Abstract
Tobacco control has made strides in prevention and cessation, but deaths will not decline rapidly without massive behavior change. Currently, inhaled smoke from combusting tobacco is chiefly responsible for prematurely killing 7.2 million people worldwide and 530,000 in the United States annually. An array of noncombustible nicotine products (NNPs) has emerged and has disrupted the marketplace. Saving lives more speedily will require societal acceptance of locating a “sweet spot” within a three-dimensional framework where NNPs are simultaneously: 1. Less toxic, 2. Appealing (can reach smokers at scale), and 3. Satisfying (adequate nicotine delivery) to displace smoking. For this harm minimization framework to eliminate smoking, a laser focus on “smoking control” (not general tobacco control) is needed. By adopting these economically viable NNPs as part of the solution, NNPs can be smoking control’s valued ally. Synthesis of the science indicates that policy and regulation can sufficiently protect youth while speeding the switch away from smoking. Despite some risks of nicotine dependence that can be mitigated but not eliminated, no credible evidence counters the assertion that NNPs will save lives if they displace smoking. But scientific evidence and advocacy has selectively exaggerated NNP harms over benefits. Accurate communication is crucial to dispel the misperception of NNPs harms and reassure smokers they can successfully replace smoking cigarettes with NNPs. Saving more lives now is an attainable and pragmatic way to call for alignment of all stakeholders and factions within traditional tobacco control rather than perpetuate the unrealized and unrealizable perfection of nicotine prohibition.
Keywords: Harm reduction, tobacco, nicotine, electronic nicotine delivery systems, non-combusted tobacco, smoking, mortality, harm minimization, public health impact
1. INTRODUCTION
1.1. Reframe nicotine use in society or stay the course?
We often attribute smoking’s incredible toll on public health to tobacco products in general. However, the overwhelming majority of tobacco-related deaths are caused by inhaling lethal smoke chiefly from cigarettes as well as from all types of cigars, hookah, roll your own, pipes and bidis. In 2017, smoking prematurely killed over 7 million people worldwide.1 At this rate, over 1 billion premature deaths will accrue globally during the 21st century.2 In the United States (US), 530,000 smokers per year die prematurely, and about 16 million more smokers suffer debilitating chronic disease burdens.3 Despite 50 years of concerted and successful tobacco control efforts to eliminate all tobacco products, the death caused by smoking persists at unacceptable levels.4 Several endgame strategies have been proposed to stay the course, eliminate all tobacco use, and destroy the tobacco industry.5 The stay-the-course framework strives to protect non-users, especially youth at any costs, and also expects all smokers to quit in this Utopian vision of a world without nicotine. But the implementation of this endgame is slow, difficult to attain and remains unrealized.
1.2. Recent developments in tobacco control
There have been enormous changes in the tobacco and nicotine product landscape over the last decade, culminating in a fundamental re-thinking of the role of nicotine and tobacco in society. In July 2017, the US Food and Drug Administration (FDA) announced a new national comprehensive nicotine management strategy: “The FDA agency’s new tobacco strategy has two primary parts: reducing the addictiveness of combustible cigarettes while recognizing and clarifying the role that potentially less harmful tobacco products could play in improving public health…The availability of potentially less harmful tobacco products could reduce risk while delivering satisfying levels of nicotine for adults who still need or want it [emphasis added].”6 (p.1). Strategies to reduce the addictiveness of combustible tobacco products are discussed in detail elsewhere,7,8 but it is important to note that the two parts are complementary. Reduced risk noncombustible nicotine products (NNPs) can provide smokers with an alternative source of enjoyable nicotine and preferably some time before introducing a product standard for reducing addictiveness in combustibles to accelerate a mass-migration away from smoked tobacco/cigarettes.8,9
The last 10 years have witnessed other unprecedented changes in the nicotine and tobacco product marketplace.10 New innovations in electronic cigarettes, heat-not-burn tobacco products and other substantially less harmful products are emerging. The world has not seen such technology-driven disruption in nicotine delivery since the 1880’s, with the invention of the cigarette rolling machine.4,11
Another recent development is the emergence of the new field of tobacco and nicotine regulatory science,12,13 which focuses on research directly relevant to informing policy and regulation of tobacco and nicotine products. Regulation of tobacco-derived nicotine (both medicinal cessation therapy and consumer products for adult recreational use) by the US FDA14 is now a critical part of any reframing of nicotine and tobacco use in society. In 2018, Public Health England (PHE)15 and the US National Academies of Sciences, Engineering and Medicine (NASEM)16 updated and synthesized the science base. There was increasing convergence in the science with some differences in emphasis derived from different predisposing ideological conviction (i.e., stay-the-course or harm reduction) in the interpretation of some of the scientific data. Warner summarized differences as being possibly driven more by emotion rather than rationality in his Doll-Winder Public Health Theme Address: How to Think - Not Feel - about Tobacco Harm Reduction.17
Rapid technological innovation in the nicotine and tobacco product marketplace, the new regulatory climate, and the stronger science focus is on maximizing benefits and minimizing harms for public health at a population level.
1.3. Division in the tobacco control community
A troubling divisiveness has emerged about rethinking the tobacco control framework. When disruptive change occurs, diffusion of innovation (theory about how new technologies spread) involves multiple streams of influence (e.g., Kingdon’s model where policy, politics, and problem focus converge in a “window of opportunity”18). During the early stages of responding to disruption, hypothetical fears about unknown consequences abound, coupled with an instinctive resistance to changing course.19,20 Over 400 years ago, Sir Francis Bacon warned about divisiveness based on prior ideological beliefs of the types being experienced by the tobacco/nicotine community today:21 “The human understanding when it has once adopted an opinion draws all things else to support and agree with it. And though there be a greater number and weight of instances to be found on the other side, yet these it either neglects and despises, or else by some distinction sets aside and rejects, in order that by this great and pernicious predetermination the authority of its former conclusion may remain inviolate.” As scientific evidence accumulates, reason prevails over emotional attachment to prior preconceived ideology. Tobacco control’s struggle with change is no different than in other fields.
Divisiveness and uncertainty aside, the opportunity lost by not changing course must also be considered. In light of the dramatic changes in the product landscape, by not taking some risks to speed the demise of deadly smoked tobacco, then worldwide over the next century the lives of a billion smokers are ultimately at stake. While all agree that saving lives from smoked tobacco is paramount, the tactics of how to move forward are unclear as long as the differences in the core underlying framework remain unresolved.15,16 The deep question boils down to whether one can accept that NNPs are less harmful, can displace smoked tobacco and that the makers and marketers of NNPs can profit from a legal product provided they comply with reasonable rules of the road (e.g., are regulated, sell to adults only, do not sell or engage in marketing to underage youth). In the next sections we explore what specific frameworks and scientific evidence provide a roadmap for maximizing the benefits and minimizes the risks of NNPs.
2. A New Framework
2.1. Overview
In considering a new framework for harm minimization, some prior tobacco control strategies will be continued, others modified, and some abandoned as iatrogenic. For example, effective policies such as taxing cigarettes, smoke free indoor air laws and reimbursement of pharmacotherapies for cessation treatment would remain. But if smokers receive deceptive information about exaggerated NNP harms or that all products are harmful (absolute risk) without direct comparison to the much greater (relative) harms of smoking, then smokers who have switched to NNPs may go back to smoking, or smokers planning to switch may not even try. Bauld (2017) stated: “Although not harmless, the evidence is unequivocal that (e-cigarette) vaping is much safer than smoking. But misinformation and scaremongering could still be putting people off switching.”22 Using the precautionary principle, the principle that a product with unknown long-term effects should be resisted, to withhold accurate information that NNPs are much less harmful than smoking is therefore iatrogenic.17 Treating all tobacco or nicotine products as equally harmful and regulating them as such supports the long-term viability and continued sale of cigarettes and the associated deaths [for details, see Royal College of Physicians23 (2016; pp.187)], Abrams et. al (2018)24 and Warner (2018)17.
A harm minimization framework requires a strategic alignment of action by all stakeholders (manufacturers, regulators, policy makers, scientists, advocates, politicians) and clear communication that risk is proportional to the harms of different nicotine products.17,24 This is an overarching paramount principle that must be adopted without ambivalence and with enthusiasm. The principle of regulation and policy being proportionate to product risk is the cornerstone of the proposed framework going forward.24,25 This core principle is covered in more detail by Fairchild and colleagues25 who outline a continuum where various action steps will differ on the degree that each action step supports harm reduction with less or more conviction.
