Abstract
Background
Sodium intake is excessive among Spanish children, but the salt use behaviors of parents and children are unknown. This study aims to determine behaviors related to salt intake in both schoolchildren and parents and the relationship between parental behaviors and 24 h urinary sodium excretion (UNa-24h) in children.
Subjects and methods
A convenience sample was taken from a cross-sectional analysis. Parents completed a self-reported questionnaire about their behaviors related to salt, and their responses were compared with the UNa-24h of their own children. The median test was used to identify differences in UNa-24h according to behaviors. Logistic regression was used to assess the relationship between the behaviors of parents and high sodium excretion in the children and the risk of children’s use of table salt, adjusting for age, sex, and BMI. Multinomial logistic regression models, adjusted by the covariates, were used to study the children’s salt preferences.
Results
A total of 329 schoolchildren from different Spanish provinces were included in the study (mean age: 9.0 ± 1.2 years, 157 girls). The majority of families (parents mean age: 42.0 ± 5.2 years) reported adding salt to food during cooking (92%), and 59% of them never looked at the sodium content on food labels. However, none of these behaviors were related to UNa-24h (p > 0.05). The use of iodized salt (53%), the presence of a salt shaker on the table (6%), and the use of table salt by fathers (57%), mothers (52%) or children (17%) increased the odds (p < 0.05) of children having a higher UNa-24h. Checking sodium content on food labels and the use of table salt by the children or father was associated with a lower preference for salty foods (p < 0.05).
Conclusions
It is important to make parents aware of the relationship between their behaviors regarding the use of discretionary salt and their children's sodium intake. Our data suggest that salt-specific education programs on how to reduce salt both in-home and outside the home should be implemented to improve behavior skills related to salt consumption in parents and children.
Introduction
Cardiovascular diseases (CVDs) are the leading cause of death in Europe, with more than 3.9 million deaths per year due to CVD [1]. Excess sodium intake is linked to raised blood pressure, which is a major risk factor for cardiovascular-related conditions such as stroke and heart attacks [2]. In addition, it has been suggested blood pressure tracks with age [3,4], and for this reason, there is a need to reduce the dietary risk factors such as sodium consumption associated with high blood pressure in children [3]. The adoption of a healthy diet at an early age is crucial for the prevention of CVD [5].
In Spain, nationally representative data shows that the population exceeded sodium intake [6–8]. Partearroyo et al. have recently studied sodium intake in the Spanish ANIBES population, with adults and children aged 9–75 years (n = 2009). The authors reported a dietary sodium intake of 2025 ± 805 mg/day, using a 3-day dietary record and excluding salt added at table or during cooking [6]. Although there is extensive scientific evidence regarding the consumption of excessive sodium in various populations, there are fewer data available for children than for adults [9]. In a recently conducted analysis by Aparicio et al. [10], the researchers found that, on average, Spanish children aged 7–11 years excreted 3052 ± 1182 mg/day of sodium. According to WHO criteria, the results of this study imply that 84% of the subjects who were under 10 years old consumed >4 g of salt per day and that 67% of those aged >10 years had an intake of >5 g salt/day [11].
Dietary behaviors, including the choice of foods that are high in sodium content, are established during childhood and track over time [12,13]. However, it has been observed that children have a greater preference for sweet and salty foods than adults [14], making them particularly vulnerable in an environment in which salt and sugar dominate the food supply.
Food patterns are established in childhood, and the “family environment” and parents’ behavior (as food choices or feeding practices) determine the children's food preferences and diet [15]. Given the knowledge of the current excessive sodium intake in Spanish children and the benefits of decreasing salt intake from an early age to reduce the likelihood of a diet high in salt during adulthood or to prevent cardiovascular disease [16], it is useful to try to understand current patterns involving schoolchildren and their environment regarding behaviors related to salt intake. These behaviors include the use of discretionary salt (adding salt at the table or when cooking) and checking the sodium content on food labeling [17]. Furthermore, given children’s vulnerability to developing a preference for salty foods, it would be interesting to analyze whether these behaviors are related to children’s greater preference for salty foods.
Currently, there are no available data about the behaviors of Spanish parents and their children related to the use of discretionary salt and the relationship between parental behaviors to their children’s sodium intake. Our main objective is to determine parents’ and their own children’s salt-related behaviors and their association with the children’s 24 h urinary sodium excretion (UNa-24h). Our secondary objectives are to examine the association between parental salt-related behaviors and (1) high sodium excretion among children, (2) children’s table salt use, and (3) children’s preference for “salty foods” in the context of Spanish schoolchildren.
Material and methods
Study design
A convenience sample was used from an ongoing cross-sectional trial registered at clinicaltrials.gov as NCT03465657. The main aim of this study is to establish the average sodium intake in Spanish schoolchildren according to sodium excretion in a 24 h urine sample and analyze the relationship between salt intake and health parameters. Another goal is to identify the use of discretionary salt in parents and children. The data reported in this manuscript were collected between February 2014 and February 2018. The study design has been previously described in other papers [10,18]. The study protocol was approved by the Ethics Committee of the San Carlos Hospital (Ref 12/319-E and 15/522-E), which is part of the Complutense University of Madrid [19]. Coding was used to identify samples and forms.
Data collection and subjects
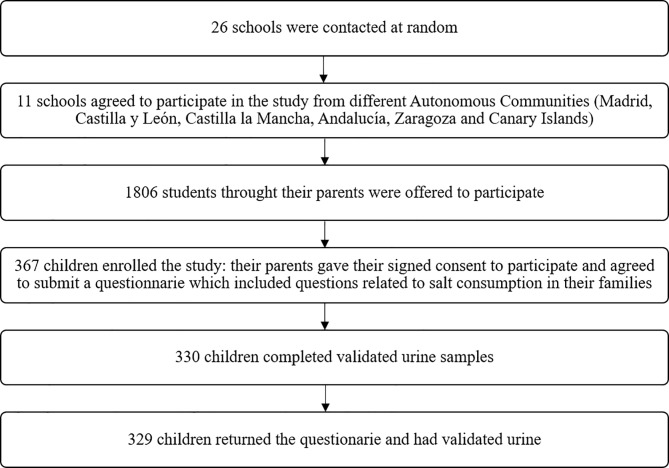
This convenience sample involved primary schools from six Spanish provinces (Madrid, Córdoba, Segovia, Ciudad Real, Zaragoza, and Tenerife), including the capital of the province and a semi-urban/rural city (<50000 inhabitants). A number was assigned to the schools, and schools were chosen randomly from each location. The random selection of schools was conducted using Microsoft Office Excel 2013 and the RANDBETWEEN function [20]. Of the twenty-six schools contacted by telephone, eleven accepted the invitation to be included in the study (Fig 1).
Fig 1. Recruitment of the population.
First, the schools were contacted by telephone. Once the Director agreed to the school's participation, an appointment was arranged with the parents, who were invited to an informative talk during which the details of the study were explained. Once any questions posed by the parents were answered, the interested parents signed a written informed consent form. On the second day, the researchers visited the school to collect the questionnaires completed by the parents and children. Subsequently, the children gave a urine sample, and several anthropometric measurements were made. Of the 1806 children and their own parents that were offered to participate, 367 parents gave their informed consent and enrolled with their children in the study (20.3%).
The inclusion criteria were schoolchildren aged 7–11 years, attending between the second and fifth grades of primary school, and compliance with the informed consent via parents or legal guardians. The exclusion criteria were absence on the day of the visit to the school, having a disease that could affect the excretion of sodium (metabolic or chronic diseases, such as diabetes, kidney disease, or liver disease), altered eating habits (when the child has to modify his or her diet in a timely manner for some reason, e.g., a soft diet), and not providing a urine sample.
Sociodemographic and anthropometric data
Parents completed a self-administered questionnaire at home about the children and family health status. The questionnaire also included sociodemographic data, such as the working situation of the father and mother (details of the questionnaire described below).
Anthropometric measurements were carried out in the schools. Weight and height were determined with a digital balance (range 0.1–150 kg, accuracy 100 g; Alpha; Seca, Igni, France) and a digital stadiometer (70–205 cm, 1 mm; Harpenden Pfifter, Carlstadt, NJ, USA), respectively. For these measurements, the children were barefooted and wore only their underwear. For the analyses, the mean value of three measurements was used. Their body mass index (BMI) was then calculated.
Twenty-four-hour urine sample
A 24-hour urine sample was analyzed to study the urinary parameters. To ensure compliance with 24-hour urine collection, the schoolchildren and their parents were instructed in the collection procedure and given written instructions. The children were requested to urinate at 8 p.m. (start of the collection), and this urine sample was excluded. Then, the subsequent voiding was included in the collection. From that moment onward, all urine was collected to obtain the complete sample at the scheduled time (24 hours after the start time of the collection), with instructions that the last urine sample be collected at the same hour as the first sample. This protocol was adapted from Neubert et al. [21]. The 24-hour sampling began Saturday night and continued until Sunday at the same time to facilitate the collection of urine samples on a non-teaching day. Parents were asked to write down any urinations that were not collected during the 24 hours.
