whitney U-tests was conducted with a significance level set at P ≤ .0007 (ie, .05/66). None of the related set of pairs showed statistically significant differences although many showed a P value < .05.
T2-w TSE Images
Kruskal-Wallis tests showed statistically significant differences in WM-Ls SI (P = .006) and NAWM ROIs SI (P ≤ .0001), but not CNR (P = .055) depending upon which TE was used for T2-w TSE images (Fig 3). For this set of comparisons, the significance level was set at P ≤ .017 (ie, .05/3).
For WM-Ls SI and NAWM-ROIs-SI post hoc analysis with Mann-Whitney U-tests were conducted with a significance level set at P ≤ .0007 (ie, .05/72, ie, 36 comparisons for the WM-Ls SI and 36 comparisons for the NAWM ROIs SI). The results disclosed no significant differences in WM-Ls SI between any pair, although several pairs showed a P value < .05. Conversely, statistically significant differences in NAWM ROIs SI were seen in several pairs (see Table 2).
Table 2.
Differences in NAWM-ROIs SI among T2-w TSE Images with different TES
| TE (ms) | P Value |
|---|---|
| 35 | <0.0001 vs. all TEs but TE = 40 and 45 ms |
| 40 | <0.0001 vs. all TEs but TE = 35 and 45 ms |
| 45 | <0.0001 only vs. TE = 60–75 ms |
| 50 | <0.0001 only vs. TE = 65–75 ms |
Significant level at P ≤ 0.0007.
Comparisons between T2-w GRASE and T2-w TSE Images
On the basis of the achieved results, T2-w TSE sequences with TE = 35, TE = 45, and TE = 75 were selected to enter the second set of comparisons. The selection was based upon the fact that T2-w SE sequences with TE = 35 ms, TE = 45 ms, and TE = 75 ms differed in NAWM ROIs SI compared to the majority of the other sequences. In addition, although not statistically significant, the T2-w TSE sequence with TE=45 ms presented the highest CNR value.
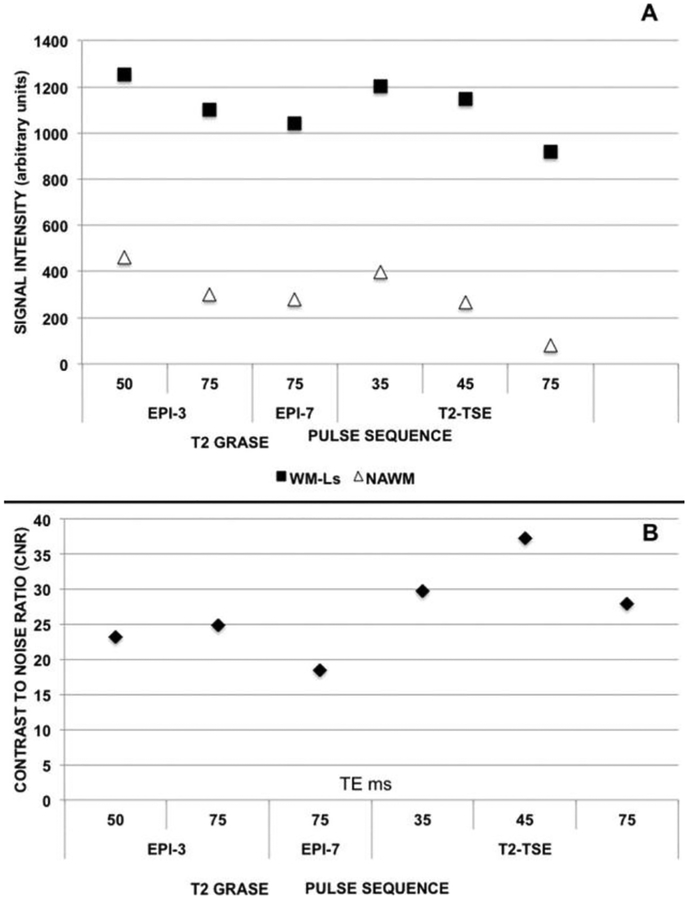
For T2-w GRASE images, no statistically significant differences were seen in any of the measures across sequences upon Bonferroni adjustment. Thus, an arbitrary selection of 3 sequences representative of the entire group was made. Similar to the procedure applied to T2-w TSE images, the selection was based upon NAWM ROIs SI and CNR. The selected sequences corresponded to the T2-w GRASE protocols with EPI/TE = 3/50 ms and EPI/TE = 7/75 ms because those protocols yielded relatively higher and lower NAWM ROIs SI as well as the T2-w GRASE protocols with EPI/TE = 3/75 ms because that protocol produced the relatively higher CNR. Figure 4 shows WM-Ls SI, NAWM ROI SI (Fig 4A), and CNR (Fig 4B) of these 6 sequences side-by-side.
