Summary
Objective
Determine prevalence of hyperfiltration (high estimated glomerular filtration rate "eGFR" >95th percentile for age/sex) among youth and association with BMI classification.
Methods
With the use of 1999 to 2016 National Health and Nutrition Examination Survey data from 12‐ to 29‐year‐olds, data for serum creatinine and thresholds for high eGFR were normed using a metabolically healthy subsample (no albuminuria, healthy weights, normal blood pressures, blood glucoses, lipids, and liver enzymes). Logistic regression examined the association of BMI classification (healthy weight, overweight, and obesity classes 1‐3) with hyperfiltration (eGFR > 95th percentile for age/sex), adjusted for diabetes and other covariates.
Results
Of 12‐ to 29‐year‐olds (N = 18 698), 27.4% (n = 5493) met criteria for entry into the "healthy subsample" and contributed data to derive normative values for serum creatinine/hyperfiltration thresholds. In the full sample, hyperfiltration prevalence in 12‐ to 29‐year‐olds classified as healthy‐weight, overweight, and obesity classes 1 to 3 was 4.9%, 4.7%, 6.5%, 8.7%, and 11.8%, respectively (P < .001). In multivariable analysis, obesity classes 2 and 3 were associated with greater likelihood of hyperfiltration (adjusted ORs for class 2: 1.5, 95% CI, 1.1‐2.1; and for class 3, 2.1, 95% CI, 1.5‐2.9). Diabetes also was associated with hyperfiltration (AOR, 4.0; 95% CI, 2.2‐7.4).
Conclusion
Obesity classes 2 to 3 are associated with hyperfiltration in youth. Age/sex‐specific norms for creatinine and hyperfiltration thresholds may aid recognition of kidney dysfunction early.
Keywords: hyperfiltration, kidney disease, obesity, overweight
1. INTRODUCTION
Primordial prevention of kidney disease requires understanding modifiable risk factors for kidney disease early in life. Whereas the prevalence of chronic kidney disease (CKD) in adults is 15%, the prevalence in children is less than 0.01% (1.5‐3.0 per one million).1, 2
Glomerular hyperfiltration is one of the earliest markers of CKD that predicts progressive kidney‐function decline in adults with and without diabetes.3, 4, 5, 6, 7, 8 Hyperfiltration is reversible.3, 4, 5, 6, 7, 8 The definition of hyperfiltration in epidemiological studies is an estimated glomerular filtration rate (eGFR) greater than 95th percentile for sex and age among a healthy population.9, 10 Causes are primarily pathological (including hyperglycemia and nephron loss11) with a couple notable exceptions (pregnancy and immediately after a high‐protein meal). Prevalence of hyperfiltration increases directly with increases in blood glucose and blood pressure in adults with prediabetes and prehypertension,9 and in adults with diabetes, hyperfiltration precedes development of microalbuminuria and predicts rapid kidney‐function decline.3, 7
Obesity in youth may be an important target for the identification of glomerular hyperfiltration and prevention of kidney disease. Obesity begins early in life, affects 20.6% of adolescents and 39.8% of adults, increases blood volume, which increases renal blood flow, and increases both development of kidney‐disease risk factors (for example, diabetes and hypertension) and likelihood of end‐stage renal disease (ESRD).12, 13, 14, 15, 16, 17 Data from a longitudinal cohort study of 1.2 million adolescents indicate that obesity at age 17 to 18 years increases the likelihood of ESRD eight‐fold.15 In adults, greater weight gain over time increases the likelihood of CKD, independent of body mass index (BMI).18 Little is known about whether overweight and obesity impact kidney function in youth.
Because of the high prevalence of obesity and potential reversibility of hyperfiltration, determining population‐based thresholds for hyperfiltration and prevalence of hyperfiltration by BMI classification in youth may guide the design and development of alternative CKD prevention strategies targeted toward high‐risk youth who otherwise would not undergo screening for kidney function (eg, those without diabetes or hypertension).
The primary objective of this study was to determine if higher BMI classification (eg, overweight/obesity) among US adolescents and young adults is associated with higher prevalence of hyperfiltration, after establishing population‐based normative data for serum creatinine, creatinine‐based eGFR, and thresholds for hyperfiltration among metabolically healthy 12‐ to 29‐year‐olds without albuminuria. A secondary objective was to test whether obesity‐associated comorbidities for which kidney‐function screening is recommended (diabetes or hypertension) are associated with hyperfiltration.
2. METHODS
This study used cross‐sectional, population‐based data from the National Health and Nutrition Examination Survey (NHANES) from 1999 to 2016 among 12‐ to 29‐year‐olds. To adequately represent the US population, NHANES collects data from a stratified, multistage probability sample in 2‐year cycles. Each participant completes interviews, physical examinations, laboratory studies, and surveys at a single time point. Data regarding the representativeness of NHANES data are published.19 To determine prevalence of hyperfiltration, data were needed to define "normal" serum‐creatinine levels and creatinine‐based eGFR thresholds for hyperfiltration indexed to population‐based data from healthy youth and young adults—that is, those with no proteinuria, healthy BMIs, and no other recognized risk factors for kidney disease.
Data were used to create normative values for serum creatinine, eGFR, and thresholds for hyperfiltration. Exclusions applied to ensure complete data for analyses were missing values for key study measures, underweight, and pregnancy (Figure 1). To create normative values, a “healthy subsample” was identified within the full sample, defining health as free from known risk factors for kidney or cardiovascular disease in adolescents/adults.12, 13, 14, 20, 21, 22 Inclusion required a healthy BMI (defined below), plus the following covariates (each described in Covariates below): no albuminuria and no evidence of abnormal blood pressure, lipids, blood glucose, or fatty liver (using liver‐enzyme alanine aminotransferase [ALT]). Creatinine values in the full sample (including those not meeting "healthy‐subsample" criteria) also were examined.
Figure 1.
