Abstract
Background:
Little is known about the long-term effects of high-deductible insurance on care for chronic medical conditions.
Objective:
To determine whether a transition from low-deductible to high-deductible insurance is associated with delayed medical care for macrovascular complications of diabetes.
Design:
Observational longitudinal comparison of matched groups.
Setting:
A large national health insurer during 2003 to 2012.
Participants:
The intervention group comprised 33 957 persons with diabetes who were continuously enrolled in low-deductible (≤$500) insurance plans during a baseline year followed by up to 4 years in high-deductible (≥$1000) plans. The control group included 294 942 persons with diabetes who were enrolled in low-deductible plans contemporaneously with matched intervention group members.
Intervention:
Employer-mandated transition to a high-deductible plan.
Measurements:
The number of months it took for persons in each study group to seek care for their first major macrovascular symptom, have their first major diagnostic test for macrovascular disease, and have their first major procedure-based treatment was determined. Between-group differences in time to reach a midpoint event rate were then calculated.
Results:
No baseline differences were found between groups. During follow-up, the delay for the high-deductible group was 1.5 months (95% CI, 0.8 to 2.3 months) for seeking care for the first major symptom, 1.9 months (CI, 1.4 to 2.3 months) for the first diagnostic test, and 3.1 months (CI, 0.5 to 5.8 months) for the first procedure-based treatment.
Limitation:
Health outcomes were not examined.
Conclusion:
Among persons with diabetes, mandated enrollment in a high-deductible insurance plan was associated with delays in seeking care for the first major symptoms of macrovascular disease, the first diagnostic test, and the first procedure-based treatment.
Patients with diabetes are at risk for macrovascular disease, including coronary heart disease, cerebrovascular disease, and peripheral artery disease (1–5). Macrovascular disease causes 70% of deaths and can profoundly affect patient well-being (6–17). Access to primary care, acute care, diagnostic tests, preventive medications, and advanced interventions can help prevent or delay macrovascular complications, such as myocardial infarction, stroke, and amputation (3, 18–26).
The RAND Health Insurance Experiment (27) and a study of a single employer (28) found that high levels of cost sharing reduce use of many health services, but other studies have found that such reductions do not occur in all clinical situations (27, 29–32) or subgroups of people (27, 31, 32). High-deductible plans, which require potential out-of-pocket spending of approximately $1000 to $7000 per person per year for most nonpreventive care, have become an increasingly common feature of U.S. commercial health insurance. In 2018, 58% of workers with individual plans had deductibles of $1000 or more, and 26% had deductibles of $2000 or more (33).
Recent research has found that low-income (but not high-income) patients with diabetes in high-deductible health insurance plans have short-term increases in emergency department visits for acute complications of diabetes (32) and high-severity conditions (31). We hypothesized that patients with diabetes might also experience changes over a longer period after an employer-mandated switch from a low-deductible to a high-deductible plan.
Methods
Study Population
Our study population comprised commercially insured persons in the Optum database who were enrolled between 1 January 2003 and 31 December 2012. This database includes enrollment information and all medical, pharmacy, and hospitalization claims for approximately 43 million members of 1 large national health insurance plan. We included only members with employer-sponsored insurance; we excluded those with individually purchased insurance because of concerns about selection.
We considered an insurance plan to have a low deductible if the annual amount was $500 or less and a high deductible if the annual amount was $1000 or more. For smaller employers, we determined the deductible from a benefits table obtained from the health insurer. This table mostly included employers with fewer than 100 employees but also included a modest number of larger employers. For employers not represented in the benefits table (mostly large employers), we used an algorithm with a sensitivity and specificity greater than 96% to impute deductible amounts from actual out-of-pocket spending by persons who used health services (Table 1 of the Supplement, available at Annals.org).
Persons in this study were not able to choose a low- versus a high-deductible plan because each employer provided only 1 level of deductible each year. Some employers offered a low-deductible plan throughout the study, and others that offered a low-deductible plan early in the study switched to a high-deductible plan for the rest of the study.
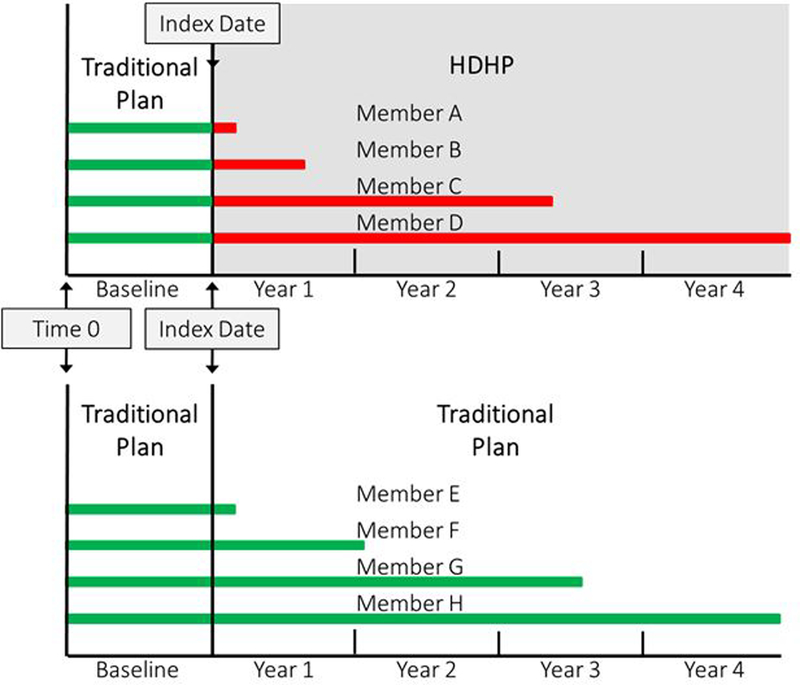
We defined the index date for employers who switched to high-deductible plans as the first day of the month when the switch occurred. We defined the index date for employers who did not switch plans as the first day of the month when their yearly account was renewed. If an employer had multiple potential index dates (for example, 5 continuous years with a low-deductible plan or 4 years with a low-deductible plan followed by 1 year with a high-deductible plan), we randomly selected 1 index date. Persons entered the study at different times because their employers had different index dates. Therefore, for each person, we defined “time zero” as 12 months before the employer’s index date and treated the interval between time zero and the index date as the baseline period. We used the employer’s index date as the beginning of follow-up (Figure 1). For each person, we measured the number of months from time zero to the first outcome measure in the baseline period and from the index date to the first outcome measure during follow-up.
Figure 1.
