Abstract
Sarcopenia is defined as skeletal muscle attenuation and has an association with metabolic syndrome. Metabolic syndrome, which includes obesity, is one of known predictive factors for gastroesophageal reflux disease (GERD). This study aimed to elucidate the association between sarcopenia and GERD. We retrospectively reviewed electronic medical records of 8,218 patients who were performed an upper gastrointestinal endoscopy at check-up center of the Gangnam Severance Hospital. GERD was diagnosed by endoscopic findings. Erosive reflux disease (ERD) included Barrett's esophagus and reflux esophagitis, with the exception of minimal change esophagitis. Sarcopenia was defined by appendicular skeletal muscle (skeletal muscle in the upper and lower limbs). Sarcopenic obesity was defined as the presence of both sarcopenia and obesity. Associations between sarcopenia and GERD, as well as between sarcopenic obesity and ERD, were analyzed. A total of 3,414 patients were diagnosed with GERD, and 574 (16.8%) had sarcopenia. Sarcopenia was independent predictive factor for GERD (odds ratio [OR] = 1.170, 95% confidence interval [CI]: 1.016–1.346, P = 0.029). In addition, male sex, smoking, alcohol, and diet, including sweets and fatty food, had a significant association with GERD. A total of 1,423 (17.3%) of 8,218 patients were diagnosed with ERD, and 302 (21.2%) had sarcopenia. Male sex, smoking, and fatty food consumption had a significant association with ERD. Moreover, sarcopenia (OR = 1.215, 95% CI: 1.019–1.449, P = 0.030), obesity (OR = 1.343, 95% CI: 1.163–1.552, P < 0.001), and sarcopenic obesity (OR = 1.406, 95% CI: 1.195–1.654, P < 0.001) were independent predictive factors for ERD. Sarcopenia is associated with GERD, and sarcopenic obesity may be predictive factor for ERD.
Subject terms: Gastro-oesophageal reflux disease, Oesophagus
Introduction
Sarcopenia is progressive skeletal muscle attenuation that occurs as a consequence of the aging process. Baumgartner et al. first defined sarcopenia by appendicular skeletal muscle mass (skeletal muscle mass in the upper and lower limbs, ASM, kg)/height2 (m2) in 19981. Although there have been several different suggestive definitions of sarcopenia2–5, consensus definition for clinical research and practice is scarce3,6. The decline in muscle mass that begins around the fourth decade of life is approximately 25–30% during lifetimes7,8. With an increase in the world's advanced age population, the prevalence of sarcopenia has been increasing but has a wide variation according to the definition, method of measurement for muscle mass, age, race, and sex9,10. Sarcopenia is considered as a major health concern, because it is associated with physical disability, low quality of life, and mortality3,11. Previous studies have shown that sarcopenia has an association with metabolic syndrome. Since skeletal muscle is the main tissue for the insulin-dependent glucose uptake, sarcopenia is associated with the systemic insulin resistance that is a component of metabolic syndrome12,13. One study reported that sarcopenic obesity, which is the presence of both sarco penia and obesity that are associated with metabolic syndrome, has a greater impact on metabolic risk and health outcome6.
Gastroesophageal reflux disease (GERD) is a more common disease in Western populations than in Asian populations. A GERD prevalence of approximately 10–20% was identified in Western populations and was less than 5% in Asian populations14. However, in Asia, the prevalence of GERD has been increasing dramatically over recent decades because of Westernized diets, extended life expectancy, and increased eradication rate of Helicobacter pylori15. Previous studies reported that metabolic syndrome and insulin resistance are one of the risk factors for GERD. One study reported that an increased triglyceride (TG) level, which is associated with metabolic syndrome, is an independent risk factor for GERD16. Interleukin (IL)−6 is a cytokine that stimulates hepatic TG secretion, has a key role in insulin resistance17,18, and is increased in Barrett's esophagus19.
Since both sarcopenia and GERD are interlinked with metabolic syndrome, we developed a hypothesis that sarcopenia is associated with GERD. However, the data demonstrating this relationship have been scarce.
The aim of our study was to investigate the predictive factors for GERD, including erosive reflux disease (ERD), with a focus on sarcopenia and sarcopenic obesity among patients who were performed a health check-up program. In addition, we evaluated the correlation between skeletal muscle index (SMI) as an estimator of sarcopenia and body mass index (BMI) that estimates obesity in patients with sarcopenic obesity.