2.2. How harmful are NNPs relative to smoking?
Summarizing the science, FDA’s Commissioner and the Director of the Center for Tobacco Products stated:6 “Nicotine, though not benign, is not directly responsible for the tobacco-caused cancer, lung disease and heart disease that kill hundreds of thousands of Americans each year” (p.1). Systematic reviews concur that NNPs are substantially less harmful than smoking.7,16,23,24,26–31 This view recognizes that nicotine per se is not a primary cause of cancers, but does contribute to a limited set of cardiovascular disease risks and risks to the unborn fetus.7,23,31–34 Consistent with the announcement6 of FDA’s nicotine management strategy to provide NNPs with satisfying levels of nicotine to smokers while reducing harmful exposures, Benowitz (July 28th 2017)9 stated:
“Without question, it is the products of combustion from tobacco that are responsible for most of the harmful effects of tobacco use on health. While nicotine is not harmless, it contributes relatively little to the harmful effects of tobacco use… The risk of using lower nicotine concentration liquids (in e-cigarettes) is that the user must consume many-fold larger amounts of aerosol, often generated at higher temperatures, to achieve desired levels of nicotine…. Larger volumes of aerosol and/or generation of aerosol at higher temperatures would result in the user being exposed to higher levels of aerosol toxicants. It might actually be safer to use e-liquids with high nicotine concentrations compared to lower concentrations.”
[emphasis added]
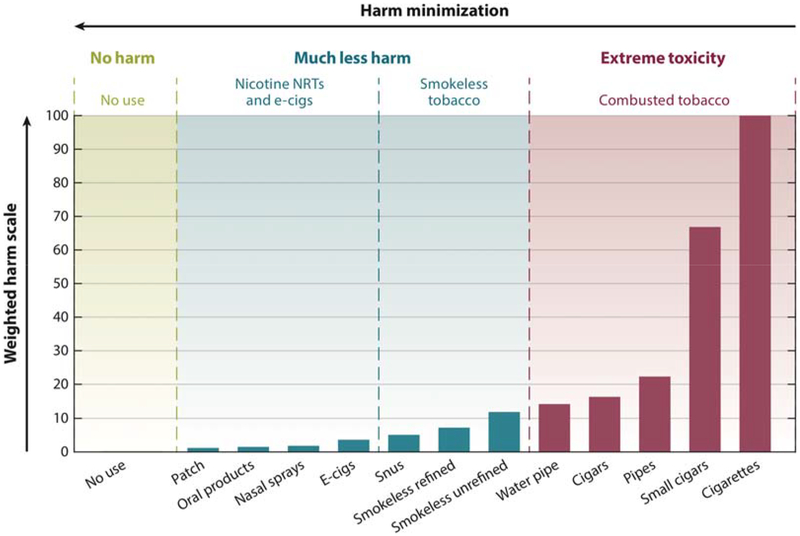
NNPs are substantially less harmful than all smoked tobacco products, and they vary in harms within and across specific products. NNPs can be framed as a four-panel supra-ordinate categorical typology (Figure 1):24 1. combusted versus non-combusted products, 2. smokeless tobacco products, 3. nicotine without tobacco products, and 4. No use and thus no exposure. Approximate product harms are depicted by bar graphs adapted from Nutt et al.35 While combusting tobacco smoke is substantially more toxic than smokeless tobacco, the bar graphs represent a weighted harm scale so the difference is not as large between unrefined smokeless tobacco and the combustible water pipe and premium cigars. The critical point is that differences within the NNPs are relatively small when compared to smoked tobacco. In terms of the harm continuum (Figure 1, explained in more detail in Abrams et al. 201824), we concur with most experts and systematic reviews15,16,28,36 summarized by West and colleagues who stated with respect to e-cigarette vapor:37
“Studies that purport to have found concentrations of some toxicants in vapor high or higher than in cigarette smoke, or physiological reactions to vapor similar to or greater than smoking, have either failed to model natural exposure conditions or overstated the clinical significance of physiological changes…. that have little or no relevance to prediction of serious illnesses in e-cigarette users.”
Figure 1.
Products along the harm minimization continuum. Adapted from Nutt et al., 201435 and reproduced from Abrams et al., 201824 The figure depicts four panels representing classes of products ranging from exceptionally low harm to exceptionally high harm. Panel 1 (left) depicts no use and thus no exposure. Panel 2 (left middle) depicts the class of nicotine delivery products without any tobacco (e-cigs/e-vapor products and nicotine replacement therapies - NRTs). Products containing tobacco are depicted as noncombusted or smokeless (panel 3, right middle) and combusted or smoked (panel 4, right). Panels 2 and 3 constitute the broader supra-ordinate category of non-combusted nicotine products (NNPs).
Some scientists and advocates have expressed concerns regarding potential cardiovascular and respiratory risks of e-vapor in certain cell preparation and acute physiological exposure studies.28,38 Extrapolation from many of these studies appears to be questionable when the studies imply direct causal links to long-term human harms equal to or greater than smoking or make no direct comparison with smoking so relative harms can be compared. Although nicotine use poses some risk for smokers with existing cardiovascular disease, risk is small relative to the risk posed by smoking cigarettes.7,31,34,36,39,40
There is less controversy about cancer risk, but there has been exaggeration of harms when NNPs are not explicitly compared with deadly smoking.38 A recent review of cancer risk41 suggests that e-cigarette emissions under normal use have about 1% of the cancer potency of tobacco smoke, even less than the Royal College of Physicians estimate of about 5%.23,41 This conclusion is consistent with others42 and puts in perspective circumstances (i.e., excessive power generated to the atomizer coil) under which some toxicants (e.g., formaldehyde, acrolein) can be produced.42–44
We suggest some of the divisiveness that paralyzes policymaking and confuses the public can be mitigated by paying closer attention to the strongest evolving scientific syntheses and not relying on select, isolated studies that exaggerate claims of harms and/or omit direct comparisons of harms relative to smoking. Strong assertions that go beyond the science (e.g., conflating correlation with causation, cherry picking results to highlight a particular viewpoint) are troubling trends that lead to greater confusion than is warranted.45–50 Adhering to good research practices (e.g., research integrity, ethics and professional standards, honesty and transparency, openness and accountability, complete expression of study limitations) is also necessary to reduce these apparent conflicts.24,51,52
The bottom line is that product standards are widely used by FDA to provide specific criteria to be met for a class of products without burdensome and expensive pre-market approval. Prudent product standards can readily eliminate or minimize many of the unnecessary potential risks of NNPs (e.g., temperature controls) and ensure quality control over devices, and purity of liquids (e.g., nicotine, propylene glycol, vegetable glycerin, flavorings) while retaining their ability to appeal to and satisfy smokers and protecting children such as with child resistant packaging.40
2.3. A Three-Dimensional Nicotine Management Framework
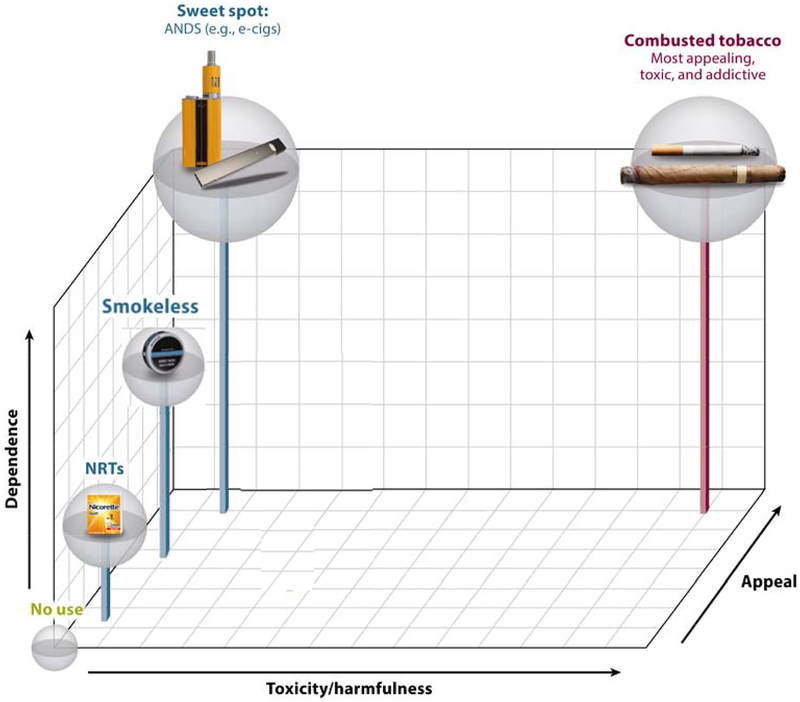
Nicotine and tobacco products can fit into a three-dimensional conceptual space [Figure 2 and in Abrams et al. (2018)24] that is not necessarily to scale: (1) harmfulness (x-axis), (2) appeal (z-axis) and (3) dependence (y-axis).24 All three dimensions must be simultaneously considered to determine how new NNP products will impact on net population health. NNPs differ substantially from smoking in their toxicity (x-axis). NNP’s appeal relates to their ability to displace smoking (z-axis), which contributes to the likelihood that the product will be adopted and its use sustained at a scale large enough to affect population health improvement (i.e., reach or market penetration).53 Appeal is complex and encompasses attractiveness of the product, sensory characteristics, and subjective satisfaction as well as cost, accessibility, and marketing practices.40,54–56 A product with minimal appeal will not be adopted or used extensively (e.g., over-the-counter NRT57,58). NNPs must be sufficiently appealing to encourage a larger portion of smokers to switch from the high- to the low-harm products.54 Dependence (y-axis) refers to the potential for the product to provide satisfaction and induce a degree of addiction, which is a function both of its pharmacological and its subjective rewarding and sensory properties. Dependence can reflect a response to withdrawal and to enjoying, liking or needing nicotine’s well-documented desirable effects, like improved alertness, concentration, mood and memory.59,60 Some degree of dependence upon less harmful NNPs may have to be acceptable to society to speed the demise of smoking and its attendant massive harms by ensuring NNPs are sufficiently enjoyable and effective at providing the experience smokers want including the beneficial effects of nicotine on cognition and memory.59,60
Figure 2.
