Abstract
The hormone leptin is a key regulator of body weight, food intake and metabolism. In mammals, leptin acts as an anorexigen and inhibits food intake centrally by affecting the appetite centres in the hypothalamus. In teleost fish, the regulatory connections between leptin and other appetite-regulating genes are largely unknown. In the present study, we used a zebrafish mutant with a loss of function leptin receptor to investigate brain expression patterns of 12 orexigenic and 24 anorexigenic genes under different feeding conditions (normal feeding, 7-day fasting, 2 and 6-hours refeeding). Expression patterns were compared to wild-type zebrafish, in order to identify leptin-dependent differentially expressed genes under different feeding conditions. We provide evidence that the transcription of certain orexigenic and anorexigenic genes is influenced by leptin signalling in the zebrafish brain. We found that the expression of orexigenic genes was not affected by impaired leptin signalling under normal feeding conditions; however, several orexigenic genes showed increased transcription during fasting and refeeding, including agrp, apln, galr1a and cnr1. This suggests an inhibitory effect of leptin signal on the transcription of these orexigenic genes during short-term fasting and refeeding in functional zebrafish. Most pronounced effects were observed in the group of anorexigenic genes, where the impairment of leptin signalling resulted in reduced gene expression in several genes, including cart family, crhb, gnrh2, mc4r, pomc and spx, in the control group. This suggests a stimulatory effect of leptin signal on the transcription of these anorexigenic genes under normal feeding condition. In addition, we found multiple gain and loss in expression correlations between the appetite-regulating genes, in zebrafish with impaired leptin signal, suggesting the presence of gene regulatory networks downstream of leptin signal in zebrafish brain. The results provide the first evidence for the effects of leptin signal on the transcription of various appetite-regulating genes in zebrafish brain, under different feeding conditions. Altogether, these transcriptional changes suggest an anorexigenic role for leptin signal, which is likely to be mediated through distinct set of appetite-regulating genes under different feeding conditions.
Subject terms: Metabolism, Fat metabolism, Feeding behaviour, Metabolic diseases, Endocrine system and metabolic diseases, Dyslipidaemias, Multihormonal system disorders, Obesity, Transcription, Molecular biology, Physiology, Endocrinology, Neuroscience, Feeding behaviour, Molecular neuroscience, Motivation, Neuronal physiology, Reward
Introduction
Feeding behaviour involves complex processes of foraging controlled by appetitive behaviours (hunger-driven activities) and food ingestion1. The central regulation of feeding behaviour in the brain is under the influence of endocrine signals, originating from the brain itself, as well as peripheral organs, after exposure to different metabolic and nutritional conditions. The regulation of food intake, in both mammals and fish, takes place in the hypothalamus in the central nervous system. In mammals, appetite regulation through hormones is mediated by their receptors, located on a group of key neurons called arcuate nucleus neurons2. In fish, similar neurons exist in the periventricular and lateral parts of the hypothalamus3. Nevertheless, it appears that neurons generating neuroendocrine signals display a wider anatomical distribution in the fish brain than in mammals, as it has been observed for appetite-regulating genes expressed in different parts of the fish brain4. The neurons mediating the appetite-regulating effects can be divided into two main types: orexigenic, stimulating food intake and/or related locomotor activity; and anorexigenic, reducing food intake and/or related locomotor activity5. At the core of these processes are the key molecular players, the so-called appetite-regulation genes, which encode a range of neuropeptides and their cognate receptors. The appetite-regulation genes can also be classified in two categories of orexigenic and anorexigenic genes based on their functions6,7. Due to the large diversity of teleost fish in diet, feeding habits, physiology and adaptation to various environmental factors influencing their feeding behaviour, the number of studies addressing molecular mechanisms underlying appetite regulation in fish has been growing in recent years8. However, transcriptional activity and regulatory connections between the appetite-regulating genes, under different feeding conditions, are still not fully explored in fish.
One of the signals, transduced by the leptin hormone and its receptor, is an appetite-regulating molecular mechanism, among its other regulatory roles, which include controlling body weight, reproduction, development and stress responses in vertebrates9,10. Zebrafish has two leptin hormone genes, lepa and lepb, which are differently expressed in several organs11. Both leptin isoforms mediate their signal through a single leptin receptor gene in zebrafish (lepr), which is expressed in several brain regions, including the hypothalamic lateral tuberal nucleus12. Most studies of leptin functions in fish are conducted on Cypriniformes (mainly goldfish and zebrafish) and Salmoniformes8. Several studies in goldfish showed that excessive level of leptin can reduce both feeding behavior and locomotor activity (anorexigenic effects)13–16. The regulatory effects of leptin signal on appetite can be exerted through stimulation of anorexigenic genes, while inhibiting orexigenic genes in the brain8,13,17,18. However, the leptin expression in the brain and other organs, in response to different feeding conditions, seems to be highly variable across fish species8,19. For instance, post-prandial increase of leptin expression has been observed in goldfish and zebrafish in different organs16,20, whereas such an increase was absent in rainbow trout21. On the other hand, fasting did not affect the hepatic expression of leptin in goldfish, common carp, pacu and Nile tilapia, while on the contrary, its hepatic expression was affected during fasting in most Perciformes and Salmoniformes examined8,22. Furthermore, in zebrafish, inhibiting lepa leads to a decrease in metabolic rate23, whereas leptin treatment can increase energy expenditure24. After feeding the expression of leptin increases in most fishes including goldfish and zebrafish16,20. Despite the fact that the number of molecular studies on leptin-dependent regulation of feeding in fish is consistently growing, little is known about regulatory connections between leptin signal and other appetite-regulating genes under different feeding conditions.
To further explore the role of leptin in appetite regulation, we compared in this study the expression levels of 36 genes, which have already been shown in other studies of Cypriniformes to have appetite-regulating functions, i.e. 12 orexigenic and 24 anorexigenic genes (Table 1), in brain samples of wild-type zebrafish and a zebrafish mutant with a loss of function leptin receptor (lepr mutant). We first identified a stably expressed gene in zebrafish brain under different feeding conditions and used it as normalization factor for the analysis of gene expression by qPCR. Next, we aimed to profile the expression dynamics of the appetite-regulating genes during different feeding conditions: normal feeding (control group), fasting and two refeeding stages (2 and 6 hours after refeeding) in wild-type zebrafish and compared the results to the expression patterns observed in the lepr mutant. This allowed us to identify genes for which transcriptional changes, in different feeding conditions, may be under the influence of functional leptin signalling. Finally, we predicted loss and gain of potential regulatory connections between the appetite regulating genes in defective leptin signalling,through pairwise expression correlation analysis. Our findings provide comprehensive information about the expression dynamics of known appetite-regulating genes in relation to different feeding conditions, as well as non-functional leptin signalling in the brain of adult zebrafish.
Table 1.
Descriptions of candidate target genes and their predicted function in appetite regulation.
| Genes | Description | Organism | Effects | References |
|---|---|---|---|---|
| agrp (agrp1) | Agouti related neuropeptide | Zebrafish | Orexigenic | 35,36 |
| apln, aplnr | Apelin, agtrl1 ligand and its receptor | Goldfish | Orexigenic | 30,32 |
| cart1-4 | Cocaine and amphetamine regulated transcripts | Zebrafish | Anorexigenic | 43,44 |
| cnr1 | Cannabinoid receptor 1 | Zebrafish | Orexigenic | 43,108 |
| crh | Corticotropin-releasing hormone | Goldfish | Anorexigenic | 55,56 |
| dbi | Diazepam binding inhibitor, octadecaneuropeptide | Goldfish | Anorexigenic | 109,110 |
| galn, galr1,2 | Galanin/GMAP prepropeptide and its receptors |
Goldfish Zebrafish |
Orexigenic | 40–42 |
|
ghrl ghsr |
Ghrelin and its receptor (growth hormone secretagogue receptor) |
Goldfish Zebrafish |
Orexigenic | 111–113 |
|
gnrh2,3 gnrhr1-4 |
Gonadotropin releasing hormone 2 and 3, and their receptors |
Goldfish Zebrafish |
Anorexigenic | 61,62 |
| hcrt | Orexin, hypocretin neuropeptide precursor |
Goldfish Zebrafish |
Orexigenic | 90–92 |
| kiss1, kiss1r | Prepro-Kisspeptin 1 and its receptor | Sea bass | Anorexigenic | 69 |
| mc4r | Melanocortin 4 receptor | Goldfish | Anorexigenic | 76 |
| mch, mchr1,2 | Pro-melanin-concentrating hormone and its receptors | Goldfish | Anorexigenic | 65,66 |
| nmu | Neuromedin U preproprotein | Goldfish | Anorexigenic | 70,71 |
| npy | Prepro-neuropeptide Y |
Goldfish Zebrafish |
Orexigenic | 93,94 |
| nucb2 | Nucleobindin 2/Nesfatin 1 |
Goldfish Zebrafish |
Anorexigenic | 72–74 |
| pacap | Pituitary adenylate cyclase activating polypeptide | Goldfish | Anorexigenic | 75 |
| pomc | Pro-opiomelanocortin preproprotein |
Goldfish Zebrafish |
Anorexigenic | 95–97 |
| pyy | Prepro-peptide YY | Goldfish | Anorexigenic | 98 |
| scg2 | Secretogranin 2/Secretoneurin | Goldfish | Orexigenic | 99,100 |
| spx | Spexin Hormone |
Goldfish Zebrafish |
Anorexigenic | 50,51 |
| trh | Thyrotropin-releasing hormone | Goldfish | Orexigenic | 49 |
Results
Validation of suitable reference gene
Identifying stably expressed reference gene(s) in every specific experimental conditions is a prerequisite for the analysis of relative gene expression levels by qPCR25–27. We quantified the expression levels of 8 candidate reference genes in the brains of wild-type and lepr mutant, exposed to different feeding conditions. The expression levels of the candidate reference genes varied considerably in the brain of zebrafish with highest expression (lowest Cq) for actb2, around 19 average Cq value, to tmem50a with lowest expression (highest Cq), around 28 average Cq value (Supplementary Fig. 1A). We found that all the three algorithms, BestKeeper, geNorm and Normfinder, suggested g6pd and rplp0, as the most and the least stably expressed genes, respectively (Table 2). However, the second and third ranks of the most stable reference genes, were variable for each of the algorithms (the ranks were interchanging between ef1a, rpl13 actb2 and tmem50a) (Table 2). Thus, we considered the expression level of only g6pd as a normalization factor to quantify the expression levels of our candidate appetite-regulating genes in each sample.