Method
Study design and population
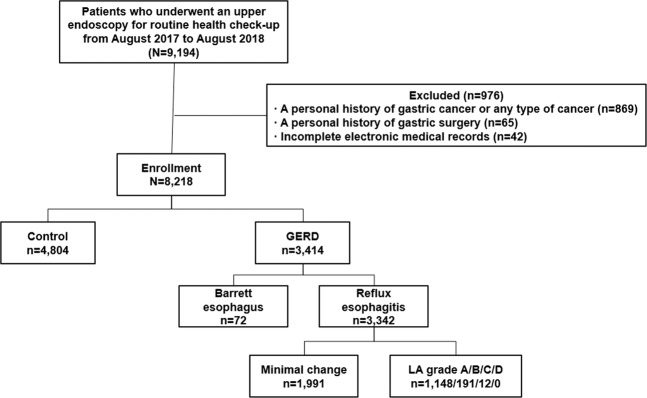
A total of 9,194 patients who underwent an upper gastrointestinal endoscopy at the Health Promotion Center of the Gangnam Severance Hospital, Seoul, South Korea as part of a routine health check-up were considered for this cross-sectional and retrospective study. We reviewed the patients’ electronic medical records from August 2017 to August 2018, when is able to measure SMI in our check-up center. The exclusion criteria were as follows: (1) a personal history of malignancy including gastric cancer (n = 869), (2) a personal history of stomach operation, such as a resection of the stomach or gastrectomy (n = 65), and (3) incomplete electronic medical records (n = 42). As a result, 8,218 patients were enrolled in our study. All enrolled patients were Korean. Figure 1 shows a flow chart of the enrolled patients.
Figure 1.
Flow chart of the enrolled patients.
The study protocol conformed to the ethical guidelines of the World Medical Association Declaration of Helsinki and was approved by the Institutional Review Board of Gangnam Severance Hospital (IRB No: 3-2018-0102). Since this study was a retrospective analysis of existing administrative and clinical data, informed consent was not required.
Anthropometric measurements
We measured anthropometric parameters in all enrolled patients. Body weight and height were measured to the nearest 0.1 kg and 0.1 cm, respectively with the patients barefoot and putting on light-weight clothing. Multi-frequency bioelectrical impedance analysis (ACCUNIQ BC 720; SELVAS healthcare, Korea) was used to measure skeletal muscle mass, which is considered an appropriate device to dual-energy X-ray absorptiometry20. ASM was calculated as the total of appendicular lean mass of both arms and legs21. BMI was defined as body weight (kg) divided by height squared (m2).
We adopted the definition of sarcopenia developed by Janssen et al.2; they used the SMI to evaluate the prevalence of sarcopenia and defined sarcopenia as a SMI less than one standard deviation below the sex-specific mean for healthy subjects aged 20 to 39 years. SMI was calculated by ASM as a percentage of body weight. In our study, the mean SMI of healthy adults was 32.9 ± 3.6% and 29.8 ± 3.1% in males and females, respectively. In our study, cutoff value for sarcopenia was 29.3% in males and 26.7% in females. Therefore, sarcopenia was diagnosed as SMI ≤ 29.3% in males and ≤ 26.7% in females. We used the definition of obesity with Asia-Pacific criteria22, which is a BMI ≥ 25 kg/m2. Sarcopenic obesity is the combination of sarcopenia and obesity in our study.
Questionnaire
All patients who underwent our health check-up program were asked to fill out a questionnaire. Smoking history, alcohol history, combined diabetes mellitus (DM), and diet, which included rapid intake, irregular intake, sweets, fatty foods, caffeinated drinks, and spicy foods, were included in the questionnaire. Rapid intake meant that it took less than 15 minutes to have one meal. Patients were asked to select “yes” or “no” for irregular intake. For the diet, which included sweets, fatty foods, caffeinated drinks, and spicy foods, a patient selected “yes” if they consumed that particular diet item over six times within seven days.
Upper gastrointestinal endoscopy
All enrolled patients were performed upper gastrointestinal endoscopic examination using an endoscope (GIF-H260; Olympus Medical Systems, Tokyo, Japan) with an electronic endoscopic system (EVIS LUCERA; Olympus Medical Systems). The diagnosis of GERD was made by endoscopists who had at least 3 years of experience. The severity of reflux esophagitis was graded according to the Los Angeles (LA) classification23. The GERD group was comprised of patients who had non-erosive reflux disease and ERD. Patients who had minimal change esophagitis were classified as non-erosive reflux disease, and patients with obvious evidence of esophageal mucosal injury on the endoscopy, such as Barrett's esophagus and LA grade A to D reflux esophagitis, were classified as ERD-(1) group. And LA grade B to D reflux esophagitis was classified as ERD-(2) group24.
Statistical analysis
Continuous variable values were reported as the mean ± standard deviation. The t-test or Wilcoxon rank-sum test was performed to compare continuous variables between the groups. Categorical variable values were reported as the number and percentage, and we used the chi-square test or Fisher’s exact test to compare categorical variables.
The Pearson's correlation analysis was used to evaluate the correlation between two continuous variables. Moreover, multivariate Cox regression analysis was performed to investigate the predictive factors for GERD and ERD. Since sarcopenic obesity is a combination of sarcopenia and obesity, two multivariate analyses were performed. We used SPSS version 23.0 (IBM Corp., Armonk, NY, USA) for statistical analysis. Statistical significance was determined by a two-tailed P-value < 0.05.
