Abstract
Diet during pregnancy has long lasting consequences on the offspring, warranting a study on the impact of early exposure to a high fat diet on the adult offspring. We hypothesized that a prenatal n-6 enriched diet will have adverse metabolic outcomes on the adult offspring that may be reversed with a postnatal n-3 enriched diet. To test this hypothesis, we examined the adult offspring from three groups: 1) n-6 group: during gestation and lactation, dams consumed an n-6 polyunsaturated fatty acid enriched diet, 2) n-3 group: gestational n-6 diet was followed by an n-3 enriched diet during lactation, and 3) a control (CD) group that received standard diet throughout gestation and lactation. Offspring from all groups weaned to a control diet ad libitum. Beginning at postnatal day 2 (p<0.03) and persisting at 360d in males (p<0.04), an increase in hypothalamic AgRP expression occurred in the n-6 and n-3 groups, with an increase in food intake (p=0.01), and the n-3 group displaying lower body (p<0.03) and brain (p<0.05) weights. At 360d, the n-6 and n-3 groups remained glucose tolerant and insulin sensitive, with increased phosphorylated-AMP-activated protein kinase (p<0.05). n-6 group developed hepatic steatosis with reduced hepatic reflected as higher plasma microRNA-122 (p<0.04) that targets pAMPK. We conclude that early life exposure to n-6 and n-3 led to hypothalamic AgRP-related higher food intake, with n-6 culminating in a fatty liver partially mitigated by postnatal n-3. While both diets preserved glucose tolerance and insulin sensitivity, postnatal n-3 displayed detrimental effects on the brain.
Keywords: rats, high-fat diet, polyunsaturated fatty acids, brain, liver
1. Introduction
There is an obesity epidemic in the United States (US), which has extended into childhood. Between the ages of 12 to 19 years, 21% of US children are obese and 8% are severely obese [1]. This health crisis has led to an increased incidence of hypertension, dyslipidemia, cardiovascular disease, insulin resistance, and non-alcoholic fatty liver disease [2-7]. These diseases cause blindness, stroke, and renal and liver failure, all of which can be fatal and tax our health care economy [8,9]. Not surprisingly, 32% of women of childbearing age are obese. Once pregnant, obese women are at high risk for gestational hypertension and diabetes mellitus, both of which have adverse, long-lasting repercussions on the woman’s health and that of the offspring extending into adulthood [10,11].
On this backdrop a considerable amount of attention has been given to the type of diet that pregnant women consume. There has been a large push to increase consumption of fat-reduced foods with the goal of reducing cholesterol concentrations, a major risk factor for cardiovascular accidents and myocardial infarctions [7,12]. However, these fat-reduced products have not abated the obesity epidemic and suggest that the replacement of fat with carbohydrates may negatively impact the adult phenotype of the offspring. For these reasons, it is important to determine the optimal diet during pregnancy and lactation [13]. The composition of fat, especially the n-6:n-3 polyunsaturated fatty acid ratio, is important for overall health. The recommended dietary n-6:n-3 fatty acid ratio is 2:1-4:1 for humans [14]. However, western diets typically exceed a ratio of 15:1 [15]. Excessive n-6 fatty acid intakes are associated with obesity, diabetes, and non-alcoholic fatty liver disease [16,17]. On the other hand, increased n-3 provisions are associated with improved glucose tolerance, insulin sensitivity and a decreased incidence of non-alcoholic fatty liver disease [18-21]. n-3 fatty acids also play a critical role in the development of the central nervous system and somatic growth [18,19].
Rodent investigations have shown that when maternal hyperglycemia due to maternal diabetes is encountered, the offspring develops glucose intolerance and insulin resistance [22-24]. More recently, a high fat diet consumed at least 2 months before conception and continued during gestation and lactation led to the adult offspring with fatty liver complicated by glucose intolerance and insulin resistance [25-27]. n-3 supplementation ameliorates this phenotype [28-30]. However, the birth weight of offspring exposed to a high fat diet was reduced as opposed to the expected increased birth weight [31,32], typically seen in humans. This observation raises the question whether the metabolic perturbations that manifest in the adult rodent offspring are because of fetal growth restriction or exposure to a high fat diet initiated prior to conception and continued during fetal life. Further, these studies support the premise that an abnormally high pre-conception body mass index signifying pre-existent obesity due to a high caloric diet has adverse effects on the fetus that may last into adult life. However in these investigations, it is difficult to separate the effects of pre-existing maternal obesity and its associated metabolic and inflammatory changes from the effect of maternal hypercaloric diet alone, on the ultimate phenotype of the adult offspring.
In our present study, we focused on diet content during pregnancy and lactation alone, rather than before conception, where a propensity towards pre-pregnancy obesity may confound the diet-induced observations. We hypothesized that an n-6 rich high fat diet during gestation initiated after conception and lasting through lactation in non-obese dams would lead to lesser obesity in the offspring, associated with milder glucose intolerance, insulin resistance, and a fatty liver phenotype. Secondly, we hypothesized that an n-6 rich high fat diet during gestation followed by an n-3 rich high fat diet during lactation would mitigate the prenatal n-6 exposure induced changes, even if milder, by maintaining glucose tolerance and insulin sensitivity, and preventing fatty liver. To test these hypotheses, we used a prenatal and postnatal dietary modified rat model employing three groups, control with ad libitum access to regular chow diet (CD group), n-6 rich high fat diet during gestation and lactation (n-6 group), and n-6 rich high fat diet during gestation followed by an n-3 enriched high fat diet during lactation (n-3 group). All three groups had ad libitum access to water. To meet our objectives, we then compared insulin sensitivity and glucose tolerance, and the state of the liver in these three groups. Because polyunsaturated fatty acids play an important role in central nervous system development, we also assessed brain weight, certain neural markers and specific energy balance regulating hypothalamic neuropeptides.
2. Methods and Materials
2.1. Animals
2.1.1. Animal Care
Sprague-Dawley rats (Charles River Laboratories, Hollister, CA) were housed under 12h light/dark cycles at ~21-23°C and allowed ad libitum access to water [33]. The care and use of all animals in this study were approved by the Animal Research Committee of the University of California Los Angeles (protocol no. 1999-104-61A), and were in keeping with the guidelines issued by the National Institutes of Health.
2.1.2. Animal Model
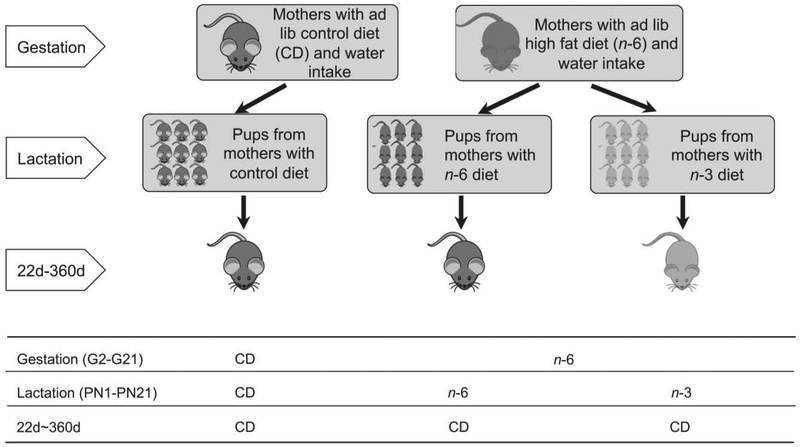
The experimental design (Figure 1) consisted of creating two pregnant high fat exposed groups following conception towards preventing pre-pregnancy obesity in dams. Gestational day 2 through day 21, rats had ad libitum access to a control regular chow diet (CD; NIH-31 modified 7013 diet: Teklad Inc., Madison, WI) with the n-6:n-3 fatty acid ratio of 8.3:1 shown in table 1, or an n-6 enriched high fat diet (n-6; D12266B: Research Diet Inc., New Brunswick, NJ), the formulation of which is shown in table 1, resulting in a n-6:n-3 fatty acid ratio of 31.3:1 (table 1).
Figure 1: Scheme demonstrating the experimental design.
Three experimental groups were created: Two groups were created with ad lib access to either control regular chow diet (CD) or dietary n-6 polyunsaturated fatty acids (n-6) during gestation (G2-G21). The CD group continued on the same diet while the n-6 exposed group was sub-divided at birth into receiving n-6 or n-3 during lactation (PN1-21). After weaning, all three groups were given CD until day 360 of age.
