It is now almost 30 years since tissue engineering has been officially established, to offer “the application of principles and methods of engineering and life sciences toward fundamental understanding of structure-function relationships in normal and pathological mammalian tissues and the development of biological substitutes to restore, maintain, or improve tissue function.” With end-stage heart and lung disease representing major medical problems worldwide, tissue engineering is focused on providing definitive solutions by repairing the heart, lung, and many other organs, in a way not limited by the shortage of donor organs. This issue of The Journal of Cardiovascular and Thoracic Surgery is focused on tissue engineering, giving us opportunity to reflect on the state of the art of the field and some recent developments.
Much has been learned during this time. Current tissue-engineering approaches are increasingly based on biologically inspired environments designed to inform and guide cell differentiation and functional assembly, towards re-establishing the organ function lost to injury or disease. While the three key components: cells (the actual “tissue engineers”), biomaterial scaffolds (structural and logistic templates for tissue formation) and the environmental signals (in vitro in bioreactors, or in vivo in the body) are still a basis of all tissue engineering systems, each of them has changed quite substantially. Young animal cells have been replaced by patient-specific stem/progenitor cells that are being derived from a number of tissues, differentiated into desired lineages, and gene-edited to study mechanisms of disease. Inert biomaterials, once considered ideal for cell culture and transplantation, have been replaced with biomaterials interacting with the cells via biochemical, mechanical and electrical signals. Bioreactors, used for culture of human cells on advanced scaffolds, are now able to capture many aspects of the native context of development or disease.
For over two decades, the main focus of cardiac tissue engineering: to develop a standard of care based on cell therapy of the heart, is still without clinical application, largely due to the lack of understanding of the underlying mechanisms of action. A most interesting result of clinical studies, that were mostly done using mesenchymal stem cells (MSC) to treat ischemic injury, is the discrepancy between the scarcity of cell engraftment and functional benefits, a finding strongly suggesting paracrine effects. In particular, the allogeneic and autologous MSCs had similar functional outcomes despite more rapid rejection of allogeneic MSCs1.
These findings bring up a logical question: is cell engraftment necessary for heart repair, and if so, for how long? The collective experience with better outcomes of cell injection into the heart compared to intravenous infusion suggests that the paracrine action requires some residence time for the cells in the zone of injury. It is possible that (i) improving cell retention (e.g., using a hydrogel to inject cells into the heart) and (ii) providing transient immunosuppression to allogeneic cells (derived from donors with high expression of CD34 and used off-the-shelf) may be an interesting option to explore.
In parallel, cardiac progenitor cells are being explored in primate studies2 and the first clinical trials of heart repair. Again, the question is if these cells need to engraft and form new muscle, or just provide paracrine signals that would initiate repair. Clearly, if cells are not used, or not required to engraft, this would also help avoid arrhythmias encountered in animal studies.
Other approaches are focused on cell-free therapies: if cells are acting through the factors they secrete, can we just use factors? There is growing evidence that paracrine factors are clustered in cell-secreted vesicles that are rich in μRNAs and other biological cargo. Notably, a study comparing the effects of cardiac cells and secreted vesicles on heart repair could not find much difference3, in support of the “paracrine hypothesis”. This opens a possibility of introducing an entirely new paradigm: cell-free heart repair using cocktails of cell-secreted factors which can be mass-produced in cell culture, isolated from culture medium, and delivered in a hydrogel patch. It would be of great interest to evaluate in more detail the paracrine effects of cardiac and non-cardiac therapeutic cells, and learn more about the key factors that can induce heart repair.
A related question is if biomaterials alone could be functionalized to provide the necessary regulatory cues. A recent study showed that epicardially-derived follistatin could rebuild heart muscle4. This simple and elegant approach utilized a collagen patch loaded with a single cardiokine that has multiple developmental and cardiogenic roles and is depleted from the heart following myocardial infarction. While previous studies showed the cardioprotective and vasculogenic effects of follistatin, this study resulted in an unexpected finding that follistatin secreted from the hydrogel patch induced proliferation of resident cardiomyocytes. One can envision a new cell-free approach to sustained delivery of regulatory factors, over times controlled by degradation and release kinetics of the collagen patch. Clearly, patch delivery could be extended to clusters of cardioprotective, cardiogenic and vasculogenic factors; to the delivery of cell-secreted vesicles to provide more comprehensive paracrine effects; and to studies of paracrine effects from various cell types and mechanisms of their paracrine action.
