Abstract
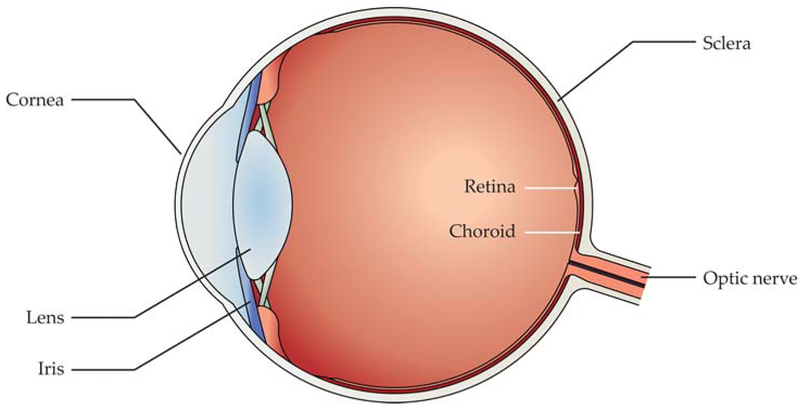
Vision begins when the eye’s optical system—the cornea, iris, and crystalline lens—projects an image onto the retina, the thin and nearly transparent sheet of neural tissue that lines the back of the eye (see figure 1). Photoreceptors located at the back of the retina transduce incident photons into neural signals that are relayed to the brain. Those signals form the basis for visual perception. In humans, cone photoreceptors, which number about 6 million, dominate the central region of the visual field and are responsible for color and high-resolution day vision. Rod photoreceptors, which number about 120 million, dominate the periphery and mediate night vision.
Like other neural cells, photoreceptors encode, process, and transmit information by means of electrical and chemical processes. In a resting state, the neurons maintain an intracellular electric potential of approximately −70 mV relative to the extracellular medium. That potential is controlled by transmembrane proteins called ion pumps, which keep the intracellular concentrations of sodium and chloride ions about a factor of 10 lower than in the extracellular medium and potassium ion concentrations about 30 times as large. A change in incident illumination alters the membrane potential and changes the rate of release of chemicals known as neurotransmitters from photoreceptors onto input synapses of secondary neurons located in the retina’s inner nuclear layer (see figure 2a). By converting light into electrochemical signals, photo -receptors act as the “camera pixels” of the eye.
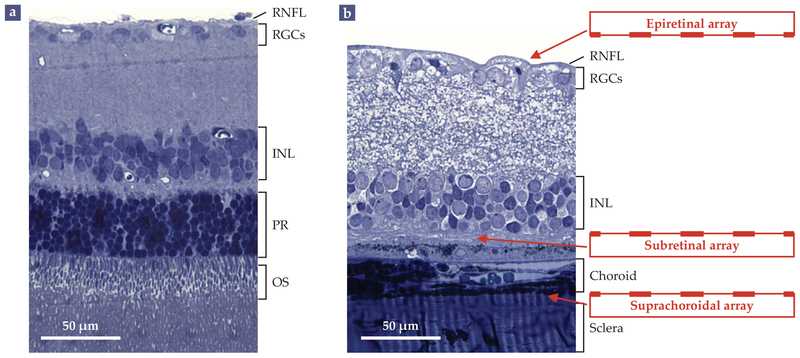
FIGURE 2. RETINAL TISSUE, FOR BETTER AND WORSE.
(a) A histological cross section shows the layers of a healthy rat retina. Absorption of light (incident from the top) by the outer segments (OS) of photoreceptor cells (PR) at the back of the retina generates electrical and chemical signals that are processed by the neurons of the inner nuclear layer (INL) and by the retinal ganglion cells (RGCs). The RGCs generate spike-like signals known as action potentials that propagate via the retinal nerve-fiber layer (RNFL) to the brain. (b) In a rat with retinal degeneration, the photoreceptor cells have all but wasted away. Vision can be partially restored by introducing electrode arrays on top of the nerve-fiber layer (epiretinal array), below the inner nuclear layer (subretinal array), or between the underlying choroid and sclera layers (suprachoroidal array). (Adapted from G. A. Goetz, D. V. Palanker, Rep. Prog. Phys. 79, 096701, 2016.)
