Abstract
Esophageal, esophago-gastric, and gastric cancers are major causes of cancer morbidity and cancer death. For patients with potentially resectable disease, multimodality treatment is recommended as it provides the best chance of survival. However, quality of life may be adversely affected by therapy, and with a wide variation in outcome despite multi-modality therapy, there is a clear need to improve patient stratification. Radiomic approaches provide an opportunity to improve tumor phenotyping. In this review we assess the evidence to date and discuss how these approaches could improve outcome in esophageal, esophago-gastric, and gastric cancer.
Keywords: Esophageal cancer, Esophagogastric junction cancer, Radiomics, Computed tomography, Magnetic resonance imaging, Positron emission tomography
The need for better patient stratification
Esophageal or esophago-gastric cancer (456,000 new cases annually) and gastric cancer (952,000 new cases annually) are leading causes of cancer deaths worldwide [1]. Above 50% of presenting patients are diagnosed with stage IV disease, precluding curative treatment. For patients with early stage disease, surgery, often combined with neoadjuvant chemotherapy or chemoradiotherapy, offers the best chance of cure [2–7].
As an example, data from the OEO2 and MAGIC trials for esophageal and esophago-gastric cancer have shown a 6% [3] and 13% [4] improvement in 5-year overall-survival, respectively. Trimodality therapy for esophageal and esophago-gastric cancer combining neoadjuvant chemo- and radiation-therapy in addition to surgery may also be superior to neoadjuvant chemotherapy alone in a selected patient population [8]. The CROSS trial [6] comparing neoadjuvant chemoradiotherapy plus surgery with surgery alone in patients with esophageal and esophago-gastric cancer showed a superior overall-survival of 49 vs. 24 months, hazard ratio 0.657, p = 0.003, and a pathological complete response rate of 29%, for patients with multi-modality treatment with no increase in surgical mortality (4% in surgery and trimodality groups, respectively).
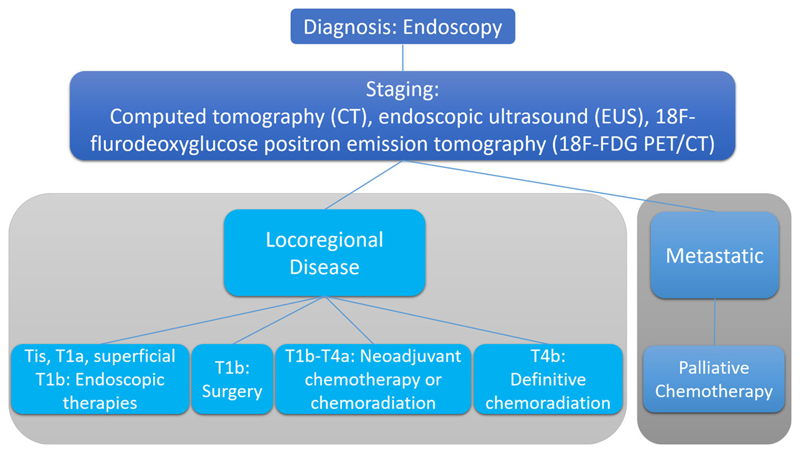
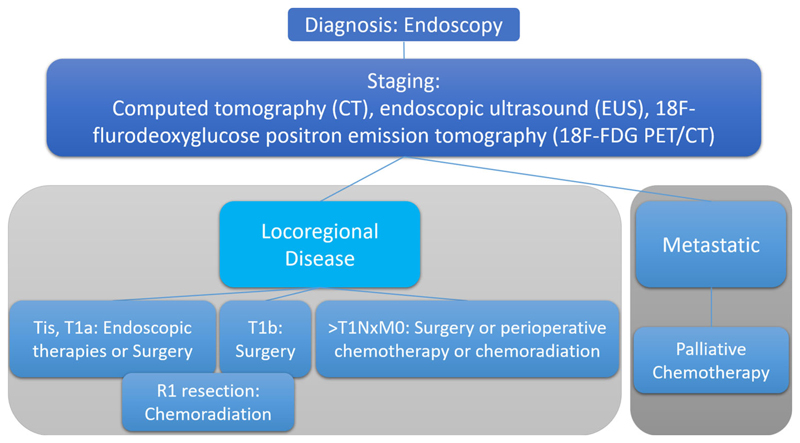
A recent systematic review and meta-analysis of neoadjuvant chemotherapy in patients with gastric cancer has also found improved 3-year survival rates (relative risk 1.30; 95% CI 1.06–1.59, p < 0.01) [9]. Typical management pathways are shown in Figs. 1 and 2 for esophageal, esophago-gastric, and gastric cancer, respectively. Nevertheless overall-survival remains poor despite these improvements in patient care.
Fig. 1.
Typical pathways for the management of patients with newly diagnosed esophageal and esophago-gastric cancer.
Fig. 2.
Typical pathways for the management of patients with newly diagnosed gastric cancer.
Recent genomic analyses have highlighted the genetic heterogeneity present in esophageal, esophago-gastric [10], and gastric cancer [11, 12] as an underlying cause for the differences in outcome and heterogeneity of response to therapy. Quality of life also remains poor for many patients post-surgery, taking up to 3 years to return to pre-therapy levels in patients undergoing esophageal resection [13]. Better patient stratification remains a key challenge for patients with upper gastrointestinal tract cancers.
The imaging pathway at staging
For esophageal and esophago-gastric cancer, contrast-enhanced computed tomography (CT) remains the most commonly performed first step in staging due to the high prevalence of metastatic disease at presentation [14–16]. For patients being considered for a curative pathway, endoscopic ultrasound (EUS) and 18F-fluorodeoxyglucose positron emission tomography/CT (18F-FDG PET/CT) are performed due to the high sensitivity and specificity of EUS for local tumor and nodal staging; and 18F-FDG PET for distant metastases [17–19]. This aims to reduce the futile surgery rate.
