Abstract
GVHD is a frequent complication following allo-HCT. The NIH consensus group established new guidelines for the evaluation of chronic GVHD. However, GVHD assessment remains challenging due its complexity and requirement for laborious evaluation. We, therefore, established a standardized approach for the assessment of chronic GVHD in accordance with the NCC guidelines. At a single institution, all allograft recipients were evaluated for GVHD within the first-year post allo-HCT following a 3-step workflow (real-time assessment, consensus review, and documentation). A GVHD adjudication committee was created and a dynamic electronic GVHD data capture form was developed guiding the clinician through a comprehensive review of systems following the NCC guidelines. We found that the assessment and reporting of GVHD reached 100% compliance. The establishment of an institutional GVHD adjudication committee enabled standardized assessment of GVHD. Our workflow can be adopted by other centers to create a similar framework for dedicated GVHD evaluation.
Keywords: GVHD, quality improvement, data management
INTRODUCTION
Graft-versus-host Disease (GVHD) is a common complication after allogeneic stem cell transplantation (allo-HCT)1–5. Historically, the Seattle grading system was widely used for the assessment of chronic GVHD6. However, this grading system did not recognize that acute and chronic GVHD have distinctly different clinical manifestations from one another that are not time dependent. In addition, the Seattle grading system resulted in a largely heterogeneous population limiting its utility for the prediction of non-relapse mortality. Therefore in 2005, the National Institute of Health (NIH) consensus group proposed a new classification system which included standardized criteria for the clinical diagnosis, individual organ scoring, and global overall severity of chronic GVHD7. The new classification emphasized that distinct clinical manifestations existed between acute and chronic GVHD and recognized that acute and chronic GVHD can exist at the same time by defining a new category of overlap syndrome. The NIH further refined these guidelines in 20148. In recent years, the prognostic value of the NIH consensus criteria (NCC) has been validated supporting the use of this new system4,9–19. While most of Bone Marrow Transplant (BMT) centers have adopted these guidelines into their routine clinical assessments, GVHD evaluation requires laborious time-consuming assessments and can lead to clinician inter-variability in both diagnosis and scoring of GVHD. An international survey of the European Society for Blood and Marrow Transplantation and the National Cancer Institute identified barriers to greater use of the NIH recommendations that included lack of time, unfamiliarity with the recommendations, insufficient training or experience in chronic GVHD20. We, therefore, established a systematic approach for data collection, assessment, and documentation of chronic GVHD in effort to adopt and uniformly apply the NCC guidelines to all allo-HCT recipients. This report outlines our center’s framework for systematically assessing all patients for GVHD through 1-year post allo-HCT following the updated 2014 NIH Consensus Conference guidelines8.
METHODS
GVHD evaluation was conducted as standard of care and in accordance to Memorial Sloan Kettering Cancer Center (MSKCC) bone marrow transplant (BMT) practice service and institutional guidelines from 09/2014 to 01/2017. Allo-HCT recipients were systematically assessed for signs and symptoms of GVHD from day 100 to 365 post allo-HCT. The BMT Service followed the NIH guidelines of evaluating patients for chronic GVHD every 3 months, or more often as clinically indicated. If a patient required a second allo-HCT, further GVHD assessments were obtained according to their most recent allograft treatment.
GVHD Adjudication Committee
A GVHD adjudication committee was responsible for thoroughly evaluating each patient’s GVHD course to reach a consensus regarding the diagnosis, staging, and maximum global scoring following the NCC guidelines8. The purpose of using a centralized committee was to decrease the inter-variability of diagnosis and scoring across all allo-HCT recipients to achieve homogenous evaluation of GVHD. The committee consisted of at least one BMT physician, a nurse practitioner, physician assistant, research nurse, and research data manager. All members were trained in GVHD, as well as the NCC guidelines. The clinicians served as a subject matter professional and were tasked with adjudicating each patient, as well as completing source documentation to capture the adjudicated GVHD scoring for each patient. All source documentation was authored under the lead BMT physician who was responsible for signing off on all Electronic Medical Records (EMR) documentation. The data manager was responsible for coordinating adjudication meetings, compiling meeting notes, and tracking allo-HCT patients to ensure each patient was evaluated and appropriate documentation was completed.