Inner retinal neurons process visual signals in analog fashion: The release rate of neurotransmitters at the output synapses is a gradually varying function of the uptake rate of neurotransmitters at the input synapses. The cells transmit the results of their signal processing to the retinal ganglion cells at the retina’s surface. If the neurotransmitter signal received by a ganglion cell is sufficiently large, the cell responds with a spike-like variation known as an action potential: Na+ channels open, generating a rapid, roughly 100 mV increase in the cell potential; those channels then close and K+ channels open, causing the potential to plunge below its resting level; and within a few milliseconds, the ion pumps restore normal intracellular concentrations. The visual information digitally encoded in those all-or-nothing responses propagates to the brain along the ganglion cells’ long, slender axons, which form the optic nerve.
Any disturbance in the finely tuned process that converts incident light into trains of action potentials in the retinal ganglion cells can lead to blindness. For example, the clouding of the lens, called a cataract, can prevent the formation of sharp retinal images and thereby impair vision. Replacement of the nat ural lens with an artificial one restores normal vision.
Several diseases that afflict ocular tissues can lead to permanent blindness. A category of diseases called retinal degeneration is the leading cause of incurable blindness in the developed world today. Retinal degeneration leads to a gradual loss of photoreceptors and irreversibly impairs the ability of the visual system to convert light into neural signals.
Over the past decade, retinal prostheses have emerged as a promising technology for restoring vision lost due to retinal degeneration. The prostheses effectively function as artificial photoreceptors or even as artificial retinas; they use systems of cameras, computers, and electrodes to convert light into electronic signals that can be processed or transmitted by retinal cells. No retinal implant has yet fully restored a patient’s sight. But proof-of-concept devices are already demonstrating an ability to partially revive visual sensation, even in patients who’ve experienced decades of profound blindness.
Failing photoreceptors
The most common type of retinal degeneration, age-related macular degeneration (AMD), primarily affects older patients. It largely leaves peripheral vision intact; patients can navigate their surroundings but have difficulty reading, recognizing faces, and performing other tasks that require high visual acuity. A less common class of retinal degeneration, called retinitis pigmentosa (RP), originates from various genetic disorders and afflicts approximately 1 in 3500 people.1 It typically affects patients in their twenties or thirties and leads to profound blindness.
Both AMD and RP are characterized by a significant loss in spatial resolution, or visual acuity. In the US, visual acuity is quantified in the form 20/x, which signifies that the subject sees an object as clearly from 20 ft (6 m) as would a person with normal visual acuity from x ft. A visual acuity of 20/10 is twice as good, and 20/40 half as good, as normal. In the US, a person with a visual acuity of less than 20/200 is considered legally blind, though the World Health Organization sets the limit at 20/400.
A person with normal, 20/20 visual acuity can resolve lines spaced 1.75 mm apart from 20 ft away, which corresponds to a visual angle of 1 arcminute, or 5 μm on the retina. In addition to acuity, a sufficiently large field of vision is also important for localizing and recognizing objects. A normal visual field is approximately 160° in the horizontal direction, with about 140° of that range corresponding to peripheral vision. A person with a visual field below 20°—about two fists’ width at arm’s length or 6 mm on the retina—is considered legally blind in the US. Such “tunnel vision” commonly develops in patients with RP before they completely lose their sight.
Inner retinal neurons and ganglion cells largely survive retinal degeneration. They therefore provide an entry point to artificially introduce visual information that can no longer be generated by the damaged photoreceptors. Because neurons process information by means of electrical signals, their activity can be stimulated with electric current delivered either directly into the cell or into the surrounding medium. In that way, retinal prostheses restore sight—by effectively writing information into the visual system of a blind patient in a manner that emulates the neural activity of the retina.