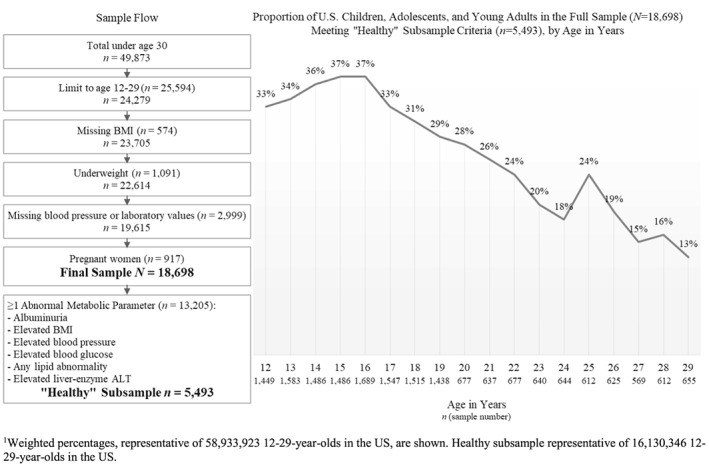
Sample flow (on the left) alongside the proportion of U.S. children, adolescents, and young adults (by age) in the full sample (N=18,698) meeting criteria for entry into the “healthy” subsample (n=5,493). Criteria for entry into the healthy subsample were: no albuminuria, a healthy body mass index, normal blood pressure, normal blood glucose, no abnormal lipids, and no evidence of fatty liver disease (per liver‐enzyme alanine aminotransferase [ALT]). Weighted percentages are shown: the full sample represents 58,933,923 12‐29‐year‐olds in the US, and the healthy subsample represents 16,130,346 12‐29‐year‐olds in the US
2.1. Measures
The primary independent predictor was BMI classification. BMI was calculated using measured height and weight. For 12‐ to 17‐year‐olds, Centers for Disease Control growth charts were used to define five categories: healthy weight, BMI% ≥10 to <85 (inclusion criterion for 12‐ to 17‐year‐olds to enter the healthy subsample); overweight, BMI% ≥85 to < 95; class 1 obesity, BMI% ≥95 to <120th %‐of‐95th BMI‐percentile (BMI95), class 2 obesity, BMI% ≥120 to <140th of BMI95, and class 3 obesity, BMI% ≥140th of BMI95.23 For 18‐ to 29‐year‐olds, the corresponding categories were healthy weight, BMI ≥18.5 to 24.9 kg/m2 (inclusion criterion for entry into the healthy subsample); overweight, 25.0 to 29.9 kg/m2; and obesity classes 1 to 3, defined respectively as BMI ≥30.0 to 34.9, 35.0 to 39.9, and ≥40 kg/m2.
The primary study outcome was prevalence of hyperfiltration. We derived normative eGFR estimates in the metabolically healthy subsample to define hyperfiltration as eGFR > 95th percentile for sex and age category. The rationale for defining hyperfiltration as eGFR > 95th percentile for age and sex is that adult studies that defined hyperfiltration similarly have reported associations of hyperfiltration and adverse outcomes (REF). The rationale for indexing the greater than 95th percentile eGFR threshold in a metabolically healthy population‐based sample is that diabetes, hypertension, and other metabolic risk factors are associated both with overweight/obesity and abnormal kidney function.
Serum creatinine was measured using a kinetic‐rate Jaffe method in NHANES.24 All measurements were recalibrated to standardized creatinine measurements obtained at Cleveland Clinic Research Laboratory (Cleveland, Ohio), per published methods.25
2.1.1. Glomerular filtration rate
Although inulin‐clearance‐measured GFR (mGFR) is considered the gold standard of GFR measurement, NHANES lacks mGFR or sufficient cystatin‐C data for cystatin‐C‐based‐GFR estimates. Moreover, data for creatinine‐based GFR estimation have come from separate investigations of children (<18 y old) and adults (≥18 y old).26, 27, 28, 29 Recognizing this, a preceding study characterized a method to calculate eGFR using the mean of established pediatric and adult creatinine‐based eGFR equations in young adults (≥18 y old).30 This was the approach used in the present study to derive normative eGFR estimates for sex and four peri‐pubertal age groups: 12 to 14 years, 15 to 17 years, 18 to 21 years, and 22 to 29 years (to ensure sufficient sample size for estimation within each group).
Using inputs for eGFR equations of height (in centimeters), serum creatinine (S Cr, mg/dL), and blood urea nitrogen (BUN, mg/dL), this study estimated serum‐creatinine‐based eGFR using the following two equations:
-
1
For 12‐ to 17‐year‐olds, the pediatric creatinine‐based Chronic Kidney Disease in Children (CKiD) equation:27
-
2
For 18‐29‐year‐olds, the mean of eGFRpediatric (above) and the adult CKD‐Epidemiology Collaboration (CKD‐EPI) equation:26, 30
2.2. Covariates
2.2.1. Urinary albumin/creatinine ratio
A single random spot urine sample was obtained from participants in mobile‐examination centers to measure urine albumin and creatinine.24 Albuminuria was defined as greater than or equal to 30‐mg albumin/gram creatinine.4
Dyslipidemia was defined using standard measures, choosing conservative definitions when recommendations were inconsistent: total cholesterol ≥ 200 mg/dL, LDL cholesterol ≥ 110 mg/dL, HDL cholesterol < 35 mg/dL, or triglycerides ≥ 150 mg/dL.31, 32
2.2.2. Prediabetes/diabetes
Prediabetes was defined as HbA1c ≥ 5.7 to <6.5% or fasting glucose ≥ 100 to <126 mg/dL; and diabetes, HbA1c ≥ 6.5%, fasting glucose ≥ 126 mg/dL, or random glucose ≥ 200 mg/dL (using American Diabetes Association criteria).17
Elevated ALT was determined using assay‐recommended thresholds: for less than 20‐year‐olds, greater than or equal to 36 mg/dL for males, and greater than or equal to 29 mg/dL for females; for greater than or equal to 20‐year‐olds, greater than or equal to 47 mg/dL for males and greater than or equal to 30 mg/dL for females.21, 24
2.2.3. Elevated blood pressure/hypertension
Blood pressure was measured three times by trained observers using a standard protocol.19 Individual participant's blood pressures were averaged using all readings. For less than 18‐year‐olds, standardized blood‐pressure tables were used to define “elevated blood pressure” as average systolic or diastolic blood pressure ≥90th to <95th percentile (or systolic ≥120 mmHg/diastolic ≥80 mmHg, if the 90th‐percentile thresholds exceeded 120/80 mmHg, per Fourth Report guidelines used at the time data were collected33) and “hypertension” as average systolic or diastolic blood pressure ≥95th percentile (with the caveat that diagnosis of hypertension requires three separate visits with ≥95th‐percentile elevations). For 18‐ to 29‐year‐olds, elevated blood pressure was defined as systolic ≥120 to <140 mmHg or diastolic blood pressure ≥80 to <90 mmHg, and hypertension as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg.34
2.3. Statistical analysis
2.3.1. Norms
Normative charts were developed for serum creatinine and eGFR by estimating percentile distributions of each for sex and age category, applying each eGFR equation to data from the metabolically healthy subsample and estimating the fifth, 50th, and 95th percentiles for serum creatinine and eGFR. Thresholds for hyperfiltration by sex and age category were defined using greater than 95th percentile.