Table 1:
Fatty acid and ingredient composition employed in the study
| Fatty acid (wt%) | Dietary treatments a,b,c,d | ||
|---|---|---|---|
| CD | n-6 | n-3 | |
| 4:0 | 0 | 0.9 | 0.3 |
| 6:0 | 0 | 0.5 | 0.2 |
| 8:0 | 0 | 0.3 | 0.1 |
| 10:0 | 0 | 0.7 | 0.3 |
| 12:0 | 0 | 0.8 | 0.3 |
| 14:0 | 0 | 2.8 | 5.9 |
| 14:1n-9 | 0 | 0.4 | 0.1 |
| 15:0 | 0 | 0 | 0.3 |
| 16:0 | 17.3 | 15.4 | 16.1 |
| 16:1n-9 | 0 | 0.6 | 7.0 |
| 16:2n-4 | 0 | 0 | 1.1 |
| 16:3n-9 | 0 | 0 | 1.1 |
| 16:4n-4 | 0 | 0 | 1.1 |
| 17:0 | 0 | 0 | 0.3 |
| 18:0 | 3.8 | 4.8 | 3.6 |
| 18:1n-9 | 25 | 25.6 | 16.3 |
| 18:2n-6 | 48.1 | 45.4 | 18.5 |
| 18:3n-3 | 5.8 | 1.5 | 1.6 |
| 18:4n-3 | 0 | 0 | 2.1 |
| 20:0 | 0 | 0.3 | 0.3 |
| 20:1 | 0 | 0 | 1.1 |
| 20:2 | 0 | 0 | 0.1 |
| 20:3n-6 | 0 | 0 | 0.3 |
| 20:4n-6 | 0 | 0 | 1.5 |
| 20:5n-3 | 0 | 0 | 9.9 |
| 21:5n-3 | 0 | 0 | 0.5 |
| 22:0 | 0 | 0 | 0.1 |
| 22:1 | 0 | 0 | 0.2 |
| 22:4n-6 | 0 | 0 | 0.1 |
| 22:5n-3 | 0 | 0 | 1.9 |
| 22:6n-3 | 0 | 0 | 7.1 |
| 24 | 0 | 0 | 0.4 |
| 24:1 | 0 | 0 | 0.1 |
| SAT | 21.1 | 26.4 | 27.7 |
| MONO | 25.0 | 26.7 | 24.9 |
| PUFA | 53.9 | 46.9 | 47.0 |
| n-6 PUFA | 48.1 | 45.4 | 20.4 |
| n-3 PUFA | 5.8 | 1.5 | 23.2 |
| n-6:n-3 | 8.3 | 31.3 | 0.9 |
The semipurified basal diet contains the following (g/kg): for n-6 and n-3, casein, 182; corn starch, 206.2; sucrose, 278.1; cellulose, 28.8; choline bitartrate, 1.9; salt mix, 38.4; vitamin mix, 10.5 (based on Research diet). For CD, corn starch, 193.52; oats, 100; wheat, 355; choline chloride, 60%, 1.48 NIH-31 vitamin mix, 3.5; NIH-31 mineral mix, 1.5 (based on Teklad diets).
Mineral mix provides (mg/kg diet): for n-6 and n-3, CaHPO4, 19200; MgO, 921.6; C6H5K3O7·H2O, 8448; K2SO4, 1996.8; NaCl, 2841.6; CrK(SO4)2·12H2O, 21.12; CUCO3, 11.52; KIO3, 0.38; C6H5FeO7, 230.4; MnCO3, 134.4; Na2SeO3, 0.38; ZnCO3, 61.4. For CD, Fe, 174; Cu, 13.1; Zn, 205; Se, 0.4; Mn, 139; I, 1.4.
Vitamin mix provides (mg/kg diet): for n-6 and n-3 diets, thiamine HCl, 6.6; riboflavin, 6.6; pyridoxine HCl, 7.7; niacin, 33; calcium pantothenate, 17.6; folic acid, 2.2; biotin, 0.22; cyanocobalamin (B12), 11; vitamin A palmitate (500,000 IU/g), 8.8; vitamin E acetate (500 IU/g), 110; vitamin D3 (100,000 IU/g), 11. For CD: thiamine HCl, 66; riboflavin, 7; pyridoxine HCl, 9; calcium pantothenate, 36; folic acid, 70; biotin, 0.2; cyanocobalamin (B12), 1.54; vitamin A (25,560IU/Kg), vitamin E (a tocopherol acetate), 31, vitamin D3 (cholecalciferol, 3620 IU/Kg).
Dietary fat treatments include butter fat and corn oil for n-6 and n-3, soy oil for control diet (CD). Only n-3 diet includes 10% Menhaden oil. Total fat content is 155.6 g/kg for n-6 and n-3 diet, 62g/kg for CD.
SAT, total saturated fatty acids; MONO, total monounsaturated fatty acids; PUFA, total polyunsaturated fatty acids.
0 = not detected
After birth, three groups of pups each maintained at 6 pups/litter, were created by sub-dividing the high fat n-6 enriched diet fed pregnant dams into either 1) continuing to receive a high fat n-6 enriched diet (n-6) or 2) replaced with a high fat n-3 enriched diet (n-3; D09101401: Research diets Inc., New Brunswick, NJ) with 10% menhaden oil (table 1), resulting in a n-6:n-3 fatty acid ratio of 0.9:1 (table 1), while 3) the CD group continued to receive the regular chow diet, in all groups through the period of lactation (PN1-PN21). We opted to study these ratios of n-6:n-3 fatty acids (n-6 being ~4 times greater and n-3 being ~8 times lower than that found in the standard regular chow diet, which in turn was two to four times that recommended for humans) so we could determine the presence of phenotypic differences in the offspring between the groups both in early suckling and subsequent adult life. After weaning at 22d, males and females in all three groups were placed on a regular chow diet until the adult age of 360d (12 months).
2.2. Morphometric Measurements
Body weights, nose-rump, nose-tail lengths and organ weights were measured longitudinally through the life course of male and female offspring beginning from PN2 until 360d of age. At 360d of age, following euthanasia with intraperitoneal administration of phenobarbital (100 mg/kg) using a 27G 1/2 needle [34,35], organ weights were assessed.
2.3. Food and Water Intake
At adult ages of 30d, 90d, 180d and 360d of age, food intake was longitudinally assessed over a 24-hour period at each age by calculating the difference in the measured weight of food that was placed in the food container before and remaining after the 24-hour period (g-food/g-body weight). Similarly, water intake was also assessed over a 24-hour period by calculating the difference in the volume of water placed in the water container before and remaining after the 24-hour period (g-water/g-body weight). Evaporative losses were assessed by placing a water and food container attached to an empty cage with no animal in the same room, and these losses subtracted from the final amounts of food and water intake over a 24-hour period. All these measurements were performed first thing early in the morning soon after the maximal nocturnal ingestive phase as previously described [35,36].
2.4. Analysis of Brain Samples
2.4.1. Hypothalamic Neuropeptide mRNA Analysis
Hypothalamus from whole brain was removed under dissecting microscope (Olympus SZ40, Center Valley, PA ) using established anatomical landmarks [37]. Total RNA from hypothalamus was extracted using RNAeasy lipid tissue kit (Qiagen, Valencia, CA). First strand cDNA was synthesized from 1 μg of DNase treated total RNA using Superscript II reverse transcriptase (Invitrogen Life Technologies, Carlsbad, CA), as previously described [35]. Quantitative real time PCR was performed as previously described [22,35,36]. Primers and Taqman probes for detection of specific genes in hypothalamus were designed using Primer Express Software (Applied Biosystems, Foster, CA) and are listed [36]. These designed forward and reverse primers generate corresponding DNA fragments after amplification. Taqman probes were synthesized and labeled with fluorescent dye, 6-carboxyfluorescein (FAM) on the 5’-end and N,N,N’,N’-tetramethyl-6-carboxyrhodamine (TAMRA) on the 3’-end (Applied Biosystems, Foster CA). Taqman PCR was carried out using a StepOnePlus™ real-time PCR system (Applied Biosystems, Foster, CA). Real time PCR quantification was then performed using Taqman glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or eukaryotic 18S rRNA (Applied Biosystems, Foster, CA) as internal controls. PCR amplifications were performed in triplicates. The amplification cycles consisted of 12 min at 95°C (hot start), followed by 40 cycles at 95°C for 30 sec (denaturation), 56°C for long isoform of the leptin receptor (ObRb) and melanocortin isoform 3 receptor (MC3-R); 58°C for cocaine and amphetamine regulated transcript (CART), neuropeptide Y (NPY) and melanocortin isoform 4 receptor (MC4-R); 60°C for AgRP and proopiomelanocortin (POMC) over 30 sec (annealing), and 72°C for 30 sec (extension) [35,36], using reagents from Applied Biosystems (Foster, CA). Relative quantification of PCR products were based on value differences between the target and GAPDH or 18S rRNA control using the comparative CT method, as previously described [22,35,36].
2.4.2. Brain Protein Analysis
Whole brains except hypothalamus were homogenized either in phosphate-buffered saline (PBS) containing protease inhibitors (20 μg/ml pepstatin A, 20 μg/ml leupeptin, 30 μg/ml aprotinin and 2 mM PMSF), 1% Nonidet P-40 and 5 mM EDTA or in cell lysis buffer (Cell Signaling Technology, Danvers, MA) as previously described [35,36]. Protein content was measured by the Bio-Rad Protein Assay (Bio-Rad, Hercules, CA). Homogenates (30 μg of protein) were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis and the separated proteins transferred to nitrocellulose membrane filters (Bio-Rad, Hercules, CA). The blotted membranes were sequentially incubated in 3% bovine serum albumin or 5% nonfat dry milk and the primary antibody consisting of the rabbit anti postsynaptic density 95 (PSD95) and rabbit anti glucose transporter isoform 1 (Glut1, abcam, Cambridge, MA), rabbit anti Synaptophysin (SYP) (Millipore, Burlington, MA), rabbit anti glucose transporter isoform 3 (Glut3, a gift from Dr. Takata in Japan) and mouse anti-vinculin as an internal loading control (Sigma, St. Louis, MO). The proteins were visualized in Typhoon 9410 Phosphorimager (GE Healthcare Biosciences, Piscataway, NJ) by blotting with the enhanced chemiluminescence (ECL) plus detection kit (GE Healthcare BioSciences Corp., Piscataway, NJ) following horseradish peroxidase-labeled anti-rabbit IgG for anti PSD95, SYP, Glut1 and Glut3 or anti-mouse IgG for vinculin (GE Healthcare Biosciences Corp., Piscataway, NJ). Each protein was quantified by using Image Quant 5.2 software (GE Healthcare Biosciences, Piscataway, NJ), and normalized to vinculin.
