Abstract
Purpose
In chronic myeloid leukaemia, tyrosine kinase inhibitor treatment is traditionally given continuously for life. However, these drugs produce excellent responses for many patients, and this is accompanied by survival that is close to normal. This has prompted studies of whether it is possible to stop treatment, thus achieving a treatment-free remission (TFR).
Recent Findings
Most TFR studies have focussed on abrupt cessation in patients with long-standing deep remissions, but recent data suggest that more gradual treatment de-escalation may improve TFR success, and that it may be possible to extend TFR attempts to patients who are in stable major molecular response but not necessarily MR4.
Summary
Further data are badly needed on TFR for patients whose remission is less than stable MR4 and on the importance of prior interferon-alpha treatment. Funding TFR trials in a disease with such an excellent outlook is an increasing challenge.
Keywords: Chronic myeloid leukaemia, CML, Discontinuing treatment, TKI, Tyrosine kinase inhibitors
Introduction
Myelosuppressive therapy in the form of busulphan was introduced for the treatment of chronic myeloid leukaemia (CML) in the early 1950s, and it and its more convenient successor hydroxycarbamide remained the centrepiece of CML therapy for the next 30 years. It was assumed that these agents should be given continually, perhaps in the belief that it was important to sustain suppression of an overactive marrow. Trials were done to examine the optimal timing of busulphan delivery (reviewed in [1]), but none considered whether it might be feasible to stop treatment in good responders, presumably because the Philadelphia (Ph) t(9:22)(q34:q11) translocation that typifies CML remains readily detectable in the marrow of even the best responders to busulphan/hydroxycarbamide. Interferon-alpha (IFN) became available in the early 1980s, and serial cytogenetic studies in IFN recipients revealed for the first time that around half could achieve some degree of marrow cytogenetic remission. A small minority (~ 5%) of patients may achieve enduring complete cytogenetic remission (CCyR; none of at least 20 marrow metaphases are Ph positive), and about half the patients in long-standing CCyR on IFN may successfully stop treatment [2].
The tyrosine kinase inhibitor (TKI) imatinib entered clinical trial in 1998 and became generally available over the next few years. The IRIS trial of first-line therapy recruited during 2000-01, and rapidly established that imatinib was more efficacious and less toxic than IFN. A 10-year follow-up of the imatinib recipients in this study revealed an overall survival of 83%, with approximately half remaining on imatinib throughout and 82% achieving CCyR [3]. This high cytogenetic remission rate led to the adoption of BCR-ABL1 quantitation by real-time quantitative reverse transcriptase polymerase chain reaction (PCR) for monitoring responses to TKI treatment, and prompted the introduction of deeper remission yardsticks; major molecular response (MMR or MR3; BCR-ABL1/ABL1 ratio ≤ 0.1%), MR4 (BCR-ABL1/ABL1 ratio ≤ 0.01%) and MR4.5 (BCR-ABL1/ABL1 ratio ≤ 0.0032%). There is a vogue to refer to MR4 or lower as ‘deep molecular response’.
Some patients receiving imatinib achieve not only CCyR but also molecular negativity. After an encouraging pilot study by the French CML group of stopping imatinib in 12 patients [4], this group carried out STIM, a bold study in 100 patients with undetectable disease by the molecular techniques of ~ 2006. Twelve months after stopping treatment, 41% remained molecularly negative [5], and at 5 years 38% remained recurrence free [6]. The vast majority of recurrences occurred in the first 6 months or so after stopping. This concentration of recurrences in the first few months after stopping treatment is a feature seen in virtually all subsequent studies.
Studies of Treatment-Free Remission
Earlier [7•, 8•] and more recent [9•] studies of treatment-free remission (TFR) have been ably reviewed. Table 1 summarizes the main studies of TFR. During the course of these studies, the definition of recurrence has evolved from the emergence of BCR-ABL1 positivity as used in STIM and the almost contemporary Australian TWISTER study [10, 11], to loss of MMR. This latter definition was first used in the A-STIM study (12) which had similar entry criteria to STIM but reported a recurrence free survival (RFS) of 61% at 3 years. Loss of MMR has been widely used as the definition of recurrence in almost all the majority of more recent studies (see Table 1).
Table 1.
A non-exhaustive list of the key studies of TKI discontinuation. CMR = complete molecular remission; DMR = deep molecular remission, as defined in the text. a: CMR was defined as undetectable BCR-ABL1 transcripts with sensitivity ≥ 40,000 amplified copies of the ABL control gene. b: All cases had an initial 12 months of half-dose treatment. c: Retrospective observational study
| Study acronym | Ref | Entry requirements | No | Definition of recurrence | Recurrence-free survival after stopping | Factors predicting lower recurrence risk |
|---|---|---|---|---|---|---|
| STIM1a | [5, 6] | CMR; Undetectable for ≥ 2 yrs | 100 | BCR-ABL1 positivity | 38% at 5 years | Lower Sokal score and longer TKI duration |
| TWISTER | [10, 11] | Imatinib for ≥3 yrs. Undetectable for ≥ 2 yrs | 40 | Loss of MMR OR 2 consecutive positives | 47.1% at 2 years; 45% at 8.6 years | Prior IFN |
| A-STIM a | [12] | CMR; Undetectable for ≥ 2 yrs | 80 | Loss of MMR | 61% at 3 years | |
| STOP 2G-TKI | [13] | 2G TKI ≥ 3 yrs.: MR4.5 for ≥ 2 yrs | 60 | Loss of MMR | 54% at 4 years | Lack of TKI resistance to first line treatment |
| EURO-SKI | [14••] | TKI ≥ 3 yrs.; MR4 for ≥ 12 months | 755 | Loss of MMR | 50% at 2 years | Longer treatment and DMR duration |
| DESTINY b | [15, 16••] | TKI ≥ 3 yrs.; MR4 or MMR | 174 | Loss of MMR | 72% (MR4)/36% (MMR) at 2 yrs | Longer treatment duration |
| Unnamedc | 17 | Unrestricted; ≥ MR4 on three occasions | 293 | Variable (retrospective) | 62% at 34 months | Retrospective study, not a trial |
| Unnamedc | 18 | TKI ≥ 3 yrs.; MR4.5 ≥ 2 yrs | 236 | Loss of MMR or > 1 log rise | 64% at 4 years | Retrospective study, not a trial |
| ISAV | 19 | Imatinib > 2 yrs.; CMR; Undetectable ≥ 18 months | 108 | Loss of MMR | 48% at 3 years | Older age |
| KID | 20 | Imatinib for ≥ 3 yrs. Undetectable for ≥ 2 years | 156 | Loss of MMR | 58.5% at 24 months | Longer duration of imatinib and presence of withdrawal syndrome |
| RE-STIM | 21 | In DMR after a first unsuccessful TFR attempt | 70 | Loss of MMR | 35% at 3 years | Slower speed of molecular relapse after the first TKI discontinuation attempt |
| ENESTop | 22 | Imatinib then nilotinib ≥ 3 yrs.; MR4.5 | 126 | Loss of MMR or confirmed loss of MR4 | 53% at 96 weeks | |
| DASFREE | 23 | Dasatinib > 2 yrs. (first-line or later); MR4.5 | 84 | Loss of MMR | 48% at 1 year | Includes patients resistant to first line TKI |
| DADI | 24 | Dasatinib as second or subsequent line; MR4 | 63 | Loss of MR4 | 44% at 36 months | Lack of imatinib resistance |
| ENESTfreedom | 25 | Nilotinib ≥ 3 years (first line); MR4.5 at entry | 190 | Loss of MMR | 48.8% at 96 weeks | |
| ENESTpath | 26 | Nilotinib (second line); MR4 | 620 | Los of MR4 | Yet to report TFR phase | |
| ENESTgoal | 27 | Nilotinib (second line); MR4.5 | 59 | Loss of MMR | Yet to report TFR phase | (Only 17 of 59 entrants entered the TFR phase) |
Most studies have focussed on patients with excellent molecular responses to their first-line TKI (usually imatinib), and report an RFS of 48–61% at 3 years of TKI discontinuation. There are considerable differences in the detailed eligibility for study entry, which complicates comparisons between studies. Most academic-led studies have used the presence of at least MR4 sustained for 1–2 years before entry, while studies led by pharmaceutical companies such as DASFREE [23] or ENESTfreedom [25] are typically more cautious, requiring MR4.5, either sustained or at least at study entry. There is no evidence that those studies restricted to patients in MR4.5 produce a better RFS than those allowing patients in only MR4. The ISAV study [19] found that TFR was more likely to be successful in older patients. The reasons for this are not clear.