Table 2.
Expression stability ranking of reference genes in zebrafish brain across wild type and lepr mutant adults during the fasting-refeeding experiment.
| BestKeeper | geNorm | NormFinder | |||||
|---|---|---|---|---|---|---|---|
| Ranking | r values | Ranking | SD values | Ranking | M values | Ranking | S values |
| g6pd | 0.676 | g6pd | 0.237 | g6pd | 0.466 | g6pd | 0.124 |
| ef1a | 0.655 | rpl13 | 0.246 | actb2 | 0.489 | ef1a | 0.158 |
| actb2 | 0.626 | tmem50a | 0.255 | tmem50a | 0.493 | rpl13 | 0.158 |
| b2m | 0.615 | actb2 | 0.268 | rpl13 | 0.507 | actb2 | 0.161 |
| rpl13 | 0.604 | ube2a | 0.270 | ef1a | 0.524 | tmem50a | 0.169 |
| tmem50a | 0.531 | ef1a | 0.283 | ube2a | 0.537 | ube2a | 0.173 |
| ube2a | 0.404 | b2m | 0.418 | b2m | 0.625 | b2m | 0.237 |
| rplp0 | 0.375 | rplp0 | 0.577 | rplp0 | 0.842 | rplp0 | 0.316 |
Abbreviations: SD = standard deviation, r = Pearson product-moment correlation coefficient, S = stability value, M = mean value of stability.
Differential expression of orexigenic genes in wild-type versus lepr mutant
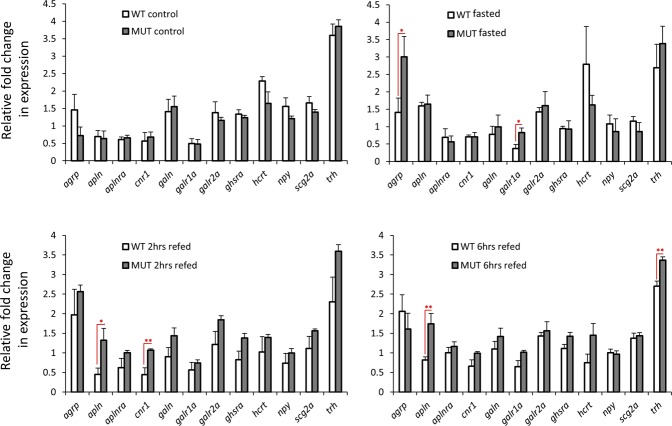
We investigated the expression levels of 12 orexigenic genes, within wild-type or lepr mutant brain during the fasting-refeeding experiment, and found expression changes in a few of the genes for each genotype (Fig. 1). We observed expression differences in wild-type for only 2 genes; apln and hcrt; whereas in lepr mutant, 4 genes showed differences: agrp (previously called agrp1), aplnra, galr1a and galr2a. Among these DE genes, none of them displayed changes in both genotypes, and even their expression dynamics were not following similar patterns between the genotypes (Fig. 1). These reveal clear differences between wild-type and the genotype with defective leptin signalling in transcriptional regulation of orexigenic genes in brain during fasting and refeeding. We found that one of the DE genes in wild-type zebrafish, hcrt, had reduced expression levels in a refeeding group compared to the control, but such a decrease in expression was not observed for any of the DE genes in lepr mutant. On the contrary, all of the DE genes in lepr mutant displayed increased expression in at least one of the refeeding group compared to the control group, which means stronger appetite stimulation during refeeding when the leptin signalling is impeded (Fig. 1). We also found that agrp exhibit an expression induction in lepr mutant, and the expression of apln is increased in wild-type after fasting. The induced apln and agrp expressions seem to be the most prominent brain responses among the tested orexigenic genes during fasting in wild-type and lepr mutant, respectively.
Figure 1.
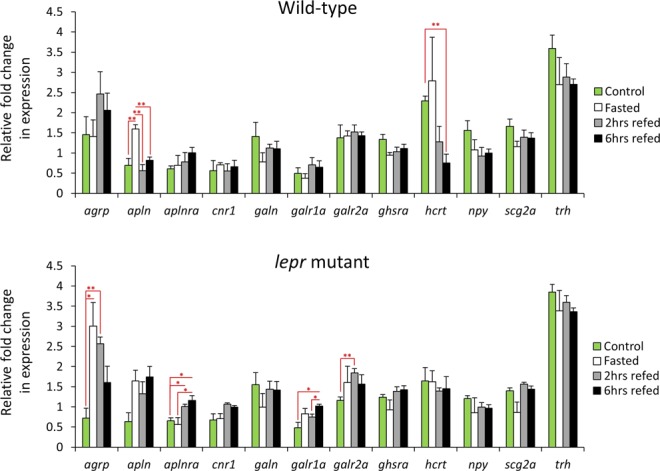
Expression dynamics of orexigenic genes in the brain of wild-type and lepr mutant zebrafish during the fasting-refeeding experiment. Means and standard errors of fold changes in expression of five biological replicates are shown for each experimental group. Significant differences between the treatment groups for each genotype are indicated by red asterisks (*P < 0.05; **P < 0.01;).
Next, we compared the expression of the orexigenic genes, between wild-type and lepr mutant, and did not find any difference in control groups. Thus, under normal feeding conditions, the defective leptin signal does not affect the brain expression of orexigenic genes (Fig. 2). Interestingly, we found lepr mutants to have higher expression of several orexigenic genes compared to the control group within the three treatment groups (fasting: agrp and galr1a; 2 hrs refeeding: apln and cnr1; 6hrs refeeding: apln and trh). This shows more appetite stimulation by orexigenic genes, during changes in feeding condition, in defective leptin signalling. Furthermore, we only found one of the genes, apln, with increased brain expression in both refeeding groups of lepr mutant. Altogether, these findings demonstrate noticeable differences in transcriptional regulation of orexigenic genes in the brain between wild-type and lepr mutant strains, under different feeding conditions.
Figure 2.
Expression differences of orexigenic genes in the brain of wild-type versus lepr mutant zebrafish within each experimental group. Means and standard errors of fold changes in expression of five biological replicates are shown for each experimental group. Significant differences between the wild-type and lepr mutant for each treatment are indicated by red asterisks (*P < 0.05; **P < 0.01).
Differential expression of anorexigenic genes in wild-type versus lepr mutant
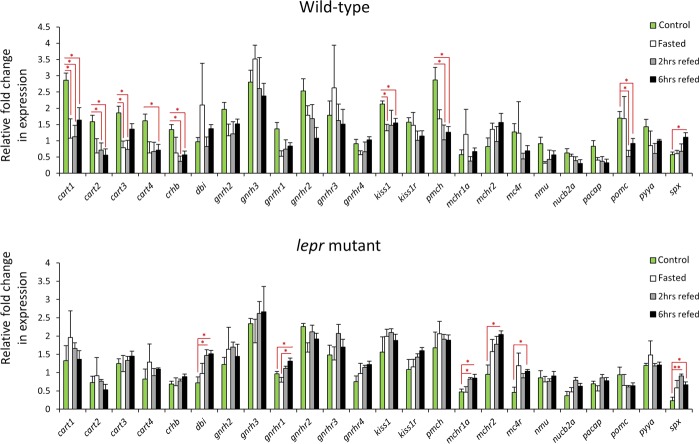
We investigated the expression levels of 24 anorexigenic genes between wild-type and lepr mutant during the fasting-refeeding experiment, and found 15 genes showing expression changes in at least one of the genotypes (Fig. 3). We identified expression differences of 9 genes in the wild-type samples, whereas in lepr mutant samples 6 genes displayed differences (Fig. 3). In wild-type zebrafish, all the DE genes, except for spx, showed reduced expression in at least one treatment group compared to the control, but all of the DE genes in lepr mutant had almost opposite expression patterns with increased expression in at least one treatment group (mainly a refeeding group). Interestingly, we found all cart genes displaying similar changes within the wild-type group while no difference was identified in the mutant. Spx was the only gene showing similar differential expression pattern in both genotypes; however, it seems that in zebrafish with impaired leptin signal, its induction appeared earlier during refeeding. These findings demonstrate weakened transcriptional regulation for certain anorexigenic genes in functional or defective leptin signalling during the fasting-refeeding experiment (Fig. 3).
Figure 3.
Expression dynamics of anorexigenic genes in the brain of wild-type and lepr mutant zebrafish during the fasting-refeeding experiment. Means and standard errors of fold changes in expression of five biological replicates are shown for each experimental group. Significant differences between the treatment groups for each genotype are indicated by red asterisks (*P < 0.05; **P < 0.01).
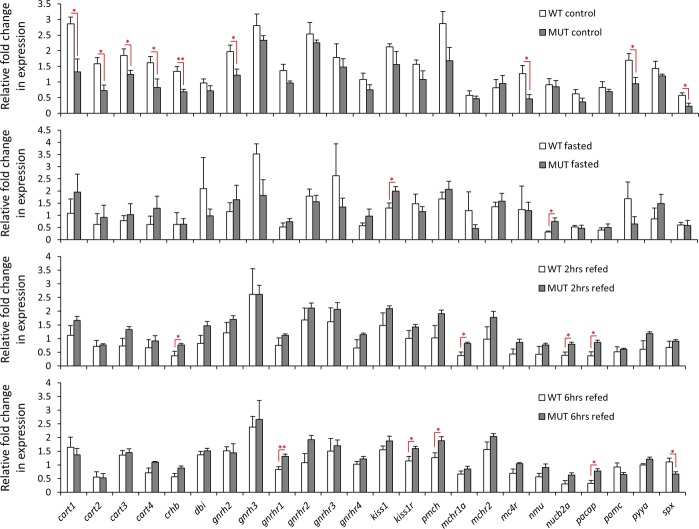
We directly compared the expression of the anorexigenic genes between the genotypes, and observed expression changes opposite to the orexigenic genes. For instance, while no difference was observed in the control group for orexigenic genes, for the anorexigenic genes, most differences were found in the control group (Fig. 4). All of the DE anorexigenic genes in the control group followed the same pattern of higher expression in wild-type than lepr mutant. This indicates that, in contrast to orexigenic genes, defective leptin signal leads to reduction in expression of certain anorexigenic genes, which in turn possibly increases the appetite during normal feeding condition. In the mutant group, we found slight but significant increase in expression of 2 genes during fasting; kiss1 and nmu, 4 genes during 2 hrs refeeding; crhb, mchr1a, nucb2a and pacap, and 4 genes during 6 hrs refeeding; gnrhr1, kiss1r, mch and pacap (only spx had reduced expression in 6 hrs refeeding in the mutant). These findings, during fasting and refeeding conditions, are similar to the orexigenic genes, where only increased expression of the genes was found in the mutant compared to wild-type zebrafish. Therefore, the results demonstrate that functional leptin signalling is required for transcriptional suppression of certain anorexigenic and orexigenic genes, in changes of feeding condition.