2.5. Glucose (GTT) and Insulin (ITT) Tolerance Tests
360d old male and female offspring were lightly restrained in a plastic holder and allowed to acclimatize. Tail vein basal blood glucose was assessed after an overnight fast in the case of the GTT and non-fasting basal blood glucose in the case of the ITT [33]. The animals received a dose of glucose (1g/kg body weight) via the tail vein in the case of GTT or an intraperitoneal dose of insulin (0.75U/kg body weight) in the case of ITT [33]. Tail vein blood to assess glucose concentrations were drawn at various time points following either the glucose or the insulin administration to assess glucose tolerance and insulin sensitivity.
2.6. Analysis of Liver Samples
2.6.1. Liver Histology
360d old male animals were anesthetized with inhalational isoflurane. Liver tissues were taken and fixed in 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 and washed with PBS, infused in 20% sucrose in 0.1 M sodium phosphate buffer, pH 7.4, containing 0.02% sodium azide, and embedded in OCT compound as previously described [38]. Specimens were sectioned (10-μm thickness) with a cryostat Leica CM 1850 (Nussloch, Germany). Hematoxylin and Eosin stain and Oil red O stain were performed as previously described [39-41]. After nuclear stain with Hematoxylin (Vector Laboratories, Burlingame, USA), tissue samples were immersed briefly (30 sec) in 60% isopropanol and stained with Oil Red O (Sigma, St. Louis, Mo) for 15 min and briefly washed with 60% isopropanol followed by running tap water for 10 min and mounted. m thickness) washed with PBS and hydrated in distilled water. Separate sections were incubated in Picro Sirius Red solution (Abcam, Cambridge, MA) for 60 min following washing with PBS. The sections were next washed briefly with acetic acid solution, dehydrated through graded alcohol, and mounted. Images were visualized using a Nikon Eclipse E-600 Microscope (Nikon, Melville, NY, USA) or a Leica DM1000 microscope equipped with the MC170 HD camera (Leica Microsystems; Heerbrugg, Switzerland), as previously described [35,42].
2.6.2. Hepatic Protein Analysis
As previously described [35,36], the blotted membranes from homogenates of liver (30 μg of protein) were sequentially incubated in the primary antibody consisting of the mouse anti-fatty acid synthetase (FAS, BD Biosciences, San Jose, CA), the rabbit anti-acetyl coenzyme-A carboxylase (ACC), 5 AMP-activated protein kinase (AMPK) and phosphorylated 5 AMP-activated protein kinase (pAMPK) (Cell Signaling Technology, Beverly, MA), and mouse anti-vinculin (internal loading control; Sigma, St. Louis, MO). The protein bands were visualized in Typhoon 9410 Phosphorimager (GE Healthcare Biosciences, Piscataway, NJ) by blotting with the enhanced chemiluminescence (ECL) plus detection kit (GE Healthcare BioSciences Corp., Piscataway, NJ) following horseradish peroxidase-labeled anti-rabbit IgG for anti ACC, AMPK, pAMPK, or anti-mouse IgG for anti-FAS and vinculin (GE Healthcare Biosciences Corp., Piscataway, NJ). Each protein band was quantified by using Image Quant 5.2 software (GE Healthcare Biosciences, Piscataway, NJ), and normalized to the vinculin protein bands.
2.6.3. Hepatic and Plasma miR-122 Analysis
Liver was collected and snap frozen in liquid nitrogen. Samples were stored at −80°C until the time of analysis. Tissue RNA was extracted using Zymo Direct-zol RNA miniprep kit (Irvine, CA) according to the manufacturer’s instructions. miRNA specific cDNA was synthesized using TaqMan MicroRNA reverse transcription kit (Applied Biosystems, Foster City, CA). To analyze the relative expression of mature microRNA-122 (miR-122) in various samples, qPCR was performed using Taqman MicroRNA Assay kit specific for rno-miR-122 (Applied Biosystem assay, Foster City, CA). Expression of tissue miRNA was normalized using U6 RNA (Applied Biosystem assay, Foster City, CA ) as an internal control as previously described [43].
For cell free miRNA, blood samples were collected and plasma was separated by centrifugation. Samples were stored at −80°C until further analysis. Plasma miRNA was isolated using miRNA easy Serum/Plasma kit from Qiagen according to the manufacturer’s instructions (Hilden, Germany). miRNA specific cDNA synthesis and qPCR was done as previously described [43]. To analyze the concentration of cell free/secreted miR-122, synthetic Ce-miR-39 (Applied Biosystem assay, Foster City, CA) from C elegans was used as a spike-in control during the isolation of miRNA from serum, as recommended by the manufacturer.
2.7. Statistical analyses
Sample size was determined to be n=6/group by using the two-tailed unpaired t-test at 80% power to detect a difference (delta) of 1.4 - 2.0 with a significance level (alpha) of 0.05 (Graphpad Statmate version 2.0; San Diego, CA). All data was expressed as means±SEM. All experimental groups were compared following establishment of normality. Analysis of variance (ANOVA) was employed and F values determined. Once significance was established, inter-group differences were validated by the Fisher’s paired least significance difference test. Final p values were considered significant at < 0.05. Statistical analyses were performed using StatView Version 5.0 (SAS institute Inc, Cary, NC) or GraphPad Prism version 7 (San Diego, CA) [35,44].
3. Results
3.1. Body Weights
Body weights for males (A) and females (B) are presented in table 2 for all three groups, CD, n-6 and n-3 spanning from PN2, PN21, 30d, 90d, 180d and 360d of age. For males and females, no inter-group differences were observed in body weights at PN2 and body weights for n-6 were comparable to CD at subsequent time points. However, at PN21, n-3 males and females weighed less than CD and n-6. This reduction in weight persisted until 30d of age. Thereafter, n-3 group was no different from the CD or n-6 groups at 90d, 180d and 360d (Table 2).
Table 2:
Body Weight (g)
| PN 2 |
PN21 |
30d |
90D |
180D |
360D |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD | n-6 | n-3 | CD | n-6 | n-3 | CD | n-6 | n-3 | CD | n-6 | n-3 | CD | n-6 | n-3 | CD | n-6 | n-3 | |
| Male | 7.02 ± 0.14 | 7.25 ± 0.14 | 7.25 ± 0.14 | 58.58 ± 1.59 | 61.33 ± 0.76 | 54.00 ± 1.39*, ** | 103.70 ± 2.17 | 109.25 ± 2.54 | 100.13 ± 3.70** | 572.50 ± 11.76 | 557.42 ± 17.46 | 570.75 ± 25.33 | 714.92 ± 17.42 | 696.33 ± 27.17 | 715.00 ± 37.58 | 867.50 ± 27.92 | 800.25 ± 37.04 | 815.86 ± 46.12 |
| Female | 6.57 ± 0.16 | 6.68 ± 0.11 | 6.68 ± 0.11 | 56.83 ± 2.35 | 58.75 ± 0.82 | 49.88 ± 0.64*, ** | 95.40 ± 3.48 | 96.92 ± 2.46 | 85.00 ± 0.76*, ** | 311.25 ± 12.38 | 310.92 ± 12.66 | 303.50 ± 10.28 | 377.58 ± 16.60 | 377.33 ± 23.86 | 377.50 ± 22.80 | 481.13 ± 25.68 | 498.42 ± 43.75 | 503.25 ± 31.60 |
Body weights of male (A) and female (B) offspring from three experimental groups: CD, n-6, and n-3 from PN2 to 360d are shown. In both males and females, data are shown as means ± SE (n=8-12)
p<0.02 vs CD
p<0.03 vs n-6 by one-way ANOVA and Fisher's PLSD test.
3.2. Morphometric measurements
As previously described, nose-rump, nose-tail lengths and organ weights at 360d of age for males and females are depicted in table 3 [33]. No differences were noted in either sex for length. When compared to CD, a significant reduction in brain weights was evident in both high fat groups in males, and in n-3 females only. When brain weights were expressed per average body weights (g-brain/g-body weight), brain weights of males in n-3 (brain:body weight ratio: 0.0026±2.49×10−5) were significantly lower than those in n-6 (0.0027±3.22×10−5; p=0.03) with no significant difference when compared to CD (0.0026±4.71×10−5, p=0.1). Both n-3 (0.0037±8.93×10−5; p=0.002) and n-6 (0.0039±5.97×10−5; p=0.02) exposed female brain weights were significantly lower than that of CD (0.0042±7.49×10−5). While the male pancreas weighed less in n-6 versus the CD and n-3 groups, the female kidney weighed less in n-3 versus the n-6 group. There were no other weight differences observed in liver, brown adipose and white adipose tissues.