Changes in mean serum creatinine over 1999 to 2016 NHANES cycles also were examined in the full sample and in the healthy subsample using adjusted Wald tests.
Cross‐tabulations were used to examine prevalence of hyperfiltration by covariates and tested for differences using Pearson's chi square with a second‐order Rao and Scott correction. Adjusted Wald tests were used to compare prevalence of hyperfiltration in the full sample of 12‐ to 29‐year‐olds by BMI classification and by BMI classification within each age category.
For the multivariable analysis, a logistic regression model was used to estimate odds of hyperfiltration associated with higher BMI classification (primary independent variable), blood glucose (normal, prediabetes, or diabetes), and blood pressure (normal, elevated blood pressure, or hypertensive blood pressure). The model adjusted for age category, sex, race/ethnicity (categorized into White/non‐Hispanic, Black/non‐Hispanic, Hispanic, or other/non‐Hispanic race/ethnicity), abnormal lipids, elevated ALT, and NHANES cycle (as recommended by the National Center for Health Statistics). All analyses were weighted to account for the complex survey design and performed using Stata svy commands (version 15.0).
The study was exempted from human‐subjects review by Duke University's Institutional Review Board (Federal Regulation 45CFR §46.101[b]).
3. RESULTS
3.1. Sample characteristics
Of 18 698 youth and young adults in the full sample, representative of 58 933 923 US 12‐to 29‐year‐olds, one‐third were youth 12 to 17 years old, and two‐thirds were young adults 18 to 29 years old (Table 1). Half had a BMI classification of healthy weight. The remainder had overweight or obesity. Rates of metabolic abnormalities precluding entry into the metabolically healthy subsample ranged from 0.9% for diabetes to 31.7% for abnormal lipids.
Table 1.
Sample characteristics and weighted percentages for 12‐ to 29‐year‐olds in the United States: full NHANES sample (N = 18 698) and “healthy subset” (n = 5493) used to derive normative serum creatinine and eGFR values
| Full samplea | Healthy subsetb | |||
|---|---|---|---|---|
| Characteristic | N | % | n | % |
| Age category, y | ||||
| 12‐14 | 4675 | 16.4 | 1613 | 22.1 |
| 15‐17 | 4722 | 17.5 | 1694 | 23.3 |
| 18‐21 | 4267 | 21.6 | 1242 | 23.2 |
| 22‐29 | 5034 | 44.5 | 944 | 31.4 |
| Sex | ||||
| Female | 9063 | 47.5 | 2872 | 54.5 |
| Male | 9635 | 52.5 | 2621 | 45.5 |
| Race/ethnicity | ||||
| White, non‐Hispanic | 5639 | 59.2 | 1729 | 62.4 |
| Black, non‐Hispanic | 4913 | 13.3 | 1368 | 11.5 |
| Hispanic | 6671 | 20.2 | 1883 | 17.5 |
| Other, non‐Hispanic | 1475 | 7.3 | 513 | 8.7 |
| BMI classificationc | ||||
| Healthy weight | 9977 | 51.0 | 5493 | 100 |
| Overweight | 4228 | 25.0 | ||
| Class 1 obesity | 2637 | 14.0 | ||
| Class 2 obesity | 1130 | 6.0 | ||
| Class 3 obesity | 726 | 4.0 | ||
| Albuminuriad | ||||
| Absent | 16 945 | 91.7 | 5493 | 100 |
| Present | 1753 | 8.3 | ||
| Blood pressuree | ||||
| Normal | 14 409 | 74.4 | 5493 | 100 |
| Elevated | 3675 | 22.5 | ||
| Hypertension | 614 | 3.1 | ||
| Blood glucosef | ||||
| Normal | 16 742 | 89.9 | 5493 | 100 |
| Prediabetes | 1796 | 9.2 | ||
| Diabetes | 160 | 0.9 | ||
| Lipidsg | ||||
| Normal | 13 541 | 68.3 | 5493 | 100 |
| Abnormal | 5157 | 31.7 | ||
| Liver enzyme ALTh | ||||
| Normal | 17 031 | 90.9 | 5493 | 100 |
| Elevated | 1667 | 9.1 | ||
Representative of 58 933 923 12‐ to 29‐year‐olds in the United States. Weighted percentages are shown.
Healthy subset defined as healthy weight with no evidence of albuminuria, prediabetes/diabetes, elevated blood pressure/hypertension, abnormal lipids, or abnormal ALT; representative of 16 130 346 12‐ to 29‐year‐olds in the United States.
