Abstract
Background:
A growing literature suggests deficient emotional facial expression (EFE) processing among recently abstinent individuals with alcohol use disorders (AUDs). Further investigation is needed to clarify valence-related discrepancies and elucidate neural and psychosocial correlates. We examined neurobehavioral indices of EFE processing and interpersonal problems in treatment seekers with AUDs and healthy community controls (CC).
Methods:
Thirty-four individuals with AUDs and 39 CCs completed an emotion judgement task (EJT), requiring discrimination between happy, angry, and sad EFEs. A second task requiring discrimination of male and female faces with neutral expressions served as the control task (i.e., sex judgement task, SJT). Neurophysiological (i.e., N170 and P3) and behavioral measures were analyzed using GLMM. Interpersonal problems were assessed with the Inventory of Interpersonal Problems-64 (IIP-64). The relationship of IIP-64 and EJT performance was investigated via within-group correlations.
Results:
Analysis of the SJT revealed no group differences on behavioral measures, N170 amplitude or P3 latency. P3 amplitudes, however, were significantly lower in the AUD group. For the EJT, initial observations of group differences in P3 amplitude were accounted for by differences in the control task. Behavioral analyses indicated that the AUD group was significantly less accurate than the CC group. Hypothesis driven analyses using GLMM-estimated group differences indicated that anger processing was affected to a greater degree than were other emotions. Significant EJT/IIP-64 correlations were observed for anger processing within the AUD group and were confined to IIP-64 subscales with relatively high ratings on the affiliation dimension.
Conclusions:
Findings provide partial support for an emotion-specific processing deficit in persons with AUDs. Anger processing was more robustly affected than other emotions and was associated with interpersonal problems characterized by being overly needy, non-assertive and overly accommodating. Results extend prior reports and reinforce the need for comprehensive study of emotion processing and its real-world implications.
Keywords: Emotion Processing, Emotional Face Expression, Alcohol Use Disorder, Interpersonal Problems, Neurophysiology
Introduction
Alcohol use disorders (AUDs) are frequently characterized by compromise in traditional neurocognitive domains that may persist for months to years following the initiation of recovery (e.g., Nixon et al., 2014; Bates et al., 2002; Chanraud, et al, 2007; Fein et al., 2006). This literature also demonstrates that neuropsychological/ cognitive domains vary significantly in their vulnerability. Overall, neuropsychological domains/cognitive abilities dependent on prefrontal cortices and related networks, such as inhibitory processing, decision-making, cognitive/behavioral regulation, and including aspects of attention and working memory, demonstrate greater sensitivity to alcohol (e.g., Le Berre and Sullivan, 2014).
Critically, but generally underappreciated (but see Oscar-Berman et al., 1990) is alcohol-related impairment in social/emotional processing (e.g., Marinkovic et al., 2009; Nixon et al., 1992). More specifically, while interpersonal problems, emotional dysregulation, and inadequate social cognitive skills are often noted as hallmarks of alcohol and other substance use disorders, systematic investigations are less frequently reported. Within extant literature, empirical studies demonstrate that AUDs are not only associated with predictable compromise in self-reported interpersonal function (Kornreich et al, 2002, 2003), but also demonstrate objective deficits in emotion regulation, social/emotion identification, and performance on broader measures of social cognition (e.g., Bora & Zorlu, 2017, Foisy et al, 2007; Maurage et al. 2008a, Monnot et al, 2001; Townsend & Duka, 2003).
Notably, social and emotion processing rely on neural networks and component processes that overlap, albeit incompletely, with those underlying the more vulnerable cognitive domains (Muller-Oehring & Shulte, 2014). Leveraging this commonality, a growing number of studies investigating social/emotion compromise in AUDs employ neurobehavioral methods including both behavioral and neuroimaging/physiological measures (e.g., Sawyer et al., 2019; Salloum et al., 2007). Given the importance of facial expressions in interpersonal and social contexts, many investigations probe the integrity of emotion processing using variations on Emotion Face Expression (EFE) tasks. EFE tasks typically require that individuals make judgements regarding the type and/or intensity of emotions portrayed on face stimuli (e.g., angry, happy, sad; Salloum et al., 2007; Maurage, et al. 2008a, Townshend & Duka, 2003).
Integrating information regarding neural substrates underlying face processing and that regarding neurobehavioral processes commonly compromised in AUD, investigators have used both earlier components, purportedly related to face processing (e.g., N170), and later components, commonly associated with higher-order processes related to decision making (e.g., P3) (Bentin et al, 1996; Luck & Kappenman, 2012; Polich, 2004; Rossion & Jacques, 2011). Although patterns across studies are not entirely consistent, alcohol-related attenuations in amplitude and/or delayed latencies are commonly reported (Fein, et al, 2010, Maurage et al., 2007; Maurage, et al, 2008b, 2008c, 2008d). Variability in the specificity of these outcomes prohibits strong conclusions regarding differential impact on N170 or P3 measures. Similarly, whether emotional valence (i.e., positive vs. negative) differentially impacts alcohol-related compromise remains unresolved. Some studies report alterations and behavioral deficits regardless of emotional valence (Maurage et al 2008a, 2008b; Kornreich et al, 2001a, 2001b), whereas others find differential compromise in processing of negative emotion (Maurage et al, 2008c, 2008d; Kornreich et al, 2013; Quaglino et al., 2015; Salloum, et al, 2007).
Although interest has increased, published work on emotion processing in AUD derives largely, but not solely, from work in one/two laboratories (see Donadon & Osorio, 2014). Given the purported role of emotional processing on AUD progression/trajectories, recovery (Marlatt et al, 1996), and interpersonal functioning more broadly (Kornreich et al, 2002; Uekermann & Daum, 2008), this area of inquiry is of significant scientific and clinical relevance. Thus, it is imperative that seminal findings be replicated and expanded.
The current study was designed to address this need. Here, we obtained both neurophysiological and behavioral measures during the conduct of an EFE task with a sample of treatment-seeking men and women with AUD and a community comparison (CC) group. Given the focus on N170 and P3 in published EFE work and the overarching objective of the current investigation, we constrained analysis to these components. We predicted that the AUD group would exhibit altered neurophysiological responses as reflected in the N170 and/or P3, as well as previously reported behavioral compromise (Lewis et al, 2019). Given published inconsistencies, we posed no differential hypothesis regarding the two ERP components. In contrast, in considering the role of emotional valence, we felt there were sufficient data to support a preliminary hypothesis that persons with AUDs would demonstrate differential deficits when processing negative vs. positive emotions.