Figure 4.
Expression differences of anorexigenic genes in the brain of wild-type versus lepr mutant zebrafish within each experimental group. Means and standard errors of fold changes in expression of five biological replicates are shown for each experimental group. Significant differences between the wild-type and lepr mutant for each treatment are indicated by red asterisks (*P < 0.05; **P < 0.01).
Pairwise expression correlation analyses of the appetite regulating genes
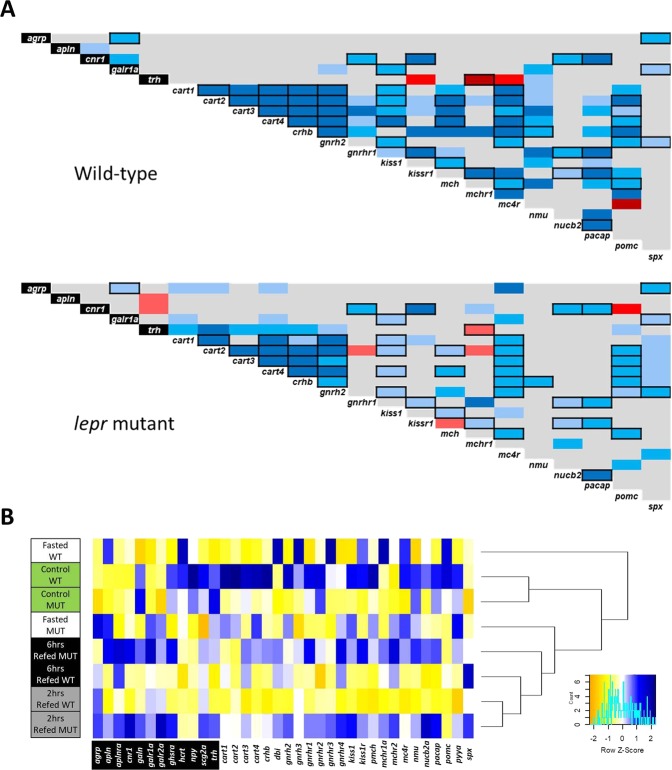
For each of the genotype, we conducted pairwise expression correlation analysis of the appetite-regulating genes, which showed differential expression in previous steps, in order to predict possible regulatory connections between the genes28. Most of the identified correlations appeared to be positive in both genotypes; however, not all of the positive correlations were similar between the genotypes, indicating gain or loss of certain regulatory connections between the genes in defective leptin signal (Fig. 5A). Among the positive correlations, which were similar between the two genotypes (i.e. unaffected by leptin signal), we observed strong connections between cart1,2,3,4/crhb/gnrh2/mc4r/pomc, cnr1/kiss1, nucb2/pacap, cnr1/pacap, and agrp/spx. We found only one negative correlation, trh/mchr1, which showed similarity between the genotypes (Fig. 5A). The overall higher number of positive correlations between and within orexigenic and anorexigenic genes in zebrafish brain demonstrates that their expression regulations have more positive co-regulatory connections in different feeding conditions.
Figure 5.
Expression correlations of appetite-regulating genes and clustering of the experimental conditions based on the gene expression patterns. (A) Pairwise expression correlations between appetite regulating genes in the brain of wild-type and lepr mutant zebrafish in the fasting-refeeding experiment. The red and blue colours respectively indicate negative and positive Pearson correlation coefficients and their light to dark shadings show significant levels of P < 0.05, P < 0.01 and P < 0.001. The genes specified with black and white backgrounds represent orexigenic and anorexigenic genes, respectively, and pairwise correlations delineated with black borders are similar between the two genotypes. (B) Dendrogram clustering of the experimental condition in each genotype based expression pattern similarities of all the appetite regulating genes in this study (the blue and yellow indicate higher and lower expression level respectively).
In addition, we also found strong pairwise correlations, which were specific to each genotype, implying that regulatory connections are influenced by leptin signalling. For instance, we observed several strong positive correlations, which were lost in the lepr mutant group compared to the wildtype group, including those between cart1/cart3, cart3/gnrhr1, crhb/kiss1r, crhb/mch, crhb/mchr1, mc4r/kiss1r, mc4r/mchr1, and nmu with cart3, gnrhr1, mch and nucb2 (Fig. 5A). On the other hand, we found a few gains of strong positive correlation in lepr mutant, including those between cart2/trh, gnrhr1/mchr1, and agrp/mc4r. Furthermore, 3 negative expression correlations were lost in lepr mutant; kiss1r/trh, mc4r/trh, and mc4r/pomc, whereas 6 negative expression correlations were gained within the mutant group; apln/trh, cnr1/trh, cart2/gnrhr1, cart2/mchr1, kiss1r/mch, and cnr1/pomc (Fig. 5A). These findings revealed that impaired leptin signalling influences potential regulatory connections between appetite-regulating genes in zebrafish brain, particularly the connections involving genes like cart3, cnr1, gnrhr1, kiss1r, mch, mchr1, mc4r, nmu and trh. It should be noted that using these genes as input for a vertebrate protein interactome prediction tool (STRING v10, http://string-db.org/) demonstrates potential molecular interactions between products of cart3, kiss1r, nmu, trh, mc4r, pomca, agrp and mchr1a in zebrafish and/or other vertebrates (Supplementary Fig. 1B).
Finally, we also preformed hierarchical clustering of the experimental groups, for both genotypes, based on the combined expression patterns of all appetite regulating genes in this study, in order to identify their similarities and divergences affected by leptin signal. Interestingly, we found that the refeeding groups were clustered distantly from the control groups, while the two genotypes were closely branched for control and refeeding groups (Fig. 5B). However, we observed distant clustering of the fasting groups, i.e. the wild-type fasting group was more similar, in overall expression pattern, to the control group, whereas the mutant fasting group was closer to the refeeding groups (Fig. 5B). Therefore, the brain expression of appetite-regulating genes shows the strongest divergence during fasting between wild-type and lepr mutant.
Discussion
The main goal of this study was to explore the effects of impaired leptin signalling on transcription of appetite regulating genes in zebrafish brain. We compared expression profiles of known appetite-regulating genes in teleost fish in the brain of zebrafish with normal and non-functional leptin signalling, and provide evidence that both orexigenic and anorexigenic genes are influenced by leptin signalling. Among the orexigenic genes, we only found two genes, belonging to the same signal, apln (encoding apelin), and its cognate receptor, aplnra, to be differentially expressed in wild-type and lepr mutant, respectively. However, the changes seemed to differ in pattern between the two genotypes. Apln encodes a peptide that functions as an endogenous ligand for the G-protein coupled apelin receptor (aplnra), which in turn activates different tissue specific signalling pathways with mainly metabolic effects29. The observed difference was due to the increase in expression of apln during fasting in wild-type and expression induction of aplnra immediately after refeeding in the brain of lepr mutant (Fig. 1). The orexigenic function of apln has been demonstrated in Cypriniformes30–32 and Characiformes33,34. However, the orexigenic role of apln in zebrafish and the regulatory connection between leptin signalling and apln-aplnra in the brain have not been investigated. In goldfish, the direct administration of leptin appeared to have no effects on apln expression in the brain during normal feeding conditions17. In mice, a regulatory connection between leptin receptor and apelin has been already described; non-functional leptin receptor mutant (db/db mice) exhibit up-regulation in Apln expression35. We did not find expression difference between the two genotypes in the control group; however, the difference emerged after fasting and in the refeeding groups. The induction of apln by fasting in the wild-type group indicates its potential orexigenic effect in zebrafish, which seems to be weakened by the impaired leptin signal. On the other hand, we found apln to have higher expression in the lepr mutant than the wild-type during refeeding (Fig. 2). This suggests potential opposing role of leptin signal in regulating apln expression, under different feeding conditions, i.e. inducing effect during fasting and suppressive during refeeding. Future studies are required to elucidate the regulatory connections between leptin signalling and apln-aplnra in zebrafish under different feeding conditions.
In addition to apln, we found another gene encoding an agouti related neuropeptide, agrp, to have increased expression in the lepr mutant during fasting and 2hrs refeeding (Fig. 1). In mice, brain expression of Agrp is inhibited by leptin signal and its expression is mainly induced during fasting as well36. The comparison of the two genotypes also revealed higher agrp expression in the lepr mutant during fasting suggesting a suppressive role of leptin signal on agrp expression during fasting in zebrafish (Fig. 2). In vertebrates, agrp is mainly expressed in the hypothalamus and acts as antagonist for the melanocortin receptors, mc3r and mc4r5. We found a gain of positive expression correlation between agrp and mc4r in the lepr mutant (Fig. 5A), and this could be due to interference of functional leptin signal in co-regulation of agrp and mc4r in the wild-type zebrafish. In Cypriniformes, including zebrafish, agrp has been shown to have orexigenic effects37–40, and its overexpression can lead to obesity and higher growth rate in zebrafish38. In medaka, knockout of lepr had induced agrp expression indicating suppressive effect of active leptin signal on agrp expression and a conserved leptin-dependent negative regulation of agrp across the two distantly related fish species41. These effects might imply an anorexigenic role of leptin signal during fasting in zebrafish through suppression of agrp expression.