All urine was stored in a 2 L plastic container without preservatives. After receiving the urine, the volume of the 24-hour urine sample was calculated, and the urine was stored in 100 mL containers at a temperature below 12°C before being transferred to the laboratory.
Urinary sodium excretion was quantified using an indirect potentiometer, which had selective solid membranes for each ion and was connected to an AU 5400 Autoanalyzer (Olympus, Mishima, Japan): the coefficient of variation (CV) was 1.0 for sodium [22]. Creatinine levels were determined according to a modification of the Jaffe reaction using the same apparatus. The color intensity was measured at 520 nm (CV = 2.8) [23]. Creatinine was expressed in mg/day and converted to mmol/kg/day with the following factor: 1 mg of creatinine × 0.0088 = 1 mmol of creatinine. We used a cut-off limit taken from Remer et al. for creatinine [24] to identify under-collected 24 h urine samples. Collections were considered incomplete if (1) there were self-reported missing voids (n = 19), (2) urinary creatinine < 0.1 mmol/kg/day (n = 18) [24], and (3) volume < 300 mL (n = 0) [25].
Behaviors related to salt consumption
Parents completed a self-administered questionnaire at home about behaviors concerning salt consumption. They answered eight closed questions regarding (1) the use of salt by the person in charge of cooking; (2) the use of table salt after cooking by the father (3) and mother; (4) the frequency of checking the salt content on food labels; (5) the type of salt used; (6) the availability of a salt shaker on the table; (7) the free use of the salt shaker by the children; (8) and the schoolchildren's preference for salty foods. S1 File shows the questionnaire used with all answer options.
Statistical processing of data
The results were summarized as the mean ± standard deviation (SD), medians with the interquartile range (IQR), or proportions (for categorical variables). The Kolmogorov–Smirnov test was used to assess the assumption of normality. To compare the main baseline characteristics of the participants according to sex, we used the Student’s t-test on homogeneous variables and the Mann–Whitney U test on non-parametric variables. The χ2 test was used for categorical variables, and the Spearman correlation (rS) was employed to assess the relationship between UNa-24h and BMI and age by sex. UNa-24h was expressed in mg/day and salt equivalent in g/day, and it was studied according to its relationship with different behaviors. Subjects who did not answer a question were excluded from the analysis of that question but not from the overall study. Because not all data followed a normal distribution, the median test for k independent samples was used to compare sodium excretion between different groups regarding dietary behaviors related to salt intake to identify differences in sodium excretion [26], and the Levene homoscedasticity test was used to determine whether variance differed significantly between groups. Pairwise multiple comparisons were used to observe the differences between groups.
Afterward, logistic regression models with calculations of the corresponding odds ratio (OR) and 95% confidence intervals (CI) were used to examine the possible association between parents’ and children’s behaviors related to salt (independent variables) with children’s sodium excretion above the median or with the use of table salt by children (dependent variables). The responses in the questions about behaviors related to salt were consolidated into two groups: ‘only if it is tasteless/sometimes’ and ‘always’ were combined into one category to compare with ‘never’. The preference for salty foods was consolidated into ‘somewhat salty/salty food’ versus ‘not salty food’, and the child’s use of table salt was grouped into ‘sometimes/always’ (when the response was from ‘Once a day’ to ‘1–3 times in a month’) versus ‘never’ (when the response was ‘Never, less than once in a month’). We evaluated the associations using three models: (a) a basic model that was not adjusted, (b) a second model that accounted for the sex, age, and BMI of the children, and (c) a third model that included the second model’s considerations plus the simultaneous effect of all explanatory variables to analyze those that had the most significant influence. Subsequently, to study the children’s preference for salty or medium-salt-content foods, we used multinomial logistic regression models adjusted by the variables described above in the three models. A p-value of p < 0.05 was considered statistically significant. The evaluation of the data obtained was completed with the SPSS® version 24.0 statistical software.
Results
Of the 367 children who enrolled in the study, three did not return the behavior questionnaire completed by their family. For the urine study, 19 participants did not complete the 24-hour collection of urine (the samples were lost during collection), while a further 18 participants had creatinine excretion rates below the cut-limit of 0.1 mmol/kg/day, resulting in a final sample of 329 children with valid urine samples and completed salt behavior questionnaires (Fig 1).
The mean age of children was 9.0 ± 1.2 years old, with 52% boys, and the mean UNa-24h was 3133 ± 1194 mg/24 h (7.8 ± 3.0 g/day of salt). There were no significant differences between the sexes in the sociodemographic characteristics shown in Table 1, but there were differences in UNa-24h (p < 0.01) and creatinine/weight (p < 0.001), which were both higher in boys than in girls. Furthermore, there were a positive correlations between sodium excretion and BMI and age in boys (rS = 0.336, p < 0.001; rS = 0.174, p < 0.05, respectively) and girls (rS = 0.193, p < 0.01; rS = 0.336, p < 0.05, respectively).
Table 1. Participants’ demographic, anthropometric, and urinary parameters by children’s sex (mean ± SD or %).
| Variables | Subcategories | Total children (n = 329) |
Girls (n = 157) |
Boys (n = 172) |
pa value |
|---|---|---|---|---|---|
| Children | |||||
| Age (years) | 9.0 ± 1.2 | 9.0 ± 1.2 | 8.9 ± 1.2 | 0.664 | |
| Geographical area (%) | 0.656 | ||||
| <50000 inhabitants | 46.5 | 45.2 | 47.7 | ||
| >50000 inhabitants | 53.5 | 54.8 | 52.3 | ||
| Anthropometric data | |||||
| Weight (kg)† | 35.6 ± 8.5 | 35.8 ± 8.8 | 35.4 ± 8.1 | 0.654 | |
| Height (cm) | 137.3 ± 8.9 | 137.0 ± 9.5 | 137.6 ± 8.2 | 0.939 | |
| BMI (kg/m2)† | 18.7 ± 3.2 | 18.9 ± 3.2 | 18.6 ± 3.2 | 0.234 | |
| Urinary parameters | |||||
| Volume 24 h (mL/24 h)† | 907.2 ± 297.2 | 886.5 ± 284.9 | 926.1 ± 307.7 | 0.228 | |
| Creatinine/weight (mg/kg)† | 20.6 ± 4.3 | 19.4 ± 4.0 | 21.6 ± 4.4 | 0.000 | |
| UNa-24h (mEq/24 h) | 135.9 ± 51.9 | 127 ± 44.4 | 144.1 ± 56.8 | 0.007 | |
| UNa-24h (mg/24 h) | 3133 ± 1194 | 2921 ± 1022 | 3314 ± 1306 | 0.007 | |
| Salt (g/day) | 7.9 ± 3.0 | 7.4 ± 2.6 | 8.4 ± 3.3 | 0.007 | |
| Na/Creatinine (mg/mg) | 4.4 ± 1.4 | 4.4 ± 1.4 | 4.4 ± 1.5 | 0.785 | |
| Parents | |||||
| Age (years)† | 42.0 ± 5.2 | 42.7 ± 5.1 | 41.5 ± 5.3 | 0.008 | |
| Father's employment status (%) | 0.659 | ||||
| Non-paid work/no work | 11.9 | 12.7 | 11.0 | ||
| Private company | 65.3 | 62.4 | 68.0 | ||
| Official position | 16.1 | 17.8 | 14.5 | ||
| Retired | 1.2 | 0.6 | 1.7 | ||
| DK, NA | 5.5 | 6.4 | 4.7 | ||
| Mother's employment status (%) | 0.195 | ||||
| Non-paid work/no work | 36.8 | 32.5 | 40.7 | ||
| Private company | 45.0 | 48.4 | 41.9 | ||
| Official position | 16.4 | 18.5 | 14.5 | ||
| Retired | 0.9 | 0.0 | 1.7 | ||
| DK, NA | 0.9 | 0.6 | 1.2 | ||
† Data do not follow a normal distribution. BMI: Body Mass Index. DK, NA: do not know, no answer. UNa-24h: 24 h urinary sodium excretion.
a Significant difference according to sex group, as shown by the Student’s t-test for continuous and parametric variables, the Mann–Whitney U test for nonparametric variables (†), and the chi-square test among the groups. Significant differences are bolded.
Use of discretionary salt and other behaviors by the families
Ninety-two percent of the families reported adding salt to their meals while cooking. Forty-three percent of the fathers replied that they never added salt at the table compared with 48% of the mothers (Table 2). More than half of the families reported using iodized salt (53%). A total of 59% of parents never checked the sodium content on food labels. Most of the families (94%) reported never having a salt shaker on the table, and 80% of the children reported never adding salt to food after it is cooked.