Study design showing example members (horizontal lines) of the high-deductible health plan group (above) and matched control group (below)
A person was eligible for the study if their employer was present in the database for at least 1 year before and 1 year after the index date (20 344 218 persons from 192 458 employers [Figure 1 of the Supplement]), they were aged 12 to 64 years and met criteria for diabetes (Table 2 of the Supplement) (716 715 persons from 61 099 employers), their first diabetes diagnosis occurred before the index date (486 208 persons from 51 585 employers), and they were continuously enrolled for at least 1 year before and 1 month after the index date (353 337 persons from 44 457 employers). These criteria yielded 34 744 persons among 11 808 employers who switched to high-deductible plans and 318 593 persons among 32 431 employers who kept low-deductible plans (Table 1).
Table 1.
Baseline Characteristics of the Intervention and Control Groups Before and After Coarsened Exact Matching*
| Characteristic | Before Coarsened Exact Matching | After Coarsened Exact Matching† | ||||
|---|---|---|---|---|---|---|
| Intervention Group | Control Group | Standardized Difference‡ |
Intervention Group | Control Group | Standardized Difference‡ |
|
| Participants (employers), n | 34 744 (11 808) | 318 593 (32 431) | – | 33 957 (11 575) | 294 942 (31 443) | – |
| Aged >40 y on index date, n (%) | 29 003 (83.5) | 262 292 (82.3) | 0.0305 | 28 337 (83.4) | 247 203 (83.8) | –0.0099 |
| Mean age on index date (SD), y | 49.4 (10.4) | 49.5 (10.8) | –0.0072 | 49.4 (10.4) | 49.7 (10.5) | –0.0340 |
| Female, n (%) | 15 788 (45.4) | 148 934 (46.7) | –0.0262 | 15 472 (45.6) | 133 738 (45.3) | 0.0044 |
| Participants, by neighborhood characteristics, n (%) | ||||||
| Proportion of residents living below poverty level | – | – | 0.0581 | – | – | 0.0321 |
| <5.0% | 12 815 (36.9) | 125 658 (39.5) | 12 511 (36.8) | 111 440 (37.8) | ||
| 5.0%–9.9% | 9204 (26.5) | 83 780 (26.3) | – | 8988 (26.5) | 79 683 (27.0) | – |
| 10.0%–19.9% | 8240 (23.7) | 70 419 (22.1) | – | 8091 (23.8) | 68 077 (23.1) | – |
| ≥20.0% | 4451 (12.8) | 38 279 (12.0) | – | 4367 (12.9) | 35 741 (12.1) | – |
| Missing | 34 (0) | 457 (0) | – | – | – | – |
| Proportion of residents with less than high school education | – | – | 0.0625 | – | – | 0.0313 |
| <15.0% | 16 468 (47.4) | 160 559 (50.5) | 16 085 (47.4) | 144 052 (48.8) | ||
| 15.0%–24.9% | 9122 (26.3) | 80 536 (25.3) | – | 8922 (26.3) | 76 271 (25.9) | – |
| 25.0%–39.9% | 6573 (18.9) | 55 571 (17.5) | – | 6456 (19.0) | 54 177 (18.4) | – |
| ≥40.0% | 2547 (7.3) | 21 470 (6.7) | – | 2494 (7.3) | 20 442 (6.9) | – |
| Missing | 34 (0) | 457 (0) | – | – | – | – |
| Race/ethnicity, n (%)§ | – | – | 0.1014 | – | – | 0.0367 |
| Hispanic | 4165 (12.0) | 38 892 (12.2) | 4072 (12.0) | 32 639 (11.1) | ||
| Asian | 839 (2.4) | 11 373 (3.6) | – | 802 (2.4) | 6941 (2.4) | – |
| From black neighborhood | 1151 (3.3) | 12 463 (3.9) | – | 1127 (3.3) | 8812 (3.0) | – |
| From mixed neighborhood | 5223 (15.1) | 54 072 (17.0) | – | 5090 (15.0) | 44 086 (14.9) | – |
| From white neighborhood | 23 311 (67.2) | 201 179 (63.3) | – | 22 866 (67.3) | 202 463 (68.6) | – |
| Missing | 55 (0) | 614 (0) | – | – | – | – |
| Mean ACG score (SD)|| | 1.9 (3.0) | 2.0 (2.9) | –0.0023 | 1.9 (2.9) | 2.0 (3.0) | –0.0307 |
| U.S. region, n (%) | – | – | 0.2225 | – | – | 0.0474 |
| West | 3159 (9.1) | 36 685 (11.5) | – | 3057 (9.0) | 28 025 (9.5) | – |
| Midwest | 11 797 (34.0) | 96 879 (30.4) | – | 11 530 (34.0) | 98 468 (33.4) | – |
| South | 17 205 (49.5) | 142 275 (44.7) | – | 16 878 (49.7) | 149 909 (50.8) | – |
| Northeast | 2571 (7.4) | 42 571 (13.4) | – | 2492 (7.3) | 18 540 (6.3) | – |
| Missing | 12 (0) | 183 (0) | – | – | – | – |
| Participants, by employer characteristics, n (%) | ||||||
| Number of employees | – | – | 1.0751 | – | – | 0 |
| 0–99 | 13 907 (40.0) | 44 136 (13.9) | 13 388 (39.4) | 116 285 (39.4) | ||
| 100–999 | 17 381 (50.0) | 107 389 (33.7) | – | 17 144 (50.5) | 148 908 (50.5) | – |
| ≥1000 | 3456 (9.9) | 167 068 (52.4) | – | 3425 (10.1) | 29 749 (10.1) | – |
| Number of employees with diabetes | – | – | 1.0774 | – | – | 0.1788 |
| 1–2 | 9009 (25.9) | 26 506 (8.3) | – | 8700 (25.6) | 61 952 (21.0) | – |
| 3–12 | 13 115 (37.7) | 53 150 (16.7) | – | 12 762 (37.6) | 103 715 (35.2) | – |
| 13–100 | 10 939 (31.5) | 111 436 (35.0) | – | 10 823 (31.9) | 104 897 (35.6) | – |
| ≥100 | 1681 (4.8) | 127 501 (40.0) | – | 1672 (4.9) | 24 378 (8.3) | – |
ACG = Adjusted Clinical Group.
Percentages may not sum to 100 due to rounding.
The coarsened exact matching algorithm created 8160 matching strata, of which 3684 were matched between groups.
Indicates the difference in means between the intervention and control groups divided by the SD of the difference in means. Lower values indicate greater similarity, and values <0.2 indicate minimal differences between groups.
See the Covariates section of the text for category definitions.
A score of 1.0 represents the mean of the population in which the score was developed.
Study Design
We conducted an observational, longitudinal, before–after study by comparing matched groups. Longitudinal study designs are less subject to bias than cross-sectional designs (34). The intervention group comprised persons who were in low-deductible insurance plans for 1 year and then were switched to high-deductible plans for an additional 1 month to 4 years. The control group consisted of matched persons who remained in low-deductible plans throughout the study (Figure 1). We matched participants on the propensity of the employer to mandate high-deductible insurance and the propensity of persons to work for such employers (divided into tertiles) (35, 36) (section I.c. of the Supplement), employer size (0 to 99, 100 to 999, or ≥1000 employees), baseline year tertile of mean out-of-pocket expenditure per member at the employer, mean out-of-pocket expenditures per person at baseline ($0 to $500, $501 to $999, $1000 to $2499, or ≥$2500), months of follow-up, and presence of a study outcome at baseline.