Table 3:
Organ weights and nose-tail or nose-rump length
| CD | n-6 | n-3 | CD | n-6 | n-3 | ||
|---|---|---|---|---|---|---|---|
| Male | Female | ||||||
| Nose-rump(cm) | 28.56 ± 0.29 | 27.94 ± 0.27 | 28.00 ± 0.45 | Nose-rump(cm) | 23.57 ± 0.37 | 24.00 ± 0.31 | 23.75 ± 0.17 |
| Nose-tail(cm) | 51.88 ± 0.58 | 51.88 ± 0.31 | 52.10 ± 0.95 | Nose-tail(cm) | 44.21 ± 0.60 | 44.86 ± 0.20 | 44.42 ± 0.63 |
| Brain(g) | 2.30 ± 0.04 | 2.19 ± 0.03* | 2.12 ± 0.02* | Brain(g) | 2.00 ± 0.04 | 1.93 ± 0.03 | 1.89 ± 0.05* |
| Liver(g) | 22.45 ± 1.04 | 22.36 ± 1.47 | 21.75 ± 2.18 | Liver(g) | 13.64 ± 1.15 | 14.14 ± 1.87 | 13.18 ± 1.47 |
| Kidney(g) | 4.75 ± 0.25 | 4.46 ± 0.19 | 4.26 ± 0.38 | Kidney(g) | 2.69 ± 0.18 | 3.14 ± 0.32 | 2.42 ± 0.10** |
| Pancreas(g) | 0.96 ± 0.05 | 1.54 ± 0.13* | 0.97 ± 0.12** | Pancreas(g) | 0.68 ± 0.03 | 0.67 ± 0.08 | 0.63 ± 0.03 |
| BAT(g) | 0.75 ± 0.09 | 0.69 ± 0.05 | 0.68 ± 0.04 | BAT(g) | 0.56 ± 0.08 | 0.48 ± 0.07 | 0.59 ± 0.05 |
| WAT(g) | 40.17 ± 2.38 | 33.54 ± 4.00 | 30.66 ± 2.30 | WAT(g) | 43.78 ± 7.32 | 50.33 ± 12.15 | 37.08 ± 1.47 |
Data are shown as means ± SE (n=5-8)
p<0.05 vs CD
p<0.05 vs n-6 by one-way ANOVA and Fisher's PLSD test. BAT, brown adipose tissue; WAT, white adipose tissue.
3.3. Food and Water Intake
Food and water intakes were examined longitudinally in males and females in the three experimental groups (Table 4 & 5). When compared to the CD group, an increase in food intake was seen in male n-3 and n-6 groups at 30d of age with no further change noted at later time points (Table 4). No differences were observed in females at the various time points. In contrast, at 30d, a significant reduction in water intake by the male n-3 group and the female n-6 group was evident when compared to the respective sex-matched CD group. In the male n-3 group, a reduction in water intake persisted at 90d of age when compared to CD and n-6 groups, while at 360d, this reduction was only evident when compared to the n-6 group (Table 5).
Table 4:
Food Intake
| 30d |
90D |
180D |
360D |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD | n-6 | n-3 | CD | n-6 | n-3 | CD | n-6 | n-3 | CD | n-6 | n-3 | |
| Male | 0.121 ± 0.009 | 0.149 ± 0.003* | 0.148 ± 0.003* | 0.049 ± 0.002 | 0.047 ± 0.001 | 0.045 ± 0.002 | 0.039 ± 0.002 | 0.037 ± 0.001 | 0.036 ± 0.002 | 0.032 ± 0.002 | 0.037 ± 0.002 | 0.031 ± 0.002# |
| Female | 0.122 ± 0.006 | 0.132 ± 0.005 | 0.124 ± 0.007 | 0.054 ± 0.004 | 0.064 ± 0.006 | 0.06 ± 0.005 | 0.041 ± 0.001 | 0.042 ± 0.004 | 0.04 ± 0.006 | 0.041 ± 0.003 | 0.033 ± 0.003 | 0.035 ± 0.006 |
Food intake (g-food/g-body weight) in male and female offspring was measured at 30d, 90d, 180d and 360d. Data are shown as means ± SE (n=8-12)
p<0.01 vs CD
p<0.06 vs n-6 by one-way ANOVA and Fisher's PLSD test.
Table 5:
Water Intake
| 30d |
90D |
180D |
360D |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CD | n-6 | n-3 | CD | n-6 | n-3 | CD | n-6 | n-3 | CD | n-6 | n-3 | |
| Male | 0.380 ± 0.033 | 0.318 ± 0.020 | 0.276 ± 0.018* | 0.063 ± 0.002 | 0.063 ± 0.002 | 0.049 ± 0.003*,** | 0.051 ± 0.008 | 0.044 ± 0.003 | 0.042 ± 0.003 | 0.027 ± 0.002 | 0.032 ± 0.002 | 0.025 ± 0.002** |
| Female | 0.391 ± .038 | 0.290 ± 0.018* | 0.324 ± 0.016 | 0.086 ± 0.017 | 0.069 ± 0.006 | 0.066 ± 0.003 | 0.062 ± 0.006 | 0.059 ± 0.014 | 0.055 ± 0.005 | 0.042 ± 0.003 | 0.034 ± 0.004 | 0.040 ± 0.001 |
Water intake (g-water/g-body weight) in male and female offspring was measured at 30d, 90d, 180d and 360d. Data are shown as means ± SE (n=8-12)
p<0.02 vs CD
p<0.03 vs n-6 by one-way ANOVA and Fisher's PLSD test.
3.4. Glucose and Insulin Tolerance including Serum Profiles
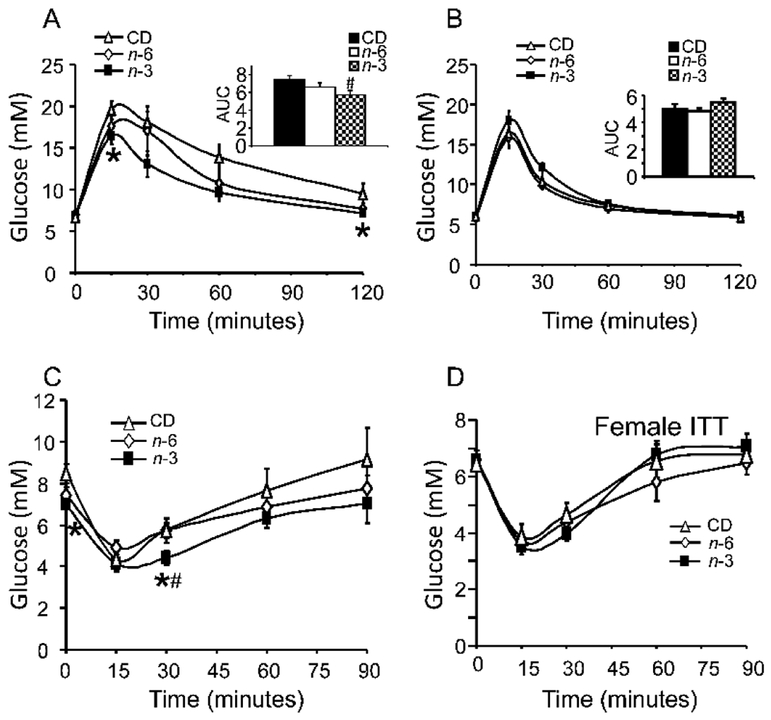
At 360d of age, glucose tolerance and insulin sensitivity were assessed by glucose and insulin tolerance tests, respectively. In males, an enhancement of glucose tolerance with increased insulin sensitivity was evident in the n-3 group versus CD, while n-6 was no different from the CD group (Figure 2A, C). In contrast, no inter-group differences were observed in glucose tolerance and insulin sensitivity in the females (Figure 2B, D). In 360d males, while total cholesterol decreased in the n-6 group when compared to CD, free fatty acids decreased in the n-3 group when compared to n-6 group (Table 6).
Figure 2: Glucose tolerance tests (A & B), and insulin tolerance tests (C & D) in CD, n-3 and n-6 groups.
A & B: GTTs in 360d male (A) and female (B) adult offspring in CD, n-3 and n-6. N= 6~7 per each group. *p<0.05 compared to CD. Inset: respective AUC calculations. #p<0.01 compared to CD by one-way ANOVA and Fisher's PLSD test. C & D: ITTs in 360d male (C) and female adult offspring (D) in CD, n-3 and n-6. N= 6 per each group. *p<0.04 compared to CD. #p<0.04 compared to n-6 by one-way ANOVA and Fisher's PLSD test.
Table 6:
Serum Lipid Profile
| CD | n-6 | n-3 | |
|---|---|---|---|
| TG mg/dl | 213.25± 30.47 | 196.25 ±29.29 | 173.6 ±14.95 |
| Tot chol. mg/dl | 129 ± 11.5 | 89.3 ±4.59* | 114.8 ±14.56 |
| HDL mg/dl | 79.75 ± 8.23 | 62.5 ±3.86 | 79.8 ±11.62 |
| UC mg/dl | 31.75 ± 3.28 | 24.375 ±1.34 | 30.4 ±4.98 |
| FFA mg/dl | 11.9 ± 0.76 | 13 ±0.68 | 9.4 ±0.67** |
Plasma concentrations of various metabolites from 360-day-old offspring exposed to Chow Diet (CD), n-6 enriched high fat diet alone or with n-3 enriched high fat diet during the postnatal period (PN1-PN21). Female serum lipid profile was not measured. Data are shown as means ± SE (n=5-8)
p<0.05 vs CD
p<0.004 vs n-6 by one-way ANOVA and Fisher's PLSD test. N=5-8 for each group.