Table 2. Frequency of dietary behaviors related to salt intake and their relationship with sodium excretion (mg/day) in 24-hour urine in Spanish schoolchildren.
| Behaviors | UNa-24h (mg/24h) | Salt equivalent (g/day) | ||||||
|---|---|---|---|---|---|---|---|---|
| Questions | Subcategories | n | % | Mean ± SD | Median (IQR) | Mean ± SD | Median (IQR) | p value† |
| In your home, do you add salt to food while cooking? | No | 27 | 8.4 | 3218 ± 1381 | 3082 (2001–4209) | 8.2 ± 3.5 | 7.8 (5.1–10.7) | 0.910 |
| Yes | 295 | 91.6 | 3126 ± 1179 | 3036 (2323–3772) | 7.9 ± 3.0 | 7.7 (5.9–9.6) | ||
| Do you add salt to food when you eat it after it is cooked? (Father) | Never | 129 | 43.0 | 3008 ± 1332 | 2806 (2116–3726) | 7.6 ± 3.4 | 7.1 (5.4–9.5) | 0.232 |
| Only if it is tasteless | 157 | 52.3 | 3166 ± 1021 | 3151 (2461–3772) | 8.0 ± 2.6 | 8.0 (6.3–9.6) | ||
| Always | 14 | 4.7 | 3343 ± 1228 | 3289 (2208–4025) | 8.5 ± 3.1 | 8.4 (5.6–10.2) | ||
| Do you add salt to food when you eat it after it is cooked? (Mother) | Never | 157 | 48.3 | 3064 ± 1313 | 2875 (2208–3772)a | 7.8 ± 3.3 | 7.3 (5.6–9.6)a | 0.048 |
| Only if it is tasteless | 153 | 47.1 | 3152 ± 1096 | 3128 (2392–3772) | 8.0 ± 2.8 | 7.9 (6.1–9.6) | ||
| Always | 15 | 4.6 | 3384 ± 941 | 3174 (2783–3910)b | 8.6 ± 2.4 | 8.1 (7.1–9.9)b | ||
| In your home, do you routinely check food labels for salt content? | Never | 192 | 59.1 | 3069 ± 1124 | 3071 (2289–3738) | 7.8 ± 2.9 | 7.8 (5.8–9.5) | 0.830 |
| Sometimes | 104 | 32.0 | 3141 ± 1224 | 2990 (2254–3876) | 8.0 ± 3.1 | 7.6 (5.7–9.8) | ||
| Always | 29 | 8.9 | 3199 ± 1262 | 3174 (2208–4209) | 8.1 ± 3.2 | 8.1 (5.6–10.7) | ||
| In your home, is the salt shaker on your table for anyone who wants it? | Never | 308 | 94.2 | 3094 ± 1191 | 2990 (2243–3749)a | 7.9 ± 3.0 | 7.6 (5.7–9.5)a | 0.025 |
| Sometimes | 13 | 4.0 | 3761 ± 1084 | 3887 (3197–4140)b | 9.6 ± 2.8 | 9.9 (8.1–10.5)b | ||
| Always | 6 | 1.8 | 3320 ± 1528 | 3370 (1817–4209) | 8.4 ± 3.9 | 8.6 (4.6–10.7) | ||
| Does your child prefer not salty or very salty food? | Not salty | 22 | 6.7 | 3078 ± 915 | 2956 (2691–3381) | 7.8 ± 2.3 | 7.5 (6.8–8.6) | 0.529 |
| Somewhat salty | 269 | 82.3 | 3125 ± 1245 | 3036 (2231–3818) | 7.9 ± 3.2 | 7.7 (5.7–9.7) | ||
| Very salty | 36 | 11.0 | 3134 ± 970 | 3151 (2461–3864) | 8.0 ± 2.5 | 8.0 (6.3–9.8) | ||
| How often does your child add salt to food after it is cooked? | Never, less than once in a month | 264 | 80.2 | 3381 ± 1850 | 3105 (1875–4888) | 8.6 ± 4.7 | 7.9 (4.8–12.4) | 0.901 |
| 1–3 times in a month | 28 | 8.5 | 3332 ± 1780 | 3289 (1426–5244) | 8.5 ± 4.5 | 8.4 (3.6–13.3) | ||
| Once a week | 8 | 2.4 | 2921 ± 763 | 2829 (2208–3726) | 7.4 ± 1.9 | 7.2 (5.6–9.5) | ||
| 2–3 times in a week | 7 | 2.1 | 2865 ± 1011 | 3013 (2047–3887) | 7.3 ± 2.6 | 7.7 (5.2–9.9) | ||
| 4–6 times in a week | 3 | 0.9 | 3134 ± 665 | 3163 (2565–3715) | 8.0 ± 1.7 | 8.0 (6.5–9.4) | ||
| Once a day | 7 | 2.1 | 3370 ± 1008 | 3232 (2783–4014) | 8.6 ± 2.6 | 8.2 (7.1–10.2) | ||
| > Once a day | 4 | 1.2 | 3090 ± 1190 | 2979 (2323–3761) | 7.9 ± 3.0 | 7.6 (5.9–9.6) | ||
| DK, NA | 8 | 2.4 | 3459 ± 1847 | 3381 (1886–4658) | 8.8 ± 4.7 | 8.6 (4.8–11.8) | ||
| In your home, do you use iodized salt or regular salt? | Regular salt | 120 | 42.4 | 3009 ± 1202 | 2829 (2208–3634)a | 7.6 ± 3.1 | 7.2 (5.6–9.2)a | 0.007 |
| Iodized salt and regular salt | 4 | 1.4 | 2266 ± 1288 | 2001 (1403–3128) | 5.8 ± 3.3 | 5.1 (3.6–7.9) | ||
| Iodized salt | 151 | 53.4 | 3277 ± 1156 | 3347 (2438–4002)b | 8.3 ± 2.9 | 8.5 (6.2–10.2)b | ||
| Others (ecological salt) | 8 | 2.8 | 2923 ± 1192 | 2933 (2507–3588) | 7.4 ± 3.0 | 7.4 (6.4–9.1) | ||
DK, NA: do not know, no answer. IQR: interquartile range. UNa: urinary sodium excretion.
†Sodium excretion for each question was compared using the median test to compare medians between groups. Values in the same category suffixed with a or b are significantly different from each other at p < 0.05 when compared by pairwise multiple comparisons. Significant differences are bolded.
When comparing the use of table salt between fathers and mothers, it was observed that both parents tended to have the same answers (S1 Fig): when the mother reported adding salt ‘always when she eats after the food is cooked’, a higher proportion of fathers did so as well (82%). The same pattern occurred when the mother reported only adding salt if the food was tasteless (74% of the fathers answered the same) or never added it (62% of the fathers did the same).
Dietary behaviors and their relationship with UNa-24h in Spanish schoolchildren
Table 2 also describes the differences in 24-hour urine sodium excretion related to behavioral questions about salt. The schoolchildren did not show differences in the urinary sodium for five of the eight questions related to the parents or children. Differences in sodium excretion were observed according to the use of table salt by the mother (p < 0.05), the presence of the salt shaker on the table (p < 0.05), and the type of salt used (p < 0.01). The children of mothers who reported never adding salt to food after it is cooked had lower sodium excretion than those who reported always adding salt. Further, in families in which the salt shaker was sometimes on the table, schoolchildren excreted more sodium than those who never had it present. In addition, in families using iodized salt, schoolchildren excreted more sodium than children in families using regular salt.
In Table 3, the multivariate analysis results show that the use of salt at the table by the father or mother, the presence of the salt shaker on the table, the use of iodized salt and the use of table salt by the children were positively associated with an increased risk of excreting sodium over the median after adjusting for sex, age, and BMI. Further, the use of iodized salt remained significant after taking into account the simultaneous effect of all the variables.
Table 3. Logistic regression models.