Electric stimulation
One way to stimulate a retinal cell is to directly inject electric charge into it with a pipette electrode. Such charge injections raise the cell potential and thereby change the conductivity of the voltage-sensitive ion channels in the cell membrane.2 If the charge injected into a ganglion cell is sufficient to raise the cell potential by some threshold amount, around a few millivolts, it will trigger the opening of Na+ channels and elicit an action potential. Current injections into nonspiking, graded-response neurons generate gradual adjustments in the cell potential rather than the all-or-nothing response of an action potential.
Intracellular activation is extremely invasive. It requires chronic access to the cell interior and therefore is not currently used clinically. More practical is to polarize the cell using electrodes placed in the extracellular medium. Because the cell membrane is highly resistive and the cytoplasm is very conductive, the applied electric field rapidly causes ions to redistribute themselves in the cell: Within a microsecond, negative ions accumulate on the side of the cell nearest the positive electrode (anode) and positive ions accumulate on the side nearest the negative electrode (cathode).
The accumulation of positive ions on the anode-facing side of the cell increases the membrane potential in that region, making it less negative. If the increase in potential, or depolarization, is large enough, it will trigger the opening of voltage-sensitive ion channels, generate an influx of cations, and, in a spiking neuron, initiate an action potential.
In retinal implants, the neuron-stimulating electrodes are typically placed in one of three locations: atop the retinal surface, below the inner nuclear layer, or beneath the 100-μm-thick vascular layer—the choroid—that surrounds the retina (see figure 2b). Electrode arrays placed atop the retinal surface are known as epiretinal implants and typically target the retinal ganglion cells.3 They are designed to directly stimulate the ganglion cells to produce spike-like signals that mimic the outputs of normal retinal signal processing.4 Epiretinal arrays can be implanted with relative ease and can be removed in case of postsurgical complications or device failure.
Electrode arrays positioned underneath the inner nuclear layer, in place of the degenerated photoreceptors, are known as subretinal implants.5,6 They induce graded responses in the inner retinal neurons that are transmitted through the retinal network to the ganglion cells, which convert the responses into trains of action potentials. Because much of the normal retinal signal processing is preserved, the encoding of the visual information by subretinal implants is simpler than with epi -retinal implants. Subretinal implants also remain in place, close to the target neurons, whereas epiretinal implants often float above the retina and shift over time. The subretinal arrays, however, are more difficult to implant and remove.
Electrodes placed outside the choroid—just inside the sclera, the hard shell that encapsulates the eye—are known as suprachoroidal implants. The extra layer between the stimulating electrodes and the retinal neurons they target limits the implants’ spatial resolution. They are therefore used primarily to help with low-resolution peripheral vision.7 A key advantage of suprachoroidal implants is that they can be placed without disturbing the sensitive retina.
Data delivery
With more than 100 million photoreceptors and 1 million retinal ganglion cells relaying information to the brain, the human visual system processes and makes sense of an enormous amount of data. Thousands of pixels are required to recognize even simple objects in a familiar environment.8 More than 3500 pixels are required to reliably recognize clocks, coffee mugs, and other familiar objects against a blank background in a 25° visual field; about twice that many are required for objects against a complex natural background. Transmitting useful amounts of data via a retinal prosthesis is a significant engineering challenge.
In a retinal prosthesis, information about a visual scene is captured with a camera and then transmitted to the electrode array implanted in the retina. Because skin-penetrating wires would introduce risks of infection and scarring, visual information—and electrical power—must be transmitted to the electrodes wirelessly. Modern implants use one of three techniques: They deliver power and visual information through inductive coils; they deliver power inductively and visual information optically through the pupil of the eye; or they deliver both visual information and power optically.
Inductive coils are widely used to transmit power and data to medical implants. Typically, an RF current in an external transmitting coil generates an oscillating magnetic field that induces an AC current in a receiving coil in the implanted device.3,8 Among the retinal prostheses that use inductive power and data transmission is the Argus II, an epiretinal implant produced for nearly a decade by the US company Second Sight Medical Products.