Results
Baseline characteristics
Baseline characteristics of the study population are showed in Table 1. Of the 8,218 patients, 4,622 (56.2%) were men, and the mean age was 48.9 ± 11.6 years. The average BMI was 23.9 ± 3.4 kg/m2. The mean SMI was 32.2 ± 2.7% in male and 28.8 ± 3.0% in female. A total of 15.1% and 13.4% of the patients were diagnosed with sarcopenia and sarcopenic obesity, respectively. The male had a higher prevalence of sarcopenia (18.7% male vs 10.5% female) and sarcopenic obesity (17.2% male vs 8.5% female) than female.
Table 1.
Baseline characteristics of the study population.
| Characteristics | All patients (N = 8,218) |
|---|---|
| Age (years, mean ± SD) | 48.9 ± 11.6 |
| Male (n, %) | 4,622 (56.2) |
| Height (cm, mean ± SD) | 166.6 ± 8.5 |
| Weight (kg, mean ± SD) | 66.6 ± 12.9 |
| BMI (kg/m2, mean ± SD) | 23.9 ± 3.4 |
| Obesity (n, %) | 2,799 (34.1) |
| SMI (%, mean ± SD) | |
| Male | 32.2 ± 2.7 |
| Female | 28.8 ± 3.0 |
| Current smoker (n, %) | 1,470 (17.9) |
| Alcohol history (n, %) | 5,482 (66.7) |
| Combined DM (n, %) | 1,190 (14.5) |
| Sarcopenia (n, %) | 1,242 (15.1) |
| Male | 864/4,622 (18.7) |
| Female | 378/3,596 (10.5) |
| Sarcopenic obesity (n, %) | 1103 (13.4) |
| Male | 797/4,622 (17.2) |
| Female | 306/3,596 (8.5) |
| Diet style (n, %) | |
| Rapid intake | 2,938 (35.8) |
| Irregular intake | 2,406 (29.3) |
| Sweets | 2,343 (28.5) |
| Fatty foods | 1,759 (21.4) |
| Caffeinated drinks | 3,894 (47.4) |
| Spicy foods | 1,139 (13.9) |
| Endoscopic finding | |
| Barrett’s esophagus | 72 |
| Minimal change esophagitis | 1,991 |
| Reflux esophagitis LA grade A/B/C/D | 1,148/191/12/0 |
BMI, body mass index; SMI, skeletal muscle index; DM, diabetes mellitus; GERD, gastroesophageal reflux disease.
A total of 3,414 patients were classified as GERD by endoscopic findings. Among these patients, 3,342 had reflux esophagitis, 1,991 had minimal change esophagitis, 1,148 had LA grade A, 191 had LA grade B, and 12 had LA grade C. Moreover, 72 had short-segment Barrett's esophagus.
Predictive factors for GERD
As a result of the univariate analysis, advanced age, male sex, obesity, smoking, alcohol, sarcopenia, sarcopenic obesity, and diet, which included sweets, fatty foods, and caffeinated drinks, had a significant association with an increased risk of GERD. In the multivariate analysis, sarcopenia was independent predictive factor for GERD (odds ratio [OR] = 1.170, 95% confidence interval [CI]: 1.016–1.346, P = 0.029). Moreover, male sex (OR = 1.493, 95% CI: 1.344–1.658, P < 0.001), smoking (OR = 1.139, 95% CI: 1.007–1.287, P = 0.038), alcohol (OR = 1.121, 95% CI: 1.013–1.241, P = 0.027), and diet, which included sweets (OR = 1.132, 95% CI: 1.024–1.251, P = 0.015) and fatty foods (OR = 1.212, 95% CI: 1.085–1.354, P = 0.001), were independent predictive factors for GERD. However, obesity was not independently associated with GERD in multivariate analysis.
Predictive factors for ERD
Predictive factors for ERD-(1) group were shown in Tables 2 and 3. As a result of the univariate analysis, advanced age, male sex, obesity, smoking, alcohol, sarcopenia, sarcopenic obesity, and diet, which included fatty foods, caffeinated drinks, and spicy foods, had a significant association with an increased risk of ERD-(1) group (Table 2). Among these factors, sarcopenia (OR = 1.215, 95% CI: 1.019–1.449, P = 0.030), obesity (OR = 1.343, 95% CI: 1.163–1.552, P < 0.001), and sarcopenic obesity (OR = 1.406, 95% CI: 1.195–1.654, P = 0.001) were independent predictive factors for ERD-(1). Moreover, male sex (OR = 2.447, 95% CI: 2.098–2.853, P < 0.001), smoking (OR = 1.259, 95% CI: 1.081–1.466, P = 0.003), and fatty foods (OR = 1.327, 95% CI: 1.148–1.536, P < 0.001) had an independent association with ERD-(1) (Table 3).