For gastric cancer, initial staging is again by contrast-enhanced CT. If curative treatment is being considered, the use of endoscopic ultrasound (EUS) is helpful in determining the proximal and distal extent of the tumor, whereas 18F-FDG PET/CT has been shown to improve staging by detecting involved lymph nodes and metastatic disease, although it can be less accurate in mucinous and diffuse tumors [20].
In esophageal cancer, PET has the potential to change management in up to a third of patients [21, 22], and is often incorporated into radiotherapy planning pathway [23, 24]. The American College of Surgeons Oncology Group reported sensitivity and specificity of 18F-FDG-PET/CT scans to be 79% and 95%, respectively [18].
Magnetic resonance imaging (MRI) is currently not recommended for the routine imaging of esophageal or gastric cancer. However, with the recent advent of hybrid PET/MRI systems in clinical practice, there has been growing interest in MRI’s ability as an assessment tool. MRI provides excellent soft-tissue contrast, and may demonstrate the esophageal wall layers and adjacent nodes. Physiological sequences (e.g., diffusion-weighted MRI) may also be included as part of the protocol. An initial staging 18F-FDG PET/MRI study with a pathology gold standard has been promising for nodal assessment with reported accuracy of 83% compared to 75% and 50% for EUS and CT, respectively [25].
A role for radiomics?
Radiomic approaches are showing promise for patient stratification. Radiomics exploit the data performed as part of the clinical management pathway. In terms of imaging, a number of parameters may be extracted and combined including standard descriptors (e.g., size, morphology, TNM (tumor, node, metastasis) stage); qualitative, semi-quantitative, or quantitative physiological parameters (e.g., contrast enhancement, diffusion characteristics, tracer uptake); and additional agnostic features which are otherwise ‘invisible’, with bioinformatic approaches. Of these, texture-based features have been investigated most commonly to date. Table 1 highlights some features that have been investigated in studies.
Table 1. Overview of features used in radiomics.
| Feature-group | Parameter examples |
|---|---|
| First-order-histogram statistics | Mean, median, skewness, kurtosis, energy (uniformity), entropy |
| Second-order gray-level co-occurrence matrix (GLCM) statistics | Entropy, homogeneity, energy (uniformity), contrast, autocorrelation, cluster shade, cluster prominence, difference entropy, difference variance, dissimilarity, inverse difference moment, maximum probability, sum average, sum entropy, sum variance |
| Second-order gray-level difference matrix (GLDM) statistics | Mean, entropy, variance, contrast |
| High-order neighborhood gray-tone difference matrix (NGTDM) statistics | Coarseness, contrast, busyness, complexity, texture strength |
| High-order gray-level run-length (GLRL or RLM) statistics | Short run emphasis, long run emphasis, gray-level nonuniformity, run-length nonuniformity, intensity variability, run-length variability |
| High-order gray-level size zone matrix (GLSZM) statistics | Short-zone emphasis, long-zone emphasis, intensity nonuniformity, zone percentage, intensity variability, size zone variability |
| Fractal analysis | Mean fractal dimension, standard deviation, lacunarity, Hurst component |
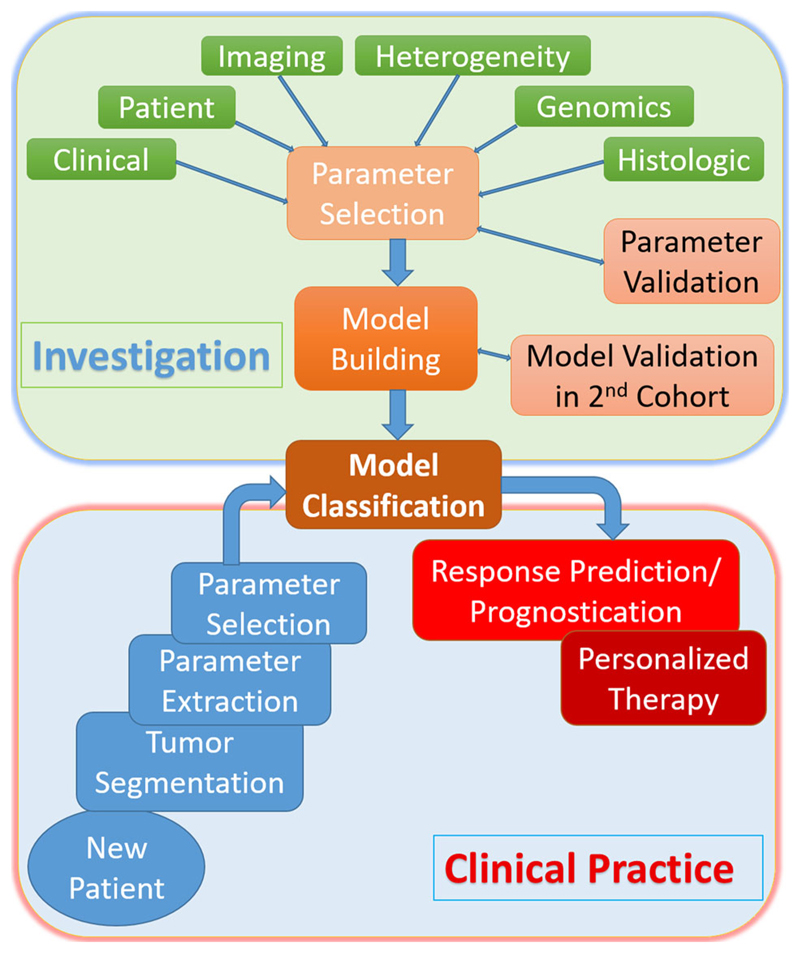
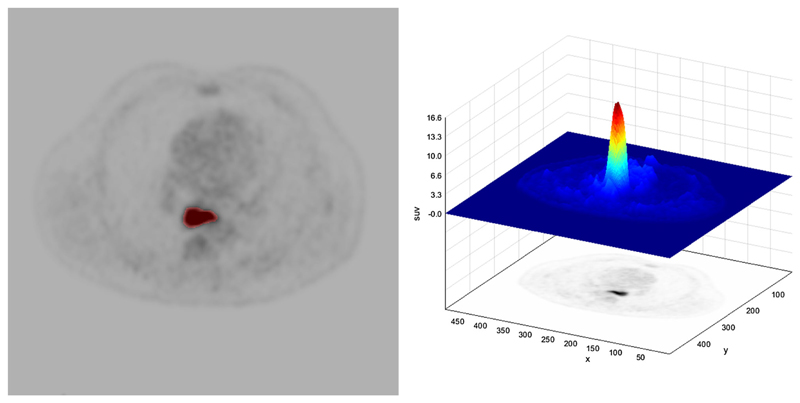
Radiomic signatures provide additional information predictive of underlying tumor biology and behavior. These signatures can be used alone or with other patient-related data (e.g., pathological data; genomic data) to improve tumor phenotyping, treatment response prediction and prognosis. Radiomic signatures may be obtained for all cross-sectional imaging modalities, including CT, PET, and MRI. figure 3 illustrates a typical radiomics pipeline.figure 4 demonstrates the process of tumor segmentation for a 18F-FDG PET image with a corresponding plot of standardized uptake value for the tumor. Initial studies in esophageal, esophago-gastric, and gastric cancer have shown promise for patient care.