We used coarsened exact matching to match participants (37–39) (sections I.d. and I.e. of the Supplement), which is similar to exact matching but differs in that it uses categories instead of exact values (for example, 5-year age groups rather than age in years). The software for this type of matching creates weights for each stratum that adjust for differences between study groups in the proportion of persons in the stratum.
Our final study groups were an intervention group of 33 957 persons from 11 575 employers matched with a control group of 294 942 persons from 31 443 employers (Table 1).
Person-Level Outcome Measures
Our principal outcome measures were the differences between groups in the time to the first major symptom, the first major diagnostic test, and the first procedure-based treatment for aggregated coronary heart disease, cerebrovascular disease, and peripheral artery disease (Tables 3 and 4 of the Supplement). We also analyzed 9 secondary measures that disaggregated the primary measures by disease type. The “major symptoms” measure was intended to include conditions that represent recognizable macrovascular disease at a stage where intervention can prevent subsequent major complications and that patients can identify themselves so that they can decide whether to delay care. We did not include myocardial infarction, stroke, and amputation in this measure because patients have much less discretion in decisions to present for care.
We defined major symptoms as angina and acute and subacute forms of ischemic heart disease for coronary heart disease; transient ischemic attack for cerebrovascular disease; and intermittent claudication, resting ischemic pain, extremity thrombosis or embolism, lower-limb ulcer, cellulitis, extremity abscess, and acute osteomyelitis for peripheral artery disease. Major diagnostic tests were defined as electrocardiographic exercise tolerance test, stress echocardiography, cardiac angiography, cardiac perfusion imaging, computed tomography angiography of coronary vessels, and cardiac magnetic resonance imaging for coronary heart disease; brain and neck vessel angiography, brain imaging, and ambulatory cardiac monitoring and echocardiography (to detect atrial fibrillation and clot) for cerebrovascular disease; and magnetic resonance angiography, arteriography, and intravascular ultrasonography for peripheral artery disease. Finally, we defined procedure-based treatment as percutaneous coronary intervention and coronary artery bypass grafting for coronary heart disease; cerebrovascular endarterectomy and stenting for cerebrovascular disease; and peripheral artery angioplasty, stenting, endarterectomy, bypass, and thrombectomy for peripheral vascular disease.
Population-Level Outcome Measure
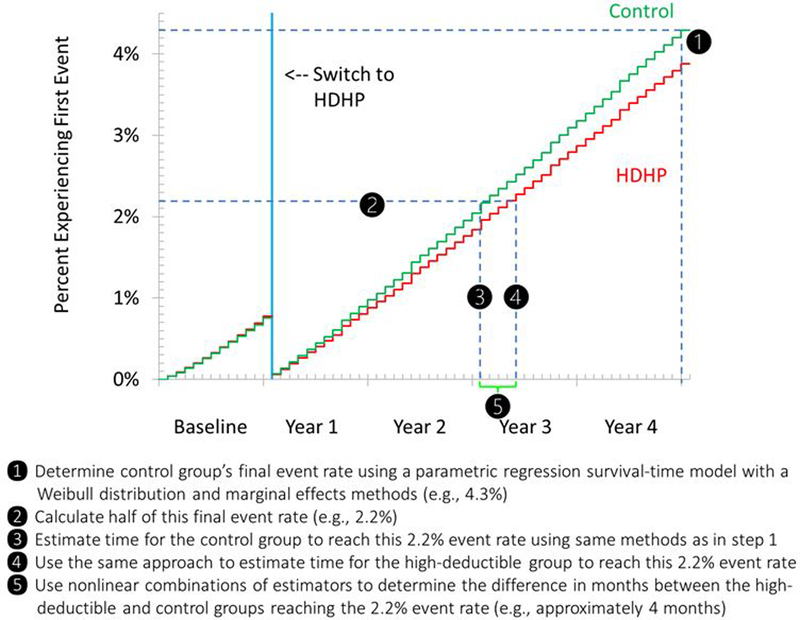
To estimate the difference between groups in the time to each outcome (Figure 2; section I.e. of the Supplement), we estimated the interval during follow-up between the index date and the date when the control group reached half its event rate at the end of follow-up, estimated the interval during follow-up between the index date and the date when the intervention group reached half the event rate that the control group achieved at the end of follow-up, and calculated the difference between these intervals. We believe this difference provides an intuitive measure of any delays that an average patient with diabetes in our sample might experience after a mandated switch to high-deductible insurance. We used the same approach to assess potential delays during the baseline year.
Figure 2.
Steps in estimating the delay for the high-deductible group to reach half of the final event rate of controls at follow up.
Covariates
We used version 10 of the Johns Hopkins ACG System (40, 41) to calculate participants’ baseline morbidity score (section I.b. of the Supplement). We used block group data from the 2000 U.S. Census (42–44) to create categories (43, 45) defining neighborhoods with less than 5%, 5% to 9.9%, 10% to 19.9%, and at least 20% of residents living below the poverty level. Similarly, we defined categories of residence in neighborhoods with less than 15%, 15% to 24.9%, 25% to 39.9%, and at least 40% of residents having less than a high school education. We used geocoding to classify participants as from white, black, Hispanic, or mixed neighborhoods and we classified participants as Hispanic or Asian based on the E-Tech system that analyzes full names and geographic location of individuals (46). Other covariates included age (12 to 39 and 40 to 64 years), sex, U.S. region (West, Midwest, South, or Northeast), employer size (as a continuous variable or with categories of 0 to 99, 100 to 999, or ≥1000 employees) (section I.b. of the Supplement), number of employees with diabetes at each employer (1 or 2, 3 to 12, 13 to 100, or >100), calendar month of the first detected diabetes diagnosis, and calendar month of the index date.