BMI classification. For 12‐ to 17‐year‐olds: healthy weight, BMI% ≥10 to <85; overweight, BMI% ≥85 to <95; class 1 obesity, BMI% ≥95 to <120th %‐of‐95th BMI‐percentile (BMI95), class 2 obesity, BMI% ≥120 to <140th of BMI95, and class 3 obesity, BMI% ≥140th of BMI95.23 For 18‐ to 29‐year‐olds, healthy weight, BMI ≥18.5 to 24.9 kg/m2; overweight, 25.0 to 29.9 kg/m2; and obesity classes 1 to 3, defined respectively as BMI ≥30.0 to 34.9, 35.0 to 39.9, and ≥40 kg/m2.
Albuminuria defined as greater than or equal to 30‐mg albumin/gram creatinine. Urine samples are not first morning samples. Some youth in the sample may have had orthostatic albuminuria (the most common cause of isolated proteinuria in children.)
Blood pressure (BP) definitions. For 12‐ to 17‐year‐olds, elevated BP: average systolic/diastolic BP 90th to <95th percentile (or systolic BP ≥120 mmHg/diastolic BP ≥80 mmHg, if 90th percentile exceeded 120/80 mmHg34). Hypertension: avg. systolic or diastolic BP ≥95th percentile. For 18‐ to 29‐year‐olds, elevated BP defined as systolic BP 120 to <140 mmHg or diastolic BP 80 to <90 mmHg, and hypertension as systolic BP ≥ 140 mmHg or diastolic ≥ 90 mmHg.34
Blood glucose. Normal: HbA1c < 5.7% and glucose fasting <100 and random <200 mg/dL. Prediabetes: HbA1c 5.7% to <6.5% or fasting glucose 100 to <126 mg/dL. Diabetes, HbA1c ≥ 6.5%, glucose fasting ≥ 126, or random ≥ 200 mg/dL.17
Lipid definition. Abnormal lipids was defined by presence of one or more of the following: total cholesterol ≥ 200 mg/dL, LDL cholesterol ≥ 110 mg/dL, HDL cholesterol < 35 mg/dL, or triglycerides ≥ 150 mg/dL.31, 32
Liver enzyme ALT definition. Elevated ALT was determined using assay‐recommended thresholds: for less than 20‐year‐olds, greater than or equal to 36 mg/dL for males and greater than or equal to 29 mg/dL for females; for greater than or equal to 20‐year‐olds, greater than or equal to 47 mg/dL for males and greater than or equal to 30 mg/dL for females.21, 24
The proportion of the full sample meeting criteria for entry into the "healthy subsample" was 27.4% (n = 5498)—that is, less than 30% of US 12‐ to 29‐year‐olds had no albuminuria, a healthy weight, and normal blood glucose, blood pressure, lipids, and liver‐enzyme ALT (Figure 1).
3.2. Normative serum creatinine level
Whereas the serum creatinine level in healthy females increased very little from age 12 to 14 years to 22 to 29 years, serum creatinine at the 50th percentile among healthy males increased sharply across each of the first three of four peri‐pubertal age categories examined (Figure 2). For example, in metabolically healthy 12‐ to 29‐year‐old females, serum creatinine at the 50th percentile ranged from 0.62 mg/dL for 12‐ to 14‐year‐olds to 0.72 mg/dL for 22‐ to 29‐year‐olds—an increase of 0.10 mg/dL (Figure 2A). In contrast, among metabolically healthy 12‐ to 29‐year‐old males, serum creatinine at the 50th percentile ranged from 0.65 mg/dL for 12‐ to 14‐year‐olds to 0.86 mg/dL for 15‐ to 17‐year‐old, to 0.95 for 18‐ to 21‐year‐olds, and to 0.96 mg/dL for 22‐ to 29‐year‐olds—an increase in serum creatinine among healthy youth/young‐adult males of 0.31 mg/dL.
Figure 2.
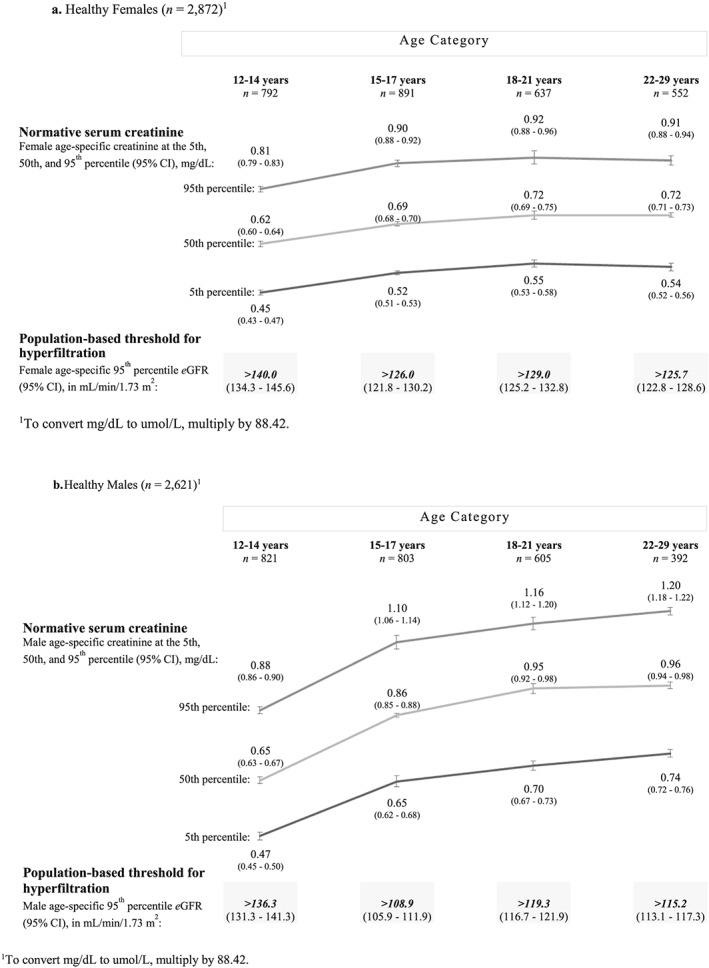
Normative serum creatinine levels (at the fifth, 50th, and 95th percentiles) and estimated glomerular filtration rate (eGFR) thresholds for hyperfiltration among metabolically healthy females (A) and males (B) by age. (A). Healthy females (n = 2872).1 (B). Healthy males (n = 2621). To convert mg/dL to umol/L, multiply by 88.42
3.3. Time trends (1999‐2016) in normative serum creatinine level
Although creatinine values in the full sample overlapped with those in the healthy subsample (data not shown), time trends differed among these groups. Over 1999 to 2016 NHANES cycles, mean serum creatinine decreased (from 0.83 mg/dL in 1999‐2000 to 0.78 mg/dL in 2015‐2016, P < .001) in the full sample of US youth and young adults. In contrast, normative mean serum creatinine did not change significantly over the same NHANES cycles among metabolically healthy 12‐ to 29‐year‐olds.