Table 2.
Univariate analysis of the predictive facfstors for ERD-(1) group.
| Univariate analysis | |||
|---|---|---|---|
| Control (n = 4,804) | ERD-(1) (n = 1,423) | P-value | |
| Age (years, mean ± SD) | 48.1 ± 11.7 | 49.5 ± 11.5 | 0.031 |
| Male (n, %) | 2,464 (51.3) | 1,088 (76.5) | <0.001 |
| Obesity (n, %) | 1,542 (32.1) | 683 (48.0) | <0.001 |
| Current smoker (n, %) | 755 (15.7) | 387 (27.2) | <0.001 |
| Alcohol history (n, %) | 3,081 (64.1) | 1,055 (74.1) | <0.001 |
| Combined DM (n, %) | 696 (14.5) | 212 (14.9) | 0.700 |
| Diet style (n, %) | |||
| Rapid intake | 1,754 (36.5) | 541 (38.0) | 0.301 |
| Irregular intake | 397 (27.9) | 1,443 (30.0) | 0.120 |
| Sweets | 382 (26.8) | 1,324 (27.6) | 0.595 |
| Fatty foods | 925 (19.3) | 394 (27.7) | <0.001 |
| Caffeinated drinks | 2,230 (46.4) | 756 (53.1) | <0.001 |
| Spicy foods | 655 (13.6) | 237 (16.7) | 0.004 |
| Sarcopenia (n, %) | 668 (13.9) | 302 (21.2) | <0.001 |
| Sarcopenic obesity (n, %) | 595 (12.4) | 274 (19.3) | <0.001 |
ERD, erosive reflux disease; BMI, body mass index; DM, diabetes mellitus.
ERD-(1) group: consisted of Barrett’s esophagus and LA grade A to D reflux esophagitis.
Table 3.
Multivariate analysis of the predictive factors for ERD-(1) group.
| Multivariate analysis 1 | Multivariate analysis 2 | |||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Male | 2.447 (2.098–2.853) | <0.001 | 2.629 (2.264–3.052) | <0.001 |
| Current smoker | 1.259 (1.081–1.466) | 0.003 | 1.265 (1.087–1.473) | 0.002 |
| Alcohol history | 1.093 (0.945–1.263) | 0.230 | 1.082 (0.936–1.250) | 0.286 |
| Diet style | ||||
| Fatty foods | 1.327 (1.148–1.536) | <0.001 | 1.339 (1.158–1.548) | <0.001 |
| Caffeinated drinks | 1.040 (0.916–1.180) | 0.546 | 1.051 (0.927–1.193) | 0.438 |
| Spicy foods | 0.981 (0.824–1.168) | 0.829 | 0.994 (0.835–1.182) | 0.944 |
| Sarcopenia | 1.215 (1.019–1.449) | 0.030 | — | — |
| Obesity | 1.343 (1.163–1.552) | <0.001 | — | — |
| Sarcopenic obesity | — | — | 1.406 (1.195–1.654) | <0.001 |
OR, Odds ratio.
ERD-(1) group: consisted of Barrett’s esophagus and LA grade A to D reflux esophagitis.
Multivariate analysis 1: Adjusted for male, smoking, alcohol, diet style, sarcopenia, and obesity. Multivariate analysis 2: Adjusted for male, smoking, alcohol, diet style, and sarcopenic obesity.
Tables 4 and 5 show the predictive factors for ERD-(2) group. As a result of the univariate analysis, male sex, obesity, smoking, alcohol, DM, sarcopenia, sarcopenic obesity, and diet, which included fatty foods and caffeinated drinks had a significant association with an increased risk of ERD-(2) group (Table 4). As a result of the multivariate analysis, male (OR = 2.514, 95% CI: 1.756–3.598, P < 0.001), smoking (OR = 1.224, 95% CI: 1.042–1.464, P = 0.015), sarcopenia (OR = 1.375, 95% CI: 1.020–1.754, P = 0.040), obesity (OR = 2.564, 95% CI: 1.972–3.322, P < 0.001), and sarcopenic obesity (OR = 2.020, 95% CI: 1.515–2.703, P < 0.001) were independently associated with ERD-(2) group (Table 5).
Table 4.