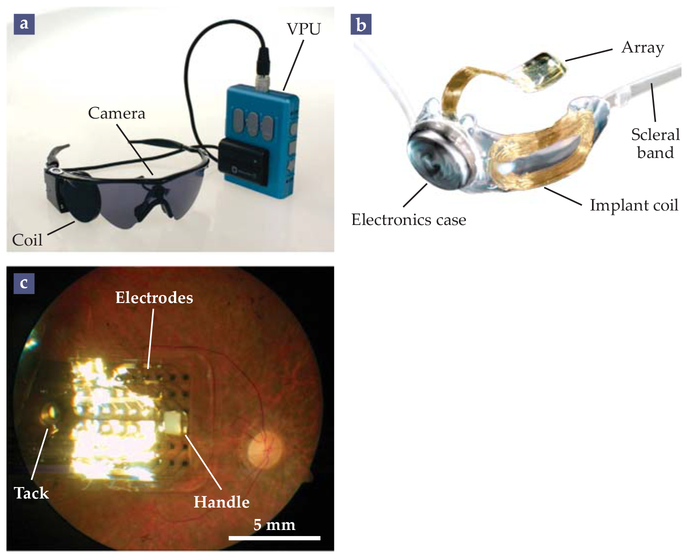
In the Argus II, shown in figure 3, the transmitting coil is mounted, along with a camera, on a pair of glasses. A video processing unit converts the camera images into AC signals that are sent to the transmitting coil and relayed to a coil affixed to the outer surface of the sclera. The inductively delivered signals are decoded and processed inside the implant before being distributed via a transscleral cable to a 6 × 10 epiretinal electrode array. The array consists of 200-μm-diameter electrodes spaced 575 μm apart and attached to the retinal surface with a flexible foil and tack.
FIGURE 3. THE ARGUS II.
(a) The external components of the Argus II epiretinal prosthesis system include a goggles-mounted video camera, a video processing unit (VPU) that converts the camera’s images into a sequence of AC signals, and an RF coil that inductively transmits those signals to the implant. (b) The implant, affixed to the outside of the eye with a silicone band, receives the signal at an implant coil, processes the signal with onboard electronics, and then transmits it to a 6 × 10 retinal electrode array. (c) An implanted array, secured to the retina with a retinal tack. The surgeon uses the white handle to position the device in the eye. (Adapted from ref. 3, M. S. Humayun et al.)
The image that the camera transmits to the implant does not depend on eye movements, which creates a perceptual mismatch: The brain expects images to shift on the retina during eye movements, and the absence of such a shift leads to unnatural visual sensations. In principle, that effect can be rectified by using eye tracking to digitally mimic natural image shifts.
Other prosthetic systems deliver power through inductive coupling but transmit visual information through the natural optics of the eye.5,9 The best known of them is the Alpha IMS, a subretinal implant built since 2010 by the German company Retina Implant AG.5 In the Alpha IMS, the implanted array includes the camera. As illustrated in figure 4, each pixel contains active circuitry—including a microphotodiode, amplifier, and electrode—that converts incident light into electrical currents, which stimulate neurons in the inner nuclear layer.
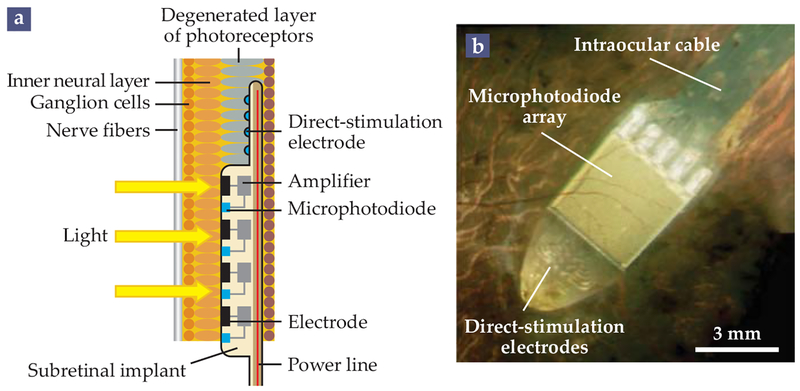
FIGURE 4. THE ALPHA IMS.