3.4. Population‐based thresholds for hyperfiltration
Hyperfiltration thresholds (eGFR > 95th percentile), indexed in metabolically healthy individuals, ranged in females from greater than 140.0 mL/min/1.73 m2 in 12‐ to 14‐year‐olds to greater than 125.7 mL/min/1.73 m2 in 22‐ to 29‐year‐olds (Figure 2A) and in males from greater than 136.3 mL/min/1.73 m2 in 12‐ to 14‐year‐olds to greater than 115.2 mL/min/1.73 m2 in 22‐ to 29‐year‐olds (Figure 2B).
3.5. Prevalence of hyperfiltration
The prevalence of hyperfiltration among those with healthy weight, overweight, and obesity classes 1 to 3 was 4.9%, 4.7%, 6.5%, 8.7%, and 11.8%, respectively (P < .001) (Figure 3). Similarly, the prevalence of hyperfiltration was significantly associated with increasing BMI classification within each of the four age categories examined.
Figure 3.
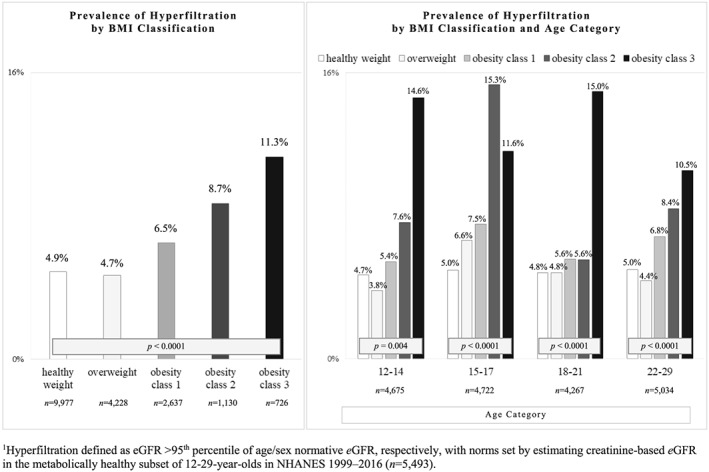
Hyperfiltration in 12‐ to 29‐year‐old US youth and young adults (N = 18 698). Hyperfiltration defined as estimated glomerular filtration rate (eGFR) greater than 95th percentile of age/sex normative eGFR, respectively, with norms set by estimating creatinine‐based eGFR in the metabolically healthy subset of 12‐ to 29‐year‐olds in NHANES 1999 to 2016 (n = 5493). Prevalence of hyperfiltration by body mass index (BMI) classification among 12‐ to 29‐year‐old US youth and young adults (N = 18 698) in the overall sample and for each of the four peri‐pubertal age groups studied
Diabetes was associated with an increased prevalence of hyperfiltration (22.7% with diabetes vs 5.6% with prediabetes and 5.4% with normal blood glucose), but elevated blood pressure/hypertension was not. Other factors associated with an increased prevalence of hyperfiltration in the bivariate (unadjusted) analysis included elevated ALT, all non‐White/non‐Hispanic races/ethnicities, and female sex (Table 2). Hyperfiltration prevalence was not associated with abnormal lipids or age category.
Table 2.
Prevalence of hyperfiltration by sample characteristics among 12‐ to 29‐year‐olds in NHANES and results of multivariable analysis of the association of BMI classification, diabetes, and hypertension with adjusted odds of hyperfiltration (defined as an eGFR > 95th percentile for sex/age in a healthy subsample)a,b
| No. | Prevalence of Hyperfiltration | Adjusted Odds c (95% CI) of Hyperfiltration | |
|---|---|---|---|
| Characteristic | N = 18 698 | 7.0% | |
| BMI classification | |||
| Healthy weight | 9977 | 4.9 | Reference |
| Overweight | 4228 | 4.7 | 0.9 (0.7‐1.1) |
| Class 1 obesity | 2637 | 6.5 | 1.2 (1.0‐1.5) |
| Class 2 obesity | 1130 | 8.7 | 1.5 (1.1‐2.1) |
| Class 3 obesity | 726 | 11.8 | 2.1 (1.5‐2.9) |
| P | <0.001 | ||
| Blood glucose | |||
| Normal | 16 742 | 5.4 | Reference |
| Prediabetes | 1796 | 5.6 | 0.9 (0.7‐1.1) |
| Diabetes | 160 | 22.7 | 4.0 (2.2‐7.4) |
| P | <0.001 | ||
| Blood pressure | |||
| Normal | 14 409 | 5.5 | Reference |
| Elevated | 3675 | 5.8 | 1.0 (0.8‐1.2) |
| Hypertensive | 614 | 5.4 | 0.8 (0.5‐1.3) |
| P | 0.8 | ||
| Liver enzyme ALT | |||
| Normal | 17 031 | 5.3 | Reference |
| Elevated | 1667 | 7.7 | 1.1 (0.9‐1.5) |
| P | 0.003 | ||
| Race/ethnicity | |||
| White, non‐Hispanic | 5639 | 3.4 | Reference |
| Black, non‐Hispanic | 4913 | 7.4 | 2.1 (1.7‐2.6) |
| Hispanic | 6671 | 10.4 | 3.1 (2.6‐3.8) |
| Other, non‐Hispanic | 1475 | 6.0 | 1.7 (1.2‐2.4) |
| P | <0.001 | ||
| Sex | |||
| Female | 9063 | 6.2 | Reference |
| Male | 9635 | 5.0 | 0.8 (0.7‐1.0) |
| P | 0.006 | ||
| Age category, y | |||
| 12‐14 | 4675 | 5.0 | Reference |
| 15‐17 | 4722 | 6.2 | 1.3 (1.0‐1.7) |
| 18‐21 | 4267 | 5.3 | 1.1 (0.9‐1.4) |
| 22‐29 | 5034 | 5.6 | 1.1 (0.9‐1.4) |
| P | 0.3 | ||
| Lipids | |||
| Normal | 13 541 | 5.7 | Reference |
| Abnormal | 5157 | 5.3 | 0.9 (0.8‐1.1) |
| P | 0.4 | ||
Weighted percentages are shown for estimates of hyperfiltration prevalence. Percentage with normal eGFR are not shown (for simplicity).