GVHD Assessment Periods
All allograft recipients were systematically evaluated by the GVHD adjudication committee for signs and symptoms of GVHD through 1-year post allo-HCT. GVHD evaluation was conducted in two assessment periods: A) Day 180, included an evaluation within day 101 to 180 post allo-HCT, and B) Day 365, which assessed GVHD features present within day 181 to 365 post allo-HCT. Assessing patients through 1 year allowed capturing most GVHD cases since approximately 90% of allo-HCT recipients who are diagnosed with GVHD will show signs or symptoms within the first-year post allo-HCT5. To note, the assessment of GVHD from day 7 to 100 post allo-HCT was conducted by the acute GVHD adjudication committee following the established criteria for the staging and grading of acute GVHD21,22. Our workflow for the evaluation and adjudication of acute GVHD was established in September 2008 and preceded our chronic GVHD assessment workflow. Consequently, all allo-HCT recipients at our center are assessed for acute and chronic GVHD from day 7 until 1-year post allo-HCT.
GVHD Assessment Workflow
The GVHD assessment workflow consisted of three steps, which included prospective (real-time) assessment, consensus review, and documentation (Figure 1).
Figure 1: Chronic GVHD assessment workflow.
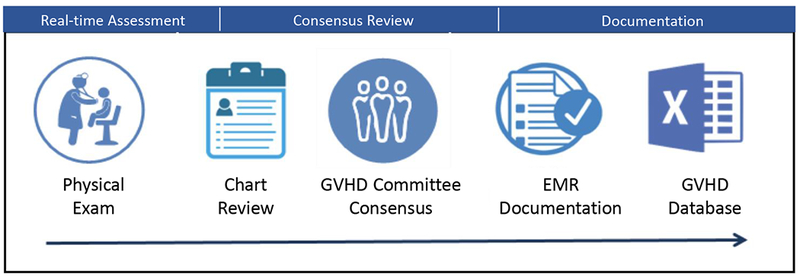
Included three steps: real time assessment, consensus review and documentation.
Real-time assessment
During BMT clinic visits, the treating provider or a member of the GVHD committee conducted a prospective (real-time) assessment that included a thorough history, review of systems, physical exam, and clinical laboratory (e.g. liver function test) for the assessment of signs and symptoms of GVHD. If a patient had signs and/or symptoms affecting a GVHD target organ, referral to a specialist was requested for further assessment (e.g. dental, ophthalmology, pulmonary, and gynecology). Pulmonary function testing was performed at baseline pre-HCT, at D100-180 and D365, per institutional guidelines. For symptomatic patients, an additional assessment was performed at the time of symptom onset. Findings were documented regardless of the differential diagnosis or other potential etiologies in the patient’s EMR. Clinicians were encouraged to document their findings in a dedicated GVHD section that was available in all post allo-BMT clinic notes. Findings could also be reported in a stand-alone GVHD assessment form.
Consensus review
Chart review.
After a patient reached day 180 or 365 post allo-HCT, a member of the GVHD committee conducted a near-real time patient chart review for the entirety of the patient’s assessment period. This review captured HCT type and donor type, patient disease status, GVHD prophylaxis, any signs or symptoms of acute and chronic GVHD for each individual organ, other possible etiologies (i.e. drug toxicity, infection), biopsy and relevant test results, immunosuppressants, corticosteroid treatment (including for non-GVHD indication), and other lines of therapy for GVHD, if applicable. The patient’s information was summarized into a concise document that was then distributed to all GVHD adjudication committee participants.
GVHD adjudication meetings.