Statistical Analysis
We compared characteristics of our study groups by using a standardized differences approach (47). A parametric regression survival time model with a Weibull distribution was used to estimate delays in care (48). For the baseline period, we modeled the interval between time zero and the first study outcome at the person level. We adjusted for age group, sex, race/ethnicity, category of number of employees per employer, and U.S. region. We used the same approach for the follow-up period to estimate the interval between the index date and the first study outcome at the person level. These models incorporated weights from the coarsened exact matching algorithm. For the baseline analyses, persons were censored when they reached the end of the baseline period. For the follow-up analyses, persons were censored if they left the sample (for example, because of disenrollment, reaching age 65 years [when Medicare coverage begins], or reaching the end of follow-up [4 years after the index date]). The coefficient of interest from the baseline and follow-up regression models was a binary variable that indicated membership in the intervention group. The coefficient for this term was an adjusted hazard ratio indicating the independent association of high-deductible group membership with the outcome of interest. These hazard ratios were used to estimate baseline and follow-up delays (Figure 2; section I.e. of the Supplement). We applied a Bonferroni–Holm correction (49) that tested each effect estimate for 6 hypotheses with a desired α level of 0.05. We conducted a sensitivity analysis that used the same approach but included baseline and follow-up events in the same model and included an interaction term between study period (baseline vs. follow-up) and study group (high- vs. low-deductible group) to determine whether adjustment for baseline differences between groups altered interpretation of the findings.
We also assessed whether a Cox proportional hazards model, which has fewer assumptions, would yield similar adjusted hazard ratios (section I.f. of the Supplement). Because the patients with diabetes in our study were nested within employers and employer effects on outcome measures might be important, we ran sensitivity analyses on a 1:1 matched sample that both included and excluded clustering of persons within employers when estimating standard errors (section I.g. of the Supplement). We calculated the E-value (50) for our adjusted hazard ratios to determine the strength of the association of unmeasured factors that would be required to make the reported association between delay and switching to a high-deductible insurance plan either zero or nonsignificant. Finally, we performed a sensitivity analysis in which we did not match on employer- or person-level out-of-pocket spending categories.
Results
After matching was done and matching-generated weights were applied, all standardized differences between the intervention and control groups at baseline were less than 0.2 (Table 1), indicating minimal differences (51). The mean age in both groups was approximately 50 years, and 45% of participants were female. Thirty-five percent to 37% lived in neighborhoods where at least 10% of residents lived below the poverty level, 25% to 26% lived in neighborhoods where at least 25% of residents had less than a high school education, and 11% to 12% were Hispanic.
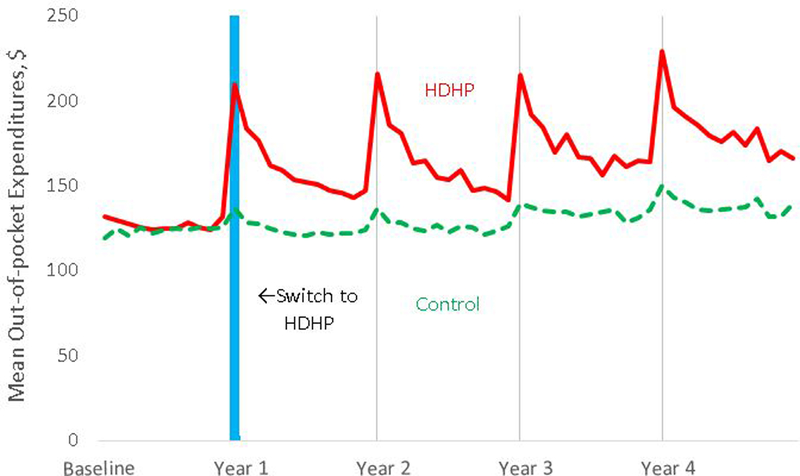
Persons with high-deductible insurance plans had increases in out-of-pocket medical expenditures ranging from 43% (95% CI, 35% to 51%) to 53% (CI, 42% to 63%) per follow-up year versus baseline and relative to those in the control group (Figure 3; Table 5 of the Supplement).
Figure 3.
Monthly mean out-of-pocket medical expenditures before and after the index date in the high-deductible health plan and control groups, indicating the extent of the actual cost sharing increase experienced by high-deductible health plan members. The HDHP group experiences peaks at the beginning each benefit year, which taper as members exceed their annual deductible.
Abbreviation: HDHP, high-deductible health plan. Vertical blue line is centered at the index month when high-deductible health plan group members were switched into high-deductible health plans.
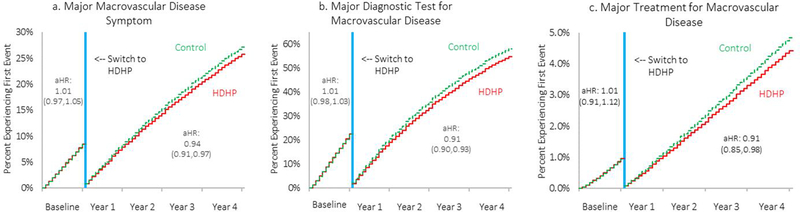
During the baseline period, no statistically significant differences in time to any measure were observed between the intervention and control groups (Table 2 and Figure 4). During follow-up, however, the delay for the intervention group was 1.5 months (CI, 0.8 to 2.3 months) for seeking care for the first major macrovascular disease symptom, 1.9 months (CI, 1.4 to 2.3 months) for the first major diagnostic test, and 3.1 months (CI, 0.5 to 5.8 months) for the first procedure-based treatment. Estimates remained statistically significant after Bonferroni–Holm adjustment, except for time to the first procedure-based treatment, which had a corrected P value of 0.074 (Table 6 of the Supplement).
Table 2.