Information on TFR is limited in patients resistant to a first-line TKI (almost always imatinib) who then achieve excellent responses to a second-line TKI. The ENESTop study [22] of patients on second-line nilotinib was cautious in that patients had to be in at least durable MR4.5 for entry, and maintain this on nilotinib during a subsequent 48-week observation period; it reported 53% RFS at 96 weeks after stopping. The DADI study [24] reported a RFS of 44% at 3 years after stopping second-line dasatinib, though used loss of MR4 as its definition of recurrence. The STOP-2G TKI [13] reported a RFS of 54% at 4 years using loss of MMR as its recurrence endpoint. Interestingly, both this and the DADI study reported better RFS for patients who were intolerant of first-line imatinib than for those with first-line TKI resistance. ENESTpath is in progress, comparing two different durations of second-line nilotinib before stopping treatment.
How Might we Improve TFR Success?
In the STIM trial, a non-significant trend for a better TFR success rate was seen in patients who had previously received IFN [5], though patients in early TFR studies who had received initial IFN tended to have received their TKI for longer, and also IFN may increase the proportion of patients achieving remissions deep enough to allow consideration of TFR. In the large multinational EURO-SKI study, patients pretreated with IFN for > 1.5 years had a 6-month RFS of 86%, which was significantly higher than the 56% seen in patients who had received IFN for ≤ 1.5 years [14••]. IFN maintenance therapy may enable high rates of treatment discontinuation success [28], and the value of pegylated IFN maintenance is currently being prospectively explored in the ENDURE study (https://clinicaltrials.gov/ct2/show/NCT03117816). The German TIGER (CML V) study is currently comparing nilotinib ± IFN as first-line treatment, followed by an IFN-only maintenance phase and then a TFR attempt where appropriate. Its first results may be available in late 2019, though data on the TFR phase may take rather longer. Similarly, the Nordic and French group are examining initial bosutinib with or without pegylated IFN, followed by TFR, in the BOSUPEG trial. All these studies require stable MR4 before attempting TFR, and over the course of the next 2–3 years will establish whether concurrent and sequential IFN with TKI improves TFR success.
It is plausible that other treatment strategies might increase the TFR success rate. Recently a small study of lenolidamide as maintenance therapy after TKI withdrawal has been reported [29].
All but one of the studies summarized in Table 1 have used an abrupt discontinuation of TKI. However, it is plausible that some patients who fail to successfully stop their TKI might nevertheless remain in good remission on lower doses, which might ameliorate TKI-related adverse events and help with the burden of drug costs. Reduction of therapy appears safe [30], and mathematical modelling of clinical trial data predicts that after an initial phase of disease bulk reduction, reduced TKI doses are likely to control disease as effectively as standard doses [31]. The British De-Escalation and Stopping Treatment of Imatinib, Nilotinib or sprYcel (DESTINY) study differs from the other studies summarized in Table 1 in that patients first receive 12 months of their TKI at half the standard dose (imatinib 200 mg daily, nilotinib 200 mg twice daily or dasatinib 50 mg daily) before outright stopping. At the end of this de-escalation phase, 98% of patients in at least stable MR4 over the year prior to entry remain recurrence-free [15], and at 36 months (i.e. after a subsequent 2 years of complete cessation), the overall RFS is 72% [16••]. These excellent results have only been equalled by a small study of interferon maintenance on imatinib withdrawal [28] and recent observational retrospective studies where patient selection may have been a factor [17, 18].
It is possible that gradual TKI withdrawal might provoke hitherto quiescent leukaemic stem cells (LSC) into a more proliferative state in which they may become vulnerable to the TKI, thus lowering the LSC burden at subsequent TKI cessation. The kinetics of residual leukaemia during de-escalated TKI doses or after stopping are difficult to study, since by definition there is very little disease present, though recent data on ten patients in the DESTINY study suggest that lower marrow baseline levels of cells positive for CD93, a potential LSC marker, may predict a better RFS [32]. An alternative possibility is that gradual TKI withdrawal in some way coerces the immune system to respond to residual leukaemia. TKIs may suppress T cell proliferation and activation and dendritic, natural killer and B cell function (reviewed in [33]), but this does not appear to have significant clinical consequences. Immune responses to BCR-ABL peptide vaccination in TKI recipients are the same as in normal subjects [34].
There are recent data about how immune responses may alter after TKI withdrawal. In DESTINY patients, CD4+ regulatory T cells and CD56dim and CD56bright NK cell subsets fall during de-escalation, though no further lymphocyte subset changes are seen after subsequent TKI cessation; furthermore, a rise in the effector memory CD8+ subset predicts recurrence [35]. Patients in the EURO-SKI trial with higher levels of NK cells and lower CD86+ plasmacytoid dendritic cell counts at the time of treatment cessation have a higher probability of successful treatment discontinuation [36, 37], and their numbers may increase further after treatment cessation in non-relapsing patients [38]. Overall, current data suggest that the degree to which the immune system is affected by TKIs may be greater in patients with disease recurrence after TKI cessation. Although this is currently of little practical use in the clinic, further study of the immune system during TKI withdrawal will be useful. Other studies of gradual TKI withdrawal are currently being planned, and it is hoped that they will incorporate laboratory studies of the immune system.