The GVHD adjudication committee met at least twice a month for the assessment in near-real time of all allograft recipients who reached day 180 and 365 post allo-HCT. To have up-to-date GVHD data, adjudication for patients occurred the month after the patient met the day 180 or 365 time-points. During adjudication, the NCC guidelines were strictly enforced for categorization of GVHD syndromes, which was conducted according to the time of symptom presentation after allo-HCT and the presence/absence of acute and chronic GVHD features. The diagnosis of GVHD was made based on identification of GVHD features according to published criteria8. The presence of GVHD symptoms without an alternative etiology and/or receiving GVHD therapy regardless of biopsy result was classified as GVHD. Negative GVHD diagnosis required unequivocal (negative) laboratory/pathologic evidence or disappearance of symptoms in the absence of GVHD treatment. All signs and symptoms of other GVHD manifestations were assessed by the GVHD adjudication committee, and findings were documented in the electronic data capture form (Appendix 1). Patients who presented exclusively with other chronic GVHD manifestations, no diagnosis of chronic GVHD was attributed in the absence of a diagnostic criteria as per NIH guidelines8, and had no impact on the final global score of cGVHD. If undefined other chronic GVHD manifestations were present concurrently with diagnostic and/or distinctive features of chronic GVHD, these were also reported but did not have an impact on the final global score. The GVHD diagnosis date was determined by the date GVHD therapy was initiated or date of positive pathology, whichever occurred earlier. In cases where GVHD therapy was not initiated and biopsy was not obtained, the date GVHD symptoms first began was used as the diagnosis date. The GVHD committee reviewed the histopathologic findings and documented whether the biopsy was positive, equivocal or negative. The GVHD committee discussed complex patient cases with the primary and/or treating BMT physician, as well as subspecialists, such as dermatologist, ophthalmologist and gastroenterologist when necessary. For skin manifestations, digital photography was reviewed when available. For patients who transferred their care to a local oncologist, clinical assessments, test results and/or other relevant documentation were requested from the local provider. If the team was unable to obtain a follow-up update, patients were censored and documented as lost to follow up. For censored patients, only information through the censored date was considered for GVHD grading and scoring.
RESULTS
After consensus was reached by the GVHD adjudication committee, a member of the committee documented the adjudicated data using an electronic GVHD data capture form created by the GVHD adjudication committee (Appendix 1). Documentation was completed for each allo-HCT recipient at each assessment time-point (day 180 and 365). The dynamic GVHD form guided the user through a comprehensive review of systems following the NCC guidelines8. Relevant signs and symptoms of GVHD were categorized for each individual organ according to the diagnostic, distinctive, and other manifestations criteria and matched the NCC guidelines. Subsequently, the user was prompted to indicate whether the patient’s symptoms met diagnostic criteria for either acute or chronic GVHD. If the patient met criteria for establishing a diagnosis of chronic GVHD, the form intuitively prompted the user to complete the individual organ scoring section and the overall global chronic severity section. If the patient had acute GVHD, then the electronic form populated a scoring section relevant to grading of acute GVHD and according to International Bone Marrow Transplant Registry (IBMTR) criteria22. If the patient was diagnosed with overlap syndrome, both the acute and chronic scoring sections would populate. The electronic form also included additional sections for GVHD prophylaxis, organ biopsy, immunosuppressant drug(s) and GVHD treatment. We collaborated with CIBMTR to ensure our data reporting would align with their revised chronic GVHD forms. Therefore, additional questions were added including whether signs or symptoms of GVHD were still present at the end of the assessment period and the date maximum overall GVHD severity was reached. The Bone Marrow Transplant Clinical Trials Network (BMT CTN) reporting requirements were also taken into consideration. An optional question was added to capture overall chronic GVHD severity based on the BMT CTN severity scale (Figure 2).
Figure 2. Chronic and late acute GVHD electronic data form and BMT CTN severity scale.