Finally, as an exploratory analysis and to supplement our recent report (Lewis et al, 2019), we examined correlations between domains of interpersonal problems using the IIP-64 (Horowitz et al, 2000) and EFE performance. In our prior report, we considered alcohol-related effects on IIP-64 total scores. The total score is derived from 8 subscales that are organized along two dimensions; affiliation and dominance. Broadly speaking, higher scores on the former domain reflect problems arising from behaviors/traits such as being overly self-sacrificing, needy and overally accommodating, while those on the latter dimension are associated with traits such as being self-centered and controlling. Here, we conducted analyses to determine if the association between EFE and IIP-64 varied by dimension or constituent subscales.
Materials and Methods
Participants.
Participants provided written informed consent prior to any data collection and were compensated for their time. The University of Florida Medical IRB approved all procedures.
Participants (N=73, 78% White/Caucasian; 18% Black, & 4% Other; AUD and CC groups were equivalent) included 34 inpatient treatment seekers meeting criteria for current alcohol use disorder (AUD; 6 women) and 39 healthy, community controls (CCs; 24 women). This sample constitutes a subset of those reported in our recent work (Lewis et al, 2019) for whom neurophysiological measures were available.To determine eligibility, participants completed paper-pencil questionnaires addressing demographics and substance-use histories including chronicity, quantity and frequency of alcohol use (Quantity Frequency Index; QFI [Cahalan et al., 1969]). The QFI generates an estimate of the average number of ounces of absolute alcohol consumed per day and can be transformed to estimate standard drinks/day. Self-reported medical histories were obtained and probablistic psychiatric disorders were assessed per DSM-IV criteria (computerized Diagnostic Interview Schedule-IV; cDIS [Robins et al., 1995, American Psychiatry Association, 1994]). A DSM 5 form of the cDIS was not available at study initiation, thus the alcohol craving questionnaire (Singleton et al., 2000) was added to confirm DSM 5 AUD status. Eligible participants were aged 25–59 years and had 10–16 years of education. Psychiatric/physical conditions deemed exclusionary across groups included: 1) significant neurologic disorder/insult, 2) medical conditions or medication use that challenged interpretations of neurobehavioral function, 3) lifetime psychotic or bipolar disorder, 4) current major depression, and 5) significant anxiety-related disorders (e.g., PTSD). Nicotine use/dependence was not exclusionary, given its prevalence in treatment populations (i.e., 76%, n=26 in the AUD group; 10%, n=4 in the CC group).
Consistent with previous research, persons in the AUD group were required to meet criteria for substantive alcohol use problems; i.e., DSM-IV criteria for dependence, equivalent DSM 5 severity. Participants in the AUD group had completed medical detoxification including any related medications (if required) and were 21–90 days abstinent at the time of testing. This time range is consistent with the conventional timeframe for assessing neurocognitive deficits in early recovery while also mitigating potential confounds associated with subclinical protracted withdrawal (see Oscar-Berman et. al, 2014). Polysubstance use is common in treatment-seeking samples. In the current sample, 82% (28/34) of those in the AUD group endorsed the use of a substance other than alcohol or nicotine in the prior six months. Of these, marijuana was most commonly reported (53%, n=15). Opiates were reported by 43% (n=12), stimulants by 36% (n=10) and benzodiazepines by 18% (n=5). Muscle relaxers and hallucinogens were reported by one person each. Because AUD diagnosis is not contingent on a specific level of alcohol consumption, only persons who met AUD clinical criteria and whose pre-treatment consumption levels were consistent with heavy drinking were included. CCs were healthy, community-residing non-problem drinkers with no current or lifetime history of any substance use disorder, with the exception of nicotine. In this sample, 10% (n=4) reported marijuana use in the prior 6 months. None reported regular use or produced a positive urine test.
Laboratory Protocol
On testing day, negative breath-alcohol, urine toxicology (for common drugs of abuse), and pregnancy tests (for women) were required for continued participation. Current nicotine users were administered a 7mg nicotine patch before testing to avoid withdrawal. This dose is well-tolerated across sexes and does not differentially affect performance between AUD and CC groups (Ceballos et al., 2005; Nixon et al., 2007). Preliminary analyses suggested no difference between nicotine users (n=30) and non-users (n=43) for any primary outcome measure (ps>0.05). Therefore, nicotine was omitted from further analyses. Given the potential influence of negative affect on the EFE task, participants also completed measures of depressive (Beck Depression Inventory, BDI, Beck et al, 1996) and state anxiety symptoms (AI; Spielberger, et al, 1983).
Assessments
Interpersonal difficulties were evaluated with the Inventory of Interpersonal Problems-64 (IIP-64 [Horowitz et al, 2000]). Overall (total) scores for interpersonal problems as well as scores for the 8 individual subscales were calculated and converted to t-scores, per standard scoring instruction (Horowitz et al., 2000).
To investigate EFE processing, two face identification tasks were administered. The experimental task was an emotion judgement task (EJT; adapted from Maurage et al., 2008b) and the control task was a sex judgement task (SJT). The SJT was included to facilitate interpretation of EJT outcome (i.e., specificity to emotion processing versus generalized face processing). For both tasks, stimuli were presented foveally, on a computer monitor (E-Prime software). Participants responded via button-press with the index finger of their dominant hand. Task instructions equally emphasized response speed and accuracy. Face stimuli were derived using the Ekman stimulus set (Ekman, 1976). Equal numbers of male and female posers (ages 30–50 years) were used.
For the SJT, eight neutral expression faces were selected and male/female (M/F) pairs were identified. For each pair, morphing software (Fantamorph) was applied to generate faces with varying proportions of masculine/feminine features. Four morph levels were created, depicting the following M/F proportions: 1) 5%/95%, 2) 35%/65%, 3) 65%/35%, and 4) 95%/5%. This process resulted in two levels, 65% or 95% for each sex. No M/F pair or morph level was repeated within four consecutive trials. In each of the 256 total trials, a single face was presented for 1500 ms, during which participants indicated whether the face appeared more male or female. A 300 ms interstimulus interval (ISI), designated by a fixation cross, signaled termination of the response window for the preceding trial.