What PCR Level Is Required?
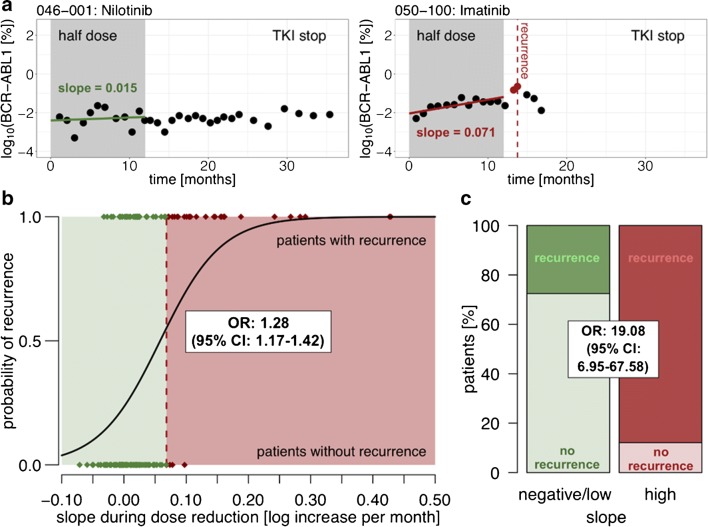
As Table 1 shows, the vast majority of studies of TFR have focussed on patients in at least stable MR4. The current National Comprehensive Cancer Network (US) guidelines recommend that TFR should only be attempted in patients who have been in at least MR4 for at least 2 years [39]. Those of the European Society for Medical Oncology recommend achievement of MR4.5 and stable in at least MR4 for at least 2 years [40], though acknowledge that less stringent criteria do not exclude successful TFR. However, absence of evidence does not constitute evidence of absence. Apart from anecdotal reports[41, 42] and a study of 12 patients [43], TFR has not been formally studied in patients achieving enduring MMR but not necessarily MR4. However, 49 patients who had received TKI for at least 3 years and were in stable MMR but not always MR4 during the previous 12 months were included in the DESTINY study cited above. Their RFS at 12 months of de-escalation was 81%, [15], and 36% at 3 years (after a subsequent 2 years of stopping) [16••]. These are inferior results to those seen in patients in at least stable MR4 (98% and 72% respectively). However, better RFS was seen in patients who had received their treatment for longer, and the RFS in the MR4 patients may be similar to MMR patients who have received their TKI for 2–3 years longer [16••]. Furthermore, the trend (slope) in the BCR-ABL1/ABL1 ratio during de-escalation may be used to predict the probability of recurrence after subsequent TKI cessation [44]. Panel A of Fig. 1 illustrates two contrasting representative patients, on the left with almost no PCR change and no recurrence and on the right with a high upward slope who did relapse. A PCR rise of greater than 0.068 log per month is associated with a high probability of recurrence on TKI stopping, and this PCR slope could be used to guide advice about PCR cessation; indeed, if TKI cessation were limited to patients with negative (falling PCR) or low positive slopes then the 2-year RFS for MMR patients exceeds 50% [40]. Overall, these data in MMR patients require confirmation in further studies, and at present it may be wise to consider TFR attempts for such patients as experimental and best confined to clinical trials.
Fig. 1.
Results from reference 44 on the DESTINY trial [16••] data, showing the relationship between the slope of the BCR-ABL1/ABL1 PCR during the initial 12-month dose reduction period and the probability of recurrence. OR = odds ratio; CI = 95% confidence interval. n = 171 throughout. A PCR dynamics of two representative patients during the dose reduction period. Left: a patient with a low positive slope (0.015 log rise per month; green line) that remains in TFR after stopping TKI. Right: a patient with a high slope (0.071 log rise per month; red line); recurrence occurred shortly after stopping TKI. B Logistic regression curve for patient-specific estimates of the individual PCR slope during dose reduction, with corresponding OR and CI per 0.01 log-increase per month. Patients are separated into cohorts with negative/low slopes (green area) or high slopes (red area) by a cut-off of 0.068 log rise per month. C Proportion of patients with and without recurrence in the negative/low and high slope cohorts during dose reduction, and corresponding OR and CI
Other Considerations
Surprisingly, failure of a TFR attempt does not necessarily preclude success at a second attempt, though information on the best approach for such patients is currently lacking. The observational RE-STIM study, of 70 patients failing a first TFR attempt who then regained a stable remission of at least MR4, reported an RFS of 42% at 2 years after a second stop, and this may be higher in patients with longer times to recurrence in the first stopping attempt [21]. These results are not dramatically inferior to those in first TFR attempts, and it is unclear why patients who fail TFR initially may succeed with essentially the same strategy a few months later. Studies of a second TFR attempt are in progress for imatinib recipients that fail a TFR attempt, in which treatment is first changed to either nilotinib (NILO post-STIM, NAUT) or dasatinib (TRAD).
There is some evidence that RFS after stopping TKI may be better in patients with the e14a2 form of BCR-ABL1 transcript than in those with the e13a2 form [45, 46], though this finding is not universal. It is unclear whether this is real or due to a PCR artefact whereby a given level of e13a2 burden may appear higher than for e14a2, thus disproportionately lowering any relapse threshold in e13a2 patients. Almost no data are available on TFR for patients with variant BCR-ABL1 transcripts (i.e. not e13a2 or e14a2) as such patients have been excluded from most studies due to the lack of parity between PCR techniques for variant transcripts versus e13a2/e14a2 and the difficulties of standardization of variant transcript PCR techniques. As a result, several guidelines do not recommend attempting TFR in such patients.
In the past few years, new molecular platforms have become available which are more sensitive than conventional RT-PCR. These include DNA-based PCR and digital and next-generation sequencing methodology (reviewed in [47]). Using such techniques, disease may remain detectable in patients who have undetectable BCR-ABL by conventional RT-PCR [10]. This has led to the suggestion that such techniques may be useful for identifying patients at higher risk of recurrence, though few studies have investigated this possibility to date. However, many patients undergoing TFR have disease detectable by conventional PCR at TKI cessation, yet can remain off treatment for months and years without losing MMR, even if PCR levels may rise. Since such patients are clearly not leukaemia-free yet can manage without treatment, this raises questions about the clonal kinetics during TFR and at recurrence. A particular difficulty is investigating the LSC compartment during TFR, since this is by definition small, may be quiescent, and if not then it is dwarfed by its differentiated progeny.