Odds ratios and 95% confidence intervals for the presence of sodium excretion greater than 3048 mg/day (50th percentile).
| Predictor Variables | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|
| Questions | Groups | OR (CI 95%) | p value | OR (CI 95%) | p value | OR (CI 95% | p value |
| In your home, do you add salt to the food while cooking? a | Sometimes/Always | 0.974 (0.443–2.143) | 0.947 | 1.246 (0.545–2.847) | 0.602 | 1.039 (0.380–2.846) | 0.940 |
| Do you add salt to food when you eat it after it is cooked? (Father) a | Only if it is tasteless /Always | 1.629 (1.028–2.583) | 0.038 | 1.719 (1.061–2.785) | 0.028 | 1.385 (0.780–2.460) | 0.267 |
| Do you add salt to food when you eat it after it is cooked? (Mother) a | Only if it is tasteless /Always | 1.617 (1.043–2.507) | 0.032 | 1.663 (1.052–2.629) | 0.029 | 1.277 (0.727–2.241) | 0.395 |
| In your home, do you routinely check food labels for salt content? a | Sometimes/Always | 0.925 (0.595–1.44) | 0.731 | 0.876 (0.551–1.392) | 0.575 | 0.969 (0.567–1.657) | 0.909 |
| In your home, is the salt shaker on your table for anyone who wants it? a | Sometimes/Always | 3.849 (1.249–11.859) | 0.019 | 4.730 (1.473–15.188) | 0.009 | .3844 (0.978–15.101) | 0.054 |
| Does your child prefer not salty or very salty food? b | Somewhat salty/Very salty | 1.273 (0.534–3.035) | 0.586 | 1.445 (0.587–3.557) | 0.423 | 1.651 (0.523–5.212) | 0.393 |
| How often does your child add salt to the food after it is cooked? a | Sometimes/Always | 1.794 (0.995–3.236) | 0.052 | 1.963 (1.060–3.635) | 0.032 | 1.485 (0.728–3.027) | 0.277 |
| In your home, do you use iodized salt or regular salt? c | Iodized salt and regular salt | 0.425 (0.043–4.184) | 0.463 | 0.638 (0.063–6.445) | 0.703 | -d | -d |
| Iodized salt | 1.901 (1.203–3.005) | 0.006 | 1.919 (1.187–3.103) | 0.008 | 1.710 (1.007–2.904) | 0.047 | |
| Others (ecological salt) | 0.849 (0.23–3.142) | 0.807 | 0.873 (0.23–3.316) | 0.841 | 1.235 (0.274–5.571) | 0.783 | |
Dependent variable: UNa-24h ≥ 50th percentile.
aThe reference is ‘never’.
bThe reference is ‘not salty food’.
cThe reference is ‘regular salt’.
dData was removed because it was statistically unreliable.
Model 1: not adjusted. Model 2: adjusted for age, BMI, and sex. Model 3: model 2 plus the rest of the predictor variables (In your home, do you add salt to the food while cooking? Do you add salt to food when you eat it after it is cooked? (father, mother); In your home, do you routinely check food labels for salt content? Does your child prefer not salty or very salty food? How often does your child add salt to the food after it is cooked? In your home, do you use iodized salt or regular salt?). Significant differences are bolded.
Behaviors and preferences of children and their relationship with the behaviors of parents
The association between the salt use behaviors of the family and the use of table salt by the children is shown in Table 4. The use of table salt by father or mother was positively associated with a higher risk of the use of table salt ‘sometimes or always’ by the children after adjusting for sex, age and BMI (p < 0.01). As opposed to never having the salt shaker on the table, its presence on some occasions or all the time was also positively associated with an increased risk of the use of table salt by the children after adjusting for sex, age, and BMI (p < 0.001). After adjusting for all factors simultaneously, only the use of table salt by the father remained significant (p < 0.05).
Table 4. Logistic regression models.
Odds ratios and 95% confidence intervals for the child's use of table salt sometimes/always versus never.
| Predictor Variables | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|
| Questions | Groups | OR (CI 95%) | p value | OR (CI 95%) | p value | OR (CI 95%) | p value |
| In your home, do you add salt to the food while cooking? a | Sometimes/Always | 1.595 (0.459–5.541) | 0.462 | 0.667 (0.190–2.340) | 0.527 | 1.329 (0.255–6.910) | 0.736 |
| Do you add salt to food when you eat it after it is cooked? (Father) a | Only if it is tasteless/Always | 5.396 (2.441–11.930) | 0.000 | 5.231 (2.359–11.603) | 0.000 | 3.813 (1.553–9.361) | 0.003 |
| Do you add salt to food when you eat it after it is cooked? (Mother) a | Only if it is tasteless/Always | 1.635 (3.094–5.853) | 0.001 | 3.231 (1.697–6.151) | 0.000 | 2.076 (0.977–4.410) | 0.057 |
| In your home, do you routinely check food labels for salt content? a | Sometimes/Always | 1.206 (0.674–2.158) | 0.528 | 1.159 (0.643–2.089) | 0.623 | 1.147 (0.576–2.283) | 0.697 |
| In your home, is the salt shaker on your table for anyone who wants it? a | Sometimes/Always | 5.292 (1.998–14.016) | 0.001 | 5.604 (2.081–15.092) | 0.001 | 2.133 (0.662–6.877) | 0.205 |
| Does your child prefer not salty or very salty food?b | Somewhat salty/Very salty | 2.133 (0.483–9.426) | 0.318 | 2.037 (0.458–9.052) | 0.350 | 2.027 (0.237–17.353) | 0.519 |
| In your home, do you use iodized salt or regular salt?c | Iodized salt and regular salt | 2.105 (0.208–21.301) | 0.528 | 1.846 (0.178–19.130) | 0.607 | 9.192 (0.664–127.299) | 0.098 |
| Iodized salt | 1.641 (0.885–3.042) | 0.116 | 1.763 (0.940–3.307) | 0.077 | 1.409 (0.703–2.823) | 0.334 | |
| Others (ecological salt) | 0.702 (0.084–5.858) | 0.774 | 0.648 (0.077–4.472) | 0.690 | 0.634 (0.068–5.866) | 0.688 | |
Dependent variable: the use of table salt sometimes/always.
aThe reference is ‘never’.
bThe reference is ‘not salty food’.
cThe reference is ‘regular salt’.
dData was removed because it was statistically unreliable.
Model 1: not adjusted. Model 2: adjusted for age, BMI, and sex. Model 3: Model 2 plus the rest of the predictor variables (In your home, do you add salt to the food while cooking? Do you add salt to food when you eat it after it is cooked? (Father, mother); In your home, do you routinely check food labels for salt content? Does your child prefer not salty or very salty food? In your home, do you use iodized salt or regular salt?). Significant differences are bolded.
Table 5 shows which behaviors are related to children's preference for somewhat salty or very salty foods over non-salty food. The father's use of table salt was associated with a higher risk of preferring somewhat salty or very salty foods by the schoolchildren (p < 0.05), the use of table salt by the children was associated with a higher risk of preferring salty food (p < 0.05), while checking the sodium content on food labels was associated with a lower risk of having a preference for salty foods (p < 0.05) after adjusting for age, sex, and BMI. After adjusting for all factors simultaneously, no one remained significant.
Table 5. Multinomial logistic regression models.
Odds ratios and 95% confidence intervals for children's preference for somewhat salty/very salty foods.
| Model 1 | Model 2 | Model 3 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Predictor Variables | Medium salt content | Salty food | Medium salt content | Salty food | Medium salt content | Salty food | |||||||
| Questions | Groups | OR (CI 95%) | p value | OR (CI 95%) | p value | OR (CI 95%) | p value | OR (CI 95%) | p value | OR (CI 95%) | p value | OR (CI 95%) | p value |
| In your home, do you add salt to the food while cooking?a | Sometimes/Always | 3.034 (0.928–9.923) | 0.066 | 2.588 (0.519–12.913) | 0.246 | 2.772 (0.833–9.219) | 0.096 | 2.124 (0.408–11.053) | 0.371 | 3.818 (0.981–14.863) | 0.053 | 3.207 (0.390–26.359) | 0.278 |
| Do you add salt to food when you eat it after it is cooked? (Father)a | Only if it is tasteless/ Always | 2.777 (1.083–7.122) | 0.034 | 3.818 (1.192–12.231) | 0.024 | 2.697 (1.044–6.969) | 0.041 | 3.330 (1.021–10.862) | 0.046 | 2.341 (0.669–8.200) | 0.183 | 1.639 (0.343–7.826) | 0.536 |
| Do you add salt to food when you eat it after it is cooked? (Mother)a | Only if it is tasteless/ Always | 2.205 (0.862–5.636) | 0.099 | 2.8 (0.910–8.611) | 0.072 | 2.234 (0.871–5.729) | 0.094 | 3.054 (0.978–9.534) | 0.055 | 1.129 (0.330–3.859) | 0.847 | 1.141 (0.248–5.247) | 0.865 |
| In your home, do you routinely check food labels for salt content?a | Sometimes/Always | 0.473 (0.195–1.146) | 0.097 | 0.305 (0.101–0.921) | 0.035 | 0.453 (0.185–1.109) | 0.083 | 0.280 (0.090–0.866) | 0.027 | 0.665 (0.222–1.994) | 0.466 | 0.293 (0.072–1.199) | 0.088 |
| In your home, is the salt shaker on your table for anyone who wants it?a | Sometimes/Always | 1.157 (0.145–9.237) | 0.89 | 2.625 (0.274–25.140) | 0.402 | 1.164 (0.145–9.354) | 0.886 | 2.710 (0.274–26.795) | 0.394 | - c | - c | - c | - c |
| How often your children add salt to the food after it is cooked?a | Sometimes/Always | 1.667 (0.374–7.434) | 0.503 | 8.382 (1.669–42.103) | 0.010 | 1.615 (0.361–7.232) | 0.531 | 7.753 (1.523–39.473) | 0.014 | 1.306 (0.153–11.152) | 0.807 | 8.029 (0.824–78.283) | 0.073 |
| In your home, do you use iodized salt or regular salt?b | Iodized salt | 2.012 (0.767–5.281) | 0.155 | 2.449 (0.771–7.778) | 0.129 | 2.123 (0.798–5.646) | 0.131 | 3.112 (0.949–10.197) | 0.061 | 2.577 (0.802–8.277) | 0.112 | 3.169 (0.760–13.218) | 0.113 |
Dependent variable: children's preference for somewhat salty/very salty foods.
aThe reference is ‘never’.
bThe reference is ‘regular salt’.
cData was removed because it was statistically unreliable.