We found that two receptors of galanin, galr1a and galr2a, showed increased expression during refeeding in the mutant (Fig. 1), but in the direct comparison between the genotypes only galr1a showed higher expression in the mutant during fasting (Fig. 2). Studies of Cypriniformes suggest an orexigenic role of galanin signal in central nervous system42–44, and fasting induces the expression of galanin receptors in zebrafish brain44. Therefore, the increased galr1a expression during fasting in lepr mutant might reflect stronger appetite stimulation, through galanin signal in zebrafish with the impaired leptin function. Similar to agrp, the expression suppression of galr1a by leptin signal during fasting suggests yet another evidence for potential anorexigenic role of leptin signal during fasting in zebrafish. Two other genes, cnr1 and trh, showed increased expression in the mutant at 2hrs and 6hrs refeeding groups, respectively (Fig. 1). The first gene encodes cannabinoid receptor 1, which appeared to have orexigenic effects in Cypriniformes45–48. A fasting experiment in zebrafish has shown that cnr1 acts as an upstream regulator of cart3 (an anorexigenic factor) in the nervous system, and is essential for suppression of cart3 expression during fasting45. We did not find expression correlation between cnr1 and cart3 in both genotypes, but we found a gain of negative expression correlation between cnr1 and pomc in the lepr mutant (Fig. 5A). Interestingly, cnr1 has been shown to increase feeding through a POMC-related mechanism in rat hypothalamus49. Furthermore, blockade of cnr1 restored leptin signaling in a leptin resistance mice through POMC/MC4R pathway50. Although such regulatory connections have not been shown in fish, our data suggest a potential leptin-dependent regulatory connection between cnr1 and pomc in the zebrafish brain. The brain expression of POMC in mice is directly dependent on the function of leptin signal and its expression is reduced in the non-functional leptin mutant (ob/ob mice)51. Among teleost fish, in both Salmoniformes and Cypriniformes, leptin increases pomc expression in the brain52,53, and in medaka, knockout of lepr decreases pomc expression in the brain as well41. These findings are consistent with our results of pomc expression, which shows expression reduction in the lepr mutant at the normal feeding condition. Furthermore, the expression suppression of cnr1 by leptin signal during 2hrs refeeding indicates potential anorexigenic role of leptin signal at early refeeding in zebrafish.
The second gene, trh, encodes thyrotropin-releasing hormone and only in goldfish it has been shown that trh can increase feeding and locomotor behaviours54. In addition to higher expression of trh in the lepr mutant at 6 hrs refeeding (Fig. 1), we observed several gains and losses of expression correlations between trh and other appetite-regulating genes, in the impaired leptin signal group. Among them were gains of positive expression correlations with cart genes. It is already known that trh acts as an upstream regulator of cart genes in goldfish hypothalamus54. Altogether, our findings demonstrate more active transcription of several orexigenic genes during fasting and refeeding in the brain of zebrafish with impaired leptin signal, as well as several potential leptin-dependent regulatory connections between these genes. This suggests that the anorexigenic role of leptin signals during changes in feeding conditions appears to be mediated through expression suppression of certain orexigenic genes.
Among the anorexigenic genes tested, we found distinct expression dynamics between the genotypes for almost all of the DE genes (except for spx). Perhaps the most striking findings about anorexigenic genes was the consistent and similar expression differences for all of the cart genes (Cocaine and amphetamine regulated transcripts) between the two genotypes. The analysis of expression dynamics for cart1,2,3,4 genes revealed their reduced expression in the wild-type zebrafish, only in the treatment groups (Fig. 3). The direct comparisons of the two genotypes displayed higher expression of cart genes in the wild-type in the control group (Fig. 4). These results are very consistent with findings in rodents, in which not only leptin administration increases the brain expression of Cart gene55, but also the non-functional leptin signal leads to total absence of Cart expression in the brain56. Furthermore, our results demonstrate that changes in feeding conditions reduce the expression of all the cart genes in the brain. This is in agreement with the conserved anorexigenic role of cart genes shown in zebrafish45,46 and other teleost fishes8, although a previous study suggests that not all cart genes follow similar expression pattern in response to fasting57. We found that the reduced cart expressions in different feeding experiments were totally lost in the impaired leptin signal, suggesting leptin-dependent response of cart genes to different feeding conditions. In goldfish, it has been shown that cart1 inhibition of feeding is regulated by leptin in the brain58, indicating a conserved regulatory connection between leptin signal and cart genes in both zebrafish and goldfish. Furthermore, we observed similar expression differences for another anorexigenic gene, crhb or corticotropin-releasing hormone59,60, could imply regulatory connections between cart genes and crhb in zebrafish brain. In particular, we found positive expression correlations between crhb and all cart genes in both genotypes (Fig. 5). In mammals and birds, it had been already shown that CRH is a downstream target of CART in the brain61,62, and in chicken CART1 induces the CRH expression in the brain63. Additionally, CRH has been shown to be a downstream target of leptin in rat, and its brain expression increases after leptin administration64. These findings suggest anorexigenic role of leptin signal in the normal feeding condition through expression induction of anorexigenic cart and crh genes. Future studies are required to unravel regulatory hierarchy of crhb, cart genes and leptin signalling, in zebrafish brain, through identification of related GRNs (gene regulatory networks)28,65.
The only gene we found to have similar changes in both genotypes was spx, with increased expression during refeeding, however, in the direct comparisons of the genotypes it showed higher expression in the wild-type, for the control and 6 hrs refeeding groups. Spexin hormone encoding gene, spx, has been shown to have anorexigenic affects in the brain of Cypriniformes66,67. In goldfish, for instance, spx inhibits feeding, supresses the expression of agrp and apln while inducing the brain expression of cart, pomc, mch and crh67. A later study on zebrafish revealed that spx exerts its anorexigenic effects by suppressing agrp expression in the brain after feeding66. The anorexigenic effects of spx in fish might be complex and involve different regulatory mechanisms, as seen in a Perciforme species, in which spx acts only as a very short-term anorexigenic factor68.
A gonadotropin releasing hormone, gnrh2, and a receptor of the same signal, gnrhr1, were also among the DE candidate anorexigenic genes in our study. In both goldfish and zebrafish, gnrh2 decreases food intake69,70, and in zebrafish overfeeding induces gnrh2 expression in brain as well69. We did not find any expression changes for gnrh2, between the experimental groups, in both genotypes, although gnrh2 and gnrhr1 were the only ones showing differences in direct comparison of the genotypes (Fig. 4). Importantly, gnrhr1 had lost many of its expression correlations with the other genes in the lepr mutant; in particular, gnrhr1 lost its positive expression correlations with cart genes in the impaired leptin signal (Fig. 5). The cart-dependent regulation of gnrh secretion by activated leptin signal had been already reported in rat71,72. Also, in a Perciforme species, it has been shown that leptin increases the brain expression of gnrh2, but not gnrh173. Our results indicate that the functional leptin signal induces the expression of anorexigenic gnrh2 in zebrafish, in normal feeding condition. Interestingly, gnrh2 is a direct downstream target of crh in goldfish74, and we also found similar expression pattern between gnrh2 and crh in zebrafish, hence the expression induction of gnrh2 by leptin signal can be through transcriptional activation of crh in zebrafish brain. It has been shown that anorexigenic effects of gnrh2 are mediated through the receptor encoded by gnrhr169. Thus, the increased expression of gnrhr1 in later feeding conditions in lepr mutant might be a compensatory mechanism for lack of gnrh2 protein, as a result of lower gnrh2 expression in the mutant, at earlier condition of normal feeding. However, such a compensatory mechanism has not been reported for gnrh-gnrhr signal in zebrafish.
We found another functionally connected group of anorexigenic genes, showing differential expression between the genotypes, including melanin concentrating hormone gene (mch/pmch) and its receptors (mchr1,2). The ligand encoding gene, mch, showed reduced expression in the treatment groups than the control group but only in the wild-type, whereas its receptors showed increased expression in the refeeding groups compared to the control group in the mutant (Fig. 3). However, in the direct comparison of the genotypes, mch and mchr1a showed higher expression in one of the refeeding groups in the lepr mutant than the wild-type (Fig. 4). In addition, both mch and mchr1 had several gains and losses of expression correlations with other appetite-regulating genes, in the impaired leptin signalling zebrafish (Fig. 5). The appetite-regulating role of mch signal has not been investigated in zebrafish, but its anorexigenic role is already described in goldfish75,76. In mice, it is already shown that leptin signal acts as an upstream regulator of MCH and MCHR1 in the brain77,78, and the absence of functional leptin signal it increases the expression of both genes in the brain77. Consistently, our results indicate that the expression of mch and its receptor is regulated by leptin signal in zebrafish; although future studies are required to understand their detailed regulatory connection with leptin signal, as well as their potential anorexigenic role in zebrafish.
Comparing the two genotypes, we also observed expression changes in genes belonging to different signals, including prepro-kisspeptin 1 and its receptor, kiss1 and kiss1r, melanocortin 4 receptor, mc4r, neuromedin U preproprotein, nmu, nucleobindin 2 or nesfatin 1, nucb2, and pituitary adenylate cyclase activating polypeptide, pacap. Except for kiss1 and kiss1r, which are only studied in sea bass (a Perciforme species)79, the rest of these genes are shown to have anorexigenic role in at least one Cypriniforme species, mainly goldfish80–86. Except for nucb2, for which the anorexignenic role already is described in zebrafish84, the transcriptional changes of the rest of the genes suggest their potential appetite-regulating role in zebrafish as well. In mice, leptin increases the brain expression of Mc4r87. Consistently, the zebrafish lepr mutant in our study shows reduced mc4r expression under normal feeding condition, indicating a conserved positive regulatory connection between leptin signal and mc4r. The potential regulatory connections between these genes and leptin singalling has not been studied in fish, but our results indicate the existence of such connections in zebrafish brain. In goldfish, nmu and pacap81,88, and in zebrafish, nmu89, act upstream of crh in the brain, and in this study, we also found positive expression correlation between nmu and crh in both genotypes, and between pacap and crh in the wild-type (Fig. 5), suggesting a similar conserved regulatory connection in the zebrafish brain. Another interesting example could be the losses of positive correlations between nmu and several other genes such as all of cart genes and pacap itself in the zebrafish with impaired leptin signalling. Altogether, these observations demonstrate the effects of leptin on the expression of several anorexigenic genes in zebrafish brain under different feeding conditions, as well as inter-dependent regulatory connections in normal functioning or impaired leptin signalling. Furthermore, the consistent lower expression level of several anorexigenic genes, in fish with impaired of leptin signal under normal feeding conditions, indicates that the potential anorexigenic role of leptin signal is mediated through these genes.