All TFR studies to date have specifically excluded patients with prior advanced phase CML, even if in excellent TKI-induced remissions for many years, presumably on the grounds that they potentially harbour clones with the proven capability of relapsing in blast crisis. This exclusion is unlikely to change and TFR is best regarded as contraindicated for such patients.
TKI Withdrawal Syndrome
Withdrawal symptoms on TKI cessation were first described in 2014 [48, 49] in 15 of 50 Swedish patients in the EURO-SKI study. In a retrospective analysis of STOP-TKI and EURO-SKI data, 23% of 427 patients developed musculoskeletal symptoms on TKI withdrawal [50]. These mainly affected the upper body joints, and required multiple symptomatic treatments in 30% of patients. The mechanism of this TKI withdrawal syndrome is not clear, though has been suggested to be due to withdrawal of an off-target effect (i.e. a target other than ABL1) of the TKI. All TKIs have off target effects, though the relative magnitude of these on a given target differ between the TKIs, whereas there is currently little evidence of differences in rate or clinical manifestation of the withdrawal syndrome between the TKIs. Duration of TKI treatment and a previous history of osteoarticular symptoms increased the risk of symptoms. Most subsequent studies have also reported similar symptoms in 25–40% of patients stopping TKI (reviewed in [51•]). In the KID study, its occurrence is associated with a greater TFR success rate, and a longer time to recurrence in those who do relapse [20], though this is not seen in other studies. The effect of musculoskeletal symptoms on quality of life is not known, as few studies have assessed this during TFR and these have typically used assessment instruments for general cancer that may be too insensitive for well-controlled CML patients. Symptoms may be more likely in patients with lower body weight and body mass index [52]. Although the symptoms may resemble other inflammatory arthropathies, rheumatoid factor, markers of systemic lupus erythematosus and radiological investigations for joint damage are typically negative [53].
The natural history appears to be gradual and complete resolution, though this may take many months. There are no data on optimal treatment. Symptomatic treatment with mild analgesics such as paracetamol and non-steroidal anti-inflammatory drugs may help; more severe cases may require a course of steroids. In the original Swedish series within EURO-SKI, symptoms resolved in all six patients who resumed imatinib because of recurrence [48] and this amelioration on resumption of TKI has been confirmed in other series, though patients are understandably reluctant to resume TKI solely for this reason.
Practical Considerations
TKIs are expensive drugs, and therefore it is not surprising that both treatment de-escalation and withdrawal have been reported to save substantial sums of money [14••, 15]. This cost saving may be less dramatic since imatinib is now generic and therefore cheaper in most countries, and may diminish further as newer TKIs follow suit in the coming years. However, more frequent molecular monitoring is required for patients who stop treatment, especially in the first few months. The National Comprehensive Cancer Network (NCCN) guidelines recommend monthly testing for the first 12 months [35], and this additional monitoring partly offsets the savings on drug costs. An interesting recent theoretical report predicts that two monthly monitoring for the first 6 months and 3 monthly thereafter will still be safe enough to capture recurrence (loss of MMR) before patients lose CCyR [54]. It will be interesting to see this bold monitoring schedule applied in future TFR trial designs.
Conclusions
It is clear that some patients with excellent responses to a few years of TKI therapy can successfully discontinue treatment. TFR appears safe; there are currently only two reports of disease progression (lymphoid blast crisis in the A-STIM study [12] and blast crisis in the KID study [55]) amongst many thousand patients attempting TFR from stable remissions of MMR or better, and almost all patients with disease recurrence after stopping TKI will regain MMR and MR4 on resuming their TKI. There is therefore an understandable current trend to regard successful TFR as the primary treatment goal, and TFR rightly features centrally in the design of the trials of most major CML groups.
However, caution is urged before adopting TFR as the sine plus ultra of CML treatment, in place of the more humble aim of at least MMR (and thus freedom from progression) without unacceptable side effects. Around 20% of otherwise eligible patients may not wish to stop treatment [16••, 56], and TKI cessation may raise levels of anxiety and depression [57]. Interestingly, a recent survey of over 1500 patients suggests that they are less concerned about TFR than haematologists, and regard other facets of the disease as of greater importance [58]. The precise definition of who might be eligible for a TFR attempt is not yet finalized, and key questions remain about the role of IFN in initial remission induction or in consolidation before attempting TFR. More work is needed on whether gradual treatment withdrawal, which clearly ameliorates symptoms, can improve TFR success, and whether TFR can be extended to patients in stable MMR but not necessarily MR4; we also do not know how best to advise patients who fail a first TFR attempt and wish to try again. Funding further investigation of TFR in a disease with survival approximating to the normal population is a challenge; nevertheless, several studies of these aspects of TFR are underway and are likely to report in the next few years.
Compliance with Ethics Guidelines
Conflict of Interest
The author declares that there is no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Chronic Myeloid Leukemias
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Goldman JM, Baughan ASJ. Chronic granulocytic leukaemia: treatment. In: Goldman JM, Preisler HD, editors. Leukemias, Butterworth International Medical Reviews, vol. 8. London: Butterworth and Co. p. 239–65.
