Summary
Background
The public health crisis of obesity leads to increasing morbidity that are even more profound in certain populations such as rural adults. Live, two‐way video‐conferencing is a modality that can potentially surmount geographic barriers and staffing shortages.
Methods
Patients from the Dartmouth‐Hitchcock Weight and Wellness Center were recruited into a pragmatic, single‐arm, nonrandomized study of a remotely delivered 16‐week evidence‐based healthy lifestyle programme. Patients were provided hardware and appropriate software allowing for remote participation in all sessions, outside of the clinic setting. Our primary outcomes were feasibility and acceptability of the telemedicine intervention, as well as potential effectiveness on anthropometric and functional measures.
Results
Of 62 participants approached, we enrolled 37, of which 27 completed at least 75% of the 16‐week programme sessions (27% attrition). Mean age was 46.9 ± 11.6 years (88.9% female), with a mean body mass index of 41.3 ± 7.1 kg/m2 and mean waist circumference of 120.7 ± 16.8 cm. Mean patient participant satisfaction regarding the telemedicine approach was favourable (4.48 ± 0.58 on 1‐5 Likert scale—low to high) and 67.6/75 on standardized questionnaire. Mean weight loss at 16 weeks was 2.22 ± 3.18 kg representing a 2.1% change (P < .001), with a loss in waist circumference of 3.4% (P = .001). Fat mass and visceral fat were significantly lower at 16 weeks (2.9% and 12.5%; both P < .05), with marginal improvement in appendicular skeletal muscle mass (1.7%). In the 30‐second sit‐to‐stand test, a mean improvement of 2.46 stands (P = .005) was observed.
Conclusion
A telemedicine‐delivered, intensive weight loss intervention is feasible, acceptable, and potentially effective in rural adults seeking weight loss.
Keywords: obesity, pragmatic, rural, telemedicine
1. INTRODUCTION
As a major public health crisis nationally and internationally, obesity rates continue to rise, exceeding1 an estimated 38%. Obesity is known to adversely impact cardiometabolic factors, including hypertension, diabetes, and dyslipidemia,2 ultimately increasing vascular risk and leading to disability3 and death.4 The escalating costs associated with obesity in the United States demonstrate a critical need to address this epidemic5 given that direct and indirect costs account for 9.3% of the gross domestic product.6 Conditions are even worse in rural areas of the United States where obesity prevalence rates are much higher7 and patients often need to travel extensive distances to access health care services and specialist providers.8, 9, 10, 11 Disparities in accessing care are especially problematic in caring for patients with obesity, where regular interactions are needed to promote health behaviour change.12
Mobile health interventions hold promise in engaging adults with obesity in behavioural change. For instance, self‐monitoring using commercial applications has demonstrated an increased likelihood of short‐term weight loss.13, 14 Goal setting through text messaging,15 automated voice response systems,16 or tailored self‐monitoring platforms17 can all enhance success and are cost‐effective strategies to at‐risk populations. However, engagement drops off after initial usage suggesting a need for more personalized approaches.18 In fact, at 12 months, there are no differences in weight loss between digital and control arms.19 Recent studies using just‐in‐time adaptive interventions also hold considerable promise in influencing patterns of behaviour for engaging and sustaining weight loss.20
The emergence of telemedicine, two‐way live video‐conferencing, has been embraced by the Centers for Medicare and Medicaid services21 as a different type of mobile health modality that can potentially surmount geographic barriers to health care delivery. With the advent of policy changes promoting rural broadband and cellular access,22 telemedicine is increasingly available to both health care entities and patients alike. In rural obesity management, telemedicine is particularly promising because it reduces demands on patients' time by reducing the need to travel long distances and spend hours away from work23 in order to attend high‐intensity visits recommended by the 2013 guidelines.12 While an initial investment is needed, the payoff is significant in that it may reduce costs.24 The affordability can allow rural patients increased access to specialists, making it a plausible method to deliver care.
Few trials have evaluated the use of telemedicine in obesity management. The Veterans Affairs MOVE! trial has implemented telemedicine in effective and sustainable approaches.25, 26 Their programme, although, focused only on veterans with obesity across the United States and was not specific to rural areas. The delivery was based on using a telehealth monitor delivering electronic modules, rather than using a clinical care provider. Other studies focus on paediatric populations with hybrid models (in‐person and remote),27, 28, 29, 30 or the potential efficacy of low‐intensity models.31, 32 Studies have demonstrated mixed results in other populations, including pregnancy33 or endometrial cancer survivors.34 While diet‐quality and obesity are strongly associated with rural health care resource use,35 there is a lack of pragmatic research strategies for delivering high‐intensity obesity therapy in rural areas. Our hypothesis in this pilot study was that an adaptation of an in‐person, 16‐week intensive lifestyle intervention could feasibly be delivered using telemedicine and would be acceptable and potentially effective for participants.
2. METHODS
2.1. Study design and setting
This was a single‐arm, non‐randomized study by enrolling participants attending the Dartmouth‐Hitchcock (D‐H) Weight and Wellness Center between November 2017 and September 2018. D‐H is a 396‐bed hospital serving over 1.5 million persons in the region and situated in Lebanon, NH, on the New Hampshire and Vermont border in Grafton County, which is classified as rural according to the 2010 census.36 Sixty five percent of persons live in a health professional shortage area or medically underserved area. The centre was initiated in 2016 and, at that time, evaluated 385 new consultations for adult obesity management yearly. At the time of the study, staffing consisted of three physicians, an advanced practice registered nurse, a behavioural psychologist, a registered nurse exercise specialist, two health coaches, two registered dietitians, and administrative staff. Outcomes were assessed at an in‐person baseline and 16‐week visit. The study was approved by the Committee for the Protection of Human Subjects at Dartmouth College and NCT03309787.