3.5. Hypothalamic Neuropeptides and Proteins in Brain
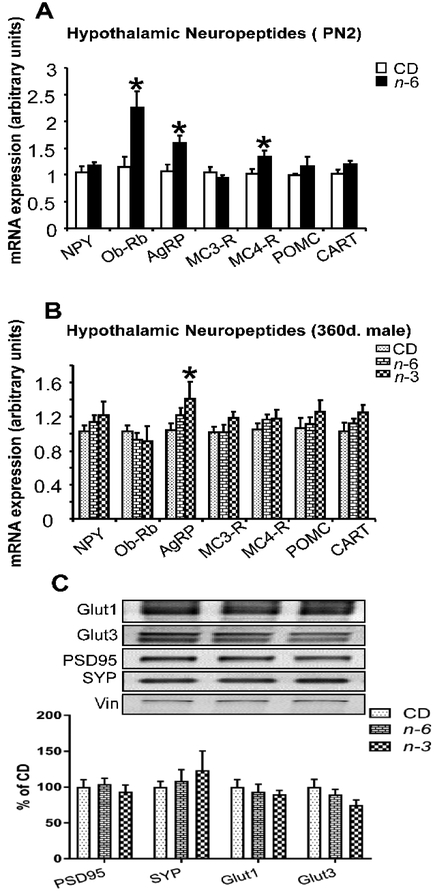
These sex-specific phenotypic observations noted mainly in males led to our subsequent brain and liver specific studies restricted to the male offspring alone. First, we investigated the hypothalamic expression of key neuropeptides and receptors that mediate energy balance in the male groups at two distinct time points, PN2 and 360d (Figure 3A). At PN2, higher expression of AgRP, ObRb and MC4-R was observed in the n-6 male group versus CD, with no change in NPY, MC3-R, POMC and CART. At 360d of age, the male offspring demonstrated a persistence in higher AgRP expression particularly in the n-3 group versus CD, with no other change in any of the other neuropeptides in both high fat male groups (Figure 3B). Since we noted a reduction in brain weights in both high fat groups in the 360d male offspring, we assessed certain synaptic markers along with glucose transporters in the adult male brain cortical regions. We noted no differences in PSD95 (post-synaptic marker) and SYP (axonal marker) expression. Further, while the blood-brain barrier and glial-cell specific glucose transporter isoform (Glut1) was not affected, there appeared to be a tendency towards lower values in the neuronal/synaptic Glut3 concentrations in the n-3 group when compared to CD and n-6 groups (Figure 3C).
Figure 3: Hypothalamic neuropeptides (A & B) and proteins in brain (C).
A & B; Real-time quantitative RT-PCR analysis of male hypothalamic NPY, ObRb, AgRP, MC3-R, MC4-R, POMC and CART expression. CD and n-6 pups (male) at PN2 (A, n=6 per each group) or 360d (B, n=5~8 per each group). *p<0.05 compared to CD. C; PSD95, SYP, Glut1 and Glut3 proteins assessed by Western blot analysis. There is no significant difference in the groups. N=5-8, each group. Unpaired t-test (A) or one-way ANOVA and Fisher's PLSD test (B & C).
3.5. Histology, Metabolic Proteins and miR-122 in Livers
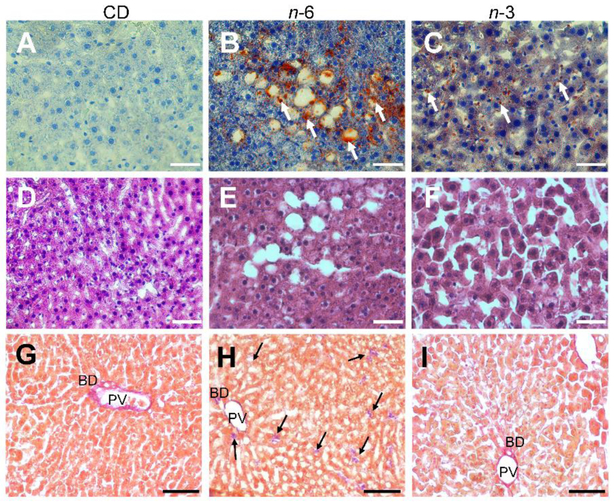
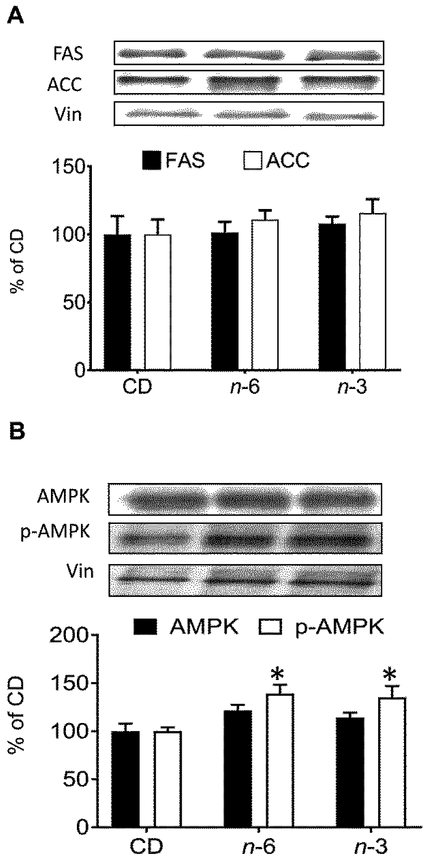
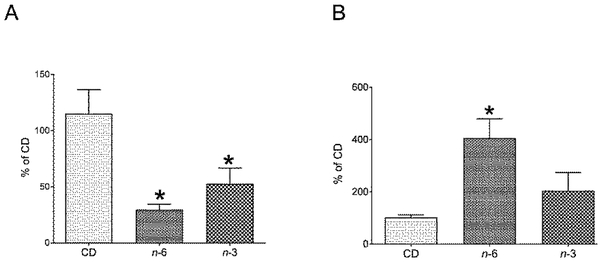
Second and next, we examined hepatic histology (Figure 4) and proteins (FAS, ACC and AMPK) (Figure 5), and hepatic and plasma miR-122 (Figure 6) in the CD, n-6, n-3 male groups. We observed a fatty liver in the n-6 and n-3 groups. However, the amount of steatosis detected visually in the n-3 group was notably less than that seen in the n-6 group (Figure 4). In addition, fibrosis assessed as collagen (type I fibers) staining was noted within the liver parenchyma in only small collections more so in the n-6 group versus the n-3 group when compared to the CD group (Figure 4). Our exploration of the 360d hepatic fatty acid synthesizing enzymes revealed no differences in FAS or ACC proteins in either high fat group compared to the CD group (Figure 5A). In contrast, while no differences in total AMPK were seen, an increase in phosphorylated (p) AMPK was noted in both the n-6 and n-3 groups when compared to CD (Figure 5B). We next evaluated the impact on a microRNA that is predominantly expressed by hepatocytes that also targets pAMPK [45], namely miR-122, and observed lower expression in hepatic miR-122 in n-6 and n-3 groups versus the CD group (Figure 6A). In contrast, miR-122 concentrations were significantly higher in the plasma of the n-6 group when compared to CD (Figure 6B). However, in the n-3 group, concentrations in-between that seen in the n-6 and CD were observed (Figure 6B).
Figure 4: Liver Histology.
In 360d male offspring, morphology by Hematoxylin and Eosin stain (A-C), fat deposition by Oil red stain O (D-F) and collagen fiber (fibrosis) by Picro Sirius Red Stain (G-I) in liver sections of CD (A,D & G), n-6 (B,E & H) and n-3 (C,F & I) groups. n-6 group shows prominent fat deposits compared to n-3. In addition, n-6 group demonstrates inter-cellular parenchymal collagen deposits (arrows) more than that seen in the n-3 group. Collagen staining is present in the portal vein (PV) and bile ducts (BD) in all three groups. Scale bars, A-F: 50 μm; G-I: 100 μm
Figure 5: Hepatic fatty acid synthesizing metabolic proteins.
In male CD, n-3 and n-6 offspring at 360d, Top: Representative Western blots. Bottom: Densitometric quantification of corresponding protein concentrations. A, Fatty acid synthase (FAS) and Acetyl CoA carboxylase (ACC); B, AMPK (5′ AMP-activated protein kinase) and phosphorylated AMPK (pAMPK). *p< 0.05 compared with CD, one-way ANOVA and Fisher's PLSD test. N=5-8, each group.
Figure 6: Hepatic and plasma miR-122.
In 360d male offspring, relative concentrations of hepatic (A) and plasma (B) miR-122 concentrations determined by RT-qPCR. Hepatic miR-122 concentrations were significantly lower in n-6 (*p=0.003) and n-3 (*p= 0.03) when compared to CD group (A), as analyzed by one-way ANOVA and Fisher's PLSD test. Plasma concentrations of miR-122 was significantly higher in n-6 (*p=0.04) but not in n-3 exposed male rat offspring when compared to the CD group, as analyzed by one-way ANOVA and Fisher's PLSD test. In the n-3 group, a tendency towards higher values compared to the CD group, and a tendency towards lower values when compared to the n-6 group is observed in plasma miR-122 concentrations (B). N=5-6 in each group.
4. Discussion:
Our results reveal that n-6 diet during pregnancy beginning after conception and during lactation, and n-3 diet during lactation alone affect growth, brain size, metabolism, and the state of the liver in a sex-specific manner. Contrary to our hypothesis and expectation, both male and female offspring exposed pre- and postnatally to n-6 diet during mother’s pregnancy and lactation period are not overweight and are in fact (much more in males) glucose tolerant and insulin sensitive, thereby proving the null hypothesis in this case. However, despite being lean, these male rats have liver disease akin to non-alcoholic fatty liver disease. On the other hand, n-6 during pregnancy followed by n-3 during lactation lowered the offspring’s body weight and conferred even further enhanced glucose tolerance and insulin sensitivity in males with some protection against later development of hepatic steatosis. Despite this relative protection, male n-6 and male and female n-3 had smaller brains compared to CD at 360d (12 months) of age.
We opted to introduce a high fat diet after conception, and not before conception, so that we could isolate the effect of maternal diet from maternal increase in preconception body mass and its associated effects on the offspring. Various investigations including our own in mice [46] have introduced a high fat diet prior to conception and continued this diet during pregnancy and lactation. This diet produces an overweight mother who gives birth to offspring who subsequently become obese and glucose intolerant. In contrast, our present study specifically questioned whether these metabolic changes were due to maternal high fat diet alone in the absence of maternal obesity. Further, we ensured a moderately high fat diet consisting of ~32 kcal% rather than the traditional ~60 kcal%, during pregnancy and lactation alone.