- 2.Mahon FX, Delbrel X, Cony-Makhoul P, Fabères C, Boiron JM, Barthe C, Bilhou-Nabéra C, Pigneux A, Marit G, Reiffers J. Follow-up of complete cytogenetic remission in patients with chronic myeloid leukemia after cessation of interferon alfa. J Clin Oncol. 2002;20:214–220. doi: 10.1200/JCO.2002.20.1.214. [DOI] [PubMed] [Google Scholar]
- 3.Hochhaus A, Larson RA, Guilhot F, Radich JP, Branford S, Hughes TP, Baccarani M, Deininger MW, Cervantes F, Fujihara S, Ortmann CE, Menssen HD, Kantarjian H, O'Brien SG, Druker BJ, IRIS investigators Long-term outcomes of imatinib treatment for chronic myeloid leukemia. N Engl J Med. 2017;376:917–927. doi: 10.1056/NEJMoa1609324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rousselot P, Huguet F, Rea D, Legros L, Cayuela JM, Maarek O, Blanchet O, Marit G, Gluckman E, Reiffers J, Gardembas M, Mahon FX. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood. 2007;109:58–60. doi: 10.1182/blood-2006-03-011239. [DOI] [PubMed] [Google Scholar]
- 5.Mahon FX, Réa D, Guilhot J, Guilhot F, Huguet F, Nicolini F, Legros L, Charbonnier A, Guerci A, Varet B, Etienne G, Reiffers J, Rousselot P, Intergroupe Français des Leucémies Myéloïdes Chroniques Discontinuation of imatinib in patients with chronic myeloid leukaemia who have maintained complete molecular remission for at least 2 years: the prospective, multicentre Stop Imatinib (STIM) trial. Lancet Oncol. 2010;11:1029–1035. doi: 10.1016/S1470-2045(10)70233-3. [DOI] [PubMed] [Google Scholar]
- 6.Etienne G, Guilhot J, Rea D, Rigal-Huguet F, Nicolini F, Charbonnier A, Guerci-Bresler A, Legros L, Varet B, Gardembas M, Dubruille V, Tulliez M, Noel MP, Ianotto JC, Villemagne B, Carré M, Guilhot F, Rousselot P, Mahon FX. Long-term follow-up of the French sStop Imatinib (STIM1) study in patients with chronic myeloid leukemia. J Clin Oncol. 2017;35:298–305. doi: 10.1200/JCO.2016.68.2914. [DOI] [PubMed] [Google Scholar]
- 7.Saussele S, Richter J, Hochhaus A, Mahon FX. The concept of treatment-free remission in chronic myeloid leukemia. Leukemia. 2016;30:1638–1647. doi: 10.1038/leu.2016.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes TP, Ross DM. Moving treatment-free remission into mainstream clinical practice in CML. Blood. 2016;128:17–23. doi: 10.1182/blood-2016-01-694265. [DOI] [PubMed] [Google Scholar]
- 9.Cortes J, Rea D, Lipton JH. Treatment-free remission with first- and second-generation tyrosine kinase inhibitors. Am J Hematol. 2019;94:346–357. doi: 10.1002/ajh.25342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross DM, Branford S, Seymour JF, Schwarer AP, Arthur C, Yeung DT, Dang P, Goyne JM, Slader C, Filshie RJ, Mills AK, Melo JV, White DL, Grigg AP, Hughes TP. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood. 2013;122:515–522. doi: 10.1182/blood-2013-02-483750. [DOI] [PubMed] [Google Scholar]
- 11.Ross DM, Pagani IS, Shanmuganathan N, Kok CH, Seymour JF, Mills AK, Filshie RJ, Arthur CK, Dang P, Saunders VA, Braley J, Yong AS, Yeung DT, White DL, Grigg AP, Schwarer AP, Branford S, Hughes TP, Australasian Leukaemia and Lymphoma Group (ALLG) Long-term treatment-free remission of chronic myeloid leukemia with falling levels of residual leukemic cells. Leukemia. 2018;32:2572–2579. doi: 10.1038/s41375-018-0264-0. [DOI] [PubMed] [Google Scholar]
- 12.Rousselot P, Charbonnier A, Cony-Makhoul P, Agape P, Nicolini FE, Varet B, Gardembas M, Etienne G, Réa D, Roy L, Escoffre-Barbe M, Guerci-Bresler A, Tulliez M, Prost S, Spentchian M, Cayuela JM, Reiffers J, Chomel JC, Turhan A, Guilhot J, Guilhot F, Mahon FX. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J Clin Oncol. 2014;32:424–430. doi: 10.1200/JCO.2012.48.5797. [DOI] [PubMed] [Google Scholar]
- 13.Réa D, Nicolini FE, Tulliez M, Guilhot F, Guilhot J, Guerci-Bresler A, Gardembas M, Coiteux V, Guillerm G, Legros L, Etienne G, Pignon JM, Villemagne B, Escoffre-Barbe M, Ianotto JC, Charbonnier A, Johnson-Ansah H, Noel MP, Rousselot P, Mahon FX, France Intergroupe des Leucémies Myéloïdes Chroniques Discontinuation of dasatinib or nilotinib in chronic myeloid leukemia: interim analysis of the STOP 2G-TKI study. Blood. 2017;129:846–854. doi: 10.1182/blood-2016-09-742205. [DOI] [PubMed] [Google Scholar]
- 14.Saussele S, Richter J, Guilhot J, Gruber FX, Hjorth-Hansen H, Almeida A, Janssen JJWM, Mayer J, Koskenvesa P, Panayiotidis P, Olsson-Strömberg U, Martinez-Lopez J, Rousselot P, Vestergaard H, Ehrencrona H, Kairisto V, Machová Poláková K, Müller MC, Mustjoki S, Berger MG, Fabarius A, Hofmann WK, Hochhaus A, Pfirrmann M, Mahon FX, EURO-SKI investigators Discontinuation of tyrosine kinase inhibitor therapy in chronic myeloid leukaemia (EURO-SKI): a prespecified interim analysis of a prospective, multicentre, non-randomised, trial. Lancet Oncol. 2018;19:747–757. doi: 10.1016/S1470-2045(18)30192-X. [DOI] [PubMed] [Google Scholar]
- 15.Clark RE, Polydoros F, Apperley JF, Milojkovic D, Pocock C, Smith G, Byrne JL, de Lavallade H, O'Brien SG, Coffey T, Foroni L, Copland M. De-escalation of tyrosine kinase inhibitor dose in patients with chronic myeloid leukaemia with stable major molecular response (DESTINY): an interim analysis of a non-randomised, phase 2 trial. Lancet Haematol. 2017;4:e310–e316. doi: 10.1016/S2352-3026(17)30066-2. [DOI] [PubMed] [Google Scholar]
- 16.Clark RE, Polydoros F, Apperley JF, Milojkovic D, Rothwell K, Pocock C, Byrne J, de Lavallade H, Osborne W, Robinson L, O'Brien SG, Read L, Foroni L, Copland M. De-escalation of tyrosine kinase inhibitor therapy before complete treatment discontinuation in patients with chronic myeloid leukaemia (DESTINY): a non-randomised, phase 2 trial. Lancet Haematol. 2019;6:e375–e383. doi: 10.1016/S2352-3026(19)30094-8. [DOI] [PubMed] [Google Scholar]