Fig 4.
SI and CNR measurements. (A) WM-Ls SI (black squares) and NAWM ROIs SI (white triangles) changes as function of EPI/TE and TE in T2-w GRASE and T2-w TSE images. (B) CNR measurements. WM-Ls/NAWM CNR as function of EPI/TE and TE in T2-w GRASE and T2-w TSE images.
Statistically significant differences were found among WM-Ls-SI (P = .002), NAWM ROIs SI (P < .0001), and CNR (P < .0001) based on which TE and combination of EPI/TE were used. For this set of comparisons, the significance level was set at P ≤ .017 (ie, .05/3). Post hoc analyses (see Table 3) were conducted with a Bonferroni correction resulting in a significance level set at P ≤ .0024 (ie, .05/21). We observed T2-w GRASE images with EPI/TE = 3/50 ms having comparable SI of WM-Ls and NAWM as well as CNR only with the acquisition with TE = 35 ms. T2-w GRASE images with EPI/TE = 3/75 ms had an intermediate SI for WM-Ls and NAWM between T2-w SE images with TE = 35 ms and TE = 75 ms. T2-w GRASE images with EPI/TE = 7/75 ms had significantly lower CNR measures than any examined T2-w SE images as well as higher NAWM ROIs SI than T2-w SE images with TE = 75 ms. T2-w SE sequences with TE = 75 had significantly lower SI in the NAWM ROIs compared to any of the examined GRASE sequences.
Table 3.
Differences in WM-Ls SI, NAWM ROIs SI, and CNR among Tw GRASE and T2-w TSE Images
| P Value | |||
|---|---|---|---|
| Sequence Pair | NAWM ROIs SI | WM‐Ls SI | CNR |
| T2-w GRASE EPI/TE = 3/50 vs. | |||
| T2-w TSE TE = 35 ms | NS | NS | NS |
| T2-w TSE TE = 45 ms | <0.0001 | NS | 0.001 |
| T2-w TSE TE = 75 ms | <0.0001 | 0.002 | NS |
| T2-w GRASE EPI/TE = 3/75 vs. | |||
| T2-w TSE TE =35 ms | NS | NS | NS |
| T2-w TSE TE = 45 ms | NS | NS | 0.002 |
| T2-w TSE TE = 75 ms | <0.0001 | NS | NS |
| T2-w GRASE EPI/TE = 7/75 vs. | |||
| T2-w TSE TE = 35 ms | NS | NS | 0.002 |
| T2-w TSE TE = 45 ms | NS | NS | <0.0001 |
| T2-w TSE TE = 75 ms | <0.0001 | NS | 0.002 |
NS = not significant (ie, P > 0.0024).