Univariate analysis of the predictive factors for ERD-(2) group.
| Univariate analysis | |||
|---|---|---|---|
| Control (n = 4,804) | ERD-(2) (n = 275) | P-value | |
| Age (years, mean ± SD) | 49.5 ± 11.5 | 49.0 ± 11.8 | 0.486 |
| Male (n, %) | 2,464 (51.3) | 223 (81.1) | <0.001 |
| BMI (kg/m2, mean ± SD) 0) | 23.7 ± 3.3 | 26.0 ± 2.9 | <0.001 |
| Obesity (n, %) | 1,542 (32.1) | 171 (62.2) | <0.001 |
| Current smoker (n, %) | 755 (15.7) | 85 (30.9) | <0.001 |
| Alcohol history (n, %) | 3,081 (64.1) | 208 (75.6) | <0.001 |
| Combined DM (n, %) | 696 (14.5) | 53 (19.3) | 0.030 |
| Diet style (n, %) | |||
| Rapid intake | 1,754 (36.5) | 104 (37.8) | 0.662 |
| Irregular intake | 397 (27.9) | 86 (31.3) | 0.371 |
| Sweets | 382 (26.8) | 76 (27.6) | 0.732 |
| Fatty foods | 925 (19.3) | 71 (25.8) | 0.008 |
| Caffeinated drinks | 2,230 (46.4) | 147 (53.5) | 0.023 |
| Spicy foods | 655 (13.6) | 35 (12.7) | 0.669 |
| Sarcopenia (n, %) | 668 (13.9) | 75 (27.3) | <0.001 |
| Sarcopenic obesity (n, %) | 595 (12.4) | 70 (25.5) | <0.001 |
ERD, erosive reflux disease; BMI, body mass index; DM, diabetes mellitus.
ERD-(2) group: consisted of Barrett’s esophagus and LA grade B to D reflux esophagitis.
Table 5.
Multivariate analysis of the predictive factors for ERD-(2) group.
| Multivariate analysis 1 | Multivariate analysis 2 | |||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Male | 2.514 (1.756–3.598) | <0.001 | 3.085 (2.174–4.379) | <0.001 |
| Current smoker | 1.224 (1.042–1.464) | 0.015 | 1.256 (1.062–1.488) | 0.008 |
| Alcohol history | 1.104 (0.812–1.499) | 0.528 | 1.086 (0.800–1.473) | 0.599 |
| DM | 1.221 (0.889–1.678) | 0.217 | 1.259 (0.918–1.727) | 0.152 |
| Diet style | ||||
| Fatty foods | 1.135 (0.850–1.517) | 0.391 | 1.175 (0.880–1.567) | 0.274 |
| Caffeinated drinks | 1.002 (0.773–1.287) | 0.987 | 1.025 (0.795–1.319) | 0.853 |
| Sarcopenia | 1.375 (1.020–1.754) | 0.040 | — | — |
| Obesity | 2.564 (1.972–3.322) | <0.001 | — | — |
| Sarcopenic obesity | — | — | 2.020 (1.515–2.703) | <0.001 |
OR, Odds ratio; DM, diabetes mellitus.
ERD-(2) group: consisted of Barrett’s esophagus and LA grade B to D reflux esophagitis.
Multivariate analysis 1: Adjusted for male, smoking, alcohol, DM, diet style, sarcopenia, and obesity.
Multivariate analysis 2: Adjusted for male, smoking, alcohol, DM, diet style, and sarcopenic obesity.
Correlation between SMI and BMI in ERD patients
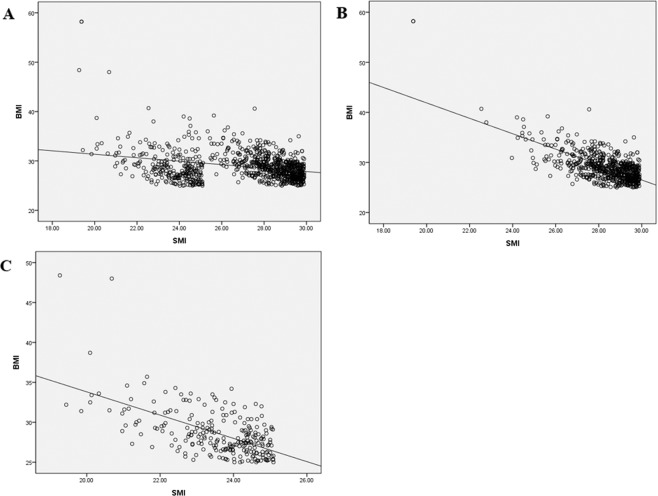
Since sarcopenia, obesity, and sarcopenic obesity were independent predictive factors for ERD, we performed an additional analysis to identify the correlation between SMI and BMI. Figure 2A shows the significantly negative correlation between SMI and BMI in the patients who were diagnosed with sarcopenic obesity in the ERD group. (Pearson’s correlation coefficient: −0.301, P < 0.001). According to sex, the male group (Pearson’s correlation coefficient: −0.682, P < 0.001, Fig. 2B) had a more strongly negative correlation than the female group (Pearson’s correlation coefficient: −0.587, P < 0.001, Fig. 2C).
Figure 2.
Correlation analysis between body mass index (BMI) and skeletal muscle index (SMI) in patients diagnosed with sarcopenic obesity in the ERD group. (A) In both the male and female groups (B) In the male group (C) In the female group.