- 17.Fava C, Rege-Cambrin G, Dogliotti I, Cerrano M, Berchialla P, Dragani M, Rosti G, Castagnetti F, Gugliotta G, Martino B, Gambacorti-Passerini C, Abruzzese E, Elena C, Pregno P, Gozzini A, Capodanno I, Bergamaschi M, Crugnola M, Bocchia M, Galimberti S, Rapezzi D, Iurlo A, Cattaneo D, Latagliata R, Breccia M, Cedrone M, Santoro M, Annunziata M, Levato L, Stagno F, Cavazzini F, Sgherza N, Giai V, Luciano L, Russo S, Musto P, Caocci G, Sorà F, Iuliano F, Lunghi F, Specchia G, Pane F, Ferrero D, Baccarani M, Saglio G. Observational study of chronic myeloid leukemia Italian patients who discontinued tyrosine kinase inhibitors in clinical practice. Haematologica. 2019;104:1589–1596. doi: 10.3324/haematol.2018.205054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernández-Boluda JC, Pereira A, Pastor-Galán I, Alvarez-Larrán A, Savchuk A, Puerta JM, Sánchez-Pina JM, Collado R, Díaz-González A, Angona A, Sagüés M, García-Gutiérrez V, Boqué C, Osorio S, Vallansot R, Palomera L, Mendizábal A, Casado LF, Pérez-Encinas M, Pérez-López R, Ferrer-Marín F, Sánchez-Guijo F, García C, Heras NL, López-Lorenzo JL, Cervantes F, Steegmann JL, Grupo Español de Leucemia Mieloide Crónica (GELMC) Feasibility of treatment discontinuation in chronic myeloid leukemia in clinical practice: results from a nationwide series of 236 patients. Blood Cancer J. 2018;8:91. doi: 10.1038/s41408-018-0125-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mori S, Vagge E, le Coutre P, Abruzzese E, Martino B, Pungolino E, Elena C, Pierri I, Assouline S, D'Emilio A, Gozzini A, Giraldo P, Stagno F, Iurlo A, Luciani M, De Riso G, Redaelli S, Kim DW, Pirola A, Mezzatesta C, Petroccione A, Lodolo D'Oria A, Crivori P, Piazza R, Gambacorti-Passerini C. Age and dPCR can predict relapse in CML patients who discontinued imatinib: the ISAV study. Am J Hematol. 2015;90:910–914. doi: 10.1002/ajh.24120. [DOI] [PubMed] [Google Scholar]
- 20.Lee SE, Choi SY, Song HY, Kim SH, Choi MY, Park JS, Kim HJ, Kim SH, Zang DY, Oh S, Kim H, Do YR, Kwak JY, Kim JA, Kim DY, Mun YC, Lee WS, Chang MH, Park J, Kwon JH, Kim DW. Imatinib withdrawal syndrome and longer duration of imatinib have a close association with a lower molecular relapse after treatment discontinuation: the KID study. Haematologica. 2016;101:717–723. doi: 10.3324/haematol.2015.139899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Legros L, Nicolini FE, Etienne G, Rousselot P, Rea D, Giraudier S, Guerci-Bresler A, Huguet F, Gardembas M, Escoffre M, Ianotto JC, Noël MP, Varet BR, Pagliardini T, Touitou I, Morisset S, Mahon FX, French Intergroup for Chronic Myeloid Leukemias Second tyrosine kinase inhibitor discontinuation attempt in patients with chronic myeloid leukemia. Cancer. 2017;123:4403–4410. doi: 10.1002/cncr.30885. [DOI] [PubMed] [Google Scholar]
- 22.Mahon F-X, Boquimpani C, Kim D-W, Benyamini N, Clementino NCD, Shuvaev V, Ailawadhi S, Lipton JH, Turkina AG, De Paz R, Moiraghi B, Nicolini FE, Dengler J, Sacha T, Takahashi N, Fellague-Chebra R, Acharya S, Wong S, Jin Y, Hughes TP. Treatment-free remission after second-line nilotinib treatment in patients with chronic myeloid leukemia in chronic phase: results from a single-group, phase 2, open-label study. Ann Intern Med. 2018;168:461–470. doi: 10.7326/M17-1094. [DOI] [PubMed] [Google Scholar]
- 23.Shah NP, Paquette R, Müller MC, Saussele S, Garcìa-Gutiérrez V, Jiménez-Velasco A, Nicolini F-E, Mauro MJ, Mahon F-X, Rea D, Martin-Regueira P, Subar M, Li L, Lipton JH. Treatment-free remission in patients with chronic phase chronic myeloid leukemia and in stable deep molecular response to dasatinib - the Dasfree study. Blood. 2016;128:1895. [Google Scholar]
- 24.Okada M, Imagawa J, Tanaka H, Nakamae H, Hino M, Murai K, Ishida Y, Kumagai T, Sato S, Ohashi K, Sakamaki H, Wakita H, Uoshima N, Nakagawa Y, Minami Y, Ogasawara M, Takeoka T, Akasaka H, Utsumi T, Uike N, Sato T, Ando S, Usuki K, Mizuta S, Hashino S, Nomura T, Shikami M, Fukutani H, Ohe Y, Kosugi H, Shibayama H, Maeda Y, Fukushima T, Yamazaki H, Tsubaki K, Kukita T, Adachi Y, Nataduka T, Sakoda H, Yokoyama H, Okamoto T, Shirasugi Y, Onishi Y, Nohgawa M, Yoshihara S, Morita S, Sakamoto J, Kimura S, DADI Trial Group, Japan Final 3-year results of the dasatinib discontinuation trial in patients with chronic myeloid leukemia who received dasatinib as a second-line treatment. Clin Lymphoma Myeloma Leuk. 2018;18:353–360. doi: 10.1016/j.clml.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 25.Ross DM, Masszi T, Gómez Casares MT, Hellmann A, Stentoft J, Conneally E, Garcia-Gutierrez V, Gattermann N, le Coutre PD, Martino B, Saussele S, Giles FJ, Radich JP, Saglio G, Deng W, Krunic N, Bédoucha V, Gopalakrishna P, Hochhaus A. Durable treatment-free remission in patients with chronic myeloid leukemia in chronic phase following frontline nilotinib: 96-week update of the ENESTfreedom study. J Cancer Res Clin Oncol. 2018;144:945–954. doi: 10.1007/s00432-018-2604-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Réa D, Rosti G, Cross NCP, Hellmann A, Niederwieser D, Pungolino E, Falzetti F, Pregno P, Orlandi EM, Almeida A, Illes A, Sagues M, Haenig J, Supekar S, Shah S, Saglio G, Steegmann JL, Baccarani M. ENESTPath: a phase 3 study to assess the effect of nilotinib treatment duration on treatment-free remission in patients with chronic myeloid leukemia in chronic phase previously treated with imatinib: 24-month analysis of the first 300 patients in the induction/consolidation phase. Presented at the American Society of Hematology Annual Meeting, 2016.
- 27.Ritchie EK, Catchatourian R, Klisovic RB, Pinilla-Ibarz J, Deininger MW, Erba HP, Radich JP, Savona MR, Dautaj I, Purkayastha D, Habucky K, Mauro MJ. Results from ENESTgoal: a phase 2 study of treatment-free remission in patients with chronic myeloid leukemia in chronic phase who switched from imatinib to nilotinib. Presented at the American Society of Hematology Annual Meeting, 2017.