Multidimensional framework for nicotine containing products, considering (1) harmfulness, (2) appeal, and (3) dependence. Reproduced from Abrams et al., 201824 The top, back, right corner depicts the most popular (appealing), highly satisfying (dependence), and toxic space (combusted products), whereas no use at all is zero on all three axes. The bottom, front, left space depicts products that have low toxicity but little appeal or satisfaction (e.g., nicotine replacement therapies - NRTs). Minimizing risk while making a net population health impact requires products to successfully compete with and replace smoking. Thus, the sweet spot, where ANDS or NNP’s products might fall, is depicted by high appeal and satisfaction but low toxicity along with products such as Swedish-type snus, which has successfully displaced cigarettes in Sweden.
The three dimensional space depicted in Figure 2 can be helpful in locating what may be the “sweet spot” of an ideal NNP. Availability of safe, appealing flavors, efficient nicotine delivery, and lower cost than cigarettes all play an important role in improving the overall appeal on a large-scale basis.55,56 Some e-cigarettes appear to be able to occupy the “sweet spot” because some smokers have found an e-cigarette to sustain use and replace smoking.28,55,56,61–64 E-cigarettes are used by more smokers than NRT in quit attempts in both the US and the UK.23,65 Evidence also suggests they can be effective in helping smokers to quit smoking.46 The more appealing and satisfying the NNP product is the greater the likelihood of switching away from smoking.
A tradeoff is raised between concerns for youth uptake among non-users who otherwise would not have smoked if NNPs did not exist and helping smokers and potential smokers to switch (including youth who would have smoked anyway). The risk to youth non-users will increase as products evolve and become better at finding that sweet spot (appeal and satisfaction) to replace smoked tobacco (e.g., better smoking cessation medication, JUUL’s use of benzoic acid salts, modern tank or modular e-cigarette devices, heat-not-burn or smokeless tobacco products). While higher nicotine dependence liability is likely for some users, this risk must be considered in the overall calculus of a harm reduction benefit for smokers and potential smokers when the nicotine is decoupled from toxic smoke.
Different products can be ordered in this space, compared to one another and evaluated on their ability to minimize net harm and maximize net benefits (finding the “sweet spot”). If NNPs can compete with and ultimately replace smoking,10 the net population toxicant exposure can be substantially reduced as has been shown in the Swedish experienced with snus use among males.66 Holding all three dimensions in consideration at the same time is critical for an overarching new framework for guiding planned action steps and provides a conceptual and visual road map to speed the demise of using deadly smoked tobacco as the preferred way to enjoy nicotine.
3. Making a Population Impact: Modeling State Transitions to Characterize Benefits over Harms
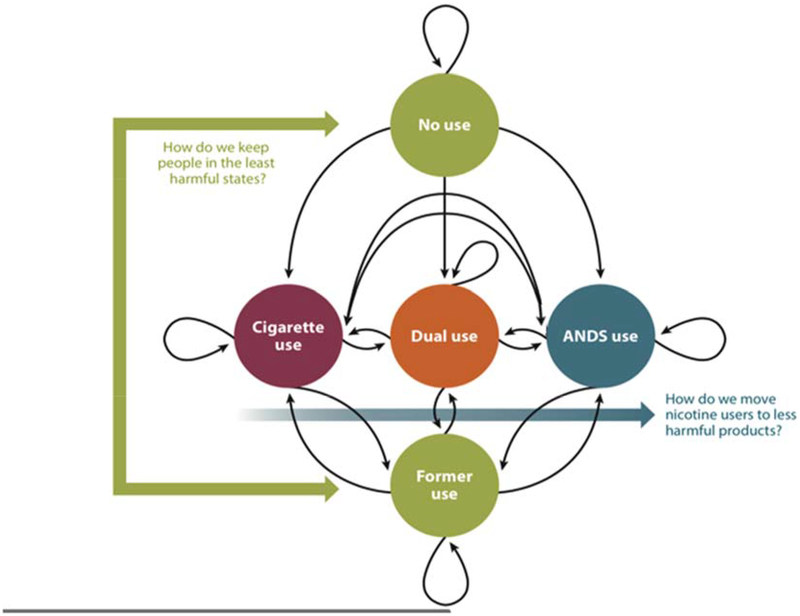
As stated previously, the core principle for the alignment of stakeholders is that regulatory, other strategies and tactics and communications are made proportional to the relative harms of each class of products and every NNP product is always compared with deadly smoking. The FDA’s Center for Tobacco Products’ public health standard implies an integrated consideration of product benefits and harms at the individual and population levels (including likelihoods of initiation and cessation). Population net toxicant exposure depends on the patterns and prevalence of product use that vary along the continuum of harm (Figures 1 and 2). Figure 3 presents a model using the example of cigarettes and NNPs (e-cigarettes) to illustrate the possible states and pathways that must be considered to optimize the framework for smoking control.24,67 Briefly, directed arrows represent transitions; looped arrows at each state represent maintenance of that state.
Figure 3.
Markov state transition model of cigarette and non-combusted nicotine products (NNPs), or alternative nicotine delivery systems (ANDS) use. Adapted from Cobb et al., 201567 and reproduced from Abrams et al., 201824 Directed arrows represent transitions, whereas looped arrows at each state represent maintenance of that state. Traditional youth prevention and smoking cessation strategies reinforce the states of noncurrent and former use depicted by green circles, and complementary new harm minimization strategies facilitate movement away from deadly combusted tobacco smoking to substantially less harmful alternative NNP/ANDS products (blue arrow).
Each strategy influences the flow from one state to another. The FDA two-part strategy6 includes policy and regulation (a) to keep non-users and former users in the no use states at the top and bottom of Figure 3; and (b) harm minimization strategies that facilitate movement away from smoking (depicted by the arrow from smoking to exclusive e-cigarette use either via dual use or directly switching and thus skipping dual use). It should be noted that one could remain in dual use with no reduction in cigarette smoking, resulting in no change in harm but no known increased harm in terms of biomarker evidence to date.68 Outcomes can be determined empirically using population prevalence rates in states and transition rates between states. Simulation modeling of policy and regulation effects on transition rates can indicate tipping points for benefits and harms, given different scenarios of product use, harmful exposure and smoking prevalence.69 Examples of these approaches could be to impose a differential tax on nicotine-containing products proportional to their degree of harm (less harmful, lower tax),70 ensure efficient nicotine delivery and appeal in NNPs,8,71 and simultaneously reduce the appeal of smoking by banning menthol or flavored cigars and reducing nicotine yields in smoked products but not in NNPs.55,56,72,73 Making combusted tobacco more expensive and less appealing and NNPs less expensive and more appealing will heighten the contrast between less and more harmful products and help steer smokers at any age away from smoking. This can be achieved through not only regulating products, but also through policies and communications that differentially incentivize those manufacturers willing to responsibly make and market much less harmful NNPs to adult consumers and phase out smoked products.
3.1. Do E-Cigarettes Attract Youth and Lead More to Smoking Over and Above the Counterfactual (the Absence of E-Cigarettes)?