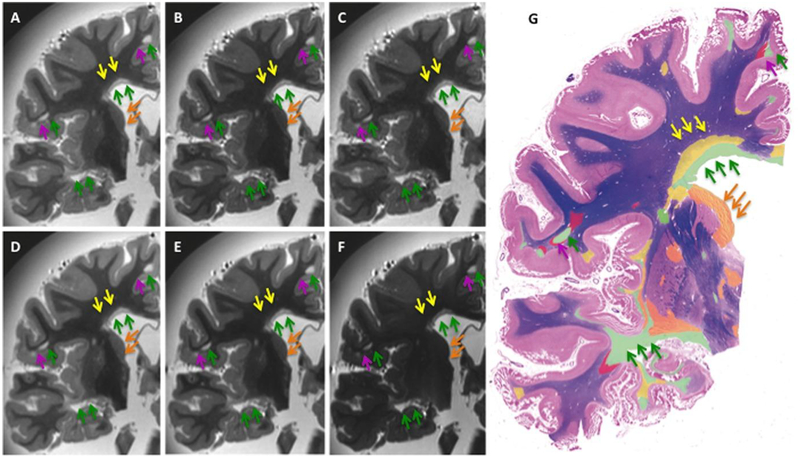
Imaging and Pathological Correlation
Side-by-side MRI-histopathological comparisons were obtained between LFB and PLP stainings and each of the above 6 sequences. An example of these comparisons is shown in Figure 5. By visual inspection, areas of complete WM demyelination as determined by histology (indicated with the green arrows in the color-coded WM map and MRIs in Fig 5) were seen in their entire extent in each of the MRIs. SI in NAWM was generally low. The latter may have limited the ability to see signal changes due to remyelination seen by histopathology (indicated with the yellow arrows in the color-coded WM map and MRIs in Fig 5). Similarly, all the sequences only minimally displayed areas of GM demyelination (indicated with the orange arrows in the color-coded WM map and MRIs in Fig 5) and/or axonal degeneration with secondary WM loss (indicated with the dark-pink arrows in the color-coded WM map and MRIs in Fig 5).
Fig 5.
Imaging/histopathology comparisons. Side-by-side LFB staining, T2-w GRASE and T2-w TSE images exhibiting the best SI and CNR in each group of 3 representative samples. In the figure, (A) T2-w GRASE sequence EPI/TE = 3/50 ms, (B) T2-w GRASE sequence EPI/TE = 3/75 ms, (C) T2-w GRASE sequence EPI/TE = 7/75 ms, (D) T2-w TSE sequence TE = 35 ms, (E) T2-w TSE sequence TE = 45 ms, (F) T2-w TSE sequence TE = 75 ms, (G) color-coded LFB staining indicating in green areas of complete WM demyelination, in yellow areas of WM remyelination, in red areas of WM Wallerian degeneration with secondary demyelination, and in orange areas of deep GM demyelination.
Discussion
Our 7T postmortem work: (1) allowed for acquisition of T2-w SE images that are not possible to be obtained in vivo due to the high SAR and (2) offered the unique opportunity to validate the MRI findings with histopathology. Validating the use of a sequence by histopathology is an important, not always achievable, step toward the understanding of its biological significance for a given disease/pathology. Two main findings were reported. The first one is that GRASE imaging and T2-w TSE imaging at 7T are comparable. The second one is that even at 7T either sequence fails in disclosing the entire amount of WM pathology seen in MS patients. The latter is a novel finding and extremely important for a better delineation on the importance of imaging MS at 7T and disease pathology in general, beyond the visibility of MRI.
GRASE Imaging and T2-w SE Imaging at 7T are Comparable
At lower magnetic strength, GRASE imaging was shown to reduce scan time for brain imaging and to have potential for clinical use in patients with brain disorders.31–34
In patients with MS, initial in vivo studies35,36 at lower magnetic strength demonstrated that GRASE scans may have lower sensitivity in the detection of WM-Ls and lower reproducibility of measured WM-Ls volume than both T2-w FLAIR and rapid acquisition relaxation-enhanced sequences also known as TSE or fast SE. A larger follow-up study,37 however, suggested that the application of a dual echo and the use of a proton density-weighted GRASE image, as opposed to T2-w GRASE sequence, could permit better WM-Ls visibility. No data are available for comparisons at higher field strength and our work is the first one of this kind.