Model 1: not adjusted. Model 2: adjusted for age, BMI, and sex. Model 3: Model 2 plus the rest of the predictor variables (In your home, do you add salt to the food while cooking? Do you add salt to food when you eat it after it is cooked? (Father, mother); In your home, do you routinely check food labels for salt content? Does your child prefer not salty or very salty food? How often does your child add salt to the food after it is cooked? In your home, do you use iodized salt or regular salt?). Significant differences are bolded.
Discussion
This study provides a novel understanding of the salt-specific behaviors of Spanish families and their association with the current levels of sodium excretion in the children, studying the parental reported behaviors compared to sodium excretion of their own children. On average, schoolchildren consumed 7.9 g/day of salt (only 15.2% of children had a salt intake below 5 g/day). This amount is similar for Portuguese schoolchildren [27], but in children from Italy [28], Germany [29], or the United Kingdom [30], the intake is lower. These differences may be due to different age ranges in the studies or differences in the quality control to exclude urine collections (for example, Cotter et al. [27] used a volume < 400 mL urine in 24 h). Besides, it could be affecting the day of the collection since, in other populations, salt intake was higher on the weekend vs. weekdays [31,32]. There was a relationship between the urinary sodium excretion in children and the use of table salt by parents or children, the use of iodized salt by the families, and the presence of the salt shaker on the table. With regard to the use of table salt, schoolchildren with families who sometimes or always had the salt shaker on the table or with fathers or mothers adding salt at the table were more likely to use table salt. Children preferred salty foods when they added salt to their food or when their father sometimes or always added table salt, and they preferred non-salty foods when the parents reported checking labels. We looked into behaviors that can be modified by educational interventions aimed at parents and children in order to change the levels of excessive salt intake in the family and childhood environment.
The proportion of children who use table salt in our study (17%) and parents who reported placing a salt shaker on the table at mealtimes (6%) was lower than in Australian children and parents (32% and 45% respectively) [33]. Meanwhile, slightly more than 50% of Spanish fathers and mothers use table salt (52–57%), a similar proportion to the 54% of the Australian parents. Differently, 92% of Spanish parents reported adding salt during cooking, a result higher than 67% of Australian parents, 65%-71% of Belgian adults or 77%-85% of Norwich adults, and it was more similar to that found it in Slovaks adults (91% 98%) [33,34]. The differences between Spanish parents and the adults of other European countries could be due to different samples (mean age in Spanish parents: 42 ± 5 years; mean age in the adults studied in the De Keyzer et al. study: 53–55 years) [34].
Regarding the type of salt used, 53% of the families consumed iodized salt, followed by those who consumed regular salt (42%). Our result is in line with the results found it in other studies, in which it was observed that 44% and 69% of the Spanish adult and child population, respectively, consumed iodized salt [35,36]. In addition, in our study, families who reported using iodized salt had children with higher sodium intake and risk of sodium excretion above the median than families who used regular salt. In contrast to these results, in the study of Nazeri et al. [37], the behavior of mothers regarding the use of iodized salt was not associated with the ingestion of salt by their relatives. WHO stated that ‘Ministries of health should make sure that the message to consume iodized salt does not promote excessive salt consumption’[38]. One possible cause of this relationship is that iodized salt is potentially considered less dangerous or healthier than regular salt because its iodine content has been promoted as a benefit [39], perhaps leading people to use it more indiscriminately. It is known that iodized salt is a vehicle for introducing iodine, which is in line with iodine intake control policies that aim to ensure adequate intake of this essential mineral—this could be useful in this population [40–42]. As Vila et al. said, there are no national initiatives in Spain regarding the consumption of iodized salt at the moment, so it would be advisable to implement them [42]. In the present study, the use of iodized salt was associated with higher sodium intake. It would be advisable to establish policies about iodine while monitoring the population’s sodium intake [39,42] to provide guidelines to limit the use of discretionary salt, promote the use of iodized salt, and encourage people to choose foods that have less hidden salt.
Regarding other behaviors, the use of salt while cooking at home was not related to the sodium excretion of the children. These results differ from those found in a study on Australian children [43], who found an inverse relationship between the addition of cooking salt by parents and the sodium excretion of their children. As mentioned by Service et al. [43], other food choices (i.e., processed food vs. unprocessed food, the frequency of parents cooking at home vs. eating out, or the seasoning of meals) could explain the lack of positive association. However, the odds for children having a sodium excretion above the median (3,048 mg/d) were higher for parents who reported adding table salt to food ‘always or only if it is tasteless’, for children who used table salt or when the salt shaker was accessible on the table. Sodium intake by schoolchildren has previously been associated with parental sodium intake, probably because of closeness in dietary habits or behaviors [27,43].
In this study, roughly 40% of the parents reported checking the sodium content on food labels (Table 2). However, this behavior was not associated with the excretion of less sodium of their children. In adults, it has been observed that consulting the salt content on the label is related to lower sodium intake [44,45]. However, no such association has been found in other studies [46,47]. In this study the lack of association could be because parents were not purchasing low-salt foods, or parents may only look at the salt content of foods that taste salty but not at other foods that contain salt but do not taste salty (such as breakfast cereals); alternatively, parents may not understand the label and maybe they don’t know how to read sodium information on food labels, or just there are other factors not picked up that are affecting such as a high frequency of eating out. Nevertheless, encouraging people to look at the label or making it easier for them to do so is essential [48] and it is important to establish early dietary practices related to salt intake. A recent report from the National Academies of Sciences, Engineering, and Medicine recommends reducing intakes of sodium in children if above 1,500 mg/d (children aged 4–8 years) or 1,800 mg/d (children aged 9–13 years) to reduce chronic disease risk [49].
Also, children’s salt taste preference was not associated with children’s 24 h urinary sodium excretion. Other studies have found that children who liked salty foods consumed more salt. For example, in a study by Matsuzuki et al. [50], children who preferred salty foods, as observed by their mothers, consumed more salt. Similarly, in a study by Mennella et al. [14], it was observed that the children’s preference for salty foods and the reported sodium intake were related. However, in our study, we found no association between the preference for salty foods and sodium excretion. One possible explanation is that these children may not eat salty foods frequently, even though they prefer them. Additionally, it should be borne in mind that part of the dietary sodium found in foods is not considered to have a salty taste, for example, saltiness in breakfast cereals is not usually obvious to the taste [51,52] and parents could be unaware of the amount of sodium in these foods. Moreover, the saltiness of food affects the preference we have for food, depending on the type of food [53], and children may like some salty foods but not all of them.
About the relationship of behaviors between parents and their child, the use of table salt by father or mother was associated with table salt use by children. Both the father and mother can potentially influence salt intake in schoolchildren because of their involvement in early feeding [15]. Moreover, the behaviors of one parent were related to those of the other parent (S1 Fig), and this could be associated with the adaptation of family members to their family environment. In addition, children who sometimes or always had a salt shaker nearby on the table were more likely to use table salt sometimes or always. The use of table salt by the parents and the presence of a salt shaker may be associated with an adaptation by children to prefer saltier foods and to use table salt.
Regarding the preference for salty foods, we found a relationship between the use of table salt by the father and the use of table salt by the children with children's preference for salty foods. Different factors could explain this relationship, maybe children living in a family environment in which table salt is used by the father could have more exposure to table salt and salty foods and therefore prefer them to non-salty foods since liking a salty taste is determined by exposure to salty foods [51,54]. Also, this preference for salty foods could be due to the presence of genetic components of taste or with the imitation of parents’ food choices [55]. On the other hand, parental label viewing of sodium content ties into children’s preference for low-salt foods. One explanation could be that parents who read the sodium value on the label may have more nutritional knowledge, because nutritional knowledge has been positively related to labeling reading and inversely related to the use of discretionary salt in adults [56–58].