2.2. Intervention description
The Healthy Lifestyle Program consisted of a 16‐week programme delivered by a health coach, registered dietitian, and nurse exercise specialist (see Table 1) focused on health‐behaviour change and based on the Diabetes Prevention Program.37 Medication management and bariatric surgery are separate programmes within the clinic. Participants are referred from their primary care physicians and complete an initial comprehensive multidisciplinary intake prior to entering the lifestyle programme. As part of the lifestyle programme, patients have the option of choosing in‐person individual or group (up to 15‐20) formats for weekly coaching visits. For the pilot, participants evaluated in the clinic were offered the opportunity to complete 30‐minute individual 1:1 coaching visits remotely via video‐conferencing (see below) after the initial evaluation, in lieu of in‐person care. Other participants were eligible for bariatric surgery or medication management and did not enter this programme. The structure and operational infrastructure paralleled that observed within on‐site care. In addition, participants were provided with a wearable fitness device during the study to enable them to track their physical activity as part of a separate research study.
Table 1.
Components of the Healthy Lifestyle Program at the Dartmouth Weight and Wellness Center
| Staff | Week | Content |
|---|---|---|
| Health coach | Week 1a | Mindfulness, goal setting |
| Week 2 | Hunger awareness, mindful eating | |
| Week 3 | Working with emotions | |
| Exercise trainer | Week 4 | Movement vs exercise |
| Health coach | Week 5 | Managing thoughts |
| Week 6 | Stress + social support | |
| Week 7 | Problem solving | |
| Exercise trainer | Week 8 | Myths and truths |
| Registered dietitian | Week 9 | Detoxing your diet and food tracking |
| Week 10 | Food label reading and serving size | |
| Week 11 | Meal planning, grocery shopping, preparing for success | |
| Exercise trainer | Week 12 | Sorting through the noise |
| Registered dietitian | Week 13 | The power of protein |
| Week 14 | Healthy carbohydrates | |
| Week 15 | Good/bad fats, review of the toolbox | |
| Exercise trainer | Week 16 | Moving forward |
Week 1 occurs after the initial visit at the center.
2.3. Telemedicine delivery
The D‐H Center for Telehealth has an extensive infrastructure to support clinical initiatives within D‐H and provided logistical and technical support for this project. All study staff (health coaches, nurse, registered dietitians) were participated in multiple, on‐site training sessions to ensure familiarity with the telehealth platform. Live, mock sessions and ongoing on‐site support was provided by the research assistant (RA) and by a technology consultant from the Center for Telehealth. All communications were conducted through an HIPAA‐compliant Vidyo software platform that includes end‐to‐end data encryption using HTTPS (browsing), TLS (signalling), and AES encryption. Coaching sessions were conducted in a private clinical space, using a T450s Lenovo laptop and Logitech H390 USB Headset with a noise‐cancelling microphone. Participants were provided with a Samsung Galaxy Tab A 10.1 tablet that was encrypted per institutional policies to conduct the intervention off‐site (ie, home) with the same software allowing them to interact with the study personnel.
2.4. Recruitment and enrolment
Clinic schedules were initially reviewed by the RA. New patients were approached by the clinician and introduced the study opportunity to assess interest. The RA then further described the study, obtained consent, and scheduled a 1‐hour individual orientation for all subjects. Inclusion criteria consisted of English‐speaking, community‐dwelling adults, aged 18 to 65 years with a body mass index (BMI) ≥ 30 kg/m2 and otherwise willing to participate in the Healthy Lifestyle Program if recommended by the clinical provider. An additional requirement was access to high‐speed internet access with Wi‐Fi. All patients required an electronic medical record patient portal account; if one was not available, the RA assisted in its creation. Participants were excluded if they were unwilling to participate, as well as those with a medical record diagnosis of dementia, life‐threatening illness, psychiatric illness (untreated serious mental illness, suicidal ideation) precluding their participation in the study, or a history of bariatric surgery. All participants required medical clearance from their primary care provider and needed to provide voluntary written consent. The lead author (JAB) was responsible for training the RA during this process. All participants received a $20 incentive at each in‐person outcome assessment.
2.5. Outcomes
Baseline measures were chosen a priori based on their validity, brevity, use in routine clinical care, and availability in the electronic medical record. The RA obtained baseline demographic information and co‐morbid health conditions from the EMR. On‐site assessments occurred at baseline and at 16 weeks, with additional surveys conducted at 4‐week intervals (data not shown). Monthly weights were acquired using an A&D Medical Bluetooth enabled scale and captured using the application. Surveys were sent electronically using REDCap, a secure, web‐based application designed to support research data capture.38
Height was measured using a wall‐mounted stadiometer (SECA 216, Hamburg, Germany), with the participant standing barefoot, against a wall, with their weight evenly distributed on both feet and heels together. Three height measurements were taken, and the average was used as the final value. Waist circumference was measured by a registered nurse using a tape measure placed around the abdomen, just above the iliac crest, snug, and not compressing the skin. The participant was asked to relax, and measurements were taken at end‐exhalation. A bioelectrical impedance analyser (SECA mBCA 514, Hamburg, Germany) was used to assess weight, body fat, muscle mass, and visceral fat. Participants were instructed to remove any outer clothing, jewelry, shoes and socks, or tights and stood on the metal electrodes on the base of the machine, facing forward. A 6‐minute walk test was conducted by a nurse according to standard protocols,39 in a 100‐ft hallway; a clinically significant difference was defined as 50 m.40, 41 Last, a 30‐second sit‐to‐stand test42 was conducted, administered using an armless chair placed against a wall. Participants were instructed to sit in the middle of the chair, with their backs straight and feet shoulder width apart, with their arms crossed at the wrists and held against their chest. The participant was encouraged to complete as many repetitions of being fully seated to standing within the 30 seconds. Participants also completed the Yip Telemedicine Scale43 that has been validated in patients with diabetes at the 16‐week timepoint only; it is a 15‐item, 5‐point scale (maximum score 75) representing satisfaction with telemedicine delivered interventions. Additional acceptability questionnaires were asked at the conclusion of the study. An exit‐interview at the end of the study was conducted that ascertained the participant's impressions of the overall programme and what they liked/disliked about the programme. These interviews were digitally audio‐recorded and transcribed by http://www.rev.com, a commercial transcription programme.