Fig. 3.
Schema demonstrating typical radiomics pipeline.
Fig. 4.
Example of tumor segmentation for extraction of radiomic features from an axial PET image. In the right image the corresponding standardized uptake values for the region-of-interest is displayed.
18F-FDG PET radiomics
Nine 18F-FDG PET studies have been performed in esophageal and esophago-gastric cancer and are summarized in Table 2. As yet no studies have been performed for gastric cancer. Studies to date have focused on the prediction of response or prognosis in comparison to standard practice. Studies have found that various first, second and high-order features have been contributory to the assessment of response, differentiating between responders and non-responders (with greater heterogeneity in non-responders), as well as being predictive of complete response. Performance has been better than conventional parameters alone. Prognostication data remain conflicting.
Table 2. Radiomic studies using PET in esophageal cancer.
| Author | PET time point | Therapy | Features assessed | Outcome and methods | Findings |
|---|---|---|---|---|---|
| Tixier et al. [26] n = 41 SCC:31 AC:10 |
Pre | CRT: 60 Gy with cisplatin or carboplatin/fluorouracil | 38 features including: First order statistics GLCM RLM GLSZM NGTDM |
Response prediction: AUROC | Tumor GLCM homogeneity, GLCM entropy, RLM intensity variability and GLSZM size zone variability can differentiate non-responders, partial responders, and complete-responders with higher sensitivity (76%–92%) than any SUV measurement |
| Beukinga et al. [27] n = 97 AC:88 SCC:9 |
Pre | CRT: 41.4 Gy with carboplatin/paclitaxel | 88 features including: First order statistics Geometry GLCM NGTDM |
Response prediction: Models constructed with least absolute shrinkage and selection operator regularized logistic regression | 18F-FDG long run low gray level emphasis higher in responders than non-responders Model including histologic type, clinical T stage, 18F-FDG long run low gray level emphasis and non-contrast CT run percentage has higher AUC than SUVmax: 0.74 vs. 0.54 (after internal validation) |
| Nakajo et al. [28] n = 52 SCC |
Pre | CRT: 41–70 Gy with cisplatin/5-flurouracil | GLCM: Entropy, homogeneity, dissimilarity; GLSZM: Intensity variability, Size-zone variability, zone percentage |
Response prediction: AUROC Prognostication: Multivariable cox analysis |
GLSZM intensity variability and GLSZM size-zone variability predictive of response No texture parameter independently associated with progression free or overall survival |
| Paul et al. [29] n = 65 SCC:57 AC:8 |
Pre | CRT: 50 Gy with platinum chemotherapy & 5-flurouracil | 84 features including: First order statistics GLCM GLSZM GLDM |
Response prediction Prognostication: Multivariable cox analysis |
Best subset of predictive variables: metabolic tumor volume, GLCM homogeneity Best subset of prognostic variables: WHO performance status, nutritional risk index, metabolic tumor volume |
| Foley et al. [17] n = 403 AC:237 + 79 SCC:65 + 22 |
Pre | NACT, NACRT, dCRT: not specified | First order statistics GLCM: homogeneity, entropy, dissimilarity; coarseness; GLSZM: intensity variability, large area emphasis, zone percentage; |
Prognostication: Multivariable cox analysis | TLG, histogram energy and histogram kurtosis are independently associated with overall survival |
| Tan et al. [31] n = 20 AC:17 SCC:3 |
Pre-post | CRT: 50.4 Gy with cisplatin/fluorouracil | 192 features including: First order statistics GLCM |
Response prediction: AUROC | SUVmean decline, SUV skewness, GLCM inertia, GLCM correlation, and GLCM cluster prominence are predictors of complete response with AUC 0.76–0.85 |
| Van Rossum et al. [32] n = 217 AC |
Pre-post | CRT: 45 or 50.4 Gy with fluoropyrimidine and either a platinum compound or taxane | 86 features including: First order statistics Geometry GLCM NGTDM |
Response prediction: Multivariable Cox analysis | Feature selection by univariable logistic regression Model including induction chemotherapy, change in RLM run percentage, change in GLCM entropy, and post –CRT roundness is better than clinical prediction model |
| Yip et al. [33] n = 45 AC:44 SCC:1 |
Pre-post | CRT: 45–50.4 Gy with cisplatin, 5-flurouracil, irinotecan/paclitaxel or carboplatin/paclitaxel | GLCM: homogeneity, entropy RLM: high gray run emphasis, short-run high gray run emphasis GLSZM: high gray zone emphasis, shortzone high gray emphasis |
Response prediction: AUROC | Response prediction: Change in run length and size zone matrix parameters differentiates non-responders from artial/complete responders (AUC: 0.71–0.76) |
| Beukinga et al. [33] n = 70 AC:65 SCC:8 |
Pre-post | CRT: 41.4 Gy in 23 fractions with carboplatin/paclitaxel | 113 features including: First order statistics Geometry Local intensity GLCM GLSZM NGTDM |
Response prediction: Models constructed with least absolute shrinkage and selection operator regularized logistic regression | Prediction model composed of clinical T-stage and post-NCRT joint maximum adds important information to the visual PET/CT evaluation of a pathologic complete response |
Studies are ordered after time point of imaging (pre therapy, or pre-post therapy imaging), evaluated outcome (response prediction, prognostication, or both), and finally chronologically dCRT definitive chemoradiation; NACT neoadjuvant chemotherapy; NACRT neoadjuvant chemoradiation; GLCM gray-level co-occurrence matrix; GLDM gray-level difference matrix, GLSZM gray-level size zone matrix; NGTDM neighborhood gray tone difference matrix; RLM gray-level run-length matrix; AUROC area under the receiver operative curve; AUC area under the curve