Estimated Intervals Between Time Zero or the Index Date and Achievement of Half the Respective Final Baseline or Follow-up Period Rate of Control Participants Among the Intervention and Control Groups
| Event | Estimated Interval During Baseline Period (95% CI), mo* | Estimated Interval During Follow-up (95% CI), mo† | ||||
|---|---|---|---|---|---|---|
| Intervention Group | Control Group | Intervention vs. Control Group‡ |
Intervention Group | Control Group | Intervention vs. Control Group‡ |
|
| First major symptom§ | 6.5 (6.2 to 6.8) | 6.6 (6.4 to 6.8) | −0.1 (–0.3 to 0.2) | 23.9 (22.9 to 24.9) | 22.3 (21.7 to 23.0) | 1.5 (0.8 to 2.3) |
| Coronary heart disease | 8.1 (7.5 to 8.7) | 8.1 (7.6 to 8.6) | 0 (–0.4 to 0.5) | 30.0 (27.8 to 32.2) | 28.5 (26.9 to 30.1) | 1.4 (–0.1 to 3.0) |
| Cerebrovascular disease | 7.3 (6.4 to 8.3) | 7.5 (6.8 to 8.3) | −0.2 (–0.9 to 0.5) | 32.3 (28.4 to 36.1) | 28.9 (26.3 to 31.5) | 3.4 (0.6 to 6.1) |
| Peripheral artery disease | 6.2 (5.9 to 6.6) | 6.4 (6.1 to 6.6) | −0.1 (–0.4 to 0.2) | 24.1 (22.8 to 25.4) | 22.3 (21.5 to 23.1) | 1.8 (0.8 to 2.9) |
| First diagnostic test§ | 6.4 (6.3 to 6.6) | 6.5 (6.3 to 6.6) | 0 (–0.2 to 0.1) | 20.9 (20.4 to 21.5) | 19.0 (18.7 to 19.4) | 1.9 (1.4 to 2.3) |
| Coronary heart disease | 7.3 (7.0 to 7.6) | 7.3 (7.1 to 7.5) | 0 (–0.2 to 0.2) | 27.1 (26.1 to 28.1) | 24.6 (24.0 to 25.3) | 2.5 (1.7 to 3.2) |
| Cerebrovascular disease | 6.5 (6.3 to 6.7) | 6.6 (6.4 to 6.7) | −0.1 (–0.2 to 0.1) | 22.2 (21.5 to 22.8) | 20.5 (20.1 to 20.9) | 1.7 (1.1 to 2.2) |
| Peripheral artery disease | 7.8 (7.2 to 8.4) | 7.6 (7.2 to 8.1) | 0.1 (–0.3 to 0.6) | 31.5 (29.3 to 33.6) | 28.2 (26.7 to 29.6) | 3.3 (1.7 to 4.9) |
| First procedure-based treatment§ | 10.2 (8.6 to 11.7) | 10.3 (8.9 to 11.6) | −0.1 (–0.9 to 0.8) | 37.6 (33.5 to 41.7) | 34.5 (31.4 to 37.5) | 3.1 (0.5 to 5.8)|| |
| Coronary heart disease | 9.9 (8.4 to 11.5) | 10.0 (8.6 to 11.3) | 0 (–0.9 to 0.8) | 37.1 (32.9 to 41.3) | 33.1 (30.2 to 36.1) | 3.9 (1.2 to 6.7) |
| Cerebrovascular disease | 8.1 (4.8 to 11.3) | 8.6 (5.8 to 11.3) | −0.5 (–2.7 to 1.8) | 38.3 (26.5 to 50.2) | 39.1 (28.7 to 49.4) | −0.7 (–7.6 to 6.2) |
| Peripheral artery disease¶ | 7.0 (5.0 to 9.0) | 7.0 (5.5 to 8.5) | 0 (–1.5 to 1.6) | 26.4 (20.1 to 32.8) | 27.0 (22.2 to 31.8) | −0.5 (–5.3 to 4.2) |
Defined as the interval between time zero and achievement of half the final baseline rate among control participants, estimated using a parametric regression survival time model with a Weibull distribution and adjusted for age group, sex, race/ethnicity, category of number of patients with diabetes per employer, and U.S. region.
Defined as the interval between the index date and achievement of half the final follow-up rate of control participants, estimated using the same modeling approach as for baseline measures.
Estimand of interest, reflecting the delay in the intervention group relative to the control group.
Primary measure; results are aggregated across coronary heart disease, cerebrovascular disease, and peripheral artery disease.
Results did not remain statistically significant after application of the Bonferroni–Holm correction for 6 primary hypotheses in which the formula was as follows: (target α level [0.05]) / (number of tests [10] − rank number of pair ranked by degree of significance − 1).
Adjustment for peripheral artery disease treatment estimates did not include race or age group because of very low event rates among Asian persons, black persons, and members of the younger age group.
Figure 4.a-c.
Weighted and adjusted time-to-event plots1 showing time to first major macrovascular disease symptom, diagnostic test, or treatment after a mandated high-deductible health plan switch, compared to contemporaneous control group members who remained in low-deductible plans.
Abbreviation: HDHP, high-deductible health plan; aHR, adjusted hazard ratio. Vertical blue line is centered at the index month when high-deductible health plan members were switched into high-deductible health plans. Major macrovascular disease symptoms included intermittent claudication, peripheral artery disease related ischemic pain, cellulitis, abscess of upper and lower limb, embolism/thrombosis, ulcer of lower limb, acute osteomyelitis, transient ischemic attack, angina, and acute and sub-acute forms of ischemic heart disease (Appendix Table 4). Major macrovascular disease diagnostic testing included magnetic resonance angiogram, angiography, intravascular ultrasound, ambulatory cardiac monitoring, brain and neck vessel angiography, brain imaging, echocardiogram, exercise tolerance tests, stress echocardiogram, cardiac catheterization angiogram, computed tomography of coronary vessels, cardiac MRI, and perfusion imaging (Appendix Table 4). Major macrovascular disease procedure-based treatments included angioplasty/stenting, endarterectomy, peripheral artery bypass, peripheral artery thrombectomy/embolectomy, endarterectomy/stenting, percutaneous coronary intervention/angioplasty, and coronary artery bypass grafting (Appendix Table 4).
1Plots derived from parametric regression survival-time models with a Weibull distribution and adjusted for age group, gender, race/ethnicity, diabetes patients per employer category, and US region; and using weights derived from the coarsened exact matching algorithm.
In analyses of secondary measures that were disaggregated by macrovascular disease type, adjusted hazard ratios for times to the first major symptom and the first diagnostic test had similar magnitudes and directions as the aggregated hazard ratios (Table 2; Figure 2 of the Supplement). In contrast, for the first procedure-based treatment, findings were not consistent when disaggregated by macrovascular disease type. The difference for the intervention group at follow-up was 3.9 months (CI, 1.2 to 6.7 months) for coronary heart disease but −0.7 month (CI, −7.6 to 6.2 months) for cerebrovascular disease and −0.5 month (CI, −5.3 to 4.2 months) for peripheral artery disease.
During follow-up, the adjusted hazard ratios were 0.94 (CI, 0.91 to 0.97) for seeking care for the first major symptom, 0.91 (CI, 0.90 to 0.93) for the first diagnostic test, and 0.91 (CI, 0.85 to 0.98) for the first procedure-based treatment. Table 7 of the Supplement shows hazard ratios disaggregated by macrovascular disease type. The E-values (and the limit of their CI closest to the null) were 1.32 (1.21) for seeking care for the first major symptom, 1.41 (1.35) for the first diagnostic test, and 1.51 (1.25) for the first procedure-based treatment of coronary heart disease.
The adjusted hazard ratios from Cox proportional hazards models and the corresponding E-values were nearly identical to those generated with our primary analytic approach (Tables 8 and 9 of the Supplement).
Results of sensitivity analyses that accounted for potential baseline differences in measures (Table 10 of the Supplement) were similar to results of the primary analysis. Comparison of 1:1 coarsened exact matching samples that did (Tables 11 and 13 of the Supplement) and did not (Tables 12 and 14 of the Supplement) account for employer-level clustering produced nearly identical results. Our sensitivity analysis in which we did not match on baseline employer- or person-level out-of-pocket spending categories yielded adjusted effect estimates that were smaller than the primary results but were in the same direction (Tables 15 and 16 of the Supplement).