- 28.Burchert A, Saussele S, Eigendorff E, Müller MC, Sohlbach K, Inselmann S, Schütz C, Metzelder SK, Ziermann J, Kostrewa P, Hoffmann J, Hehlmann R, Neubauer A, Hochhaus A. Interferon alpha 2 maintenance therapy may enable high rates of treatment discontinuation in chronic myeloid leukemia. Leukemia. 2015;29:1331–1335. doi: 10.1038/leu.2015.45. [DOI] [PubMed] [Google Scholar]
- 29.Ross DM, Pagani IS, Irani YD, Clarson J, Leclercq T, Dang P, et al. Lenalidomide maintenance treatment after imatinib discontinuation: results of a phase 1 clinical trial in chronic myeloid leukaemia. Br J Haematol. 2019. 10.1111/bjh.15894[Epub ahead of print]. [DOI] [PubMed]
- 30.Faber E, Divoká M, Skoumalová I, Novák M, Marešová I, Mičová K, Friedecký D, Adam T, Jarošová M, Indrák K. A lower dosage of imatinib is sufficient to maintain undetectable disease in patients with chronic myeloid leukemia with long-term low-grade toxicity of the treatment. Leuk Lymphoma. 2016;57:370–375. doi: 10.3109/10428194.2015.1056184. [DOI] [PubMed] [Google Scholar]
- 31.Fassoni AC, Baldow C, Roeder I, Glauche I. Reduced tyrosine kinase inhibitor dose is predicted to be as effective as standard dose in chronic myeloid leukemia: a simulation study based on phase III trial data. Haematologica. 2018;103:1825–1834. doi: 10.3324/haematol.2018.194522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kinstrie R, Horne GA, Morrison H, Irvine D, Munje C, Castañeda EG, Moka HA, Dunn K, Cassels JE, Parry N, Scott MT, Clark RE, Holyoake TL, Wheadon H, Copland M. CD93 is selectively upregulated on chronic myeloid leukemia stem cells and identifies a quiescent population which persists after tyrosine kinase inhibitor therapy. Submitted for publication.
- 33.Steegmann JL, Cervantes F, le Coutre P, Porkka K, Saglio G. Off-target effects of BCR-ABL1 inhibitors and their potential long-term implications in patients with chronic myeloid leukemia. Leuk Lymphoma. 2012;53:2351–2361. doi: 10.3109/10428194.2012.695779. [DOI] [PubMed] [Google Scholar]
- 34.Rojas JM, Knight K, Watmough S, Bell J, Wang L, Callaghan T, Clark RE. BCR-ABL peptide vaccination in healthy subjects: immunological responses are equivalent to those in chronic myeloid leukaemia patients. Leukaemia Research. 2011;35:369–372. doi: 10.1016/j.leukres.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 35.Austin GM, Knight K, Bell J, Carter A, Heartin E, Watson D, Foroni L, Christmas SE, Polydoros F, Clark RE. The effect on lymphocyte subsets of decreasing/stopping tyrosine kinase inhibitor therapy in chronic myeloid leukaemia: data from the DESTINY trial. Br J Haematol. 2019;185:791–793. doi: 10.1111/bjh.15629. [DOI] [PubMed] [Google Scholar]
- 36.Ilander M, Olsson-Strömberg U, Schlums H, Guilhot J, Brück O, Lähteenmäki H, Kasanen T, Koskenvesa P, Söderlund S, Höglund M, Markevärn B, Själander A, Lotfi K, Dreimane A, Lübking A, Holm E, Björeman M, Lehmann S, Stenke L, Ohm L, Gedde-Dahl T, Majeed W, Ehrencrona H, Koskela S, Saussele S, Mahon FX, Porkka K, Hjorth-Hansen H, Bryceson YT, Richter J, Mustjoki S. Increased proportion of mature NK cells is associated with successful imatinib discontinuation in chronic myeloid leukemia. Leukemia. 2017;31:1108–1116. doi: 10.1038/leu.2016.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schütz C, Inselmann S, Saussele S, Dietz CT, Müller MC, Eigendorff E, Brendel CA, Metzelder SK, Brümmendorf TH, Waller C, Dengler J, Goebeler ME, Herbst R, Freunek G, Hanzel S, Illmer T, Wang Y, Lange T, Finkernagel F, Hehlmann R, Huber M, Neubauer A, Hochhaus A, Guilhot J, Mahon FX, Pfirrmann M, Burchert A. Expression of the CTLA-4 ligand CD86 on plasmacytoid dendritic cells (pDC) predicts risk of disease recurrence after treatment discontinuation in CML. Leukemia. 2017;31:829–836. doi: 10.1038/leu.2017.9. [DOI] [PubMed] [Google Scholar]
- 38.Réa D, Henry G, Khaznadar Z, Etienne G, Guilhot F, Nicolini F, Guilhot J, Rousselot P, Huguet F, Legros L, Gardembas M, Dubruille V, Guerci-Bresler A, Charbonnier A, Maloisel F, Ianotto JC, Villemagne B, Mahon FX, Moins-Teisserenc H, Dulphy N, Toubert A. Natural killer-cell counts are associated with molecular relapse-free survival after imatinib discontinuation in chronic myeloid leukemia: the IMMUNOSTIM study. Haematologica. 2017;102:1368–1377. doi: 10.3324/haematol.2017.165001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Radich JP, Deininger M, Abboud CN, Altman JK, Berman E, Bhatia R, Bhatnagar B, Curtin P, DeAngelo DJ, Gotlib J, Hobbs G, Jagasia M, Kantarjian HM, Maness L, Metheny L, Moore JO, Pallera A, Pancari P, Patnaik M, Purev E, Rose MG, Shah NP, Smith BD, Snyder DS, Sweet KL, Talpaz M, Thompson J, Yang DT, Gregory KM, Sundar H. Chronic myeloid leukemia, version 1.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer NetwJ Natl Compr Canc Netw. 2018;16:1108–1135. doi: 10.6004/jnccn.2018.0071. [DOI] [PubMed] [Google Scholar]
- 40.Hochhaus A, Saussele S, Rosti G, Mahon F-X, Janssen JJWM, Hjorth-Hansen H, Richter J, Buske C, on behalf of the ESMO Guidelines Committee. Chronic myeloid leukaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncol. 2017; 28, suppl 4: iv41-iv51. [Corrigendum at Annals of Oncol. 2016; 29 supplement 4: iv261]. [DOI] [PubMed]
- 41.Goh HG, Kim YJ, Kim DW, Kim HJ, Kim SH, Jang SE, Lee J, Kim D, Kim WS, Park SH, Kweon IY. Previous best responses can be re-achieved by resumption after imatinib discontinuation in patients with chronic myeloid leukemia: implication for intermittent imatinib therapy. Leuk Lymphoma. 2009;50:944–951. doi: 10.1080/10428190902926973. [DOI] [PubMed] [Google Scholar]