Studies show that current e-cigarette use by youth consists largely of experimentation, not long-term use.24,74,75 Longitudinal studies, a meta-analysis76 (with a later correction of errors that reduced the effect size77), and a systematic review16 show as expected that some youth ever e-cigarette users will use cigarettes during a short follow-up period,78–87 raising concern about so-called “gateway” effects (i.e., e-cigarette use leading directly and causally to regular daily smoking).47 The authors duly note that finding such an association, even in longitudinal studies, does not imply causality.76 Confounding influences, such as shared vulnerability factors that predispose youth to try alcohol, marijuana, other drugs and risky experiences,50,74,75,88 cannot be easily ruled out.17 Moreover, the proportion of early users who progress beyond experimentation (e.g., use on < 5 of the past 30 days) to later daily or lifetime use has not been established. The proportion who progress to long term daily use has been extrapolated from cross-sectional studies with a wide range from about 25% to over 60 % of ever smokers possibly becoming daily users.89–91 One combined prospective and retrospective longitudinal study by Colby et al (2012)91 reported on lifetime smoking trajectories up to age approximately 40 years and found that 34% of those who ever tried a cigarette did not progress to daily smoking and an additional 27% were former smokers prior to age 40. A recent study of youth and young adults (age 15 to 24 years) in a large nationally representative sample (n = 15,275) prospectively examined product use transitions over a period of 2.5 years and showed that short-term transitions (≤1 year) between use of any product to subsequent use of any other product were equally likely, but affected only a small proportion of the population who were already product users.92 After 2.5 years, the strongest transition probabilities were from initial use of cigarettes to continuing to smoke cigarettes, and from use of any other products including e-cigarettes to no current use.92
Taken together the studies reviewed to date suggest extreme caution be exercised when attempting to make predictions from ever use or even from any past 30-day use to daily use, let alone to the likelihood of a future lifetime of smoking cigarettes. To have a net public health harm, the progression to lifetime use must be over and above those who would have smoked anyway. Moreover, even if there was a gateway effect from ever tried an e-cigarette to a lifetime of smoking, we concur with Kozlowski and Warner (2017) and others24 who conclude that overall youth smoking prevalence has dropped at faster rate during the steepest rise in e-cigarette use: while society must be vigilant, fears of hypothesized harms93 due to gateway effects among youth are unlikely to undermine the much larger benefits of discouraging smoking behavior in the whole population.47
Finally, simulation modeling with sensitivity analyses shows that the purported gateway effect (if it exists at all) would have to be implausibly large to increase the net public health harm over benefits.67,69 Both Levy et. al69 and Warner and Mendez94 independently concluded that e-cigarettes have substantial potential to improve net public health consistent with the majority of other published simulation studies including the 2018 National Academies of Science, Engineering and Medicine (NASEM), even under very conservative consumptions.16,69,95–98 The public health benefit does diminish in the models when it is assumed there is a very high relative risk of vaping compared to smoking (e.g., 50% risk) coupled with a high assumed (direct causal) gateway effect for non-using youth and/or with a low adult cessation rate. One outlier simulation model concluded that there would be a net public health harm under almost all assumptions, but this model assumed vaping would have almost no effect on current smokers as well as a very large gateway effect on youth (for every one case of cessation there would be about eight new lifetime smokers).99 The strongest scientific evidence is not consistent with these extreme assumptions.17,47,76,94 The outlier model is also based on a misleading negative correlation between e-cigarettes and smoking cessation from a meta-analysis100 of studies, many of which did not even address the cessation hypothesis. The meta-analysis has been debunked.46
In conclusion, we concur with Warner’s (2018)17 overall synthesis of the evidence that uptake of cigarettes among adolescents is declining at an unprecedented rate, and even if vaping caused some never smoking adolescents to try smoking and even if some of those triers progress to daily and then to a lifetime of smoking, then even a moderate rate of smoking cessation (see section on cessation below) still makes e-cigarettes a net public health benefit.17
3.2. Do E-Cigarettes Help Smoking Cessation or Reduction?
Randomized controlled trials (RCTs) and well-designed observational studies show that e-cigarettes can help some adult smokers to quit smoking15,28,46,101–106 at rates similar to or higher than NRT.107 Despite the increasingly positive evidence, a questionable meta-analysis100 (including observational studies, with loosely-defined measures of exposure and outcomes, inability or failure to control for potential confounders or lacking use of adequate comparison groups), reported that use of e-cigarettes was associated with no change or negative correlations with smoking cessation. But the Cochrane Handbook cautions: “meta-analysis of studies that are at risk of bias may be seriously misleading. If bias is present in each (or some) of the individual studies, meta-analysis will simply compound the errors, and produce a ‘wrong’ result that may be interpreted as having more credibility”108 (p. 247). In sharp contrast to this problematic meta-analysis, studies that take into account how and why e-cigarettes were used (e.g., frequency and duration of use, type of device, use specifically for cessation) suggest that daily vaping can facilitate quit attempts and cessation.61–64 Newer tank, mod and pod systems that are more satisfying (sweet spot) may improve outcome efficacy.109 Recent studies using large national US samples as well as the conclusions from Warner (2018) and the NASEM report15–17,110 indicate that use of e-cigarettes is associated with smoking cessation and with a greater number of quit attempts than NRT.65,111–114 Warner and Mendez (2018)94 reported that in the UK,115,116 e-cigarettes increased smoking cessation by at least 8% and in the US by at least 12% based on studies done by Zhu et. al (2017) and others.46,111–113 The recent and more methodologically sound studies (see Villanti et. al for details)46 seriously challenge and debunk the conclusions of the meta-analysis of Kalkhoran and Glantz (2016)100 and the updated meta-analysis of Glantz and Bareham (2018).117
Concerns have been raised about persistent dual use (no smoking reduction or cessation, but continued use of both products) undermining cessation in those who might otherwise have quit.100 The counterfactual case (what would the cessation rate among dual users have been if e-cigarettes had not existed) is impossible to directly determine, but many considerations mitigate concerns. Dual use even without appreciable reduction in smoking does not appear to increase biological markers of harm.26,27 Surveys of e-cigarette users indicate that quitting cigarettes is their primary reason for use,28 even among youth.118
In the years when e-cigarette use increased the most, quit attempts also increased.119–122 Studies from the UK converge with US studies indicating e-cigarettes have increased quitting smoking over and above what would have otherwise been expected.17,94,113,116,123 Some patterns of infrequent e-cigarette use or even past use measured at one point in time may be (mistakenly) called “dual use” leading to overestimates of chronic dual use.124 While “some-day” use of e-cigarettes is most common among smokers, the highest prevalence of daily e-cigarette use is seen among recent (<3 years) former smokers.125
Public Health England15 provides details about heat-not-burn NNPs, compares systematic reviews and meta-analyses, and estimates an additional 20,000 smokers’ quitting is attributed to e-cigarettes. Other reasons for e-cigarettes and smoking cessation success include that: (a) dual use was common with some users switching almost immediately while others took months to years before switching completely; (b) people are trying various products (tank models) and different nicotine strengths – perhaps to find their individual “sweet spot” (Figure 2); and (c) over time, the use of e-cigarette flavors (fruit/beverage, dessert/pastry and candy/chocolate/sweets) are favored instead of their initial use of tobacco or menthol/mint flavors.15,110,126
As is the case with using FDA-approved NRTs while still smoking (as a reduce to quit strategy), dual use of e-cigarettes either for a short period or perhaps even for a longer period of several years duration may be necessary along with finding devices, nicotine delivery levels and satisfying flavors (the sweet spot) that help vapers along the path to complete smoking cessation and possibly prevents relapse.127 There is a need to more precisely define and measure the frequency, intensity and duration of co-use at frequent time intervals128 within the same individuals to understand different types of co-use behavior and avoid the generic and confusing term “dual use”. Differences between persistent dual users and eventual switchers are not fully understood. Longitudinal studies over several years of all possible product use states, including dual use and switching (Figure 3) are needed and assumptions of negative effects of dual use on public health are premature.24,92
In summary, the accumulating evidence does not support the contention that e-cigarettes either inhibit cessation or are undermining historical “tobacco control” cessation efforts. Rather, the stronger studies suggest e-cigarettes are increasing cessation rates and quit attempts over and above the historical rates by reaching a larger proportion of smokers.15,112,113 Simulation models already reviewed above are consistent in showing that under all but the most implausible scenarios switching to safer NNPs results in net population benefits.24,46,48,67,94,129
4. Proactively Communicating Accurate, Evidence-based Information to the Public