Discussion
Patients with diabetes whose employers switched to high-deductible insurance plans had delays in seeking care for the first major symptoms of the macrovascular complications of diabetes, having their first major diagnostic test for such complications, and having their first procedure-based treatment compared with persons in the control group. These results suggest that patients with diabetes who are switched to high-deductible health plans are affected by the increased out-of-pocket costs they face for medical services. The delay in procedure-based treatments was driven by delays in coronary heart disease treatment, and we did not detect similar changes for cerebrovascular or peripheral artery disease.
Although our methods did not allow us to distinguish whether the changes we detected represent delays or ultimate reductions in the measures we studied, previous research (31, 32, 51) suggests that delays might be more likely. For example, a recent short-term study demonstrated that enrollment in high-deductible plans was associated with delayed outpatient visits for acute diabetes complications, a pattern that might have led to patients presenting to the emergency department with adverse health outcomes (32).
Our study indicates that these delays or reductions persisted over a relatively long follow-up and occurred even for services that are used for life-threatening conditions. These findings raise the possibility that patients in high-deductible plans present with more advanced disease; experience more adverse events, such as strokes, myocardial infarctions, and amputations; and have a higher death rate. However, previous research found that intermediate health end points were unchanged among persons with high cost sharing, raising the possibility that major adverse outcomes of macrovascular disease will be unchanged. For example, the RAND Health Insurance Experiment found generalized reductions in use of health services under high-level cost sharing but did not detect changes in cholesterol level and blood pressure in the overall population with high cost sharing (not a population with diabetes) (52).
We recommend that clinicians and care management teams monitor the type of insurance that patients with diabetes have and consider further outreach and education for those with high-deductible plans. Employers with high-deductible plans might also consider reduced cost sharing for patients with diabetes (53–55). Moreover, until the effects of high-deductible plans on long-term macrovascular complications of diabetes are better understood, policymakers and employers should remain cautious in encouraging uptake of such plans among vulnerable patients with diabetes, especially given recent evidence of adverse short-term health outcomes (31, 32).
Future research about high-deductible insurance and macrovascular complications of diabetes should assess whether persons with high-deductible plans ultimately require more intensive work-ups and more advanced treatments. Studies should also measure the costs of diagnosis and treatment for macrovascular complications of diabetes and measure rates of clinically meaningful outcomes, such as stroke, myocardial infarction, amputation, and death. We were unable to measure these because our sample was too small for reliable detection of such infrequent events and because obtaining complete death data after 2011 is problematic given incomplete data from the Social Security Administration (56).
Our study included 3 key elements to minimize bias. First, it was restricted to employers that mandated a low- or high-deductible insurance plan and did not allow employees to choose. Second, we used matching to balance key employer characteristics given that employers self-select into insurance types. Finally, key individual-level characteristics within employer types were also balanced because these characteristics could influence outcome measures.
Our study also had limitations. We were unable to detect adverse clinical outcomes. The study was observational, and analyses were therefore at risk for the effects of unmeasured confounders (for example, the possibility that employers in the high-deductible group might have, at the same time as their insurance switch, changed workplace policies that made it more difficult for employees to leave work to get health care). E-value calculations indicated that unmeasured confounders with hazard ratios of approximately 1.3 to 1.5 could make our primary findings nonsignificant, but this is after already controlling for some confounding through matching and adjustment. Because the duration of our baseline period was not comparable to the duration of follow-up, we were unable to make a valid comparison between delays in the baseline and follow-up periods in the intervention versus the control group. However, such a comparison of adjusted hazard ratios showed that our findings were similar when we adjusted for differences in measures at baseline. Although we knew the exact deductible level of most smaller employers, we had to impute this from claims for almost all large employers. However, we do not believe that this materially affected our results because of the high sensitivity and specificity of the imputation method (Table 1 of the Supplement). We did not have access to some information about individual persons’ health insurance expenses (such as premiums and health savings account balances). Our diagnostic testing measures were not always specific to the relevant macrovascular complication. Our analyses were unable to account for competing risks because of incomplete death data, the complexity of mapping all possible transition states, and the uncertainty that a given sequence of events was correctly constructed in claims data. Our findings are not generalizable to persons with uncommonly high deductibles, newly insured persons, or patients with newly diagnosed diabetes. Finally, our measures did not distinguish appropriate care from unnecessary care, and a proportion of the changes we detected could represent forgoing unnecessary or low-value services.
In conclusion, mandated enrollment in a high-deductible insurance plan among persons with diabetes was associated with delays in seeking care for the first major symptoms of macrovascular disease, the first diagnostic test, and the first procedure-based treatment over 4 years of follow-up.
Supplementary Material
Acknowledgment:
The authors thank Robert LeCates, MA; Beverly Adade, MBE; and Katherine Callaway, MPH, of Harvard Pilgrim Health Care Institute for valuable assistance with literature searches, computer programming, data processing, and algorithm development. They also thank Meda E. Pavkov, MD, PhD, and Edward W. Gregg, PhD, of the Division of Diabetes Translation at the Centers for Disease Control and Prevention and Ronald T. Ackermann, MD, MPH, of the Center for Diabetes and Metabolism at Northwestern University for consultation on development of macrovascular disease outcome measures. Finally, the authors thank an anonymous reviewer for suggesting many helpful edits.
Grant Support: By grant R01-DK100304 (principal investigator: Dr. Wharam) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and grant 1P30-DK092924 from the NIDDK Health Delivery Systems Center for Diabetes Translational Research.
Institutional Approval
This study was approved by the Harvard Pilgrim Health Care Institute Institutional Review Board.
Role of the Funding Source
This study was supported by grants R01-DK100304 and 1P30-DK092924from the National Institute of Diabetes and Digestive and Kidney Diseases. The funding source had no role in the design, conduct, or reporting of the study.
Primary Funding Source: National Institute of Diabetes and Digestive and Kidney Diseases.
Footnotes
Disclosures: Dr. Ross-Degnan reports employment with Harvard Pilgrim Health Care. Dr. Newhouse reports personal fees from Aetna outside the submitted work. Authors not named here have disclosed no conflicts of interest. Disclosures can also be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M17-3365.
Drs. Wharam and Zhang primarily analyzed the data.
Contributor Information
J. Frank Wharam, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, Massachusetts.
Christine Y. Lu, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, Massachusetts.
Fang Zhang, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, Massachusetts.
Matthew Callahan, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, Massachusetts.
Xin Xu, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, Massachusetts.
Jamie Wallace, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, Massachusetts.
Stephen Soumerai, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, Massachusetts.
Dennis Ross-Degnan, Harvard Medical School and Harvard Pilgrim Health Care Institute, Boston, Massachusetts.
Joseph P. Newhouse, Harvard Medical School and Harvard T.H. Chan School of Public Health, Boston, Massachusetts, and Harvard Kennedy School and National Bureau of Economic Research, Cambridge, Massachusetts.