2.6. Analysis
All data were aggregated into REDCap. Descriptive statistics (means, standard deviations, medians, proportions, and range) were computed to assess feasibility and acceptability. The analysis focused on completers of the programme. Change in weight, percent weight loss, and waist circumference were our primary preliminary effectiveness outcomes. Paired t tests assessed change between baseline and follow‐up. All qualitative interview data were inputted into Dedoose and analysed by two researchers using thematic data analysis44, 45, 46 consisting of “open coding” of transcripts, a process of labelling text to identify concepts related to acceptability.47 This process enhances rigor by allowing for different views.48 Codes were determined both a priori and inductively derived. Text excerpts were aggregated by code to distill patterns and themes related to the intervention's acceptability. The analysis was conducted using STATA v.15, Microsoft Excel 2017, and REDCap's data output for simple quantitative data analyses. While a P value < .05 was considered statistically significant, this pilot study was intended to investigate feasibility and was not powered to detect a statistically significant difference in our outcomes.
3. RESULTS
3.1. Feasibility of recruitment and retention
Clinicians approached 62 participants seeking treatment at the centre (Figure 1) of which 58 were eligible (93.5%) based on screening demographics. There were 37 participants enrolled (63.8%) exceeding our target of 30 patients. Of the 21/58 that were eligible but declined participation, 11 (19.0%) were unable to participate due to timing/logistical reasons, and 10 were uninterested participate in a video‐delivered intervention (17.2%). A total of 27 participants of the 37 enrolled (75.7%) completed the study; a successful attrition rate was defined as less than 20%. The most common reason for study discontinuation was patient participant noncompliance despite attempted communications to reach them; the clinic's policy is to shift patients to MD directed care if they missed three visits in the lifestyle programme. Only one participant voiced that their discontinuation was due to issues pertaining to the technology.
Figure 1.
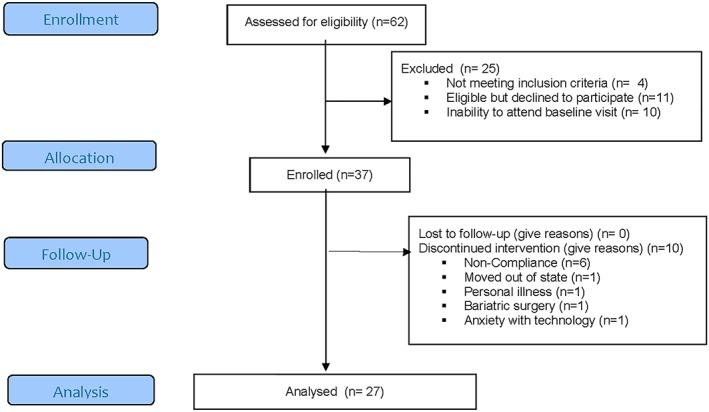
Consort diagram of all participants using telemedicine in a rural, academic, and obesity clinic
3.2. Intervention adherence
All participants completed all study measures at baseline and follow‐up points while enrolled, exceeding the a priori threshold of 80% considered as successful. The proportion of study participants completing greater than 75% of sessions was favourable among those enrolled (73%) and among those completing the study (100%). Approximately 93%, 96%, and 67% of participants attended greater than 75% of health coach, nurse, and dietitian sessions, respectively.
3.3. Baseline characteristics
Cohort characteristics, both enrolled and completers, are presented in Table 2. There were no significant differences between completers and noncompleters except for insurance status. Mean age among those completing the study was 46.9 ± 11.6 (range 27‐64 years), and the proportion of females was high (88.9%). All participants represented themselves as white and not Hispanic. Mean body mass index was 41.3 ± 7.1 kg/m2 and mean waist circumference was 120.7 ± 16.8 cm.
Table 2.
Baseline characteristics of participants
| Overall | Completers | Noncompleters | P Value | |
|---|---|---|---|---|
| N = 37 | n = 27 | n = 10 | ||
| Age, years | 46.9 ± 11.6 | 46.1 ± 12.3 | 48.9 ± 9.8 | .52 |
| Range, years | 27‐64 | 27‐64 | 27‐60 | |
| Female sex (%) | 32 (86.5) | 24 (88.9) | 8 (80.0) | .48 |
| Race, n (%) | ||||
| White | 37 (100) | 27 (100) | 10 (100) | |
| Hispanic status | 0 (0) | 0 (0) | 0 (0) | |
| Primary insurance, n (%) | ||||
| Medicare | 4 (10.8) | 2 (7.4) | 2 (20.0) | .28 |
| Medicaid | 6 (16.2) | 2 (7.4) | 4 (40.0) | .02 |
| Private | 28 (75.7) | 23 (85.2) | 5 (50.0) | .03 |
| Self‐Pay | ‐ | ‐ | ‐ | |
| Smoking status, n (%) | ||||
| Current | 1 (2.7) | 1 (3.7) | ‐ | |
| Former | 12 (32.4) | 7 (25.9) | 5 (50.0) | .17 |
| Never | 24 (64.9) | 19 (70.4) | 5 (50.0) | .25 |
| Weight, kg | 116.5 ± 28.8 | 113.1 ± 25.4 | 125.7 ± 36.5 | .24 |
| BMI, kg/m2 | ||||
| Range | 31.8‐79.9 | 31.8‐56.5 | 35.3‐79.9 | |
| Mean | 42.2 ± 9.1 | 41.3 ± 7.1 | 44.7 ± 13.3 | .32 |
| Median | 38.8 (36.2, 47.2) | 38.8 (36.0, 45.6) | 39.5 (37.2, 47.0) | |
| Waist circumference, cm | ||||
| Mean | 122.2 ± 19.1 | 120.7 ± 16.8 | 126.0 ± 24.6 | .46 |
| Range | 99.1‐185.0 | 99.1‐161.0 | 102.1‐185.0 | |
| Comorbidities, n (%) | ||||
| Anxiety | 10 (32.3) | 8 (34.8) | 2 (25.0) | .58 |
| Cognitive impairment | ‐ | ‐ | ‐ | ‐ |
| COPD | ‐ | ‐ | ‐ | ‐ |
| CAD | ‐ | ‐ | ‐ | ‐ |
| Depression | 18 (58.1) | 13 (56.5) | 5 (62.5) | .75 |
| Diabetes | 7 (22.6) | 6 (26.1) | 1 (12.5) | .38 |
| Fibromyalgia | 2 (6.5) | 1 (4.3) | 1 (12.5) | .38 |
| High cholesterol | 2 (6.5) | 2 (8.7) | ‐ | |
| Hypertension | 10 (32.3) | 9 (39.1) | 1 (12.5) | .13 |
| Nonskin cancer | ‐ | ‐ | ‐ | |
| NAFLD | 6 (19.4) | 6 (26.1) | ‐ | |
| Osteoarthritis | 3 (9.7) | 1 (4.3) | 2 (25.0) | .06 |
| Rheumatologic disease | 3 (9.7) | 3 (13.0) | ‐ | |
| OSA | 12 (38.7) | 7 (30.4) | 5 (62.5) | .08 |
| Stroke | 1 (3.2) | 1 (4.3) | ‐ |
Note. All variables indicated are represented as mean ± standard deviations, or counts (%).