Public education must ensure consumers of nicotine containing products are accurately informed about differential harms compared to deadly smoking (relative risk) and not simply compared to no use (absolute risk).24 A related need is to sharpen the language describing similarities and differences between combustible and noncombustible tobacco and NNPs along the harm continuum. Because nicotine is primarily derived from the tobacco plant, legal definitions of tobacco products in the US include all forms of tobacco-derived nicotine and conflate their harms. Legal contortions permit tobacco-derived nicotine in the form of nicotine replacement products to be classified as therapeutics while nicotine delivery products with similar, negligible risks are classified as consumer products, resulting in regulatory confusion. In the end, tobacco and nicotine product consumers are the most important victims of this lack of clarity.48,49,130–133 The potential positive impact of e-cigarettes may have therefore been slowed by overstated claims of their harms.47,48 Only 5.3% of Americans correctly believe e-cigarettes are “much less harmful” than cigarettes, 37% believe they are the same or worse than smoking, and 34% don’t know.134,135 Misperceptions of harms have increased in recent years.48,135–137 Misinformation deprives individuals of the opportunity to take health-protective action and is deceptive to consumers.48,131 Accurate public education is needed to communicate the importance of smoking cessation and nicotine’s relative safety when de-coupled from smoke.6
5. Conclusions: Reaffirming Harm Minimization and Smoking Control as the New Tobacco Control
Charting a new course in tobacco control via harm reduction must be seriously considered. Innovations in technology and accelerating adoption of NNPs have taken the “tobacco control” community, policymakers and cigarette companies by storm and surprise.10,24 In many other areas, technological advances transform behaviors at the population level. New products consistently, although not always predictably, make old ones obsolete. In light of NNPs, which themselves are undergoing transformation and evolution to minimize toxic exposures, the logic of smoking harm minimization is simple and compelling. As Michael Russell, a pioneering tobacco control scientist, put it, “People smoke for nicotine but they die from the tar.”138 The safest course is to stop smoking or, better, never to start. But a harm minimization framework recognizes that demanding the unrealistic and unrealized utopian dream (i.e., elimination of any and all consumer nicotine or tobacco products regardless of their relative harms and the related destruction of the entire tobacco and nicotine consumer product industry) actually undercuts the realistic benefits of pragmatism. When a harmful behavior cannot be eliminated, it is necessary to reduce its adverse health consequences to the greatest extent possible among any users of nicotine or tobacco containing consumer products.10,23,30,130,139
As stated several times, a critical organizing harm minimization principle is that policy, regulation, science and advocacy should be evidence-based and aligned proportional to the degree of product harm. The two-part regulatory scheme proposed for FDA should in spirit and in action place priority on ensuring accurate communication about the appeal, safety and quality for less harmful NNPs and speed their approval with prudent but not overly burdensome product standards and approve their ability to make truthful claims that their products are substantially less harmful than inhaled smoke from combusting tobacco.6
The status quo, unfortunately, is now upside down. Staying the course now risks perpetuation of smoked tobacco, prolongs unnecessary excessive deaths and slows adoption of much less harmful NNPs. Harm minimization strategies have the potential to realign market forces and economic incentives for consumers and those manufacturers willing to responsibly make and market much less harmful NNPs to adult consumers.10,53,70,140–142 Even if the minimal risk of harm to some youth who otherwise would not have smoked is marginally increased, such risks must be weighed against the substantial and immediate benefits of displacing smoking with safer nicotine products among both mostly those youth who will use tobacco anyway and any already smoking adults.10,23,24,30,32,47,53,70,130,143
The FDA’s new comprehensive nicotine framework6,24,144 acknowledges that there are now satisfying and enjoyable nicotine-containing products that are acceptable alternatives for adult smokers that these products could displace smoking.10 Within a nicotine management reframing of strategy,24 all industries that make and market different forms of nicotine products (e.g., pharmaceutical, e-cigarette, smokeless tobacco, and even the combusted tobacco makers –the so called “Big Tobacco” industry) can be politically and economically aligned with regulators, public health advocates, scientists and health care practice to speedily phase out smoked tobacco products.24,145,146 Current and future smokers’ lives are at stake.
A laser-like focus on making smoked tobacco products obsolete suggests the overall framework for the future is to focus policy, regulation, communication and practice on smoking control rather than on general tobacco control while discouraging use of any products by underage youth as much as possible.10,24 The three-dimensional framework provides a road map to find the “sweet spot” to maximize the replacement of smoked tobacco with NNPs. The model of all the stocks and flows coupled with survey data and simulation modeling provides a basis for post market tracking of the impact of NNPs on the population. Harm minimization can complement traditional tobacco control strategies that are effective. Given tectonic changes in the product landscape, some of these strategies may remain effective, but others may now be more harmful than helpful to public health because by opposing harm reduction alternatives to deadly smoked tobacco one is inadvertently helping to perpetuate smoking rather than speeding the replacement of smoking with NNPs.130 Opposing harm reduction in effect slows the speedy demise of using deadly smoked tobacco products. Going forward, both old and new strategies need to be carefully aligned using the paramount principle of having regulation, policy, advocacy and communications be proportional to the risk ratio of each class of tobacco or nicotine product. If most smokers in the US switched within the next 10 years to NNPs, it is estimated that over 6 million premature deaths and 86 million lost life years would be averted.24,147
ACKNOWLEDGEMENTS
ACV was supported by the Centers of Biomedical Research Excellence P20GM103644 award from the National Institute of General Medical Sciences. The content is solely the responsibility of the author and does not necessarily represent the official view of the National Institutes of Health.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.World Health Organization. Tobacco Fact Sheet 2017; http://www.who.int/mediacentre/factsheets/fs339/en/. Accessed August 9, 2017.
- 2.World Health Organization. WHO Report on the Global Tobacco Epidemic, 2008: The MPOWER package. Geneva, Switzerland: World Health Organization;2008. [Google Scholar]
- 3.Centers for Disease Control and Prevention. Smoking & Tobacco Use: Fast Facts - Diseases and Death. 2017; https://www.cdc.gov/tobacco/data_statistics/fact_sheets/fast_facts/index.htm. Accessed January 16, 2018.
- 4.Abrams DB, Glasser AM, Villanti AC, Niaura R. Cigarettes: The Rise, Decline but not the Demise of the Greatest Behavioral Health Disaster of the 20th Century In: Kaplan R, Spittel M, David D, eds. Emerging Behavioral and Social Science Perspectives on Population Health. Vol AHRQ Publiation No. 15–0002 Rockville, MD: Agency for Healthcare Research and Quality, Office of Behavioral and Social Sciences Research, National Institutes of Health; 2015:143–168. [Google Scholar]
- 5.Warner KE. An endgame for tobacco? Tob Control 2013;22 Suppl 1:i3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gottlieb S, Zeller M. A Nicotine-Focused Framework for Public Health. N Engl J Med 2017;377(12):1111–1114. [DOI] [PubMed] [Google Scholar]
- 7.Benowitz NL, Fraiman JB. Cardiovascular effects of electronic cigarettes. Nat Rev Cardiol 2017;14(8):447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benowitz NL, Donny EC, Hatsukami DK. Reduced nicotine content cigarettes, e-cigarettes and the cigarette end game. Addiction (Abingdon, England). 2017;112(1):6–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benowitz NL. Re: Vaporised Nicotine Products Bill 2017. Letter to Senate Community Affairs Legislation Committee:; 2017. [Google Scholar]
- 10.Abrams DB. Promise and peril of e-cigarettes: can disruptive technology make cigarettes obsolete? JAMA 2014;311(2):135–136. [DOI] [PubMed] [Google Scholar]
- 11.Brandt AM. The cigarette century: the rise, fall, and deadly persistence of the product that defined America. New York, NY: Basic Books; 2007. [Google Scholar]
- 12.Wipfli HL, Berman M, Hanson K, et al. Defining Tobacco Regulatory Science Competencies. Nicotine Tob Res 2017;19(2):222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashley DL, Backinger CL, van Bemmel DM, Neveleff DJ. Tobacco regulatory science: research to inform regulatory action at the Food and Drug Administration’s Center for Tobacco Products. Nicotine Tob Res 2014;16(8):1045–1049. [DOI] [PubMed] [Google Scholar]
- 14.U.S. Food and Drug Administration. Division A--Family Smoking Prevention and Tobacco Control Act. In: Department of Health and Human Services US, ed. Public Law 111–31 Vol 12562009. [Google Scholar]
- 15.McNeill A, Brose LS, Calder R, Bauld L, Robson D. Evidence review of e-cigarettes and heated tobacco products 2018: A report commissioned by Public Health England. London: Public Health England;2018. [Google Scholar]
- 16.National Academies of Sciences E, and Medicine. Public health consequences of e-cigarettes. Washington, DC: The National Academies Press;2018. [PubMed] [Google Scholar]
- 17.Warner KE. How to Think - Not Feel - about Tobacco Harm Reduction. Nicotine Tob Res. 2018. [DOI] [PubMed] [Google Scholar]
- 18.Kingdon JW. Agendas, Alternatives, and Public Policies. New York, NY: Addison-Wesley Educational Publishers, Inc.; 2003. [Google Scholar]
- 19.Kuhn TS. The Structure of Scientific Revolutions: 50th Anniversary Edition. Fourth ed. Chicago IL: University of Chicago Press; 2012. [Google Scholar]
- 20.Abrams DB, Niaura R. The importance of science-informed policy and what the data really tell us about e-cigarettes. Isr J Health Policy Res 2015;4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bacon F. The New Organon and Related Writings. New York, NY: Liberal Arts Press; 1960. [Google Scholar]
- 22.Bauld L. The evidence keeps piling up: e-cigarettes are definitely safer than smoking. 2017; https://www.theguardian.com/science/sifting-the-evidence/2017/dec/29/e-cigarettes-vaping-safer-than-smoking. Accessed January 16, 2018.