Healthy subset defined as healthy weight with no evidence of albuminuria, prediabetes/diabetes, elevated blood pressure/hypertension, abnormal lipids, or abnormal ALT.
Odds ratios are adjusted for covariates shown in the table plus NHANES cycle.
3.6. Multivariable analysis
In multivariable logistic regression analysis, obesity classes 2 and 3 were independently associated with 50% and 210% higher likelihoods of hyperfiltration, respectively (Table 2). Diabetes was associated with a 400% higher likelihood of hyperfiltration. Hypertension was not associated with hyperfiltration prevalence. An additional factor associated with increased likelihood of hyperfiltration was race/ethnicity (all race/ethnicities had increased adjusted odds compared with White/non‐Hispanics).
4. DISCUSSION
These nationally representative data indicate that the prevalence of hyperfiltration—an antecedent of future CKD—is higher among US youth and young adults with obesity classes 2 and 3, and those with diabetes, though not those with hypertension. Specifically, the prevalence of hyperfiltration is 8.7% among those with obesity class 2, 11.3% among those with obesity class 3, and 22.7% among those with diabetes. To give context to these estimates, the prevalence of obesity classes 2 and 3 is relatively high (10% in this population‐based sample) whereas the prevalence of diabetes is low (0.9%—an estimate comparable with ones from a meta‐analysis of diabetes‐prevalence studies in youth.35)
Given this finding, together with longitudinal cohort‐study data that document obesity in adolescence is an independent risk factor for ESRD 20 to 25 years later,12, 13, 15 the authors suspect that obesity‐related hyperfiltration represents early evidence of future renal‐function decline. Thus, the ideal time to communicate about kidney disease risk and optimize modifiable risk factors may be at onset of hyperfiltration. Although this study's cross‐sectional data do not provide evidence that obesity‐associated hyperfiltration predicts kidney failure over time, the age/sex‐specific thresholds for hyperfiltration may aid this question's future investigation.
Several possible explanations link obesity and kidney disease. Increases in fat mass increase blood volume, which increases renal blood flow.36 Obesity in preterm children (who have decreased nephron mass) increases risk of progressive kidney disease.37, 38 Premature and low birth weight children exhibit elevations in (a) biomarkers of adiposity, (b) blood pressure, and (c) GFR at 1 to 2 years of age—the co‐occurrence of high blood pressure and GFR indicates that renal autoregulation is impaired.39, 40 By 10 to 12 years old, prematurity and low birth weight are associated with obesity, hypertension, and hypofiltration.41, 42, 43 These observations highlight that obesity alone, and especially combined with prematurity/low birth weight, may place an extra burden on nephrons, contribute to loss of reno‐protective autoregulation, and promote renal failure over time.
Another important study finding is that diabetes is associated with hyperfiltration in youth. Diabetes, though rare in childhood, is more prevalent among adolescents with obesity, and childhood obesity guidelines recommend screening for it.31, 44 Once diabetes is identified, serial evaluations of kidney function are recommended. These study data give thresholds for understanding if hyperfiltration is present.
Although the current study does not indicate that hypertension is associated with hyperfiltration, hypertension is a potent risk factor for future kidney failure and rates of diagnosis/treatment of hypertension in children are low.16, 34 Moreover, the recommendation to determine kidney function in youth with hypertension is to evaluate evidence of kidney failure as a secondary underlying cause of hypertension.16, 33
Study data add sex and age‐specific normative data for serum creatinine in metabolically healthy US youth and young adults. These data depict serum creatinine in males rising by approximately 50% peri‐pubertally versus less than 20% in females. Prior data on typical creatinine values had indicated mean serum creatinine was 0.71 mg/dL in 12 to 17‐year‐olds (with no sex‐specific differences reported), 0.96 mg/dL in adult women, and 1.16 mg/dL in men.28, 29 Per data reported here, mean serum creatinine of 0.71 mg/dL falls outside the 50th percentile and associated 95% CI for both 12 and 14‐year‐old females (50th percentile, 0.62 mg/dL; 95% CI, 0.60‐0.64) and males (0.65 mg/dL; 95% CI, 0.63‐0.67). In this era of electronic health records, health care providers caring for adults typically receive a patient's creatinine‐based eGFR value when eGFR is less than 60 mL/min/1.73m2, whereas no eGFR value is provided if the eGFR is greater than 60 mL/min/1.73 m2. In contrast, pediatric providers must interpret raw serum creatinine values in growing children/adolescents, because pediatric electronic health records typically do not provide an estimate of GFR when serum creatinine is measured in children. Thus, providers caring for 12‐ to 29‐year‐olds may find our study's creatinine data by sex and age useful at the point‐of‐care to consider whether a patient has a normal creatinine level or is exhibiting hyperfiltration.