Conclusions
The study provides evidence that both orexigenic and anorexigenic genes in the zebrafish brain are influenced by leptin signalling. We observed the most pronounced effect in the group of anorexigenic genes, where impaired leptin signalling resulted in reduced gene expression in several genes of the control group. This suggests a stimulatory effect of leptin on transcription of these anorexigenic genes in wild-type zebrafish under normal feeding conditions. We also observed effects of the impaired leptin signal on expression correlations between appetite regulating genes in zebrafish brain, implicating the existence of complex gene regulatory networks, which are under the influence of leptin signal. The study further indicates the potential appetite-regulating role of several of the investigated genes; however, additional studies are required to confirm the role of these genes in appetite regulation in zebrafish. Taken together, based on the expression changes of the appetite regulating genes, in the impaired leptin receptor signal transduction, we propose an anorexigenic role for leptin signalling in zebrafish. It should be emphasized, however, that all the observed transcriptional changes might not necessarily be translated into changes at the protein level. Furthermore, functional investigations, such as leptin-dependent phosphorylation of Stat3 (a conserved leptin function between mammals and fish90–92), are useful to evaluate the level of impairment of leptin signal in the lepr mutant used in this study.
Methods
Fish breeding, fasting-refeeding scheme and sampling
In this study, we used zebrafish strain leptin receptor sa12593, obtained from European Zebrafish Resource Centre. The mutation was created by the Sanger Institute for the Zebrafish Mutation Project by replacing a cytosine with an adenine on chromosome 6, leading to the formation of a premature codon stop, causing an incomplete translation. For this study, we kept the lepr mutants and their sibling wild-type zebrafish in 3-liter recirculating tanks at 28.4 °C at the SciLife lab zebrafish facility at Uppsala University. The water parameters were checked regularly and maintained by facility staff members. We set up the dark-light conditions to 10 and 14 hours, respectively. Before the experiment, we fed the fishes with dry pellets once in the morning and with Artemia twice a day (middays and evenings). We divided a total number of 40 zebrafish of similar age, into four categories: fish fed by the facility as control group and fish fasted for a week; fish fasted for a week and sampled 2 hours after the refeeding or sampled 6 hours after the refeeding. Each group was subdivided into two categories: homozygote fish for the mutation on the leptin receptor and wild type fish, with 5 fish of each genotype per treatment group. It should be noted that within each group and its counterpart in the other genotype, we tried to include similar female/male ratios (1–2 females and 3–4 males) (Supplementary Data 1). We observed no significant differences in standard body length and net weight, as well as the hepato-somatic index (HSI) between the genotypes (Supplementary Data 1). Fasting resulted in a weight loss of around 10% in both genotypes. We conducted the feeding and sampling at similar time (during the day) within and between the genotypes, and sampling of the control groups was done 2 hours after feeding at the same time during the day. We anaesthetized the fish by immersion in a tricaine solution (MS-222) at a concentration of 0.4 mg/ml and euthanatized them by an immersion in an ice bath. Zebrafish were decapitated and each brain was carefully dissected and transferred into 200 μl of RNAlater, an RNA extraction stabilization solution (Ambion Inc, Austin Texas), at 4 °C for one day and at −20 °C the next day until RNA isolation step.
RNA isolation and cDNA synthesis
We isolated the RNA of sampled brains using Trizol (Ambion). The dissected brains were removed from RNAlater (Ambion) and were put into a new 1.5 ml Eppendorf tube containing 200 μl of Trizol. Then, we homogenized the samples using a syringe needle (25 G Terumo needle and BD Plastipak 1 mL syringe). A quantity of 40 μl of chloroform (Sigma-Aldrich) was added, followed by room temperature incubation for 5 minutes. After incubation, we centrifuged the samples at 12 000 g/min for 20 minutes at 4 °C. The aqueous upper phase was carefully transferred into new RNAse-free microfuge tubes, and 1 μl of glycoblue (Ambion) and 100 μl of ice cold (−20 °C) isopropyl alcohol (Sigma-Aldrich) were added to the tubes. Again, we centrifuged the samples at 13 000 g/min for 20 minutes at 4 °C. The upper phase was removed and the RNA pellet was washed by adding 200 μl of ice cold (−20 °C) ethanol (VWR) at 75% mixed with DMSO solution, followed by centrifugation at 9 000 g/min for 5 minutes at 4 °C. Subsequently, a washing step was performed with 75% ethanol and repeated 3 times. The samples were then dried by opening the tube under a fume hood and solubilized by adding 10 μl of Nuclease-free water (Ambion). We performed a DNAse treatment on each sample to remove genomic DNA by Turbo DNA-free kit (Ambion) according to the manufacturer’s instructions. The quantity and quality of RNA was measured with a NanoDrop 1000 v3.7 (the absorption 260/280 nm ratios were all above 1.85) and 1000 ng of RNA input was used for the reverse transcription (RT). For the RT step, we used Superscript III First-Strand Synthesis System for RT-PCR (Invitrogen). In brief, the RNA samples were mixed with 0.5 μl of random primers (50 ng/μl) and 0.5 dNTP (10 nM), incubated at 65 °C for 5 minutes and then cooled down on ice for a minute. A mix composed of 2 μl of 5X First-Strand Buffer, 0.5 μl of DTT (0.1 M), 0.5 μl of RNase OUT (40 U/μl) and 0.5 μl of Superscript III RT (200 U/μl) was made and added to each sample. An incubation time of 5 minutes at 25 °C was set up, followed by an enzyme reaction time of 50 minutes at 50 °C and an enzyme inhibition time of 15 minutes at 70 °C. The final volume of 10 μl of cDNA was stored at −20 °C until the qPCR step.
Candidate genes, designing primers and qPCR
To validate stably expressed reference gene(s), we selected 8 candidate reference genes based on studies of zebrafish, which had investigated suitable references genes across different experimental conditions, developmental stages and tissues93–95. As target genes, we selected 36 candidates involved in appetite regulation in Cypriniformes (mainly studied on goldfish and zebrafish) with the exception of kiss1 and kiss1r which are only studied in sea bass (Perciformes) (Table 1). In order to design qPCR primers, we first obtain the gene sequences from Blastn, through a zebrafish database search engine (zfin.org)96. Then, we imported the sequences to CLC Genomic Workbench (CLC Bio, Denmark) and specified the exon/exon boundaries using annotated Danio rerio genome in the Ensembl database, http://www.ensembl.org 97. The primers with short amplicon sizes (<200 bp) were designed by Primer Express 3.0 (Applied Biosystems, CA, USA) and their dimerization and secondary structure formation were evaluated using OligoAnalyzer 3.1 (Integrated DNA Technology) (Supplementary Data 1).
For the qPCR step, 1 μl of diluted cDNA (1:20) of each sample was mixed with 7.5 μl of qPCR Master mix called PowerUp SYBR Green Master Mix (Thermo Fischer Scientific), 0.3 μl of forward and reverse primers (10 uM) and 6.2 μl of RNA-free water, for a total volume of 15 μl per tube. The qPCR instrument used was MxPro-3000 PCR machine (Stratagene, La Jolla, CA) together with MxPro software (Stratagene) for data mining. We conducted each biological replicate in two technical replicates for each gene and we followed a sample maximization method98 to have an optimal experimental set-up in each run. The reaction program was as follow: 50 °C for 2 minutes (1 cycle), 95 °C for 2 minutes (1 cycle), 95 °C for 15 second and 62 °C for 1 minute (40 cycles). A dissociation step (60–95 °C) was performed at the end of the amplification step. We estimated threshold cycles, number of copies and efficiencies by the software. To calculate primer efficiencies (E values), we used generated standard curves using serial dilution steps of pooled cDNA samples. For each gene, we ran standard curves in triplicates, and calculated the results using the following formula: E = 10[−1/slope]. R2 values were higher than 0.990 and efficiencies were ranging between 94.1–108.7% for all assays (Supplementary Data 1).
Analysis of gene expression data
To identify the most suitable reference gene(s), we used three different software for calculating the expression stabilities; BestKeeper99, NormFinder100 and geNorm101. BestKeeper uses two algorithms for ranking of candidate reference genes; first one is based on the standard deviation (SD) of Cq values across all the samples and the second one considers expression correlations or BestKeeper index (r)99. NormFinder determines the most stable genes, through calculating expression stability values, which are based on analysis of inter- and intra-group variation in expression100. Finally, geNorm calculates mean pairwise variations in a stepwise manner between each gene and the other candidates (M value)101. The ranking outcomes, from each of these alone, cannot be reliable unless consistency of the results are observed between the software for the top ranked genes102,103. In this study, we used the Cq value of the most stable reference gene across both genotypes and different feeding conditions, Cqreference, to normalize Cq values of target genes for each sample (ΔCqtarget = Cqtarget − Cqreference). To calculate ΔΔCq values, we chose a biological replicate with lowest expression (highest Cq value) across all the samples for each gene (i.e. each gene can have its own calibrator sample), and then subtracted the ΔCq from the calibrator ΔCq value (ΔCqtarget − ΔCqcalibrator). We calculated the relative expression quantities (RQ values) through 2−ΔΔCq, and their logarithmic values (or fold changes) were used for statistical analysis104. We used student t-tests for the direct comparison gene expression levels of a target gene between wild-type and lepr mutant in each feeding condition. To analyse the expression dynamic of a target gene for each genotype, we implemented analysis of variance (ANOVA) test across the different feeding conditions followed by Tukey’s honest significant difference (HSD) post hoc tests. We used Benjamini-Hochberg procedure to correct for the false positive rate in the multiple comparisons105. Moreover, to identify the similarities in expression patterns between the target genes, we used pairwise Pearson correlation coefficients across the feeding conditions in each genotype. We implemented a dendrogram hierarchical clustering of expression values of the target genes to identify overall similarities between the feeding conditions and the genotypes. R (http://www.r-project.org) was used for all statistical analyses106. We explored the knowledge based interactions between the gene products by STRING v10 (http://string-db.org/), using zebrafish database for protein interactome107.