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; CAD, coronary artery disease; NAFLD, nonalcoholic fatty liver disease; OSA, obstructive sleep apnoea.
3.4. Participant acceptability of telemedicine
Figure 2 presents data on the acceptability of telemedicine as a delivery modality. All responses were favourable (Table S2). Specifically, the mean level of satisfaction with the overall intervention was 4.48 ± 0.58 (median 5; range 3‐5), and individuals reported that the programme helped them to achieve their goals (4.44 ± 0.64, 5; range 3‐5). Overall, 92.6% (n = 25/27) of completers would have recommended the intervention to their family/friends. Video‐conferencing was considered an acceptable modality in allowing individuals to achieve their goals (mean 4.30 ± 0.95, median 5, range 1‐5). The Yip Telemedicine questionnaire (Table S1), a marker of telemedicine satisfaction, also suggested that the delivery modality was favourable to participants (mean 67.6 ± 6.95 range 53‐75). The staff did not experience any software or technical issues. Of the 430 sessions, 15 (3.5%) were delayed and only three (0.7%) were cancelled due to technical issues that included bandwidth issues or that a tablet was not charged.
Figure 2.
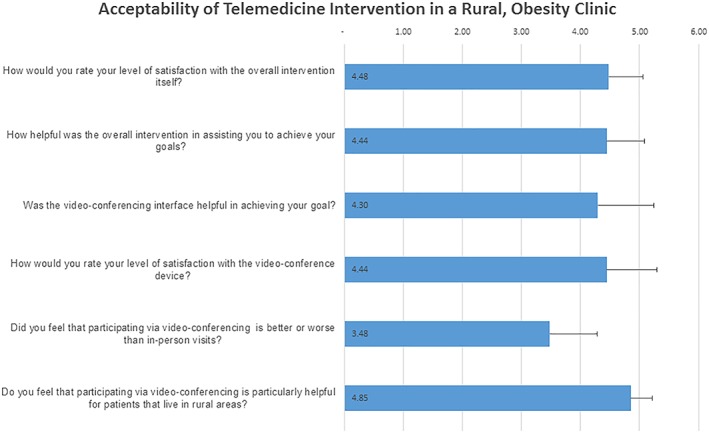
Select questions asked to participants on the acceptability of the intervention. Each question was rated from strongly disagree/dissatisfied (1) to strongly agree/satisfied (5). Mean scores are indicated with error bars representing standard deviations
3.5. Qualitative inquiry on the programme's acceptability
Many themes emerged through our participant end‐of‐study interviews (Table 3). The importance of time‐savings was observed throughout many of the conversations. Participants were highly positive about video‐conferencing rather than commuting for an in‐person evaluation. This enhanced control of their time, reduced anxiety and hassles, notably in enabling, and allowed for health consultations to occur within the context of a demanding job. Another theme included the simplicity of the video‐conferencing technology. The information delivery was helpful to all participants, and the programme provided considerable resources to enhance nutritional and behavioural strategies. In contrast, a significant criticism was the lack of peer‐support by participants and that a programme wholly based on video‐conferencing felt depersonalizing.
Table 3.
Representative quotes highlighting acceptability
| Theme | Representative Quotation |
|---|---|
| Delivery remotely vs in‐person | You can be in your pajamas if you want to and do it [telemedicine] |
| [I can] live my natural life, without little to no disruption | |
| Time‐savings | Time. There's no commute … You can do it in less than 24 hours, as long as it's set up on this side. It can be very flexible. There's no charge for gas, there's none of that stuff, which is fantastic. |
| Definitely the saving on my time and my travel, because I live in Vermont, at least two hours away, two and a half hours away, and I have to leave work half a day at least to get here in order to be here on time before you guys are done for the day. | |
| Simplicity of use | User interface [is really] quite simple |
| Oh, this was by far the easiest, the most user‐friendly [intervention]; I feel like I can do this on my own. | |
| Lack of face‐to‐face | I felt very much like an island, like I'm out there struggling all by myself and I can't do it. |
| A face group where people who are doing the same thing can communicate | |
| You need more face‐to‐face, in‐person, and groups type things that you get via telemedicine. |
3.6. Preliminary effectiveness
Table 4 demonstrates the preliminary effectiveness outcomes of the 27 completers. Over the 16‐week study period, completers lost 2.22 ± 3.18 kg, representing a 2.1% change (P < .001) from baseline to follow‐up. Of the completers, 19% lost more than 5% of their weight and waist circumference dropped 3.4% (−4.1 ± 5.9 cm; P = .001). Body composition measures were all significantly different at follow‐up (P < .05), with reductions in fat mass (2.9%), visceral fat (12.5%), and marginal improvements in appendicular skeletal muscle mass (1.7%). There were improvements (P = .005) in the 30‐second sit‐to‐stand test (39% with an improvement of 2) but no significant changes in 6‐minute walk test (P = .23).