Discussion
Sarcopenia is a growing health concern because of its negative outcomes from functional decline to death, as well as the increasing worldwide prevalence25. There are several known risk factors for GERD, including obesity, structural abnormalities such as hiatal hernia, and specific diets and medications26. However, there have beeTablen no studies that link GERD and sarcopenia. Recent studies have reported that sarcopenic obesity has an influence on cardiometabolic diseases27–31. In these studies, sarcopenic obesity is a strongly associated with metabolic syndrome and has a higher impact on metabolic diseases and mortality than obesity or sarcopenia alone. To the best of our knowledge, this study is the first to identify an association between GERD and sarcopenia. Notably, the strength of our study is that we performed additional analysis between ERD and the “new concept” of sarcopenic obesity.
In the conclusion of our study, sarcopenia is associated with GERD. This association can be explained by metabolic syndrome. Previous study reported that volume of skeletal muscle fiber is related to the expression of glucose transporter type 4 (GLUT4), which enables the uptake of glucose32. Therefore, sarcopenia can induce an insulin intolerance that is a component of metabolic syndrome. There was a vicious cycle between sarcopenia and metabolic syndrome33. Muscle mass attenuation from sarcopenia induces lower physical activity that causes visceral f at to increase in the body. Since skeletal muscle is an insulin-response target tissue, patients with sarcopenia develop progressive metabolic syndrome. Although precise mechanisms of association between sarcopenia and GERD are unclear, we guess that sarcopenia might be affect to mechanical barrier, such as function of esophagus and stomach, thereby induce GERD. Moreover, cytokine signaling can be one of mechanisms as mentioned in the section of introduction. Previous study reported that cytokine such as TNF-α, TWEAK, and IL-6 were related to skeletal muscle wasting34.
The prevalence of GERD in the Asian population has been reported as generally less than 10%35. However, the prevalence of GERD in our study was much higher at 41.5%. In addition, obesity, which is a known potential risk factor for GERD, did not have a statistical significance in our multivariate analysis36. These results were due to the following: First, since the questionnaire of our check-up center did not have symptoms for GERD, such as heartburn and regurgitation, non-erosive reflux disease was diagnosed by only endoscopic findings of minimal change esophagitis, which is ambiguous; second, the characteristics of the non-erosive reflux disease group was heterogeneous, and they may have had functional dyspepsia or psychological comorbidities.
Thus, we performed an additional analysis on 1,423 (17.3%) patients who had ERD-(1), which has a more definitive mucosal injury and is more specific for GERD. Sarcopenia, obesity, and sarcopenic obesity were independent predictive factors for ERD-(1) group. Interestingly, sarcopenic obesity (OR 1.406) was more predictive than sarcopenia (OR 1.215) or obesity (OR 1,343) for ERD-(1) group. This result suggests that sarcopenia and obesity may have a synergistic influence on ERD-(1) group. Moreover, there was a significantly negative correlation between SMI and BMI, especially in the male patients. LA grade B to D reflux esophagitis (ERD-(2) group, n = 203) are more specific endoscopic findings for GERD. In this analysis, sarcopenia (OR 1.493), obesity (OR 1.229), and sarcopenic obesity (OR 1.616) also associated with ERD-(2) group. This result overcame our study’s limitation and support conclusion of our study.
As we mentioned above, the prevalence of sarcopenia is different according to race. In the result of meta- analysis, the prevalence of sarcopenia was different according to the method to measure muscle mass9. When method BIA used, non-Asian (19%) was higher than Asian (10%) in male, and non-Asian (20%) was higher than Asian (11%) in female. When method DXA used, Asian (9%) was higher than non-Asian (6%) in male, and non-Asian (10%) was higher than Asian in female (6%). Therefore, it is necessary to make consensus diagnostic tool for sarcopenia, and further study is warranted.
There were several limitations to our study. First, GERD was diagnosed by endoscopic finding with mucosal injury. We retrospectively reviewed the result section for upper endoscopy of our electronic medical records. Moreover, we also diagnosed Barrett’s esophagus by endoscopic finding, not histologic confirmation. Second, since our study design was cross-sectional for the check-up cohort, there was a selection bias that included the individual’s food habits and socioeconomic status. Third, our cross-sectional study design made it hard to emphasize the temporal relationship between sarcopenia and the development of GERD. Fourth, despite current consensus definitions such as the European Working Group on Sarcopenia in Older People (EWGSOP), the European Society for Clinical Nutrition and Metabolism Special Interest Groups (ESPEN-SIG), and the International Working Group on Sarcopenia (IWGS) adopted muscle mass and function to define sarcopenia3,37–39, our study used only muscle mass. This was because our check-up center did not have an equipment to evaluate muscle function. In addition, routine program of most check-up centers only includes evaluation for muscle mass. Therefore, the data of our study may be enough worth predicting GERD in healthy subjects. Fifth, the patients’ information, such as combined DM, alcohol, smoking, and diet, were collected from a questionnaire. However, it is difficult to evaluate an exact amount of alcohol, frequency for smoking, and amount and concentration of food components. Moreover, taking acid suppressive drugs, such as proton pump inhibitor, is a confounding factor affecting GERD. However, questionnaire of our check-up center did not include history of medication for acid suppressive drug. Finally, because of the retrospective aspect of our study, we may have collected some incomplete data.
