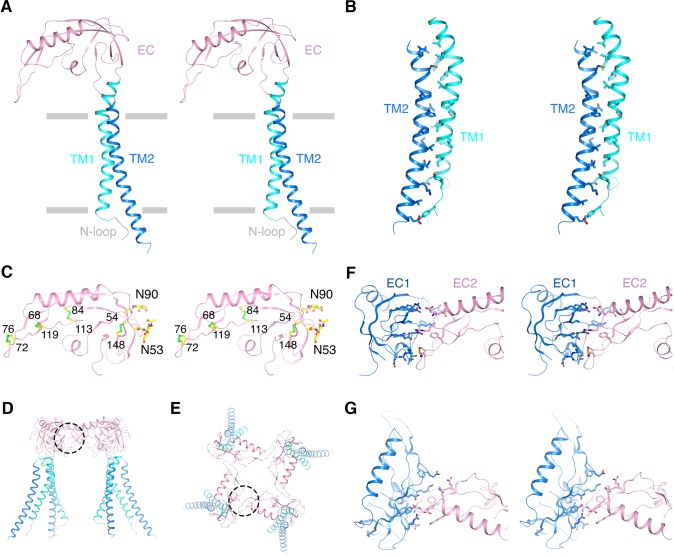
Figure 2. Architecture of the β4 subunit.
(A) Stereo view of the β4 subunit monomer in ribbon representation with the extracellular side up. The N-terminal loop (‘N-loop’), TM1, extracellular domain (‘EC’) and TM2 are discretely colored gray, cyan, pink and blue. The gray bars delimit the membrane boundaries. (B) TM1 and TM2 within one β4 subunit interact extensively with each other. TM1 and TM2 are shown as ribbons in stereo view. Residues involved in the interactions are shown in sticks and colored according to atom type. (C) The EC domain of β4 subunit in stereo, viewed parallel to the membrane. The protein is shown as ribbons and colored as in panel (A). Four disulfide bonds and 2 N-glycosylation sugar groups are shown as sticks and colored according to atom type. (D, E) The β4 subunit tetramer in ribbon representation viewed parallel to the membrane (D) or from the extracellular side (E). Interfaces between two neighboring EC domains are highlighted by dotted circles. (F, G) The interface between the EC domain of two neighboring β4 subunits viewed parallel to the membrane (F) or from the extracellular side (G). The two EC domains are colored blue and pink. Sidechains of residues at the interface are shown as sticks and colored according to atom type.
Figure 2—figure supplement 1. Sequence alignments.