Study strengths include use of a nationally representative sample with high‐quality laboratory and examination data and oversampling of racial and ethnic minorities. An important study limitation is that longitudinal data are needed to understand the natural history of hyperfiltration and how to use eGFR clinically over time and beyond young adulthood. Another limitation is that urine samples were not first morning samples (some youth may have had orthostatic albuminuria, which is the most common cause of isolated proteinuria in children), and albuminuria data came from single urine‐creatinine/urine‐albumin measurements.24 These limitations were permitted, because no one had ever examined the prevalence of hyperfiltration by BMI classification in a population‐based healthy sample of youth and young adults.
5. CONCLUSION
This is the first nationally representative study to identify that obesity classes 2 and 3 are associated with a higher prevalence of hyperfiltration among US youth and young adults and provide normative serum creatinine levels and eGFR thresholds for hyperfiltration by sex and age. Findings provide needed data regarding abnormally low creatinine values that herald hyperfiltration in youth and young adults. Intensive weight management of youth and young adults with obesity and hyperfiltration may prove useful for preventing kidney disease.
CONFLICT OF INTEREST
The authors declared no conflict of interest.
AUTHOR CONTRIBUTIONS
Dr Skinner had full access to all NHANES data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
Study concept and design: Turer, Skinner. Acquisition of data: Skinner. Analysis and interpretation of data: Turer, Baum, Selistre, Dubourg, Skinner. Drafting of the manuscript: Turer and Skinner. Critical revision for important intellectual content: Turer, Baum, Selistre, Dubourg, Skinner. Statistical analysis: Skinner. Administrative, technical, or material support: Turer, Selistre, Dubourg, Skinner. Study supervision: Turer, Baum, Skinner.
FUNDING
This study is supported in part by Awards #K23HL118152 and #R03HL144811 from the National Heart, Lung, and Blood Institute (NHLBI; to Dr. Turer); Award #R21DK114764 from the National Institutes of Diabetes and Digestive and Kidney Diseases (NIDDK; to Dr. Turer); Award # UL1TR001105 from the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH); and Award #R24HS022418 from the Agency for Healthcare Research and Quality (AHRQ). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NHLBI, NIDDK, NIH, or AHRQ.
Supporting information
Data S1: Supporting Information
Turer CB, Baum M, Dubourg L, Selistre LS, Skinner AC. Prevalence of hyperfiltration among US youth/young adults with overweight and obesity: A population‐based association study. Obes Sci Pract. 2019;5:570–580. 10.1002/osp4.365.
Clinical Trial Registration: not applicable
REFERENCES
- 1. Murphy D, McCulloch CE, Lin F, et al. Trends in prevalence of chronic kidney disease in the United States. Ann. Intern. Med. 2016;165:473‐481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saydah SH, Xie H, Imperatore G, Burrows NR, Pavkov ME. Trends in albuminuria and GFR among adolescents in the United States, 1988‐2014. Am. J. Kidney Dis. 2018;72:644‐652. [DOI] [PubMed] [Google Scholar]
- 3. Low S, Zhang X, Wang J, et al. Long‐term prospective observation suggests that glomerular hyperfiltration is associated with rapid decline in renal filtration function: a multiethnic study. Diab. Vasc. Dis. Res. 2018;15:417‐423. [DOI] [PubMed] [Google Scholar]
- 4. Matsushita K, Coresh J, Sang Y, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta‐analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3:514‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ruggenenti P, Abbate M, Ruggiero B, et al. Renal and systemic effects of calorie restriction in patients with type 2 diabetes with abdominal obesity: a randomized controlled trial. Diabetes. 2017;66:75‐86. [DOI] [PubMed] [Google Scholar]
- 6. Lee SM, Park JY, Park MS, Park JH, Park M, Yoon HJ. Association of renal hyperfiltration with incident proteinuria ‐ A nationwide registry study. PLoS ONE. 2018;13:e0195784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Magee GM, Bilous RW, Cardwell CR, Hunter SJ, Kee F, Fogarty DG. Is hyperfiltration associated with the future risk of developing diabetic nephropathy? A meta‐analysis. Diabetologia. 2009;52:691‐697. [DOI] [PubMed] [Google Scholar]
- 8. Melsom T, Nair V, Schei J, et al. Correlation between baseline GFR and subsequent change in GFR in Norwegian adults without diabetes and in Pima Indians. Am. J. Kidney Dis. 2019;73:777‐785. [DOI] [PubMed] [Google Scholar]
- 9. Okada R, Yasuda Y, Tsushita K, Wakai K, Hamajima N, Matsuo S. Glomerular hyperfiltration in prediabetes and prehypertension. Nephrol. Dial. Transplant. 2012;27:1821‐1825. [DOI] [PubMed] [Google Scholar]
- 10. Park M, Yoon E, Lim YH, Kim H, Choi J, Yoon HJ. Renal hyperfiltration as a novel marker of all‐cause mortality. J. Am. Soc. Nephrol. 2015;26:1426‐1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cozzi DA, Ceccanti S, Frediani S, Mele E, Cozzi F. Renal function adaptation up to the fifth decade after treatment of children with unilateral renal tumor: a cross‐sectional and longitudinal study. Pediatr. Blood Cancer. 2013;60:1534‐1538. [DOI] [PubMed] [Google Scholar]
- 12. Chang Y, Ryu S, Choi Y, et al. Metabolically healthy obesity and development of chronic kidney disease: a cohort study. Ann. Intern. Med. 2016;164:305‐312. [DOI] [PubMed] [Google Scholar]
- 13. Herrington WG, Smith M, Bankhead C, et al. Body‐mass index and risk of advanced chronic kidney disease: Prospective analyses from a primary care cohort of 1.4 million adults in England. PLoS ONE. 2017;12:e0173515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sundin PO, Udumyan R, Sjostrom P, Montgomery S. Predictors in adolescence of ESRD in middle‐aged men. Am. J. Kidney Dis. 2014;64:723‐729. [DOI] [PubMed] [Google Scholar]