EJT stimuli were developed using four facial expressions from each of eight posers, including neutral, happy, angry, and sad expressions. Within-poser morphing procedures were performed to create EFEs of varying emotional intensity. Each EFE was morphed with its respective neutral expression to represent 35%, 65%, and 95% intensities of the target emotion, producing 72 unique task stimuli. On each trial, a single face was shown and participants were asked to judge whether the face was one of two emotions (e.g., sad vs. angry). Thus, consistent with the SJT, each trial required a binary decision regarding one face stimulus. The two emotions to be discriminated were held constant within a task block and all emotion combinations were tested (sad vs. angry; happy vs. sad; happy vs. angry). Five blocks for each pair discrimination with 48 trials/block (to accommodate the intensity morphing) were administered. No stimulus was repeated within a given block and block order was counterbalanced. Preceding each block, participants were informed of the two emotions to be discriminated and their corresponding button assignment, which alternated across blocks. EJT stimulus and ISI timing were identical to the SJT. Assaying combinations of the three emotions dictated the number of trials in the EJT and SJT tasks, producing a ratio of ~ 3/1.
Event-Related Potentials
The electroencephalogram (EEG) was recorded using a 64-electrode array mounted in an expanded International 10–20 System configuration (Electro-Cap International), linked earlobe references, and a mid-forehead ground. Supraorbital and infraorbital electrodes monitored eye blinks/movements and a chin rest was used to minimize artifacts. Impedances were maintained below 10 kOhms. The continuous EEG was amplified at a gain of 10,000x and subjected to an analog 0.1–100 Hz band-pass filter (Neuroscan 4.4 Acquire). Data were analog-to-digital converted at a 1000 Hz sampling rate.
Offline, data were processed using EEGLAB Toolbox and subject to a 30 Hz low-pass filter and 24 dB attenuation. Epochs started 200 ms prior to face-stimulus onset (baseline) and ended 1500 ms after. Trials with incorrect responses were omitted from EEG analyses. Epochs containing gross artifacts (> ±150μV) were rejected. Participants producing less than 40 percent of the total possible accepted epochs for a given task were excluded. After participant exclusion (SJT: nCC=4, nAD=7; EJT: nCC=6, nAD=6), an average of 199 SJT and 542 EJT epochs/participant were deemed suitable for ERP analysis. Epochs were subjected to an independent component analysis (Jung et al., 2001) and an automated technique for identifying and removing artifacts (ADJUST [Mognon et al., 2011]). For each participant, epochs were averaged and baseline corrected for each of the four SJT morph levels and each of the nine EJT emotion/morph combinations.
Using conventional procedures (Luck, 2014), N170 and P3 measurement windows and electrode sites were determined via examination of group-specific grand average waveforms. This approach resulted in group-specific windows for measurement of the N170 and challenged meaningful between group analysis, therefore N170 analyses were confined to amplitude. For CCs the window was 110–180 ms after stimulus onset, for the AUD group, 130–200 ms. Activity at T5/T6 was averaged for measurement of the N170. For both groups the P3 was assessed 350–500 ms following stimulus onset at PZ. After determining ERP coordinates, individual mean amplitudes and 50% area latencies were utilized instead of peak measures, given their superior reliability and suitability to handle between-group variability (Luck, 2014). To ensure components were accurately captured, amplitudes were assessed for group- and task-specific outliers (±2 SD) and waveforms were examined for occurrences outside the designated measurement window. Based on these examinations, three participants (CCs) were excluded from N170 SJT analyses; six (4 CCs/2 AUD) from N170 EJT analyses. No P3 exclusions were required.
Data Analysis
Statistical analyses were performed using SAS Version 9.4. Significance was defined as p≤0.05. Descriptive variables were analyzed for group differences using independent t-tests and chi-square analyses. When appropriate, descriptive and dependent measures of interest were subjected to Pearson correlations to identify potentially confounding relationships.
Generalized linear mixed models (GLMM) were used to analyze SJT and EJT outcomes. GLMM compared to other models (e.g., ANOVA models), allows application of data-driven covariance structures and more accurate analysis when violations of normality/homoscedasticity occurr. For each of the tasks, the ERP parameters and behavioral measures were considered in separate analyses. Given a priori hypotheses that persons with AUD would demonstrate differential dysregulation in the processing of negative emotions, partitioned analyses of group effects, where significant, were conducted within each emotion condition.
Morph level was designated as the within-participant factor in SJT analyses. Emotion and morph were within-participant factors in EJT models. Although retained in EEG analyses, the 35% morph level was omitted from the behavioral models due to an inconsistent pattern of below-chance responding.
As an initial analysis, IIP-64 total and subscale scores were investigated for group differences using one-way and mixed-effects ANOVAs, respectively. Relationships between IIP-64 total scores and task accuracy by emotion were assessed within each group using Pearson correlations. Where a significant association between a specific emotion and the IIP was observed, correlations between that emotion and the individual subscales were produced.
Results
Demographics, Affective, and Drinking Measures
Demographic data are presented in Table 1. A significant group difference emerged for education (t(71)=3.24, p=0.002). This difference was small (~1 year) and education did not significantly correlate with dependent measures of interest. Therefore, it was not given further consideration. Consistent with related work, persons with AUD endorsed greater depressive and anxiety symptoms (ts≥1.94, ps≤.056). No correlations between affective measures and either behavioral (rs≤|.17|, ps≥.14) or electrophysiological indices (rs≤|.15|, ps≥.21) were noted. Therefore, these measures were not considered further.
Table 1.
Descriptive Measures (By Group)
| CC | AUD | |
|---|---|---|
| Measure | M (SD) / % (n) | M (SD) / % (n) |
| Age (yrs) | 43.79 (12.12) | 40.82 (8.84) |
| Education (yrs)** | 14.69 (1.56) | 13.5 (1.58) |
| Depressive Symptoms | 3.74 (4.04) | 8.73 (7.74) |
| Anxiety Symptoms | 42.02 (7.06) | 45.97 (10.18) |
| Quantity/Frequency Index†** | 0.30 (0.41) | 15.69 (10.39) |
| Previous Treatments | - | 2.24 (1.33) |
| Alc Problem Chronicity (yrs) | - | 14.91 (10.90) |
| Days Abstinent | - | 41.00 (12.63) |
| Race Caucasian | 74 (29) | 82 (28) |
| African American | 21 (8) | 15 (5) |
| Other | 5 (2) | 3 (1) |
| Sex Male | 38 (15) | 82 (28) |
| Female | 62 (24) | 18 (6) |
average daily consumption (oz. absolute alcohol) over last 6 months (or 6 mo. before treatment for AUD group). .6=1 standard drink/day (adapted from Cahalan et al., 1969), CCs< 1 drink/day; AUD ~26 drinks/day.