In greater detail, five studies have investigated the prediction of response to therapy alone (n = 2); prognosis alone (n = 1) and the prediction of response to therapy and prognosis (n = 2) from pre-therapy imaging. One of the earliest studies by Tixier et al. showed in 41 patients that gray-level co-occurrence matrix (GLCM) homogeneity, GLCM entropy, gray-level size-zone matrix (GLSZM) size-zone variability and run length matrix (RLM) intensity variability differentiated non-responders, partial-responders, and complete-responders with sensitivities of 76%–92% [26]. Beukinga et al. showed in 97 patients that a clinical model including PET-derived gray-level run length (GLRL) long run low gray level emphasis and CT-derived run percentage had a higher area under the receiver operator curve (AUROC) compared to maximum standardized uptake value (SUVmax) in predicting therapy response [27].
In a study of 52 patients with squamous cancers, Nakajo et al. found that 18F-FDG PET/CT GLSZM intensity variability, and GLSZM size-zone variability, as well as standard volumetric parameters, such as metabolic tumor volume (MTV) and total lesion glycolysis (TLG), were predictors of tumor response but not of progression free or overall-survival [28]. Non-responders showed significantly higher intensity variability and size-zone variability. Similarly, in a study of 65 patients Paul et al. found that a model incorporating GLCM homogeneity was a predictor of response (with an AUROC value of 0.823) but not of survival [29]. However, in a larger study with 403 patients, Foley et al. found that total lesion glycolysis, histogram energy and kurtosis were independently associated with overall-survival [30].
Four studies have assessed pre- and post-therapy 18F-FDG PET imaging. An initial study by Tan et al. found in 20 patients that 2 SUVmean parameters, SUVmean decline and SUVmean skewness, and 3 texture features GLCM inertia, GLCM correlation, and GLCM cluster prominence, were significant predictors of complete response with an AUROC of 0.76 [31]. In 217 patients with adenocarcinoma, Van Rossum et al. developed a prediction model which included change in run length matrix (RLM) run percentage, change in GLCM entropy, and post–chemoradiation roundness, and increased the corrected c-index (concordance-index, comparable to AUROC) from 0.67 to 0.77, compared to the clinical model alone [32]. Yip et al. found that a change in run length and size-zone matrix differentiated responders from non-responders [33]. More recently, Beukinga et al. found that clinical T-staging combined with post-chemoradiotherapy 18F-FDG PET orderliness provided high discriminatory accuracy in predicting pathologic complete response compared to clinical variables or SUVmax alone [34].
CT radiomics
Nine studies have investigated the ability of CT-derived heterogeneity parameters for classification, prediction of response and overall-survival in patients with esophageal or gastric cancer. Three studies have been performed for esophageal cancer in terms of prediction of response or prognosis (Table 3). These have found that greater heterogeneity is present in non-responders and those with poorer outcome.
Table 3. Radiomic studies using CT in esophageal cancer.
| Author | CT time point & type | Therapy | Features assessed | Outcome and methods | Findings |
|---|---|---|---|---|---|
| Hou et al. [35] n = 49 SCC |
Pre Contrast enhanced |
CRT: 60 Gy with nedaplatin/docetaxel or nedaplatin/paclitaxel | 214 features including: First order statistics Geometry GLCM RLM GLSZM NGTDM with Gabor transformation or Gaussian filtration |
Response prediction Feature selection: AUROC Prediction model: support vector machine and artificial neural network |
Features discriminating non-responders from responders: skewness, kurtosis, GLSZM long zone emphasis, Gabor_MSA-54, Gabor2D_MSE-54 |
| Ganeshan et al. [36] n = 21 AC:14 SCC:7 |
Pre Non-contrast from PET/CT |
No information available | First order statistics with Gaussian filtration | Prognostication: Kaplan–Meier analysis | High uniformity is an independent predictor of survival. Lower uniformity is associated with a poorer overall survival |
| Yip C. et al. [37] n = 36 AC:9 SCC:26 |
Pre-post Contrast enhanced Arterial Portal venous |
CRT: 50 Gy with cisplatin/5-flurouracil or single agent platinum/5-flurouracil | First order statistics with Gaussian filtration | Prognostication: Kaplan-Meier analysis Cox analysis |
Higher post treatment entropy (medium/coarse) independently associated with poorer overall survival |
CRT neoadjuvant chemo-radiotherapy; NACT neoadjuvant chemotherapy; AC adenocarcinoma; SCC squamous cell carcinoma; Th thorax; Ab abdomen; RLM gray-level run-length matrix; GLCM gray-level co-occurrence matrix; GLSZM gray-level size zone matrix; NGTDM neighborhood gray tone difference matrix; OS overall survival
The largest study in 49 patients found that histogram skewness, histogram kurtosis, GLSZM long-zone emphasis, and 2 Gabor transformed parameters MSA-54 and MSE-54, discriminated non-responders from responders using an artificial neural network-derived prediction model [35]. The two remaining studies have assessed prognostication. Ganeshan et al. found that lower histogram uniformity (with Gaussian filtration) from unenhanced CT images before start of treatment was an independent predictor for poorer overall-survival [36].Yip et al. analyzed contrast-enhanced images of 36 patients before and after treatment and found a significant decrease in histogram entropy and increase in uniformity (with Gaussian filtration) between the two time points. Higher post treatment entropy was associated with poorer overall-survival [37].