In our study, the sequences were selected with the goal to investigate the effects of varying TEs on TSE and GRASE in disease visibility both within and outside lesions. All GRASE scans used the same acceleration factor (TSE factor = 4) used by the TSE scans. However, GRASE scans had additional acceleration from gradient echoes collected between SEs. As GRASE TE was allowed to increase, more space was available for additional gradient echoes. To isolate the effect of changing from TSE to GRASE by adding gradient echoes between SEs, we kept TR, voxel size, and number of signal averages constant. This caused GRASE scans to be faster than TSE scans by a factor equal to the number of gradient echoes per SE. Similarly, when TSE or GRASE scans were designed with different TEs, the number of SEs, TR, voxel size, and number of signal averages were held constant in order to isolate the T2-weighting effect of the extended TE. Our results show that 7T high-resolution T2-w GRASE images with EPI/TE = 3/50 ms and 3/75 ms yield CNR between WM-Ls and NAWM-ROIs comparable to T2-w TSE sequences. T2-w GRASE images with EPI/TE = 3/50 ms also yield to higher SI in WM-Ls and NAWM-ROIs compared to T2-w TSE sequences with TEs of 45–75 ms. SI of WM-Ls and NAWM decreased as a function of increasing TE for both T2-w GRASE and T2-w TSE sequences. However, somewhat surprisingly, SI levels of both WM-Ls and NAWM drop dramatically for longer TEs in the T2-w TSE protocols. Normally, the signal refocusing in a TSE approach should preserve signal longer compared to a GRE approach. We postulate that imperfect refocusing RF pulses (caused by B1 inhomogeneity) enhances the WM-Ls and NAWM signal suppression. Therefore, the lower signal suppression in the T2-w GRASE images is explained by the lower TSE factor (lower number of imperfect refocusing pulses) compared to the T2-w TSE sequences.
Even at 7T, T2-w Sequences Fail in Disclosing the Entire Amount of WM Pathology Seen in MS Patients
From side-by-side comparison of MRI and histopathological stainings, we first observed that all sequences accurately demarcated WM-Ls in their entire extent where the degree of demyelination was defined as complete by histopathology. We have indicated these areas with green arrows and green-color coding in the example MRIs and WM-L map, respectively (Fig 5). The results substantiate the notion that GRASE imaging can replace the use of T2-w SE imaging for clinical use at 7T. Disappointingly, however, all sequences failed to reveal areas of WM remyelination, WM Wallerian axonal degeneration with secondary demyelination, and GM demyelination indicated by histopathology. The results indicate that even at 7T, T2-w sequences are sensitive to areas where myelin loss is advanced and it is the predominant pathological feature. When remyelination has occurred and/or secondary demyelination induced by Wallerian degeneration is ongoing, T2-w techniques do not have enough sensitivity in depicting these processes. Similarly, in areas of GM where less myelin is present, T2-w techniques fail in demonstrating small signal changes induced by GM demyelination.
From Postmortem Findings into In Vivo Application
A combined imaging-pathological study postmortem is as far as one can go in comparing lesion detectability between T2-w TSE and other sequences at 7T. Acquiring 7T T2-w TSE in vivo remains impractical.
Notwithstanding the above consideration, one should keep in mind that MRI properties of postmortem tissue differ from in vivo tissue. Formaldehyde fixation may lead to a reduction of the T2 relaxation time38 by facilitating protein cross-linking.39 Natural decomposition of the tissue may lead to increased water content or mobility, which may increase T2 values. Overall, postmortem samples have reduced T2 values compared to in vivo tissues.40 The cumulative effect of changes in T2 relaxation time caused by tissue fixation may thus affect the visibility of WM abnormalities and impair a direct translation of postmortem results into in vivo application.