Ethical approval
The fish handling procedures were approved by the Swedish Ethical Committee on Animal Research in Uppsala (permit C10/16). All methods were carried out in accordance with the guidelines and regulations of the Swedish Ethical Committee on Animal Research in Uppsala.
Supplementary information
Acknowledgements
The authors thank the SciLife lab zebrafish facility of Uppsala University for its responsible management of our fish. Special thanks to Chrysoula Zalamitsou for her technical assistance through the study and Laurene A. Lecaudey for proof reading of our manuscript. The work was supported by the Carl Trygger Foundation for scientific research (CTS 16:413).
Author contributions
M.S., M.B. and E.P.A. designed the study. M.B. performed the fish breeding, feeding experiments, tissue sampling and RNA extraction. E.P.A. designed the qPCR primers and studied literature for selection of candidate genes. E.P.A., M.B. and E.T. conducted the laboratory work for the gene expression step. E.P.A. analysed the gene expression data and prepared the graphs. E.P.A., M.B. and M.S. wrote the manuscript. All authors reviewed the manuscript and approve its content.
Data availability
All the data represented in this study are provided within the main manuscript or in the supplementary materials.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-56779-z.
References
- 1.Keen-Rhinehart E, Ondek K, Schneider JE. Neuroendocrine regulation of appetitive ingestive behavior. Front. Neurosci. 2013;7:213. doi: 10.3389/fnins.2013.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Opazo R, et al. Fasting Upregulates npy, agrp, and ghsr Without Increasing Ghrelin Levels in Zebrafish (Danio rerio) Larvae. Front. Physiol. 2019;9:1901. doi: 10.3389/fphys.2018.01901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jeong I, et al. mRNA expression and metabolic regulation of npy and agrp1/2 in the zebrafish brain. Neurosci. Lett. 2018;668:73–79. doi: 10.1016/j.neulet.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Cerdá‐Reverter JM, Canosa LF. Neuroendocrine Systems of the Fish Brain. Fish Physiol. 2009;28:3–74. doi: 10.1016/S1546-5098(09)28001-0. [DOI] [Google Scholar]
- 5.Sohn J-W. Network of hypothalamic neurons that control appetite. BMB Rep. 2015;48:229–33. doi: 10.5483/BMBRep.2015.48.4.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parker JA, Bloom SR. Hypothalamic neuropeptides and the regulation of appetite. Neuropharmacology. 2012;63:18–30. doi: 10.1016/j.neuropharm.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Arora S, Anubhuti Role of neuropeptides in appetite regulation and obesity – A review. Neuropeptides. 2006;40:375–401. doi: 10.1016/j.npep.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 8.Volkoff H. The Neuroendocrine Regulation of Food Intake in Fish: A Review of Current Knowledge. Front. Neurosci. 2016;10:540. doi: 10.3389/fnins.2016.00540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorissen M, Flik G. Leptin in teleostean fish, towards the origins of leptin physiology. J. Chem. Neuroanat. 2014;61–62:200–206. doi: 10.1016/j.jchemneu.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Park H-K, Ahima RS. Physiology of leptin: energy homeostasis, neuroendocrine function and metabolism. Metabolism. 2015;64:24–34. doi: 10.1016/j.metabol.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorissen M, Bernier NJ, Nabuurs SB, Flik G, Huising MO. Two divergent leptin paralogues in zebrafish (Danio rerio) that originate early in teleostean evolution. J. Endocrinol. 2009;201:329–339. doi: 10.1677/JOE-09-0034. [DOI] [PubMed] [Google Scholar]
- 12.Liu Q, et al. Expression of leptin receptor gene in developing and adult zebrafish. Gen. Comp. Endocrinol. 2010;166:346–355. doi: 10.1016/j.ygcen.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volkoff H, Joy Eykelbosh A, Ector Peter R. Role of leptin in the control of feeding of goldfish Carassius auratus: interactions with cholecystokinin, neuropeptide Y and orexin A, and modulation by fasting. Brain Res. 2003;972:90–109. doi: 10.1016/S0006-8993(03)02507-1. [DOI] [PubMed] [Google Scholar]
- 14.de Pedro N, Martínez-Alvarez R, Delgado MJ. Acute and chronic leptin reduces food intake and body weight in goldfish (Carassius auratus) J. Endocrinol. 2006;188:513–520. doi: 10.1677/joe.1.06349. [DOI] [PubMed] [Google Scholar]
- 15.Vivas Y, et al. Time-dependent effects of leptin on food intake and locomotor activity in goldfish. Peptides. 2011;32:989–995. doi: 10.1016/j.peptides.2011.01.028. [DOI] [PubMed] [Google Scholar]
- 16.Tinoco AB, Nisembaum LG, Isorna E, Delgado MJ, de Pedro N. Leptins and leptin receptor expression in the goldfish (Carassius auratus). Regulation by food intake and fasting/overfeeding conditions. Peptides. 2012;34:329–335. doi: 10.1016/j.peptides.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Yan A-F, et al. Goldfish Leptin-AI and Leptin-AII: Function and Central Mechanism in Feeding Control. Int. J. Mol. Sci. 2016;17:783. doi: 10.3390/ijms17060783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Imperatore R, et al. Overlapping Distribution of Orexin and Endocannabinoid Receptors and Their Functional Interaction in the Brain of Adult Zebrafish. Front. Neuroanat. 2018;12:62. doi: 10.3389/fnana.2018.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahima RS, et al. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 20.Tian J, He G, Mai K, Liu C. Effects of postprandial starvation on mRNA expression of endocrine-, amino acid and peptide transporter-, and metabolic enzyme-related genes in zebrafish (Danio rerio) Fish Physiol. Biochem. 2015;41:773–787. doi: 10.1007/s10695-015-0045-x. [DOI] [PubMed] [Google Scholar]
- 21.Johansson M, Björnsson BT. Elevated plasma leptin levels of fasted rainbow trout decrease rapidly in response to feed intake. Gen. Comp. Endocrinol. 2015;214:24–29. doi: 10.1016/j.ygcen.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 22.Trombley S, Maugars G, Kling P, Björnsson BT, Schmitz M. Effects of long-term restricted feeding on plasma leptin, hepatic leptin expression and leptin receptor expression in juvenile Atlantic salmon (Salmo salar L.) Gen. Comp. Endocrinol. 2012;175:92–99. doi: 10.1016/j.ygcen.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Dalman MR, Liu Q, King MD, Bagatto B, Londraville RL. Leptin expression affects metabolic rate in zebrafish embryos (D. rerio) Front. Physiol. 2013;4:160. doi: 10.3389/fphys.2013.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Renquist BJ, Zhang C, Williams SY, Cone RD. Development of an Assay for High-Throughput Energy Expenditure Monitoring in the Zebrafish. Zebrafish. 2013;10:343–352. doi: 10.1089/zeb.2012.0841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kubista M, et al. The real-time polymerase chain reaction. Mol. Aspects Med. 2006;27:95–125. doi: 10.1016/j.mam.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Ahi EP, Richter F, Sefc KM. A gene expression study of ornamental fin shape in Neolamprologus brichardi, an African cichlid species. Sci. Rep. 2017;7:17398. doi: 10.1038/s41598-017-17778-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ahi EP, Singh P, Lecaudey LA, Gessl W, Sturmbauer C. Maternal mRNA input of growth and stress-response-related genes in cichlids in relation to egg size and trophic specialization. Evodevo. 2018;9:23. doi: 10.1186/s13227-018-0112-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahi EP, Sefc KM. Towards a gene regulatory network shaping the fins of the Princess cichlid. Sci. Rep. 2018;8:9602. doi: 10.1038/s41598-018-27977-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bertrand C, Valet P, Castan-Laurell I. Apelin and energy metabolism. Front. Physiol. 2015;6:115. doi: 10.3389/fphys.2015.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Volkoff H, Wyatt JL. Apelin in goldfish (Carassius auratus): Cloning, distribution and role in appetite regulation. Peptides. 2009;30:1434–1440. doi: 10.1016/j.peptides.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 31.Lin F, et al. Molecular and physiological evidences for the role in appetite regulation of apelin and its receptor APJ in Ya-fish (Schizothorax prenanti) Mol. Cell. Endocrinol. 2014;396:46–57. doi: 10.1016/j.mce.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 32.Zhang J, Sun P, Yang F, Kong T, Zhang R. Tributyltin disrupts feeding and energy metabolism in the goldfish (Carassius auratus) Chemosphere. 2016;152:221–228. doi: 10.1016/j.chemosphere.2016.02.127. [DOI] [PubMed] [Google Scholar]
- 33.Penney CC, Volkoff H. Peripheral injections of cholecystokinin, apelin, ghrelin and orexin in cavefish (Astyanax fasciatus mexicanus): Effects on feeding and on the brain expression levels of tyrosine hydroxylase, mechanistic target of rapamycin and appetite-related hormones. Gen. Comp. Endocrinol. 2014;196:34–40. doi: 10.1016/j.ygcen.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 34.Volkoff H. Appetite regulating peptides in red-bellied piranha, Pygocentrus nattereri: Cloning, tissue distribution and effect of fasting on mRNA expression levels. Peptides. 2014;56:116–124. doi: 10.1016/j.peptides.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 35.Ringström C, et al. Apelin is a novel islet peptide. Regul. Pept. 2010;162:44–51. doi: 10.1016/j.regpep.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Mizuno TM, Mobbs CV. Hypothalamic Agouti-Related Protein Messenger Ribonucleic Acid Is Inhibited by Leptin and Stimulated by Fasting*. Endocrinology. 1999;140:814–817. doi: 10.1210/endo.140.2.6491. [DOI] [PubMed] [Google Scholar]