Prenatal exposure to n-6 enriched diet stimulated the male hypothalamic AgRP expression noted a day after birth. AgRP is orexigenic and increases the intake of fat enriched foods [26,47] as opposed to NPY which is also orexigenic, but targets carbohydrate enriched foods [26,48]. Along with the increase in AgRP, the expression of its associated receptor MC4-R (melanocortin isoform 4 receptor) also increased. Reduction in MC4-R due to mutations is associated with obesity [49,50], while an increase may signify a loss of weight. Similarly, mutations of ObRb (long form of the leptin receptor) cause mild obesity [51,52], while the increase observed in our study would mediate a lean phenotype. These hypothalamic changes that were examined only in the male were associated with a transient reduction in body weight, despite an increase in food intake in the male n-6 and n-3 groups. While the AgRP increase lasted until 360d in males, other hypothalamic changes reverted to normal without later body weight changes.
The high fat groups revealed glucose tolerance and insulin sensitivity, with the n-3 group revealing exaggerated glucose tolerance with heightened insulin sensitivity, particularly in the adult male offspring. This was accompanied by a decrease in free fatty acids in the male n-3 group when compared to CD. Additional confirmation was forthcoming when no changes in the liver fatty acid synthesizing enzymes were seen in males. An increase in hepatic pAMPK, a heterotrimeric serine-threonine kinase, further supports this lean phenotype, since pAMPK is a master regulator of cellular energy homeostasis by promoting catabolic pathways towards producing adenosine triphosphate. Thus, pAMPK promotes glycolysis, inhibits glycogen synthesis, enhances glucose transport and fatty acid oxidation. This change in hepatic pAMPK is in keeping with a high energy producing phenotype perhaps intended to match enhanced energy expenditure (not measured in our study).
Given these changes, we sought further confirmation, by examining the liver thoroughly. Other and our groups have demonstrated that miR-122 is a liver-specific non-coding RNA post-translationally regulating gene expression [53]. Its function was noted to be in regulation of fatty acid and cholesterol metabolism, in replication of hepatitis C virus, as a tumor suppressor gene in hepatocellular carcinoma, and in regulation of iron homeostasis by modulating activators of the hormone hepcidin [54]. However, others have demonstrated that miR-122 targets the 3’-UTR of the cation amino acid transporter isoform 1 (CAT-1), which provides bidirectional transport for the essential amino acids lysine and arginine. Recovery occurs when nutritional stressors disappear and human antigen R, an RNA-binding protein arrives in the cytoplasm from the nucleus and rescues CAT-1 from reductive regulation by miR-122 [55]. In addition, pAMPK is specifically targeted in hepatocytes by miR-122 suppressing its expression [45]. Given this background information, we considered miR-122 to be an important indicator of hepatic cellular health. We found that the n-6 and n-3 groups had lower hepatic miR-122 with simultaneous higher plasma (secreted by the hepatocytes) miR-122 concentrations. Although the biological significance of these changes is not entirely uncovered by our present study suggesting a limitation, prior studies provide some clues. In non-alcoholic fatty liver disease, murine hepatic miR-122 was down-regulated [56,57], while serum miR-122 was up-regulated [58-60]. In other studies, upregulation of human serum miR-122 predicted liver fibrosis [61]. In our present study, a high fat diet decreased liver specific miR-122 concentrations and led to fatty liver changes with n-6 being more pronounced than that seen with n-3. In addition, some amount of fibrosis was noted in the n-6 group scattered within the parenchyma, that was notably absent in the n-3 group. These histological changes are despite the offspring being lean, glucose tolerant, and insulin sensitive. Changes in miR-122 may also signify changes in cholesterol biosynthesis and compromise to essential amino acid transport. In an independent study, we have shown that a western diet administered to pregnant mice led to an amino acid deficiency post-partum [62]. We speculate that a high fat diet exposure may have compromised the availability of essential amino acids causing a miR-122 mediated reduction in amino acid transport as well. These concepts need future investigation, being a limitation of our present study. We did however observe higher hepatic pAMPK concentrations in the n-6 group in the presence of lower hepatic miR-122 expression, supporting prior evidence [45], that a reduction in miR-122 may release pAMPK from its inhibitory regulation. In this study, introduction of an n-3 diet postnatally led to a partial reversal of the adult phenotypic changes encountered in response to prenatal n-6 dietary exposure, particularly in the male offspring.
Our findings are novel and attest to the fact that a high fat diet only during pregnancy and lactation can be protective of the offspring with respect to the metabolic phenotype, maintaining body weight and glucose-insulin homeostasis. Our present observation is in keeping with a previous report where the process of insulin resistance was averted in the male rat adult offspring under n-3 supplementation during maternal pregnancy [18]. This lean glucose tolerant and insulin sensitive phenotype was noted by us despite a propensity towards higher hypothalamic AgRP induced food intake during early adult years. Thus, this higher food intake is more suggestive of an attempt at matching and supporting the presumed enhanced energy expenditure (indirect evidence being the elevated pAMPK). Further, our present study’s high fat diet exposure during gestation and lactation, affording protection to the offspring may also be related to reduced carbohydrate transfer from mother to fetus during a critical window of fetal (via placenta)/postnatal (via milk) development.
However, while both n-6 and n-3 diets during this window proved to be protective on the metabolic phenotype of the offspring, the detection of fatty liver and adverse brain findings were concerning. While our present study was not meant to fully examine brain development, the reduction in brain weight, while other organs for the most part remained unchanged, is concerning. Previous reports describing abnormal neuronal development and sensory function in offspring born to pregnant dams consuming n-3 further supports this concern [16,63].
While specific brain markers were not perturbed in our study, the subtle sign of a reduction of neuronal Glut3 concentrations, known for fueling neurotransmission [64-66], is concerning and requires future investigation. If this reduction in brain weight serves as a surrogate for inadequate neurogenesis, gliosis and/or myelination, there should be concern regarding the neurobehavior of these offspring. It is known that a pre-pregnancy and gestational high fat diet causes anxiolysis and other adverse changes in neurobehavior expressed by the offspring [67,68]. Further, investigations during embryonic day 21 revealed defects in peri-ventricular stem cell migration in the fetal brain [26] due to a high fat dietary exposure, supporting our current findings of aberrant brain growth in the offspring exposed prenatally and postnatally to a high fat diet. Thus, prior to recommending a high fat diet during gestation that is overlaid by postnatal n-6 or even n-3 (known to enhance infant visual and neural function) [69], one must weigh the potential metabolic (glucose tolerance and insulin sensitivity) benefits with the potential neurodevelopmental risks. Caution is recommended in ensuring that brain development and neurobehavior are optimized so that all offspring achieve their full potential.
We conclude that a high fat diet only during gestation and lactation (n-6 or n-3) has the propensity of affording metabolic protection to the adult offspring. This protection, results in lean, glucose tolerant and insulin sensitive male and female adult offspring. At the same time, the adult male offspring develops fatty liver. Non-alcoholic fatty liver disease is the most common chronic pediatric liver disorder [70], and predicted to become an indication for liver transplant, with increasing frequency. This same diet during gestation and lactation also detrimentally affected the brain weight of the adult offspring. It remains unclear currently how this change in brain weight impacts the neurological status, warranting future studies. However, like non-alcoholic fatty liver disease, the incidence of childhood neurodevelopmental disorders, including autism spectrum disorders, continues to grow [63]. Large epidemiological studies have made an association between maternal obesity and non-alcoholic fatty liver disease along with adverse neurological outcomes in the offspring [71]. In summary, we have shown that a high fat diet limited to n-6 in gestation and lactation, and n-3 in lactation only, produces a glucose tolerant and insulin sensitive offspring, but causes fatty liver and reduces brain weight.
Highlights:
Gestational n-6 diet results in a glucose tolerant and insulin sensitive adult offspring.
Postnatal n-3 superimposition on gestational n-6 diet enhances insulin sensitivity in offspring.
Gestational n-6 diet enhances hypothalamic AgRP related food intake in the offspring.
Gestational n-6 diet results in adult onset fatty liver disease in male offspring.
Gestational n-6 with or without postnatal n-3 diet reduces brain weight in offspring.
Acknowledgments:
This work was supported by grants from NIH, HD-41230 and HD-81206. All authors have nothing to disclose, except for Kara L, Calkins.
Abbreviations
- ACC
acetyl coenzyme-A carboxylase
- AgRP
agouti-related peptide
- AMPK
5 AMP-activated protein kinase
- ANOVA
analysis of variance
- CART
cocaine and amphetamine regulated transcript
- CAT-1
cation amino acid transporter isoform 1
- CD
control regular chow diet
- FAS
fatty acid synthetase
- G
gestation
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- Glut1
glucose transporter isoform 1
- Glut3
glucose transporter isoform 3
- GTT
glucose tolerance test
- ITT
insulin tolerance test
- MC3-R
melanocortin isoform 3 receptor
- MC4-R
melanocortin isoform 4 receptor
- miR-122
microRNA-122
- n-3
dietary n-3 polyunsaturated fatty acids
- n-6
dietary n-6 polyunsaturated fatty acids
- NPY
neuropeptide Y
- ObRb
long isoform of the leptin receptor
- PBS
phosphate-buffered saline
- POMC
proopiomelanocortin
- PN
postnatal day
- PSD95
postsynaptic density 95
- pAMPK
phosphorylated 5 AMP-activated protein kinase
- SYP
synaptophysin
Footnotes
Kara L. Calkins served on the advisory boards of Mead Johnson, Baxter and Fresenius Kabi, and is a consultant for Fresenius Kabi. None of these activities or products are part of the work cited in this manuscript. The authors declare no other conflicts of interest.