Study Limitations
Some study limitations must be acknowledged before drawing conclusions from our findings. First, a drawback associated with our study is that the difference in WM-Ls volume among different sequences was not quantitatively evaluated. We reasoned that since automated tools to measure WM-Ls volume in postmortem images have not been standardized, our results could not be generalizable. Furthermore, the similar visual detection of WM-Ls minimizes the impact of not computing WM-L load. A second limitation is given by the fact that only 3 MS brain specimens were analyzed in our study due to limited availability of samples. Our group is already expanding the study to a larger number of samples with the additional goal of investigating in greater detail the pathological significance of signal alteration by T2-GRASE images at 7T.
Conclusions
Despite these limitations, we believe that our results provide a solid preliminary support to the notion that T2-w GRASE images may be of high value in replacing T2-w TSE images for clinical imaging at 7T. Specifically, T2-w GRASE imaging may replace T2-w TSE imaging for the use that T2-w imaging currently has in MS patients at both the clinical and research settings. Even at ultrahigh field MRI, however, both sequence approaches are still far from: (1) reporting on the extent of damage induced by primary or secondary demyelinating mechanisms and (2) depicting zones of MS tissue where restoration of myelin has occurred.
Coupling T2-w imaging with MRI sequences sensitive to other mechanisms of contrast remains crucial to visualize WM disease in its entirety in patients with MS.
Acknowledgments
The Investigators are grateful to all the patients for the precious donation of the brain for research studies and to the Rocky Mountain MS Center for providing the specimen material. We are indebted to the MRI technical personnel of the Vanderbilt University Institute of Imaging Science Mrs. Debbie Boner, Mrs. Leslie McIntosh, and Mrs. Donna Butler for their assistance with scanning postmortem brains. The investigators gratefully acknowledge the help of Mr. Christopher J. Cannistraci with IRB protocols. We express our gratitude to Dr. Sriram Subramian for supporting this project and permitting the team to image part of the brain samples. We gratefully acknowledge the operational support of the VUIIS Human Imaging Core by the Vanderbilt Institute for Clinical and Translational Research (VICTR) and the VICTR Clinical and Translation Science Award (UL1 TR000445 from NCATS/NIH). Dr. Francesca Bagnato’s contribution to this work was sustained in part by the Intramural Research Program of the NINDS, NIH, Bethesda, MD, USA.
References
- 1.Hammond KE, Metcalf M, Carvajal L, et al. Quantitative in vivo magnetic resonance imaging of multiple sclerosis at 7 Tesla with sensitivity to iron. Ann Neurol 2008;64:707–13. [DOI] [PubMed] [Google Scholar]
- 2.Ge Y, Zohrabian VM, Grossman RI. Seven-Tesla magnetic resonance imaging: new vision of microvascular abnormalities in multiple sclerosis. Arch Neurol 2008;65:812–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammond KE, Lupo JM, Xu D, et al. Development of a robust method for generating 7 T multichannel phase images of the brain with application to normal volunteers and patients with neurological diseases. Neuroimage 2008;39:1682–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tallantyre EC, Brookes MJ, Dixon JE, et al. Demonstrating the perivascular distribution of MS lesions in vivo with 7-Tesla MRI. Neurology 2008;70:2076–8. [DOI] [PubMed] [Google Scholar]