It is known that there is an excessive intake of sodium in Spanish adults and children [7,10]. The primary sources of dietary sodium (excluding salt added at the table or during cooking) in the Spanish population are meat, meat products, cereals, and cereal products [6,7,10]. Further, most dietary sodium comes from discretionary foods (foods and beverages that are high in saturated fats, sugars, salt, and/or alcohol and nutrient-poor) [18]. Besides that, in Spanish adults, discretionary salt intake contributes to the total sodium intake with 21.0 ± 10.3% (1.5 ± 0.8 g/day of salt) [59]. The most likely explanation for the high salt intake in Spain could be barriers to behavioral change. The adverse nature of the food environment, which includes food and beverage advertising [60], high salt content in processed foods [18], and labeling that is challenging to understand [48], could impede the reduction of salt intake. Among the strategies to reduce sodium consumption in the population, agreements have been established with the industry to reduce the salt content of some foods [61]. However, effective public communication is also required to help populations reduce their sodium intake levels [62]. As indicated by the WHO in the guide SHAKE designed to help to implement strategies to reduce salt consumption in populations [63], it is important to implement integrated education and communication messages with other public strategies to raise public awareness of health risks and sources of salt in the diet to change behaviors. For parents, strategies could be implemented to improve their salt-related behaviors, including label checking, the use of iodized salt instead of regular salt/non-fortified salt [64], the promotion of a decrease in the use of table salt, and the improvement in the choice of foods with lower salt content. Future studies have an opportunity to determine the attitudes and parents' knowledge or motivation to change salt behaviors and then launch salt campaigns that aim to change salt-related behaviors in schoolchildren and parents. Additional information that is required includes whether parents and children know which foods have hidden salt, whether the salt content of the food has actually been reduced, and whether the population will accept foods with lower salt content.
This study has some limitations. First, because it is a cross-sectional study, cause–effect relationships cannot be established. Furthermore, the study sample is not a representative sample of the Spanish population because not all regions of Spain are represented. If we want to generalize our findings, we should do so with caution. However, it should be noted that a large sample was used, and it comprised subjects with a diverse socioeconomic status and was representative of several regions, including both rural/semi-urban and urban areas, in Spain. In addition, the study results may be subject to selection bias; from the total number of participants invited to participate in the study, only a small number finally entered. On the other hand, a 24-hour urine collection per participant is subject to high intra-individual and inter-individual variability [65]. However, single 24-hour urine collection per individual can be used to characterize differences in group mean intake and is not subject to systematic error when it is completed. In addition, 24-hour urine creatinine excretion was used as a marker to ensure sample completeness and quality [66]. The questionnaire that was used to determine the behaviors of parents and children was not a validated questionnaire because there is no one available for this population. However, some of the questions have been used in other studies [33,43,45,67–70], allowing their comparison with those in previous research. On the other hand, it is essential to bear in mind that the responses of the participants may be biased toward responses that they considered to be favorable. Among the strengths of our study is (1) the constancy of the significance in most of the factors in the unadjusted and UNa-24h-adjusted models, indicating that the findings are quite solid; (2) the use of an objective measure of sodium intake; and (3) the large sample size.
Conclusions
The current levels of sodium intake are high in Spanish schoolchildren, and they have been associated with some parents’ salt-related behaviors. The use of iodized salt and table salt by the parents and children and the presence of a salt shaker on the table were associated with children’s sodium intake. It is important to make parents aware of the relationship between their behaviors regarding the use of discretionary salt and their children's sodium intake. Additionally, the low proportion of parents who looked at the sodium content on the label and the lack of a relationship between this practice and children’s sodium intake highlight a need for greater awareness and practical guidance to interpreting food labels. Educational messages, including reading food labels to select foods reduced in sodium, replace cooking salt using herbs or spices to flavor food, or avoid adding extra table salt will be useful to promote behavioral skills in parents and children related to salt intake.
Supporting information
(PDF)
Spanish and English versions.
(PDF)
Acknowledgments
The authors wish to thank the children, parents, and schools for their kind collaboration.
Data Availability
Due to ethical restrictions and participant confidentiality, individual data cannot be made publicly available. Data from this study are available upon request from the Complutense University of Madrid (UCM), for researchers who meet the criteria for access to confidential data. Data requests can be sent to UCM through the Principal investigator of the study (Ana M. López-Sobaler, asobaler@ucm.es) or non-author the Vice-dean of Research from the Pharmacy Faculty (Pilar Gómez-Serranillos, vdipfarm@ucm.es).
Funding Statement
This work was supported by Santander-UCM project (PR6/13-18866); "Creation and Consolidation Program Study Group at the Complutense University of Madrid, Madrid” (REF: GR3/14, GR58/08 and GR15/17) and the Complutense University Research Group VALORNUT-920030 thorough FEI16/127. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Wilkins E, Wilson L, Wickramasinghe K, Bhatnagar P, Leal J, Luengo-Fernandez R, et al. European Cardiovascular Disease Statistics 2017 edition [Internet]. Løgstrup S, European Heart Network, editors. Brussels, Belgium: European Heart Network; 2017. Available: https://www.bhf.org.uk/informationsupport/publications/statistics/european-cardiovascular-disease-statistics-2017 [Google Scholar]
- 2.World Health Organization. Sodium intake for adults and children [Internet]. Geneva, Switzerland; 2012. Available: http://www.who.int/nutrition/publications/guidelines/sodium_intake/en/
- 3.Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: A systematic review and meta-regression analysis. Circulation. 2008;117: 3171–3180. 10.1161/CIRCULATIONAHA.107.730366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Juhola J, Magnussen CG, Viikari JSA, Kähönen M, Hutri-Kähönen N, Jula A, et al. Tracking of Serum Lipid Levels, Blood Pressure, and Body Mass Index from Childhood to Adulthood: The Cardiovascular Risk in Young Finns Study. J Pediatr. 2011;159: 584–590. 10.1016/j.jpeds.2011.03.021 [DOI] [PubMed] [Google Scholar]
- 5.Funtikova AN, Navarro E, Bawaked RA, Fíto M, Schröder H. Impact of diet on cardiometabolic health in children and adolescents. Nutr J. 2015;14: 118 10.1186/s12937-015-0107-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Partearroyo T, Samaniego-Vaesken M de L, Ruiz E, Aranceta-Bartrina J, Gil Á, González-Gross M, et al. Sodium Intake from Foods Exceeds Recommended Limits in the Spanish Population: The ANIBES Study. Nutrients. 2019;11: 2451 10.3390/nu11102451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ortega RM, López-Sobaler AM, Ballesteros JM, Pérez-Farinós N, Rodríguez-Rodríguez E, Aparicio A, et al. Estimation of salt intake by 24h urinary sodium excretion in a representative sample of Spanish adults. Br J Nutr. 2011;105: 787–794. 10.1017/S000711451000423X [DOI] [PubMed] [Google Scholar]
- 8.López-Sobaler AM, Aparicio A, González-Rodríguez LG, Cuadrado-Soto E, Rubio J, Marcos V, et al. Adequacy of Usual Vitamin and Mineral Intake in Spanish Children and Adolescents: ENALIA Study. Nutrients. Switzerland; 2017;9: 131 10.3390/nu9020131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization. Reducing salt intake in populations: report of a WHO forum and technical meeting [Internet]. Paris, France; 2006. 10.1017/CBO9781107415324.004 [DOI]
- 10.Aparicio A, Rodríguez-Rodríguez E, Cuadrado-Soto E, Navia B, López-Sobaler AM, Ortega RM. Estimation of salt intake assessed by urinary excretion of sodium over 24 h in Spanish subjects aged 7–11 years [Internet]. European Journal of Nutrition. 2017. February 10.1007/s00394-015-1067-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Strategies to monitor and evaluate population sodium consumption and sources of sodium in the diet Report of a joint technical meeting convened by WHO and the Government of Canada [Internet]. Geneva, Switzerland: World Health Organization; 2011. Available: https://apps.who.int/iris/bitstream/handle/10665/44614/9789241501699_eng.pdf?sequence=1 [Google Scholar]
- 12.Lioret S, McNaughton SA, Spence AC, Crawford D, Campbell KJ. Tracking of dietary intakes in early childhood: The Melbourne InFANT Program. Eur J Clin Nutr. 2013;67: 275–281. 10.1038/ejcn.2012.218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Movassagh E, Baxter-Jones A, Kontulainen S, Whiting S, Vatanparast H. Tracking Dietary Patterns over 20 Years from Childhood through Adolescence into Young Adulthood: The Saskatchewan Pediatric Bone Mineral Accrual Study. Nutrients. 2017;9: 990 10.3390/nu9090990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mennella JA, Finkbeiner S, Lipchock S V, Hwang L-D, Reed DR. Preferences for salty and sweet tastes are elevated and related to each other during childhood. PLoS One. 2014;9: e92201 10.1371/journal.pone.0092201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scaglioni S, De Cosmi V, Ciappolino V, Parazzini F, Brambilla P, Agostoni C. Factors Influencing Children’s Eating Behaviours. Nutrients. Multidisciplinary Digital Publishing Institute (MDPI); 2018;10: 706 10.3390/nu10060706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Appel LJ, Lichtenstein AH, Callahan EA, Sinaiko A, Van Horn L, Whitsel L. Reducing Sodium Intake in Children: A Public Health Investment. J Clin Hypertens. 2015;17: 657–62. 10.1111/jch.12615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khokhar D, Nowson C, Margerison C, Bolam B, Grimes C. Comparison of salt-related knowledge, attitudes and behaviours between parents and caregivers of children under 18 years of age and other adults who do not care for children under 18 years of age in Victoria, Australia. BMJ Nutr Prev Heal. 2019;0: bmjnph-2018-000018. 10.1136/bmjnph-2018-000018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cuadrado-Soto E, Peral-Suarez Á, Aparicio A, Perea JM, Ortega RM, López-Sobaler AM. Sources of Dietary Sodium in Food and Beverages Consumed by Spanish Schoolchildren between 7 and 11 Years Old by the Degree of Processing and the Nutritional Profile. Nutrients. 2018;10: 1880 10.3390/nu10121880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perales-García A, Ortega RM, Urrialde R, López-Sobaler AM. Physical activity and sedentary behavior impacts on dietary water intake and hydration status in Spanish schoolchildren: A cross-sectional study. PLoS One. 2018;13: e0208748 10.1371/journal.pone.0208748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Microsoft. Microsoft Excel. Redmond, Washington: Microsoft; 2013.