In conclusion, sarcopenia is associated with GERD. Moreover, sarcopenia and sarcopenic obesity are predictive factors for ERD. Our check-up center measured the patients’ anthropometric profile, which included body weight, height, and skeletal muscle mass. Therefore, our study is meaningful because the patients’ anthropometric measurements proved to be a useful data to predict GERD and ERD. Since the clinical presentations of sarcopenia are not specific, early detection by a routine health check-up is important. This study identified the association between sarcopenia and GERD, and proved our hypothesis. Further studies should be warranted to evaluate the mechanism for this relationship.
Author contributions
Y.M.K. substantial contributions to conception and design, or analysis and interpretation of data and drafting the article or revising it critically for important intellectual content; J.-H.K. substantial contributions to conception and design, final approval of the version to be published and agreement to be accountable for all aspects of the work; S.J.B. Acquisition of data; D.H.J., J.J.P., Y.H.Y., and H.P. revising the article critically for important intellectual content; All authors reviewed the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Baumgartner RN, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am. J. Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
- 2.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002;50:889–896. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 3.Cruz-Jentoft AJ, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman AB, et al. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J. Am. Geriatr. Soc. 2003;51:323–330. doi: 10.1046/j.1532-5415.2003.51105.x. [DOI] [PubMed] [Google Scholar]
- 5.Studenski SA, et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wannamethee SG, Atkins JL. Muscle loss and obesity: the health implications of sarcopenia and sarcopenic obesity. Proc. Nutr. Soc. 2015;74:405–412. doi: 10.1017/S002966511500169X. [DOI] [PubMed] [Google Scholar]
- 7.Balagopal P, Rooyackers OE, Adey DB, Ades PA, Nair KS. Effects of aging on in vivo synthesis of skeletal muscle myosin heavy-chain and sarcoplasmic protein in humans. Am. J. Physiol. 1997;273:E790–800. doi: 10.1152/ajpcell.1997.273.3.C790. [DOI] [PubMed] [Google Scholar]
- 8.Keller K, Engelhardt M. Strength and muscle mass loss with aging process. Age and strength loss. Muscles Ligaments Tendons J. 2013;3:346–350. doi: 10.32098/mltj.04.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shafiee G, et al. Prevalence of sarcopenia in the world: a systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017;16:21. doi: 10.1186/s40200-017-0302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pongchaiyakul C, Limpawattana P, Kotruchin P, Rajatanavin R. Prevalence of sarcopenia and associated factors among Thai population. J. Bone Min. Metab. 2013;31:346–350. doi: 10.1007/s00774-013-0422-4. [DOI] [PubMed] [Google Scholar]
- 11.Arango-Lopera VE, Arroyo P, Gutierrez-Robledo LM, Perez-Zepeda MU, Cesari M. Mortality as an adverse outcome of sarcopenia. J. Nutr. Health & Aging. 2013;17:259–262. doi: 10.1007/s12603-012-0434-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klip A, Paquet MR. Glucose transport and glucose transporters in muscle and their metabolic regulation. Diabetes Care. 1990;13:228–243. doi: 10.2337/diacare.13.3.228. [DOI] [PubMed] [Google Scholar]
- 13.Cleasby ME, Jamieson PM, Atherton PJ. Insulin resistance and sarcopenia: mechanistic links between common co-morbidities. J. Endocrinol. 2016;229:R67–R81. doi: 10.1530/JOE-15-0533. [DOI] [PubMed] [Google Scholar]
- 14.Dent J, El-Serag HB, Wallander MA, Johansson S. Epidemiology of gastro-oesophageal reflux disease: a systematic review. Gut. 2005;54:710–717. doi: 10.1136/gut.2004.051821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kinoshita Y, Adachi K, Hongo M, Haruma K. Systematic review of the epidemiology of gastroesophageal reflux disease in Japan. J. Gastroenterology. 2011;46:1092–1103. doi: 10.1007/s00535-011-0429-3. [DOI] [PubMed] [Google Scholar]
- 16.Chung SJ, et al. Metabolic syndrome and visceral obesity as risk factors for reflux oesophagitis: a cross-sectional case-control study of 7078 Koreans undergoing health check-ups. Gut. 2008;57:1360–1365. doi: 10.1136/gut.2007.147090. [DOI] [PubMed] [Google Scholar]