Table 4.
Effectiveness outcomes (completers n = 27)
| Baseline | Follow‐up | Deltaa | % Change | Range | P Value | |
|---|---|---|---|---|---|---|
| Weighta, kg | 113.07 ± 25.4 | 110.8 ± 25.8 | 2.22 ± 3.18 | −2.1 ± 3.0 | −3.8, 9.6 | <.001 |
| Body mass index, kg/m2 | 41.3 ± 7.1 | 40.5 ± 7.3 | −0.88 ±1.2 | −2.1 ± 3.0% | −1.65, 3.43 | <.001 |
| Waist circumferenceb, cm | 120.6 ± 16.7 | 116.5 ± 17.0 | −4.1 ± 5.9 | −3.4 ± 5.0 | −16.0, 8.0 | .001 |
| % pre/post Δ WC | ‐ | ‐ | +7.5, −14.6 | ‐ | ||
| Body composition | ||||||
| Fat mass, % | 49.2 ± 6.0 | 47.9 ± 6.6 | −1.33 ± 1.13 | −2.87 ± 2.53 | −3.8, 1.0 | <.001 |
| Muscle mass, kg/height2 | 50.8 ± 6.0 | 52.1 ± 6.6 | 1.33 ± 1.13 | +2.55 ± 2.17 | −1, 3.8 | <.001 |
| ASM, kg | 15.4 ± 3.35 | 15.7 ± 3.6 | 0.26 ± 0.57 | 1.7 ± 4.0% | −3.7, 15.1 | .03 |
| Visceral fat, L | 5.08 ± 3.16 | 4.48 ± 2.79 | −0.60 ± 0.86 | −12.4 ± 17.5 | −2.8, 1.0 | .001 |
| 6‐minute walk test, m | 466.6 ± 105.4 | 484.6 ± 98.8 | 18.0 ± 59.5 | 4.9 ± 12.9 | −16.7, 30.7 | .55 |
| % with >50 m improvement | 7 (29.2) | |||||
| 30s sit‐to‐stand, # stands | 16.2 ± 4.96 | 19.1 ± 7.3 | 2.46 ± 3.90 | 14.2 ± 20.1 | −18.2, 50 | .005 |
Delta represents only data on full data (baseline, follow‐up) of completers.
Missing data in n = 4 STS and n = 3 6mwt.
4. DISCUSSION
An evidence‐based weight loss intervention delivered using telemedicine was feasible and acceptable to rural adults with obesity. Importantly, the intervention led not only to weight loss but also to significant changes in visceral fat as measured by bioelectrical impedance with maintenance in appendicular muscle mass and improvements in strength measures. These results suggest that a future intervention using this delivery modality within a clinical setting can potentially overcome many hurdles/barriers to delivering high‐quality, intensive obesity care in this rural population.
Many previously published obesity interventions occur within primary care environments49 or in research centres.50 Interventions such as the diabetes prevention programme are effective,51 but their reach and dissemination, particularly in rural areas, are limited.52 The importance of novel delivery methods such as telemedicine is that it can overcome geographic and operational barriers that have previously impeded the delivery of evidence‐based interventions widely. Among other pragmatic obesity trials in the literature, the majority of obesity trials using telemedicine have focused on paediatric,27, 30, 53, 54 workplace,55 or veterans affairs populations.26, 56 Others have used telemedicine in disparity populations for weight maintenance.57 To our knowledge, this telemedicine‐delivered multicomponent intervention is the first delivered from a rural, tertiary care medical centre that provides specialty obesity care. Findings from this pilot project demonstrate that participants felt positively about video‐conferencing and that telemedicine could be effective and feasible in obesity management programmes in rural settings.
While adherence to the intervention, as represented by attendance and completion of outcome assessments, was high, the programme suffers from similar engagement issues that plague other obesity programmes, both in research and clinical settings. The clinical programme was specifically designed so that the first 8 weeks are health‐coach intensive and the last 8 weeks are predominantly dietitian related. While weight loss was observed, only 19% lost more than 5% of their body weight. This success rate is partially attributed to many factors. First, most comprehensive weight loss interventions last a minimum of 6 months rather than 16 weeks and such weight loss is represented in this period of time; long‐term outcomes and maintenance are needed. Second, while behaviours are critical to long‐standing behavioural change, a caloric reduction is the key component to losing weight. The educational materials and tools related to nutrition were presented in the latter parts of the intervention; hence, the extent of weight loss that would have expected if this information was delivered earlier in the study period may not have been observed. Future studies could alter the order of the sessions. Third, it is unclear whether the telemedicine modality impacted weight loss and if a hybrid (in‐person and remote) model is needed to augment and enhance weight loss that take advantage of peer and group leader relationships for support. The attrition rate parallels those observed in other studies. The study population's readiness to change, ascertained through qualitative inquiry, which is known to impact willingness to engage in health promotion programmes, may have also played a factor. Marginal improvements in appendicular skeletal muscle mass could also account for improvements in function. During weight loss efforts, not only fat is lost but also muscle58; it is also possible that the nurse‐led resistance exercise sessions may have had a positive impact on body composition. These results suggest that further testing of the dose‐dependence of exercise training during weight loss interventions is warranted for this middle‐aged adult population within a clinical setting.