In the subretinal Alpha IMS implant, powered via inductive coils, a camera and electrode array is inserted behind the retina, in the place of the eye’s degenerated photoreceptors. (a) Each pixel in the array contains a microphotodiode that converts light to electric current, an amplifier, and an electrode that stimulates neurons in the inner neural layer. Effectively, the device takes the place of the eye’s degenerate photoreceptors. The Alpha IMS also contains direct-stimulation electrodes, which can be manipulated by an external controller even in the absence of a light stimulus. (b) The implant as imaged through a patient’s pupil. (Adapted from ref. 5, CC BY 3.0.)
Such implants are scalable to thousands of electrodes; the Alpha IMS consists of 1500 pixels, each 72 μm × 72 μm in area.10 The light-sensitive implants retain the natural relation between the direction of a subject’s gaze and the image on the implant. However, power must be delivered via a cable that runs underneath the retina, through the sclera, and under the skin to a receiving inductive coil located just behind the ear. The implantation of the Alpha IMS is therefore difficult and prone to complications.
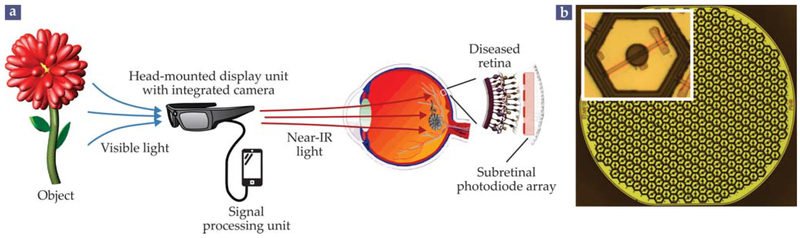
A third category of implants uses photovoltaic (PV) pixels; each pixel converts incident light directly into a neuron-stimulating electric current, with no need for external power.6 One such implant, the PRIMA-photovoltaic subretinal pros-thesis developed at Stanford University since 2005 and recently commercialized by the French company Pixium Vision, is shown in figure 5. Visual scenes are captured by an external camera and then projected onto the eye with augmented- reality video goggles. Because bright, pulsed illumination is required to elicit neural activity with PV pixels, images are delivered at near-IR wavelengths, around 880 nm, to avoid over-stimulating the remaining healthy photoreceptors. A pocket computer can be used to enhance the captured images before projecting them onto the implant.
FIGURE 5. A PRIMA PROSTHESIS.
(a) In the photovoltaic (PV) PRIMA system, a visual scene is captured with a head-mounted camera, processed with a mobile computer, and relayed to a patient’s eye in intense bursts of near-IR light. The images are projected via the natural optics of the eye onto a subretinal PV array that takes the place of the photoreceptor layer in the retina. (Adapted from G. A. Goetz, D. V. Palanker, Rep. Prog. Phys. 79, 096701, 2016.) (b) A single 1 mm module of the PV array consisting of about 250 pixels, 55 μm across, arranged in a hexagonal pattern. The inset shows a close-up of a single pixel, composed of two photodiodes and two electrodes. Larger or mulitple modules can be placed to cover a larger visual field.
The PV elements in the implanted array can be fashioned from silicon, as commonly implemented in solar panels, or from light-sensitive polymers. Because PV implants do not require wires, several independent electrode arrays can be arranged to tile the visual field.11 The arrays can be inserted via a small incision and tiled to follow the curvature of the eye, so that the surgery is minimally traumatic.
Mimicking the neural code
Ideally, the spiking activity that an implant elicits from ganglion cells for a given visual scene should match the natural retinal response to the scene. Two competing approaches are being developed toward that end.
One approach is to use epiretinal electrode arrays to directly activate retinal ganglion cells. Because those ganglion cells respond to electrical stimulation quickly, within 1–3 ms of the stimulus, the timing of their action potentials can be precisely controlled. However, different types of ganglion cells encode different aspects of an image; some signal an increase or decrease in brightness, others signal the direction of an object’s motion, and so forth. Hence different ganglion cells require different codes. It is difficult to identify and selectively activate the different cell types in a diseased retina, especially considering that epiretinal electrodes stimulate not just nearby ganglion cells but the many axons that pass through the nerve fiber layer.