- 17.Mohamed-Ali V, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J. Clin. Endocrinol. Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 18.Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J. Biol. Chem. 2003;278:45777–45784. doi: 10.1074/jbc.M301977200. [DOI] [PubMed] [Google Scholar]
- 19.Dvorak K, Dvorak B. Role of interleukin-6 in Barrett’s esophagus pathogenesis. World J. Gastroentero. 2013;19:2307–2312. doi: 10.3748/wjg.v19.i15.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson LJ, Erceg DN, Schroeder ET. Utility of multifrequency bioelectrical impedance compared with dual-energy x-ray absorptiometry for assessment of total and regional body composition varies between men and women. Nutr. Res. 2012;32:479–485. doi: 10.1016/j.nutres.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Delmonico MJ, et al. Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J. Am. Geriatr. Soc. 2007;55:769–774. doi: 10.1111/j.1532-5415.2007.01140.x. [DOI] [PubMed] [Google Scholar]
- 22.Pan WH, Yeh WT. How to define obesity? Evidence-based multiple action points for public awareness, screening, and treatment: an extension of Asian-Pacific recommendations. Asia Pac. J. Clin. Nutr. 2008;17:370–374. [PubMed] [Google Scholar]
- 23.Armstrong D, et al. The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology. 1996;111:85–92. doi: 10.1053/gast.1996.v111.pm8698230. [DOI] [PubMed] [Google Scholar]
- 24.Gyawali CP, et al. Modern diagnosis of GERD: the Lyon Consensus. Gut. 2018;67:1351–1362. doi: 10.1136/gutjnl-2017-314722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beaudart C, Zaaria M, Pasleau F, Reginster JY, Bruyere O. Health Outcomes of Sarcopenia: A Systematic Review and Meta-Analysis. PLoS One. 2017;12:e0169548. doi: 10.1371/journal.pone.0169548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hallan A, Bomme M, Hveem K, Moller-Hansen J, Ness-Jensen E. Risk factors on the development of new-onset gastroesophageal reflux symptoms. A population-based prospective cohort study: the HUNT study. Am. J. Gastroenterol. 2015;110:393–400. doi: 10.1038/ajg.2015.18. [DOI] [PubMed] [Google Scholar]
- 27.Baumgartner RN. Body composition in healthy aging. Ann. N. Y. Acad. Sci. 2000;904:437–448. doi: 10.1111/j.1749-6632.2000.tb06498.x. [DOI] [PubMed] [Google Scholar]
- 28.Kohara K. Sarcopenic obesity in aging population: current status and future directions for research. Endocrine. 2014;45:15–25. doi: 10.1007/s12020-013-9992-0. [DOI] [PubMed] [Google Scholar]
- 29.Stenholm S, et al. Sarcopenic obesity: definition, cause and consequences. Curr. Opin. Clin. Nutr. Metab. Care. 2008;11:693–700. doi: 10.1097/MCO.0b013e328312c37d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu CW, et al. Sarcopenic obesity is closely associated with metabolic syndrome. Obes. Res. Clin. Pract. 2013;7:E301–E307. doi: 10.1016/j.orcp.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Zamboni M, Mazzali G, Fantin F, Rossi A, Di Francesco V. Sarcopenic obesity: a new category of obesity in the elderly. Nutr. Metab. Cardiovasc. Dis. 2008;18:388–395. doi: 10.1016/j.numecd.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Gaster M, Vach W, Beck-Nielsen H, Schroder HD. GLUT4 expression at the plasma membrane is related to fibre volume in human skeletal muscle fibres. Apmis. 2002;110:611–619. doi: 10.1034/j.1600-0463.2002.1100903.x. [DOI] [PubMed] [Google Scholar]
- 33.Choi KM. Sarcopenia and sarcopenic obesity. Endocrinol. Metab. (Seoul.) 2013;28:86–89. doi: 10.3803/EnM.2013.28.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou J, Liu B, Liang C, Li Y, Song YH. Cytokine Signaling in Skeletal Muscle Wasting. Trends Endocrinol. Metab. 2016;27:335–347. doi: 10.1016/j.tem.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Wu JC. Gastroesophageal reflux disease: an Asian perspective. J. Gastroenterol. Hepatol. 2008;23:1785–1793. doi: 10.1111/j.1440-1746.2008.05684.x. [DOI] [PubMed] [Google Scholar]
- 36.El-Serag HB, Graham DY, Satia JA, Rabeneck L. Obesity is an independent risk factor for GERD symptoms and erosive esophagitis. Am. J. Gastroenterology. 2005;100:1243–1250. doi: 10.1111/j.1572-0241.2005.41703.x. [DOI] [PubMed] [Google Scholar]
- 37.Muscaritoli M, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: Joint d ocument elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin. Nutr. 2010;29:154–159. doi: 10.1016/j.clnu.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 38.Fielding RA, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rizzoli R, et al. Quality of Life in Sarcopenia and Frailty. Calcif. Tissue Int. 2013;93:101–120. doi: 10.1007/s00223-013-9758-y. [DOI] [PMC free article] [PubMed] [Google Scholar]