Such pilot findings should be interpreted with caution as the design was not randomized. The staff was also limited in space and resources. The evaluated intervention lasted only 16 weeks rather than the recommended minimum of 6 months and 14 encounters for high‐intensity obesity interventions.12 Strengths include the team's ability to recruit within the centre, the completion of outcome assessments by participants, and the acceptability of telemedicine to rural adults. It is unknown whether telemedicine may be as acceptable to urban‐dwelling populations, other rural populations, or parts of the country with ready access to services in delivering obesity care. Such an at‐risk, rural population with obesity at risk for health disparities would not ordinarily have access to these services.
5. CONCLUSION
A multicomponent, telemedicine‐based obesity lifestyle programme appears to be feasible and acceptable to patients and is thus a promising approach for weight and visceral fat loss in rural populations. A randomized controlled trial is needed to evaluate this modality for future implementation and effectiveness as part of their routine practice.
CONFLICT OF INTEREST
There are no conflicts of interest pertaining to this manuscript.
AUTHOR CONTRIBUTIONS
J.A.B., A.C.M., A.B.W., and D.G.D. analysed and interpreted the data. J.A.B., A.C.M., D.F.K., S.R., S.B.C., D.G.D., and R.I.R. were involved in conceiving the study design. All authors read and approved the final manuscript and provided critical input in the revision of the manuscript.
FUNDING
Dr Batsis receives funding from the National Institute on Aging of the National Institutes of Health under Award Number K23AG051681 and from the Friends of the Norris Cotton Cancer Center at Dartmouth and National Cancer Institute Cancer Center Support Grant 5P30 CA023108‐37 Developmental Funds. Dr Batsis also receives funding from the Patient Centered Oriented Research Institute. Dr Batsis has also received honoraria from the Royal College of Physicians of Ireland, Endocrine Society, and Dinse, Knapp, McAndrew LLC, legal firm. Support was also provided by the Department of Medicine and the Dartmouth Health Promotion and Disease Prevention Research Center supported by Cooperative Agreement Number U48DP005018 from the Centers for Disease Control and Prevention. The Dartmouth Clinical and Translational Science Institute, under award number UL1TR001086 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH).
Supporting information
Table S1. Telemedicine Satisfaction Questionnaire
Table S2. Acceptability of Telemedicine Intervention
ACKNOWLEDGEMENTS
This study was approved by the Committee for the Protection of Human Subjects #30240. All participants consented to participate. The authors approve publication if accepted. The data that support the findings of this study are available from Dartmouth‐Hitchcock, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Dartmouth‐Hitchcock.
We thank the Center for Telehealth (Mary Lowry, Vanessa Brown, Fredric Glazer) for their assistance in developing the telemedicine component, and Tara Efstathiou, Laurie Gelb, Eugene Soboleski, Jane Brewer, Martha Catalona, Philip Oman, and Kaitlyn Christian, for their administrative assistance at the Weight and Wellness Center.
Batsis JA, McClure AC, Weintraub AB, et al. Feasibility and acceptability of a rural, pragmatic, telemedicine‐delivered healthy lifestyle programme. Obes Sci Pract. 2019;5:521–530. 10.1002/osp4.366
REFERENCES
- 1. Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in obesity and severe obesity prevalence in US youth and adults by sex and age, 2007‐2008 to 2015‐2016. JAMA. 2018;319:1723‐1725. 2018/03/24. 10.1001/jama.2018.3060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gregg EW, Cheng YJ, Cadwell BL, et al. Secular trends in cardiovascular disease risk factors according to body mass index in US adults. JAMA. 2005;293:1868‐1874. 2005/04/21. 10.1001/jama.293.15.1868 [DOI] [PubMed] [Google Scholar]
- 3. Houston DK, Ding J, Nicklas BJ, et al. Overweight and obesity over the adult life course and incident mobility limitation in older adults: the health, aging and body composition study. Am J Epidemiol. 2009;169:927‐936. Research Support, N.I.H., Intramural 2009/03/10. 10.1093/aje/kwp007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all‐cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta‐analysis. JAMA. 2013;309:71‐82. 2013/01/03. 10.1001/jama.2012.113905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tremmel M, Gerdtham U‐G, Nilsson PM, Saha S. Economic burden of obesity: a systematic literature review. Int J Environ Res Public Health. 2017;14:435 10.3390/ijerph14040435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Waters H, Graf M. America's obesity crisis: the health and economic costs of excess weight: Milken Institute. 2018.