The other approach relies on the surviving retinal network to transmit and shape the signals introduced by subretinal implants. That approach preserves many of the features of natural retinal signal processing. The amplitude of the retinal response sharply diminishes with increasing activation frequency.6 (The effect, known as flicker fusion, is what causes us to perceive fast stroboscopic illumination as a continuous movie.) The size of a retinal ganglion cell’s receptive field, the region of the visual field the cell responds to, is about the same as it would be under natural retinal signal processing. And the receptive fields retain their antagonistic center–surround organization: If a light stimulus at the center of a ganglion cell’s receptive field increases the cell’s response, then a stimulus at the periphery of the field inhibits it, and vice versa.12
Like their ganglion counterparts, however, inner retinal neurons come in different cell types. For instance, so-called ON cells are activated by an increase in illumination, whereas OFF cells are activated by a decrease. Indiscriminate activation of the different cell types limits the accuracy with which the natural retinal code can be reproduced. Signal processing may also be affected by the reorganization of the retinal network during degeneration, especially during the end stage of RP.13 Proper interpretation of signals from retinal implants therefore relies on brain plasticity: The brain must learn the new prosthetic language of the retina in order to generate meaningful interpretations, or percepts, of the visual signals.
Recordings of prosthesis-induced brain signals, called visually evoked potentials, provide important insight into the quality of prosthetic vision. Such measurements helped establish that the brightness of a visual percept can be modulated with subretinal prostheses by changing the duration and amplitude of the stimulus, as is the case with natural vision. That result was important for understanding how to encode levels of gray in an image. Measurements of the visual response to alternating gratings demonstrated that subretinal implants with 70 μm pixels can deliver images with a spatial resolution matching the interpixel distance, or pitch. In a human eye, a 70 μm pitch corresponds to about 20/280 visual acuity6—a bit below the US limit for legal blindness.
Behavioral measurements in rodents have also provided valuable insights. They’ve demonstrated, for instance, that the contrast sensitivity of PV prosthetic vision is only about onefifth that of natural vision, though image processing between the camera and the implant can partially compensate for the deficiency.
Clinical promise
The ultimate measure of a retinal prosthesis is psychophysical evaluation in patients. Indeed, clinical studies of various prosthetic technologies have demonstrated that retinal implants can elicit meaningful percepts in patients blinded by severe retinal degeneration.
The only retinal prosthesis currently approved for commercial use in the US is the Argus II epiretinal implant, which has now been placed in more than 200 patients. While wearing the implant, patients are typically able to perceive light and some times can detect the direction of an object’s motion. Percepts of light typically appear as bright flashes across the visual field. The device tends to improve patients’ spatial mobility despite its low resolution: To date, the best grating-based visual acuity reported with the implant14 is 20/1260.
A significant limitation of the Argus II is that the epiretinal electrodes inevitably stimulate axons from distant ganglion cells. As a result, patients who view round, localized spots of light instead see distorted, bow-shaped visual percepts.15 However, the use of 20 ms and longer electrical pulses, which stimulate inner neurons rather than ganglion cells or their axons, recently led to improved localization of the retinal responses.16 Nonetheless, the implant’s large, 575 μm electrode spacing severely limits visual acuity.
In 2013 the subretinal implant Alpha IMS received the CE mark, indicating conformity with European health, safety, and environmental standards, and became the first commercially available subretinal implant in the European Union. Patients have demonstrated improved light perception and visual acuity, and some even managed to identify and count objects and read large fonts. The best reported visual acuity with the Alpha IMS to date is 20/550, a significant improvement over the ARGUS II, albeit still below the limit for legal blindness. Because the implanted photodiode arrays retain the natural link between eye movements and visual percept, patients with the Alpha IMS have been able to fixate on a target and redirect their gaze toward new stimuli.17
Other systems, though not yet commercially available, are undergoing clinical tests. Suprachoroidal implants developed by the Australian company Bionic Vision have been implanted in three patients,7 and suprachoroidal implants developed at Japan’s Osaka University have been implanted in two.18 Because the electrode spacing in those devices is extremely large, 2 mm, reported visual acuities effectively range from 20/4000 to 20/20000—deep in the realm of ultralow vision.