References
- [1].Ogden CL, Fryar CD, Hales CM, Carroll MD, Aoki Y, Freedman DS. Differences in Obesity Prevalence by Demographics and Urbanization in US Children and Adolescents, 2013-2016. JAMA 2018;319:2410–8. doi: 10.1001/jama.2018.5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Leong KS, Wilding JP. Obesity and diabetes. Baillieres Best Pr Res Clin Endocrinol Metab 1999;13:221–37. [DOI] [PubMed] [Google Scholar]
- [3].Barnes AS. The epidemic of obesity and diabetes: trends and treatments. Tex Hear Inst J 2011;38:142–4. [PMC free article] [PubMed] [Google Scholar]
- [4].Verma S, Hussain ME. Obesity and diabetes: An update. Diabetes Metab Syndr 2017;11:73–9. doi: 10.1016/j.dsx.2016.06.017. [DOI] [PubMed] [Google Scholar]
- [5].Bhupathiraju SN, Hu FB. Epidemiology of Obesity and Diabetes and Their Cardiovascular Complications. Circ Res 2016;118:1723–35. doi: 10.1161/CIRCRESAHA.115.306825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chapman MJ, Ginsberg HN, Amarenco P, Andreotti F, Boren J, Catapano AL, et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur Hear J 2011;32:1345–61. doi: 10.1093/eurheartj/ehr112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mennitti L V, Oliveira JL, Morais CA, Estadella D, Oyama LM, Oller do Nascimento CM, et al. Type of fatty acids in maternal diets during pregnancy and/or lactation and metabolic consequences of the offspring. J Nutr Biochem 2015;26:99–111. doi: 10.1016/j.jnutbio.2014.10.001. [DOI] [PubMed] [Google Scholar]
- [8].Garcia-Compean D, Jaquez-Quintana JO, Gonzalez-Gonzalez JA, Maldonado-Garza H. Liver cirrhosis and diabetes: risk factors, pathophysiology, clinical implications and management. World J Gastroenterol 2009;15:280–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shapiro D, Vaiyani D, Horlbeck D, Pattishall S. Case 1: Otorrhea, Otalgia, and Blurry Vision in an 11-year-old Girl. Pediatr Rev 2017;38:566. doi: 10.1542/pir.2016-0091. [DOI] [PubMed] [Google Scholar]
- [10].Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005;115:e290–6. doi: 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- [11].Sathyapalan T, Mellor D, Atkin SL. Obesity and gestational diabetes. Semin Fetal Neonatal Med 2010;15:89–93. doi: 10.1016/j.siny.2009.09.002. [DOI] [PubMed] [Google Scholar]
- [12].JI Harland. Food combinations for cholesterol lowering. Nutr Res Rev 2012;25:249–66. doi: 10.1017/S0954422412000170. [DOI] [PubMed] [Google Scholar]
- [13].Astrup A The American paradox: The role of energy-dense fat-reduced food in the increasing prevalence of obesity. Curr Opin Clin Nutr Metab Care 1998. doi: 10.1097/00075197-199811000-00016. [DOI] [PubMed] [Google Scholar]
- [14].Simopoulos AP. Importance of the omega-6/omega-3 balance in health and disease: evolutionary aspects of diet. World Rev Nutr Diet 2011;102:10–21. doi: 10.1159/000327785. [DOI] [PubMed] [Google Scholar]
- [15].Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother 2002;56:365–79. [DOI] [PubMed] [Google Scholar]
- [16].Jen KL, Church MW, Wang C, Moghaddam M, Dowhan L, Laja F, et al. Perinatal n-3 fatty acid imbalance affects fatty acid composition in rat offspring. Physiol Behav 2009;98:17–24. doi: 10.1016/j.physbeh.2009.03.031. [DOI] [PubMed] [Google Scholar]
- [17].Simopoulos AP. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016;8:128. doi: 10.3390/nu8030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Albert BB, Vickers MH, Gray C, Reynolds CM, Segovia SA, Derraik JGB, et al. Fish oil supplementation to rats fed high-fat diet during pregnancy prevents development of impaired insulin sensitivity in male adult offspring. Sci Rep 2017;7:5595. doi: 10.1038/s41598-017-05793-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Coletta JM, Bell SJ, Roman AS. Omega-3 Fatty acids and pregnancy. Rev Obs Gynecol 2010;3:163–71. [PMC free article] [PubMed] [Google Scholar]
- [20].Greenberg JA, Bell SJ, Ausdal W V. Omega-3 Fatty Acid supplementation during pregnancy. Rev Obs Gynecol 2008;1:162–9. [PMC free article] [PubMed] [Google Scholar]
- [21].Kim J, Carlson ME, Kuchel GA, Newman JW, Watkins BA. Dietary DHA reduces downstream endocannabinoid and inflammatory gene expression and epididymal fat mass while improving aspects of glucose use in muscle in C57BL/6J mice. Int J Obes 2016;40:129–37. doi: 10.1038/ijo.2015.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Thamotharan M, Shin BC, Suddirikku DT, Thamotharan S, Garg M, Devaskar SU. GLUT4 expression and subcellular localization in the intrauterine growth-restricted adult rat female offspring. Am J Physiol Endocrinol Metab 2005;288:E935–47. doi: 10.1152/ajpendo.00342.2004. [DOI] [PubMed] [Google Scholar]
- [23].Isganaitis E, Woo M, Ma H, Chen M, Kong W, Lytras A, et al. Developmental programming by maternal insulin resistance: hyperinsulinemia, glucose intolerance, and dysregulated lipid metabolism in male offspring of insulin-resistant mice. Diabetes 2014;63:688–700. doi: 10.2337/db13-0558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Garcia-Vargas L, Addison SS, Nistala R, Kurukulasuriya D, Sowers JR. Gestational Diabetes and the Offspring: Implications in the Development of the Cardiorenal Metabolic Syndrome in Offspring. Cardiorenal Med 2012;2:134–42. doi:000337734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Desai M, Jellyman JK, Han G, Beall M, Lane RH, Ross MG. Maternal obesity and high-fat diet program offspring metabolic syndrome. Am J Obs Gynecol 2014;211:237 e1–237 e13. doi:S0002-9378(14)00235-X[pii] 10.1016/j.ajog.2014.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Stachowiak EK, Srinivasan M, Stachowiak MK, Patel MS. Maternal obesity induced by a high fat diet causes altered cellular development in fetal brains suggestive of a predisposition of offspring to neurological disorders in later life. Metab Brain Dis 2013;28:721–5. doi: 10.1007/s11011-013-9437-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Edlow AG, Guedj F, Pennings JL, Sverdlov D, Neri C, Bianchi DW. Males are from Mars, and females are from Venus: sex-specific fetal brain gene expression signatures in a mouse model of maternal diet-induced obesity. Am J Obs Gynecol 2016;214:623 e1–623 e10. doi:S0002-9378(16)00396-3[pii] 10.1016/j.ajog.2016.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Huang CW, Chien YS, Chen YJ, Ajuwon KM, Mersmann HM, Ding ST. Role of n-3 polyunsaturated fatty acids in ameliorating the obesity-induced metabolic syndrome in animal models and humans. Int J Mol Sci 2016. doi: 10.3390/ijms17101689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li JJ, Huang CJ, Xie D. Anti-obesity effects of conjugated linoleic acid, docosahexaenoic acid, and eicosapentaenoic acid. Mol Nutr Food Res 2008. doi: 10.1002/mnfr.200700399. [DOI] [PubMed] [Google Scholar]
- [30].Purushotham A, Shrode GE, Wendel AA, Liu LF, Belury MA. Conjugated linoleic acid does not reduce body fat but decreases hepatic steatosis in adult Wistar rats. J Nutr Biochem 2007. doi: 10.1016/j.jnutbio.2006.10.008. [DOI] [PubMed] [Google Scholar]
- [31].Yang Y, Smith DL Jr., Keating KD, Allison DB, Nagy TR. Variations in body weight, food intake and body composition after long-term high-fat diet feeding in C57BL/6J mice. Obes (Silver Spring) 2014;22:2147–55. doi: 10.1002/oby.20811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hartil K, Vuguin PM, Kruse M, Schmuel E, Fiallo A, Vargas C, et al. Maternal substrate utilization programs the development of the metabolic syndrome in male mice exposed to high fat in utero. Pediatr Res 2009;66:368–73. doi: 10.1203/PDR.0b013e3181b33375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Thamotharan M, Shin B-C, Suddirikku DT, Thamotharan S, Garg M, Devaskar SU. GLUT4 expression and subcellular localization in the intrauterine growth-restricted adult rat female offspring. Am J Physiol Metab 2005. doi: 10.1152/ajpendo.00342.2004. [DOI] [PubMed] [Google Scholar]
- [34].Tomi M, Zhao Y, Thamotharan S, Shin BC, Devaskar SU. Early life nutrient restriction impairs blood-brain metabolic profile and neurobehavior predisposing to Alzheimer’s disease with aging. Brain Res 2013. doi: 10.1016/j.brainres.2012.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shin BC, Dai Y, Thamotharan M, Gibson LC, Devaskar SU. Pre- and postnatal calorie restriction perturbs early hypothalamic neuropeptide and energy balance. J Neurosci Res 2012;90:1169–82. doi: 10.1002/jnr.23013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gibson LC, Shin BC, Dai Y, Freije W, Kositamongkol S, Cho J, et al. Early leptin intervention reverses perturbed energy balance regulating hypothalamic neuropeptides in the pre- and postnatal calorie-restricted female rat offspring. J Neurosci Res 2015;93:902–12. doi: 10.1002/jnr.23560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Acad Press 1998. [DOI] [PubMed] [Google Scholar]