- 42.Benjamini O, Kantarjian H, Rios MB, Jabbour E, O'Brien S, Jain P, Cardenas-Turanzas M, Faderl S, Garcia-Manero G, Ravandi F, Borthakur G, Quintas-Cardama A, Cortes J. Patient-driven discontinuation of tyrosine kinase inhibitors: single institution experience. Leuk Lymphoma. 2014;55:2879–2886. doi: 10.3109/10428194.2013.831092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koskenvesa P, Kreutzman A, Rohon P, Pihlman M, Vakkila E, Räsänen A, Vapaatalo M, Remes K, Lundán T, Hjorth-Hansen H, Vakkila J, Simonsson B, Mustjoki S, Porkka K. Imatinib and pegylated IFN-α2b discontinuation in first-line chronic myeloid leukemia patients following a major molecular response. Eur J Haematol. 2014;92:413–420. doi: 10.1111/ejh.12258. [DOI] [PubMed] [Google Scholar]
- 44.Gottschalk A., Glauche I., Clark R., Röder I. INDIVIDUAL RESPONSE OF CML PATIENTS TO TKI DOSE REDUCTION CAN RELIABLY IDENTIFY PATIENTS WITH HIGH RISK OF MOLECULAR RELAPSE AFTER TREATMENT CESSATION. HemaSphere. 2019;3:530. [Google Scholar]
- 45.Claudiani S, Apperley JF, Gale RP, Clark R, Szydlo R, Deplano S, Palanicawandar R, Khorashad J, Foroni L, Milojkovic D. E14a2 BCR-ABL1 transcript is associated with a higher rate of treatment-free remission in individuals with chronic myeloid leukemia after stopping tyrosine kinase inhibitor therapy. Haematologica. 2017;102:e297–e299. doi: 10.3324/haematol.2017.168740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D'Adda M, Farina M, Schieppati F, Borlenghi E, Bottelli C, Cerqui E, Ferrari S, Gramegna D, Pagani C, Passi A, Maifredi A, Tucci A, Capucci MA, Ruggeri G, Rossi G. The e13a2 BCR-ABL transcript negatively affects sustained deep molecular response and the achievement of treatment-free remission in patients with chronic myeloid leukemia who receive tyrosine kinase inhibitors. Cancer. 2019;125:1674–1682. doi: 10.1002/cncr.31977. [DOI] [PubMed] [Google Scholar]
- 47.Alikian M, Gale RP, Apperley JF, Foroni L. Molecular techniques for the personalised management of patients with chronic myeloid leukaemia. Biomol Detect Quantif. 2017;11:4–20. doi: 10.1016/j.bdq.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richter J, Söderlund S, Lübking A, Dreimane A, Lotfi K, Markevärn B, Själander A, Saussele S, Olsson-Strömberg U, Stenke L. Musculoskeletal pain in patients with chronic myeloid leukemia after discontinuation of imatinib: a tyrosine kinase inhibitor withdrawal syndrome? J Clin Oncol. 2014;32:2821–2823. doi: 10.1200/JCO.2014.55.6910. [DOI] [PubMed] [Google Scholar]
- 49.Rousselot P, Charbonnier A, Cony-Makhoul P, Agape P, Nicolini FE, Varet B, Gardembas M, Etienne G, Réa D, Roy L, Escoffre-Barbe M, Guerci-Bresler A, Tulliez M, Prost S, Spentchian M, Cayuela JM, Reiffers J, Chomel JC, Turhan A, Guilhot J, Guilhot F, Mahon FX. Reply to J. Richter et al. J Clin Oncol. 2014;32:2823–2825. doi: 10.1200/JCO.2014.56.3858. [DOI] [PubMed] [Google Scholar]
- 50.Berger Marc G., Pereira Bruno, Rousselot Philippe, Cony‐Makhoul Pascale, Gardembas Martine, Legros Laurence, Escoffre‐Barbe Martine, Nicolini Franck‐Emmanuel, Saugues Sandrine, Lambert Céline, Réa Delphine, Guerci‐Bresler Agnès, Giraudier Stéphane, Guilhot Joëlle, Saussele Susanne, Mahon François‐Xavier. Longer treatment duration and history of osteoarticular symptoms predispose to tyrosine kinase inhibitor withdrawal syndrome. British Journal of Haematology. 2019;187(3):337–346. doi: 10.1111/bjh.16083. [DOI] [PubMed] [Google Scholar]
- 51.• Kota V, Atallah E. Musculoskeletal pain in patients with chronic myeloid leukemia after tyrosine kinase inhibitor therapy cessation. Clin Lymphoma Myeloma Leuk. 2019. A recent review of the TKI withdrawal syndrome. [DOI] [PubMed]
- 52.Katagiri S, Tauchi T, Ando K, Okabe S, Gotoh M, Ohyashiki K. Low body weight and body mass index may be associated with musculoskeletal pain following imatinib discontinuation in chronic myeloid leukemia. Leuk Res Rep. 2017;7:33–35. doi: 10.1016/j.lrr.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Diab M, Schiffer CA. The spectrum of musculoskeletal symptoms in patients with chronic myeloid leukemia after stopping tyrosine kinase inhibitors. Leuk Res. 2019;79:1–2. doi: 10.1016/j.leukres.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 54.Shanmuganathan N, Braley JA, Yong ASM, Hiwase DK, Yeung DT, Ross DM, Hughes TP, Branford S. Modelling the safe minimum frequency of molecular monitoring for CML patients attempting treatment-free remission. Blood. 2019;134:85–89. doi: 10.1182/blood.2019000120. [DOI] [PubMed] [Google Scholar]
- 55.Zang DY, Lee WS, Mun Y-C, Do YR, Oh S, Lee S-E, Choi SY, Kim D-W. Long-term follow-up after treatment discontinuation in patients with chronic myeloid leukemia: the Korean imatinib discontinuation (KID) study. Blood. 2018;132:4252. [Google Scholar]
- 56.Villemagne Sanchez LA, O'Callaghan C, Gough K, Hall K, Kashima Y, Seymour JF, Schofield P, Ross DM. Patient perceptions of treatment-free remission in chronic myeloid leukemia. Leuk Lymphoma. 2018;59:406–415. doi: 10.1080/10428194.2017.1337114. [DOI] [PubMed] [Google Scholar]
- 57.Sogawa R, Kimura S, Yakabe R, Mizokami Y, Tasaki M, Sueoka-Aragane N, Narisawa Y, Kimura S. Anxiety and depression associated with tyrosine kinase inhibitor discontinuation in patients with chronic myeloid leukemia. Int J Clin Oncol. 2018;23:974–979. doi: 10.1007/s10147-018-1275-6. [DOI] [PubMed] [Google Scholar]
- 58.Jiang Q, Yu L, Gale RP. Patients’ and hematologists’ concerns regarding tyrosine kinase-inhibitor therapy in chronic myeloid leukemia. J Cancer Res Clin Oncol. 2018;144:735–741. doi: 10.1007/s00432-018-2594-8. [DOI] [PMC free article] [PubMed] [Google Scholar]