- 7. Befort CN, Nazir N, Perri MG. Prevalence of obesity among adults from rural and urban areas of the united states: findings from NHANES (2005‐2008). J Rural Health. 2012;28:392‐397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DesRoches CM, Buerhaus P, Dittus RS, et al. Primary care workforce shortages and career recommendations from practicing clinicians. Acad Med. 2015;90:671‐677. Research Support, Non‐U.S. Gov't 2014/12/30. 10.1097/ACM.0000000000000591 [DOI] [PubMed] [Google Scholar]
- 9. Gamm L, Hutchison L, Bellamy G, Dabney BJ. Rural healthy people 2010: identifying rural health priorities and models for practice. J Rural Health. 2002;18:9‐14. 2002/06/05 [DOI] [PubMed] [Google Scholar]
- 10. Goins RT, Williams KA, Carter MW, Spencer SM, Solovieva T. Perceived barriers to health care access among rural older adults: a qualitative study. J Rural Health. 2005;21:206‐213. 2005/08/12 [DOI] [PubMed] [Google Scholar]
- 11. MacDowell M, Glasser M, Fitts M, Nielsen K, Hunsaker M. A national view of rural health workforce issues in the USA. Rural Remote Health. 2010;10:1531. 2010/07/28 [PMC free article] [PubMed] [Google Scholar]
- 12. Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults. Circulation. 2014;129:S102‐S138. 2013/11/14. 10.1161/01.cir.0000437739.71477.ee [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Patel ML, Brooks TL, Bennett GG. Consistent self‐monitoring in a commercial app‐based intervention for weight loss: results from a randomized trial. J Behav Med. 2019. 2019/08/10. 10.1007/s10865-019-00091-8 [DOI] [PubMed] [Google Scholar]
- 14. Patel ML, Hopkins CM, Bennett GG. Early weight loss in a standalone mHealth intervention predicting treatment success. Obes Sci Pract. 2019;5:231‐237. 2019/07/06. 10.1002/osp4.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McVay M, Steinberg D, Askew S, Bennett GG. Provider counseling and weight loss outcomes in a primary care‐based digital obesity treatment. J Gen Intern Med. 2019;34:992‐998. 2019/03/21. 10.1007/s11606-019-04944-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Krishnan A, Finkelstein EA, Levine E, et al. A digital behavioral weight gain prevention intervention in primary care practice: cost and cost‐effectiveness analysis. J Med Internet Res. 2019;21:e12201 2019/05/19. 10.2196/12201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Steinberg D, Kay M, Burroughs J, Svetkey LP, Bennett GG. The effect of a digital behavioral weight loss intervention on adherence to the dietary approaches to stop hypertension (DASH) dietary pattern in medically vulnerable primary care patients: results from a randomized controlled trial. J Acad Nutr Diet. 2019;119:574‐584. 2019/03/25. 10.1016/j.jand.2018.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lin PH, Grambow S, Intille S, et al. The association between engagement and weight loss through personal coaching and cell phone interventions in young adults: randomized controlled trial. JMIR Mhealth Uhealth. 2018;6:e10471 2018/10/21. 10.2196/10471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Thomas JG, Raynor HA, Bond DS, et al. Weight loss in weight watchers online with and without an activity tracking device compared to control: a randomized trial. Obesity (Silver Spring). 2017;25:1014‐1021. 2017/04/25. 10.1002/oby.21846 [DOI] [PubMed] [Google Scholar]
- 20. Thomas JG, Bond DS. Behavioral response to a just‐in‐time adaptive intervention (JITAI) to reduce sedentary behavior in obese adults: Implications for JITAI optimization. Health Psychol. 2015;34s:1261‐1267. 2015/12/15. 10.1037/hea0000304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Expansion of Medicare Telehealth Services for CY 2012 . 2011.: U.S. Department of Health and Human Services, Centers for Medicare and Medicaid Services.
- 22. 2018 Broadband Deployment Report, https://www.fcc.gov/reports-research/reports/broadband-progress-reports/2018-broadband-deployment-report (2018, ).
- 23. Batsis JA, Pletcher SN, Stahl JE. Telemedicine and primary care obesity management in rural areas—innovative approach for older adults? BMC Geriatr. 2017;17:6 2017/01/07. 10.1186/s12877-016-0396-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grustam AS, Severens JL, De Massari D, Buyukkaramikli N, Koymans R, Vrijhoef HJM. Cost‐effectiveness analysis in telehealth: a comparison between home telemonitoring, nurse telephone support, and usual care in chronic heart failure management. Value Health. 2018;21:772‐782. 2018/07/15. 10.1016/j.jval.2017.11.011 [DOI] [PubMed] [Google Scholar]
- 25. Damschroder LJ, Goodrich DE, Robinson CH, Fletcher CE, Lowery JC. A systematic exploration of differences in contextual factors related to implementing the MOVE! weight management program in VA: a mixed methods study. BMC Health Serv Res. 2011;11:248 2011/10/04. 10.1186/1472-6963-11-248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ahrendt AD, Kattelmann KK, Rector TS, Maddox DA. The effectiveness of telemedicine for weight management in the MOVE! Program. J Rural Health. 2014;30:113‐119. 2013/10/12. 10.1111/jrh.12049 [DOI] [PubMed] [Google Scholar]
- 27. Cohen GM, Irby MB, Boles K, Jordan C, Skelton JA. Telemedicine and pediatric obesity treatment: review of the literature and lessons learned. Clin Obes. 2012;2:103‐111. 2012/12/12. 10.1111/j.1758-8111.2012.00050.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lipana LS, Bindal D, Nettiksimmons J, Shaikh U. Telemedicine and face‐to‐face care for pediatric obesity. Telemed J E Health. 2013;19:806‐808. Comparative Study 2013/08/29. 10.1089/tmj.2012.0292 [DOI] [PubMed] [Google Scholar]
- 29. Shaikh U, Nettiksimmons J, Romano P. Pediatric obesity management in rural clinics in California and the role of telehealth in distance education. J Rural Health. 2011;27:263‐269. 2011/07/07. 10.1111/j.1748-0361.2010.00335.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Slusser W, Whitley M, Izadpanah N, Kim SL, Ponturo D. Multidisciplinary pediatric obesity clinic via telemedicine within the Los Angeles metropolitan area: lessons learned. Clin Pediatr (Phila). 2016;55:251‐259. 2015/07/19. 10.1177/0009922815594359 [DOI] [PubMed] [Google Scholar]
- 31. Brown JD, Hales S, Evans TE, et al. Description, utilisation and results from a telehealth primary care weight management intervention for adults with obesity in South Carolina. J Telemed Telecare. 2018: 1357633x18789562; 2018/07/27. 10.1177/1357633x18789562 [DOI] [PubMed] [Google Scholar]
- 32. Whitlock E, O'Connor E, Williams S, Beil T, Lutz K. Effectiveness of weight management programs in children and adolescents. Evid Rep Technol Assess (Full Rep). 2008;1‐308. 2009/05/05 [PMC free article] [PubMed] [Google Scholar]
- 33. Chao AM, Srinivas SK, Studt SK, Diewald LK, Sarwer DB, Allison KC. A pilot randomized controlled trial of a technology‐based approach for preventing excess weight gain during pregnancy among women with overweight. Front Nutr. 2017;4:57 2017/12/08. 10.3389/fnut.2017.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haggerty AF, Hagemann A, Barnett M, et al. A randomized, controlled, multicenter study of technology‐based weight loss interventions among endometrial cancer survivors. Obesity (Silver Spring). 2017;25:S102‐s108. 2017/11/01. 10.1002/oby.22021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ford DW, Hartman TJ, Still C, et al. Diet quality and body mass index are associated with health care resource use in rural older adults. J Acad Nutr Diet. 2014;114:1932‐1938. 2014/04/22. 10.1016/j.jand.2014.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Government US. Census Bureau Statistics, (2012, ).