References
- 1.Fox CS, Sullivan L, D’Agostino RB Sr, Wilson PW; Framingham Heart Study. The significant effect of diabetes duration on coronary heart disease mortality: the Framingham Heart Study. Diabetes Care. 2004;27:704–8. [PMID: 14988289] [DOI] [PubMed] [Google Scholar]
- 2.Hu FB, Stampfer MJ, Solomon CG, Liu S, Willett WC, Speizer FE, et al. The impact of diabetes mellitus on mortality from all causes and coronary heart disease in women: 20 years of follow-up. Arch Intern Med. 2001;161:1717–23. [PMID: 11485504] [DOI] [PubMed] [Google Scholar]
- 3.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–6. [PMID: 19171871] doi: 10.1161/CIRCULATIONAHA.108.191259 [DOI] [PubMed] [Google Scholar]
- 4.Selvin E, Coresh J, Brancati FL. The burden and treatment of diabetes in elderly individuals in the U.S. Diabetes Care. 2006;29:2415–9. [PMID: 17065677] [DOI] [PubMed] [Google Scholar]
- 5.Towfighi A, Markovic D, Ovbiagele B. Current national patterns of comorbid diabetes among acute ischemic stroke patients. Cerebrovasc Dis. 2012;33:411–8. [PMID: 22456491] doi: 10.1159/000334192 [DOI] [PubMed] [Google Scholar]
- 6.Abbott RD, Curb JD, Rodriguez BL, Masaki KH, Popper JS, Ross GW, et al. Age-related changes in risk factor effects on the incidence of thromboembolic and hemorrhagic stroke. J Clin Epidemiol. 2003;56:479–86. [PMID: 12812823] [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes Association. Standards of medical care in diabetes—2013. Diabetes Care. 2013;36 Suppl 1:S11–66. [PMID: 23264422] doi: 10.2337/dc13-S011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anand DV, Lim E, Hopkins D, Corder R, Shaw LJ, Sharp P, et al. Risk stratification in uncomplicated type 2 diabetes: prospective evaluation of the combined use of coronary artery calcium imaging and selective myocardial perfusion scintigraphy. Eur Heart J. 2006;27:713–21. [PMID: 16497686] [DOI] [PubMed] [Google Scholar]
- 9.Charles M, Ejskjaer N, Witte DR, Borch-Johnsen K, Lauritzen T, Sandbaek A. Prevalence of neuropathy and peripheral arterial disease and the impact of treatment in people with screen-detected type 2 diabetes: the ADDITION-Denmark study. Diabetes Care. 2011;34:2244–9. [PMID: 21816977] doi: 10.2337/dc11-0903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, et al. ; Emerging Risk Factors Collaboration. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–22. [PMID: 20609967] doi: 10.1016/S0140-6736(10)60484-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hammoud T, Tanguay JF, Bourassa MG. Management of coronary artery disease: therapeutic options in patients with diabetes. J Am Coll Cardiol. 2000;36:355–65. [PMID: 10933343] [DOI] [PubMed] [Google Scholar]
- 12.Laakso M Hyperglycemia as a risk factor for cardiovascular disease in type 2 diabetes. Prim Care. 1999;26:829–39. [PMID: 10523462] [DOI] [PubMed] [Google Scholar]
- 13.Lièvre MM, Moulin P, Thivolet C, Rodier M, Rigalleau V, Penfornis A, et al. ; DYNAMIT investigators. Detection of silent myocardial ischemia in asymptomatic patients with diabetes: results of a randomized trial and meta-analysis assessing the effectiveness of systematic screening. Trials. 2011;12:23. [PMID: 21269454] doi: 10.1186/1745-6215-12-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moss SE, Klein R, Klein BE. Cause-specific mortality in a population-based study of diabetes. Am J Public Health. 1991;81:1158–62. [PMID: 1951827] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008;88:1254–64. [PMID: 18801858] doi: 10.2522/ptj.20080020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohira T, Shahar E, Chambless LE, Rosamond WD, Mosley TH Jr, Folsom AR. Risk factors for ischemic stroke subtypes: the Atherosclerosis Risk in Communities study. Stroke. 2006;37:2493–8. [PMID: 16931783] [DOI] [PubMed] [Google Scholar]
- 17.Van de Werf F, Ardissino D, Betriu A, Cokkinos DV, Falk E, Fox KA, et al. ; Task Force on the Management of Acute Myocardial Infarction of the European Society of Cardiology. Management of acute myocardial infarction in patients presenting with ST-segment elevation. The Task Force on the Management of Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2003;24:28–66. [PMID: 12559937] [DOI] [PubMed] [Google Scholar]
- 18.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, et al. ; American College of Cardiology. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients with Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50:e1–157. [PMID: 17692738] [DOI] [PubMed] [Google Scholar]
- 19.Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, et al. ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients with Acute Myocardial Infarction). Circulation. 2004;110:588–636. [PMID: 15289388] [DOI] [PubMed] [Google Scholar]
- 20.Antman EM, Hand M, Armstrong PW, Bates ER, Green LA, Halasyamani LK, et al. ; Canadian Cardiovascular Society. 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2008;51:210–47. [PMID: 18191746] doi: 10.1016/j.jacc.2007.10.001 [DOI] [PubMed] [Google Scholar]
- 21.Ayanian JZ, Weissman JS, Schneider EC, Ginsburg JA, Zaslavsky AM. Unmet health needs of uninsured adults in the United States. JAMA. 2000;284:2061–9. [PMID: 11042754] [DOI] [PubMed] [Google Scholar]
- 22.Beckles GL, Engelgau MM, Narayan KM, Herman WH, Aubert RE, Williamson DF. Population-based assessment of the level of care among adults with diabetes in the U.S. Diabetes Care. 1998;21:1432–8. [PMID: 9727887] [DOI] [PubMed] [Google Scholar]
- 23.Fang J, Alderman MH. Does supplemental private insurance affect care of Medicare recipients hospitalized for myocardial infarction? Am J Public Health. 2004;94:778–82. [PMID: 15117700] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibbons LW, Mitchell TL, Wei M, Blair SN, Cooper KH. Maximal exercise test as a predictor of risk for mortality from coronary heart disease in asymptomatic men. Am J Cardiol. 2000;86:53–8. [PMID: 10867092] [DOI] [PubMed] [Google Scholar]
- 25.Pearson TA, Blair SN, Daniels SR, Eckel RH, Fair JM, Fortmann SP, et al. AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update: consensus panel guide to comprehensive risk reduction for adult patients without coronary or other atherosclerotic vascular diseases. American Heart Association Science Advisory and Coordinating Committee. Circulation. 2002;106:388–91. [PMID: 12119259] [DOI] [PubMed] [Google Scholar]
- 26.Powell-Griner E, Bolen J, Bland S. Health care coverage and use of preventive services among the near elderly in the United States. Am J Public Health. 1999;89:882–6. [PMID: 10358679] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newhouse JP. Free for All? Lessons from the RAND Health Insurance Experiment. Cambridge, MA: Harvard Univ Pr; 1996. [Google Scholar]
- 28.Brot-Goldberg ZC, Chandra A, Handel BR, Kolstad JT. What does a deductible do? The impact of cost-sharing on health care prices, quantities, and spending dynamics. Q J Econ. 2017;132:1261–318. [Google Scholar]
- 29.Wharam JF, Landon BE, Galbraith AA, Kleinman KP, Soumerai SB, Ross-Degnan D. Emergency department use and subsequent hospitalizations among members of a high-deductible health plan. JAMA. 2007;297:1093–102. [PMID: 17356030] [DOI] [PubMed] [Google Scholar]
- 30.Selby JV, Fireman BH, Swain BE. Effect of a copayment on use of the emergency department in a health maintenance organization. N Engl J Med. 1996;334:635–41. [PMID: 8592528] [DOI] [PubMed] [Google Scholar]
- 31.Wharam JF, Zhang F, Eggleston EM, Lu CY, Soumerai SB, Ross-Degnan D. Effect of high-deductible insurance on high-acuity outcomes in diabetes: a Natural Experiment for Translation in Diabetes (NEXT-d) study. Diabetes Care. 2018;41:940–8. [PMID: 29382660] doi: 10.2337/dc17-1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wharam JF, Zhang F, Eggleston EM, Lu CY, Soumerai S, Ross-Degnan D. Diabetes outpatient care and acute complications before and after high-deductible insurance enrollment: a Natural Experiment for Translation in Diabetes (NEXT-d) study. JAMA Intern Med. 2017;177:358–68. [PMID: 28097328] doi: 10.1001/jamainternmed.2016.8411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Claxton G, Rae M, Long M, Damico A, Sawyer B. The Kaiser Family Foundation Employer Health Benefits 2018 Annual Survey. 3 October 2018. Accessed at http://files.kff.org/attachment/Report-Employer-Health-Benefits-Annual-Survey-2018 on 23 October 2018.