Significant group effect (p<.01). AUD =Alcohol Use Disorder (n=34), CC=Community Controls (n=39)
As expected, the AUD group reported higher QFIs than the CC group (t(71)=8.63, p<0.0001). Pre-treatment QFIs for the AD group reflected consumption of ~26 standard drinks per day, whereas CCs consumed an average of less than one drink per day.
Neurobehavioral Measures
SJT Behavior and Neurophysiology.
No significant main or interaction effects were observed for either accuracy or reaction time (Fs≤1.34, ps≥.251) measures (Table 2). Electrophysiological analyses revealed diminished P3 amplitudes among individuals with AUDs (F(1,60)=5.32, p=.02; see Table 3), but no group difference or interactions for N170 amplitudes (Fs≤0.17, ps≥.682). No group effects/interactions were observed for P3 latency (Fs≤0.86, ps≥.465).
Table 2.
Behavioral Task Performance (By Group)
| 2a. Sex Judgement Task: Accuracy/Reaction Time | ||||
| CC | AUD | |||
| Sex/Morph | ACC (%) | RT (ms) | ACC (%) | RT (ms) |
| Female95 | 91.39 (9.49) | 775.01 (78.73) | 92.84 (9.92) | 774.95 (102.61) |
| Female65 | 83.79 (14.53) | 814.21 (76.46) | 85.97 (11.99) | 813.87 (108.53) |
| Male95 | 96.47 (5.60) | 748.61 (90.22) | 97.43 (3.85) | 765.65 (90.04) |
| Male65 | 84.22 (14.23) | 855.78 (103.22) | 84.07 (9.68) | 879.20 (95.64) |
| 2b. Emotion Judgement Task: Accuracy/Reaction Time1,2 | ||||
| CC | AUD | |||
| Emotion/Morph | ACC (%) | RT (ms) | ACC (%) | RT (ms) |
| Happy65 | 97.07 (3.46) | 746.66 (96.70) | 95.56 (4.96) | 732.16 (80.26) |
| Angry65 | 89.44 (6.88) | 853.08 (82.01) | 85.12 (10.67) | 845.85 (87.18) |
| Sad65 | 87.95 (5.66) | 829.06 (82.90) | 87.59 (5.43) | 820.73 (81.47) |
| Happy95 | 96.70 (3.40) | 718.60 (93.59) | 95.96 (4.18) | 711.02 (79.16) |
| Angry95 | 92.98 (5.10) | 816.74 (82.58) | 90.21 (7.66) | 805.92 (77.99) |
| Sad95 | 88.33 (6.53) | 819.18 (84.22) | 87.37 (6.56) | 809.09 (82.05) |
Values are M(SD) for accuracy (ACC) & reaction time (RT). Labels indicate the dominant sex (SJT) or emotion (EJT) portrayed in each stimulus; numerals indicate the morph level. CC=Community Controls, AUD =Alcohol Use Disorder.
Group difference in accuracy (AUD < CCs)
Group difference persisted for Angry, but not Happy or Sad, trials
Table 3.
Electrophysiological Measures
| 3a. Sex Judgement Task: P3 Amplitude/Latency1 | ||||
| CC | AUD | |||
| Sex/Morph | Amp (μV) | Lat (ms) | Amp (μV) | Lat (ms) |
| Female95 | 5.73 (3.98) | 434.69 (15.86) | 4.00 (3.23) | 429.67 (17.24) |
| Female65 | 5.62 (3.77) | 431.83 (19.38) | 3.22 (3.14) | 428.07 (17.77) |
| Male95 | 5.52 (3.47) | 431.26 (19.01) | 3.79 (3.21) | 431.26 (17.22) |
| Male65 | 4.80 (3.72) | 426.00 (17.51) | 3.30 (2.81) | 425.96 (17.93) |
| 3b. Emotion Judgement Task: P3 Amplitude/Latency2 | ||||
| CC | AUD | |||
| Emotion/Morph | Amp (μV) | Lat (ms) | Amp (μV) | Lat (ms) |
| Happy35 | 7.54 (4.00) | 431.61 (11.30) | 5.06 (3.60) | 428.82 (14.97) |
| Angry35 | 7.17 (3.40) | 429.73 (9.08) | 5.18 (3.95) | 427.14 (15.26) |
| Sad35 | 6.74 (3.80) | 430.73 (10.16) | 4.74 (3.48) | 429.17 (15.60) |
| Happy65 | 8.19 (3.87) | 430.45 (9.46) | 6.20 (4.22) | 428.50 (10.25) |
| Angry65 | 8.07 (4.30) | 431.18 (8.07) | 5.38 (3.80) | 432.54 (18.38) |
| Sad65 | 7.69 (3.73) | 431.06 (9.78) | 5.24 (3.72) | 429.18 (14.12) |
| Happy95 | 9.12 (3.95) | 430.73 (8.85) | 6.50 (4.03) | 433.54 (13.83) |
| Angry95 | 8.55 (4.04) | 433.36 (10.42) | 6.21 (4.02) | 430.11 (11.65) |
| Sad95 | 7.88 (3.54) | 432.18 (8.27) | 5.52 (4.02) | 430.32 (15.59) |
Values are M(SD) for amplitude (Amp) & latency (Lat). Labels indicate the dominant sex (SJT) or emotion (EJT) portrayed in each stimulus; numerals indicate the morph level. CC=Community Control, AUD= Alcohol Use Disorder.
Group difference in SJT P3 amplitude (AUD < CCs)
Group difference in EJT P3 amplitude (AUD < CCs) that failed to persist when SJT differences considered
EJT Neurophysiology
Preliminary EJT ERP analyses revealed diminished P3 amplitudes among AUD individuals relative to CCs (F(1,59)=15.30, p<.001) with no group by emotion interaction (F(2,118)=0.01, p=.986). A main effect of morph was observed (F(2,118)=11.07, p<.001), such that across groups P3 amplitudes increased with higher morph levels (ts≥1.98, ps≤.050). A main effect of emotion was observed (F(2,118)=4.37, p=.015), apparently driven by greater amplitudes to happy, relative to sad, stimuli (t=2.94, p=.004). Amplitudes to angry stimuli were intermediate, and did not differ from either happy or sad. Given the significant effect of group on P3 amplitude in the control (SJT) task, we repeated the EJT P3 amplitude analysis using SJT P3 amplitude as a covariate. This analysis eliminated the group effect (F(1,52) =0.0, p=.99).