- 37.Song Y, Golling G, Thacker TL, Cone RD. Agouti-Related Protein (AGRP) Is Conserved and Regulated by Metabolic State in the Zebrafish, Danio rerio. Endocrine. 2003;22:257–266. doi: 10.1385/ENDO:22:3:257. [DOI] [PubMed] [Google Scholar]
- 38.Song Y, Cone RD. Creation of a genetic model of obesity in a teleost. FASEB J. 2007;21:2042–2049. doi: 10.1096/fj.06-7503com. [DOI] [PubMed] [Google Scholar]
- 39.Cerdá-Reverter JM, Peter RE. Endogenous Melanocortin Antagonist in Fish: Structure, Brain Mapping, and Regulation by Fasting of the Goldfish Agouti-Related Protein Gene. Endocrinology. 2003;144:4552–4561. doi: 10.1210/en.2003-0453. [DOI] [PubMed] [Google Scholar]
- 40.Wei R, et al. Characterization, tissue distribution and regulation of agouti-related protein (AgRP) in a cyprinid fish (Schizothorax prenanti) Gene. 2013;527:193–200. doi: 10.1016/j.gene.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Chisada S, et al. Leptin receptor-deficient (knockout) medaka, Oryzias latipes, show chronical up-regulated levels of orexigenic neuropeptides, elevated food intake and stage specific effects on growth and fat allocation. Gen. Comp. Endocrinol. 2014;195:9–20. doi: 10.1016/j.ygcen.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 42.de Pedro N, Céspedes MV, Delgado MJ, Alonso-Bedate M. The galanin-induced feeding stimulation is mediated via α2-adrenergic receptors in goldfish. Regul. Pept. 1995;57:77–84. doi: 10.1016/0167-0115(95)91255-4. [DOI] [PubMed] [Google Scholar]
- 43.Volkoff H, Peter RE. Interactions between orexin A, NPY and galanin in the control of food intake of the goldfish, Carassius auratus. Regul. Pept. 2001;101:59–72. doi: 10.1016/S0167-0115(01)00261-0. [DOI] [PubMed] [Google Scholar]
- 44.Li L, et al. A novel galanin receptor 1a gene in zebrafish: Tissue distribution, developmental expression roles in nutrition regulation. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2013;164:159–167. doi: 10.1016/j.cbpb.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 45.Nishio S-I, et al. Fasting Induces CART Down-Regulation in the Zebrafish Nervous System in a Cannabinoid Receptor 1-Dependent Manner. Mol. Endocrinol. 2012;26:1316–1326. doi: 10.1210/me.2011-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guillot R, et al. Behind melanocortin antagonist overexpression in the zebrafish brain: A behavioral and transcriptomic approach. Horm. Behav. 2016;82:87–100. doi: 10.1016/j.yhbeh.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 47.Valenti M, et al. The endocannabinoid system in the brain of Carassius auratus and its possible role in the control of food intake. J. Neurochem. 2005;95:662–672. doi: 10.1111/j.1471-4159.2005.03406.x. [DOI] [PubMed] [Google Scholar]
- 48.Cottone E, et al. Interplay of the Endocannabinoid System with Neuropeptide Y and Corticotropin-releasing Factor in the Goldfish Forebrain. Ann. N. Y. Acad. Sci. 2009;1163:372–375. doi: 10.1111/j.1749-6632.2009.04432.x. [DOI] [PubMed] [Google Scholar]
- 49.Koch M, et al. Hypothalamic POMC neurons promote cannabinoid-induced feeding. Nature. 2015;519:45–50. doi: 10.1038/nature14260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tam J, et al. Peripheral cannabinoid-1 receptor blockade restores hypothalamic leptin signaling. Mol. Metab. 2017;6:1113–1125. doi: 10.1016/j.molmet.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thornton JE, Cheung CC, Clifton DK, Steiner RA. Regulation of Hypothalamic Proopiomelanocortin mRNA by Leptin in ob/ob Mice. Endocrinology. 1997;138:5063–5066. doi: 10.1210/endo.138.11.5651. [DOI] [PubMed] [Google Scholar]
- 52.Murashita K, et al. Leptin reduces Atlantic salmon growth through the central pro-opiomelanocortin pathway. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2011;158:79–86. doi: 10.1016/j.cbpa.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Murashita K, Uji S, Yamamoto T, Rønnestad I, Kurokawa T. Production of recombinant leptin and its effects on food intake in rainbow trout (Oncorhynchus mykiss) Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2008;150:377–384. doi: 10.1016/j.cbpb.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 54.Abbott M, Volkoff H. Thyrotropin Releasing Hormone (TRH) in goldfish (Carassius auratus): Role in the regulation of feeding and locomotor behaviors and interactions with the orexin system and cocaine- and amphetamine regulated transcript (CART) Horm. Behav. 2011;59:236–245. doi: 10.1016/j.yhbeh.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 55.Lee SJ, et al. Leptin stimulates neuropeptide Y and cocaine amphetamine-regulated transcript coexpressing neuronal activity in the dorsomedial hypothalamus in diet-induced obese mice. J. Neurosci. 2013;33:15306–17. doi: 10.1523/JNEUROSCI.0837-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kristensen P, et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature. 1998;393:72–76. doi: 10.1038/29993. [DOI] [PubMed] [Google Scholar]
- 57.Akash G, Kaniganti T, Tiwari NK, Subhedar NK, Ghose A. Differential distribution and energy status-dependent regulation of the four CART neuropeptide genes in the zebrafish brain. J. Comp. Neurol. 2014;522:2266–2285. doi: 10.1002/cne.23532. [DOI] [PubMed] [Google Scholar]
- 58.Volkoff H, Peter RE. Characterization of Two Forms of Cocaine- and Amphetamine-Regulated Transcript (CART) Peptide Precursors in Goldfish: Molecular Cloning and Distribution, Modulation of Expression by Nutritional Status, and Interactions with Leptin. Endocrinology. 2001;142:5076–5088. doi: 10.1210/endo.142.12.8519. [DOI] [PubMed] [Google Scholar]
- 59.De Pedro N, Alonso-Gómez AL, Gancedo B, Delgado MJ, Alonso-Bedate M. Role of corticotropin-releasing factor (CRF) as a food intake regulator in goldfish. Physiol. Behav. 1993;53:517–520. doi: 10.1016/0031-9384(93)90146-7. [DOI] [PubMed] [Google Scholar]
- 60.Bernier NJ. The corticotropin-releasing factor system as a mediator of the appetite-suppressing effects of stress in fish. Gen. Comp. Endocrinol. 2006;146:45–55. doi: 10.1016/j.ygcen.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 61.Sarkar S, Wittmann G, Fekete C, Lechan RM. Central administration of cocaine- and amphetamine-regulated transcript increases phosphorylation of cAMP response element binding protein in corticotropin-releasing hormone-producing neurons but not in prothyrotropin-releasing hormone-producing neurons in the hypothalamic paraventricular nucleus. Brain Res. 2004;999:181–192. doi: 10.1016/j.brainres.2003.11.062. [DOI] [PubMed] [Google Scholar]
- 62.Smith SM, et al. Cocaine- and Amphetamine-Regulated Transcript Activates the Hypothalamic-Pituitary-Adrenal Axis through a Corticotropin-Releasing Factor Receptor-Dependent Mechanism. Endocrinology. 2004;145:5202–5209. doi: 10.1210/en.2004-0708. [DOI] [PubMed] [Google Scholar]
- 63.Mo C, et al. Corticotropin-releasing hormone (CRH) stimulates cocaine- and amphetamine-regulated transcript gene (CART1) expression through CRH type 1 receptor (CRHR1) in chicken anterior pituitary. Mol. Cell. Endocrinol. 2015;417:166–177. doi: 10.1016/j.mce.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 64.Schwartz MW, Seeley RJ, Campfield LA, Burn P, Baskin DG. Identification of targets of leptin action in rat hypothalamus. J. Clin. Invest. 1996;98:1101–6. doi: 10.1172/JCI118891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ahi EP, Sefc KM. Anterior-posterior gene expression differences in three Lake Malawi cichlid fishes with variation in body stripe orientation. PeerJ. 2017;5:e4080. doi: 10.7717/peerj.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng B, et al. Spexin Suppress Food Intake in Zebrafish: Evidence from Gene Knockout Study. Sci. Rep. 2017;7:14643. doi: 10.1038/s41598-017-15138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wong MKH, et al. Goldfish spexin: solution structure and novel function as a satiety factor in feeding control. Am. J. Physiol. Metab. 2013;305:E348–E366. doi: 10.1152/ajpcell.00108.2013. [DOI] [PubMed] [Google Scholar]
- 68.Li S, et al. Molecular cloning and functional characterization of spexin in orange-spotted grouper (Epinephelus coioides) Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2016;196–197:85–91. doi: 10.1016/j.cbpb.2016.02.009. [DOI] [PubMed] [Google Scholar]
- 69.Nishiguchi R, Azuma M, Yokobori E, Uchiyama M, Matsuda K. Gonadotropin-releasing hormone 2 suppresses food intake in the zebrafish, Danio rerio. Front. Endocrinol. (Lausanne). 2012;3:122. doi: 10.3389/fendo.2012.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoskins LJ, Xu M, Volkoff H. Interactions between gonadotropin-releasing hormone (GnRH) and orexin in the regulation of feeding and reproduction in goldfish (Carassius auratus) Horm. Behav. 2008;54:379–385. doi: 10.1016/j.yhbeh.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 71.Lebrethon MC, et al. In Vitro Stimulation of the Prepubertal Rat Gonadotropin-Releasing Hormone Pulse Generator by Leptin and Neuropeptide Y through Distinct Mechanisms 1. Endocrinology. 2000;141:1464–1469. doi: 10.1210/endo.141.4.7432. [DOI] [PubMed] [Google Scholar]
- 72.Parent AS, Lebrethon MC, Gérard A, Vandersmissen E, Bourguignon JP. Leptin effects on pulsatile gonadotropin releasing hormone secretion from the adult rat hypothalamus and interaction with cocaine and amphetamine regulated transcript peptide and neuropeptide Y. Regul. Pept. 2000;92:17–24. doi: 10.1016/S0167-0115(00)00144-0. [DOI] [PubMed] [Google Scholar]