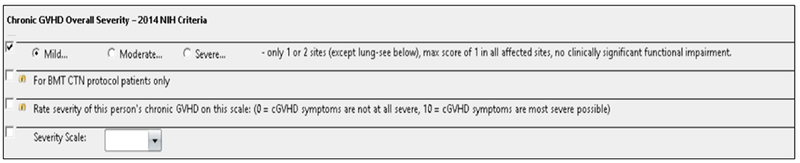
The electronic data form included an optional question to capture overall chronic GVHD severity based on the BMT CTN severity scale that is required in BMT CTN studies.
To ensure data quality assurance, different methods for error proofing, such as error notification, instructional text, radio buttons, and error checks were built into the “Chronic and Late Acute GVHD” assessment form. Except for a free-text comment field, the form utilized structured data fields, which allowed for information to be collected in a highly organized and categorized fashion. The dynamic form also utilized conditional fields that enabled the form to respond to user input, only populating fields necessary to score the specific patient case. Thus, the appropriate scoring section populated according on the patient’s specific GVHD syndrome. After the GVHD adjudication committee reviewed the patient’s data and determined the status of their GVHD diagnosis and severity, the data was transferred into the new GVHD database and became easily available when needed. Therefore, eliminating the need for clinicians to manually review each individual patient’s GVHD data from the EMR, minimizing time and effort and data inter-variability.
Prior to the establishment of the standardized chronic GVHD assessment, only 9% of allo-HSCT recipients had chronic GVHD evaluation conducted routinely from years 2000 to 2013. Of those, we identified 91% documentation errors (88% clinically non-significant, 12% had incorrect GVHD grading or missing diagnosis). Subsequently, 634 patients had day 180 and 365 acute and chronic GVHD assessments conducted by the GVHD adjudication committee. Documentation was achieved by 100% and showed that the most common GVHD syndromes were persistent or recurrent acute GVHD, followed by classical chronic GVHD and late acute GVHD.
DISCUSSION
GVHD is a complex disease. Its clinical assessment is made especially challenging by the subjectivity of clinician evaluations and attribution to competing diagnoses23–30. We established a dedicated GVHD team to ensure that consistent standards for GVHD diagnosis are applied across all patients. Our experience demonstrates the feasibility of conducting dedicated GVHD evaluations at set time-points through 1-year post allo-HCT in accordance with the NCC guidelines8. We showed that establishing an institutional GVHD adjudication committee and creating a user-friendly dynamic electronic data capture form enabled standardized internal documentation practices with a high degree of compliance. Further, centralizing GVHD assessments through an adjudication committee ensured that the NCC guidelines were uniformly applied to all allo-HCT recipients in our institution. Our data coordinators can now report consistent data to CIBMTR, BMT CTN, and other clinical trials. This framework also ensures that our center remains compliant with Foundation of the Accreditation of Cellular Therapy (FACT) accreditation standards. A current project is underway to further streamline this data capture process. Data from the Chronic and Late Acute GVHD assessment form will be electronically transferred into the GVHD database, eliminating the need to manually enter this data into the database. Notably, EBMT-NIH-CIBMTR Task Force supports the standardization of GVHD assessments as a dynamic process that incorporates progress in new diagnostic and therapeutic interventions31. Similar efforts in the standardization of GVHD data capture are underway to increase the reliability of the GVHD evaluation process including the development of new software and the eGVHD app32,33.
The additional step of capturing data within a GVHD specific database has significantly enhanced our ability to monitor GVHD incidence and response to therapy and has decreased the time and effort required providing physicians with GVHD data to support projects and manuscripts. The adjudicated GVHD data has been used in several manuscripts within our institution34–44. Improved monitoring of GVHD incidence has also enhanced our ability to assess feasibility of clinical trials in development. Adoption of similar framework for GVHD assessment and data capture across centers may improve consistency of GVHD scoring across centers and data quality in multicenter analyses. Improving the consistency of GVHD related data would assist in the design of future trials exploring novel therapies for unmet needs in GVHD prophylaxis and therapeutic interventions.