For gastric cancer (Table 4), three studies have assessed the potential of radiomic approaches for classification. Studies have found that first and second-order analysis in the contrast-enhanced images may help in differentiation of lymphoma from gastrointestinal stromal cancer [38] or adenocarcinoma [39]. Another study in 107 patients found that arterial phase standard deviation and entropy were correlated with poorer differentiation [40]. For prognostication, Yoon et al. investigated 26 HER2 + gastric cancer patients before trastuzumab-treatment. In their analysis, they found GLCM contrast, variance, correlation and angular second moment (also known as energy or uniformity) were associated with a poorer survival [41]. In another study, Giganti et al. showed in 56 patients, that first-order energy, entropy, and skewness were significantly associated with a negative prognosis [42]. Giganti et al. also assessed pre chemotherapy texture features derived from the late arterial phase of 34 patients. They found entropy and compactness were higher and uniformity lower in responders [43]. No studies have assessed prognostication or response to therapy in gastric cancer.
Table 4. Radiomic studies using CT in gastric cancer.
| Author | CT time point & type | Therapy | Features assessed | Outcome and method | Findings |
|---|---|---|---|---|---|
| Ba-Ssalamah et al. [38] n = 67 (Art) AC:47 GIST:15 Lymphoma:5 n = 73 (PV) AC:48 GIST:17 Lymphoma:5 |
Pre Contrast enhanced Arterial Portal venous |
Not applicable | First order statistics RLM GLCM Absolute gradient Autoregressive model, wavelet transformation |
Classification: linear discriminant analysis | Classification of lymphoma vs. AC or GIST feasible on arterial phase: AC vs. lymphoma: 3.1% misclassification GIST vs. lymphoma: 0% misclassification on arterial CT Classification of AC vs. GIST feasible on venous phase: 10% misclassification |
| Ma et al. [39] n = 70 AC:40 Lymphoma:30 |
Pre Contrast enhanced Portal venous |
Not applicable | First order statistics Geometry Texture analysis Wavelet transformation |
Feature selection: LASSO Classification: AUROC |
183 radiomic signature identified with potential to differentiate adenocarcinoma from lymphoma Model including histogram root mean squared, GLCM sum variance and absence of peristalsis: AUC 0.86; Sensitivity 70%, Specificity 100%, Accuracy 87% |
| Liu et al. [40] n = 107 AC:84 Signet Ring:5 Mucinous:3 Mixed:15 |
Pre Arterial Portal venous |
Surgery | First order statistics | Classification: AUROC | Arterial phase standard deviation and entropy; portal venous phase mean, max, mode, percentiles are predictive of poorer differentiation |
| Yoon et al. [41] n = 26 AC:25 Signet Ring:1 |
Pre Portal venous |
Trastuzumab-based chemotherapy | First order statistics: Kurtosis, Skewness GLCM: Angular second moment, contrast, entropy, variance, correlation |
Prognostication: AUROC Kaplan-Meier |
Lower contrast, variance and higher correlation are associated with poorer survival with AUC of 0.77, 0.75 and 0.77 respectively |
| Giganti et al. [42] n = 56 AC:37 Signet Ring:19 |
Pre Arterial |
Surgery | 107 features including: First order statistics GLCM RLM Geometry with Gaussian filtration |
Prognostication: Kaplan-Meier, Multivariable Cox analysis | Energy, entropy, skewness are associated with poorer prognosis |
| Giganti et al. [43] n = 34 AC:25 Signet Ring:6 |
Pre Arterial |
NACT: cisplatin/epirubicin/adriamycin/fluoruracil or cisplatin/epirubicin/aadriamycin/capecitabine | First order statistics GLCM Geometry |
Response prediction: Multivariable logistic model | Entropy and compactness are higher in responders and uniformity is lower in responders |
AC adenocarcinoma; GIST gastrointestinal stromal tumor; NACT neoadjuvant chemotherapy; RLM gray-level run-length matrix; GLCM gray-level co-occurrence matrix; AUROC area under the receiver operative curve
MRI radiomics
To date there have been little data for MRI in this tumor group as MRI is not performed routinely in the clinical pathway. There have been some exploratory data of pre-therapeutic ADC-maps of gastric cancer (Table 5). Liu et al. found that first-order statistics skewness may differ from node positive to node negative patients, and are associated with pathological characteristics including perineural and vascular invasion [44–47]. However, no studies so far have investigated prognostication or response assessment.