- 73.Schaefer FJ, Wuertz S. Insights into kisspeptin- and leptin-signalling on GnRH mRNA expression in hypothalamic organ cultures of immature pikeperch Sander lucioperca. Int. Aquat. Res. 2016;8:191–196. doi: 10.1007/s40071-016-0134-1. [DOI] [Google Scholar]
- 74.Kang KS, et al. Gonadotropin-releasing hormone II (GnRH II) mediates the anorexigenic actions of α-melanocyte-stimulating hormone (α-MSH) and corticotropin-releasing hormone (CRH) in goldfish. Peptides. 2011;32:31–35. doi: 10.1016/j.peptides.2010.10.013. [DOI] [PubMed] [Google Scholar]
- 75.Matsuda K, et al. Central administration of melanin-concentrating hormone (MCH) suppresses food intake, but not locomotor activity, in the goldfish, Carassius auratus. Neurosci. Lett. 2006;399:259–263. doi: 10.1016/j.neulet.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 76.Matsuda K, et al. Feeding-induced changes of melanin-concentrating hormone (MCH)-like immunoreactivity in goldfish brain. Cell Tissue Res. 2007;328:375–382. doi: 10.1007/s00441-006-0347-5. [DOI] [PubMed] [Google Scholar]
- 77.Kokkotou EG, Tritos NA, Mastaitis JW, Slieker L, Maratos-Flier E. Melanin-Concentrating Hormone Receptor Is a Target of Leptin Action in the Mouse Brain. Endocrinology. 2001;142:680–686. doi: 10.1210/endo.142.2.7981. [DOI] [PubMed] [Google Scholar]
- 78.Segal-Lieberman G, et al. Melanin-concentrating hormone is a critical mediator of the leptin-deficient phenotype. Proc. Natl. Acad. Sci. USA. 2003;100:10085–90. doi: 10.1073/pnas.1633636100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Escobar S, Felip A, Zanuy S, Carrillo M. Is the kisspeptin system involved in responses to food restriction in order to preserve reproduction in pubertal male sea bass (Dicentrarchus labrax)? Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016;199:38–46. doi: 10.1016/j.cbpa.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 80.Maruyama Keisuke, Konno Norifumi, Ishiguro Kotaro, Wakasugi Tatsuya, Uchiyama Minoru, Shioda Seiji, Matsuda Kouhei. Isolation and characterisation of four cDNAs encoding neuromedin U (NMU) from the brain and gut of goldfish, and inhibitory effect of a deduced NMU on food intake and locomotor activity. Journal of Neuroendocrinology. 2007;ja(ja):070927052250004. doi: 10.1111/j.1365-2826.2007.01615.x. [DOI] [PubMed] [Google Scholar]
- 81.Maruyama K, et al. Neuromedin U-induced anorexigenic action is mediated by the corticotropin-releasing hormone receptor-signaling pathway in goldfish. Peptides. 2009;30:2483–2486. doi: 10.1016/j.peptides.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 82.Kerbel B, Unniappan S. Nesfatin-1 Suppresses Energy Intake, Co-localises Ghrelin in the Brain and Gut, and Alters Ghrelin, Cholecystokinin and Orexin mRNA Expression in Goldfish. J. Neuroendocrinol. 2012;24:366–377. doi: 10.1111/j.1365-2826.2011.02246.x. [DOI] [PubMed] [Google Scholar]
- 83.Gonzalez R, Kerbel B, Chun A, Unniappan S. Molecular, Cellular and Physiological Evidences for the Anorexigenic Actions of Nesfatin-1 in Goldfish. PLoS One. 2010;5:e15201. doi: 10.1371/journal.pone.0015201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hatef A, Shajan S, Unniappan S. Nutrient status modulates the expression of nesfatin-1 encoding nucleobindin 2A and 2B mRNAs in zebrafish gut, liver and brain. Gen. Comp. Endocrinol. 2015;215:51–60. doi: 10.1016/j.ygcen.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 85.Matsuda K, et al. Inhibitory effects of pituitary adenylate cyclase-activating polypeptide (PACAP) and vasoactive intestinal peptide (VIP) on food intake in the goldfish, Carassius auratus. Peptides. 2005;26:1611–1616. doi: 10.1016/j.peptides.2005.02.022. [DOI] [PubMed] [Google Scholar]
- 86.Cerdá-Reverter JM, Ringholm A, Schiöth HB, Peter RE. Molecular Cloning, Pharmacological Characterization, and Brain Mapping of the Melanocortin 4 Receptor in the Goldfish: Involvement in the Control of Food Intake. Endocrinology. 2003;144:2336–2349. doi: 10.1210/en.2002-0213. [DOI] [PubMed] [Google Scholar]
- 87.Ghamari-Langroudi M, Srisai D, Cone RD. Multinodal regulation of the arcuate/paraventricular nucleus circuit by leptin. Proc. Natl. Acad. Sci. USA. 2011;108:355–60. doi: 10.1073/pnas.1016785108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Matsuda K. Regulation of feeding behavior and psychomotor activity by corticotropin-releasing hormone (CRH) in fish. Front. Neurosci. 2013;7:91. doi: 10.3389/fnins.2013.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chiu CN, et al. A Zebrafish Genetic Screen Identifies Neuromedin U as a Regulator of Sleep/Wake States. Neuron. 2016;89:842–856. doi: 10.1016/j.neuron.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Frontini A, et al. Leptin-dependent STAT3 phosphorylation in postnatal mouse hypothalamus. Brain Res. 2008;1215:105–115. doi: 10.1016/j.brainres.2008.03.078. [DOI] [PubMed] [Google Scholar]
- 91.Matsuoka T, et al. Tyrosine Phosphorylation of STAT3 by Leptin through Leptin Receptor in Mouse Metaphase 2 Stage Oocyte. Biochem. Biophys. Res. Commun. 1999;256:480–484. doi: 10.1006/bbrc.1999.0365. [DOI] [PubMed] [Google Scholar]
- 92.Douros JD, et al. Leptin Stimulates Cellular Glycolysis Through a STAT3 Dependent Mechanism in Tilapia. Front. Endocrinol. (Lausanne). 2018;9:465. doi: 10.3389/fendo.2018.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McCurley AT, Callard GV. Characterization of housekeeping genes in zebrafish: male-female differences and effects of tissue type, developmental stage and chemical treatment. BMC Mol. Biol. 2008;9:102. doi: 10.1186/1471-2199-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Casadei R, et al. Identification of housekeeping genes suitable for gene expression analysis in the zebrafish. Gene Expr. Patterns. 2011;11:271–276. doi: 10.1016/j.gep.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 95.Xu H, et al. Genome-wide identification of suitable zebrafish Danio rerio reference genes for normalization of gene expression data by RT-qPCR. J. Fish Biol. 2016;88:2095–2110. doi: 10.1111/jfb.12915. [DOI] [PubMed] [Google Scholar]
- 96.Howe DG, et al. ZFIN, the Zebrafish Model Organism Database: increased support for mutants and transgenics. Nucleic Acids Res. 2013;41:D854–60. doi: 10.1093/nar/gks938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Flicek P, et al. Ensembl 2012. Nucleic Acids Res. 2012;40:D84–90. doi: 10.1093/nar/gkr991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007;8:R19. doi: 10.1186/gb-2007-8-2-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–Excel-based tool using pair-wise correlations. Biotechnol. Lett. 2004;26:509–15. doi: 10.1023/B:BILE.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- 100.Andersen CL, Jensen JL, Ørntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245–50. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- 101.Vandesompele J, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ahi, E. P., Richter, F., Lecaudey, L. A. & Sefc, K. M. Gene expression profiling suggests differences in molecular mechanisms of fin elongation between cichlid species. Sci. Rep. 9 (2019). [DOI] [PMC free article] [PubMed]
- 103.Ahi EP, Singh P, Duenser A, Gessl W, Sturmbauer C. Divergence in larval jaw gene expression reflects differential trophic adaptation in haplochromine cichlids prior to foraging. BMC Evol. Biol. 2019;19:150. doi: 10.1186/s12862-019-1483-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thissen D, et al. Quick and Easy Implementation of the Benjamini-Hochberg Procedure for Controlling the False Positive Rate in Multiple Comparisons. J. Educ. Behav. Stat. 2002;27:77–83. doi: 10.3102/10769986027001077. [DOI] [Google Scholar]
- 106.Team RDC. R: A Language and Environment for Statistical Computing. R Found. Stat. Comput. Vienna, Austria (2013).
- 107.Szklarczyk D, et al. The STRING database in 2017: quality-controlled protein–protein association networks, made broadly accessible. Nucleic Acids Res. 2017;45:D362–D368. doi: 10.1093/nar/gkw937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hoekstra M, et al. Absence of HDL cholesteryl ester uptake in mice via SR-BI impairs an adequate adrenal glucocorticoid-mediated stress response to fasting. J. Lipid Res. 2008;49:738–45. doi: 10.1194/jlr.M700475-JLR200. [DOI] [PubMed] [Google Scholar]
- 109.McGill BE, et al. Enhanced anxiety and stress-induced corticosterone release are associated with increased Crh expression in a mouse model of Rett syndrome. Proc. Natl. Acad. Sci. USA. 2006;103:18267–72. doi: 10.1073/pnas.0608702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Okere B, Xu L, Roubos EW, Sonetti D, Kozicz T. Restraint stress alters the secretory activity of neurons co-expressing urocortin-1, cocaine- and amphetamine-regulated transcript peptide and nesfatin-1 in the mouse Edinger–Westphal nucleus. Brain Res. 2010;1317:92–99. doi: 10.1016/j.brainres.2009.12.053. [DOI] [PubMed] [Google Scholar]
- 111.Wu Y, Patchev AV, Daniel G, Almeida OFX, Spengler D. Early-Life Stress Reduces DNA Methylation of the Pomc Gene in Male Mice. Endocrinology. 2014;155:1751–1762. doi: 10.1210/en.2013-1868. [DOI] [PubMed] [Google Scholar]
- 112.Yamano Y, et al. Regulation of CRF, POMC and MC4R Gene Expression after Electrical Foot Shock Stress in the Rat Amygdala and Hypothalamus. J. Vet. Med. Sci. 2004;66:1323–1327. doi: 10.1292/jvms.66.1323. [DOI] [PubMed] [Google Scholar]
- 113.Chaki S, Okuyama S. Involvement of melanocortin-4 receptor in anxiety and depression. Peptides. 2005;26:1952–1964. doi: 10.1016/j.peptides.2004.11.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data represented in this study are provided within the main manuscript or in the supplementary materials.