Supplementary Material
Appendix 1. Chronic and late acute GVHD electronic data form. This dynamic form responds to user input.
Ethics Statement:
This manuscript was developed ensuring quality and integrity. Confidentiality and anonymity of the subjects was maintained, and the information presented is independent and impartial.
Clinical Implications Statement:
Establishing a framework for dedicated assessment in GVHD enabled standardized documentation practices with a high degree of compliance. This workflow can be adopted by other centers that may improve consistency of GVHD scoring across centers and data quality in multicenter analyses. Improving the consistency of GVHD related data would assist in the design of future trials exploring novel therapies for unmet needs in GVHD.
Acknowledgements
This work was supported in part by the National Institutes of Health award number P01 CA23766 and NIH/NCI Cancer Center Support Grant P30 CA008748 by the NCI Core Grant Core Grant. We would like to acknowledge the GVHD research team, nursing staff and mid-level providers who greatly contributed to this work.
Footnotes
Conflict of Interest: The authors have no relevant conflicts of interest to declare.
References
- 1.Gilman AL, Schultz KR. Treatment of chronic GVHD. Bone Marrow Transplant. 2000;26(4):460–462. [DOI] [PubMed] [Google Scholar]
- 2.Goerner M, Gooley T, Flowers ME, et al. Morbidity and mortality of chronic GVHD after hematopoietic stem cell transplantation from HLA-identical siblings for patients with aplastic or refractory anemias. Biol Blood Marrow Transplant. 2002;8(1):47–56. [DOI] [PubMed] [Google Scholar]
- 3.Lee SJ, Klein JP, Barrett AJ, et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100(2):406–414. [DOI] [PubMed] [Google Scholar]
- 4.Omer AK, Weisdorf DJ, Lazaryan A, et al. Late Acute Graft-versus-Host Disease after Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2016;22(5):879–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9(4):215–233. [DOI] [PubMed] [Google Scholar]
- 6.Shulman HM, Sullivan KM, Weiden PL, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69(2):204–217. [DOI] [PubMed] [Google Scholar]
- 7.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. [DOI] [PubMed] [Google Scholar]
- 8.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389–401 e381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perez-Simon JA, Encinas C, Silva F, et al. Prognostic factors of chronic graft-versus-host disease following allogeneic peripheral blood stem cell transplantation: The national institutes health scale plus the type of onset can predict survival rates and the duration of immunosuppressive therapy. Biology of Blood and Marrow Transplantation. 2008;14(10):1163–1171. [DOI] [PubMed] [Google Scholar]
- 10.Cho BS, Min CK, Eom KS, et al. Feasibility of NIH consensus criteria for chronic graft-versus-host disease. Leukemia. 2009;23(1):78–84. [DOI] [PubMed] [Google Scholar]
- 11.Perez-Simon JA, Afram G, Martino R, et al. Evaluation of prognostic factors among patients with chronic graft-versus-host disease. Haematologica. 2012;97(8):1187–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SE, Cho BS, Kim JH, et al. Risk and prognostic factors for acute GVHD based on NIH consensus criteria. Bone Marrow Transplant. 2013;48(4):587–592. [DOI] [PubMed] [Google Scholar]
- 13.Liu YC, Chien SH, Fan NW, et al. Prognostic Factors on the Graft-versus-Host Disease-Free and Relapse-Free Survival after Adult Allogeneic Hematopoietic Stem Cell Transplantation. Stem Cells Int. 2016;2016:5143071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon JH, Sohn SK, Lambie A, et al. Validation of National Institutes of Health global scoring system for chronic graft-versus-host disease (GVHD) according to overall and GVHD-specific survival. Biol Blood Marrow Transplant. 2014;20(4):556–563. [DOI] [PubMed] [Google Scholar]