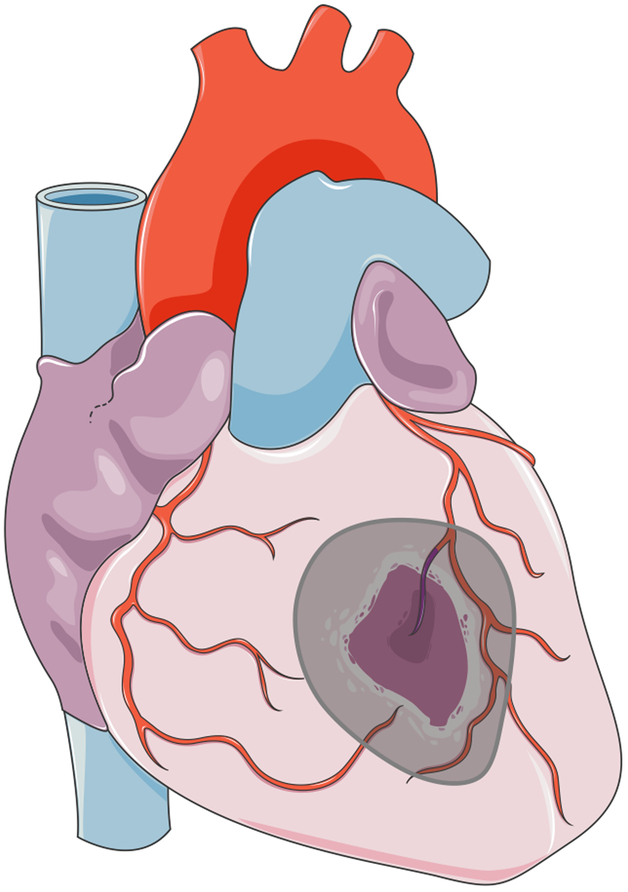
As many times in the past, cardiac field is being informed by advances in other fields of tissue engineering. A recent study5 reported development of an elastic “second skin” that can be applied topically to reduce skin herniation, re-establish barrier function, or deliver drugs. One can envision that a similar “epicardial skin” could be used for sustained delivery of biological cargo (such as the secretome from immature cardiomyocytes) (Figure 1). The most attractive features are the adherence of such “second skin”, the possibility of using both the encapsulation and immobilization chemistry, and the possibility of implementing minimally invasive treatment modalities. Taken together, these two articles4,5 from two very different areas of tissue engineering show us that tissue regeneration could indeed be achieved using simple methods that evolved from lessons learned in the past.
Figuere 1.
A patch for heart repair: cell therapy, or induction of endogenous repair?
In summary, regeneration of myocardium is certainly an area of great potential for therapeutic applications. Interestingly, the initial focus in the field is moving from remuscularization into revascularization, and from myocardial infarction into noinshemic cardiac diseases. However, cell and tissue therapies also carry risks – most notably arrhythmia, a complication observed in primate studies of cardiac repair using human stem cells. To avoid arrhythmia, the implanted tissue (or cells) would need to be matured to cease beating spontaneously, and instead start to display synchronized contractions in response to electrical signals. Development of protocols for maturation and synchronization of cardiac cells is one of the key challenges in the application of tissue engineering methodologies for heart repair. Another challenge of cell/tissue therapies is the incorporation of a vascularized network that can be anastomosed to the circulatory system of the host to provide immediate blood supply needed for cell survival. These increased risks, and the higher cost of cell-based treatment modalities – during all phases of their development, preclinical and clinical studies and implementation in patients – suggest that the clinical experience will continue to evolve using cell-free approaches, whenever possible. In principle, one can envision the use of just a biomaterial incorporating biologic factors that activate endogenous cells (cardiac and vascular) by triggering specific signaling pathways. Finally, the treatment modalities need to be simple in order to be adopted, while being able to support efficient use of therapeutic agents: cells, factors, or biomaterials, alone or in combinations. One of the future challenges will be the combination of tissue-engineering and endocardial delivery.
Perspective statement:
The state of the art of cardiac tissue engineering is an evolving paradigm: from cell therapy to the induction of endogenous repair mechanisms by bioactive factors and functionalized biomaterials.
Funding:
The author’s work in the area of cardiac tissue engineering has been supported by NIH (grants HL076485, EB17103, EB002520) and New York State Stem Cell Initiative (grant C028119).
Footnotes
Disclosure:
Author has nothing to disclose with regard to commercial support
References
- 1.Tano N, Kaneko M, Ichihara Y, Ikebe C, Coppen SR, Shiraishi M et al. Allogeneic Mesenchymal Stromal Cells Transplanted Onto the Heart Surface Achieve Therapeutic Myocardial Repair Despite Immunologic Responses in Rats. J Am Heart Assoc. 2016. 18:5(2). pii: e002815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chong JJ, Yang X, Don CW, Minami E, Liu YW, Weyers JJ et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014; 510: 273–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kervadec A, Bellamy V, El Harane N, Arakélian L, Vanneaux V, Cacciapuoti I et al. Cardiovascular progenitor-derived extracellular vesicles recapitulate the beneficial effects of their parent cells in the treatment of chronic heart failure. J Heart Lung Transplant. 2016; 35(6):795–807. [DOI] [PubMed] [Google Scholar]
- 4.Wei K, Serpooshan V, Hurtado C, Diez-Cuñado M, Zhao M, Maruyama S et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature. 2015; 525(7570):479–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu B, Kang SY, Akthakul A, Ramadurai N, Pilkenton M, Patel A et al. An elastic second skin. Nat Mater. 2016; May 9 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]