- 21.Neubert A, Remer T. The impact of dietary protein intake on urinary creatinine excretion in a healthy pediatric population. J Pediatr. 1998;133: 655–9. Available: http://www.ncbi.nlm.nih.gov/pubmed/9821424 10.1016/s0022-3476(98)70107-6 [DOI] [PubMed] [Google Scholar]
- 22.Ng RH, Altaffer M, Ito R, Statland BE. The Technicon RA-1000 evaluated for measuring sodium, potassium, chloride, and carbon dioxide. Clin Chem. 1985;31 Available: http://clinchem.aaccjnls.org/content/31/3/435.short [PubMed] [Google Scholar]
- 23.Kroll MH, Chesler R, Hagengruber C, Blank DW, Kestner J, Rawe M. Automated determination of urinary creatinine without sample dilution: theory and practice. Clin Chem. 1986;32 Available: http://clinchem.aaccjnls.org/content/32/3/446.short [PubMed] [Google Scholar]
- 24.Remer T, Neubert A, Maser-Gluth C. Anthropometry-based reference values for 24-h urinary creatinine excretion during growth and their use in endocrine and nutritional research. Am J Clin Nutr. 2002;75: 561–9. Available: http://www.ncbi.nlm.nih.gov/pubmed/11864864 10.1093/ajcn/75.3.561 [DOI] [PubMed] [Google Scholar]
- 25.Grimes CA, Baxter JR, Campbell KJ, Riddell LJ, Rigo M, Liem DG, et al. Cross-Sectional Study of 24-Hour Urinary Electrolyte Excretion and Associated Health Outcomes in a Convenience Sample of Australian Primary Schoolchildren: The Salt and Other Nutrients in Children (SONIC) Study Protocol. JMIR Res Protoc. 2015;4: e7 10.2196/resprot.3994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris JE, Boushey C, Bruemmer B, Archer SL. Publishing Nutrition Research: A Review of Nonparametric Methods, Part 3. J Am Diet Assoc. 2008;108: 1488–1496. 10.1016/j.jada.2008.06.426 [DOI] [PubMed] [Google Scholar]
- 27.Cotter J, Cotter MJ, Oliveira P, Cunha P, Torres E, Polonia J. Comparison of Salt Intake in Children to that of their Parents. Nephron. 2019;142: 284–290. 10.1159/000499344 [DOI] [PubMed] [Google Scholar]
- 28.Campanozzi A, Avallone S, Barbato A, Iacone R, Russo O, De Filippo G, et al. High sodium and low potassium intake among Italian children: relationship with age, body mass and blood pressure. Gong Y, editor. PLoS One. Editrice Gastroenterologica Italiana; 2015;10: e0121183 10.1371/journal.pone.0121183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alexy U, Cheng G, Libuda L, Hilbig A, Kersting M. 24h-Sodium excretion and hydration status in children and adolescents—Results of the DONALD Study. Clin Nutr. Elsevier Ltd; 2012;31: 78–84. 10.1016/j.clnu.2011.08.014 [DOI] [PubMed] [Google Scholar]
- 30.Marrero NM, He FJ, Whincup P, MacGregor GA. Salt intake of children and adolescents in South London consumption levels and dietary sources. Hypertension. 2014;63: 1026–1032. 10.1161/HYPERTENSIONAHA.113.02264 [DOI] [PubMed] [Google Scholar]
- 31.Grimes CA, Riddell LJ, Campbell KJ, Beckford K, Baxter JR, He FJ, et al. Dietary intake and sources of sodium and potassium among Australian schoolchildren: results from the cross-sectional Salt and Other Nutrients in Children (SONIC) study. BMJ Open. British Medical Journal Publishing Group; 2017;7: e016639 10.1136/bmjopen-2017-016639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nowson C, Lim K, Land MA, Webster J, Shaw JE, Chalmers J, et al. Salt intake and dietary sources of salt on weekdays and weekend days in Australian adults. Public Health Nutr. 2018; 10.1017/S1368980017004104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khokhar D, Nowson C, Margerison C, Bolam B, Grimes C. Knowledge and Attitudes Are Related to Selected Salt-Specific Behaviours among Australian Parents. Nutrients. 2018;10: 720 10.3390/nu10060720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Keyzer W, Dofková M, Lillegaard ITL, De Maeyer M, Andersen LF, Ruprich J, et al. Reporting accuracy of population dietary sodium intake using duplicate 24 h dietary recalls and a salt questionnaire. Br J Nutr. Cambridge University Press; 2015;113: 488–497. 10.1017/S0007114514003791 [DOI] [PubMed] [Google Scholar]
- 35.Soriguer F, García-Fuentes E, Gutierrez-Repiso C, Rojo-Martínez G, Velasco I, Goday A, et al. Iodine intake in the adult population. Di@bet.es study. Clin Nutr. 2012; 10.1016/j.clnu.2012.04.004 [DOI] [PubMed] [Google Scholar]
- 36.Vila L, Donnay S, Arena J, Arrizabalaga JJ, Pineda J, Garcia-Fuentes E, et al. Iodine status and thyroid function among Spanish schoolchildren aged 6–7 years: the Tirokid study. Br J Nutr. 2016;115: 1623–1631. 10.1017/S0007114516000660 [DOI] [PubMed] [Google Scholar]
- 37.Nazeri P, Mirmiran P, Asghari G, Shiva N, Mehrabi Y, Azizi F. Mothers’ behaviour suppoutes to suboptimal iodine status of family members: Findings from an iodine-sufficient area. Public Health Nutr. Cambridge University Press; 2015;18: 686–694. 10.1017/S1368980014000743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.World Health Organization. Salt as a vehicle for fortification [Internet]. Luxe: World Health Organization; 2007. Available: https://www.who.int/dietphysicalactivity/LUXsaltreport2008.pdf
- 39.García-Ascaso MT, Ares-Segura S, Ros-Pérez P. Is iodine nutrition in the Spanish pediatric population adequate? Historical review and current situation. Endocrinol Diabetes y Nutr. SEEN y SED; 2018;65: 458–467. 10.1016/j.endinu.2018.05.011 [DOI] [PubMed] [Google Scholar]
- 40.García Ascaso MT, Pérez PR, Alcol EC, López AL, de Lucas Collantes C, Santos IM, et al. Nutritional status of iodine in children: When appropriateness relies on milk consumption and not adequate coverage of iodized salt in households. Clin Nutr ESPEN. 2019;30: 52–58. 10.1016/j.clnesp.2019.02.007 [DOI] [PubMed] [Google Scholar]
- 41.Arena Ansótegui J, Ares Segura S. Iodine deficiency in Spain: A circumstantially significant ingestion but with no clear public health strategy that ensures sustainability. An Pediatr. Elsevier; 2010;72: 297–301. 10.1016/j.anpedi.2010.02.003 [DOI] [PubMed] [Google Scholar]
- 42.Vila L, Lucas A, Donnay S, de la Vieja A, Wengrovicz S, Santiago P, et al. The nutrition of iodine in Spain. Needs for the future. Endocrinol Diabetes y Nutr. Elsevier Doyma; 2019; 10.1016/j.endinu.2019.02.009 [DOI] [PubMed] [Google Scholar]
- 43.Service C, Grimes C, Riddell L, He F, Campbell K, Nowson C. Association between Parent and Child Dietary Sodium and Potassium Intakes as Assessed by 24-h Urinary Excretion. Nutrients. 2016;8: 191 10.3390/nu8040191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ollberding NJ, Wolf RL, Contento I. Food Label Use and Its Relation to Dietary Intake among US Adults. J Am Diet Assoc. 2010;110: 1233–1237. 10.1016/j.jada.2010.05.007 [DOI] [PubMed] [Google Scholar]
- 45.Uechi K, Asakura K, Sasaki Y, Masayasu S, Sasaki S. Simple questions in salt intake behavior assessment: Comparison with urinary sodium excretion in Japanese adults. Asia Pac J Clin Nutr. 2017;26: 769–780. 10.6133/apjcn.092016.05 [DOI] [PubMed] [Google Scholar]