- 15.Pidala J, Vogelsang G, Martin P, et al. Overlap subtype of chronic graft-versus-host disease is associated with an adverse prognosis, functional impairment, and inferior patient-reported outcomes: a Chronic Graft-versus-Host Disease Consortium study. Haematologica. 2012;97(3):451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wingard JR, Majhail NS, Brazauskas R, et al. Long-term survival and late deaths after allogeneic hematopoietic cell transplantation. J Clin Oncol. 2011;29(16):2230–2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grube M, Holler E, Weber D, Holler B, Herr W, Wolff D. Risk Factors and Outcome of Chronic Graft-versus-Host Disease after Allogeneic Stem Cell Transplantation-Results from a Single-Center Observational Study. Biol Blood Marrow Transplant. 2016;22(10):1781–1791. [DOI] [PubMed] [Google Scholar]
- 18.Pidala J, Kim J, Anasetti C, et al. The global severity of chronic graft-versus-host disease, determined by National Institutes of Health consensus criteria, is associated with overall survival and non-relapse mortality. Haematologica. 2011;96(11):1678–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jagasia M, Giglia J, Chinratanalab W, et al. Incidence and outcome of chronic graft-versus-host disease using National Institutes of Health consensus criteria. Biol Blood Marrow Transplant. 2007;13(10):1207–1215. [DOI] [PubMed] [Google Scholar]
- 20.Duarte RF, Greinix H, Rabin B, et al. Uptake and use of recommendations for the diagnosis, severity scoring and management of chronic GVHD: an international survey of the EBMT-NCI Chronic GVHD Task Force. Bone Marrow Transplantation. 2014;49(1):49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glucksberg H, Storb R, Fefer A, et al. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HL-A-matched sibling donors. Transplantation. 1974;18(4):295–304. [DOI] [PubMed] [Google Scholar]
- 22.Rowlings PA, Przepiorka D, Klein JP, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. British Journal of Haematology. 1997;97(4):855–864. [DOI] [PubMed] [Google Scholar]
- 23.Phatak UP, Seo-Mayer P, Jain D, Selbst M, Husain S, Pashankar DS. Mycophenolate mofetil-induced colitis in children. J Clin Gastroenterol. 2009;43(10):967–969. [DOI] [PubMed] [Google Scholar]
- 24.Alonso CD, Treadway SB, Hanna DB, et al. Epidemiology and outcomes of Clostridium difficile infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2012;54(8):1053–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snover DC. Mucosal damage simulating acute graft-versus-host reaction in cytomegalovirus colitis. Transplantation. 1985;39(6):669–670. [PubMed] [Google Scholar]
- 26.Legrand F, Berrebi D, Houhou N, et al. Early diagnosis of adenovirus infection and treatment with cidofovir after bone marrow transplantation in children. Bone Marrow Transplant. 2001;27(6):621–626. [DOI] [PubMed] [Google Scholar]
- 27.Roddie C, Paul JP, Benjamin R, et al. Allogeneic hematopoietic stem cell transplantation and norovirus gastroenteritis: a previously unrecognized cause of morbidity. Clin Infect Dis. 2009;49(7):1061–1068. [DOI] [PubMed] [Google Scholar]
- 28.Chang L, Frame D, Braun T, et al. Engraftment syndrome after allogeneic hematopoietic cell transplantation predicts poor outcomes. Biol Blood Marrow Transplant. 2014;20(9):1407–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsukuma KE, Wei D, Sun K, Ramsamooj R, Chen M. Diagnosis and differential diagnosis of hepatic graft versus host disease (GVHD). J Gastrointest Oncol. 2016;7(Suppl 1):S21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Byun HJ, Yang JI, Kim BK, Cho KH. Clinical differentiation of acute cutaneous graft-versus-host disease from drug hypersensitivity reactions. J Am Acad Dermatol. 2011;65(4):726–732. [DOI] [PubMed] [Google Scholar]