Table 5. Radiomic studies using MRI in gastric cancer.
| Author | MRI time point & sequence | Therapy | Features assessed | Outcome and methods | Findings |
|---|---|---|---|---|---|
| Liu et al. [44]a n = 80 AC:57 Signet ring:10 Mucinous:1 Mixed:12 |
Pre ADC-map |
Surgery | First order statistics | Staging: Prediction of tumor & nodal stage: AUROC analysis |
ADC histogram analysis may differentiate node positive from node negative disease e.g., Percentile ADC10 has an AUC of 0.79 and sensitivity, specificity and accuracy of 72%, 81% and 74% respectively No ability to differentiate T stage |
| Liu et al. [45]a n = 87 AC:60 Signet ring:11 Mucinous:1 Mixed:15 |
Pre ADC-map |
Surgery | First order statistics | Staging: Prediction of tumor & nodal stage: AUROC analysis |
Skewness yields a sensitivity and specificity of 76% and 81%, and an AUC of 0.80 for differentiating node positive from node negative gastric cancers |
| Zhang et al. [47]a n = 78 AC:58 Signet ring:11 Mucinous:1 Mixed:8 |
Pre ADC-map |
Surgery | First order statistics | Classification: AUROC analysis |
ADC histogram parameters differ between histological grades but with an AUROC < 0.70 this may not be useful in clinical practice |
| Liu et al. [46]a n = 64 AC:45 Signet ring:8 Mucinous:2 Mixed:9 |
Pre ADC-map |
Surgery | First-order Entropy GLCM Entropy |
Classification (Grade): AUROC analysis |
First-order entropy may differentiate between gastric cancers with vascular invasion with a sensitivity, specificity, accuracy of 86%, 75%, 81% and AUC of 0.82 |
AC adenocarcinoma; AUROC area under the receiver operative curve; GLCM gray-level co-occurrence matrix
Same institution data
Discussion
To date 22 imaging studies have been published investigating radiomic approaches in esophageal, esophagogastric, and gastric cancer, predominantly focused on texture analysis. Preliminary data for esophageal and esophago-gastric cancer suggest that there is potential for radiomic approaches in improving patient stratification for therapy. Eight 18F-FDG PET studies investigated the feasibility of heterogeneity analysis for response prediction (four studies with pre-therapy imaging only). Among the most often reported significant feature was GLCM entropy, reflecting the local randomness (irregularity) within the image, and where low GLCM entropy represents a more homogeneous texture. The reported accuracy for successful classification of therapy response ranged from 0.7 to 1.0 (AUC).
Nearly all published studies incorporated “classical” PET parameters e.g., SUVmax, total lesion glycolysis and metabolic tumor volume into predictive models. In general radiomic parameters contributed to predictive models and provided additional information to standard parameters. Three CT studies of esophageal cancer have also suggested that greater tumoral heterogeneity is associated with poor response and outcome.
PET-studies investigating texture features as a prognosticator were more mixed. Only two studies found associations with overall-survival. The CT and MRI data for gastric cancer were also varied. Two studies found several features to be associated with survival time, however, for some parameters, e.g., histogram entropy and energy, it was surprising to find both parameters to be associated in the same direction given what they represent mathematically.
A challenge for interpretation of studies to date is the use of retrospective datasets with different imaging techniques across different scanners and/or institutions; different methodologies for feature selection; the focus on different feature sets; the lack of transparency in methodology with the use of different in-house software; as well as varying statistical and bioinformatics approaches for data analysis and interpretation. This has been highlighted by researchers in the field [48].
Moving forward in the context of esophageal and esophago-gastric cancer, it is important to improve our data quality. Planned prospective studies incorporating quality control is a step in the right direction to improving data curation and ensuring prediction models are fit for purpose and fulfill the promise of radiomics for improving patient stratification.
Acknowledgements
The authors acknowledge support from the Department of Health via the National Institute for Health Research Comprehensive Biomedical Research Centre award to Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust; from the King’s College London/University College London Comprehensive Cancer Imaging Centre funded by Cancer Research UK and Engineering and Physical Sciences Research Council (EPSRC) in association with the Medical Research Council and Department of Health [(C1519/A16463)]; and Wellcome EPSRC Centre for Medical Engineering at King’s College London (WT 203148/Z/16/Z). Bert-Ram Sah received a research Grant from the Swiss National Science Foundation.
Footnotes
Vicky Goh: 0000-0002-2321-8091
Conflict of interest Bert-Ram Sah, Kasia Owczarczyk, Musib Siddique and Gary Cook declare that he/she have no conflicts of interest. Vicky Goh receives research support from Siemens Healthcare; this is not related to the topic of this manuscript.
Human and Animal Participants This review article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1.Globocan (2012) Retrieved from www.globocan.iarc.fr.