- 15. Vivante A, Golan E, Tzur D, et al. Body mass index in 1.2 million adolescents and risk for end‐stage renal disease. Arch. Intern. Med. 2012;172:1644‐1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Flynn JT, Kaelber DC, Baker‐Smith CM, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. 2017;140:e20171904. [DOI] [PubMed] [Google Scholar]
- 17. Fox CS, Golden SH, Anderson C, et al. Update on prevention of cardiovascular disease in adults with type 2 diabetes mellitus in light of recent evidence: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2015;132:691‐718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ryu S, Chang Y, Woo HY, et al. Changes in body weight predict CKD in healthy men. J. Am. Soc. Nephrol. 2008;19:1798‐1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Johnson CL, Dohrmann SM, Burt VL, Mohadjer LK. National health and nutrition examination survey: sample design, 2011‐2014. Vital Health Stat 2. 2014;162:1‐33. [PubMed] [Google Scholar]
- 20. Coresh J, Turin TC, Matsushita K, et al. Decline in estimated glomerular filtration rate and subsequent risk of end‐stage renal disease and mortality. JAMA. 2014;311:2518‐2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Targher G, Day CP, Bonora E. Risk of cardiovascular disease in patients with nonalcoholic fatty liver disease. N. Engl. J. Med. 2010;363:1341‐1350. [DOI] [PubMed] [Google Scholar]
- 22. Twig G, Yaniv G, Levine H, et al. Body‐mass index in 2.3 million adolescents and cardiovascular death in adulthood. N. Engl. J. Med. 2016;374:2430‐2440. [DOI] [PubMed] [Google Scholar]
- 23. Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat. 2002;11:1‐190. [PubMed] [Google Scholar]
- 24. Centers for Disease Control . National Health and Nutrition Examination Survey: Laboratory data. https://wwwn.cdc.gov/nchs/nhanes/search/datapage.aspx? Component = Laboratory. Accessed July 19, 2019.
- 25. Selvin E, Manzi J, Stevens LA, et al. Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988‐1994, 1999‐2004. Am. J. Kidney Dis. 2007;50:918‐926. [DOI] [PubMed] [Google Scholar]
- 26. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009;150:604‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J. Am. Soc. Nephrol. 2009;20:629‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chavers BM, Rheault MN, Foley RN. Kidney function reference values in US adolescents: national health and nutrition examination survey 1999‐2008. Clin. J. Am. Soc. Nephrol. 2011;6:1956‐1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jones CA, McQuillan GM, Kusek JW, et al. Serum creatinine levels in the US population: third National Health and Nutrition Examination Survey. Am. J. Kidney Dis. 1998;32:992‐999. [DOI] [PubMed] [Google Scholar]
- 30. Ng DK, Schwartz GJ, Schneider MF, Furth SL, Warady BA. Combination of pediatric and adult formulas yield valid glomerular filtration rate estimates in young adults with a history of pediatric chronic kidney disease. Kidney Int. 2018;94:170‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents, National Heart, Lung and Blood Institute . Expert panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents: Summary report. Pediatrics. 2011;128:s213‐s256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kavey RE, Daniels SR, Lauer RM, Atkins DL, Hayman LL, Taubert K. American Heart Association guidelines for primary prevention of atherosclerotic cardiovascular disease beginning in childhood. Circulation. 2003;107:1562‐1566. [DOI] [PubMed] [Google Scholar]
- 33. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents . The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Pediatrics. 2004;114:555‐576. [PubMed] [Google Scholar]
- 34. Chobanian AV, Bakris GL, Black HR, et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560‐2572. [DOI] [PubMed] [Google Scholar]
- 35. Fazeli Farsani S, van der Aa MP, van der Vorst MM, Knibbe CA, de Boer A. Global trends in the incidence and prevalence of type 2 diabetes in children and adolescents: a systematic review and evaluation of methodological approaches. Diabetologia. 2013;56:1471‐1488. [DOI] [PubMed] [Google Scholar]
- 36. Reisin E, Messerli FG, Ventura HO, Frohlich ED. Renal haemodynamic studies in obesity hypertension. J. Hypertens. 1987;5:397‐400. [PubMed] [Google Scholar]
- 37. Abitbol CL, Chandar J, Rodriguez MM, et al. Obesity and preterm birth: additive risks in the progression of kidney disease in children. Pediatr. Nephrol. 2009;24:1363‐1370. [DOI] [PubMed] [Google Scholar]
- 38. Carmody JB, Charlton JR. Short‐term gestation, long‐term risk: prematurity and chronic kidney disease. Pediatrics. 2013;131:1168‐1179. [DOI] [PubMed] [Google Scholar]
- 39. Duncan AF, Frankfurt JA, Heyne RJ, Rosenfeld CR. Biomarkers of adiposity are elevated in preterm very‐low‐birth‐weight infants at 1, 2, and 3 y of age. Pediatr. Res. 2017;81:780‐786. [DOI] [PubMed] [Google Scholar]
- 40. Frankfurt JA, Duncan AF, Heyne RJ, Rosenfeld CR. Renal function and systolic blood pressure in very‐low‐birth‐weight infants 1‐3 years of age. Pediatr. Nephrol. 2012;27:2285‐2291. [DOI] [PubMed] [Google Scholar]
- 41. South AM, Nixon PA, Chappell MC, et al. Renal function and blood pressure are altered in adolescents born preterm. Pediatr. Nephrol. 2019;34:137‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. de Jong F, Monuteaux MC, van Elburg RM, Gillman MW, Belfort MB. Systematic review and meta‐analysis of preterm birth and later systolic blood pressure. Hypertension. 2012;59:226‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Belfort MB, Gillman MW, McCormick MC. Prenatal and perinatal predictors of blood pressure at school age in former preterm, low birth weight infants. J. Perinatol. 2012;32:265‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barlow SE, Expert Committee . Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics. 2007;120:S164‐S192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1: Supporting Information