- 31.Schoemans HM, Lee SJ, Ferrara JL, et al. EBMT-NIH-CIBMTR Task Force position statement on standardized terminology & guidance for graft-versus-host disease assessment. Bone Marrow Transplant. 2018;53(11):1401–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schoemans HM, Goris K, Van Durm R, et al. Accuracy and usability of the eGVHD app in assessing the severity of graft-versus-host disease at the 2017 EBMT annual congress. Bone Marrow Transplant. 2018;53(4):490–494. [DOI] [PubMed] [Google Scholar]
- 33.Mancini G, Frulla R, Vico M, et al. A new software for evaluating scoring and response in cGVHD according to the new NIH criteria. Bone Marrow Transplantation. 2016;51:S183–S183. [Google Scholar]
- 34.Taur Y, Jenq RR, Perales MA, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. 2014;124(7):1174–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jenq RR, Taur Y, Devlin SM, et al. Intestinal Blautia Is Associated with Reduced Death from Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2015;21(8):1373–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamari R, Chung SS, Papadopoulos EB, et al. CD34-Selected Hematopoietic Stem Cell Transplants Conditioned with Myeloablative Regimens and Antithymocyte Globulin for Advanced Myelodysplastic Syndrome: Limited Graft-versus-Host Disease without Increased Relapse. Biol Blood Marrow Transplant. 2015;21(12):2106–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hobbs GS, Hamdi A, Hilden PD, et al. Comparison of outcomes at two institutions of patients with ALL receiving ex vivo T-cell-depleted or unmodified allografts. Bone Marrow Transplant. 2015;50(4):493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ponce DM, Hilden P, Mumaw C, et al. High day 28 ST2 levels predict for acute graft-versus-host disease and transplant-related mortality after cord blood transplantation. Blood. 2015;125(1):199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harnicar S, Ponce DM, Hilden P, et al. Intensified Mycophenolate Mofetil Dosing and Higher Mycophenolic Acid Trough Levels Reduce Severe Acute Graft-versus-Host Disease after Double-Unit Cord Blood Transplantation. Biol Blood Marrow Transplant. 2015;21(5):920–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ponce DM, Hilden P, Devlin SM, et al. High Disease-Free Survival with Enhanced Protection against Relapse after Double-Unit Cord Blood Transplantation When Compared with T Cell-Depleted Unrelated Donor Transplantation in Patients with Acute Leukemia and Chronic Myelogenous Leukemia. Biol Blood Marrow Transplant. 2015;21(11):1985–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ceberio I, Devlin SM, Sauter C, et al. Sirolimus, tacrolimus and low-dose methotrexate based graft-versus-host disease prophylaxis after non-ablative or reduced intensity conditioning in related and unrelated donor allogeneic hematopoietic cell transplant. Leuk Lymphoma. 2015;56(3):663–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peled JU, Devlin SM, Staffas A, et al. Intestinal Microbiota and Relapse After Hematopoietic-Cell Transplantation. J Clin Oncol. 2017;35(15):1650–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barba P, Hilden P, Devlin SM, et al. Ex Vivo CD34(+)-Selected T Cell-Depleted Peripheral Blood Stem Cell Grafts for Allogeneic Hematopoietic Stem Cell Transplantation in Acute Leukemia and Myelodysplastic Syndrome Is Associated with Low Incidence of Acute and Chronic Graft-versus-Host Disease and High Treatment Response. Biol Blood Marrow Transplant. 2017;23(3):452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cho C, Hsu M, Barba P, et al. Long-term prognosis for 1-year relapse-free survivors of CD34+ cell-selected allogeneic hematopoietic stem cell transplantation: a landmark analysis. Bone Marrow Transplant. 2017;52(12):1629–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1. Chronic and late acute GVHD electronic data form. This dynamic form responds to user input.