- 23.Royal College Physicians. Nicotine without smoke: Tobacco harm reduction. London April 2016. [Google Scholar]
- 24.Abrams DB, Glasser AM, Pearson JL, Villanti AC, Collins LK, Niaura RS. Harm Minimization and Tobacco Control: Reframing Societal Views of Nicotine Use to Rapidly Save Lives. Annu Rev Public Health 2018;39:193–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fairchild AL, Lee JS, Bayer R, Curran J. E-Cigarettes and the Harm-Reduction Continuum. N Engl J Med 2018;378(3):216–219. [DOI] [PubMed] [Google Scholar]
- 26.Goniewicz ML, Gawron M, Smith DM, Peng M, Jacob P 3rd, Benowitz NL. Exposure to Nicotine and Selected Toxicants in Cigarette Smokers Who Switched to Electronic Cigarettes: A Longitudinal Within-Subjects Observational Study. Nicotine Tob Res 2017;19(2):160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goniewicz ML, Knysak J, Gawron M, et al. Levels of selected carcinogens and toxicants in vapour from electronic cigarettes. Tob Control 2014;23(2):133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glasser AM, Collins L, Pearson JL, et al. Overview of Electronic Nicotine Delivery Systems: A Systematic Review. Am J Prev Med 2017;52(2):e33–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hecht SS, Carmella SG, Kotandeniya D, et al. Evaluation of toxicant and carcinogen metabolites in the urine of e-cigarette users versus cigarette smokers. Nicotine Tob Res 2015;17(6):704–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McNeill A, Brose LS, Calder R, Hitchman S, Hajek P, McRobbie H. E-cigarettes: an evidence update -- A report commissioned by Public Health England. London, England: Public Health England;2015. [DOI] [PubMed] [Google Scholar]
- 31.Benowitz NL, Burbank AD. Cardiovascular toxicity of nicotine: Implications for electronic cigarette use. Trends in cardiovascular medicine 2016;26(6):515–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Britton J, Bogdanovica I, McNeill A, Bauld L. Commentary on WHO Report on Electronic Nicotine Delivery Systems and Electronic Non-Nicotine Delivery Systems. 2016; http://ukctas.net/pdfs/UKCTAS-response-to-WHO-ENDS-report-26.10.2016.pdf. Accessed May 31, 2017.
- 33.Fairchild AL, Bayer R, Colgrove J. The renormalization of smoking? E-cigarettes and the tobacco “endgame”. N Engl J Med 2014;370(4):293–295. [DOI] [PubMed] [Google Scholar]
- 34.Niaura R. Re-thinking nicotine and its effects. 2016; https://truthinitiative.org/sites/default/files/ReThinking-Nicotine.pdf.
- 35.Nutt DJ, Phillips LD, Balfour D, et al. Estimating the harms of nicotine-containing products using the MCDA approach. Eur Addict Res 2014;20(5):218–225. [DOI] [PubMed] [Google Scholar]
- 36.Farsalinos K. Electronic cigarettes: an aid in smoking cessation, or a new health hazard? Ther Adv Respir Dis 2017:1753465817744960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.West R, Brown J, Shahab L. Written evidence submitted by University College London, Tobacco and Alcohol Research Group (UTARG) (ECG0047). UK Parliament; December 12 2017. [Google Scholar]
- 38.Glantz S, Bareham D. E-cigarettes: Use, Effects on Smoking, Risks, and Policy Implications. Annual Review of Public Health. 2018;39:28.21–28.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fagerstrom K, Etter JF, Unger JB. E-cigarettes: a disruptive technology that revolutionizes our field? Nicotine Tob Res 2015;17(2):125–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fagerstrom KO, Bridgman K. Tobacco harm reduction: the need for new products that can compete with cigarettes. Addict Behav 2014;39(3):507–511. [DOI] [PubMed] [Google Scholar]
- 41.Stephens WE. Comparing the cancer potencies of emissions from vapourised nicotine products including e-cigarettes with those of tobacco smoke. Tob Control 2017. [DOI] [PubMed] [Google Scholar]
- 42.Farsalinos KE, Gilman G. Carbonyl Emissions in E-cigarette Aerosol: A Systematic Review and Methodological Considerations. Frontiers in Physiology 2018;8:1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Ther Adv Drug Saf 2014;5(2):67–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farsalinos KE, Voudris V, Poulas K. E-cigarettes generate high levels of aldehydes only in ‘dry puff’ conditions. Addiction 2015;110(8):1352–1356. [DOI] [PubMed] [Google Scholar]
- 45.Baicker K, Chandra A. Evidence-Based Health Policy. N Engl J Med 2017;377(25):2413–2415. [DOI] [PubMed] [Google Scholar]
- 46.Villanti AC, Feirman SP, Niaura RS, et al. How do we determine the impact of e-cigarettes on cigarette smoking cessation or reduction? Review and recommendations for answering the research question with scientific rigor. Addiction. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kozlowski LT, Warner KE. Adolescents and e-cigarettes: Objects of concern may appear larger than they are. Drug and Alcohol Dependence. 2017;174:209–214. [DOI] [PubMed] [Google Scholar]
- 48.Kozlowski LT, Sweanor D. Withholding differential risk information on legal consumer nicotine/tobacco products: The public health ethics of health information quarantines. Int J Drug Policy 2016;32:17–23. [DOI] [PubMed] [Google Scholar]
- 49.Kozlowski LT, Edwards BQ. “Not safe” is not enough: smokers have a right to know more than there is no safe tobacco product. Tob Control 2005;14 Suppl 2:ii3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niaura RS, Glynn TJ, Abrams DB. Youth experimentation with e-cigarettes: Another interpretation of the data. JAMA Pediatrics 2014;312(6):1–2. [DOI] [PubMed] [Google Scholar]
- 51.Robson D, McNeill A. Answering the question or questioning the answer? Addiction 2017. [DOI] [PubMed] [Google Scholar]
- 52.West R. Improving the quality of research on e-cigarettes The E-Cigarette Summit; November 17, 2017; London, England. [Google Scholar]
- 53.Cobb NK, Abrams DB. The FDA, e-cigarettes, and the demise of combusted tobacco. N Engl J Med 2014;371(16):1469–1471. [DOI] [PubMed] [Google Scholar]
- 54.Smiley SL, DeAtley T, Rubin LF, et al. Early Subjective Sensory Experiences with “cigalike” E-cigarettes Among African American Menthol Smokers: A Qualitative Study. Nicotine Tob Res 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Spyrou A, Voudris V. Impact of flavour variability on electronic cigarette use experience: an internet survey. Int J Environ Res Public Health 2013;10(12):7272–7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farsalinos KE, Poulas K, Voudris V, Le Houezec J. Prevalence and correlates of current daily use of electronic cigarettes in the European Union: analysis of the 2014 Eurobarometer survey. Intern Emerg Med 2017. [DOI] [PubMed] [Google Scholar]
- 57.Hammond D, McDonald PW, Fong GT, Borland R. Do smokers know how to quit? Knowledge and perceived effectiveness of cessation assistance as predictors of cessation behaviour. Addiction 2004;99(8):1042–1048. [DOI] [PubMed] [Google Scholar]
- 58.West R, Hajek P, Foulds J, Nilsson F, May S, Meadows A. A comparison of the abuse liability and dependence potential of nicotine patch, gum, spray and inhaler. Psychopharmacology (Berl) 2000;149(3):198–202. [DOI] [PubMed] [Google Scholar]
- 59.Talati A, Keyes KM, Hasin DS. Changing relationships between smoking and psychiatric disorders across twentieth century birth cohorts: clinical and research implications. Mol Psychiatry 2016;21(4):464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl) 2010;210(4):453–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Manzoli L, Flacco ME, Fiore M, et al. Electronic Cigarettes Efficacy and Safety at 12 Months: Cohort Study. PLoS One 2015;10(6):e0129443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Biener L, Hargraves JL. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine Tob Res 2015;17(2):127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brose LS, Hitchman SC, Brown J, West R, McNeill A. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction 2015;110(7):1160–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hitchman SC, Brose LS, Brown J, Robson D, McNeill A. Associations Between E-Cigarette Type, Frequency of Use, and Quitting Smoking: Findings From a Longitudinal Online Panel Survey in Great Britain. Nicotine Tob Res 2015;17(10):1187–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Caraballo RS, Shafer PR, Patel D, Davis KC, McAfee TA. Quit Methods Used by US Adult Cigarette Smokers, 2014–2016. Prev Chronic Dis 2017;14:E32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee PN. Epidemiological evidence relating snus to health--an updated review based on recent publications. Harm Reduct J 2013;10:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cobb CO, Villanti AC, Graham AL, et al. Markov Modeling to Estimate the Population Impact of Emerging Tobacco Products: A Proof-Of-Concept Study. Tobacco Regulatory Science 2015;1(2):121–141.26236764 [Google Scholar]