- 2.Ajani JA, Winter K, Okawara GS, et al. Phase II trial of preoperative chemoradiation in patients with localized gastric adenocarcinoma (RTOG 9904): quality of combined modality therapy and pathologic response. J Clin Oncol. 2006;24(24):3953–3958. doi: 10.1200/JCO.2006.06.4840. [DOI] [PubMed] [Google Scholar]
- 3.Allum WH, Stenning SP, Bancewicz J, Clark PI, Langley RE. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27(30):5062–5067. doi: 10.1200/JCO.2009.22.2083. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 5.Claassen YHM, Hartgrink HH, Dikken JL, et al. Surgical morbidity and mortality after neoadjuvant chemotherapy in the CRITICS gastric cancer trial. Eur J Surg Oncol. 2018;44(5):613–619. doi: 10.1016/j.ejso.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 6.van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366(22):2074–2084. doi: 10.1056/NEJMoa1112088. [DOI] [PubMed] [Google Scholar]
- 7.Al-Batran SE, Hofheinz RD, Pauligk C, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016;17(12):1697–1708. doi: 10.1016/S1470-2045(16)30531-9. [DOI] [PubMed] [Google Scholar]
- 8.Reynolds JV, Preston SR, O’Neill B, et al. ICORG 10-14: NEOadjuvant trial in adenocarcinoma of the oEsophagus and oesophagoGastric junction International Study (Neo-AEGIS) BMC Cancer. 2017;17(1):401. doi: 10.1186/s12885-017-3386-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miao ZF, Liu XY, Wang ZN, et al. Effect of neoadjuvant chemotherapy in patients with gastric cancer: a PRISMA-compliant systematic review and meta-analysis. BMC Cancer. 2018;18(1):118. doi: 10.1186/s12885-018-4027-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Secrier M, Li X, de Silva N, et al. Mutational signatures in esophageal adenocarcinoma define etiologically distinct subgroups with therapeutic relevance. Nat Genet. 2016;48(10):1131–1141. doi: 10.1038/ng.3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katona BW, Rustgi AK. Gastric cancer genomics: advances and future directions. Cell Mol Gastroenterol Hepatol. 2017;3(2):211–217. doi: 10.1016/j.jcmgh.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ichikawa H, Nagahashi M, Shimada Y, et al. Actionable gene-based classification toward precision medicine in gastric cancer. Genome Med. 2017;9(1):93. doi: 10.1186/s13073-017-0484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lagergren P, Avery KN, Hughes R, et al. Health-related quality of life among patients cured by surgery for esophageal cancer. Cancer. 2007;110(3):686–693. doi: 10.1002/cncr.22833. [DOI] [PubMed] [Google Scholar]
- 14.Hayes T, Smyth E, Riddell A, Allum W. Staging in esophageal and gastric cancers. Hematol Oncol Clin North Am. 2017;31(3):427–440. doi: 10.1016/j.hoc.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Findlay JM, Bradley KM, Maile EJ, et al. Pragmatic staging of oesophageal cancer using decision theory involving selective endoscopic ultrasonography, PET and laparoscopy. Br J Surg. 2015;102(12):1488–1499. doi: 10.1002/bjs.9905. [DOI] [PubMed] [Google Scholar]
- 16.Cancers NCCNGfEaEJ. National Comprehensive Cancer Network Guidelines for Esophageal and Esophagogastric Junction Cancers. Retrieved from: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf.
- 17.Foley KG, Christian A, Fielding P, Lewis WG, Roberts SA. Accuracy of contemporary oesophageal cancer lymph node staging with radiological-pathological correlation. Clin Radiol. 2017;72(8):693e1–693e7. doi: 10.1016/j.crad.2017.02.022. [DOI] [PubMed] [Google Scholar]
- 18.Meyers BF, Downey RJ, Decker PA, et al. The utility of positron emission tomography in staging of potentially operable carcinoma of the thoracic esophagus: results of the American College of Surgeons Oncology Group Z0060 trial. J Thorac Cardiovasc Surg. 2007;133(3):738–745. doi: 10.1016/j.jtcvs.2006.09.079. [DOI] [PubMed] [Google Scholar]
- 19.Walker AJ, Spier BJ, Perlman SB, et al. Integrated PET/CT fusion imaging and endoscopic ultrasound in the pre-operative staging and evaluation of esophageal cancer. Mol Imaging Biol. 2011;13(1):166–171. doi: 10.1007/s11307-010-0306-0. [DOI] [PubMed] [Google Scholar]
- 20.Lordick F, Mariette C, Haustermans K, Obermannova R, Arnold D. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v50–v57. doi: 10.1093/annonc/mdw329. [DOI] [PubMed] [Google Scholar]
- 21.Barber TW, Duong CP, Leong T, et al. 18F-FDG PET/CT has a high impact on patient management and provides powerful prognostic stratification in the primary staging of esophageal cancer: a prospective study with mature survival data. J Nucl Med. 2012;53(6):864–871. doi: 10.2967/jnumed.111.101568. [DOI] [PubMed] [Google Scholar]
- 22.Goel R, Subramaniam RM, Wachsmann JW. PET/computed tomography scanning and precision medicine: esophageal cancer. PET Clin. 2017;12(4):373–391. doi: 10.1016/j.cpet.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Seol KH, Lee JE. PET/CT planning during chemoradiotherapy for esophageal cancer. Radiat Oncol J. 2014;32(1):31–42. doi: 10.3857/roj.2014.32.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.le Grange F, Wickers S, Warry A, et al. Defining the target in cancer of the oesophagus: direct radiotherapy planning with fluorodeoxyglucose positron emission tomography-computed tomography. Clin Oncol (Royal College of Radiologists (Great Britain)) 2015;27(3):160–167. doi: 10.1016/j.clon.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Lee G, Hoseok I, Kim SJ, et al. Clinical implication of PET/MR imaging in preoperative esophageal cancer staging: comparison with PET/CT, endoscopic ultrasonography, and CT. J Nucl Med. 2014;55(8):1242–1247. doi: 10.2967/jnumed.114.138974. [DOI] [PubMed] [Google Scholar]
- 26.Tixier F, Le Rest CC, Hatt M, et al. Intratumor heterogeneity characterized by textural features on baseline 18F-FDG PET images predicts response to concomitant radiochemotherapy in esophageal cancer. J Nucl Med. 2011;52(3):369–378. doi: 10.2967/jnumed.110.082404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beukinga RJ, Hulshoff JB, van Dijk LV, et al. Predicting response to neoadjuvant chemoradiotherapy in esophageal cancer with textural features derived from pretreatment (18)F-FDG PET/CT imaging. J Nucl Med. 2017;58(5):723–729. doi: 10.2967/jnumed.116.180299. [DOI] [PubMed] [Google Scholar]