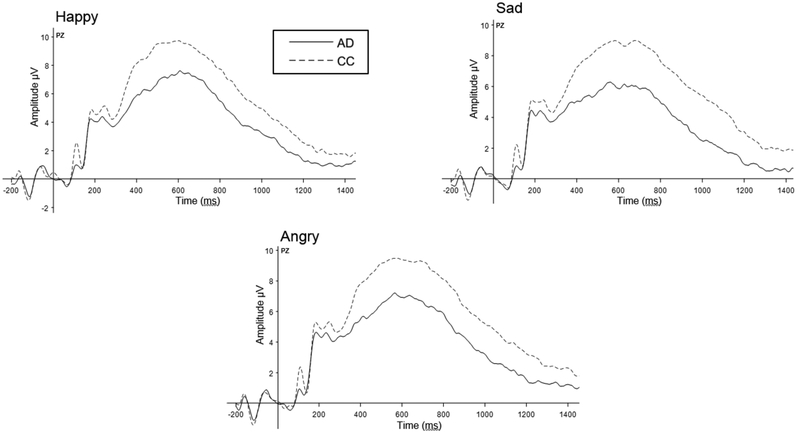
No significant main effects or interactions were observed for N170 amplitude (Fs≤0.50, ps≥.609) or P3 latency (Fs≤1.47, ps≥.233). Averaged wavefoms for the uncorrected P3 EJT data by group and for each emotion are presented in Table 3 and depicted in Figure 1.
Figure 1. Grand Average EEG Waveforms: Emotion Judgement Task (Electrode PZ).
Analyses revealed attenuated P3 amplitudes among participants with AUD, relative to community controls (F(1,59)=15.30, p<0.001). Subsequent analyses covarying for SJT amplitude found these differences were not specific to emotion processing.
EJT Behavioral Performance
Consistent with the larger sample reported in Lewis et al (2019), GLMM analysis revealed a significant effect of group (F(1,71)=4.03, p=.048), with less accurate EFE identification among AUDs relative to CCs. There was also a) an emotion main effect (F(2,142)=128.76, p<.001) with accuracy being the highest for happy stimuli and lowest for sad stimuli (ts≥118.19, ps<.001) and b) an effect of morph level with higher accuracy scores at the 95% morph (F(1,71)=15.30, p<.001). Hypothesis-driven contrasts partitioned by emotion revealed AUD-associated compromise on anger (F(1,142)=5.49, p=.020), but not happy or sad trials (Fs≤1.91, ps≥.168). No group effects or interactions were observed for reaction time measures (Fs≤1.06, ps≥.348). Similar to accuracy, reaction time was advantaged at higher morphs (F(1,71)=23.65, p<.001). A main effect of emotion was also noted (F(2,142)=233.08, p<.001). Reaction times were fastest to happy and slowest to angry (ts≥1.94, ps≤.054). (See Table 2).
Correlations were conducted to characterize relationships between behavioral and electrophysiological measures. Consistent with a priori hypotheses, and given the lack of group by morph interactions, analyses were conducted within each emotion but collapsed across morph. Greater P3 amplitude was associated with more rapid behavioral responding, although significance varied across emotions (Happy: r=−.26, p=.038; Angry: r=−.24, p=.058; Sad: r=−.20, p=.118). No other correlations were noted between outcome measures (rs≤|.19|, ps≥.136).
Interpersonal Functioning
As anticipated on the basis of the larger sample (Lewis, et al., 2019), higher IIP-64 total scores were obtained in the AUD vs. CC group (F(1,71)=13.17, p<0.001). Novel to the current study are analyses conducted to explore group differences across the individual subscales. AUD individuals reported greater interpersonal problems on all eight subscales (ts≥1.97, ps≤.053).
Given our interest in the association of emotion processing and interpersonal function, we conducted correlational analyses between the subscale scores and behavioral performance. To limit the overall number of statistical tests and because no group by morph interactions were obtained, these analyses were collapsed across morph. No associations were observed between IIP-64 total score and SJT accuracy (rs≤|.148|, ps≥.403). Within-group correlations between EJT accuracy (by emotion) and IIP-64 scores indicated a significant negative relationship between emotion processing of angry stimuli and interpersonal problems (r=−.373, p=.030). This relationship persisted only among AUD individuals; no other significant relationships were detected among either group (rs≤|.233|, ps≥.465).
To further probe these associations, anger accuracy was correlated with each of the IIP-64 subscales for AUD participants. These analyses showed that lower accuracy in identifying angry faces was associated with higher scores on three of the eight scales; “intrusive/needy”, “non-assertive”, and the overly-accommodating” (rs = −.31 to −.39, ps ≤. 07). (See Table 4).
Table 4.
Pearson Correlations Between IIP-64 Subscales and Anger Processing Accuracy among Individuals with AUD.
| IIP-64 Subscale | Anger Accuracy Correlation [r(p)] |
|---|---|
| Intrusive / Needy | −.388 (.023) |
| Nonassertive | −.354 (.040) |
| Overly Accommodating | −.313 (.071) |
| Vindictive / Self Centered | −.285 (.102) |
| Domineering / Controlling | −.270 (.122) |
| Socially Inhibited | −.264 (.131) |
| Cold / Distant | −.243 (.166) |
| Self-Sacrificing | −.168 (.342) |
Discussion
Building on an emerging empirical literature on alcohol-related compromise in emotion processing, this study sought to replicate and extend previous work by investigating brain electrophysiology and behavioral outcomes during EFE processing, evaluating the role of valence, and exploring correlates of self-reported interpersonal problems. The inclusion of both behavioral and neurophysiological measures is novel, represents a substantive extension of our prior work, and is directly responsive to existing gaps in the literature. Our current findings offer partial support for a specific deficit in emotion processing in AUDs. As hypothesized, we identified an alcohol-related deficit in accuracy on a two-choice emotion discrimination. Also consistent with predictions and published work (e.g., Carmona-Perera et al., 2014; Frigerio et al., 2002; Kornreich et al, 2013; Quaglino et al., 2015; Salloum, et al, 2007 but see Maurage et al 2008a, 2008b; Kornreich et al, 2001a, 2001b), we found some support for the prediction that negative emotions are more sensitive to alcohol effects than are positive emotions. Interestingly, we observed an effect largely limited to anger processing, a finding consistent with outcomes of a recent recent meta-analysis (Bora & Zorlu, 2017). Finally, although reported by others (e.g., Philippot et al, 1999) performance in our AUD group was not differentially contingent on emotion intensity. As anticipated,the SJT, administered to control for differences in face processing did not reveal group differences on behavioral measures. Thus, behavioral deficits in emotion identification evidenced by the AUD group could not be attributed to generalized compromise in face processing.