- 46.Sugimoto M, Asakura K, Masayasu S, Sasaki S. Relationship of nutrition knowledge and self-reported dietary behaviors with urinary excretion of sodium and potassium: comparison between dietitians and nondietitians. Nutr Res. Elsevier B.V.; 2016;36: 440–451. 10.1016/j.nutres.2015.12.012 [DOI] [PubMed] [Google Scholar]
- 47.Kim M-G, Oh S-W, Han N-R, Song D-J, Um J-Y, Bae S-H, et al. Association between Nutrition Label Reading and Nutrient Intake in Korean Adults: Korea National Health and Nutritional Examination Survey, 2007–2009 (KNHANES IV). Korean J Fam Med. 2014;35: 190 10.4082/kjfm.2014.35.4.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanz-Valero J, Sebastián-Ponce M, Wanden-Berghe C. Interventions to reduce salt consumption through labeling. Rev Panam Salud Publica. 2012;31: 332–337. 10.1590/s1020-49892012000400010 [DOI] [PubMed] [Google Scholar]
- 49.National Academies of Sciences, Engineering and M 2019. Dietary Reference Intakes for Sodium and Potassium [Internet]. Washington D.C.: The National Academies Press; 2019. 10.17226/25353 [DOI] [PubMed] [Google Scholar]
- 50.Matsuzuki H, Muto T, Haruyama Y. School Children’s Salt Intake Is Correlated with Salty Taste Preference Assessed by Their Mothers. Tohoku J Exp Med. 2008;215: 71–77. 10.1620/tjem.215.71 [DOI] [PubMed] [Google Scholar]
- 51.Liem DG. Infants’ and Children’s Salt Taste Perception and Liking: A Review. Nutrients. 2017;9: 1011 10.3390/nu9091011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Man CMD. Technological functions of salt in food products [Internet]. Reducing Salt in Foods: Practical Strategies. Woodhead Publishing Limited; 2007. 10.1533/9781845693046.2.157 [DOI] [Google Scholar]
- 53.Bouhlal S, Chabanet C, Issanchou S, Nicklaus S. Salt Content Impacts Food Preferences and Intake among Children. PLoS One. Public Library of Science; 2013;8: e53971 10.1371/journal.pone.0053971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stein LJ, Cowart BJ, Beauchamp GK. The development of salty taste acceptance is related to dietary experience in human infants: A prospective study. Am J Clin Nutr. 2012;95: 123–129. 10.3945/ajcn.111.014282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Adamo KB, Brett KE. Parental perceptions and childhood dietary quality. Matern Child Health J. Kluwer Academic/Plenum Press New York; 2014;18: 978–995. 10.1007/s10995-013-1326-6 [DOI] [PubMed] [Google Scholar]
- 56.Sarmugam R, Worsley A, Wang W. An examination of the mediating role of salt knowledge and beliefs on the relationship between socio-demographic factors and discretionary salt use: A cross-sectional study. Int J Behav Nutr Phys Act. BioMed Central; 2013;10: 25 10.1186/1479-5868-10-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barreiro-Hurlé J, Gracia A, De-Magistris T. Does nutrition information on food products lead to healthier food choices? Food Policy. 2010;35: 221–229. 10.1016/j.foodpol.2009.12.006 [DOI] [Google Scholar]
- 58.Carrillo E, Varela P, Fiszman S. Influence of nutritional knowledge on the use and interpretation of Spanish nutritional food labels. J Food Sci. John Wiley & Sons, Ltd (10.1111); 2012;77: H1–H8. 10.1111/j.1750-3841.2011.02479.x [DOI] [PubMed] [Google Scholar]
- 59.Agencia Española de Seguridad Alimentaria y Nutrición. Plan de reducción del consumo de sal. Jornadas de debate [Internet]. La Granja de San Ildefonso; 2009. Available: http://www.aecosan.msssi.gob.es/AECOSAN/docs/documentos/nutricion/jornadas_debate.pdf
- 60.León-Flández K, Rico-Gómez A, Moya-Geromin MÁA, Romero-Fernández M, Bosqued-Estefania MJJ, Damián J, et al. Evaluation of compliance with the Spanish Code of self-regulation of food and drinks advertising directed at children under the age of 12 years in Spain, 2012. Public Health. 2017;150: 121–129. 10.1016/j.puhe.2017.05.013 [DOI] [PubMed] [Google Scholar]
- 61.Agencia Española de Consumo, Seguridad Alimentaria y Nutrición,. Plan De Colaboración Para La Mejora De La Composición De Los Alimentos Y Bebidas Y Otras Medidas 2017–2020. Madrid; 2018; 1–83. Available: http://www.aecosan.msssi.gob.es/AECOSAN/docs/documentos/nutricion/PLAN_COLABORACION_2017-2020.pdf
- 62.Chariton K, Yeatman H, Houweling F, Guenon S. Urinary sodium excretion, dietary sources of sodium intake and knowledge and practices around salt use in a group of healthy Australian women. Aust N Z J Public Health. 2010;34: 356–363. 10.1111/j.1753-6405.2010.00566.x [DOI] [PubMed] [Google Scholar]
- 63.World Health Organization. The SHAKE Technical Package for Salt Reduction [Internet]. Geneva, Switzerland: World Health Organization; 2016. Available: https://apps.who.int/iris/bitstream/handle/10665/250135/9789241511346-eng.pdf;jsessionid=44689598891164FA8AF19ACD3858C381?sequence=1 [Google Scholar]
- 64.Lobato CB, Machado A, Mesquita RBR, Lima L, Bordalo AA. Can non-fortified marine salt cover human needs for iodine? Int J Food Sci Nutr. 2018; 1–6. 10.1080/09637486.2018.1498066 [DOI] [PubMed] [Google Scholar]
- 65.McLean RM. Measuring population sodium intake: a review of methods. Nutrients. 2014;6: 4651–62. 10.3390/nu6114651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.John KA, Cogswell ME, Campbell NR, Nowson CA, Legetic B, Hennis AJM, et al. Accuracy and Usefulness of Select Methods for Assessing Complete Collection of 24-Hour Urine: A Systematic Review. J Clin Hypertens. 2016;18: 456–467. 10.1111/jch.12763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Patel D, Cogswell ME, John K, Creel S, Ayala C. Knowledge, Attitudes, and Behaviors Related to Sodium Intake and Reduction Among Adult Consumers in the United States. Am J Heal Promot. 2017;31: 68–75. 10.4278/ajhp.150102-QUAN-650 [DOI] [PubMed] [Google Scholar]
- 68.Nowson C, Lim K, Grimes C, O’Halloran S, Land MA, Webster J, et al. Dietary Salt Intake and Discretionary Salt Use in Two General Population Samples in Australia: 2011 and 2014. Nutrients. 2015;7: 10501–12. 10.3390/nu7125545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Land M-A, Webster J, Christoforou A, Johnson C, Trevena H, Hodgins F, et al. The association of knowledge, attitudes and behaviours related to salt with 24-hour urinary sodium excretion. Int J Behav Nutr Phys Act. 2014;11: 11–27. 10.1186/1479-5868-11-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quader ZS, Patel S, Gillespie C, Cogswell ME, Gunn JP, Perrine CG, et al. Trends and determinants of discretionary salt use: National Health and Nutrition Examination Survey 2003–2012. Public Health Nutr. 2016;19: 2195–2203. 10.1017/S1368980016000392 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Spanish and English versions.
(PDF)
Data Availability Statement
Due to ethical restrictions and participant confidentiality, individual data cannot be made publicly available. Data from this study are available upon request from the Complutense University of Madrid (UCM), for researchers who meet the criteria for access to confidential data. Data requests can be sent to UCM through the Principal investigator of the study (Ana M. López-Sobaler, asobaler@ucm.es) or non-author the Vice-dean of Research from the Pharmacy Faculty (Pilar Gómez-Serranillos, vdipfarm@ucm.es).