- 37. Knowler WC, Barrett‐Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393‐403. 2002/02/08. 10.1056/NEJMoa012512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377‐381. 2008/10/22. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Laboratories ATSCoPSfCPF . ATS statement: guidelines for the six‐minute walk test. Am J Respir Crit Care Med. 2002;166:111‐117. 2002/07/02. 10.1164/ajrccm.166.1.at1102 [DOI] [PubMed] [Google Scholar]
- 40. Harada ND, Chiu V, Stewart AL. Mobility‐related function in older adults: assessment with a 6‐minute walk test. Arch Phys Med Rehabil. 1999;80:837‐841. 1999/07/22 [DOI] [PubMed] [Google Scholar]
- 41. Steffen TM, Hacker TA, Mollinger L. Age‐ and gender‐related test performance in community‐dwelling elderly people: six‐minute walk test, berg balance scale, timed up & go test, and gait speeds. Phys Ther. 2002;82:128‐137. 2002/02/22 [DOI] [PubMed] [Google Scholar]
- 42. Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334‐1359. 2011/06/23. 10.1249/MSS.0b013e318213fefb [DOI] [PubMed] [Google Scholar]
- 43. Yip MP, Mackenzie A, Chan J. Patient satisfaction with telediabetes education in Hong Kong. J Telemed Telecare. 2002;8:48‐51. 2002/01/26. 10.1258/1357633021937460 [DOI] [PubMed] [Google Scholar]
- 44. Boyatzis RE. Transforming qualitative information: Thematic analysis and code development. Thousand Oaks, London & New Delhi: SAGE Publications; 1998. [Google Scholar]
- 45. Braun V, Clarke V. Using thematic analysis in psychology. Qualitative Research in Psychology. 2006;3:77‐101. 10.1191/1478088706qp063oa [DOI] [Google Scholar]
- 46. Braun V, Clarke V. What can “thematic analysis” offer health and wellbeing researchers? Int J Qual Stud Health Well‐being. 2014;9:26152 Editorial 2014/10/19. 10.3402/qhw.v9.26152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Emerson R, Fretz R, Shaw L. Writing Ethnographic Fieldnotes. Chicago, IL: University of Chicago Press; 1995. [Google Scholar]
- 48. Shenton AK. Strategies for ensuring trustworthiness in qualitative research projects. Education for Information. 2004;22:63‐75. [Google Scholar]
- 49. Wadden TA, Butryn ML, Hong PS, Tsai AG. Behavioral treatment of obesity in patients encountered in primary care settings: a systematic review. JAMA. 2014;312:1779‐1791. Research Support, N.I.H., Extramural Review 2014/11/05. 10.1001/jama.2014.14173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Look AHEAD Research Group . Eight‐year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obesity (Silver Spring). 2014;22:5‐13. Research Support, N.I.H., Extramural Research Support, U.S. Gov't, Non‐P.H.S. Research Support, U.S. Gov't, P.H.S. 2013/12/07. 10.1002/oby.20662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. The Diabetes Prevention Program (DPP) . The Diabetes Prevention Program (DPP): Description of lifestyle intervention. Diabetes Care. 2002;25:2165‐2171. 10.2337/diacare.25.12.2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Perri MG, Ariel‐Donges AH, Shankar MN, et al. Design of the Rural LEAP randomized trial: an evaluation of extended‐care programs for weight management delivered via group or individual telephone counseling. Contemp Clin Trials. 2019;76:55‐63. 2018/11/09. 10.1016/j.cct.2018.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Davis AM, Sampilo M, Gallagher KS, et al. Treating rural paediatric obesity through telemedicine vs. telephone: outcomes from a cluster randomized controlled trial. J Telemed Telecare. 2016;22:86‐95. 2015/05/31. 10.1177/1357633x15586642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Coles N, Patel BP, Li P, et al. Breaking barriers: adjunctive use of the Ontario Telemedicine Network (OTN) to reach adolescents with obesity living in remote locations. J Telemed Telecare. 2018;1357633x18816254 2018/12/12. 10.1177/1357633x18816254 [DOI] [PubMed] [Google Scholar]
- 55. Das SK, Brown C, Urban LE, et al. Weight loss in videoconference and in‐person iDiet weight loss programs in worksites and community groups. Obesity (Silver Spring). 2017;25:1033‐1041. 2017/04/30. 10.1002/oby.21854 [DOI] [PubMed] [Google Scholar]
- 56. Crowley MJ, Edelman D, McAndrew AT, et al. Practical telemedicine for veterans with persistently poor diabetes control: a randomized pilot trial. Telemed J E Health. 2016;22:376‐384. 2015/11/06. 10.1089/tmj.2015.0145 [DOI] [PubMed] [Google Scholar]
- 57. Gerber BS, Schiffer L, Brown AA, et al. Video telehealth for weight maintenance of African‐American women. J Telemed Telecare. 2013;19:266‐272. Randomized Controlled Trial Research Support, N.I.H., Extramural 2013/10/29. 10.1177/1357633X13490901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Heymsfield SB, Gonzalez MC, Shen W, Redman L, Thomas D. Weight loss composition is one‐fourth fat‐free mass: a critical review and critique of this widely cited rule. Obes Rev. 2014;15:310‐321. 2014/01/23. 10.1111/obr.12143 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Telemedicine Satisfaction Questionnaire
Table S2. Acceptability of Telemedicine Intervention