- [38].Shin BC, Fujikura K, Suzuki T, Tanaka S, Takata K. Glucose transporter GLUT3 in the rat placental barrier: a possible machinery for the transplacental transfer of glucose. Endocrinology 1997;138:3997–4004. doi: 10.1210/endo.138.9.5369. [DOI] [PubMed] [Google Scholar]
- [39].Fung C, Evans E, Shin D, Shin BC, Zhao Y, Sankar R, et al. Hypoxic-ischemic brain injury exacerbates neuronal apoptosis and precipitates spontaneous seizures in glucose transporter isoform 3 heterozygous null mice. J Neurosci Res 2010. doi: 10.1002/jnr.22487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Calkins KL, Thamotharan S, Dai Y, Shin BC, Kalhan SC, Devaskar SU. Early dietary restriction in rats alters skeletal muscle tuberous sclerosis complex, ribosomal s6 and mitogen-activated protein kinase. Nutr Res 2018;54:93–104. doi: 10.1016/j.nutres.2018.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mehlem A, Hagberg CE, Muhl L, Eriksson U, Falkevall A. Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat Protoc 2013. doi: 10.1038/nprot.2013.055. [DOI] [PubMed] [Google Scholar]
- [42].Shin B-C, Cepeda C, Estrada-Sánchez AM, Levine MS, Hodaei L, Dai Y, et al. Neural Deletion of Glucose Transporter Isoform 3 Creates Distinct Postnatal and Adult Neurobehavioral Phenotypes. J Neurosci 2018;38:9579–99. doi: 10.1523/JNEUROSCI.0503-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Chen Y, Shin BC, Thamotharan S, Devaskar SU. Differential methylation of the micro-RNA 7b gene targets postnatal maturation of murine neuronal Mecp2 gene expression. Dev Neurobiol 2014;74:407–25. doi: 10.1002/dneu.22126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Shin B-C, Cepeda C, Estrada-Sánchez AM, Levine MS, Hodaei L, Dai Y, et al. Neural Deletion of Glucose Transporter Isoform 3 Creates Distinct Postnatal and Adult Neurobehavioral Phenotypes. J Neurosci 2018;38:9579–99. doi: 10.1523/JNEUROSCI.0503-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kwon IG, Ha TK, Ryu SW, Ha E. Roux-en-Y gastric bypass stimulates hypothalamic miR-122 and inhibits cardiac and hepatic miR-122 expressions. J Surg Res 2015. doi: 10.1016/j.jss.2015.05.055. [DOI] [PubMed] [Google Scholar]
- [46].Ganguly A, Devaskar SU. High-fat diet affects pregestational adiposity and glucose tolerance perturbing gestational placental macronutrient transporters culminating in an obese offspring in wild-type and glucose transporter isoform 3 heterozygous null mice. J Nutr Biochem 2018;62:192–201. doi: 10.1016/j.jnutbio.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tracy AL, Clegg DJ, Johnson JD, Davidson TL, Benoit SC. The melanocortin antagonist AgRP (83-132) increases appetitive responding for a fat, but not a carbohydrate, reinforcer. Pharmacol Biochem Behav 2008. doi: 10.1016/j.pbb.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Poon K, Barson JR, Fagan SE, Leibowitz SF. Developmental changes in embryonic hypothalamic neurons during prenatal fat exposure. Am J Physiol Endocrinol Metab 2012;303:E432–41. doi: 10.1152/ajpendo.00238.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Vaisse C, Clement K, Durand E, Hercberg S, Guy-Grand B, Froguel P. Melanocortin-4 receptor mutations are a frequent and heterogeneous cause of morbid obesity. J Clin Invest 2000;106:253–62. doi: 10.1172/JCI9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Marti A, Corbalan MS, Forga L, Martinez JA, Hinney A, Hebebrand J. A novel nonsense mutation in the melanocortin-4 receptor associated with obesity in a Spanish population. Int J Obes Relat Metab Disord 2003;27:385–8. doi: 10.1038/sj.ijo.0802244. [DOI] [PubMed] [Google Scholar]
- [51].Bjorbaek C Central leptin receptor action and resistance in obesity. J Investig Med 2009;57:789–94. doi: 10.2310/JIM.0b013e3181bb0d49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Cui H, Lopez M, Rahmouni K. The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nat Rev Endocrinol 2017;13:338–51. doi: 10.1038/nrendo.2016.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Tsai W-C, Hsu S-D, Hsu C-S, Lai T-C, Chen S-J, Shen R, et al. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J Clin Invest 2012;122:2884–97. doi: 10.1172/JCI63455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Rivkin M, Simerzin A, Zorde-Khvalevsky E, Chai C, Yuval JB, Rosenberg N, et al. Inflammation-Induced Expression and Secretion of MicroRNA 122 Leads to Reduced Blood Levels of Kidney-Derived Erythropoietin and Anemia. Gastroenterology 2016;151:999–1010.e3. doi: 10.1053/j.gastro.2016.07.031. [DOI] [PubMed] [Google Scholar]
- [55].Bhattacharyya SN, Habermacher R, Martine U, Closs EI, Filipowicz W. Relief of microRNA-Mediated Translational Repression in Human Cells Subjected to Stress. Cell 2006. doi: 10.1016/j.cell.2006.04.031. [DOI] [PubMed] [Google Scholar]
- [56].Alisi A, Da Sacco L, Bruscalupi G, Piemonte F, Panera N, De Vito R, et al. Mirnome analysis reveals novel molecular determinants in the pathogenesis of diet-induced nonalcoholic fatty liver disease. Lab Invest 2011;91:283–93. doi: 10.1038/labinvest.2010.166. [DOI] [PubMed] [Google Scholar]
- [57].Li S, Chen X, Zhang H, Liang X, Xiang Y, Yu C, et al. Differential expression of microRNAs in mouse liver under aberrant energy metabolic status. J Lipid Res 2009;50:1756–65. doi: 10.1194/jlr.M800509-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Tryndyak VP, Latendresse JR, Montgomery B, Ross SA, Beland FA, Rusyn I, et al. Plasma microRNAs are sensitive indicators of inter-strain differences in the severity of liver injury induced in mice by a choline- and folate-deficient diet. Toxicol Appl Pharmacol 2012;262:52–9. doi: 10.1016/j.taap.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Yamada H, Ohashi K, Suzuki K, Munetsuna E, Ando Y, Yamazaki M, et al. Longitudinal study of circulating miR-122 in a rat model of non-alcoholic fatty liver disease. Clin Chim Acta 2015;446:267–71. doi: 10.1016/j.cca.2015.05.002. [DOI] [PubMed] [Google Scholar]
- [60].Povero D, Eguchi A, Li H, Johnson CD, Papouchado BG, Wree A, et al. Circulating extracellular vesicles with specific proteome and liver microRNAs are potential biomarkers for liver injury in experimental fatty liver disease. PLoS One 2014;9:e113651. doi: 10.1371/journal.pone.0113651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Miyaaki H, Ichikawa T, Kamo Y, Taura N, Honda T, Shibata H, et al. Significance of serum and hepatic microRNA-122 levels in patients with non-alcoholic fatty liver disease. Liver Int 2014;34:e302–7. doi: 10.1111/liv.12429. [DOI] [PubMed] [Google Scholar]
- [62].Bhasin KK, van Nas A, Martin LJ, Davis RC, Devaskar SU, Lusis AJ. Maternal low-protein diet or hypercholesterolemia reduces circulating essential amino acids and leads to intrauterine growth restriction. Diabetes 2009;58:559–66. doi: 10.2337/db07-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Church MW, Jen KL, Jackson DA, Adams BR, Hotra JW. Abnormal neurological responses in young adult offspring caused by excess omega-3 fatty acid (fish oil) consumption by the mother during pregnancy and lactation. Neurotoxicol Teratol 2009;31:26–33. doi: 10.1016/j.ntt.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Rajakumar A, Thamotharan S, Raychaudhuri N, Menon RK, Devaskar SU. Transactivators regulating neuronal glucose transporter isoform-3 gene expression in mammalian neurons. J Biol Chem 2004;279:26768–79. doi: 10.1074/jbc.M402735200. [DOI] [PubMed] [Google Scholar]
- [65].Simpson IA, Dwyer D, Malide D, Moley KH, Travis A, Vannucci SJ. The facilitative glucose transporter GLUT3: 20 years of distinction. Am J Physiol Endocrinol Metab 2008;295:E242–53. doi: 10.1152/ajpendo.90388.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Takata K, Hirano H, Kasahara M. Transport of glucose across the blood-tissue barriers. Int Rev Cytol 1997;172:1–53. [DOI] [PubMed] [Google Scholar]
- [67].Perani C V, Neumann ID, Reber SO, Slattery DA. High-fat diet prevents adaptive peripartum-associated adrenal gland plasticity and anxiolysis. Sci Rep 2015;5:14821. doi: 10.1038/srep14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Li M, Fallin MD, Riley A, Landa R, Walker SO, Silverstein M, et al. The Association of Maternal Obesity and Diabetes With Autism and Other Developmental Disabilities. Pediatrics 2016;137:e20152206. doi:peds.2015-2206[pii] 10.1542/peds.2015-2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Uauy R, Birch E, Birch D, Peirano P. Visual and brain function measurements in studies of n-3 fatty acid requirements of infants. J Pediatr 1992;120:S168–80. [DOI] [PubMed] [Google Scholar]
- [70].Kerkar N, Lakhole A. Pediatric liver transplantation: a North American perspective. Expert Rev Gastroenterol Hepatol 2016;10:949–59. doi: 10.1586/17474124.2016.1166951. [DOI] [PubMed] [Google Scholar]
- [71].Edlow AG. Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat Diagn 2017;37:95–110. doi: 10.1002/pd.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]