ERPs, having played a critical role in clarifying alcohol-related alterations in underlying neural activity for decades (Rangaswamy & Porjesz, 2014; Porjesz & Begleiter, 2003), are receiving renewed attention as their relevance to functional measures is better appreciated (Campanella, et al, 2018). While recognizing that diverse ERP components might be interrogated, we constrained our current focus. Here, to provide better comparison with extant literature and establish the groundwork for our own developing work in the area, we considered only the N170 and P3 components. Contrary to much published work (see Kornreich et al, 2001, 2003; Maurage et al, 2008b) and our own predictions, we failed to observe alcohol-related ERP deficits specific to emotion processing. In fact, the N170 amplitude and P3 latency were fully uninformative. The P3 amplitude was sensitive to intensity, emotional valence and suggested a group difference with the AUD group producing lower amplitudes. However, group differences in P3 amplitude were also obtained in the SJT. When this effect was accounted for, the group difference in emotion processing was eliminated. Thus, in contrast to the accuracy data, the ERP data fail to support predictions of an emotion-specific processing deficit. Rather, they are consistent with a large literature demonstrating an alcohol-related generalized dysregulation in the processing of task-relevant stimuli (see Rangaswamy & Porjesz, 2014).
Characterizing emotion processing in persons with AUDs has important implications. Interpersonal functioning plays a crucial role in AUD recovery (Marlatt, 1996) and decrements in function are associated with increased likelihood of treatment drop-out (Foisy et al., 2007). Although current literature is limited, direct associations between poorer emotion recognition and interpersonal problems are also reported (Maurage et al., 2009; Kornreich et al., 2002).
In the current study, among individuals with AUDs, accuracy in anger identification was negatively correlated with self-reported interpersonal problems, as reflected on the IIP-64 (Horowitz et al, 2000). In completing the IIP-64, respondents endorse the extent to which each of 64 individual statements apply to themselves. Examples include items such as “It is hard for me to be firm when I need to be” and “I tell personal things to other people too much”. After completion, numerical scores for each of 8 subscales are obtained. Among the eight subscales, only three were meaningfully related to accurately identifying anger; the intrusive/needy, nonassertive, and overly-accommodating subscales. As noted earlier, scores on IIP-64 subscales represent a relative position along the affiliation and dominance dimensions. The three subcales that were significantly related to anger accuracy vary in their relative position on the dominance dimension. However, they share relatively high positions (loadings) on the affiliation subscale (Horowitz et al, 2000). For example, persons who score high on the intrusive/needy subscale have a strong need for social engagement and sufficient difficulty being alone that they may impose themselves in other’s business and take responsibility for solving others’ problems. Their poor boundaries eventually lead to interpersonal problems. Persons with higher scores on the nonassertive subscale are characterized by low self-esteem and difficulty in taking the initiative or assuming leadership roles. Because they fear disapproval, they avoid expressing their own opinions or desires. Finally, higher scores on the overly accommodating scale are associated with difficulty in saying “no” to others and in expressing anger, as well as avoiding interactions that might create hostility. Identifying the commonalities underlying these scales and appreciating their relationship to anger processing may suggest potential targets for behavioral interventions directed to enhancing functional outcomes.
Limitations
Despite its strengths, the paper has limitations. 1) The clinical sample was limited to treatment-seekers with AUDs, but without significant psychiatric and medical comorbidities. This restriction constrains generalization and may have contributed to the relatively subtle group differences. Although generalization may be limited, these restrictions afford better opportunity to examine the impact on neurobehavioral processes of the target substance, per se. 2) Relatedly, polysubstance use was prevalent and may have constrained interpretation. To address this issue, participants in the AUD group were required to meet clinical criteria for an AUD diagnosis and to report a level of alcohol consumption at least equivalent to a pattern of heavy drinking. Table 1 reflects the fact that our sample met this criterion. In larger samples, subgroups, defined by “other” drug, can be compared. Unfortunately, study limitations constrained such options. 3) Study design factors such as sample size, the range of tested emotions, and the limited number of assessments limits generalization and interpretation. Although generalization is constrained, we believe that this well-controlled study, which examines the link between emotion processing and functional measures as well as overarching group differences, informs the larger question and directs further work. 4) Sex differences were not studied. Given our continuing interest in sex differences (Lewis et al, 2019; Nixon et al, 2014), we regret that we did not have sufficient numbers to explore sex differences in this study. Given the presumed role of emotion and distress in women’s drinking, this is a critical area of study.
Conclusion
In summary, this investigation provides a substantive contribution to the literature focusing on alcohol-related compromise in emotion processing and interpersonal function. As predicted, the AUD group was less accurate in identifying emotion. Of particular note were provocative findings suggesting that processing angry faces was differentially challenging among persons with AUD. Further, the association identified between difficulty in anger processing and interpersonal problems appears to be largely driven by relationships in the affiliation rather than dominance subscales. Somewhat surprising was the absence of emotion-specific alterations in ERP measures, although non-specific deficits were observed. Taken together, these data suggest that expanded study that incorporates more complex emotional stimuli and interrogates related aspects of social cognition is needed.
Acknowledgements and Disclosures
The authors thank the participating treatment facilities, their clients, and the community participants. Additional thanks to previous and current laboratory members who assisted with study conduct including Robert Prather, B.A., Jeff Boissoneault, Ph.D., Alfredo Sklar M.D., Ph.D., Ian Frazier, M.S., Layla Lincoln, B.A., and Christian Garcia, M.S., who assisted in task development, data collection, and initial analyses.
Study conduct and data collection were supported by NIAAA grant R01AA022456–01 (PI: S.J. Nixon) and represents a component of Dr. Hoffman’s graduate work (UF Department of Psychology). Additional support was provided by K01AA026893 (PI: B. Lewis) and R03AA025430 (PI: S.J. Nixon). None of the authors have any financial or intellectual conflict of interest. NIAAA was not involved in the analysis, interpretation, or conclusions and the work does not necessarily represent the opinions or policy of the NIAAA.
Funding: Study conduct and data collection were supported by NIAAA grant R01AA022456–01 (PI: S.J. Nixon). Additional support was provided by K01AA026893 (PI: B. Lewis) and R03AA025430 (PI: S.J. Nixon). NIAAA was not involved in the analysis, interpretation or conclusions. The work does not necessarily represent the opinions or policy of the Institute.