In 2018 the PRIMA subretinal prosthesis began pilot clinical tests with patients who had an advanced form of AMD known as geographic atrophy. Implants 2 mm across—outfitted with 30-μm-thick, 100-μm-diameter pixels—are being tested for feasibility. To date, the PV arrays have been successfully placed in three patients. All three were able to perceive light patterns projected onto previously degenerate areas of the visual field, with the percepts mapping to the correct retinal locations. Ranges of perceived light intensity and pulse duration matched the values expected from preclinical studies, and the resolution limit of the perceived patterns matched the expectations based on pixel size. In the future, the pixel pitch is expected to decrease to 75 μm and ultimately to below 50 μm, which would provide visual acuity better than the threshold of legal blindness.
Clinical tests of retinal implants represent an important proof of the concept that sight can be restored even after decades of profound blindness caused by retinal degeneration. Signifi- cant research efforts are under way to increase the pixel counts to the thousands, improve the localization of electric stimulation, and better encode neural activity. The development of three-dimensional electroneural interfaces, novel electrode materials, and new image-processing techniques should help to accelerate technological advancement. It may not be long before prosthetic vision can truly restore functional sight to the blind.
FIGURE 1. THE HUMAN VISUAL SYSTEM.
Visual perception begins in the eye, where the lens, iris, and cornea project an inverted image of the world onto the retina, the thin layer of neural tissue at the back of the eye. The retina converts incident photons into neural action potentials, which are relayed to the brain along the optic nerve. Encasing the retina are a thin vascular layer known as the choroid and a protective outer shell called the sclera. (Adapted from G. A. Goetz, D. V. Palanker, Rep. Prog. Phys. 79, 096701, 2016.)
Biographies
Daniel Palanker is a professor in the department of ophthalmology and director of the Hansen Experimental Physics Laboratory at Stanford University in California.

Georges Goetz was formerly a postdoctoral fellow in the department of neurosurgery at Stanford.

REFERENCES
- 1.Haim M, Acta Ophthalmol. Scand 80(s233), 1 (2002). [DOI] [PubMed] [Google Scholar]
- 2.Malmivuo J, Plonsey R, Bioelectromagnetism: Principles and Applications of Bioelectric and Biomagnetic Fields, Oxford U. Press; (1995),p. 44. [Google Scholar]
- 3.Ahuja AK et al. , Transl. Vision Sci. Technol 2(4), 1 (2013); [DOI] [PMC free article] [PubMed] [Google Scholar]; Humayun MS et al. , Ophthalmology 119, 779 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jepson LH et al. , J. Neurosci 33, 7194 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stingl K et al. , Proc. R. Soc. B 280, 20130077 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorach H et al. , Nat. Med 21, 476 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayton LN et al. , PLOS One 9, e115239 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung J-H et al. , Vision Res. 111, 182 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang F et al. , J. Micro/Nanolithogr. MEMS MOEMS 15, 015002 (2016). [Google Scholar]
- 10.Zrenner E et al. , Proc. R. Soc. B 278, 1489 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee DY, Ophthalmic Surg. Lasers Imaging Retina 47, 171 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ho E et al. , J. Neurophysiol 119, 389 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones BW, Marc RE, Exp. Eye Res. 81, 123 (2005). [DOI] [PubMed] [Google Scholar]
- 14.Ho AC et al. , Ophthalmology 122, 1547 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nanduri D et al. , Invest. Ophthalmol. Visual Sci. 53, 205 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weitz AC et al. , Sci. Transl. Med 7, 318ra203 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hafed ZM et al. , Vision Res. 118, 119 (2016). [DOI] [PubMed] [Google Scholar]
- 18.Fujikado T et al. , Invest. Ophthalmol. Visual Sci. 52, 4726 (2011). [DOI] [PubMed] [Google Scholar]