- 34.Shadish WR, Cook TD, Campbell DT. Experimental and Quasi-Experimental Designs for Generalized Causal Inference. Boston: Houghton Mifflin; 2001. [Google Scholar]
- 35.Schreyögg J, Stargardt T, Tiemann O. Costs and quality of hospitals in different health care systems: a multi-level approach with propensity score matching. Health Econ. 2011;20:85–100. [PMID: 20084662] doi: 10.1002/hec.1568 [DOI] [PubMed] [Google Scholar]
- 36.Wharam JF, Zhang F, Landon BE, LeCates R, Soumerai S, Ross-Degnan D. Colorectal cancer screening in a nationwide high-deductible health plan before and after the Affordable Care Act. Med Care. 2016;54:466–73. [PMID: 27078821] doi: 10.1097/MLR.0000000000000521 [DOI] [PubMed] [Google Scholar]
- 37.Iacus SM, King G, Porro G. Multivariate matching methods that are monotonic imbalance bounding. J Am Stat Assoc. 2011;106:345–61. [Google Scholar]
- 38.Iacus S, King G, Porro G. CEM: Coarsened Exact Matching Software. 2017. Accessed at https://gking.harvard.edu/cem on 19 May 2017. [Google Scholar]
- 39.Iacus SM, King G, Porro G. Causal inference without balance checking: coarsened exact matching. Polit Anal. 2012;20:1–24. [Google Scholar]
- 40.Reid RJ, Roos NP, MacWilliam L, Frohlich N, Black C. Assessing population health care need using a claims-based ACG morbidity measure: a validation analysis in the Province of Manitoba. Health Serv Res. 2002;37:1345–64. [PMID: 12479500] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.The Johns Hopkins University. The Johns Hopkins ACG System. 2018. Accessed at www.hopkinsacg.org/advantage on 2 June 2018.
- 42.Krieger N Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82:703–10. [PMID: 1566949] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures—the Public Health Disparities Geocoding Project. Am J Public Health. 2003;93:1655–71. [PMID: 14534218] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.United States Census Bureau. Geographic Areas Reference Manual. 1994. Accessed at www.census.gov/geo/reference/garm.html on 22 October 2018.
- 45.United States Census Bureau. Census 2000 Gateway. Accessed at www.census.gov/main/www/cen2000.html on 2 June 2018.
- 46.Ethnic Technologies. Accessed at www.ethnictechnologies.com/faq/ on 24 October 2018.
- 47.Yang D, Dalton JE. A unified approach to measuring the effect size between two groups using SAS®. Presented at SAS Global Forum 2012, Orlando, Florida, 22–25 April 2012. Paper no. 335–2012. [Google Scholar]
- 48.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. 2nd ed. Hoboken, NJ: J Wiley; 2002. (Wiley Series in Probability and Statistics.) [Google Scholar]
- 49.Holm S A simple sequentially rejective multiple test procedure. Scandinavian Journal of Statistics. 1979;6:65–70. [Google Scholar]
- 50.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167:268–74. [PMID: 28693043] doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 51.Wharam JF, Zhang F, Lu CY, Wagner AK, Nekhlyudov L, Earle CC, et al. Breast cancer diagnosis and treatment after high-deductible insurance enrollment. J Clin Oncol. 2018;36:1121–7. [PMID: 29489428] doi: 10.1200/JCO.2017.75.2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Newhouse JP; Insurance Experiment Group. Free for All? Lessons from the RAND Health Insurance Experiment. Cambridge, MA: Harvard Univ Pr; 1993. [Google Scholar]
- 53.Duru OK, Turk N, Ettner SL, Neugebauer R, Moin T, Li J, et al. Adherence to metformin, statins, and ACE/ARBs within the Diabetes Health Plan (DHP). J Gen Intern Med. 2015;30:1645–50. [PMID: 25944019] doi: 10.1007/s11606-015-3284-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chernew ME, Shah MR, Wegh A, Rosenberg SN, Juster IA, Rosen AB, et al. Impact of decreasing copayments on medication adherence within a disease management environment. Health Aff (Millwood). 2008;27:103–12. [PMID: 18180484] doi: 10.1377/hlthaff.27.1.103 [DOI] [PubMed] [Google Scholar]
- 55.Lee JL, Maciejewski M, Raju S, Shrank WH, Choudhry NK. Value-based insurance design: quality improvement but no cost savings. Health Aff (Millwood). 2013;32:1251–7. [PMID: 23836741] doi: 10.1377/hlthaff.2012.0902 [DOI] [PubMed] [Google Scholar]
- 56.da Graca B, Filardo G, Nicewander D. Consequences for healthcare quality and research of the exclusion of records from the Death Master File. Circ Cardiovasc Qual Outcomes. 2013;6:124–8. [PMID: 23322808] doi: 10.1161/CIRCOUTCOMES.112.968826 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.