References
- American Psychiatric Association & American Psychiatric Association Task Force on DSM-IV 1994. Diagnostic and statistical manual of mental disorders: DSM-IV, Washington, DC, American Psychiatric Association. [Google Scholar]
- Bates ME, Bowden SC & Barry D (2002) Neurocognitive impairment associated with alcohol use disorders: Implications for treatment. Exp Clin Psychopharmacol 10: 193–212. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA & Brown GK (1996) Beck depression inventory, second edition, San Antonio, The Psychological Corporation [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E & Mccarthy G (1996) Electrophysiological studies of face perception in humans J Cogn Neurosci 8: 551–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E & Zorlu N (2017) Social cognition in alcohol use disorder: A meta-analysis Addiction 112: 40–48. [DOI] [PubMed] [Google Scholar]
- Cahalan D, Cissin L & Crossley H 1969. American drinking practices: A national study of drinking behaviors and attitudes. (Monograph no 6), New Brunswick, NJ, Rutgers Center of Alcohol Studies. [Google Scholar]
- Campanella S, Schroder E, Kajosch H, Noel X & Kornreich C (2018) Why cognitive event-related potentials (ERPs) should have a role in the management of alcohol disorders. Neurosci Biobehav Rev. (Epub) [DOI] [PubMed] [Google Scholar]
- Carmona-Perera M, Clark L, Young L, Perez-Garcia M & Verdejo-Garcia A (2014) Impaired decoding of fear and disgust predicts utilitarian moral judgment in alcohol-dependent individuals. Alcohol Clin Exp Res 38: 179–185. [DOI] [PubMed] [Google Scholar]
- Ceballos NA, Tivis R, Lawton-Craddock A & Nixon SJ (2005) Visual-spatial attention in alcoholics and illicit stimulant abusers: Effects of nicotine replacement. Prog Neuropsychopharmacol Biol Psychiatry 29: 97–107. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, Reynaud M & Martinot JL (2007) Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology 32: 429–438. [DOI] [PubMed] [Google Scholar]
- Donadon MF & Osorio Fde L (2014) Recognition of facial expressions by alcoholic patients: A systematic literature review. Neuropsychiatr Dis Treat 10: 1655–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman PFW (1976) Pictures of facial affect, Palo Alto (CA), Consulting Psychologists Press. [Google Scholar]
- Fein G, Key K & Szymanski MD (2010) Erp and rt delays in long-term abstinent alcoholics in processing of emotional facial expressions during gender and emotion categorization tasks. Alcohol Clin Exp Res 34: 1127–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foisy ML, Kornreich C, Fobe A, D’hondt L, Pelc I, Hanak C, Verbanck P & Philippot P (2007) Impaired emotional facial expression recognition in alcohol dependence: Do these deficits persist with midterm abstinence? Alcohol Clin Exp Res 31: 404–410. [DOI] [PubMed] [Google Scholar]
- Frigerio E, Burt DM, Montagne B, Murray LK & Perrett DI (2002) Facial affect perception in alcoholics. Psychiatry Res 113: 161–171. [DOI] [PubMed] [Google Scholar]
- Horowitz Lm AL, Wiggins Js 2000. Manual for the inventory of interpersonal problems, San Antonio (TX), Psychological Corporation. [Google Scholar]
- Jung TP, Makeig S, Mckeown MJ, Bell AJ, Lee TW & Sejnowski TJ (2001) Imaging brain dynamics using independent component analysis. Proceedings of the IEEE Institute of Electrical and Electronics Engineers 89: 1107–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornreich C, Blairy S, Philippot P, Dan B, Foisy M, Hess U, Le Bon O, Pelc I & Verbanck P (2001) Impaired emotional facial expression recognition in alcoholism compared with obsessive-compulsive disorder and normal controls. Psychiatry Res 102: 235–248. [DOI] [PubMed] [Google Scholar]
- Kornreich C, Blairy S, Philippot P, Hess U, Noel X, Streel E, Le Bon O, Dan B, Pelc I & Verbanck P (2001) Deficits in recognition of emotional facial expression are still present in alcoholics after mid- to long-term abstinence. J Stud Alcohol 62: 533–542. [DOI] [PubMed] [Google Scholar]
- Kornreich C, Brevers D, Canivet D, Ermer E, Naranjo C, Constant E, Verbanck P, Campanella S & Noel X (2013) Impaired processing of emotion in music, faces and voices supports a generalized emotional decoding deficit in alcoholism. Addiction 108: 80–88. [DOI] [PubMed] [Google Scholar]
- Kornreich C, Foisy ML, Philippot P, Dan B, Tecco J, Noel X, Hess U, Pelc I & Verbanck P (2003) Impaired emotional facial expression recognition in alcoholics, opiate dependence subjects, methadone maintained subjects and mixed alcohol-opiate antecedents subjects compared with normal controls. Psychiatry Res 119: 251–260. [DOI] [PubMed] [Google Scholar]
- Kornreich C, Philippot P, Foisy ML, Blairy S, Raynaud E, Dan B, Hess U, Noel X, Pelc I & Verbanck P (2002) Impaired emotional facial expression recognition is associated with interpersonal problems in alcoholism. Alcohol Alcohol 37: 394–400. [DOI] [PubMed] [Google Scholar]
- Le Berre AP, Pitel AL, Chanraud S, Beaunieux H, Eustache F, Martinot JL, Reynaud M, Martelli C, Rohlfing T, Sullivan EV & Pfefferbaum A (2014) Chronic alcohol consumption and its effect on nodes of frontocerebellar and limbic circuitry: Comparison of effects in france and the united states. Hum Brain Mapp 35: 4635–4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B, Price JL, Garcia CC & Nixon SJ (2019) Emotional face processing among treatment-seeking individuals with alcohol use disorders: Investigating sex differences and relationships with interpersonal functioning. Alcohol Alcohol (Epub). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ (2014) An introduction to the event-related potential technique, Cambridge, Massachusetts, The MIT Press. [Google Scholar]
- Luck SJ & Kappenman ES (2012) Oxford handbook of event-related potential components, Oxford, Oxford University Press. [Google Scholar]