- 5.Tallantyre EC, Morgan PS, Dixon JE, et al. A comparison of 3T and 7T in the detection of small parenchymal veins within MS lesions. Invest Radiol 2009;44:491–4. [DOI] [PubMed] [Google Scholar]
- 6.Haacke EM, Makki M, Ge Y, et al. Characterizing iron deposition in multiple sclerosis lesions using susceptibility weighted imaging. J Magn Reson Imaging 2009;29:537–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mainero C, Benner T, Radding A, et al. In vivo imaging of cortical pathology using ulra-high field MRI. Neurology 2009;73:941–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madelin G, Oesingmann N, Inglese M. Double inversion recovery MRI with fat suppression at 7 tesla: initial experience. J Neuroimaging 2010;20:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metcalf M, Xu D, Okuda DT, et al. High-resolution phased-array MRI of the human brain at 7 tesla: initial experience in multiple sclerosis patients. J Neuroimaging 2010;20: 141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tallantyre EC, Morgan PS, Dixon JE, et al. 3 Tesla and 7 Tesla MRI of multiple sclerosis cortical lesions. J Magn Reson Imaging 2010;32:971–7. [DOI] [PubMed] [Google Scholar]
- 11.Tallantyre EC, Dixon JE, Donaldson I, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology 2011;76:534–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mistry N, Tallantyre EC, Dixon JE, et al. Focal multiple sclerosis lesions abound in ‘normal appearing white matter’. Mult Scler 2011;17:1313–23. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen AS, Kinkel RP, Tinelli E, et al. Focal cortical lesion detection in multiple sclerosis: 3 Tesla DIR versus 7 Tesla FLASH-T2. J Magn Reson Imaging 2012;35:537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Graaf WL, Zwanenburg JJ, Visser F, et al. Lesion detection at seven Tesla in multiple sclerosis using magnetisation prepared 3D-FLAIR and 3D-DIR. Eur Radiol 2012;22:221–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yao B, Bagnato F, Matsuura E, et al. Chronic multiple sclerosis lesions: characterization with high-field-strength MR imaging. Radiology 2012;262:206–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinnercker T, Bozin I, Dorr J, et al. Periventricular venous density in multiple sclerosis is inversely associated with T2 lesion count: a 7 Tesla MRI study. Mult Scler 2013;3:316–25. [DOI] [PubMed] [Google Scholar]
- 17.Wuerfel J, Sinnecker T, Ringelstein EB, et al. Lesion morphology at 7 Tesla MRI differentiates Susac syndrome from multiple sclerosis. Mult Scler 2012;11:1592–9. [DOI] [PubMed] [Google Scholar]
- 18.Poloni G, Minagar A, Haacke EM, et al. Recent developments in imaging of multiple sclerosis. Neurologist 2011;17:185–204. [DOI] [PubMed] [Google Scholar]
- 19.Filippi M, Horsfield MA, Tofts PS, et al. Quantitative assessment of MRI lesion load in monitoring the evolution of multiple sclerosis. Brain 1995;118:1601–12. [DOI] [PubMed] [Google Scholar]
- 20.Miller DH, Albert PS, Barkhof F, et al. Guidelines for the use of magnetic resonance techniques in monitoring the treatment of multiple sclerosis. Ann Neurol 1996:39:6–16. [DOI] [PubMed] [Google Scholar]
- 21.Norris DG, Boyacioğlu R, Schulz J, et al. Application of PINS radiofrequency pulses to reduce power deposition in RARE/turbo spin echo imaging of the human head. Magn Reson Med 2014;71:44–9. [DOI] [PubMed] [Google Scholar]
- 22.Feinberg DA. GRASE imaging provides image quality and speed. Diagn Imaging (San Franc) 1993;15:95–8, 101–3. [PubMed] [Google Scholar]
- 23.Fellner F, Fellner C, Held P, et al. Comparison of spin-echo MR pulse sequences for imaging of the brain. Am J Neuroradiol 1997;18:1617–25. [PMC free article] [PubMed] [Google Scholar]