- 68.Shahab L, Goniewicz ML, Blount BC, et al. Nicotine, Carcinogen, and Toxin Exposure in Long-Term E-Cigarette and Nicotine Replacement Therapy Users: A Cross-sectional Study. Ann Intern Med 2017;166(6):390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Levy DT, Borland R, Villanti AC, et al. The Application of a Decision-Theoretic Model to Estimate the Public Health Impact of Vaporized Nicotine Product Initiation in the United States. Nicotine Tob Res 2017;19(2):149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chaloupka FJ, Sweanor D, Warner KE. Differential Taxes for Differential Risks--Toward Reduced Harm from Nicotine-Yielding Products. N Engl J Med 2015;373(7):594–597. [DOI] [PubMed] [Google Scholar]
- 71.Donny EC, Denlinger RL, Tidey JW, et al. Randomized Trial of Reduced-Nicotine Standards for Cigarettes. N Engl J Med 2015;373(14):1340–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Villanti AC, Giovino GA, Burns DM, Abrams DB. Menthol cigarettes and mortality: keeping focus on the public health standard. Nicotine Tob Res 2013;15(2):617–618. [DOI] [PubMed] [Google Scholar]
- 73.Tobacco Products Scientific Advisory Committee. Menthol Cigarettes and Public Health: Review of the Scientific Evidence and Recommendations Rockville, MD: U.S. Food and Drug Administration, Center for Tobacco Products;2011. [Google Scholar]
- 74.Collins LK, Villanti AC, Pearson JL, et al. Frequency of Youth E-Cigarette, Tobacco, and Poly-Use in the United States, 2015: Update to Villanti et al., “Frequency of Youth E-Cigarette and Tobacco Use Patterns in the United States: Measurement Precision Is Critical to Inform Public Health”. Nicotine Tob Res 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Villanti AC, Pearson JL, Glasser AM, et al. Frequency of youth e-cigarette and tobacco use patterns in the U.S.: Measurement precision is critical to inform public health. Nicotine Tob Res 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Soneji S, Barrington-Trimis JL, Wills TA, et al. Association Between Initial Use of e-Cigarettes and Subsequent Cigarette Smoking Among Adolescents and Young Adults: A Systematic Review and Meta-analysis. JAMA Pediatr 2017;171(8):788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Soneji S. Errors in Data Input in Meta-analysis on Association Between Initial Use of e-Cigarettes and Subsequent Cigarette Smoking Among Adolescents and Young Adults. JAMA Pediatr 2018;172(1):92–93. [DOI] [PubMed] [Google Scholar]
- 78.Huh J, Leventhal AM. Progression of Poly-tobacco Product Use Patterns in Adolescents. Am J Prev Med 2016;51(4):513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Barrington-Trimis JL, Urman R, Berhane K, et al. E-Cigarettes and Future Cigarette Use. Pediatrics. 2016;138(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leventhal AM, Stone MD, Andrabi N, et al. Association of e-Cigarette Vaping and Progression to Heavier Patterns of Cigarette Smoking. JAMA 2016;316(18):1918–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leventhal AM, Strong DR, Kirkpatrick MG, et al. Association of Electronic Cigarette Use With Initiation of Combustible Tobacco Product Smoking in Early Adolescence. Jama 2015;314(7):700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Miech R, Patrick ME, O’Malley PM, Johnston LD. E-cigarette use as a predictor of cigarette smoking: results from a 1-year follow-up of a national sample of 12th grade students. Tob Control 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Primack BA, Soneji S, Stoolmiller M, Fine MJ, Sargent JD. Progression to Traditional Cigarette Smoking After Electronic Cigarette Use Among US Adolescents and Young Adults. JAMA Pediatr 2015;169(11):1018–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wills TA, Gibbons FX, Sargent JD, Schweitzer RJ. How is the effect of adolescent e-cigarette use on smoking onset mediated: A longitudinal analysis. Psychol Addict Behav 2016;30(8):876–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wills TA, Knight R, Sargent JD, Gibbons FX, Pagano I, Williams RJ. Longitudinal study of e-cigarette use and onset of cigarette smoking among high school students in Hawaii. Tob Control 2017;26(1):34–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wills TA, Sargent JD, Gibbons FX, Pagano I, Schweitzer R. E-cigarette use is differentially related to smoking onset among lower risk adolescents. Tob Control 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Spindle TR, Hiler MM, Cooke ME, Eissenberg T, Kendler KS, Dick DM. Electronic cigarette use and uptake of cigarette smoking: A longitudinal examination of U.S. college students. Addict Behav 2017;67:66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Vanyukov MM, Tarter RE, Kirillova GP, et al. Common liability to addiction and “gateway hypothesis”: theoretical, empirical and evolutionary perspective. Drug and Alcohol Dependence 2012;123 Suppl 1:S3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kozlowski L, Giovino G. Softening of monthly cigarette use in youth and the need to harden measures in surveillance. Preventive Medicine Reports 2014;1:53–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Birge M, Duffy S, Miler JA, Hajek P. What proportion of people who try one cigarette become daily smokers? A meta analysis of representative surveys. Nicotine Tob Res 2017. [DOI] [PubMed] [Google Scholar]
- 91.Colby SM, Clark MA, Rogers ML, et al. Development and reliability of the lifetime interview on smoking trajectories. Nicotine Tob Res 2012;14(3):290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hair E, Bennett M, Williams V, et al. Progression to established patterns of cigarette smoking among young adults. Drug Alcohol Depend 2017;177:77–83. [DOI] [PubMed] [Google Scholar]
- 93.U.S. Department of Health and Human Services. E-Cigarette Use Among Youth and Young Adults. A Report of the Surgeon General. Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health;2016. [Google Scholar]
- 94.Warner KE, Mendez D. E-cigarettes: Comparing the Possible Risks of Increasing Smoking Initiation with the Potential Benefits of Increasing Smoking Cessation. Nicotine Tob Res 2018. [DOI] [PubMed] [Google Scholar]
- 95.Bachand AM, Sulsky SI, Curtin GM. Assessing the Likelihood and Magnitude of a Population Health Benefit Following the Market Introduction of a Modified-Risk Tobacco Product: Enhancements to the Dynamic Population Modeler, DPM(+1). Risk Anal 2018;38(1):151–162. [DOI] [PubMed] [Google Scholar]
- 96.Vugrin ED, Rostron BL, Verzi SJ, et al. Modeling the potential effects of new tobacco products and policies: a dynamic population model for multiple product use and harm. PLoS One 2015;10(3):e0121008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kalkhoran S, Glantz SA. Modeling the Health Effects of Expanding e-Cigarette Sales in the United States and United Kingdom: A Monte Carlo Analysis. JAMA Intern Med 2015;175(10):1671–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cherng ST, Tam J, Christine PJ, Meza R. Modeling the Effects of E-Cigarettes on Smoking Behavior: Implications for Future Adult Smoking Prevalence. Epidemiology (Cambridge, Mass). 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Soneji SS, Sung HY, Primack BA, Pierce JP, Sargent JD. Quantifying population-level health benefits and harms of e-cigarette use in the United States. PLoS One 2018;13(3):e0193328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kalkhoran S, Glantz SA. E-cigarettes and smoking cessation in real-world and clinical settings: a systematic review and meta-analysis. Lancet Respir Med 2016;4(2):116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bullen C, Howe C, Laugesen M, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet 2013;382(9905):1629–1637. [DOI] [PubMed] [Google Scholar]
- 102.Caponnetto P, Campagna D, Cibella F, et al. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PLoS One 2013;8(6):e66317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Adriaens K, Van Gucht D, Declerck P, Baeyens F. Effectiveness of the electronic cigarette: An eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. Int J Environ Res Public Health. 2014;11(11):11220–11248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.O’Brien B, Knight-West O, Walker N, Parag V, Bullen C. E-cigarettes versus NRT for smoking reduction or cessation in people with mental illness: secondary analysis of data from the ASCEND trial. Tob Induc Dis 2015;13(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tseng TY, Ostroff JS, Campo A, et al. A Randomized Trial Comparing the Effect of Nicotine Versus Placebo Electronic Cigarettes on Smoking Reduction Among Young Adult Smokers. Nicotine Tob Res 2016;18(10):1937–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.McRobbie H, Bullen C, Hartmann-Boyce J, Hajek P. Electronic cigarettes for smoking cessation and reduction. Cochrane Database Syst Rev 2014;12:Cd010216. [DOI] [PubMed] [Google Scholar]