- Marinkovic K, Oscar-Berman M, Urban T, O’reilly CE, Howard JA, Sawyer K & Harris GJ (2009) Alcoholism and dampened temporal limbic activation to emotional faces. Alcohol Clin Exp Res 33: 1880–1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlatt GA (1996) Taxonomy of high-risk situations for alcohol relapse: Evolution and development of a cognitive-behavioral model. Addiction 91 Suppl: S37–49. [PubMed] [Google Scholar]
- Maurage P, Campanella S, Philippot P, Charest I, Martin S & De Timary P (2009) Impaired emotional facial expression decoding in alcoholism is also present for emotional prosody and body postures. Alcohol Alcohol 44: 476–485. [DOI] [PubMed] [Google Scholar]
- Maurage P, Campanella S, Philippot P, De Timary P, Constant E, Gauthier S, Micciche ML, Kornreich C, Hanak C, Noel X & Verbanck P (2008b) Alcoholism leads to early perceptive alterations, independently of comorbid depressed state: An erp study. Neurophysiol Clin 38: 83–97. [DOI] [PubMed] [Google Scholar]
- Maurage P, Campanella S, Philippot P, Martin S & De Timary P (2008a) Face processing in chronic alcoholism: A specific deficit for emotional features. Alcohol Clin Exp Res 32: 600–606. [DOI] [PubMed] [Google Scholar]
- Maurage P, Campanella S, Philippot P, Vermeulen N, Constant E, Luminet O & De Timary P (2008c) Electrophysiological correlates of the disrupted processing of anger in alcoholism. Int J Psychophysiol 70: 50–62. [DOI] [PubMed] [Google Scholar]
- Maurage P, Philippot P, Joassin F, Pauwels L, Pham T, Prieto EA, Palmero-Soler E, Zanow F & Campanella S (2008d) The auditory-visual integration of anger is impaired in alcoholism: An event-related potentials study. J Psychiatry Neurosci 33: 111–122. [PMC free article] [PubMed] [Google Scholar]
- Maurage P, Philippot P, Verbanck P, Noel X, Kornreich C, Hanak C & Campanella S (2007) Is the P300 deficit in alcoholism associated with early visual impairments (P100, N170)? An oddball paradigm. Clin Neurophysiol 118: 633–644. [DOI] [PubMed] [Google Scholar]
- Mognon A, Jovicich J, Bruzzone L & Buiatti M (2011) Adjust: An automatic eeg artifact detector based on the joint use of spatial and temporal features. Psychophysiology 48: 229–240. [DOI] [PubMed] [Google Scholar]
- Monnot M, Nixon S, Lovallo W & Ross E (2001) Altered emotional perception in alcoholics: Deficits in affective prosody comprehension. Alcohol Clin Exp Res 25: 362–369. [PubMed] [Google Scholar]
- Muller-Oehring EM & Schulte T (2014) Cognition, emotion, and attention. Handb Clin Neurol 125: 341–354. [DOI] [PubMed] [Google Scholar]
- Nixon SJ, Lawton-Craddock A, Tivis RD & Ceballos NA (2007) Nicotine’s effects on attentional efficiency in alcoholics. Alcohol Clin Exp Res 31: 2083–2091. [DOI] [PubMed] [Google Scholar]
- Nixon SJ, Prather R & Lewis B (2014) Sex differences in alcohol-related neurobehavioral consequences Handb Clin Neurol 125: 253–272. [DOI] [PubMed] [Google Scholar]
- Nixon SJ, Tivis R & Parsons OA (1992) Interpersonal problem-solving in male and female alcoholics. Alcohol Clin Exp Res 16: 684–687. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Hancock M, Mildworf B, Hutner N & Weber DA (1990) Emotional perception and memory in alcoholism and aging. Alcohol Clin Exp Res 14: 383–393. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Valmas MM, Sawyer KS, Ruiz SM, Luhar RB & Gravitz ZR (2014) Profiles of impaired, spared, and recovered neuropsychologic processes in alcoholism. Handb Clin Neurol 125: 183–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippot P, Kornreich C, Blairy S, Baert I, Den Dulk A, Le Bon O, Streel E, Hess U, Pelc I & Verbanck P (1999) Alcoholics’ deficits in the decoding of emotional facial expression. Alcohol Clin Exp Res 23: 1031–1038. [PubMed] [Google Scholar]
- Polich J (2004) Clinical application of the p300 event-related brain potential. Phys Med Rehabil Clin N Am 15: 133–161. [DOI] [PubMed] [Google Scholar]
- Porjesz B & Begleiter H (2003) Alcoholism and human electrophysiology. Alcohol Res Health 27: 153–160. [PMC free article] [PubMed] [Google Scholar]
- Quaglino V, De Wever E & Maurage P (2015) Relations between cognitive abilities, drinking characteristics, and emotional recognition in alcohol dependence: A preliminary exploration. Alcohol Clin Exp Res 39: 2032–2038. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M & Porjesz B (2014) Understanding alcohol use disorders with neuroelectrophysiology. Handb Clin Neurol 125: 383–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN, Cottler L, Bucholz KK & Compton W (1995) The diagnostic interview schedule, version IV, St Louis, Washington University. [Google Scholar]
- Rossion B, Corentin J (2011) The N170: Understanding the time-course of face perception in the human brain, in The Oxford handbook of event-related potential components (Luck SJ, Kappenman ES eds), pp 115–142. Oxford University Press, New York. [Google Scholar]
- Salloum JB, Ramchandani VA, Bodurka J, Rawlings R, Momenan R, George D & Hommer DW (2007) Blunted rostral anterior cingulate response during a simplified decoding task of negative emotional facial expressions in alcoholic patients. Alcohol Clin Exp Res 31: 1490–1504. [DOI] [PubMed] [Google Scholar]
- Sawyer KS, Maleki N, Urban T, Marinkovic K, Karson S, Ruiz SM, Harris GJ & Oscar-Berman M (2019) Alcoholism gender differences in brain responsivity to emotional stimuli. eLife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton E, Tiffany St, Henningfield Je (2000) Alcohol craving questionnaire (acq-now): Background, scoring, and administration (manual), Baltimore, MD, Intramural Research Program, National Institute on Drug Abuse. [Google Scholar]
- Spielberger CD 1983. Manual for state-trait anxiety inventory, Palo Alto, CA, Consulting Psychologists Press. [Google Scholar]
- Townshend JM & Duka T (2003) Mixed emotions: Alcoholics’ impairments in the recognition of specific emotional facial expressions. Neuropsychologia 41: 773–782. [DOI] [PubMed] [Google Scholar]
- Uekermann J & Daum I (2008) Social cognition in alcoholism: A link to prefrontal cortex dysfunction? Addiction 103: 726–735. [DOI] [PubMed] [Google Scholar]