- 28.Nakajo M, Jinguji M, Nakabeppu Y, et al. Texture analysis of (18)F-FDG PET/CT to predict tumour response and prognosis of patients with esophageal cancer treated by chemoradiotherapy. Eur J Nucl Med Mol Imaging. 2017;44(2):206–214. doi: 10.1007/s00259-016-3506-2. [DOI] [PubMed] [Google Scholar]
- 29.Paul D, Su R, Romain M, et al. Feature selection for outcome prediction in oesophageal cancer using genetic algorithm and random forest classifier. Comput Med Imaging Graph. 2017;60:42–49. doi: 10.1016/j.compmedimag.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Foley KG, Hills RK, Berthon B, et al. Development and validation of a prognostic model incorporating texture analysis derived from standardised segmentation of PET in patients with oesophageal cancer. Eur Radiol. 2018;28(1):428–436. doi: 10.1007/s00330-017-4973-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan S, Kligerman S, Chen W, et al. Spatial-temporal [(1)(8)F]FDG-PET features for predicting pathologic response of esophageal cancer to neoadjuvant chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2013;85(5):1375–1382. doi: 10.1016/j.ijrobp.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Rossum PS, Fried DV, Zhang L, et al. The incremental value of subjective and quantitative assessment of 18F-FDG PET for the prediction of pathologic complete response to preoperative chemoradiotherapy in esophageal cancer. J Nucl Med. 2016;57(5):691–700. doi: 10.2967/jnumed.115.163766. [DOI] [PubMed] [Google Scholar]
- 33.Yip SS, Coroller TP, Sanford NN, et al. Use of registration-based contour propagation in texture analysis for esophageal cancer pathologic response prediction. Phys Med Biol. 2016;61(2):906–922. doi: 10.1088/0031-9155/61/2/906. [DOI] [PubMed] [Google Scholar]
- 34.Beukinga RJ, Hulshoff JB, Mul VEM, et al. Prediction of response to neoadjuvant chemotherapy and radiation therapy with baseline and restaging (18)F-FDG PET imaging biomarkers in patients with esophageal cancer. Radiology. 2018;287(3):983–992. doi: 10.1148/radiol.2018172229. [DOI] [PubMed] [Google Scholar]
- 35.Hou Z, Ren W, Li S, et al. Radiomic analysis in contrast-enhanced CT: predict treatment response to chemoradiotherapy in esophageal carcinoma. Oncotarget. 2017;86(1):104444–104454. doi: 10.18632/oncotarget.22304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ganeshan B, Skogen K, Pressney I, Coutroubis D, Miles K. Tumour heterogeneity in oesophageal cancer assessed by CT texture analysis: preliminary evidence of an association with tumour metabolism, stage, and survival. Clin Radiol. 2012;67(2):157–164. doi: 10.1016/j.crad.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Yip C, Landau D, Kozarski R, et al. Primary esophageal cancer: heterogeneity as potential prognostic biomarker in patients treated with definitive chemotherapy and radiation therapy. Radiology. 2014;270(1):141–148. doi: 10.1148/radiol.13122869. [DOI] [PubMed] [Google Scholar]
- 38.Ba-Ssalamah A, Muin D, Schernthaner R, et al. Texture-based classification of different gastric tumors at contrast-enhanced CT. Eur J Radiol. 2013;82(10):e537–e543. doi: 10.1016/j.ejrad.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 39.Ma Z, Fang M, Huang Y, et al. CT-based radiomics signature for differentiating Borrmann type IV gastric cancer from primary gastric lymphoma. Eur J Radiol. 2017;91:142–147. doi: 10.1016/j.ejrad.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 40.Liu S, Liu S, Ji C, et al. Application of CT texture analysis in predicting histopathological characteristics of gastric cancers. Eur Radiol. 2017;27(12):4951–4959. doi: 10.1007/s00330-017-4881-1. [DOI] [PubMed] [Google Scholar]
- 41.Yoon SH, Kim YH, Lee YJ, et al. Tumor heterogeneity in human epidermal growth factor receptor 2 (HER2)-positive advanced gastric cancer assessed by CT texture analysis: association with survival after trastuzumab treatment. PLloS ONE. 2016;11(8):e0161278. doi: 10.1371/journal.pone.0161278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giganti F, Antunes S, Salerno A, et al. Gastric cancer: texture analysis from multidetector computed tomography as a potential preoperative prognostic biomarker. Eur Radiol. 2017;27(5):1831–1839. doi: 10.1007/s00330-016-4540-y. [DOI] [PubMed] [Google Scholar]
- 43.Giganti F, Marra P, Ambrosi A, et al. Pre-treatment MDCT-based texture analysis for therapy response prediction in gastric cancer: Comparison with tumour regression grade at final histology. Eur J Radiol. 2017;90:129–137. doi: 10.1016/j.ejrad.2017.02.043. [DOI] [PubMed] [Google Scholar]
- 44.Liu S, Zhang Y, Chen L, et al. Whole-lesion apparent diffusion coefficient histogram analysis: significance in T and N staging of gastric cancers. BMC Cancer. 2017;17(1):665. doi: 10.1186/s12885-017-3622-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu S, Zhang Y, Xia J, et al. Predicting the nodal status in gastric cancers: the role of apparent diffusion coefficient histogram characteristic analysis. Magn Reson Imaging. 2017;42:144–151. doi: 10.1016/j.mri.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 46.Liu S, Zheng H, Zhang Y, et al. Whole-volume apparent diffusion coefficient-based entropy parameters for assessment of gastric cancer aggressiveness. J Magn Reson Imaging. 2018;47(1):168–175. doi: 10.1002/jmri.25752. [DOI] [PubMed] [Google Scholar]
- 47.Zhang Y, Chen J, Liu S, et al. Assessment of histological differentiation in gastric cancers using whole-volume histogram analysis of apparent diffusion coefficient maps. J Magn Reson Imaging. 2017;45(2):440–449. doi: 10.1002/jmri.25360. [DOI] [PubMed] [Google Scholar]
- 48.Lambin P, Leijenaar RTH, Deist TM, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14(12):749–762. doi: 10.1038/nrclinonc.2017.141. [DOI] [PubMed] [Google Scholar]