- 107.Tobacco Use and Dependence Guideline Panel Treating Tobacco Use and Dependence: 2008 Update. Rockville (MD): US Department of Health and Human Services; 2008. [Google Scholar]
- 108.The Cochrane Handbook for Systematic Reviews of Interventions. Vol Version 5.1.0 West Sussex, England: John Wiley & Sons Ltd; 2011. [Google Scholar]
- 109.O’Leary R, MacDonald M, Stockwell T, Reist D. Clearing the Air: A systematic review on the harms and benefits of e-cigarettes and vapour devices. Victoria, British Columbia: University of Victoria Centre for Addictions Research of BC;2017. [Google Scholar]
- 110.Russell C, McKeganey N, Dickson T, Nides M. Changing patterns of first e-cigarette flavor used and current flabors used by 20,836 adult frequent e-cigarette users in the United States Harm Reduct J. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Giovenco DP, Delnevo CD. Prevalence of population smoking cessation by electronic cigarette use status in a national sample of recent smokers. Addict Behav 2018;76:129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Levy DT, Yuan Z, Luo Y, Abrams DB. The Relationship of E-Cigarette Use to Cigarette Quit Attempts and Cessation: Insights From a Large, Nationally Representative U.S. Survey. Nicotine Tob Res 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhu SH, Zhuang YL, Wong S, Cummins SE, Tedeschi GJ. E-cigarette use and associated changes in population smoking cessation: evidence from US current population surveys. BMJ 2017;358:j3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Parks SH, Duncan DT, Shahawy OE, et al. Characteristics of adults who switched from cigarette smoking to e-cigarettes. Am J Prev Med 2017;53(5):652–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Beard E, Shahab L, Cummings DM, Michie S, West R. New Pharmacological Agents to Aid Smoking Cessation and Tobacco Harm Reduction: What Has Been Investigated, and What Is in the Pipeline? CNS drugs 2016;30(10):951–983. [DOI] [PubMed] [Google Scholar]
- 116.West R, Shahab L, Brown J. Estimating the population impact of e-cigarettes on smoking cessation in England. Addiction 2016;111(6):1118–1119. [DOI] [PubMed] [Google Scholar]
- 117.Glantz SA, Bareham DW. E-Cigarettes: Use, Effects on Smoking, Risks, and Policy Implications. Annu Rev Public Health 2018;39:215–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Villanti AC, Johnson AL, Ambrose BK, et al. Flavored Tobacco Product Use in Youth and Adults: Findings From the First Wave of the PATH Study (2013–2014). Am J Prev Med 2017;53(2):139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gitchell JG, Shiffman S, Sembower MA. Trends in serious quit attempts in the United States, 2009–14. Addiction 2017;112(5):897–900. [DOI] [PubMed] [Google Scholar]
- 120.Babb S, Malarcher A, Schauer G, Asman K, Jamal A. Quitting Smoking Among Adults - United States, 2000–2015. MMWR Morb Mortal Wkly Rep 2017;65(52):1457–1464. [DOI] [PubMed] [Google Scholar]
- 121.Monitoring the Future - The University of Michigan Table 2: Trends in Prevalence of Use of Cigarettes in Grades, 8, 10, and 12. Ann Arbor: 2017. [Google Scholar]
- 122.Centers for Disease Control and Prevention. Early Release of Selected Estimates Based on Data From the National Health Interview Survey, January–March 2016 U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Health Statistics;2016. [Google Scholar]
- 123.Pechacek TF, Nayak P, Gregory KR, Weaver SR, Eriksen MP. The Potential That Electronic Nicotine Delivery Systems Can be a Disruptive Technology: Results From a National Survey. Nicotine Tob Res 2016;18(10):1989–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Amato MS, Boyle RG, Levy D. How to define e-cigarette prevalence? Finding clues in the use frequency distribution. Tob Control 2016;25(e1):e24–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Delnevo CD, Giovenco DP, Steinberg MB, et al. Patterns of Electronic Cigarette Use Among Adults in the United States. Nicotine Tob Res 2016;18(5):715–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gucht DV, Adriaens K, Baeyens F. Online Vape Shop Customers Who Use E-Cigarettes Report Abstinence from Smoking and Improved Quality of Life, But a Substantial Minority Still Have Vaping-Related Health Concerns. Int J Environ Res Public Health 2017;14(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fucito LM, Bars MP, Forray A, et al. Addressing the evidence for FDA nicotine replacement therapy label changes: a policy statement of the Association for the Treatment of Tobacco use and Dependence and the Society for Research on Nicotine and Tobacco. Nicotine Tob Res 2014;16(7):909–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kirchner TR, Shiffman S. Spatio-temporal determinants of mental health and well-being: advances in geographically-explicit ecological momentary assessment (GEMA). Soc Psychiatry Psychiatr Epidemiol 2016;51(9):1211–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Levy DT, Cummings KM, Villanti AC, et al. A framework for evaluating the public health impact of e-cigarettes and other vaporized nicotine products. Addiction 2017;112(1):8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kozlowski LT, Abrams DB. Obsolete tobacco control themes can be hazardous to public health: the need for updating views on absolute product risks and harm reduction. BMC public health 2016;16(1):432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kozlowski LT, O’Connor RJ. Apply federal research rules on deception to misleading health information: an example on smokeless tobacco and cigarettes. Public Health Rep 2003;118(3):187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kozlowski LT, Strasser AA, Giovino GA, Erickson PA, Terza JV. Applying the risk/use equilibrium: use medicinal nicotine now for harm reduction. Tob Control 2001;10(3):201–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Miller T. AG Tom Miller - E-Cigarette Summit 2016. 2016; https://vimeo.com/199643660. Accessed January 11, 2018.
- 134.National Cancer Institute. Health Information National Trends Survey: Compared to smoking cigarettes, would you say that electronic cigarettes are… 2015; https://hints.cancer.gov/question-details.aspx?PK_Cycle=8&qid=1282. Accessed May 31, 2017.
- 135.Majeed BA, Weaver SR, Gregory KR, et al. Changing Perceptions of Harm of E-Cigarettes Among U.S. Adults, 2012–2015. Am J Prev Med 2017;52(3):331–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Brose LS, Brown J, Hitchman SC, McNeill A. Perceived relative harm of electronic cigarettes over time and impact on subsequent use. A survey with 1-year and 2-year follow-ups. Drug Alcohol Depend 2015;157:106–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Huerta TR, Walker DM, Mullen D, Johnson TJ, Ford EW. Trends in E-Cigarette Awareness and Perceived Harmfulness in the U.S. Am J Prev Med 2017;52(3):339–346. [DOI] [PubMed] [Google Scholar]
- 138.Russell MA. Low-tar medium-nicotine cigarettes: a new approach to safer smoking. Br Med J 1976;1(6023):1430–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Harm Reduction International. What is harm reduction? https://www.hri.global/what-is-harm-reduction Accessed May 10, 2017.
- 140.Fairchild A, Niaura R, Abrams DB. America needs a candid smoking control champion. 2017; http://thehill.com/opinion/healthcare/360111-america-needs-a-candid-smoking-control-champion. Accessed January 11, 2017.
- 141.Lee PN, Hamling J. Systematic review of the relation between smokeless tobacco and cancer in Europe and North America. BMC Med 2009;7:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yach D. Foundation for a smoke-free world. Lancet 2017;390(10104):1807–1810. [DOI] [PubMed] [Google Scholar]
- 143.Abrams DB. Potential and pitfalls of e-cigarettes--reply. JAMA 2014;311(18):1922–1923. [DOI] [PubMed] [Google Scholar]
- 144.Levy DT, Borland R, Fong GT, et al. Developing Consistent and Transparent Models of E-cigarette Use: Reply to Glantz and Soneji et al. Nicotine & tobacco research : official journal of the Society for Research on Nicotine and Tobacco 2017;19(2):268–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Proctor RN. Golden holocaust: origins of the cigarette catastrophe and the case for abolition. Oakland, CA: University of California Press; 2011. [Google Scholar]
- 146.U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General Atlanta, GA: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health;2014. [Google Scholar]
- 147.Levy DT, Borland R, Lindblom EN, et al. Potential deaths averted in USA by replacing cigarettes with e-cigarettes. Tob Control 2018;27(1):18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]