- 24.Rockwell DT, Melhem ER, Bhatia RG. GRASE (gradient- and spin-echo) MR of the brain. Am J Neuroradiol 1997;18:1923–48. [PMC free article] [PubMed] [Google Scholar]
- 25.Bagnato F, Yao B, Cantor F, et al. Multisequence-imaging protocols to detect cortical lesions of patients with multiple sclerosis: observations from a post-mortem 3 Tesla imaging study. J Neurol Sci 2009;282:80–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eapen M, Zald DH, Gatenby JC, et al. Using high-resolution MR imaging at 7T to evaluate the anatomy of the midbrain dopaminergic system. Am J Neuroradiol 2011;32:688–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dietrich O, Raya JG, Reeder SB, et al. Measurement of signal-to-noise ratios in MR images: influence of multichannel coils, parallel imaging, and reconstruction filters. J Magn Reson Imaging 2007;26:375–85. [DOI] [PubMed] [Google Scholar]
- 28.Fazekas F, Barkhof F, Filippi M. Unenhanced and enhanced magnetic resonance imaging in the diagnosis of multiple sclerosis. J Neurol Neurosurg Psychiatry 1998;64(1):2–5. [PubMed] [Google Scholar]
- 29.Riva M, Ikonomidou VN, Ostuni JJ, et al. Tissue-specific imaging is a robust methodology to differentiate in vivo T1 black holes with advanced multiple sclerosis-induced damage. Am J Neuroradiol 2009;30:1394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bradl M, Lassmann H. Progressive multiple sclerosis. Semin Immunopathol 2009;31:455–65. [DOI] [PubMed] [Google Scholar]
- 31.Umek W, Ba-Ssalamah A, Prokesch R, et al. Imaging of the brain using the fast spin-echo and gradient spin-echo techniques. Eur Radiol 1998;8:409–15. [DOI] [PubMed] [Google Scholar]
- 32.Fellner F, Schmitt R, Trenkler J, et al. Turbo gradient spin-echo (GRASE): first clinical experiences with a fast T2-weighted sequence in MRI of the brain. Eur J Radiol 1995;19: 171–6. [DOI] [PubMed] [Google Scholar]
- 33.Maubon AJ, Pothin A, Ferru JM, et al. Unselected brain 0.5 T MR imaging: comparison of lesion detection and characterization with three T2-weighted sequences. Radiology 1998;208: 671–8. [DOI] [PubMed] [Google Scholar]
- 34.Rockwell DT, Melhem ER, Bhatia RG. GRASE (gradient and spin-echo) MR of the brain. Am J Neuroradiol 1997;18:1923–8. [PMC free article] [PubMed] [Google Scholar]
- 35.Rovaris M, Yousry T, Calori G, et al. Sensitivity and reproducibility of fast-FLAIR, FSE and TGSE sequences for the assessment of brain MRI lesion load in multiple sclerosis: a preliminary study. J Neuroimaging 1997;7:98–102. [DOI] [PubMed] [Google Scholar]
- 36.Yousry TA, Filippi M, Becker C, et al. Comparison of SE, FSE, fast-FLAIR and TGSE sequences in detecting multiple sclerosis lesions. Am J Neuroradiol 1997;18:959–63. [PMC free article] [PubMed] [Google Scholar]
- 37.Filippi M, Rocca MA, Yousri I, et al. Lesion load and quantification on fast-flair, rapid acquisition relaxation-enhanced, and gradient spin echo MRI scans from multiple sclerosis patients. Magn Reson Imag 1999;7:1105–10. [DOI] [PubMed] [Google Scholar]
- 38.Fishbein KW, Gluzband YA, Kaku M, et al. Effects of formalin fixation and collagen cross-linking on T2 and magnetization transfer in bovine nasal cartilage. Magn Reson Med 2007;57: 1000–11. [DOI] [PubMed] [Google Scholar]
- 39.Kennan RP, Richardson KA, Zhong J, et al. The effects of cross-link density and chemical exchange on magnetization transfer in polyacrylamide gels. J Magn Reson B 1996;110:267–77. [DOI] [PubMed] [Google Scholar]
- 40.Dawe RJ, Bernnett DA, Schneider JA, et al. Postmortem MRI of human brain hemispheres: T2 relaxation times during formaldehyde fixation. Magn Reson Med 2009;6:810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]